Abstract
Infections caused by multidrug-resistant (MDR) bacteria are becoming a serious threat to public health worldwide. With an ever-reducing pipeline of last-resort drugs further complicating the current dire situation arising due to antibiotic resistance, there has never been a greater urgency to attempt to discover potential new antibiotics. The use of nanotechnology, encompassing a broad range of organic and inorganic nanomaterials, offers promising solutions. Organic nanomaterials, including lipid-, polymer-, and carbon-based nanomaterials, have inherent antibacterial activity or can act as nanocarriers in delivering antibacterial agents. Nanocarriers, owing to the protection and enhanced bioavailability of the encapsulated drugs, have the ability to enable an increased concentration of a drug to be delivered to an infected site and reduce the associated toxicity elsewhere. On the other hand, inorganic metal-based nanomaterials exhibit multivalent antibacterial mechanisms that combat MDR bacteria effectively and reduce the occurrence of bacterial resistance. These nanomaterials have great potential for the prevention and treatment of MDR bacterial infection. Recent advances in the field of nanotechnology are enabling researchers to utilize nanomaterial building blocks in intriguing ways to create multi-functional nanocomposite materials. These nanocomposite materials, formed by lipid-, polymer-, carbon-, and metal-based nanomaterial building blocks, have opened a new avenue for researchers due to the unprecedented physiochemical properties and enhanced antibacterial activities being observed when compared to their mono-constituent parts. This review covers the latest advances of nanotechnologies used in the design and development of nano- and nanocomposite materials to fight MDR bacteria with different purposes. Our aim is to discuss and summarize these recently established nanomaterials and the respective nanocomposites, their current application, and challenges for use in applications treating MDR bacteria. In addition, we discuss the prospects for antimicrobial nanomaterials and look forward to further develop these materials, emphasizing their potential for clinical translation.
1. Introduction
Antibiotics have been the primary treatment choice for use on bacterial infections due to their cost efficiency and powerful and fast-acting outcomes. However, bacteria possess the intrinsic ability to evolve rapidly through mutations in developing resistance to these treatments. In addition, bacteria can transfer drug-resistant genes among their community through horizontal gene transfer, resulting in the emergence of multidrug-resistant (MDR) bacteria, which are widely known as superbugs as defined by the medical and research communities [1]. Since bacterial resistance emerges and spreads via the acquisition of genetic material from resistant bacterial cells, the evolution of antibiotic resistance is unstoppable [2]. Recent projections indicate that a post-antibiotic era is approaching, and this will result in approximately 10 million annual deaths by 2050 from MDR bacterial infections [3]. Studies have shown that infections caused by multidrug-resistant bacteria cause more harm and higher patient mortality than infections caused by susceptible strains of the same species [4]. A continual increase in the numbers of infections resulting from such resistant strains poses a serious threat globally [5].
The antibiotic resistance crisis is further complicated by a lack of new antibacterial agents to act as last-line defenders for the treatment of MDR bacterial infections. For instance, the World Health Organization (WHO) has identified 80 antibacterial agents that are under clinical development to treat top-priority MDR bacteria up to November 2021, but most of these are modifications of current antibiotics and will act merely as short-term solutions [6]. Only seven of these antibacterial agents are novel chemical entities that will contribute to expanding the current antibiotic pipeline [6]. Due to economic and regulatory hurdles, the biopharmaceutical industry has largely withdrawn from developing new antibiotics, further exacerbating the situation [7]. This has triggered initiatives worldwide to discover and exploit novel antibacterial agents in order to prevent these infections from happening and to overcome the current challenges faced from MDR infections [8]. Promising solutions for the prevention and treatment of MDR bacterial infections are under investigation, such as nanotechnology and biomaterials [9].
Nanotechnology serves as an alternative promising solution for the prevention and treatment of MDR bacterial infection. Nanotechnology plays an important role in this area by covering a broad range of nanostructured materials that possess inherent antibacterial activity. Nanomaterials also show significant potential for delivering drugs to specific targeted sites in vivo [10]. Nanomaterials have at least one dimension in the nano range (1–100 nm) that convey particular and variable physiochemical properties from their bulk constituents [11]. The nanosized scale of these nanomaterials can result in multivalent interactions with bacteria, including electrostatic attractions, hydrophobic and receptor–ligand interactions, and van der Waals (hydrophobic) forces [12]. This offers particular advantages compared to small molecule antibiotics that typically result in a single mode of interaction. The ease of functionalization and engineering of nanomaterials confers them with additional advantages for mechanistically overcoming bacterial resistance [13].
Nanomaterials can be broadly classified into organic nanomaterials and inorganic nanomaterials [14]. Recent advances of nanotechnology have brought novel understandings in using nanosized building blocks to design and create new nanocomposites or nanohybrid materials with unprecedented physical properties and enhanced antibacterial activity [15]. A variety of nanomaterials can be combined to develop new nanocomposite materials, with the most-established examples being depicted in the section below. In this review, we illustrate that each category of these antibacterial nanomaterials has its own distinctive characteristics and properties which are being applied to various antibacterial applications. We present recent advances in developing the use of these nanomaterials in combating MDR bacterial infections. However, the use of nanocomposites is still at an early stage and more research and investment is needed towards these efforts before we start seeing outcomes from their clinical translation. Based on these, this review summarizes previous research progress on nanotechnology in antibacterial aspects which focuses on the last 5 years, including a detailed summary and comparison of the most promising and interesting nanomaterials (Table 1). The aim is to inspire future research ideas in this field by identifying gaps or inconsistencies in the body of knowledge.

Table 1.
Summary table of the nanomaterials.
2. Organic Nanomaterials
Organic nanomaterials usually comprise carbon and hydrogen atoms that form, most simply, hydrocarbon-based molecules. Organic nanomaterials can be designed to those that may self-assemble into nanostructures with different dimensionalities or desired characteristics by utilizing the weak intermolecular interactions of organic molecular structures [28]. Organic nanomaterials can be classified into lipid-based, polymer-based, and carbon-based nanomaterials, and these nanomaterials can be designed to act as nanocarriers or antibacterial agents in antibacterial applications.
