Novel Nanosized Spinel MnCoFeO4 for Low-Temperature Hydrocarbon Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Catalytic Tests
3. Results
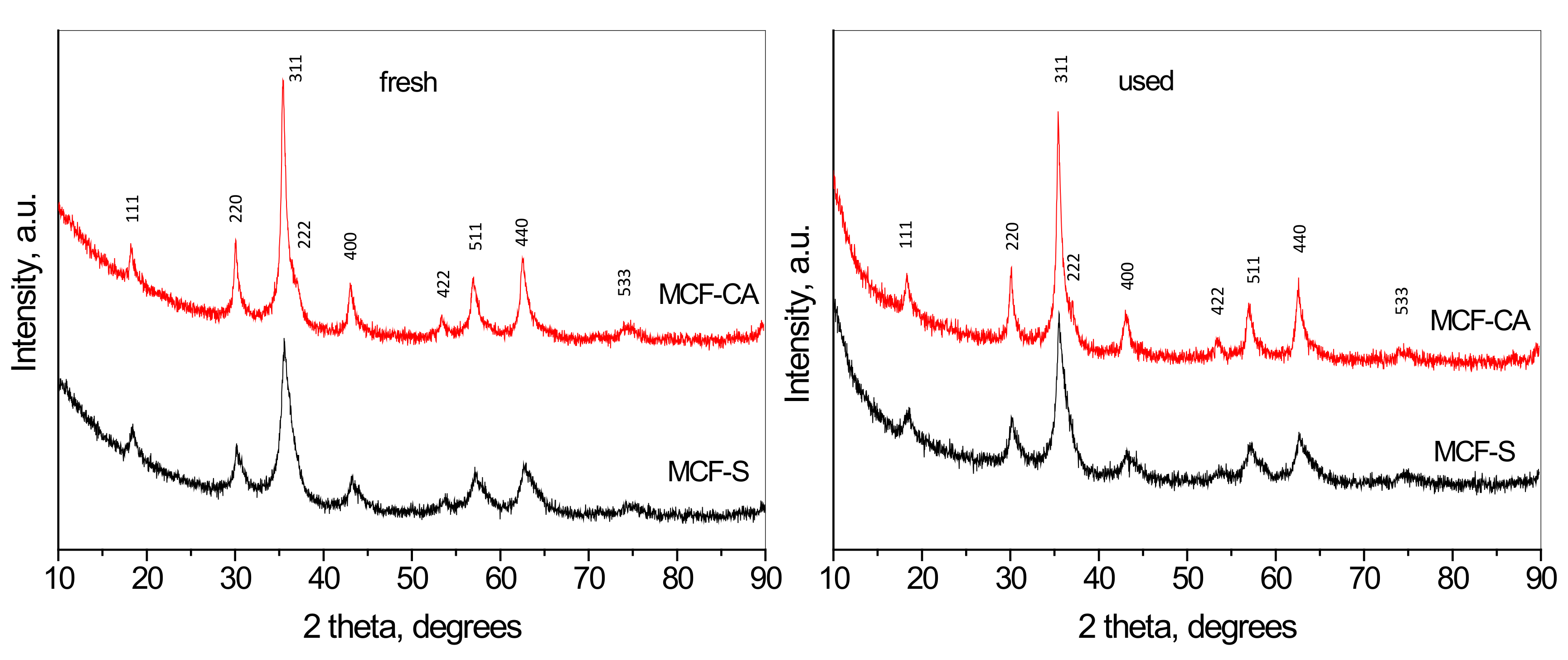
3.1. X-ray Diffraction
3.2. Nitrogen Adsorption
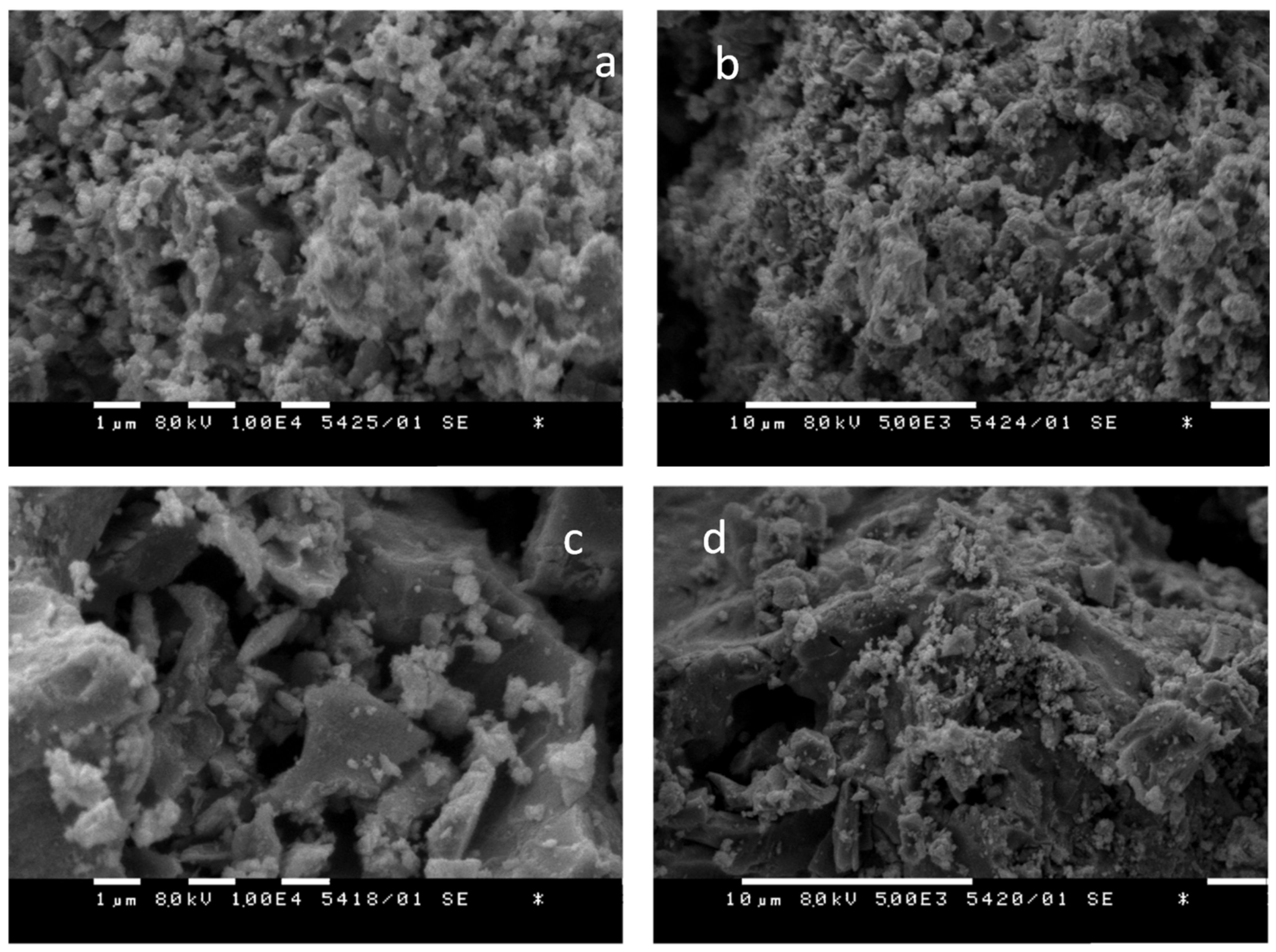
3.3. Scanning Electron Microscopy
3.4. Thermal Analysis
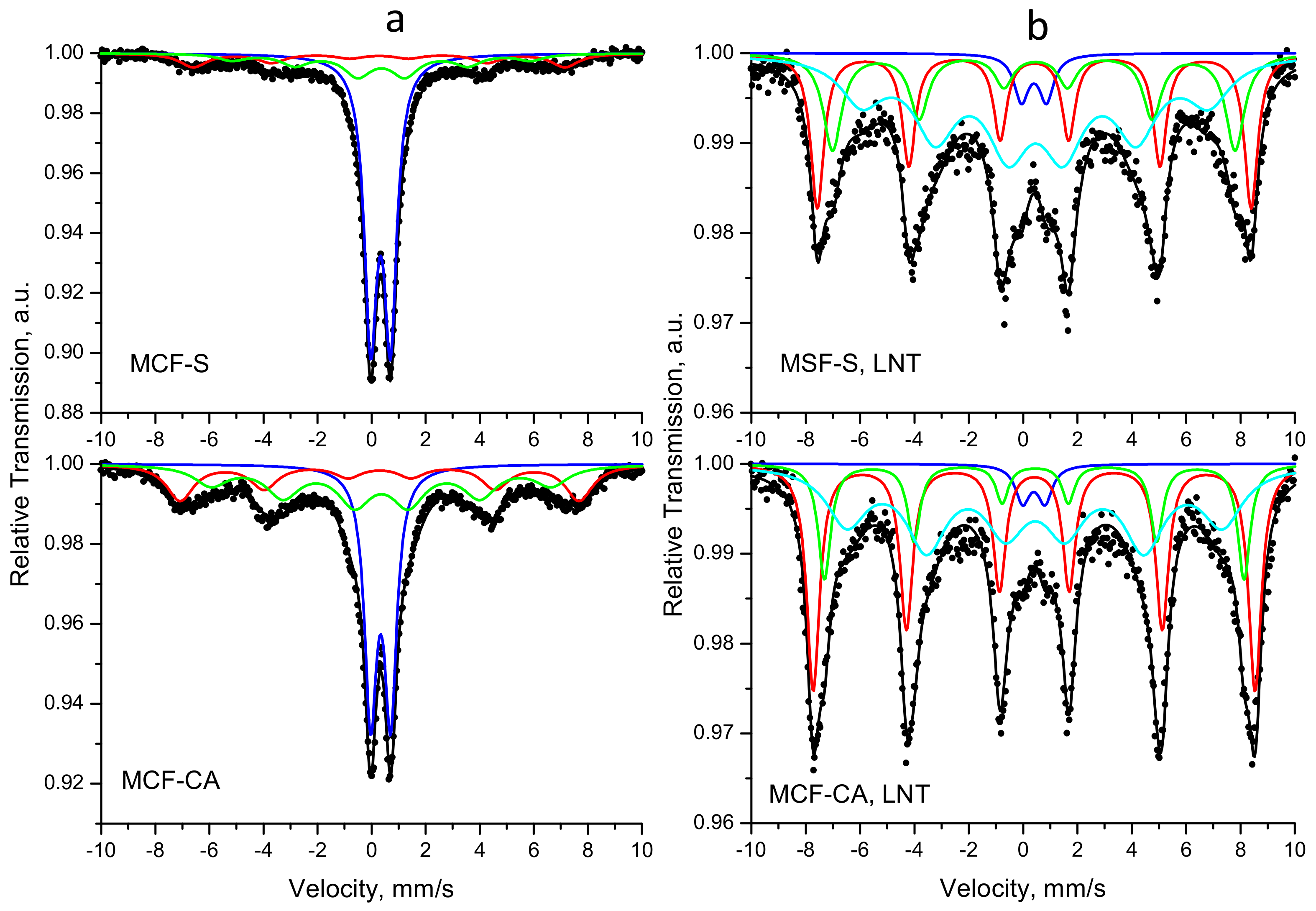
3.5. Mössbauer Study
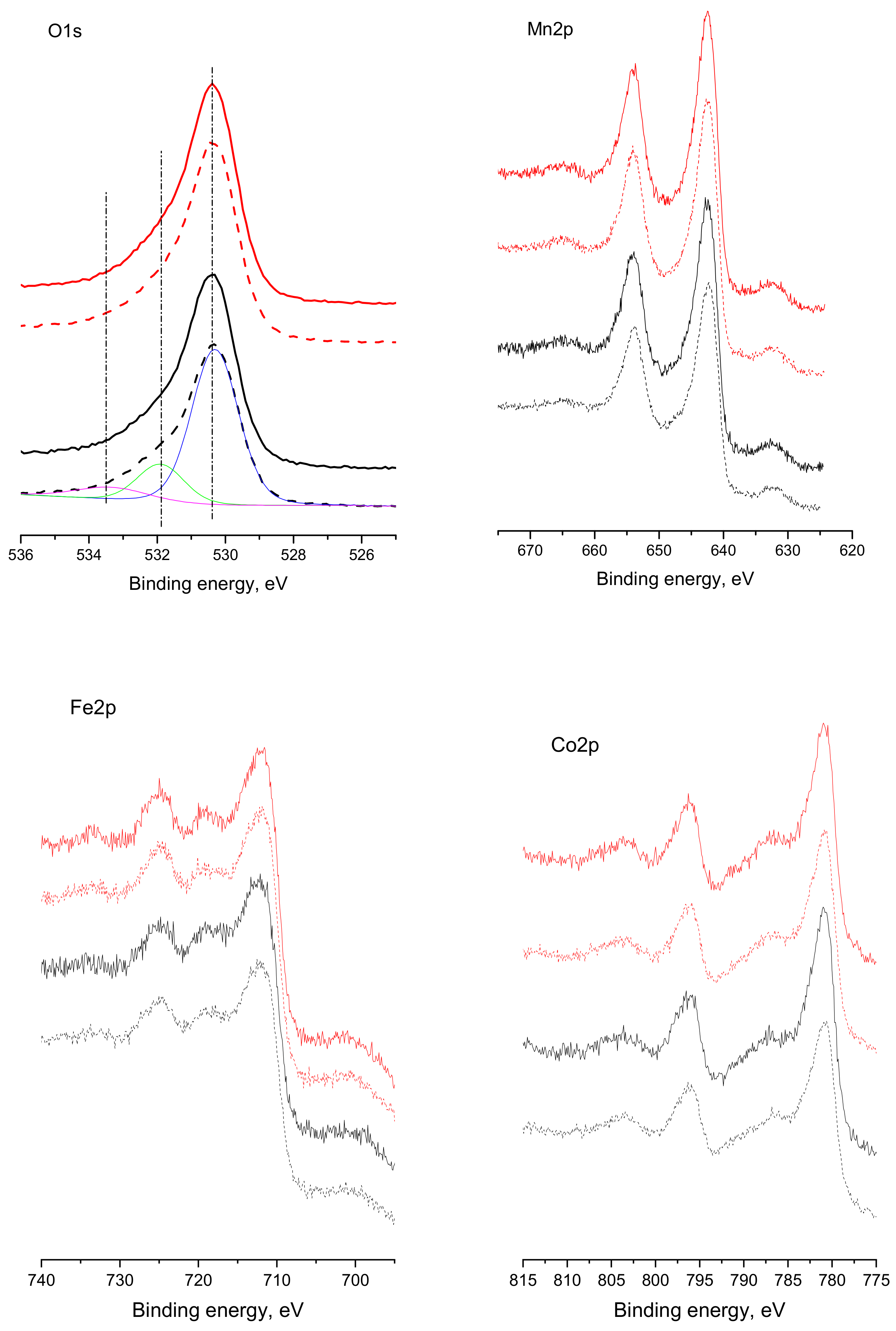
3.6. X-ray Photoelectron Spectroscopy
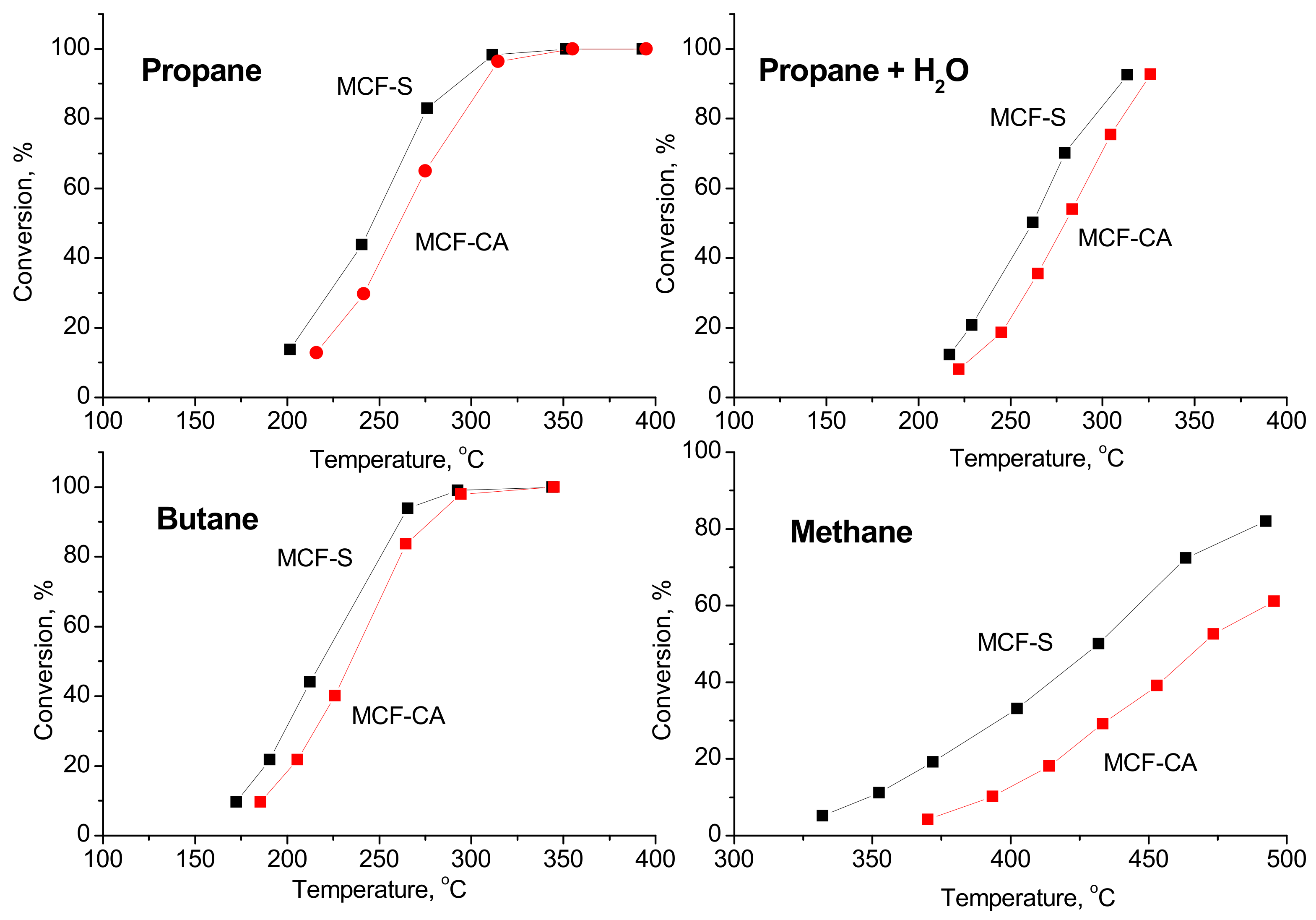
3.7. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, Y.; Yoshida, H.; Takagi, N.; Komai, S.I.; Satsuma, A.; Hattoriy, T. Acid Strength of Support Materials as a Factor Controlling Oxidation State of Palladium Catalyst for Propane Combustion. J. Catal. 1999, 187, 15–23. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K.; Urbano, F.J. Some Aspects of Hydrocarbon Activation on Platinum Group Metal Combustion Catalysts. Catal. Today 1996, 27, 243–248. [Google Scholar] [CrossRef]
- McCarty, J. Durable catalysts for cleaner air. Nature 2000, 403, 35–36. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Kim, J.-H.; Kim, J.-H.; Kang, S.-H.; Ryu, J.-H.; Park, N.-K.; Yun, D.-S.; Bae, J.-W. Noble-Metal-Based Catalytic Oxidation Technology Trends for Volatile Organic Compound (VOC) Removal. Catalysts 2022, 12, 63. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts. Chin. J. Catal. