Dual-Responsive Amphiphilic P(DMAEMA-co-LMA-co-OEGMA) Terpolymer Nano-Assemblies in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
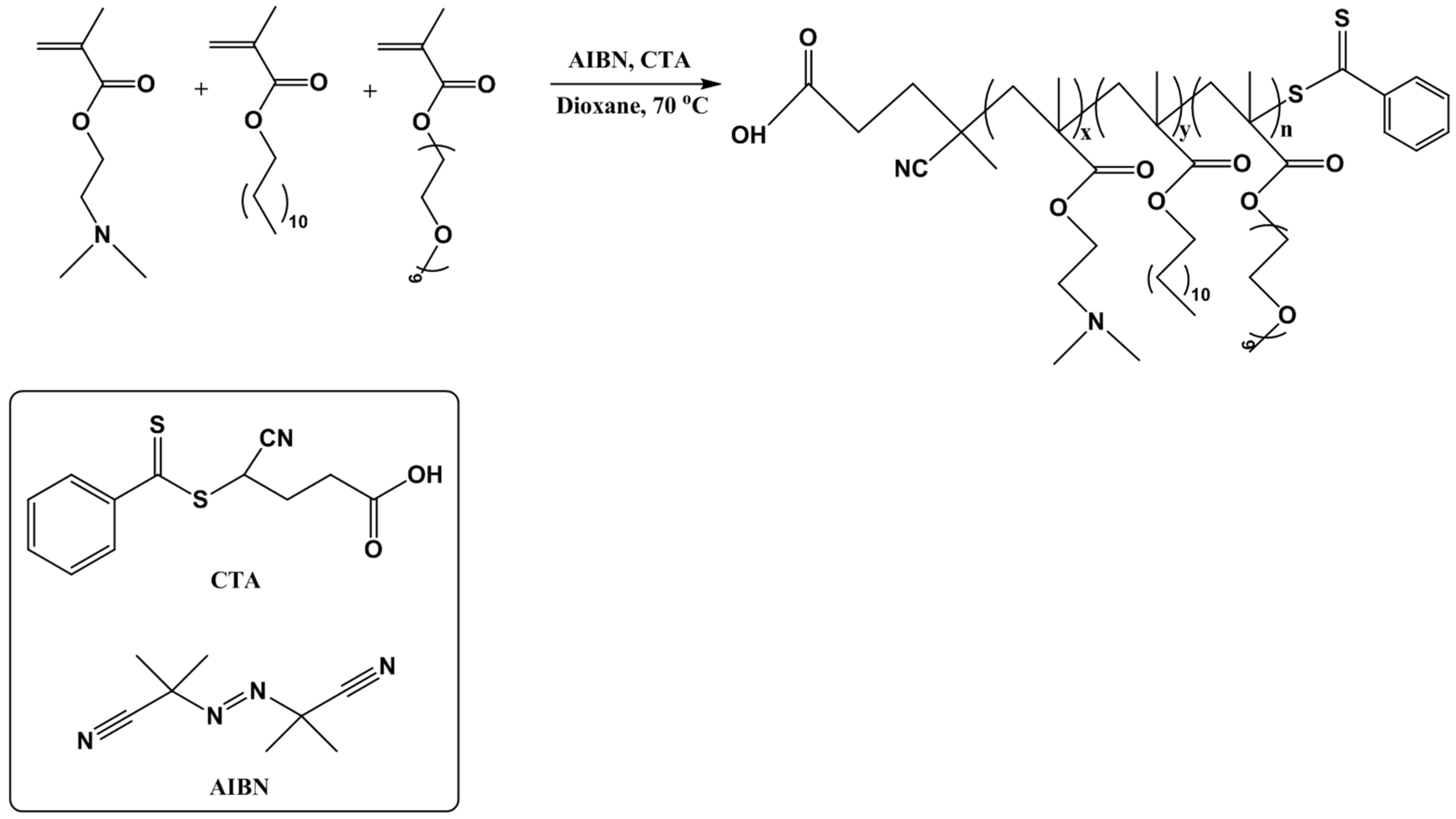
2.2. P(DMAEMA-co-LMA-co-OEGMA) Synthesis
2.3. Self-Assembly of P(DMAEMA-co-LMA-co-OEGMA) in Aqueous Media
2.4. Ionic Strength Study
2.5. Curcumin Encapsulation into P(DMAEMA-co-LMA-co-OEGMA) Aggregates
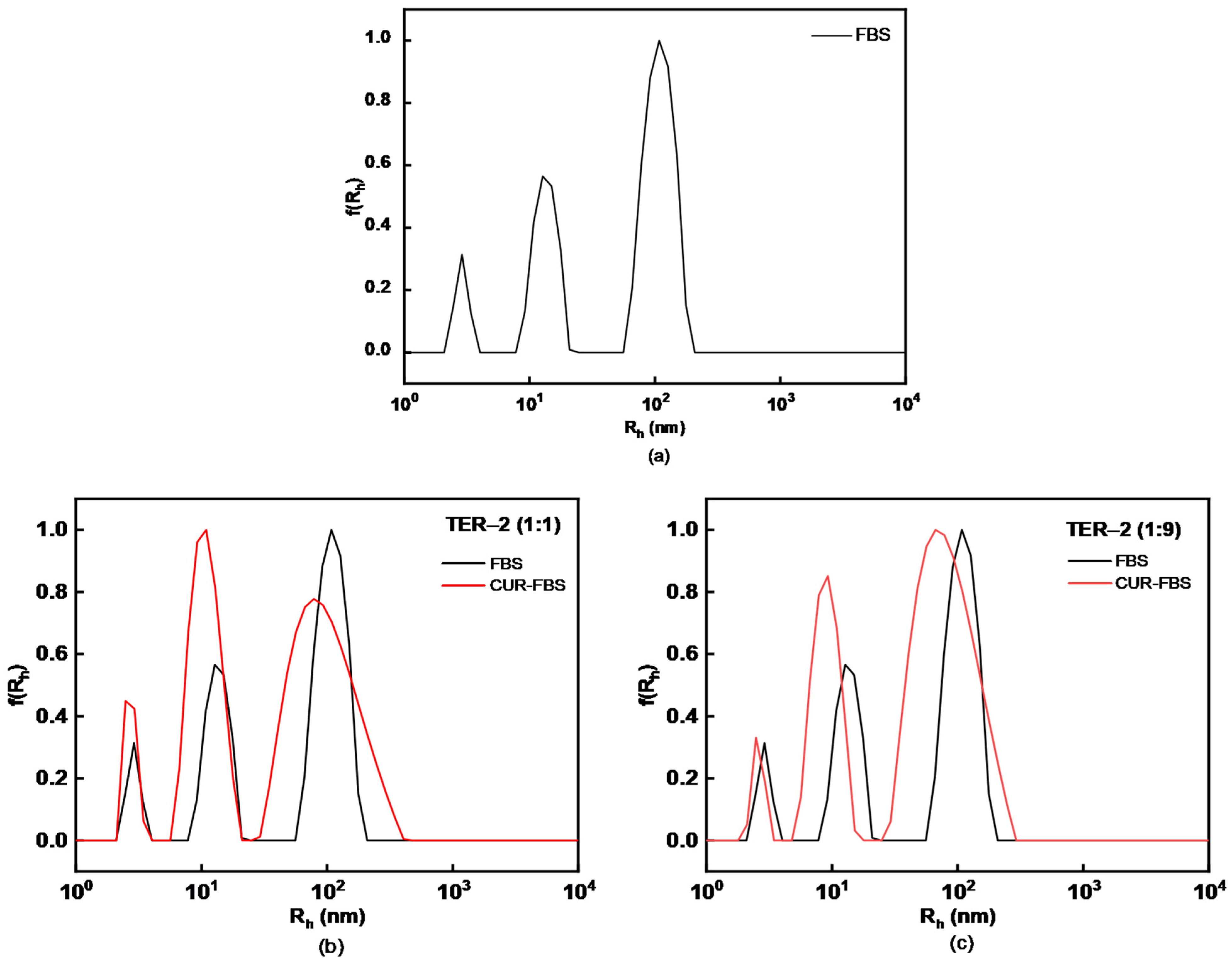
2.6. FBS Interaction Study
2.7. Characterization Methods
2.7.1. Size Exclusion Chromatography (SEC)
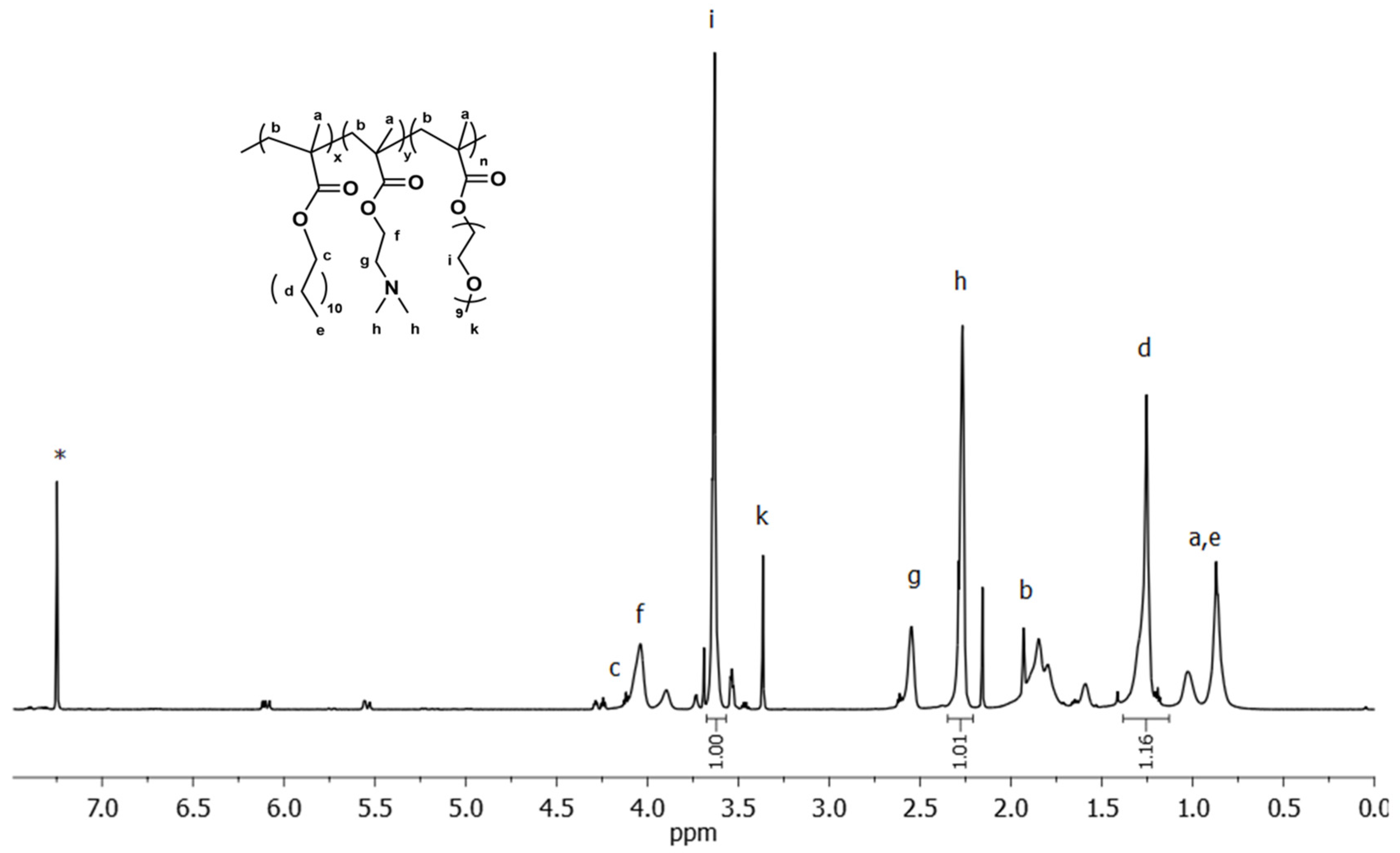
2.7.2. 1H-NMR Spectroscopy
2.7.3. Dynamic Light Scattering (DLS)
2.7.4. Fluorescence Spectroscopy (FS)
2.7.5. UV-Vis Spectroscopy
2.7.6. Electrophoretic Light Scattering (ζ-Potential)
3. Results and Discussion
3.1. Synthesis and Molecular Characterization
3.2. Self-Assembly in Aqueous Media
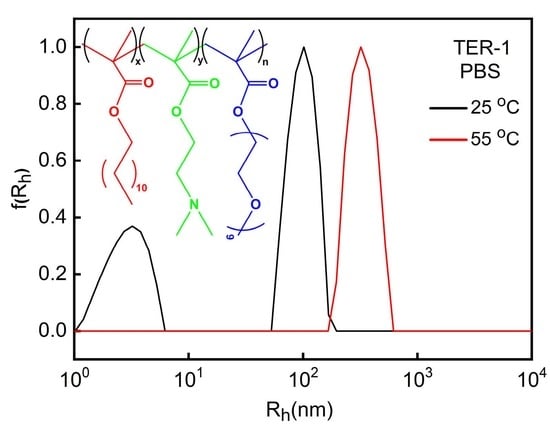
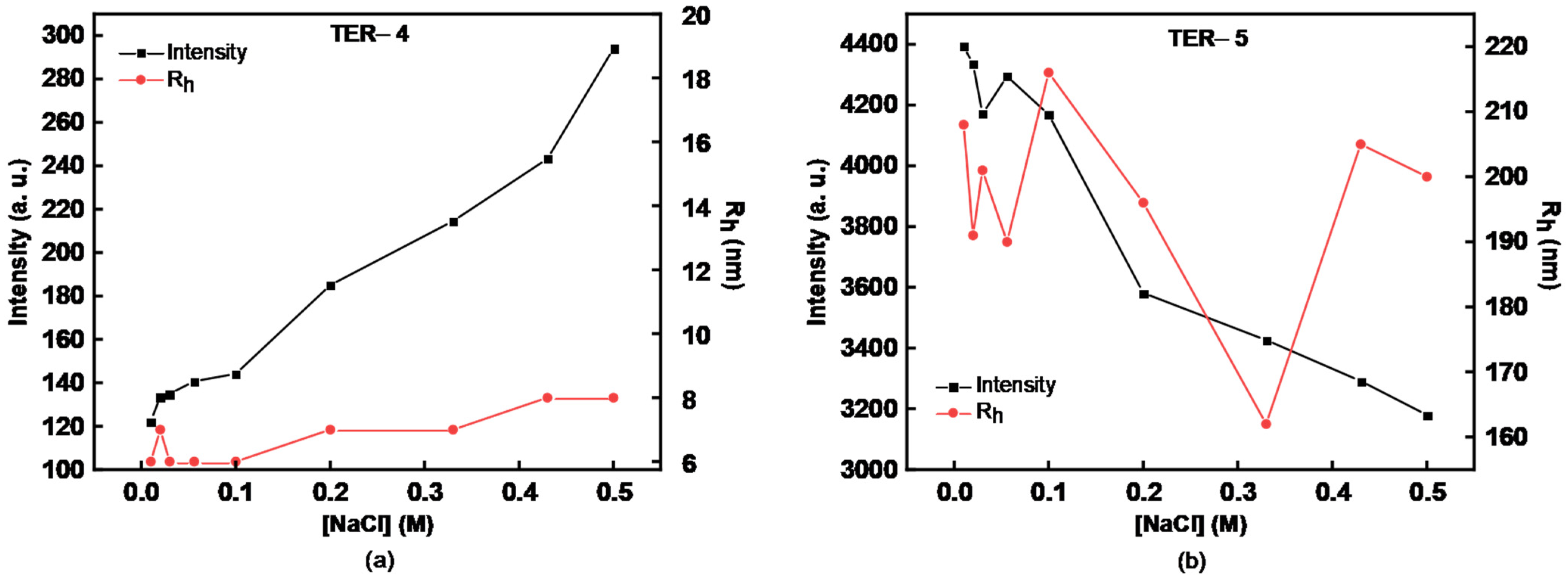
3.3. Effect of Ionic Strength on P(DMAEMA-co-LMA-co-OEGMA) Solution Assembly
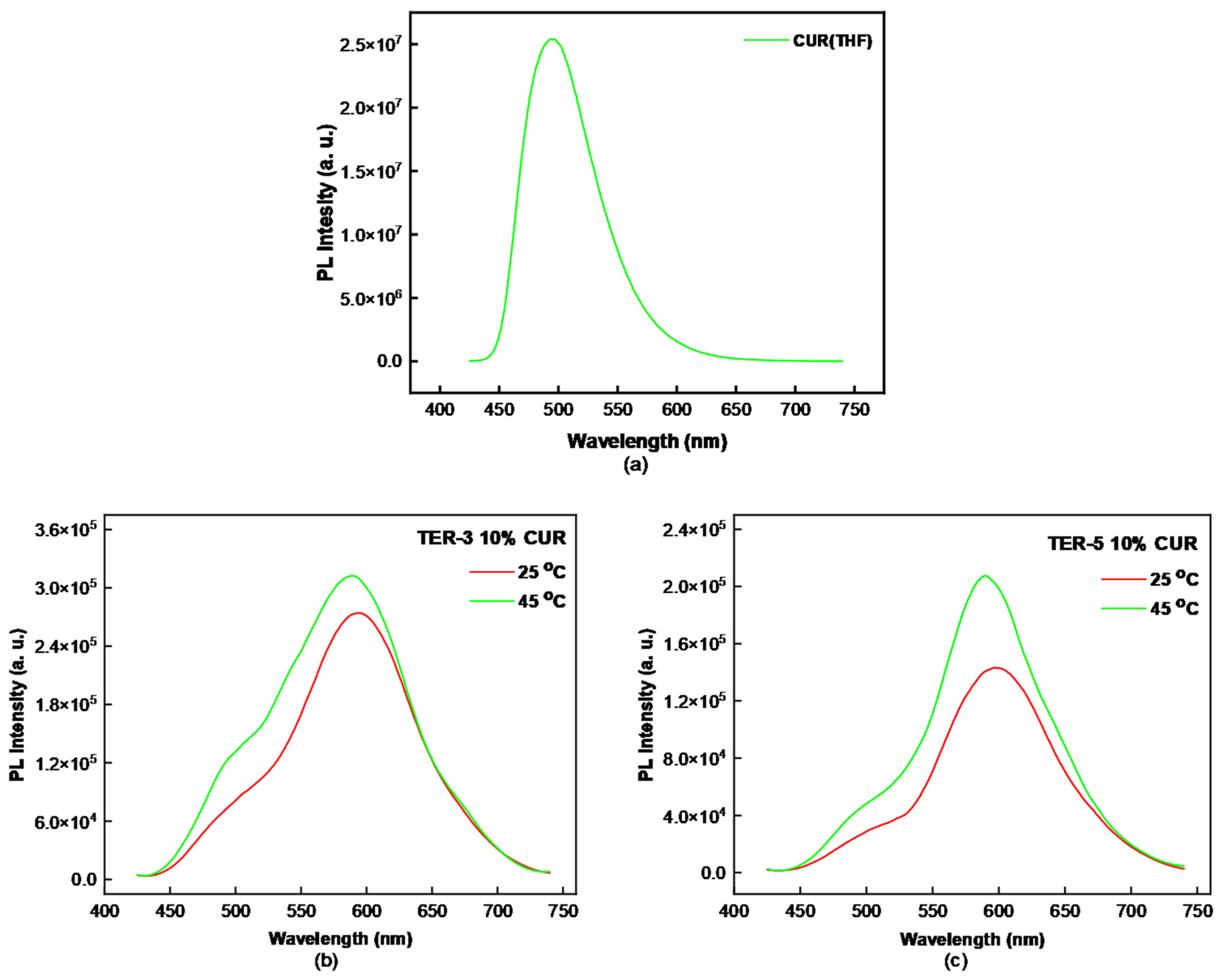
3.4. Curcumin Encapsulation in P(DMAEMA-co-LMA-co-OEGMA) Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef]