2.1. Lipid-Based Nanomaterials
A variety of lipid candidates, including free fatty acids, phospholipids, glycolipids, sphingolipids, fatty alcohols, glycerol esters, and waxes, can be utilized to nanoformulate into different classes of lipid-based nanoparticles including liposomes, emulsions, solid-lipid nanoparticles, and nanostructured lipid carriers [29]. A detailed review of these aforementioned nanoparticles has been described elsewhere [30,31,32] and will not be discussed here. Lipid-based nanoparticles are the most-established nanocarriers investigated for the delivery of a variety of pharmaceutical agents with different solubilities and pharmacokinetic behaviors. In addition to the role of nanocarrier, lipid-based nanoparticles have an emerging role as antibacterial agents against MDR bacteria. In short, these can be classified into lipidic nanocarriers and lipidic nanoparticles. Lipidic nanocarriers contain and deliver antibacterial agents including antibiotics and antimicrobial peptides, whilst lipidic nanoparticles themselves display inherent antibacterial properties.
2.1.1. Lipidic Nanocarriers as Delivery Vehicles for Antimicrobial Agents
Lipidic nanocarriers are the most-established nanocarriers utilized for delivery of a variety of pharmaceutical agents with different solubilities. Lipidic nanocarriers are composed of colloidal dispersions of physiological or physiological-related lipids (natural or synthetic lipids that have the similar chemical structure to physiological lipids) in aqueous solution. Generally, these dispersions are stabilized by an emulsifier or surfactant which intercalates on the lipid nanoparticle’s surfaces. This provides the nanoparticle stability by conferring steric stabilization in between the nanoparticles and reducing the interfacial energy between the lipidic nanoparticles and the aqueous phase [33]. In brief, lipidic nanocarriers such as liposomes [16,34,35,36,37], micelles [38,39,40], nanocapsules [41,42,43,44], emulsions [45,46], and solid lipid nanoparticles [29,47] have several advantages for delivering antimicrobial agents [48]. Lipidic nanocarriers can exhibit good biocompatibility and non-immunogenic properties due to the analogous behavior of the physiological or physiological-related lipids to biological membranes as seen in the new SARS-CoV-2 lipid-based mRNA vaccines. The encapsulation of drugs enhances their bioavailability, increases the feasibility for various routes of administration, reduces associated drug toxicity, and protects the drugs from metabolic degradation. Furthermore, drug encapsulation into lipid-based nanoparticles also improves the pharmacokinetic and pharmacodynamic profiles, which lowers the required dosages and improves the therapeutic index. Lastly, surface modifications of lipid-based nanoparticles can be achieved for various purposes, such as targeted therapies, improved cellular uptake, and increased circulation times and half-lives.
The use of these lipidic nanocarriers for delivery can have some limitations, including occasional poor colloidal or thermodynamic/kinetic stability for long-term storage, high membrane permeability that accounts for drug leakage, and low entrapment efficiency for certain hydrophobic drugs [17]. This often leads to costly and restricted preparation conditions that allows reconstitution of lipidic nanocarriers in solution prior to administration [49]. One of the potential solutions is to combine lipid-based nanoparticles with polymeric nanomaterials, forming lipid–polymer hybrid nanoparticles for delivery purposes, which will be described in Section 4.5. Despite being the most widely explored nanoparticulate delivery system for various pharmaceutical products, the role of lipidic nanocarriers in antibacterial application is limited. Currently, only one liposomal formulation (amikacin liposome inhalation suspension, Arikayce) is approved by the Food and Drug Administration (FDA) (ClinicalTrials.gov Identifier: NCT01316276) for the treatment of mycobacterial lung infection. In addition, there are a few liposomal nanoformulations delivering antibacterial agents that are undergoing clinical trials, with the details shown in Table 2.

Table 2.
Liposomal nanoformulation in clinical development for antibacterial therapy.
As compared with liposomes, other lipidic nanocarriers are still in the early stages of development [50,51,52,53]. Recently, non-lamellar lyotropic liquid crystalline nanoparticles including cubosomes and hexosomes have emerged to be the next generation of smart lipidic nanoparticles [54,55,56,57] for antimicrobial therapeutics. The antibiotic potential of cubosomes with a series of magnetite (Fe3O4), copper oxide (Cu2O), and silver (Ag) nanocrystals were developed by Meikle et al. [58]. The results showed that Ag nanocrystal-embedded cubosomes displayed exhibitory activity against both Gram-positive and Gram-negative bacteria, with observed minimum inhibitory concentration values ranging from 15.6–250 μg/mL. Recent studies have shown that polymyxin-loaded cubosomes can enhance antibacterial potency against Gram-negative bacteria, including polymyxin-resistant strains, and enable an alternative strategy for treating pathogens by combining cubosomes with polymyxins as a combination therapy [57]. To overcome the difficulty of using antimicrobial peptides in antibiotic therapies due to their lack of specificity and their susceptibility to in vivo proteolysis, Boge et al. used cubosomes to topically deliver the antimicrobial peptides, LL-37, to inhibit S. aureus. They found that the pre-loading preparation where incorporation of LL-37 into liquid crystal gels followed by dispersion into nanoparticles was most effective in killing S. aureus [55]. Additional studies have been reported investigating the use of cubosomes as drug delivery vehicles for LL-37. It was observed that the cubosomes successfully protected LL-37 from proteolytic degradation with significantly enhanced bactericidal effects against Gram-negative strains [59]. Meikle et al. explored the potential of cubosomes as delivery vehicles for six different antimicrobial peptides, including gramicidin A, alamethicin, melittin, indolicidin, pexiganan, and cecropin A [60], wherein it was observed that by adding physiological concentrations of anionic lipids or NaCl to screen the electrostatic charge of peptides, the antimicrobial peptides loading efficiency of the cubosomes was significantly improved, and encapsulation in the cubosome carriers was shown to enhance the antimicrobial activity of certain formulations [60]. Notably, there are fundamental differences in the mechanism of cubosomes uptake between Gram-positive and Gram-negative bacteria. For Gram-positive bacteria, the cubosomes adhere to the exopeptidoglycan layer and slowly internalize into the bacteria, while for Gram-negative bacteria, the interaction occurs in two stages: the cubosomes fuse with the outer lipid membrane and then pass through the inner wall via diffusion [61].
