Optimization of Tumor Targeting Gold Nanoparticles for Glioblastoma Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
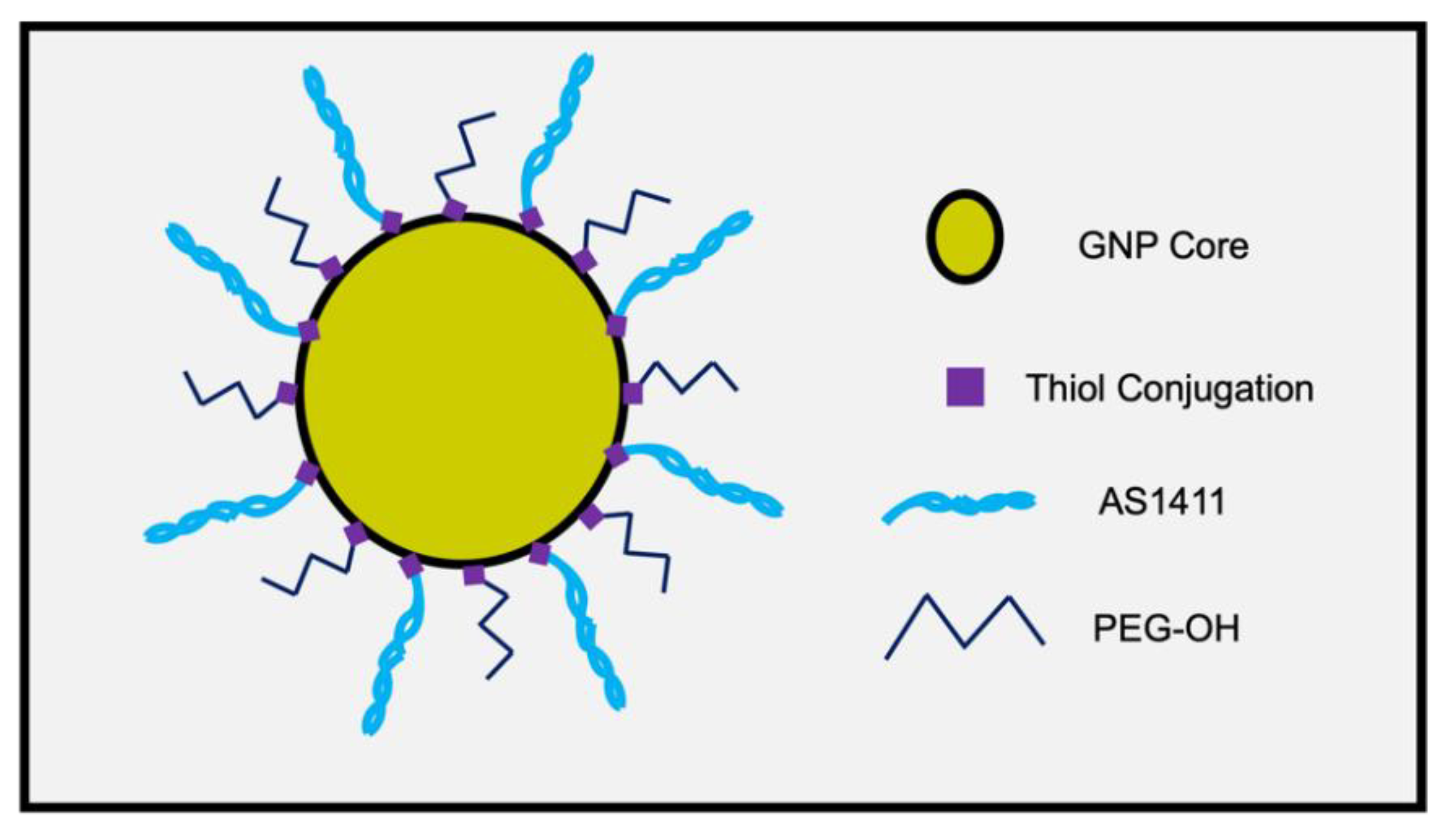
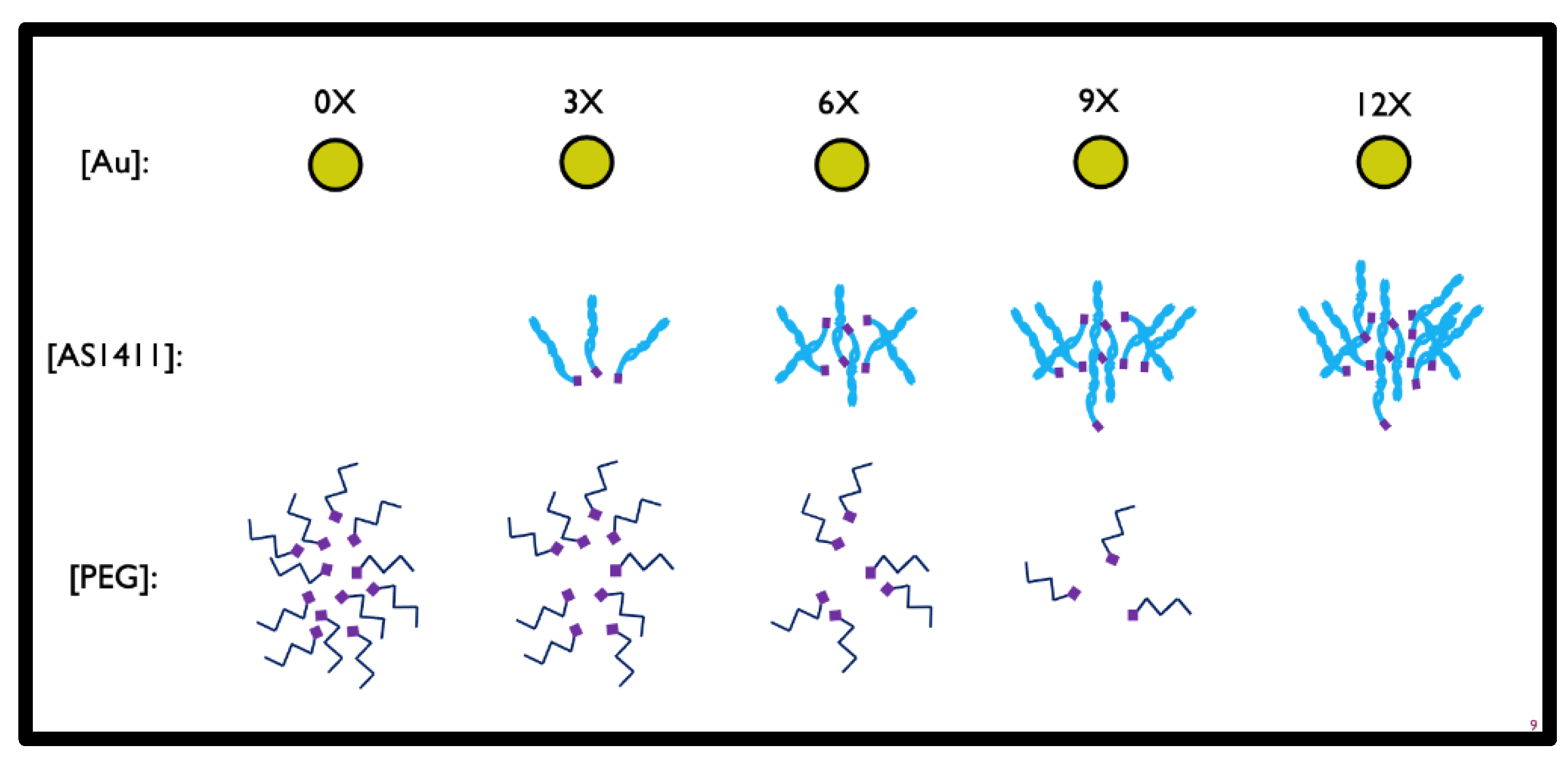
2.2. Co-Conjugated PEG and AS1411 GNP Synthesis
2.3. Nanoparticle Characterization
2.4. Cell Culture
2.5. Cytotoxicity and Specificity Studies
2.6. Microscopy Studies
2.7. Proliferation Analysis
2.8. Statistical Analysis
3. Results and Discussion
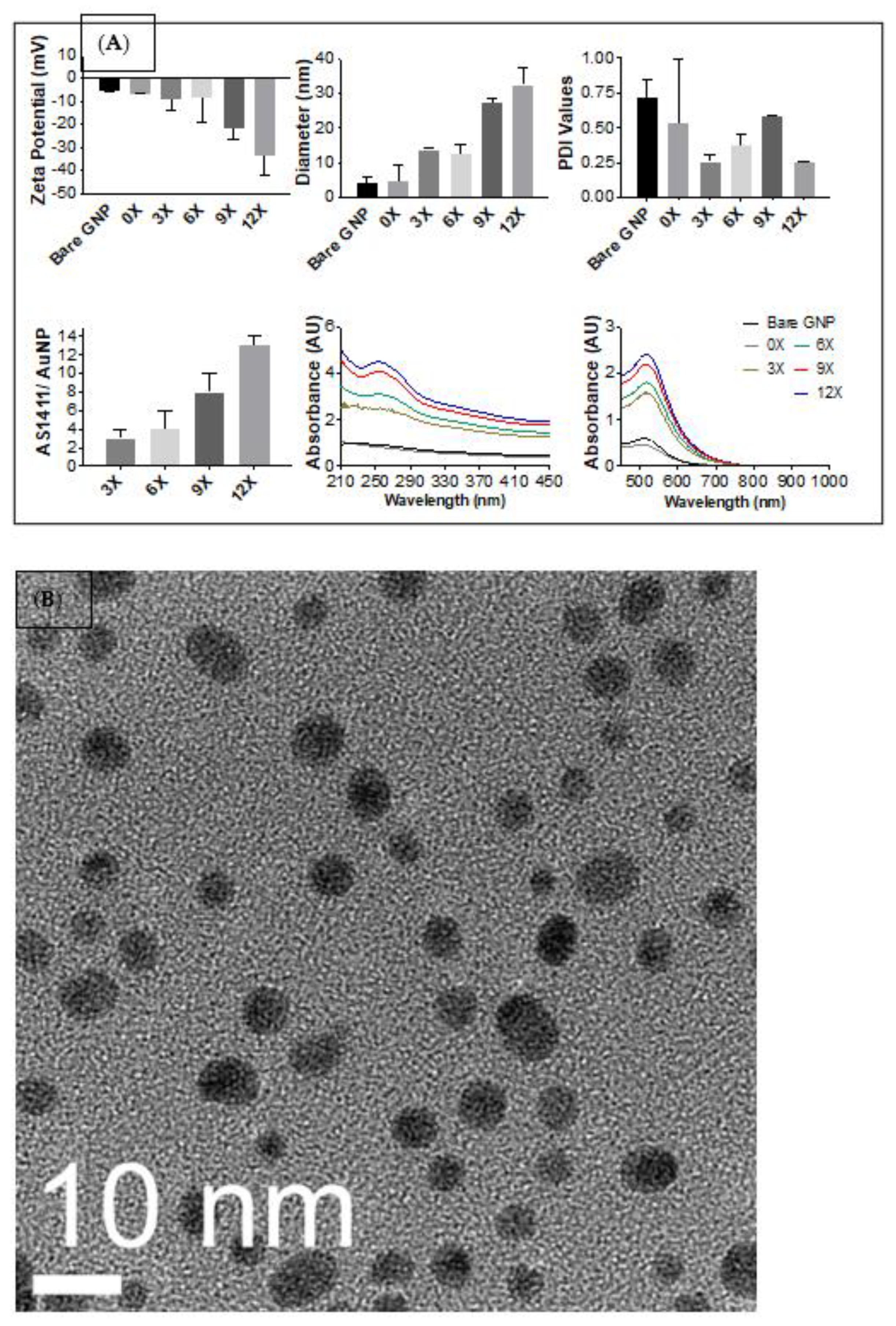
3.1. Determining Optimal Synthesis of PEG-AS1411 Co-Conjugated GNPs
3.1.1. Synthesis of PEG-AS1411 Co-Conjugated GNPs
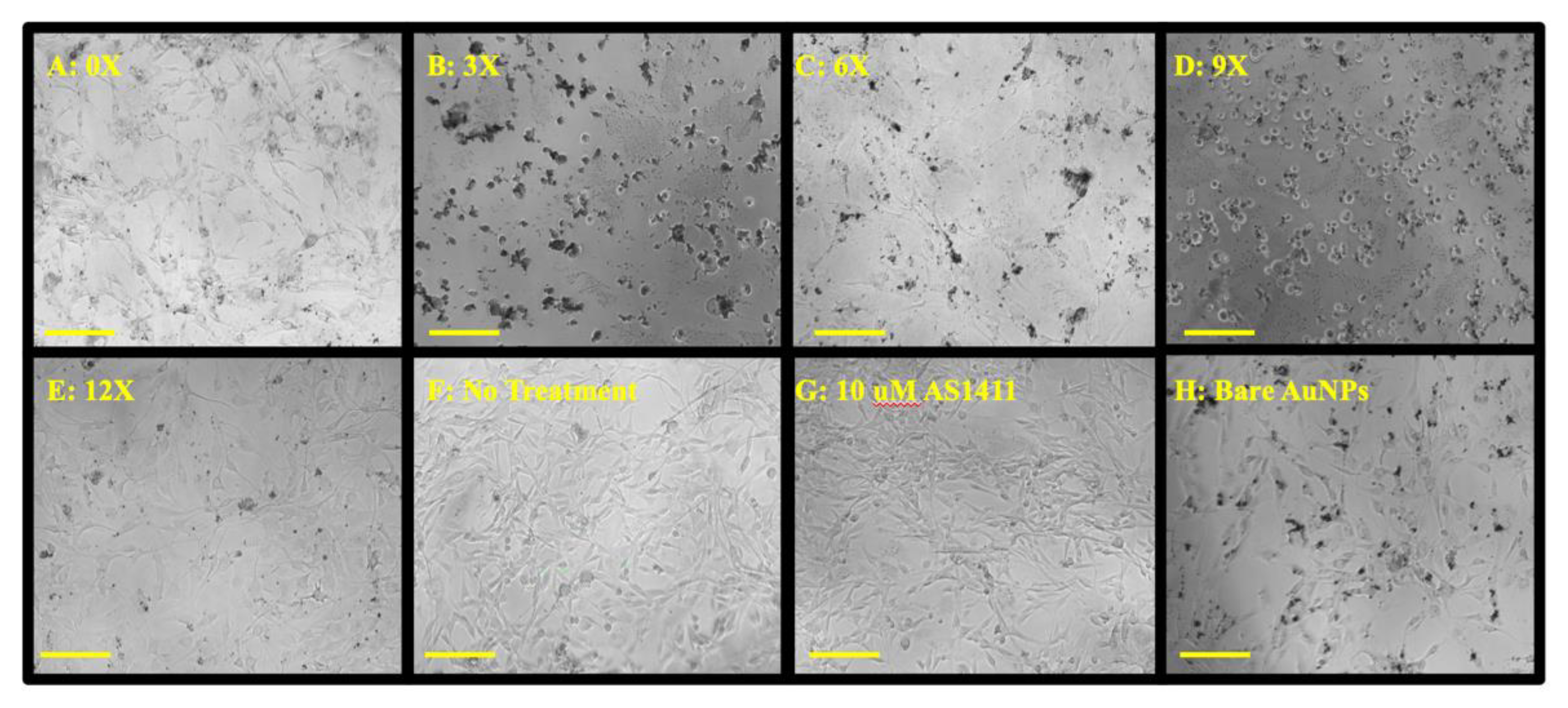
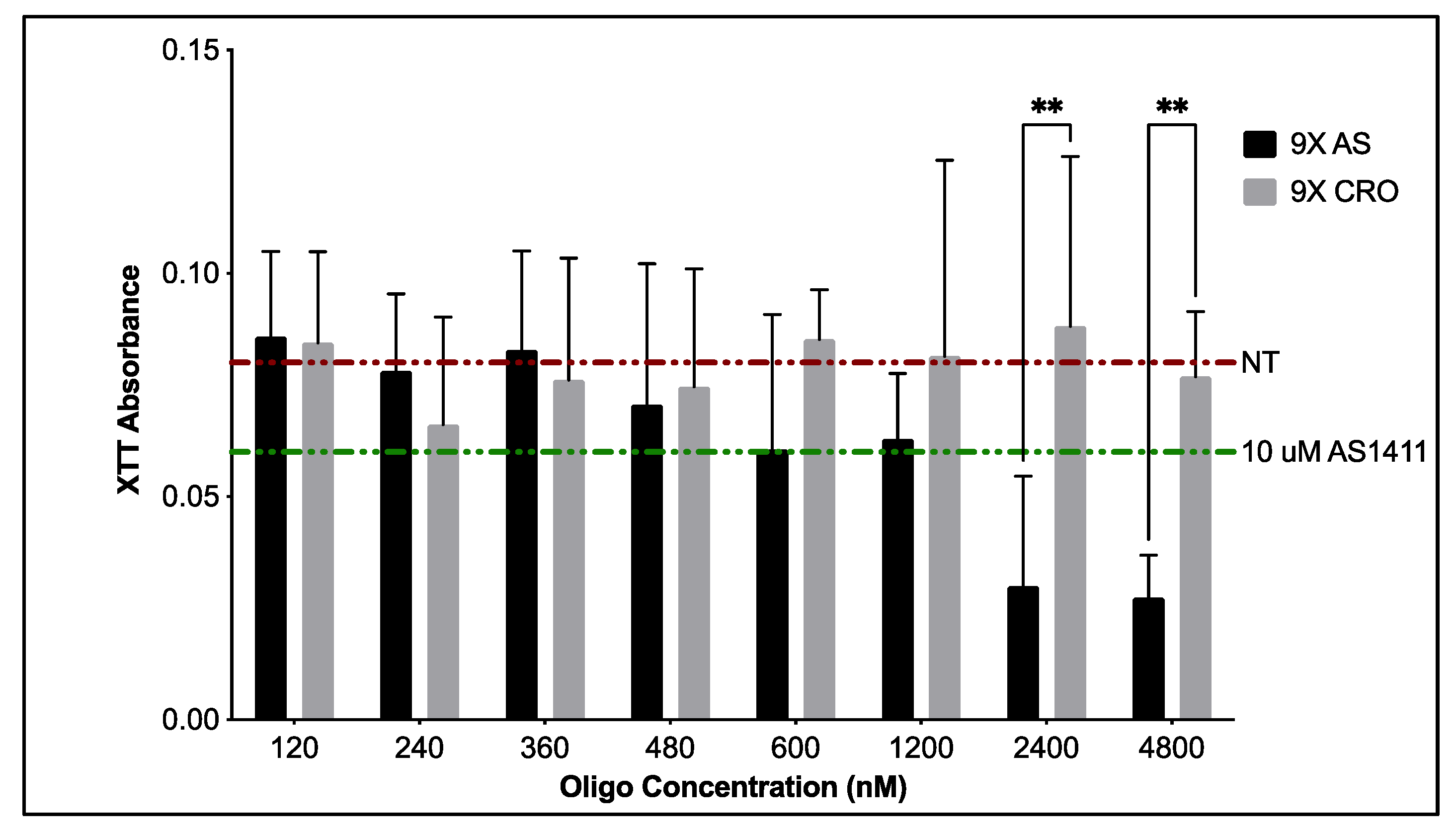
3.1.2. Cytotoxicity of PEG-AS1411 Co-Conjugated GNPs
3.2. Specificity of Optimal PEG-AS1411 Co-Conjugated GNPs
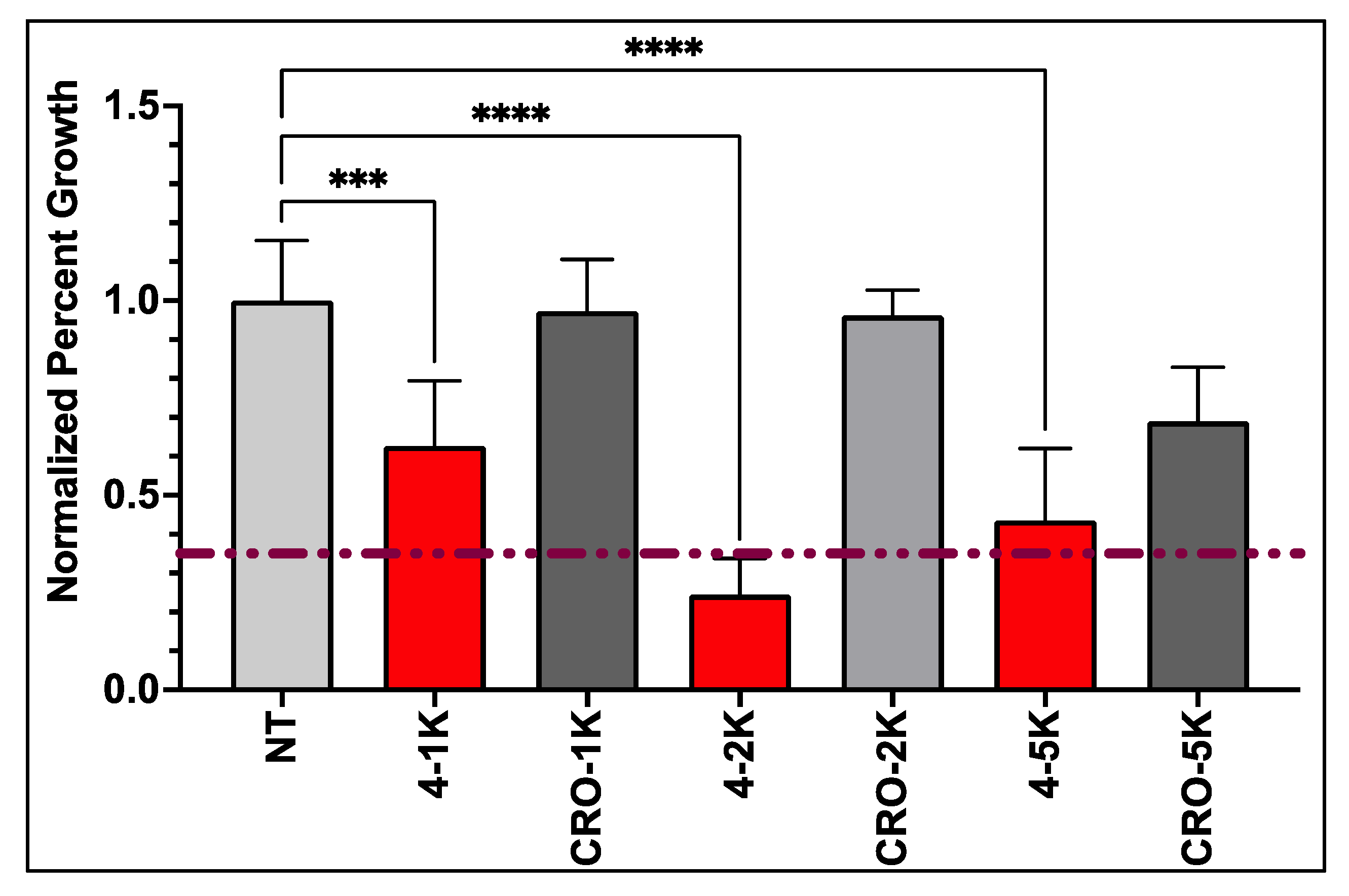
3.3. Anti-GBM Activity of PEG-AS1411 Optimal Co-Conjugated GNPs
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, D.; Wang, M.; Chen, Y.; Gong, J.; Chen, L.; Shi, X.; Lan, F.; Chen, Z.; Xiong, T.; Sun, H.; et al. Trends in Intracranial Glioma Incidence and Mortality in the United States, 1975–2018. Front. Oncol. 2021, 11, 748061. [Google Scholar] [CrossRef] [PubMed]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E.; et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neuro-Oncol. 2020, 147, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Deorah, S.; Lynch, C.F.; Sibenaller, Z.A.; Ryken, T.C. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg. Focus 2006, 20, E1. [Google Scholar] [CrossRef] [PubMed]
- Mujokoro, B. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: A review. Mater. Sci. Eng. 2016, 69, 1092–1102. [Google Scholar] [CrossRef]
- Reardon, D.A.; Herndon, J.E.; Peters, K.B.; Desjardins, A.; Coan, A.; Lou, E.; Sumrall, A.L.; Turner, S.; Lipp, E.S.; Sathornsumetee, S.; et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br. J. Cancer 2012, 107, 1481–1487. [Google Scholar] [CrossRef]
