Reduced Thermal Conductivity in Nanostructured AgSbTe2 Thermoelectric Material, Obtained by Arc-Melting
Abstract
1. Introduction
2. Materials and Methods
3. Results & Discussion
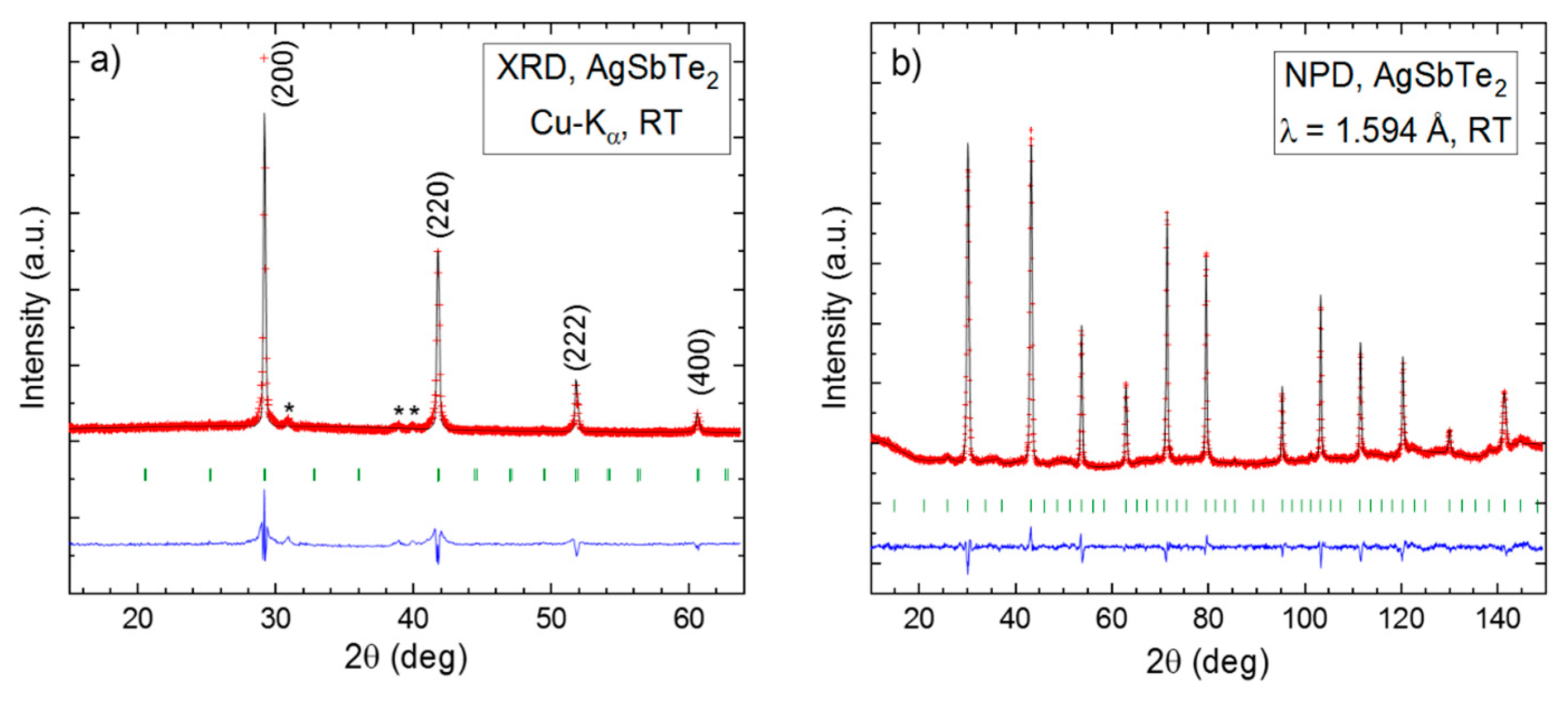
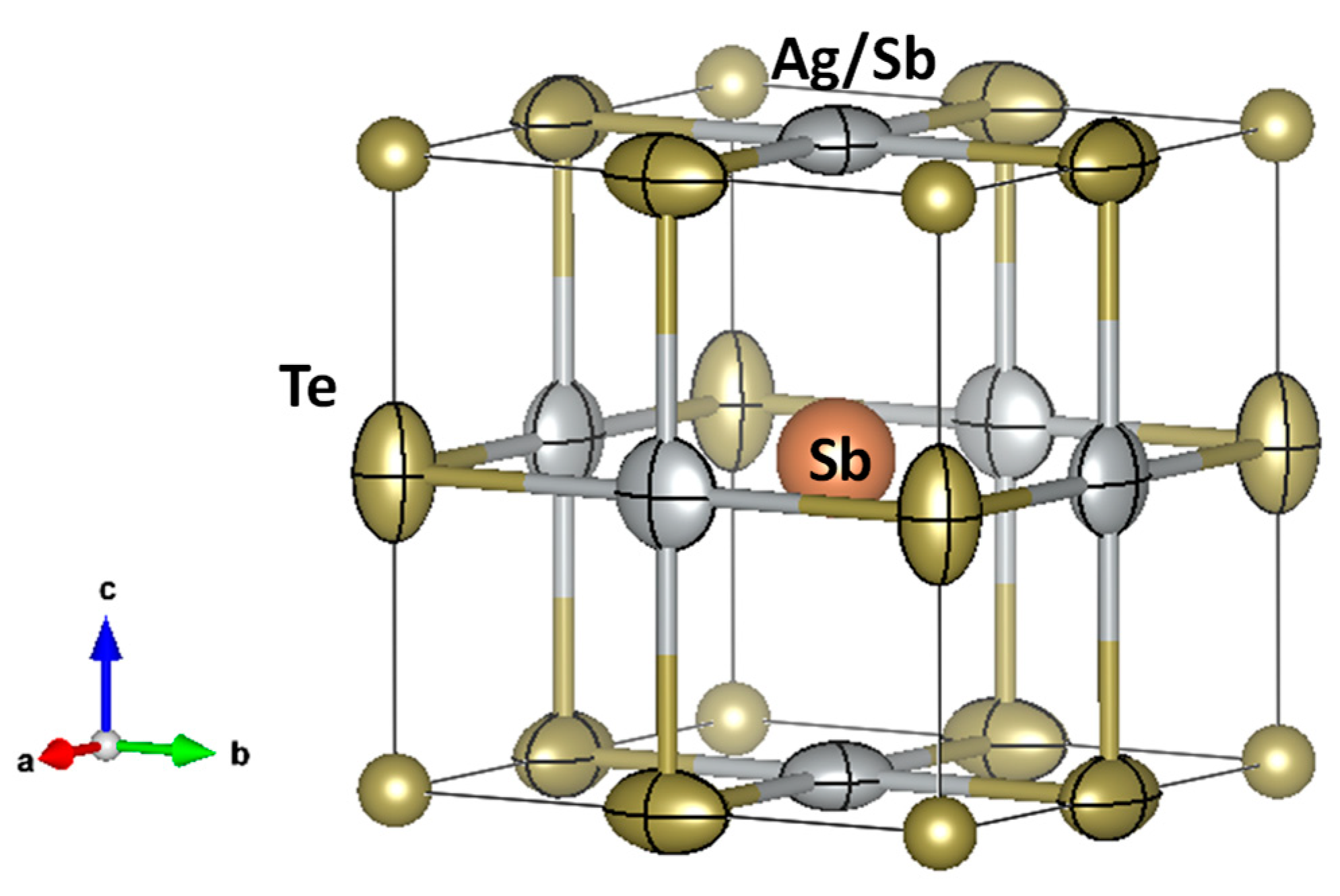
3.1. Crystallographic Analysis by the XRD and NPD
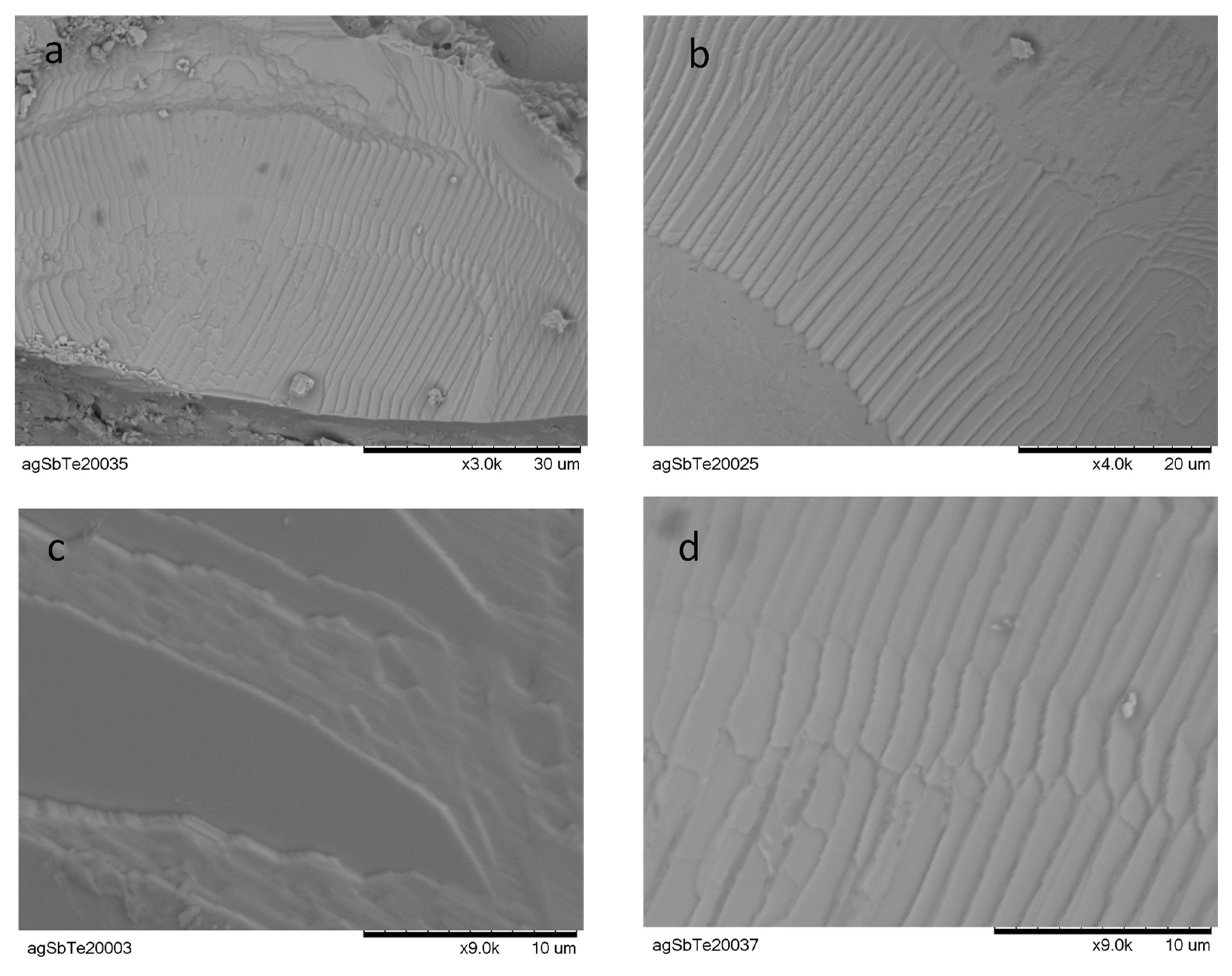
3.2. Scanning Electron Microscopy
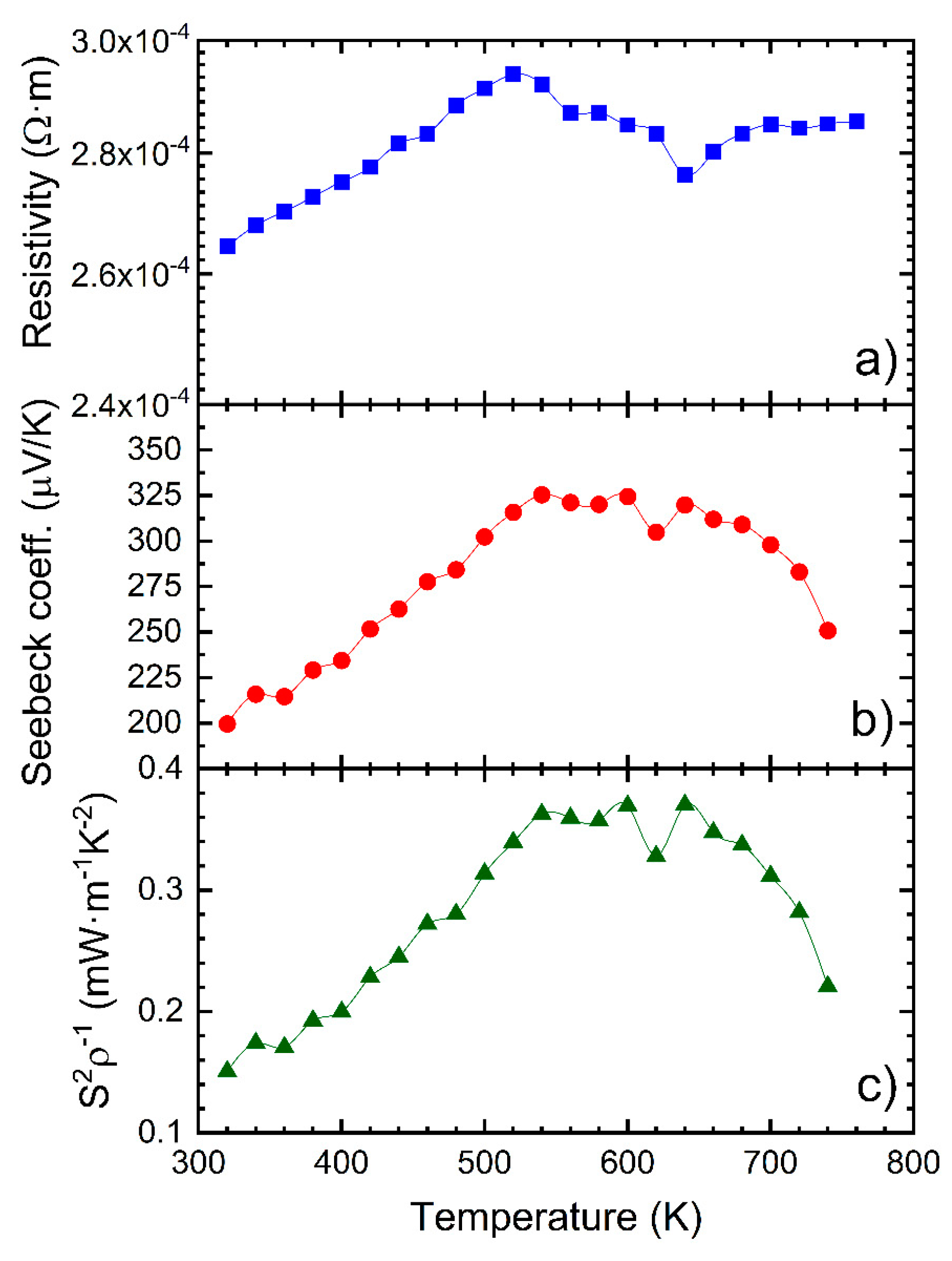
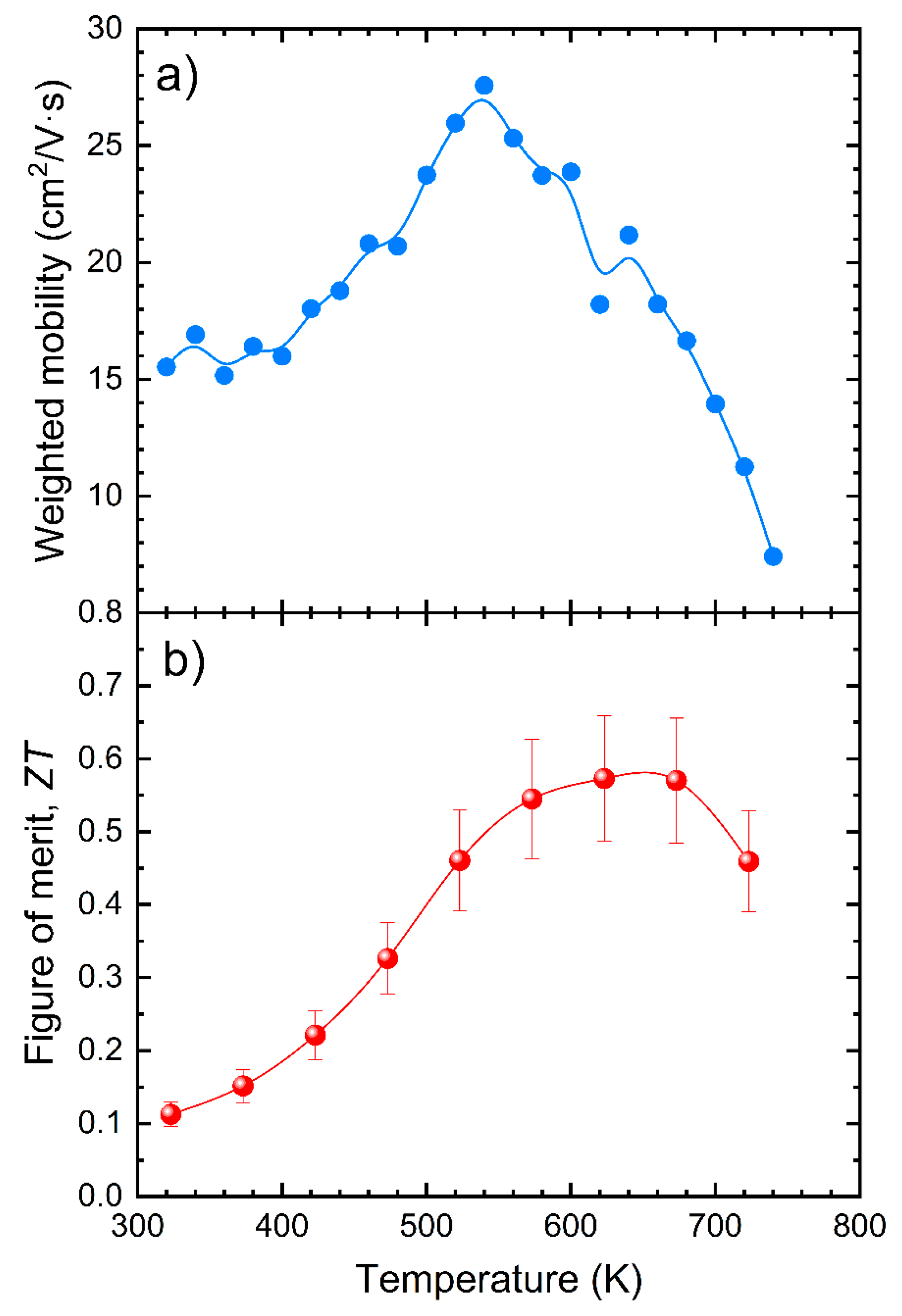
3.3. Thermoelectric Properties
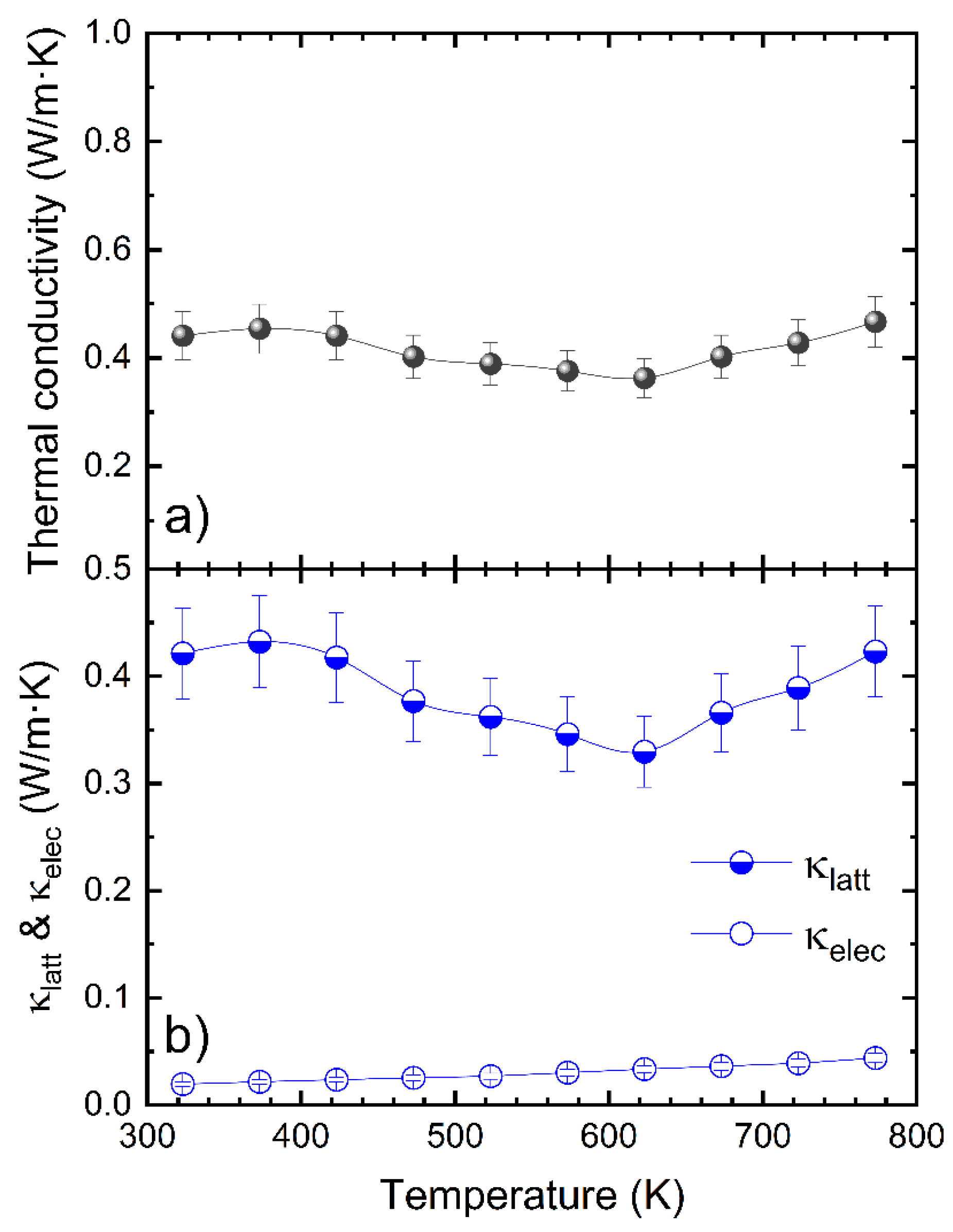
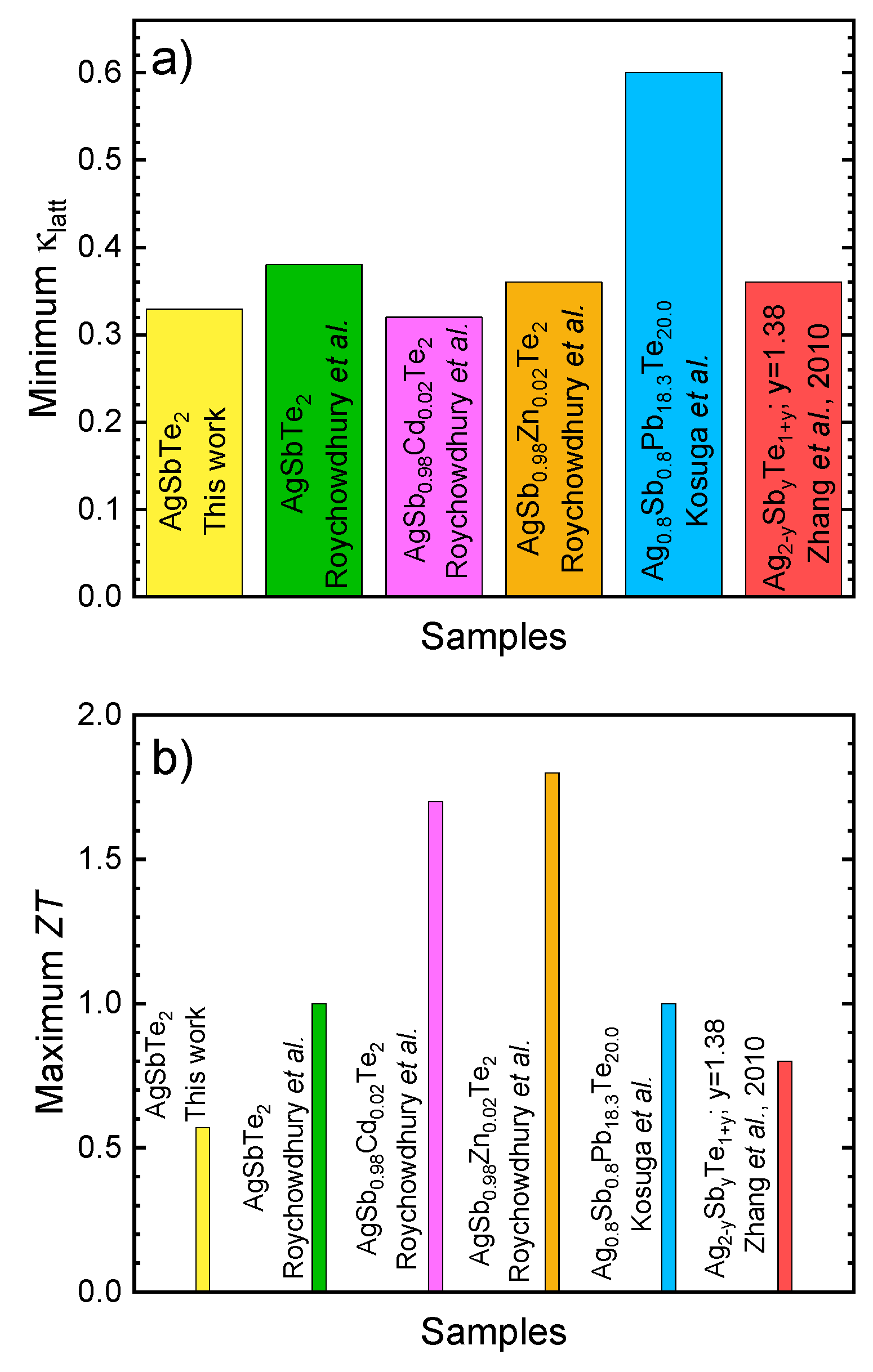
3.4. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, J.; Cui, B.; Guo, F.; Liu, Z.; Zhu, Y.; Guo, M.; Sun, Y.; Zhang, Q.; Zhang, Y.; et al. High-Performance Lead-Free Cubic GeTe-Based Thermoelectric Alloy. Cell Rep. Phys. Sci. 2022, 3, 100902. [Google Scholar] [CrossRef]
- Tan, G.; Shi, F.; Hao, S.; Chi, H.; Zhao, L.-D.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Codoping in SnTe: Enhancement of Thermoelectric Performance through Synergy of Resonance Levels and Band Convergence. J. Am. Chem. Soc. 2015, 137, 5100–5112. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, W.; Chen, Z.; Shen, J.; Ge, B.; Pei, Y. Tellurium as a High-Performance Elemental Thermoelectric. Nat. Commun. 2016, 7, 10287. [Google Scholar] [CrossRef] [PubMed]
- LaLonde, A.D.; Pei, Y.; Wang, H.; Jeffrey Snyder, G. Lead Telluride Alloy Thermoelectrics. Mater. Today 2011, 14, 526–532. [Google Scholar] [CrossRef]
- Luo, Z.-Z.; Cai, S.; Hao, S.; Bailey, T.P.; Luo, Y.; Luo, W.; Yu, Y.; Uher, C.; Wolverton, C.; Dravid, V.P.; et al. Extraordinary Role of Zn in Enhancing Thermoelectric Performance of Ga-Doped n-Type PbTe. Energy Environ. Sci. 2022, 15, 368–375. [Google Scholar] [CrossRef]
- Brod, M.K.; Toriyama, M.Y.; Snyder, G.J. Orbital Chemistry That Leads to High Valley Degeneracy in PbTe. Chem. Mater. 2020, 32, 9771–9779. [Google Scholar] [CrossRef]
- Tan, G.; Zhang, X.; Hao, S.; Chi, H.; Bailey, T.P.; Su, X.; Uher, C.; Dravid, V.P.; Wolverton, C.; Kanatzidis, M.G. Enhanced Density-of-States Effective Mass and Strained Endotaxial Nanostructures in Sb-Doped Pb0.97Cd0.03 Te Thermoelectric Alloys. ACS Appl. Mater. Interfaces 2019, 11, 9197–9204. [Google Scholar] [CrossRef]