2.1.2. Lipidic Nanoparticles with Inherent Antibacterial Activities
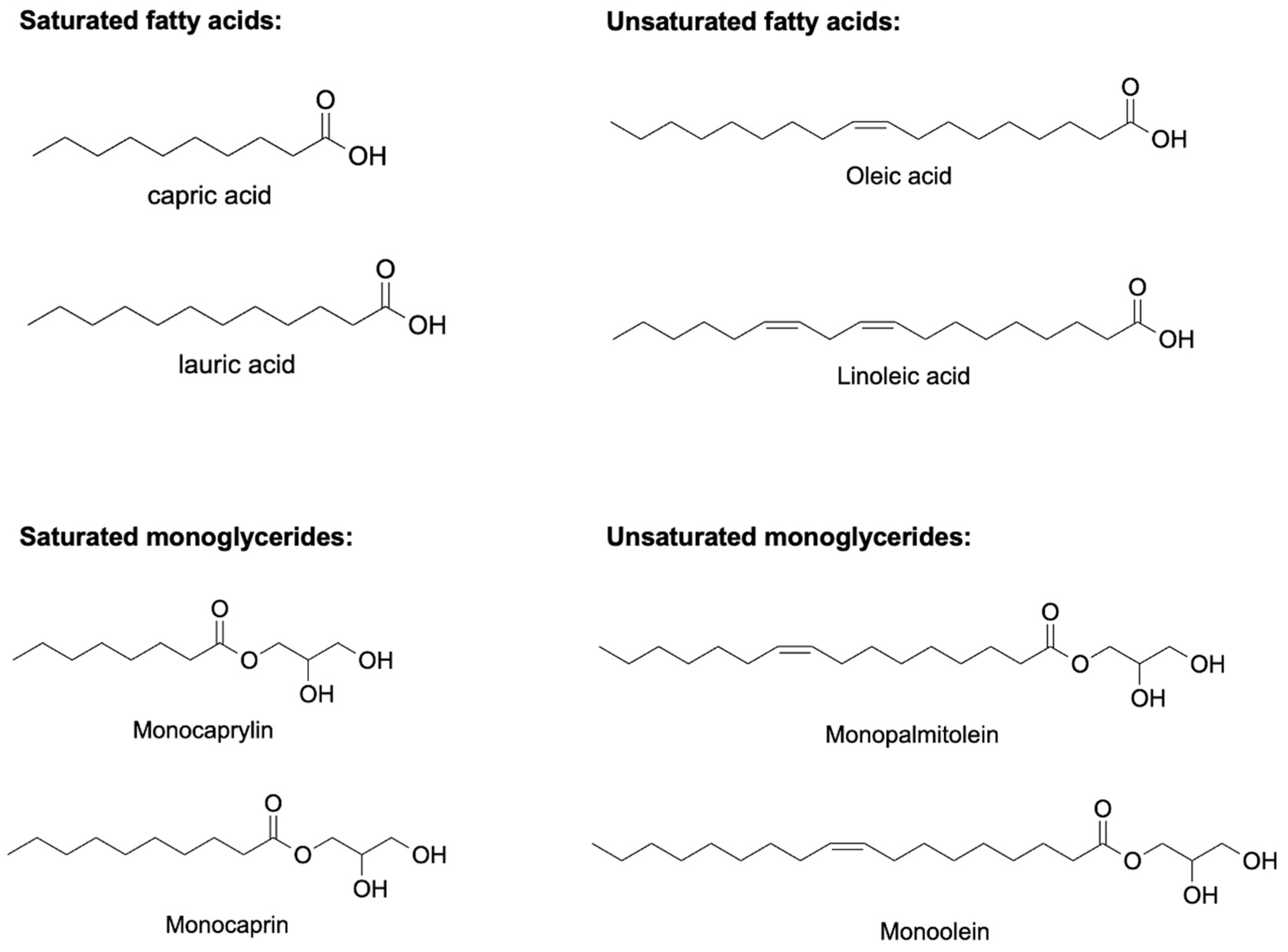
Antimicrobial lipids composed of a carboxylic acid group and a saturated or unsaturated carbon chain (Figure 1) can act as surfactants via a membrane lytic mechanism [62]. Antimicrobial lipids possess broad-spectrum antibacterial activities and serve as new and attractive candidates to fight the antibiotic resistance crisis. However, some technical challenges impede the in vivo activity of antimicrobial lipids in bulk form. These include poor aqueous solubility and weaker in vivo bactericidal activity due to in vivo oxidation, esterification, and lipid–protein complexation [10,63,64]. This can be overcome by developing lipid nanoparticle technologies to encapsulate antimicrobial lipids and convert them into different nanoformulations with inherent antimicrobial activities. The resulting antimicrobial lipidic nanoparticles using nanocarriers have excellent water solubility, can provide high concentrations of antibacterial lipids, and protect antibacterial lipids from degradation, which highlights the great potential for improving the therapeutic ability of antibacterial lipids [10,62]. Several reviews have been published elsewhere to understand the composition, mechanism, and characterization of this class of lipidic nanoparticles [65,66,67,68,69].
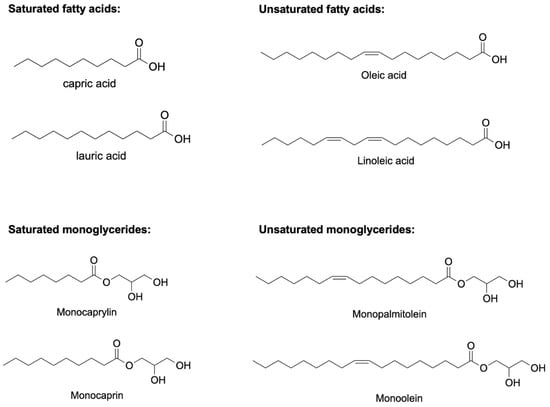
Figure 1.
Chemical structures of some potentially antimicrobial fatty acids and monoglycerides. Saturated fatty acids include capric acid and lauric acid, unsaturated fatty acids include oleic acid and elaidic acid, saturated monoglycerides include monocaprylin and monocaprin, and unsaturated monoglycerides include monopalmitolein and monoolein.