- Zhang, Y.; Cruickshanks, N.; Pahuski, M.; Yuan, F.; Dutta, A.; Schiff, D.; Purow, B.; Abounader, R. Noncoding RNAs in Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Charlotesville, VA, USA, 2017; Chapter 6. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients—Brain Cancer: Gliomas; National Comprehensive Cancer Network® (NCCN®): Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Society, A.C. Survival Rates for Selected Adult Brain and Spinal Cord Tumors; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. 4), iv1–iv62. [Google Scholar] [CrossRef]
- Kálmán, B.; Komoly, S. Pécsi Tudományegyetem, Általános Orvostudományi Kar, Klinikai Idegtudományi Doktori Iskola (D221). In Glioblastoma [Internet]; De Vleeschouwer, S., Ed.; Codon Publications: Charlotesville, VA, USA, 2017. [Google Scholar]
- Ahmed, R.; Oborski, M.J.; Hwang, M.; Lieberman, F.S.; Mountz, J.M. Malignant gliomas: Current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar]
- Bolcaen, J.; Acou, M.; Descamps, B.; Kersemans, K.; Deblaere, K.; Vanhove, C.; Goethals, I. Brisbane (AU): Codon Publications, PET for Therapy Response Assessment in Glioblastoma. In Glioblastoma [Internet]; De Vleeschouwer, S., Ed.; Codon Publications: Charlotesville, VA, USA, 2017. [Google Scholar]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma [Internet]; De Vleeschouwer, S., Ed.; Codon Publications: Charlotesville, VA, USA, 2017. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Jena, L.; McErlean, E.; McCarthy, H. Delivery across the blood-brain barrier: Nanomedicine for glioblastoma multiforme. Drug Deliv. Transl. Res. 2020, 10, 304–318. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Dancy, J.G.; Hersh, D.S.; Anastasiadis, P.; Tran, N.L.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Tumor-targeted nanotherapeutics: Overcoming treatment barriers for glioblastoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1439. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osorio, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Singapore, 2017. [Google Scholar]