- Förster, S.; Antonietti, M. Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv. Mater. 1998, 10, 195–217. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- York, A.W.; Kirkland, S.E.; McCormick, C.L. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Cansell, F.; Siove, A.; Belorgey, G. Ethylene-propylene copolymerization initiated with solubilized ziegler-natta macromolecular complexes. II. Characterization of [styrene]-b-[ethylene-co-propylene] copolymers. J. Polym. Sci. B Polym. Phys. 1990, 28, 647–654. [Google Scholar] [CrossRef]
- Li, L.; Raghupathi, K.; Song, C.; Prasad, P.; Thayumanavan, S. Self-assembly of random copolymers. Chem. Commun. 2014, 50, 13417–13432. [Google Scholar] [CrossRef] [Green Version]
- Ting, J.M.; Navale, T.S.; Bates, F.S.; Reineke, T.M. Precise compositional control and systematic preparation of multimonomeric statistical copolymers. ACS Macro Lett. 2013, 2, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Terashima, T.; Sawamoto, M. Self-Assembly of Amphiphilic Random Copolyacrylamides into Uniform and Necklace Micelles in Water. Macromol. Chem. Phys. 2017, 218, 1700230. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Terashima, T.; Matsumoto, K.; Takenaka, M.; Sawamoto, M. Compartmentalization technologies via self-assembly and cross-linking of amphiphilic random block copolymers in water. J. Am. Chem. Soc. 2017, 139, 7164–7167. [Google Scholar] [CrossRef]
- Alexandridis, P. Amphiphilic copolymers and their applications. Curr. Opin. Colloid Interface Sci. 1996, 1, 490–501. [Google Scholar] [CrossRef]
- Laaser, J.E.; Jiang, Y.; Sprouse, D.; Reineke, T.M.; Lodge, T.P. pH-and ionic-strength-induced contraction of polybasic micelles in buffered aqueous solutions. Macromolecules 2015, 48, 2677–2685. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. PDEGMA-b-PDIPAEMA copolymers via RAFT polymerization and their pH and thermoresponsive schizophrenic self-assembly in aqueous media. J. Polym. Sci. 2020, 58, 1867–1880. [Google Scholar] [CrossRef]
- Hamidi, M.; Shahbazi, M.A.; Rostamizadeh, K. Copolymers: Efficient carriers for intelligent nanoparticulate drug targeting and gene therapy. Macromol. Biosci. 2012, 12, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch it inside-out: “schizophrenic” behavior of all thermoresponsive UCST–LCST diblock copolymers. Langmuir 2019, 35, 9660–9676. [Google Scholar] [CrossRef]