- Pei, Y.; Gibbs, Z.M.; Gloskovskii, A.; Balke, B.; Zeier, W.G.; Snyder, G.J. Optimum Carrier Concentration in N-Type PbTe Thermoelectrics. Adv. Energy Mater. 2014, 4, 1400486. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of Electronic Bands for High Performance Bulk Thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, Q.; Wang, X.; Sun, J.; Zhu, Q.; Dahal, K.; Lin, X.; Cao, F.; Zhou, J.; Chen, S.; et al. Deep Defect Level Engineering: A Strategy of Optimizing the Carrier Concentration for High Thermoelectric Performance. Energy Environ. Sci. 2018, 11, 933–940. [Google Scholar] [CrossRef]
- Wang, X.-K.; Veremchuk, I.; Bobnar, M.; Zhao, J.-T.; Grin, Y. Solid Solution Pb1−xEuxTe: Constitution and Thermoelectric Behavior. Inorg. Chem. Front. 2016, 3, 1152–1159. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Nan, P.; Xiong, F.; Lin, S.; Zhang, X.; Chen, Y.; Chen, L.; Ge, B.; Pei, Y. Lattice Strain Advances Thermoelectrics. Joule 2019, 3, 1276–1288. [Google Scholar] [CrossRef]
- Luo, Z.-Z.; Cai, S.; Hao, S.; Bailey, T.P.; Su, X.; Spanopoulos, I.; Hadar, I.; Tan, G.; Luo, Y.; Xu, J.; et al. High Figure of Merit in Gallium-Doped Nanostructured n-Type PbTe-xGeTe with Midgap States. J. Am. Chem. Soc. 2019, 141, 16169–16177. [Google Scholar] [CrossRef]
- Dutta, M.; Biswas, R.K.; Pati, S.K.; Biswas, K. Discordant Gd and Electronic Band Flattening Synergistically Induce High Thermoelectric Performance in N-Type PbTe. ACS Energy Lett. 2021, 6, 1625–1632. [Google Scholar] [CrossRef]
- Zhao, L.D.; Wu, H.J.; Hao, S.Q.; Wu, C.I.; Zhou, X.Y.; Biswas, K.; He, J.Q.; Hogan, T.P.; Uher, C.; Wolverton, C.; et al. All-Scale Hierarchical Thermoelectrics: MgTe in PbTe Facilitates Valence Band Convergence and Suppresses Bipolar Thermal Transport for High Performance. Energy Enviorn. Sci. 2013, 6, 3346–3355. [Google Scholar] [CrossRef]
- Pei, Y.; Lensch-Falk, J.; Toberer, E.S.; Medlin, D.L.; Snyder, G.J. High Thermoelectric Performance in PbTe Due to Large Nanoscale Ag2Te Precipitates and La Doping. Adv. Funct. Mater. 2011, 21, 241–249. [Google Scholar] [CrossRef]
- Levin, E.M.; Cook, B.A.; Ahn, K.; Kanatzidis, M.G.; Schmidt-Rohr, K. Electronic Inhomogeneity and Ag:Sb Imbalance of Ag1-y Pb18Sb1+zTe20 High-Performance Thermoelectrics Elucidated by 125Te and 207Pb NMR. Phys. Rev. B 2009, 80, 115211. [Google Scholar] [CrossRef]