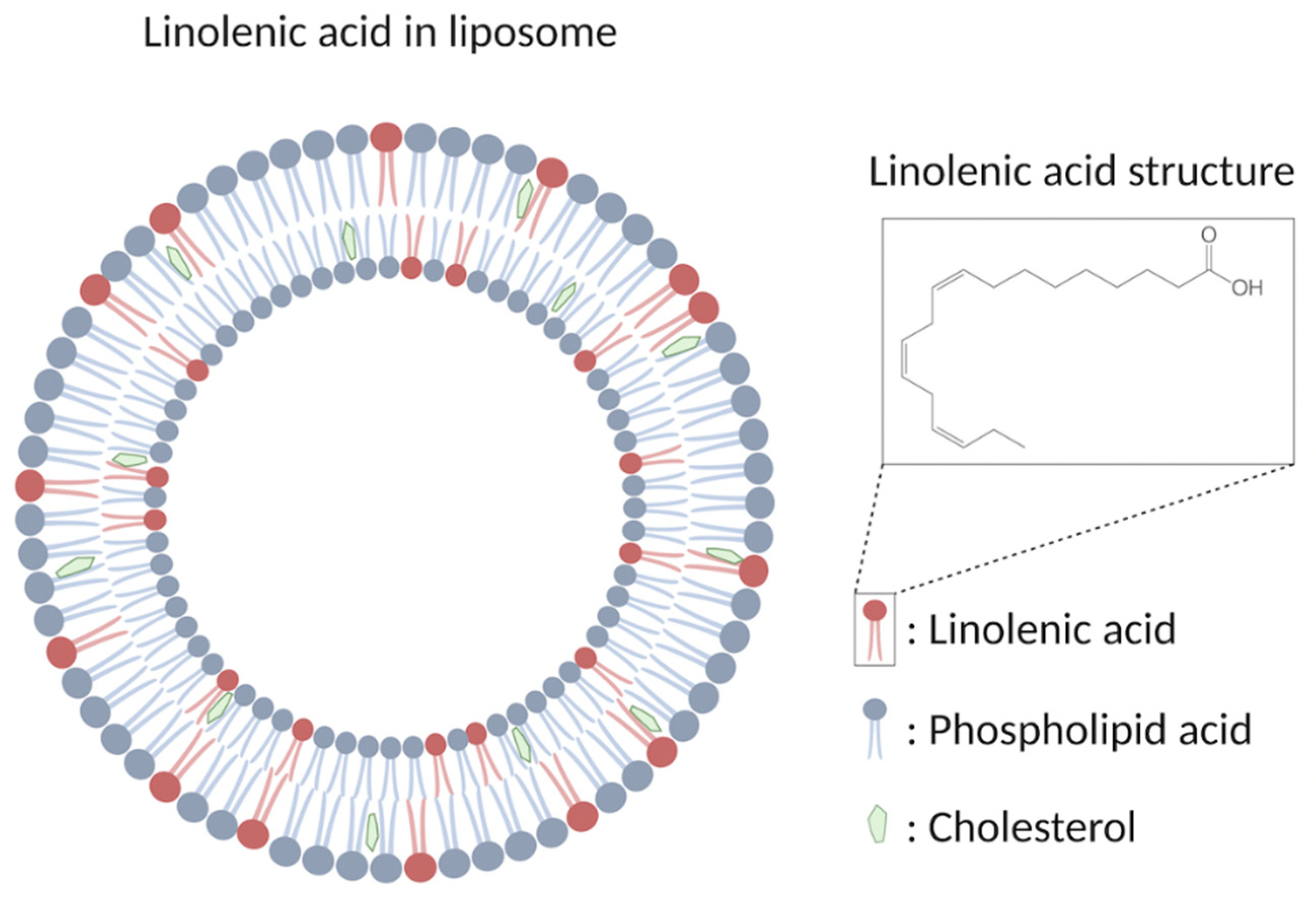
Liposomal formulations are the most-studied candidate so far for the emerging role as antimicrobial agents which are spherical closed lipid bilayers that can self-assemble in aqueous solutions and have a water core [16,70]. Antimicrobial lipids such as lauric acid and oleic acid can be incorporated to form antimicrobial liposomal formulations against Propionibacterium acnes and methicillin-resistant S. aureus (MRSA), respectively [34,71]. Among these different fatty acids, liposomal linolenic acids have received considerable attention by exhibiting particularly high levels of inhibitory activity [70]. Liposomal linolenic acid (LLA, Figure 2) that comprised liposomal nanoparticles made from linolenic acid, phospholipids, and cholesterols eradicated Helicobacter pylori clinical isolates including metronidazole-resistant H. pylori [72]. Furthermore, the bacteria did not appear to develop resistance to LLA at the sub-bactericidal concentrations used when compared with metronidazole and free linolenic acid. The fusion between the LLA and bacterial membrane, which directly inserts the linolenic acid into the bacterial membranes for subsequent membrane lysis, is suggested to be the bactericidal mechanism [72]. The in vivo efficacy of LLA in treating H. pylori infection was further investigated [73]. LLA penetrated into the mucus layer of a murine stomach, which led to reduced bacterial load and proinflammatory cytokines. In addition, a significant portion of LLA remained in the stomach at 24 h post-treatment, showing the long-last effects of LLA. Lastly, the in vivo toxicity showed no significant increase in gastric epithelial apoptosis and no changes of the murine gastric tissue under histological analysis, indicating the excellent biocompatibility of LLA in the stomach of control mice [73].
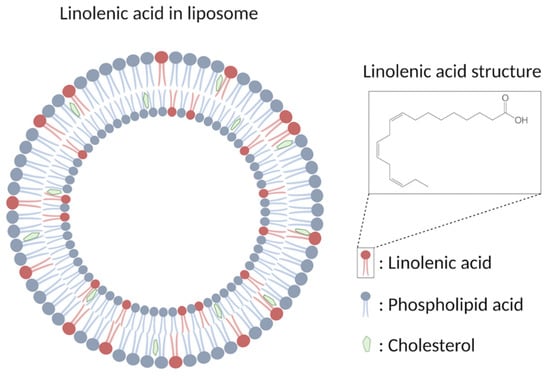
Figure 2.
Schematic drawing showing the structure of LLA.
In addition to antimicrobial liposomal formulations, other antimicrobial lipid-based nanoparticle systems, including emulsions and solid lipid nanoparticles, have shown promising antibacterial effects against MRSA and Pseudomonas aeruginosa, respectively [47,74]. Sadiq et al. encapsulated nisin in monolaurin nano-emulsions and demonstrated their ability of effectively inhibiting S. aureus in vitro [46]. Studies have found that solid lipid nanoparticles loaded with retinoic acid and lauric acid inhibited the growth of Staphylococcus epidermidis, P. acnes, and S. aureus [75]. A second generation of lipid nanoparticles that can improve the loading capacity and inhibit the excretion of bioactive compounds, called nanostructured lipid carriers, was recently developed from a mixture of solid lipids and liquid lipids [76,77,78,79,80]. Compared to the crystalline lipid core of solid lipid nanoparticles, the structural imperfections of nanostructured lipid carriers with less ordered crystalline arrangement can further improve the loading capacity and prevent the drug leakage for better antibacterial activity. Previous research comparing the antibacterial activity of docosahexaenoic acid (DHA) coated by nanostructured lipid carriers and DHA itself has found the incorporation of DHA into the nanostructured lipid carriers greatly enhanced bactericidal effect against H. pylori [81]. However, studies of emulsions, solid lipid nanoparticles, nanostructured lipid carriers, etc., as antimicrobial lipid-based nanoparticle systems are still in the early phases compared with the simplest form of liposomes.