- Van Durme, K.; Rahier, H.; Van Mele, B. Influence of additives on the thermoresponsive behavior of polymers in aqueous solution. Macromolecules 2005, 38, 10155–10163. [Google Scholar] [CrossRef]
- Boutris, C.; Chatzi, E.; Kiparissides, C. Characterization of the LCST behaviour of aqueous poly (N-isopropylacrylamide) solutions by thermal and cloud point techniques. Polymer 1997, 38, 2567–2570. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT agent design and synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Skandalis, A.; Pispas, S. PDMAEMA-b-PLMA-b-POEGMA triblock terpolymers via RAFT polymerization and their self-assembly in aqueous solutions. Polym. Chem. 2017, 8, 4538–4547. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co) polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Cotanda, P.; Wright, D.B.; Tyler, M.; O’Reilly, R.K. A comparative study of the stimuli-responsive properties of DMAEA and DMAEMA containing polymers. J. Polym. Sci. A Polym. Chem. 2013, 51, 3333–3338. [Google Scholar] [CrossRef]
- Allmeroth, M.; Moderegger, D.; Gündel, D.; Koynov, K.; Buchholz, H.-G.; Mohr, K.; Rösch, F.; Zentel, R.; Thews, O. HPMA-LMA copolymer drug carriers in oncology: An in vivo PET study to assess the tumor line-specific polymer uptake and body distribution. Biomacromolecules 2013, 14, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F. Polymerization of oligo (ethylene glycol) (meth) acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Kafetzi, M.; Pispas, S. Multifaceted pH and Temperature Induced Self-Assembly of P (DMAEMA-co-LMA)-b-POEGMA Terpolymers and Their Cationic Analogues in Aqueous Media. Macromol. Chem. Phys. 2021, 222, 2000358. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A.; Pispas, S. Self-assembly of poly (ethylene glycol-b-phenyl oxazoline) diblock copolymers in aqueous media and their interactions with proteins. Colloid Polym. Sci. 2017, 295, 1359–1369. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. P (MMA-co-HPMA)-b-POEGMA copolymers: Synthesis, micelle formation in aqueous media and drug encapsulation. Polym. Int. 2021, 70, 1508–1522. [Google Scholar] [CrossRef]
- Liu, M.; Teng, C.P.; Win, K.Y.; Chen, Y.; Zhang, X.; Yang, D.P.; Li, Z.; Ye, E. Polymeric encapsulation of turmeric extract for bioimaging and antimicrobial applications. Macromol. Rapid Commun. 2019, 40, 1800216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Ha, P.T.; Sao Nguyen, A.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025001. [Google Scholar] [CrossRef]