- Perlt, S.; Höche, T.; Dadda, J.; Müller, E.; Bauer Pereira, P.; Hermann, R.; Sarahan, M.; Pippel, E.; Brydson, R. Microstructure Analyses and Thermoelectric Properties of Ag1−xPb18Sb1+yTe20. J. Solid State Chem. 2012, 193, 58–63. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P. The Anharmonicity Blacksmith. Nat. Phys. 2015, 11, 990–991. [Google Scholar] [CrossRef]
- Chang, C.; Zhao, L.-D. Anharmoncity and Low Thermal Conductivity in Thermoelectrics. Mater. Today Phys. 2018, 4, 50–57. [Google Scholar] [CrossRef]
- Nielsen, M.D.; Ozolins, V.; Heremans, J.P. Lone Pair Electrons Minimize Lattice Thermal Conductivity. Energy Environ. Sci. 2013, 6, 570–578. [Google Scholar] [CrossRef]
- Wernick, J.H.; Benson, K.E. New Semiconducting Ternary Compounds. J. Phys. Chem. Solids 1957, 3, 157–159. [Google Scholar] [CrossRef]
- Hockings, E.F. The Thermal Conductivity of Silver Antimony Telluride. J. Phys. Chem. Solids 1959, 10, 341–342. [Google Scholar] [CrossRef]
- Irie, T.; Takahama, T.; Ono, T. The Thermoelectric Properties of AgSbTe2–AgBiTe2, AgSbTe2–PbTe and–SnTe Systems. Jpn. J. Appl. Phys. 1963, 2, 72–82. [Google Scholar] [CrossRef]
- Ghosh, T.; Roychowdhury, S.; Dutta, M.; Biswas, K. High-Performance Thermoelectric Energy Conversion: A Tale of Atomic Ordering in AgSbTe2. ACS Energy Lett. 2021, 6, 2825–2837. [Google Scholar] [CrossRef]
- Jang, H.; Toriyama, M.Y.; Abbey, S.; Frimpong, B.; Male, J.P.; Snyder, G.J.; Jung, Y.S.; Oh, M. Suppressing Charged Cation Antisites via Se Vapor Annealing Enables P-Type Dopability in AgBiSe2–SnSe Thermoelectrics. Adv. Mater. 2022, 34, 2204132. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Ghosh, T.; Arora, R.; Samanta, M.; Xie, L.; Singh, N.K.; Soni, A.; He, J.; Waghmare, U.V.; Biswas, K. Enhanced Atomic Ordering Leads to High Thermoelectric Performance in AgSbTe2. Science 2021, 371, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ibáñez, M. Tidying up the Mess. Science 2021, 371, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Korkosz, R.J.; Chasapis, T.C.; Lo, S.; Doak, J.W.; Kim, Y.J.; Wu, C.