- Hart, M.G.; Garside, R.; Rogers, G.; Stein, K.; Grant, R. Temozolomide for high grade glioma. Cochrane Database Syst. Rev. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Moyama, C.; Kojima, N.; Fujita, M.; Ohta, K.; Kohno, Y.; Ii, H.; Nakata, S. JCI-20679 suppresses autophagy and enhances temozolomide-mediated growth inhibition of glioblastoma cells. Biochem. Biophys. Res. Commun. 2022, 591, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Malek-Adamian, E.; Le, P.U.; Meng, A.; Martínez-Montero, S.; Petrecca, K.; Damha, M.J.; Shoichet, M.S. Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2018, 11, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Lee, M. Antisense oligonucleotide of microRNA-21 delivery using R3V6 peptide in glioblastoma. J. Biotechnol. 2014, 185, S107. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Yeh, M.; Kaur, B.; Lee, T.J. Targeted delivery of small noncoding RNA for glioblastoma. Cancer Lett. 2021, 500, 274–280. [Google Scholar] [CrossRef]
- Gill, J.S.; Zhu, X.; Moore, M.J.; Lu, L.; Yaszemski, M.J.; Windebank, A.J. Effects of NFκB decoy oligonucleotides released from biodegradable polymer microparticles on a glioblastoma cell line. Biomaterials 2002, 23, 2773–2781. [Google Scholar] [CrossRef]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W. CircABCC3 knockdown inhibits glioblastoma cell malignancy by regulating miR-770-5p/SOX2 axis through PI3K/AKT signaling pathway. Brain Res. 2021, 1764, 147465. [Google Scholar] [CrossRef]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R.; et al. Targeting Ephrin Receptor Tyrosine Kinase A2 with a Selective Aptamer for Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.M.; Teng, Y.; Bates, P.J. A new paradigm for aptamer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010, 70, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Trangle, S.S.; Kloog, Y.; Pinkas-Kramarski, R. Interfering with the interaction between ErbB1, nucleolin and Ras as a potential treatment for glioblastoma. Oncotarget 2014, 5, 8602–8613. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.; Antonosante, A.; d’Angelo, M.; Cristiano, L.; Galzio, R.; Destouches, D.; Florio, T.M.; Dhez, A.C.; Astarita, C.; Cinque, B.; et al. Nucleolin antagonist triggers autophagic cell death in human glioblastoma primary cells and decreased in vivo tumor growth in orthotopic brain tumor model. Oncotarget 2015, 6, 42091–42104. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, K.; Gorospe, M. RNA-binding protein nucleolin in disease. RNA Biol. 2012, 9, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Laber, D.; Taft, B.; Kloecker, G.; Bates, P.; Trent, J.; Miller, D. Extended phase I study of AS1411 in renal and non-small cell lung cancers. J. Clin. Oncol. 2006, 24 (Suppl. 18), 13098. [Google Scholar] [CrossRef]
- Stuart, R.; Stockerl-Goldstein, K.; Cooper, M.; Devetten, M.; Herzig, R.; Medeiros, B.; Schiller, G.; Wei, A.; Acton, G.; Rizzieri, D. Randomized phase II trial of the nucleolin targeting aptamer AS1411 combined with high-dose cytarabine in relapsed/refractory acute myeloid leukemia (AML). in ASCO Annual Meeting Proceedings (Post-Meeting Edition). J. Clin. Oncol. 2009, 27 (Suppl. 15), 7019. [Google Scholar] [CrossRef]
- Malik, M.T.; O’Toole, M.G.; Casson, L.K.; Thomas, S.D.; Bardi, G.T.; Reyes-Reyes, E.M.; Ng, C.K.; Kang, K.A.; Bates, P.J. AS1411-conjugated gold nanospheres and their potential for breast cancer therapy. Oncotarget 2015, 6, 22270. [Google Scholar] [CrossRef]
- Kabirian-Dehkordi, S.; Chalabi-Dchar, M.; Mertani, H.C.; Le Guellec, D.; Verrier, B.; Diaz, J.-J.; Mehrgardi, M.A.; Bouvet, P. AS1411-conjugated gold nanoparticles affect cell proliferation through a mechanism that seems independent of nucleolin. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102060. [Google Scholar] [CrossRef]
- Ai, J.; Xu, Y.; Lou, B.; Li, D.; Wang, E. Multifunctional AS1411-functionalized fluorescent gold nanoparticles for targeted cancer cell imaging and efficient photodynamic therapy. Talanta 2014, 118, 54–60. [Google Scholar] [CrossRef]
- Bates, P.J.; Reyes-Reyes, E.M.; Malik, M.T.; Murphy, E.M.; O’Toole, M.G.; Trent, J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms. Biochim. Et. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1414–1428. [Google Scholar] [CrossRef]