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.; Shi, J.; Xu, X.; Zhao, D.; Ng, Y.H.; Zhang, M.; Liu, S.; Ding, H. Recent progress on catalysts for catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2022. accepted manuscript. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Chen, Z.; An, W.; Men, Y. Boosting soot combustion efficiency of Co3O4 nanocrystals via tailoring crystal facets. Chem. Eng. J. 2018, 337, 488–498. [Google Scholar] [CrossRef]
- Bao, L.; Zhu, S.; Chen, Y.; Wang, Y.; Meng, W.; Xu, S.; Lin, Z.; Li, X.; Sun, M.; Guo, L. Anionic defects engineering of Co3O4 catalyst for toluene oxidation. Fuel 2022, 314, 122774. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Ngamou, P.H.T.; Vannier, V.; Kohse-Höinghaus, K.; Bahlawane, N. Catalytic oxidation of VOCs over mixed Co–Mn oxides. Appl. Catal. B Environ. 2012, 117–118, 125–134. [Google Scholar] [CrossRef]
- Vozniuk, O.; Tabanelli, T.; Tanchoux, N.; Millet, J.-M.M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Mixed-Oxide Catalysts with Spinel Structure for the Valorization of Biomass: The Chemical-Loop Reforming of Bioethanol. Catalysts 2018, 8, 332. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.-H.; He, J.; Zhang, X.-D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 2016, 8324826. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, L.; An, W.; Xua, J.; Men, Y. Boosting soot combustion efficiencies over CuO–CeO2 catalysts with a 3DOM structure. Catal. Sci. Technol. 2016, 6, 7342–7350. [Google Scholar] [CrossRef]