- Bechnak, L.; El Kurdi, R.; Patra, D. Fluorescence Sensing of Nucleic Acid by Curcumin Encapsulated Poly (Ethylene Oxide)-Block-Poly (Propylene Oxide)-Block-Poly (Ethylene Oxide) Based Nanocapsules. J. Fluoresc. 2020, 30, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Selianitis, D.; Pispas, S. Multi-responsive poly (oligo (ethylene glycol) methyl methacrylate)-co-poly (2-(diisopropylamino) ethyl methacrylate) hyperbranched copolymers via reversible addition fragmentation chain transfer polymerization. Polym. Chem. 2021, 12, 6582–6593. [Google Scholar] [CrossRef]
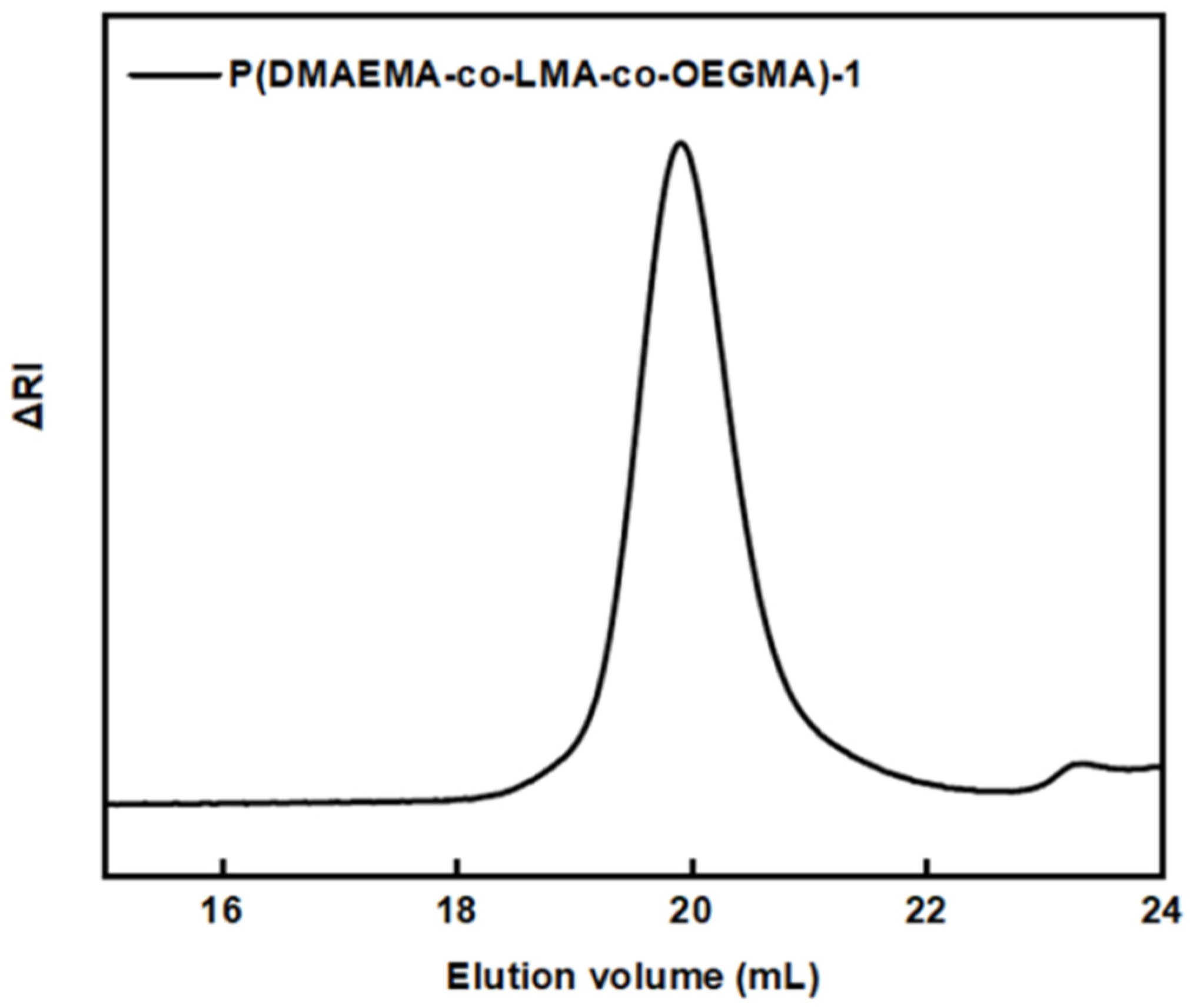
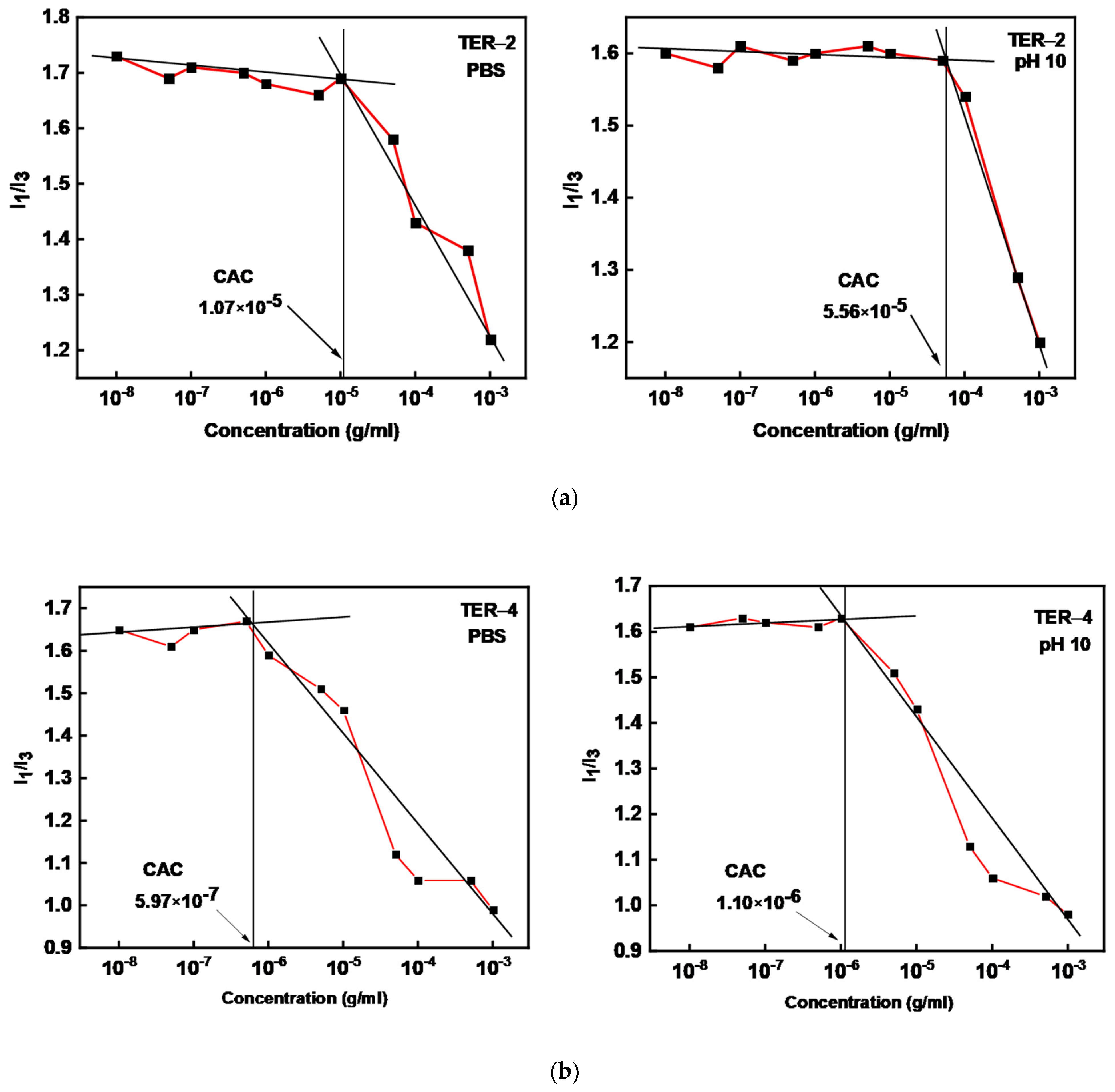
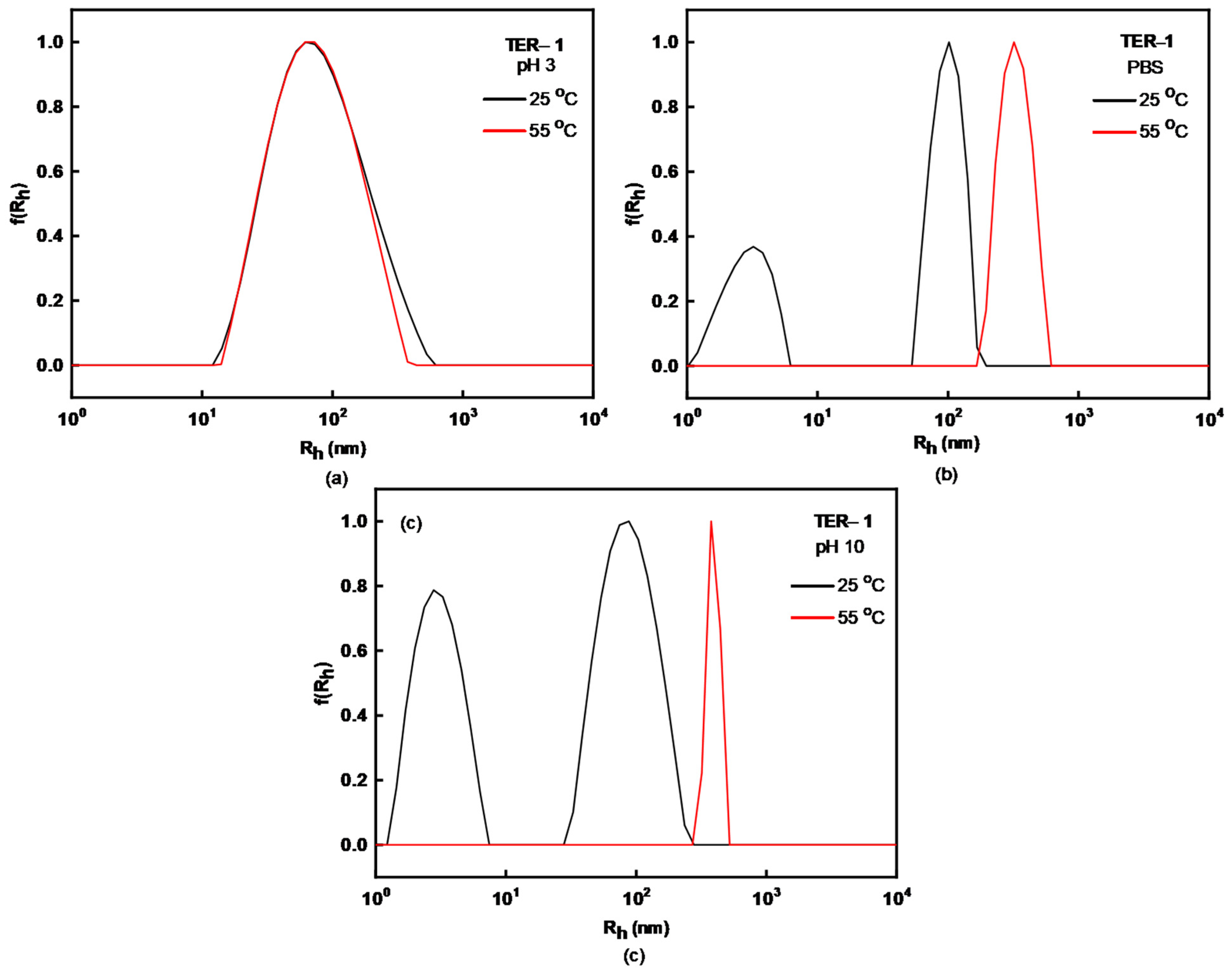
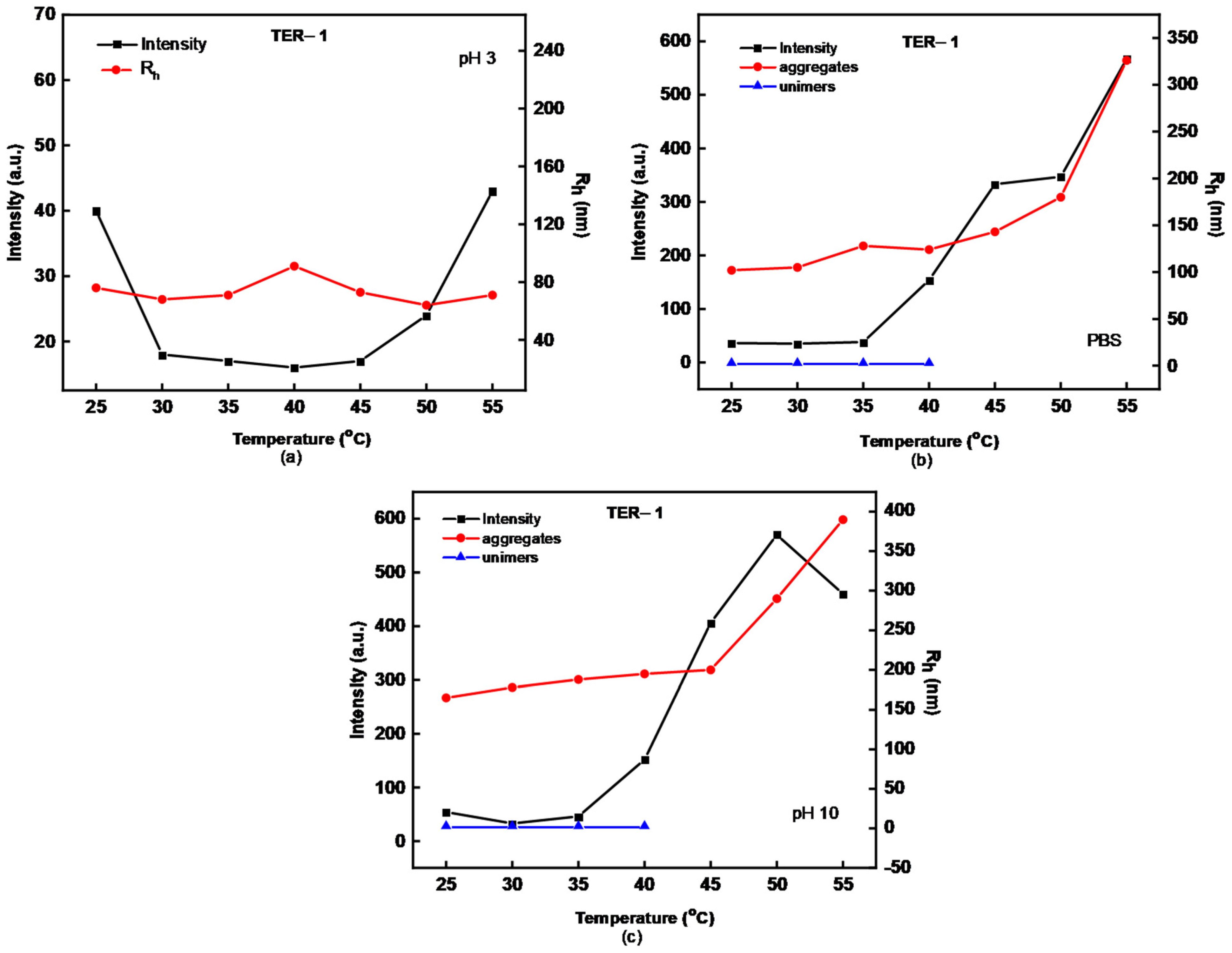

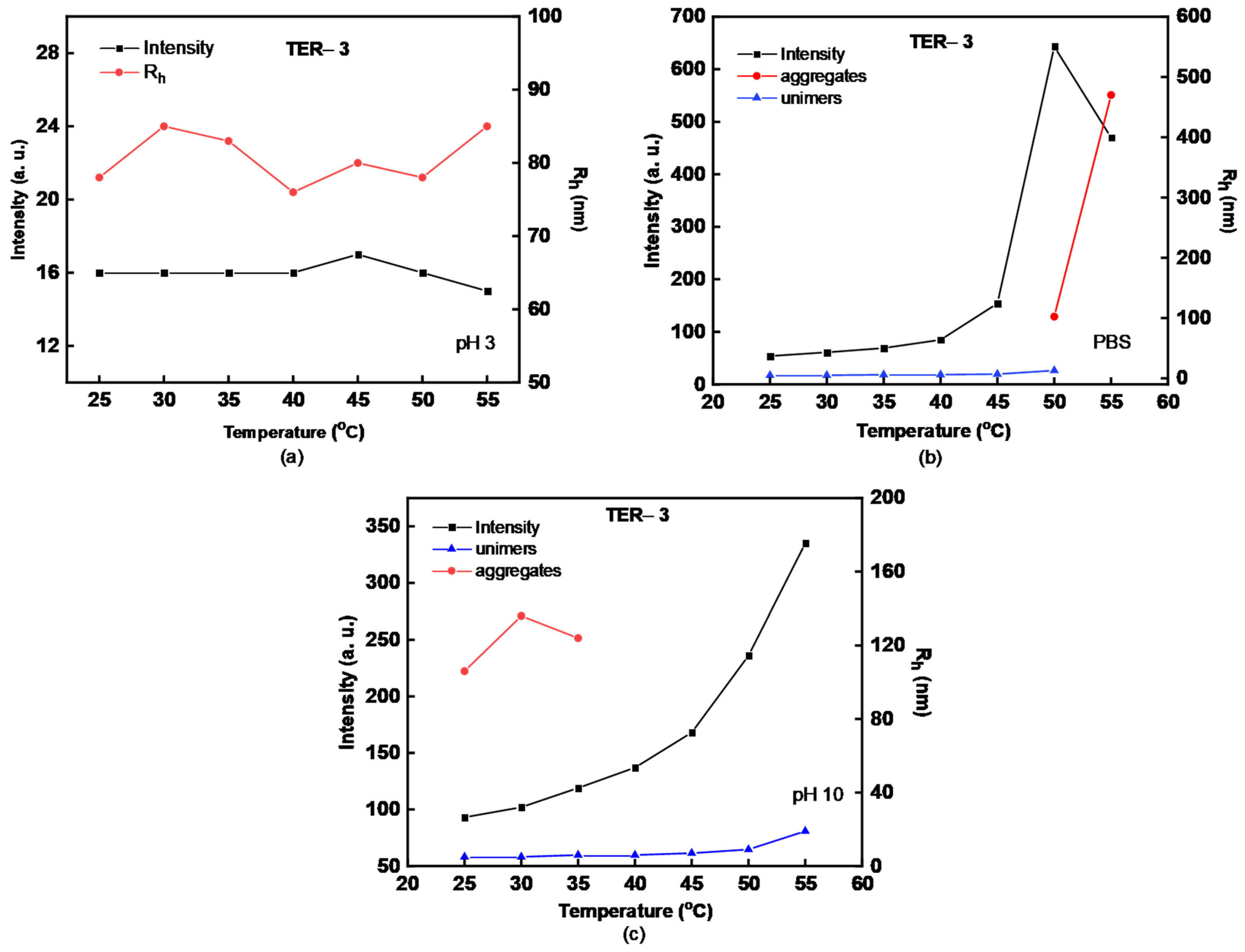


| Sample | MW (×104) (g/mol) a | MW/Mn a | %wt DMAEMA b | %wt LMA b | %wt OEGMA b | % Yield |
|---|---|---|---|---|---|---|
| TER-1 | 1.8 | 1.09 | 66 | 10 | 24 | 66.7 |
| TER-2 | 1.1 | 1.16 | 62 | 11 | 27 | 70.5 |
| TER-3 | 1.72 | 1.1 | 35 | 29 | 36 | 53.3 |