- Faulk, W.P.; Taylor, G.M. Communication to the editors: An immunocolloid method for the electron microscope. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold Nanoparticles for Nucleic Acid Delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Roca, M.; Haes, A.J. Probing cells with noble metal nanoparticle aggregates. Nanomedicine 2008, 3, 555–565. [Google Scholar] [CrossRef]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef]
- Kyriazi, M.-E.; Giust, D.; El-Sagheer, A.H.; Lackie, P.M.; Muskens, O.L.; Brown, T.; Kanaras, A. Multiplexed mRNA Sensing and Combinatorial-Targeted Drug Delivery Using DNA-Gold Nanoparticle Dimers. ACS Nano 2018, 12, 3333–3340. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Gallina, M.E.; Zhou, Y.; Johnson, C.J.; Harris-Birtill, D.; Singh, M.; Zhao, H.; Ma, D.; Cass, T.; Elson, D.S. Aptamer-conjugated, fluorescent gold nanorods as potential cancer theradiagnostic agents. Mater. Sci. Eng. C 2016, 59, 324–332. [Google Scholar] [CrossRef]
- Zhao, N.; You, J.; Zeng, Z.; Li, C.; Zu, Y. An ultra pH-sensitive and aptamer-equipped nanoscale drug-delivery system for selective killing of tumor cells. Small 2013, 9, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Dam, D.H.; Culver, K.S.; Kandela, I.; Lee, R.C.; Chandra, K.; Lee, H.; Mantis, C.; Ugolkov, A.; Mazar, A.P.; Odom, T.W. Biodistribution and in vivo toxicity of aptamer-loaded gold nanostars. Nanomedicine 2015, 11, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Shen, N.; Yang, Y.; Yu, H.; Xu, S.; Yang, Y.-W.; Liu, S.; Meguellati, K.; Yan, F. Targeting epigenetic pathway with gold nanoparticles for acute myeloid leukemia therapy. Biomaterials 2018, 167, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Kim, Y.-S.; Choi, D.G.; Shim, M.S. Cancer-targeted photothermal therapy using aptamer-conjugated gold nanoparticles. J. Ind. Eng. Chem. 2018, 67, 429–436. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Yazdian-Robati, R.; Alibolandi, M.; Taghdisi, S.M. A novel chemotherapy drug-free delivery system composed of three therapeutic aptamers for the treatment of prostate and breast cancers in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- James, K.T.; Bates, P.J.; Malik, M.T.; Keynton, R.S.; O’Toole, M.G. Gold Nanoplates as Cancer-Targeted Photothermal Actuators for Drug Delivery and Triggered Release. J. Nanomater. 2016, 2016, 2036029. [Google Scholar]
- Chauhan, R.; El-Baz, N.; Keynton, R.S.; James, K.T.; Malik, D.A.; Zhu, M.; El-Baz, A.; Ng, C.K.; Bates, P.J.; Malik, M.T.; et al. Targeted Gold Nanoparticle–Oligonucleotide Contrast Agents in Combination with a New Local Voxel-Wise MRI Analysis Algorithm for In Vitro Imaging of Triple-Negative Breast Cancer. Nanomaterials 2019, 9, 709. [Google Scholar] [CrossRef]
- Jensen, S.A.; Day, E.S.; Ko, C.H.; Hurley, L.A.; Luciano, J.P.; Kouri, F.M.; Merkel, T.J.; Luthi, A.J.; Patel, P.C.; Cutler, J.I. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013, 5, ra152–ra209. [Google Scholar] [CrossRef]
- Grafals-Ruiz, N.; Rios-Vicil, C.I.; Lozada-Delgado, E.L.; Quiñones-Díaz, B.I.; Noriega-Rivera, R.A.; Martínez-Zayas, G.; Santana-Rivera, Y.; Santiago-Sánchez, G.S.; Valiyeva, F.; Vivas-Mejía, P.E. Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. Int. J. Nanomed. 2020, 15, 2809–2828. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Masitas, R.A.; Zamborini, F.P. Oxidation of Highly Unstable <4 nm Diameter Gold Nanoparticles 850 mV Negative of the Bulk Oxidation Potential. J. Am. Chem. Soc. 2012, 134, 5014–5017. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet Chemical Synthesis of High Aspect Ratio Cylindrical Gold Nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.; Xu, Q.; Wang, J.; Wang, L.; Lou, X. Zeta-potential data reliability of gold nanoparticle biomolecular conjugates and its application in sensitive quantification of surface absorbed protein. Colloids Surf. B Biointerfaces 2016, 148, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, E.U.; Stolarczyk, K.; Łaszcz, M.; Kubiszewski, M.; Leś, A.; Michalak, O. Pemetrexed conjugated with gold nanoparticles—Synthesis, characterization and a study of noncovalent interactions. Eur. J. Pharm. Sci. 2017, 109, 13–20. [Google Scholar] [CrossRef]