- Zhang, M.; Sui, X.; Zhang, X.; Niu, M.; Li, C.; Wan, H.; Qiao, Z.-A.; Xie, H.; Li, X. Multi-defects engineering of NiCo2O4 for catalytic propane oxidation. Appl. Surf. Sci. 2022, 600, 154040. [Google Scholar] [CrossRef]
- Ramankutty, C.G.; Sugunan, S. Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods. Appl. Catal. A 2001, 218, 39–51. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Maltha, A.; Reitjes, J.G.H.; Drimal, J.; Ponec, V.; Brongersma, H.H. The surface of catalytically active spinels. J. Catal. 1994, 47, 294–300. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Zhou, Y.; Sun, S.; Hu, X.; Dai, Y.; Xi, S.; Du, Y.; Yang, Y.; Xu, Z.J. Identifying Influential Parameters of Octahedrally Coordinated Cations in Spinel ZnMnxCo2–xO4 Oxides for the Oxidation Reaction. ACS Catal. 2018, 8, 8568–8577. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, S.; Wei, C.; Sun, Y.; Xi, P.; Feng, Z.; Xu, Z.J. Significance of Engineering the Octahedral Units to Promote the Oxygen Evolution Reaction of Spinel Oxides. Adv. Mater. 2019, 31, 1902509. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Al-Najar, B.; Bououdina, M.; Ahmed, M.A.A. Catalytic and Photocatalytic Properties of Oxide Spinels. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Germany, 2019; pp. 1701–1750. [Google Scholar] [CrossRef]
- Janani, G.; Chae, Y.; Surendran, S.; Sim, Y.; Park, W.; Kim, J.K.; Sim, U. Rational design of spinel oxide nanocomposites with tailored electrochemical oxygen evolution and reduction reactions for zinc-air batteries. Appl. Sci. 2020, 10, 3165. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.; Zhang, L.; Wang, L.; Zhou, L. Controllable synthesis and bi-functional electrocatalytic performance towards oxygen electrocatalytic reactions of Co3O4 nanoflakes/nitrogen-doped modified CMK-3 nanocomposite. Inorg. Chem. Commun. 2019, 108, 107524. [Google Scholar] [CrossRef]
- Lazarova, T.; Kovacheva, D.; Georgieva, M.; Tzankov, D.; Tyuliev, G.; Spassova, I.; Naydenov, A. Tunable nanosized spinel manganese ferrites synthesized by solution combustion method. Appl. Surf. Sci. 2019, 496, 143571. [Google Scholar] [CrossRef]
- Elkholy, A.; El-Taib Heakal, F.; Allam, N.K. Nanostructured spinel manganese cobalt ferrite for high-performance supercapacitors. RSC Adv. 2017, 7, 51888–51895. [Google Scholar] [CrossRef]
- Majumdar, S.; Ray, R.; Sen, P. Anomalous intra diffusive behavior of chitosan/PVDF solid polymer electrolytes and the enhancement of effective specific capacitance with nanostructured spinel MnCoFeO4 electrode in solid-state. Electrochim. Acta 2021, 385, 138295. [Google Scholar] [CrossRef]
- Bernard, J.-L.; Baffier, N.; Huber, M. Distorsion cristalline et distribution des cations dans les spinelles de la serie CoMnxFe2−xO4. J. Solid State Chem. 1973, 8, 50–56. [Google Scholar] [CrossRef]
- Martens, J. Magneto-optical properties of substituted cobalt ferrites: CoFe2−xMexO4 (Me = Rh3+, Mn3+, Ti4++Co2+). J. Appl. Phys. 1986, 59, 3820–3823. [Google Scholar] [CrossRef]
- Laarj, M.; Kacim, S.; Gillot, B. Cationic Distribution and Oxidation Mechanism of Trivalent Manganese Ions in Submicrometer MnxCoFe2−xO4 Spinel Ferrites. J. Solid State Chem. 1996, 125, 67–74. [Google Scholar] [CrossRef]