| TER-4 | 1.67 | 1.09 | 20 | 48 | 32 | 92.4 |
| TER-5 | 1.4 | 1.1 | 49 | 27 | 24 | 76.7 |
| Sample | T (°C) | pH | Intensity | Rh (nm) | PDI | ζp (mv) |
|---|---|---|---|---|---|---|
| TER-1 | 25 °C | pH 3 | 40 | 76 | 0.6 | 26.5 |
| PBS | 36 | 3/102 | 0.6 | – | ||
| pH 10 | 54 | 3/165 | 0.6 | −35.5 | ||
| 55 °C | pH 3 | 43 | 71 | 0.5 | – | |
| PBS | 567 | 326 | 0.3 | – | ||
| pH 10 | 460 | 390 | 0.2 | – | ||
| TER-3 | 25 °C | pH 3 | 16 | 78 | 0.7 | 5.0 |
| PBS | 54 | 5/173 | 0.4 | – | ||
| pH 10 | 93 | 5/106 | 0.4 | −18.0 | ||
| 55 °C | pH 3 | 15 | 85 | 0.6 | – | |
| PBS | 470 | 532 | 3 | – | ||
| pH 10 | 335 | 19 | 0.2 | – |
| Sample * | Protocol | CUR Used (mg) | Max Encapsulation (% w/w) | % Encapsulation Efficiency (EE) | % Drug Loading (DL) |
|---|---|---|---|---|---|
| TER-1 | THF | 0.1 | 10 | 55.2 | 0.55 |
| 0.2 | 20 | 49.7 | 0.99 | ||
| Thin film | 0.1 | 10 | 83.4 | 0.83 | |
| 0.2 | 20 | 92.2 | 1.8 | ||
| TER-2 | THF | 0.1 | 10 | 68.0 | 0.75 |
| 0.2 | 20 | 47.4 | 1.04 | ||
| Thin film | 0.1 | 10 | 70.9 | 0.78 | |
| 0.2 | 20 | 95.7 | 2.11 |
| Sample * | Protocol | Rh (nm) without CUR | PDI without CUR | % CUR | Rh (nm) with CUR | PDI with CUR |
|---|---|---|---|---|---|---|