-I.; Hatzikraniotis, E.; Hogan, T.P.; Seidman, D.N.; Wolverton, C.; et al. High ZT in p-Type (PbTe)1–2x(PbSe)x(PbS)x Thermoelectric Materials. J. Am. Chem. Soc. 2014, 136, 3225–3237. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.A.; Kramer, M.J.; Harringa, J.L.; Han, M.-K.; Chung, D.-Y.; Kanatzidis, M.G. Analysis of Nanostructuring in High Figure-of-Merit Ag1−xPbmSbTe2+m Thermoelectric Materials. Adv. Funct. Mater. 2009, 19, 1254–1259. [Google Scholar] [CrossRef]
- Kanatzidis, M.G. Nanostructured Thermoelectrics: The New Paradigm? Chem. Mater. 2010, 22, 648–659. [Google Scholar] [CrossRef]
- Toprak, M.S.; Stiewe, C.; Platzek, D.; Williams, S.; Bertini, L.; Müller, E.; Gatti, C.; Zhang, Y.; Rowe, M.; Muhammed, M. The Impact of Nanostructuring on the Thermal Conductivity of Thermoelectric CoSb3. Adv. Funct. Mater. 2004, 14, 1189–1196. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, L.-D.; Shi, F.; Doak, J.W.; Lo, S.-H.; Sun, H.; Wolverton, C.; Dravid, V.P.; Uher, C.; Kanatzidis, M.G. High Thermoelectric Performance of P-Type SnTe via a Synergistic Band Engineering and Nanostructuring Approach. J. Am. Chem. Soc. 2014, 136, 7006–7017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Carrete, J.; Meng, Q.-L.; Huang, Z.; Mingo, N.; Jiang, P.; Bao, X. Independently Tuning the Power Factor and Thermal Conductivity of SnSe via Ag2S Addition and Nanostructuring. J. Mater. Chem. A Mater. 2018, 6, 7959–7966. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano-Sánchez, F.; Gharsallah, M.; Carrascoso, F.; Bermúdez, J.; Dura, O.J.; Mompean, F.J.; Biskup, N.; Meléndez, J.J.; Martínez, J.L.; et al. Evidence of Nanostructuring and Reduced Thermal Conductivity in N-Type Sb-Alloyed SnSe Thermoelectric Polycrystals. J. Appl. Phys. 2019, 126, 045105. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, S.; Hua, X.; Chen, H.; Liang, Q.; Du, C.; Zheng, Y.; Shen, J.; Xu, J.; Wolverton, C.; et al. High Thermoelectric Performance in Polycrystalline SnSe Via Dual-Doping with Ag/Na and Nanostructuring With Ag8SnSe6. Adv. Energy Mater. 2019, 9, 1803072. [Google Scholar] [CrossRef]
- Perumal, S.; Bellare, P.; Shenoy, U.S.; Waghmare, U.V.; Biswas, K. Low Thermal Conductivity and High Thermoelectric Performance in Sb and Bi Codoped GeTe: Complementary Effect of Band Convergence and Nanostructuring. Chem. Mater. 2017, 29, 10426–10435. [Google Scholar] [CrossRef]