2.2. Biodegradable Polymeric Nanomaterials
Biodegradable polymeric nanosystems can be classified into polymeric nanoparticles for the purposes of a delivery nanocarrier and antimicrobial polymers. The tailored design of polymeric chains confers versatile functions to the biodegradable polymeric nanomaterials including antibacterial activity, enhancing stability, biocompatibility, long circulation, and specific bacterial recognition of the polymeric nanomaterials [82]. Antimicrobial cationic polymers are the most-studied organic nanomaterials that have already entered clinical trials and hold great promise in replacing some antibiotics [83]. Biodegradable polymeric nanoparticles also offer an attractive delivery system which can improve the safety and efficacy of other ingredients by modulating the rate, timing, and location of release compared to lipid-based nanoparticles [84]. Furthermore, the functional groups on the polymer chain serve as a promising matrix to interact with other nanomaterials, forming polymer-based nanocomposites [85]. This paves the way for researchers to synthesize different polymer-based nanocomposites with improved or novel properties, which will be discussed further in Section 4 below.
2.2.1. Polymeric Nanoparticles as Delivery Nanocarriers
For the use of biodegradable polymeric nanoparticles with encapsulated antibacterial agents, they can be classified into four distinct classes including polymeric micelles, vesicles, nanocapsules, and nanospheres, depending on the polymer composition and the final structure of the polymeric nanosystems. A detailed review of the aforementioned nanoparticles has been described elsewhere [85,86,87,88]. Currently, over 80 clinical trials are underway or have been completed using polymeric nanoparticles in cancer therapy, highlighting the potential utility of polymeric nanoparticles in drug delivery [89].
Polymeric nanoparticles and lipid-based nanoparticles share similar advantages as drug delivery vehicles, but polymeric nanoparticles have some perceived advantages over lipid-based nanoparticles. This includes higher structural integrity and stability under biological and storage conditions, and controlled release capabilities conferred via the polymer cytoskeleton [18,90]. Among them, the use of stimuli-responsive biodegradable polymer nanoparticles to prepare drug delivery systems has great potential for controlled drug delivery [91,92]. It has been demonstrated that polymer degradation can be controlled by changing the external stimuli (e.g., pH, ultrasound, temperature, IR radiation, magnetic field, etc.), allowing stacked polymer nanoparticles to degrade in a controlled manner and release a drug on demand [90,93,94]. Qiu et al. successfully developed phosphatidylcholine–chitosan hybrid nanoparticles loaded with a gentamicin antibiotic and demonstrated that this synthetic system was able to inhibit the growth and membrane formation of Gram-positive and Gram-negative bacteria [95]. Studies have shown that by encapsulating vancomycin antibiotics in nanovesicles composed of long fatty acids grafted with hydrophilic polymers, these nanocarriers have the ability to self-assemble into spherical drug carriers and are effective against MRSA [96]. However, the polymer degradation products and clearance might cause potential toxicity as lipid-based nanoparticles typically have higher biocompatibilities than polymeric nanoparticles, which makes the application of polymeric nanoparticles in delivering antimicrobial agents a challenge. Hence, the field is still at an early development stage [19].
2.2.2. Antimicrobial Cationic Polymeric Nanoparticles
Over the last decade, synthetic biodegradable antimicrobial cationic polymers have been a promising solution to combat bacteria. The cationic charges of these synthetic polymers selectively act and are attracted to negative-charged bacterial membranes on zwitterionic mammalian cell membranes, in a mechanism similar to natural antimicrobial peptides [97,98]. Antimicrobial cationic polymers have attracted tremendous attention owing to their facile synthesis in bulk quantities at much lower costs, broad spectrum efficacy of their antibacterial activity with membrane disruptive mechanism, as well as a low propensity for inducing bacterial resistance [99,100]. Of note are the natural antimicrobial peptide-mimicking antimicrobial cationic polymers brilacidin (ClinicalTrials.gov Identifier: NCT02324335) and LTX-109 (ClinicalTrials.gov Identifier: NCT01803035), which have completed phase 2 clinical trials.