- Jain, S.R.; Adiga, K.C.; Pai Verenekar, V.R. A new approach to thermochemical calculations of condensed fuel-oxidizer mixture. Combus. Flame 1981, 40, 71–79. [Google Scholar] [CrossRef]
- Bruker, A.X.S. TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008; Available online: http://algol.fis.uc.pt/jap/TOPAS%204-2%20Users%20Manual.pdf (accessed on 1 October 2022).
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Rel. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorp. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Hirono, T.; Kaneki, S.; Ishikawa, T.; Kameda, J.; Tonoike, N.; Ito, A.; Miyazaki, Y. Generation of sintered fault rock and its implications for earthquake energetics and fault healing. Commun. Earth Environ. 2020, 1, 3. [Google Scholar] [CrossRef]
- Vila, E.; Rojas, R.M.; Martín de Vidales, J.L.; García-Martínez, O. Structural and Thermal Properties of the Tetragonal Cobalt Manganese Spinels MnxCo3−xO4 (1.4 < x < 2.0). Chem Mater. 1996, 8, 1078–1083. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Song, W.; Lin, H.-J.; Wang, S.; Biswas, S.; Mollahosseini, M.; Kuo, C.-H.; Gao, P.-X.; Suib, S.L. Manganese Oxide Nanoarray-Based Monolithic Catalysts: Tunable Morphology and High Efficiency for CO Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Beji, Z.; Sun, M.; Smiri, L.S.; Herbst, F.; Mangeney, C.; Ammar, S. Polyol synthesis of non-stoichiometric Mn-Zn ferrite nanocrystals: Structural microstructural characterization and catalytic application. RSC. Adv. 2015, 5, 65010–65022. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Fierro, G.; Lo Jacono, M.; Inversi, M.; Dragone, R.; Porta, P. TPR and XPS study of cobalt–copper mixed oxide catalysts: Evidence of a strong Co–Cu interaction. Top. Catal. 2000, 10, 39–48. [Google Scholar] [CrossRef]
- Liang, X.L.; He, Z.S.; Wei, G.L.; Liu, P.; Zhong, Y.H.; Tan, W.; Du, P.X.; Zhu, J.X.; He, H.P.; Zhang, J. The distinct effects of Mn substitution on the reactivity of magnetite in heterogeneous Fenton reaction and Pb(II) adsorption. J. Colloid Interface Sci. 2014, 426, 181–189. [Google Scholar] [CrossRef]
- Liang, X.L.; He, Z.S.; Tan, W.; Liu, P.; Zhu, J.X.; Zhang, J.; He, H.P. The oxidation state and microstructural environment of transition metals (V, Co, and Ni) in magnetite: An XAFS study. Phys. Chem. Miner. 2015, 42, 373–383. [Google Scholar] [CrossRef]
- Bratan, V.; Vasile, A.; Chesler, P.; Hornoiu, C. Insights into the Redox and Structural Properties of CoOx and MnOx: Fundamental Factors Affecting the Catalytic Performance in the Oxidation Process of VOCs. Catalysts 2022, 12, 1134. [Google Scholar] [CrossRef]
- Todorova, S.; Blin, J.L.; Naydenov, A.; Lebeau, B.; Kolev, H.; Gaudin, P.; Dotzeva, A.; Velinova, R.; Filkova, D.; Ivanova, I.; et al. Co3O4-MnOx oxides supported on SBA-15 for CO and VOCs oxidation. Catal. Today 2020, 357, 602–612. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Lv, L.; Li, D.; Yue, X.; Wang, S. Insights into the Behaviors of the Catalytic Combustion of Propane over Spinel Catalysts. Catal. Lett. 2020, 150, 3617–3625. [Google Scholar] [CrossRef]