- Vaqueiro, P.; Powell, A.V. Recent Developments in Nanostructured Materials for High-Performance Thermoelectrics. J. Mater. Chem. 2010, 20, 9577. [Google Scholar] [CrossRef]
- Li, J.-F.; Liu, W.-S.; Zhao, L.-D.; Zhou, M. High-Performance Nanostructured Thermoelectric Materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Ma, J.; Delaire, O.; May, A.F.; Carlton, C.E.; McGuire, M.A.; VanBebber, L.H.; Abernathy, D.L.; Ehlers, G.; Hong, T.; Huq, A.; et al. Glass-like Phonon Scattering from a Spontaneous Nanostructure in AgSbTe2. Nat. Nanotechnol. 2013, 8, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Gainza, J.; Serrano-Sánchez, F.; Biskup, N.; Nemes, N.M.; Martínez, J.L.; Fernández-Díaz, M.T.; Alonso, J.A. Influence of Nanostructuration on PbTe Alloys Synthesized by Arc-Melting. Materials 2019, 12, 3783. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Funes, M.; Nemes, N.M.; Dura, O.J.; Martínez, J.L.; Prado-Gonjal, J.; Fernández-Díaz, M.T.; Alonso, J.A. Low Lattice Thermal Conductivity in Arc-Melted GeTe with Ge-Deficient Crystal Structure. Appl. Phys. Lett. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano-Sánchez, F.; Nemes, N.M.; Martínez, J.L.; Fernández-Díaz, M.T.; Alonso, J.A. Features of the High-Temperature Structural Evolution of GeTe Thermoelectric Probed by Neutron and Synchrotron Powder Diffraction. Metals 2019, 10, 48. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano-Sánchez, F.; Nemes, N.M.; Dura, O.J.; Martínez, J.L.; Alonso, J.A. Lower Temperature of the Structural Transition, and Thermoelectric Properties in Sn-Substituted GeTe. Mater. Today Proc. 2021, 44, 3450–3457. [Google Scholar] [CrossRef]
- Gharsallah, M.; Serrano-Sánchez, F.; Bermúdez, J.; Nemes, N.M.; Martínez, J.L.; Elhalouani, F.; Alonso, J.A. Nanostructured Bi2Te3 Prepared by a Straightforward Arc-Melting Method. Nanoscale Res. Lett. 2016, 11, 4–10. [Google Scholar] [CrossRef]