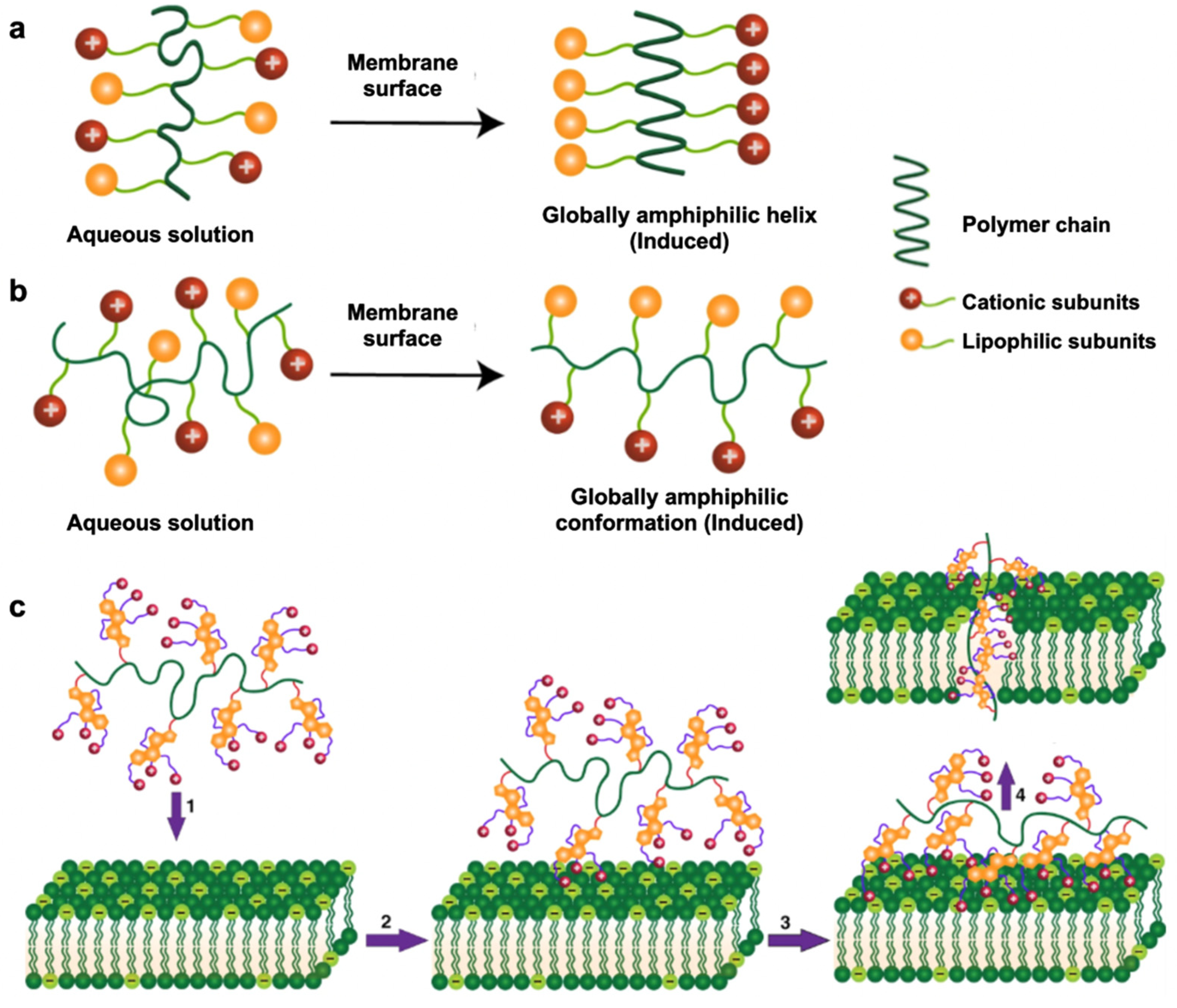
The antibacterial mechanism of cationic polymers requires contact with a bacterial membrane’s outer surfaces, which induces a globally amphiphilic conformational change to sequester cationic and lipophilic side chains [101]. This property is known as facial amphiphilicity and is shown in Figure 3. The cationic subunits are responsible for interacting with the bacterial membrane, whereas the lipophilic side chains insert into bacterial membranes for subsequent membrane disruption. [102]. This leads to cytoplasmic leakage, membrane depolarization, lysis, and ultimately cell death, showing the promising antibacterial activity of these polymers [103]. It remains challenging to achieve proper facial amphiphilicity of cationic polymers. The majority of antimicrobial cationic polymers that are generated from uncontrolled polymeric self-assembly do not comprise truly facial amphiphilicity, which greatly affects antibacterial activity and can lead to nonspecific toxicity in mammalian cells [104]. Manipulation of the sequence of hydrophobic and hydrophilic subunits of antimicrobial polymers is an important factor in achieving facial amphiphilicity for antibacterial activity. A recent study combining vancomycin with the cationic polymer Eudragit E100 ® (Eu) against P. aeruginosa showed that P. aeruginosa was eradicated within 3–6 h of exposure with this combination treatment [105]. Although bacterial envelope permeabilization and morphological changes after exposure to Eu were not sufficient to cause bacterial death, they allowed vancomycin to enter the target site, thereby enhancing the activity of an otherwise inactive vancomycin against P. aeruginosa.
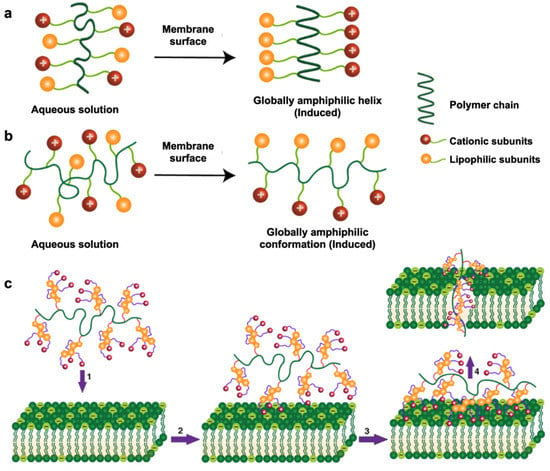
Figure 3.
Modes of action upon contact with bacterial membrane surfaces. (a) Global amphiphilic helical conformation adopted by host-defense peptides; (b) global amphiphilic random conformation adopted by synthetic antimicrobial polymers. (c) Proposed antibacterial mechanism of synthetic antimicrobial polymers: (1) diffusion, (2) surface binding via cationic subunits, (3) membrane insertion via lipophilic subunits and (4) membrane disruption. (Adapted from Rahman et al., [104] 2018 with modifications.)
The formulation of antimicrobial polymeric nanoparticles has overcome the aforementioned problems associated with antimicrobial polymers. The first antimicrobial polymer that self-assembled into cationic micellar nanoparticles by dissolution in water was reported by Nederberg [106]. A strong bactericidal activity of the cationic micellar nanoparticles was observed against MRSA and Enterococcus faecalis [106]. The polymeric nano-architecture was critical for effective bactericidal activity of the antimicrobial polymer molecules [106]. Unlike conventional antimicrobial polymers, the self-assembled antimicrobial polymeric nanoparticle does not require contact with the bacterial membrane for the formation of the secondary structure. It is hypothesized that the nanoparticle architecture increases the local concentration of cationic charge and polymer mass, leading to strong interactions between the polymer and cell membrane, which translate into effective antibacterial activities. Self-assembled antimicrobial polymeric nanoparticles have demonstrated minimal toxicity along with promising antibacterial activity, highlighting their potential in antibacterial applications and clinically relevant therapies [107,108,109,110]. Chin and colleagues reported a class of degradable guanidine-functionalized polycarbonates with a unique mechanism that does not induce drug resistance, which has great potential in the prevention and treatment of multidrug-resistant systemic infections [111]. The team optimized the structure of the polymer for treating multidrug-resistant Klebsiella pneumoniae pulmonary infections. In vivo experiments showed that the polymer backbone (pEt_20) self-assembles into micelles at high concentrations, which can alleviate lung infection with K. pneumoniae without causing damage to the major organs in mammals [112].