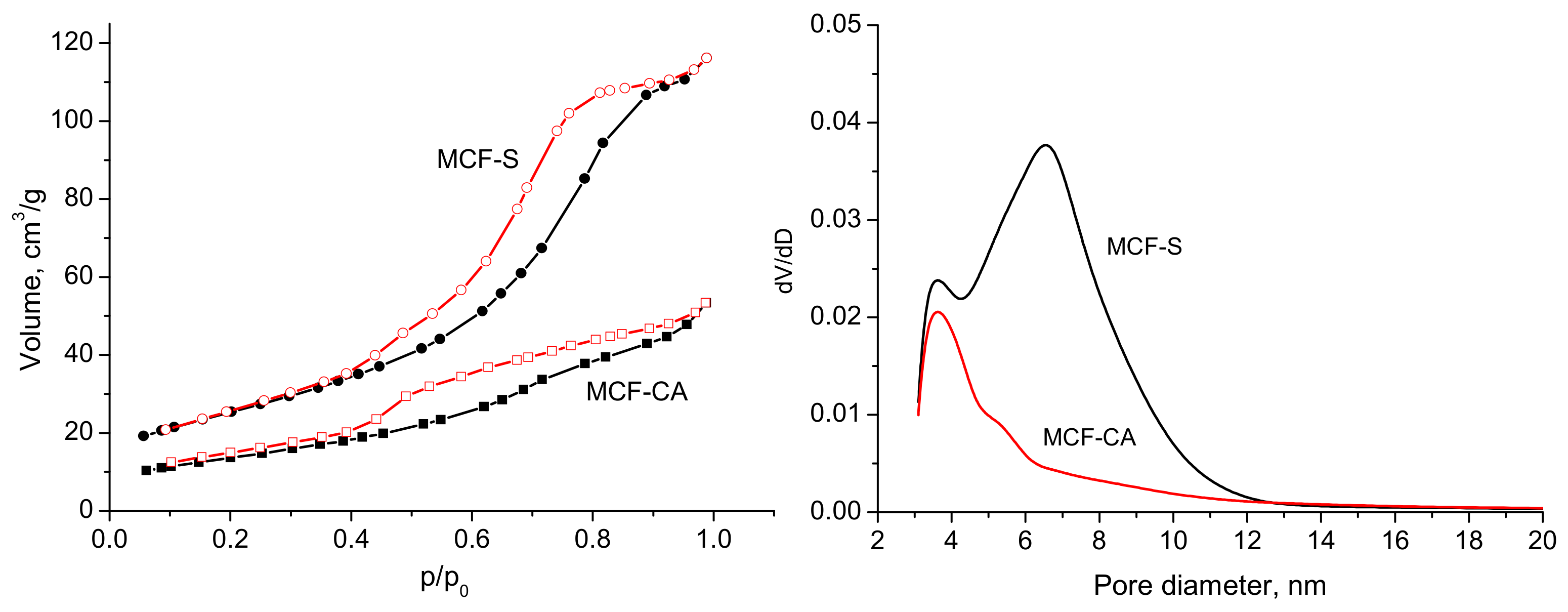

| Sample | SBET, m2/g | Vt, cm3/g | Dav, nm |
|---|---|---|---|
| MCF-S | 93 | 0.18 | 7.8 |
| MCF-CA | 50 | 0.08 | 6.7 |
| Sample | Components | δ, mm/s | Δ (2ε), mm/s | Bhf, T | Γexp, mm/s | G, % |
|---|---|---|---|---|---|---|
| MCF-CA | Sx1-Fe3+tetra | 0.30 | 0.00 | 45.8 | 1.22 | 25 |
| Sx2-Fe3+octa | 0.36 | −0.01 | 39.0 | 1.50 | 37 | |
| Db-Fe3+ | 0.34 | 0.74 | - | 0.55 | 37 | |
| MCF-S | Sx1-Fe3+tetra | 0.28 | 0.00 | 42.6 | 1.22 | 14 |
| Sx2-Fe3+octa | 0.36 | 0.00 | 34.3 | 1.22 | 21 | |
| Db-Fe3+ | 0.33 | 0.74 | - | 0.57 | 65 | |
| MCF-CA, LNT | Sx1-Fe3+tetra | 0.41 | −0.01 | 50.4 | 0.57 | 37 |
| Sx2-Fe3+octa | 0.43 | −0.03 | 47.9 | 0.60 | 16 | |
| Sx3-Fe3+ | 0.44 | −0.03 | 42.9 | 1.79 | 44 | |
| Db-Fe3+ | 0.40 | 0.82 | - | 0.65 | 3 | |
| MCF-S, LNT | Sx1-Fe3+tetra | 0.41 | −0.01 | 49.5 | 0.60 | 27 |
| Sx2-Fe3+octa | 0.43 | −0.07 | 45.9 | 0.82 | 20 | |
| Sx3-Fe3+ | 0.47 | −0.02 | 39.6 | 1.88 | 49 | |
| Db-Fe3+ | 0.40 | 0.92 | - | 0.65 | 4 |
| Sample | Mn, at. % | Co, at. % | Fe, at. % | O, at. % | O/Me | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theor. 14.33 | Theor. 14.33 | Theor. 14.33 | Theor. 57.32 | Theor. 1.33 | ||||||
| EDS | XPS | EDS | XPS | EDS | XPS | EDS | XPS | EDS | XPS | |
| MCF-S | 15.1 | 17.0 | 15.0 | 15.5 | 14.1 | 15.4 | 55.8 | 52.8 | 1.26 | 1.10 |