- Gharsallah, M.; Serrano-Sanchez, F.; Nemes, N.M.; Martinez, J.L.; Alonso, J.A. Influence of Doping and Nanostructuration on N-Type Bi2(Te0.8Se0.2)3 Alloys Synthesized by Arc Melting. Nanoscale Res. Lett. 2017, 12, 47. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Gharsallah, M.; Nemes, N.M.; Biskup, N.; Varela, M.; Martínez, J.L.; Fernández-Díaz, M.T.; Alonso, J.A. Enhanced Figure of Merit in Nanostructured (Bi, Sb)2Te3 with Optimized Composition, Prepared by a Straightforward Arc-Melting Procedure. Sci. Rep. 2017, 7, 6277. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Gharsallah, M.; Nemes, N.M.; Mompean, F.J.; Martínez, J.L.; Alonso, J.A. Record Seebeck Coefficient and Extremely Low Thermal Conductivity in Nanostructured SnSe. Appl. Phys. Lett. 2015, 106, 083902. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Nemes, N.M.; Dura, O.J.; Fernandez-Diaz, M.T.; Martínez, J.L.; Alonso, J.A. Structural Phase Transition in Polycrystalline SnSe: A Neutron Diffraction Study in Correlation with Thermoelectric Properties. J. Appl. Cryst. 2016, 49, 2138–2144. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano-Sánchez, F.; Rodrigues, J.E.F.S.; Huttel, Y.; Dura, O.J.; Koza, M.M.; Fernández-Díaz, M.T.; Meléndez, J.J.; Márkus, B.G.; Simon, F.; et al. High-Performance n-Type SnSe Thermoelectric Polycrystal Prepared by Arc-Melting. Cell Rep. Phys. Sci. 2020, 1, 100263. [Google Scholar] [CrossRef]
- Gharsallah, M.; Serrano-Sánchez, F.; Nemes, N.M.; Mompeán, F.J.; Martínez, J.L.; Fernández-Díaz, M.T.; Elhalouani, F.; Alonso, J.A. Giant Seebeck Effect in Ge-Doped SnSe. Sci. Rep. 2016, 6, 26774. [Google Scholar] [CrossRef]
- Gainza, J.; Moltó, S.; Serrano-Sánchez, F.; Dura, O.J.; Fernández-Díaz, M.T.; Biškup, N.; Martínez, J.L.; Alonso, J.A.; Nemes, N.M. SnSe:Kx Intermetallic Thermoelectric Polycrystals Prepared by Arc-Melting. J. Mater. Sci. 2022, 57, 8489–8503. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Gharsallah, M.; Nemes, N.M.; Mompeán, F.J.; Martínez, J.L.; Alonso, J.A. Facile Preparation of SnSe Derivatives in Nanostructured Polycrystalline Form by Arc-Melting Synthesis. Mater. Today Proc. 2018, 5, 10218–10226. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano-Sánchez, F.; Gharsallah, M.; Funes, M.; Carrascoso, F.; Nemes, N.M.; Dura, O.J.; Martínez, J.L.; Alonso, J.A. Nanostructured Thermoelectric Chalcogenides. In Bringing Thermoelectricity into Reality; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Medlin, D.L.; Snyder, G.J. Interfaces in Bulk Thermoelectric Materials. Curr. Opin. Colloid. Interface Sci. 2009, 14, 226–235. [Google Scholar] [CrossRef]