- Subramaniam, V.D.; Ramachandran, M.; Marotta, F.; Banerjee, A.; Sun, X.F.; Pathak, S. Comparative study on anti-proliferative potentials of zinc oxide and aluminium oxide nanoparticles in colon cancer cells. Acta Biomed. 2019, 90, 241–247. [Google Scholar]
- Khademi, Z.; Lavaee, P.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Co-delivery of doxorubicin and aptamer against Forkhead box M1 using chitosan-gold nanoparticles coated with nucleolin aptamer for synergistic treatment of cancer cells. Carbohydr. Polym. 2020, 248, 116735. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Farcas, A.; Janosi, L.; Astilean, S. Size and surface coverage density are major factors in determining thiol modified gold nanoparticles characteristics. Comput. Theor. Chem. 2022, 1209, 113581. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Liaw, J.-W.; Kao, Y.-C.; Huang, M.-Y.; Lee, C.-Y.; Rau, L.-R.; Huang, C.-Y.; Wei, K.-C.; Ye, T.-C. Internalized Gold Nanoparticles Do Not Affect the Osteogenesis and Apoptosis of MG63 Osteoblast-Like Cells: A Quantitative, In Vitro Study. PLoS ONE 2013, 8, e76545. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Jerard, C.; Katiyar, N.; Raju, G.; Shankarappa, S.A. Effect of gold nanoparticle treated dorsal root ganglion cells on peripheral neurite differentiation. Toxicol. Vitr. 2021, 74, 105175. [Google Scholar] [CrossRef]
- Kharazian, B.; Lohse, S.E.; Ghasemi, F.; Raoufi, M.; Saei, A.A.; Hashemi, F.; Farvadi, F.; Alimohamadi, R.; Jalali, S.A.; Shokrgozar, M.A.; et al. Bare surface of gold nanoparticle induces inflammation through unfolding of plasma fibrinogen. Sci. Rep. 2018, 8, 12557. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Šalipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.J. Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol. Oncol. 2015, 9, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Overmeyer, J.H.; Young, A.M.; Bhanot, H.; Maltese, W.A. A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol. Cancer 2011, 10, 69. [Google Scholar] [CrossRef]