Another breakthrough study that benefits from this nanotechnology is star-shaped peptide polymer nanoparticles [113]. This is the first example of a synthetic antimicrobial polymer that efficiently kills colistin-resistant and multidrug-resistant Gram-negative pathogens, including Acinetobacter baumannii, K. pneumoniae, and P. aeruginosa [113]. The star-shaped peptide polymer nanoparticles eradicate these Gram-negative bacteria via destabilization and fragmentation of the bacterial outer membrane, disruption of cytoplasmic membrane, and induction of bacterial apoptosis [113]. Singh and her colleagues investigated the antimicrobial activities against clinical and drug-resistant strains (MDR-PA and MRSA) through indole-3-butyryl-polyethyleneimine nanostructured self-assembly in aqueous systems [114]. The amphiphilic indole-3-butyryl-polyethyleneimine polymer nanostructures have positively charged hydrophilic polyethyleneimine on the surface, while the hydrophobic indole-3-butyryl moiety is located inside the core, which showed enhanced antibacterial effects against all drug-resistant strains [114].
2.3. Carbon-Based Antimicrobial Nanomaterials
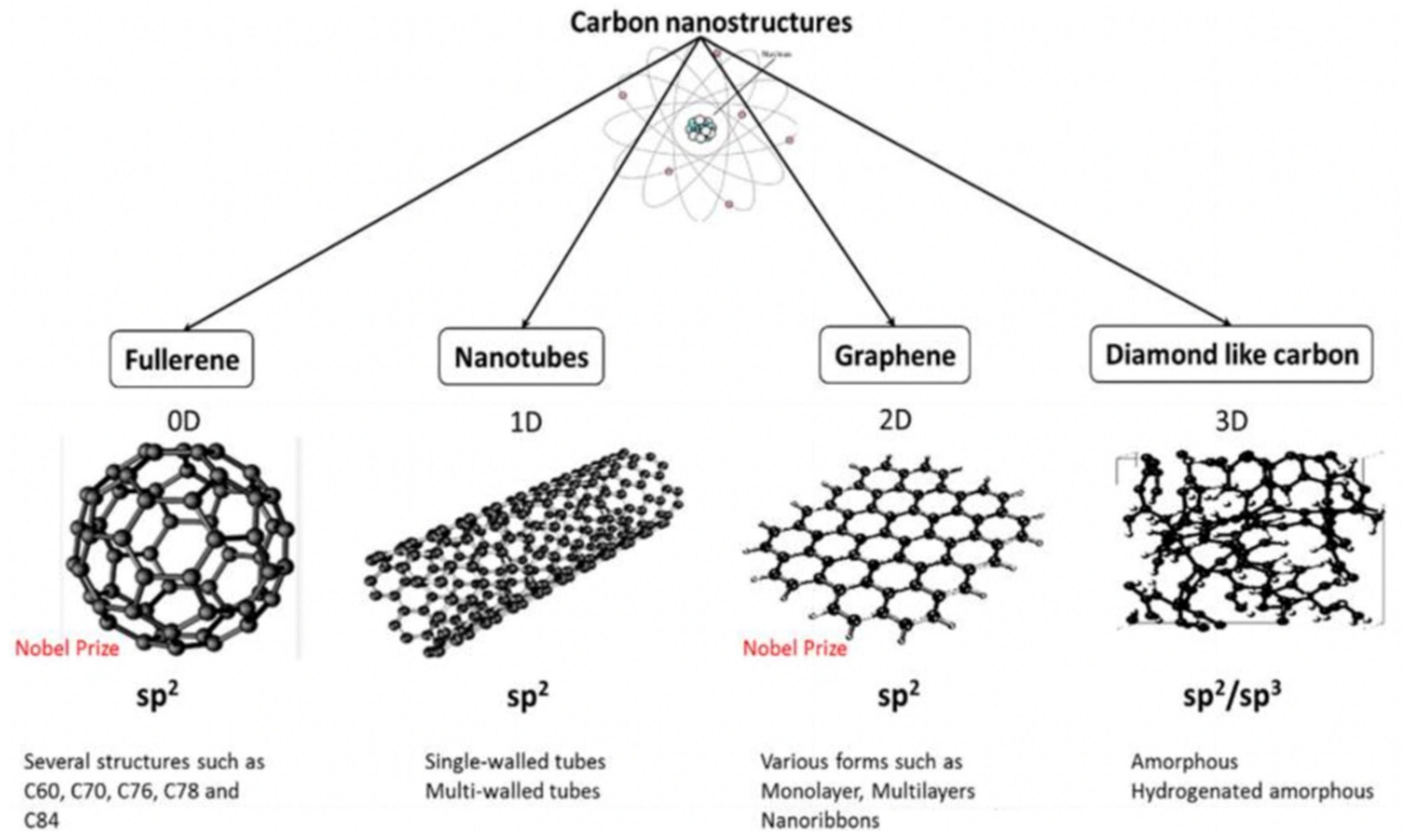
As a novel class of nanomaterials, carbon-based nanomaterials (CNMs) have received significant interest due to their remarkable properties including inherent antibacterial effects, extraordinary mechanical properties, excellent electrical conductivity and thermal conductivity, incredibly high surface area to volume ratios, photoluminescent and photocatalytic activities, and good stabilities [115]. These unique properties make carbon nanoarchitectures promising for a wide range of antibacterial applications including drug delivery, bone and tissue engineering, biosensors, photothermal therapy, and potential new antibacterial agents, which have been discussed elsewhere [116,117,118,119,120,121]. Due to its valency, carbon is able to form several allotropes that leads to a broad range of nanostructures of different dimensions, shapes, and properties, as depicted in Figure 4.
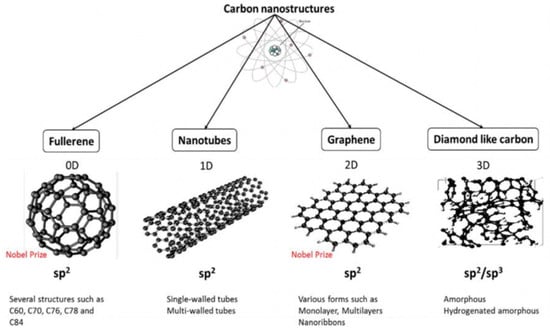
Figure 4.
Carbon-based nanomaterials categorized via different dimensionalities (D). (Adapted with permission from Ref. [116]. 2017, Al-Jumaili et al. More details on “Copyright and Licensing” are available via the following link: https://www.mdpi.com/ethics#10 (accessed on 10 October 2022).)