- Quarez, E.; Hsu, K.-F.; Pcionek, R.; Frangis, N.; Polychroniadis, E.K.; Kanatzidis, M.G. Nanostructuring, Compositional Fluctuations, and Atomic Ordering in the Thermoelectric Materials AgPbmSbTe2+m. The Myth of Solid Solutions. J. Am. Chem. Soc. 2005, 127, 9177–9190. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Panigrahi, R.; Perumal, S.; Biswas, K. Ultrahigh Thermoelectric Figure of Merit and Enhanced Mechanical Stability of p-type AgSb1−xZnxTe2. ACS Energy Lett. 2017, 2, 349–356. [Google Scholar] [CrossRef]
- Du, B.; Li, H.; Xu, J.; Tang, X.; Uher, C. Enhanced Figure-of-Merit in Se-Doped p-Type AgSbTe2 Thermoelectric Compound. Chem. Mater. 2010, 22, 5521–5527. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.-F.; Zou, M.; Sui, T. Synthesis and Transport Property of AgSbTe2 as a Promising Thermoelectric Compound. Appl. Phys. Lett. 2008, 93, 202106. [Google Scholar] [CrossRef]
- Gallo, C.F.; Chandrasekhar, B.S.; Sutter, P.H. Transport Properties of Bismuth Single Crystals. J. Appl. Phys. 1963, 34, 144–152. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Du, B.; Tang, X.; Zhang, Q.; Uher, C. High Thermoelectric Figure of Merit and Nanostructuring in Bulk AgSbTe2. J. Mater. Chem. 2010, 20, 6138–6143. [Google Scholar] [CrossRef]
- Wojciechowski, K.T.; Schmidt, M. Structural and Thermoelectric Properties of AgSbTe-AgSbSe2 Pseudobinary System. Phys. Rev. B 2009, 79, 184202. [Google Scholar] [CrossRef]
- Kosuga, A.; Uno, M.; Kurosaki, K.; Yamanaka, S. Thermoelectric properties of stoichiometric Ag1−xPb18SbTe20 (x = 0, 0.1, 0.2). J. Alloys Compd. 2005, 391, 288–291. [Google Scholar] [CrossRef]
- Zhang, S.N.; Zhu, T.J.; Yang, S.H.; Yu, C.; Zhao, X.B. Phase Compositions, Nanoscale Microstructures and Thermoelectric Properties in Ag2−ySbyTe1+y Alloys with Precipitated Sb2Te3 Plates. Acta Mater. 2010, 58, 4160–4169. [Google Scholar] [CrossRef]
| Diffraction Parameters | |
| Wavelength (Å) | 1.594 |
| 2θ range (°) | 0.07–159.97 |
| 2θ step (°) | 0.05 |
| Temperature (K) | 295 |
| Rietveld Refinement | 3199 data points |
| No. of Parameters | 67 |
| Structural parameters | |
| Formula | AgSbTe2 |
| Space Group | Pmm |
| Z | 2 |