- Li, C.; MacDonald, J.I.; Hryciw, T.; Meakin, S.O. Nerve growth factor activation of the TrkA receptor induces cell death, by macropinocytosis, in medulloblastoma Daoy cells. J. Neurochem. 2010, 112, 882–899. [Google Scholar] [CrossRef] [PubMed]
- Overmeyer, J.H.; Kaul, A.; Johnson, E.E.; Maltese, W.A. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. 2008, 6, 965–977. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, G.; Zhang, S.; Nigim, F.; Zhou, G.; Yu, Z.; Song, Y.; Chen, Y.; Li, Y. AS1411-Induced Growth Inhibition of Glioma Cells by Up-Regulation of p53 and Down-Regulation of Bcl-2 and Akt1 via Nucleolin. PLoS ONE 2016, 11, e0167094. [Google Scholar] [CrossRef]
- Khademi, Z.; Ramezani, M.; Alibolandi, M.; Zirak, M.R.; Salmasi, Z.; Abnous, K.; Taghdisi, S.M. A novel dual-targeting delivery system for specific delivery of CRISPR/Cas9 using hyaluronic acid, chitosan and AS1411. Carbohydr. Polym. 2022, 292, 119691. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, N.C.; Chauhan, R.; Bates, P.J.; O’Toole, M.G. Optimization of Tumor Targeting Gold Nanoparticles for Glioblastoma Applications. Nanomaterials 2022, 12, 3869. https://doi.org/10.3390/nano12213869
Allen NC, Chauhan R, Bates PJ, O’Toole MG. Optimization of Tumor Targeting Gold Nanoparticles for Glioblastoma Applications. Nanomaterials. 2022; 12(21):3869. https://doi.org/10.3390/nano12213869
Chicago/Turabian StyleAllen, Nicholas C., Rajat Chauhan, Paula J. Bates, and Martin G. O’Toole. 2022. "Optimization of Tumor Targeting Gold Nanoparticles for Glioblastoma Applications" Nanomaterials 12, no. 21: 3869. https://doi.org/10.3390/nano12213869
APA StyleAllen, N. C., Chauhan, R., Bates, P. J., & O’Toole, M. G. (2022). Optimization of Tumor Targeting Gold Nanoparticles for Glioblastoma Applications. Nanomaterials, 12(21), 3869. https://doi.org/10.3390/nano12213869