| TER-1 | THF | 3/102 | 0.6 | 10 | 3/112 | 0.5 |
| 20 | 99 | 0.5 | ||||
| Thin film | 3/100 | 0.5 | 10 | 3/149 | 0.4 | |
| 20 | 6/288 | 0.5 | ||||
| TER-2 | THF | 3/108 | 0.5 | 10 | 93 | 0.3 |
| 20 | 73 | 0.2 | ||||
| Thin film | 72 | 0.2 | 10 | 3/108 | 0.6 | |
| 20 | 15.2 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomara, M.; Selianitis, D.; Pispas, S. Dual-Responsive Amphiphilic P(DMAEMA-co-LMA-co-OEGMA) Terpolymer Nano-Assemblies in Aqueous Media. Nanomaterials 2022, 12, 3791. https://doi.org/10.3390/nano12213791
Tomara M, Selianitis D, Pispas S. Dual-Responsive Amphiphilic P(DMAEMA-co-LMA-co-OEGMA) Terpolymer Nano-Assemblies in Aqueous Media. Nanomaterials. 2022; 12(21):3791. https://doi.org/10.3390/nano12213791
Chicago/Turabian StyleTomara, Maria, Dimitrios Selianitis, and Stergios Pispas. 2022. "Dual-Responsive Amphiphilic P(DMAEMA-co-LMA-co-OEGMA) Terpolymer Nano-Assemblies in Aqueous Media" Nanomaterials 12, no. 21: 3791. https://doi.org/10.3390/nano12213791
APA StyleTomara, M., Selianitis, D., & Pispas, S. (2022). Dual-Responsive Amphiphilic P(DMAEMA-co-LMA-co-OEGMA) Terpolymer Nano-Assemblies in Aqueous Media. Nanomaterials, 12(21), 3791. https://doi.org/10.3390/nano12213791