| MCF-S-u | - | 16.4 | - | 14.8 | - | 16.4 | - | 52.4 | - | 1.10 |
| MCF-CA | 14.8 | 16.9 | 14.1 | 13.2 | 13.7 | 15.8 | 57.5 | 54.1 | 1.35 | 1.18 |
| MCF-CA-u | - | 16.8 | - | 13.7 | - | 15.4 | - | 52.0 | - | 1.13 |
| Catalyst | Eapp | ko | m (C3H8) | n (O2) | p (H2O) | RSS | R2 |
|---|---|---|---|---|---|---|---|
| MCF-S | 71.3 | 7.06 × 107 | 0.77 | 0.18 | −0.12 | 3.5 | 0.99 |
| MCF-CA | 70.9 | 8.32 × 105 | 0.72 | 0.15 | −0.09 | 9.9 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumbalev, V.; Kovacheva, D.; Spassova, I.; Velinova, R.; Tyuliev, G.; Velinov, N.; Naydenov, A. Novel Nanosized Spinel MnCoFeO4 for Low-Temperature Hydrocarbon Oxidation. Nanomaterials 2022, 12, 3900. https://doi.org/10.3390/nano12213900
Tumbalev V, Kovacheva D, Spassova I, Velinova R, Tyuliev G, Velinov N, Naydenov A. Novel Nanosized Spinel MnCoFeO4 for Low-Temperature Hydrocarbon Oxidation. Nanomaterials. 2022; 12(21):3900. https://doi.org/10.3390/nano12213900
Chicago/Turabian StyleTumbalev, Vencislav, Daniela Kovacheva, Ivanka Spassova, Ralitsa Velinova, Georgi Tyuliev, Nikolay Velinov, and Anton Naydenov. 2022. "Novel Nanosized Spinel MnCoFeO4 for Low-Temperature Hydrocarbon Oxidation" Nanomaterials 12, no. 21: 3900. https://doi.org/10.3390/nano12213900
APA StyleTumbalev, V., Kovacheva, D., Spassova, I., Velinova, R., Tyuliev, G., Velinov, N., & Naydenov, A. (2022). Novel Nanosized Spinel MnCoFeO4 for Low-Temperature Hydrocarbon Oxidation. Nanomaterials, 12(21), 3900. https://doi.org/10.3390/nano12213900