| a (Å) | 6.0788(1) |
| V(Å3) | 224.619(7) |
| Theoretical Density (g·cm−3) | 7.168 |
| Atomic Parameters | ||||||
| Atom | Wyckoff site | x | y | z | Ueq (Å2) | Occ. (<1) |
| Te1 | 3d | 0.00000 | 0.50000 | 0.00000 | 0.027 (7) | 0.82 (3) |
| Te2 | 1a | 0.00000 | 0.00000 | 0.00000 | 0.014 (6) | 1.0 (1) |
| Sb1 | 1b | 0.50000 | 0.50000 | 0.50000 | 0.038 (8) | 1.0 (1) |
| Ag | 3c | 0.50000 | 0.50000 | 0.00000 | 0.026 (5) | 0.6667 |
| Sb2 | 3c | 0.50000 | 0.50000 | 0.00000 | 0.026 (5) | 0.3333 |
| Atomic Displacement Parameters (Å2) | ||||||
| U11 | U22 | U33 | U12 | U13 | ||
| Te1 | 0.022 (5) | 0.04 (1) | 0.022 (5) | 0.00000 | 0.00000 | |
| Sb | 0.038 (8) | 0.038 (8) | 0.038 (8) | 0.00000 | 0.00000 | |
| Ag | 0.036 (5) | 0.036 (5) | 0.005 (6) | 0.00000 | 0.00000 | |
| Sb2 | 0.036 (5) | 0.036 (5) | 0.005 (6) | 0.00000 | 0.00000 | |
| Agreement Factors | Bond Distance (Å) | |||||
| RI(%) | Rp(%) | Rwp(%) | Rexp(%) | χ2 | d(Ag/Sb-Te) | |
| 5.2 | 3.2 | 4.1 | 2.8 | 2.1 | 3.03939(6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gainza, J.; Serrano-Sánchez, F.; Dura, O.J.; Nemes, N.M.; Martínez, J.L.; Fernández-Díaz, M.T.; Alonso, J.A. Reduced Thermal Conductivity in Nanostructured AgSbTe2 Thermoelectric Material, Obtained by Arc-Melting. Nanomaterials 2022, 12, 3910. https://doi.org/10.3390/nano12213910
Gainza J, Serrano-Sánchez F, Dura OJ, Nemes NM, Martínez JL, Fernández-Díaz MT, Alonso JA. Reduced Thermal Conductivity in Nanostructured AgSbTe2 Thermoelectric Material, Obtained by Arc-Melting. Nanomaterials. 2022; 12(21):3910. https://doi.org/10.3390/nano12213910
Chicago/Turabian StyleGainza, Javier, Federico Serrano-Sánchez, Oscar J. Dura, Norbert M. Nemes, Jose Luis Martínez, María Teresa Fernández-Díaz, and José Antonio Alonso. 2022. "Reduced Thermal Conductivity in Nanostructured AgSbTe2 Thermoelectric Material, Obtained by Arc-Melting" Nanomaterials 12, no. 21: 3910. https://doi.org/10.3390/nano12213910
APA StyleGainza, J., Serrano-Sánchez, F., Dura, O. J., Nemes, N. M., Martínez, J. L., Fernández-Díaz, M. T., & Alonso, J. A. (2022). Reduced Thermal Conductivity in Nanostructured AgSbTe2 Thermoelectric Material, Obtained by Arc-Melting. Nanomaterials, 12(21), 3910. https://doi.org/10.3390/nano12213910