Among carbon-based nanomaterials, the preferential use of graphene-based materials, especially graphene oxide (GO), for antibacterial applications is due to the following reasons: The highly oxygenated surface of GO, bearing hydroxyl, epoxide, diol, and carbonyl functional groups, provides a versatile platform for drug delivery applications or further functionalization [122]. The good aqueous solubility of GO makes it suitable for in vivo antibacterial applications compared with poorly water-soluble fullerenes and nanotubes [20,123]. Another advantage of graphene oxide is its ability to act as a barrier or overlay, delaying and controlling the release of biomolecules over time [124,125,126]. Lastly, GO synthesis can be devoid of any metallic impurities and these materials can exhibit tolerable toxicity [127,128].
2.3.1. Graphene Oxide as an Antimicrobial Delivery Nanocarrier
The use of graphene oxide as a nanocarrier for drug delivery has received significant attention for several reasons, such as its good biocompatibility with tolerable toxicities [129,130]. In addition, the ease of functionalization provides possibilities in synthesizing novel functional nanohybrids or nanocomposites for specific purposes including targeted drug delivery, as shown in Figure 5 [131]. The extremely large surface area coupled with a two-dimensional planar structure provides a huge drug-loading capacity. In fact, a significant rate of drug loading has been reported previously with a GO-based delivery system [21]. The high mechanical and chemical stability of GO makes it particularly suitable for different delivery environments [132]. Currently, GO nanoparticles have been experimentally used in various biomedical applications including gene delivery [133], drug delivery [134,135], photodynamic therapy [136], anticancer therapies [137,138], and antibacterial therapies [139]. Nevertheless, studies of GO-based delivery systems with bacterial infection are still at a preliminary stage, involving investigations in antibiotic absorption efficacy and the respective in vitro antibacterial activity [140,141]. In a recent study, polyethylene-glycol-functionalized GO nanoparticles loaded with Nigella sativa seed extract were tested as a drug delivery system to disrupt bacteria by penetrating bacterial nucleic acid and cytoplasmic membranes, successfully demonstrating potential antibacterial activity against S. aureus and Escherichia coli [142]. Pan et al. used GO as a carrier to load N-halamine compounds, which not only displayed an antibacterial effect against S. aureus and E. coli, but also had slow-release properties and good storage stability [143]. In general, with the aforementioned advantages conferred by GO-based delivery systems, the potential of GO as a delivery platform for antimicrobial agents should not be neglected.
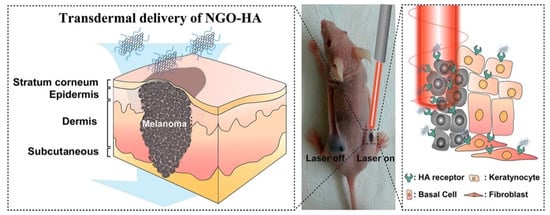
Figure 5.
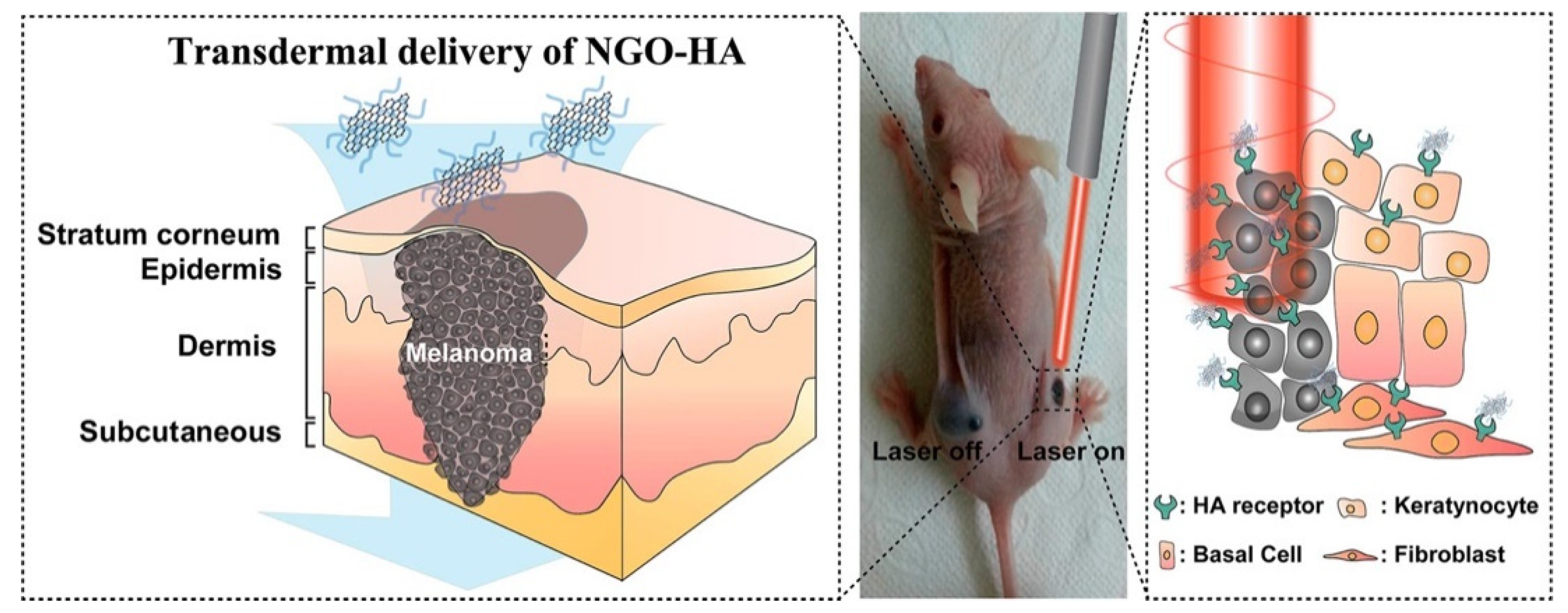
Schematic illustration for the transdermal delivery of nanographene oxide–hyaluronic acid (NGO–HA) conjugates into melanoma skin cancer cells and the following photothermal ablation therapy using a near-infrared laser. (Adapted with permission from Ref. [131]. 2014, Jung et al. More details on “Copyright and Licensing” are available via the following link: https://www.mdpi.com/ethics#10 (accessed on 10 October 2022).)
2.3.2. Graphene Oxide with Inherent Antibacterial Properties
GO has received tremendous attention as a novel antibacterial agent compared with its role as a delivery nanocarrier in antibacterial applications due to its broad-spectrum antibacterial activity and low cytotoxicity at low concentrations [144]. GO has been reported to exhibit strong antibacterial activity against a variety of Gram-positive and Gram-negative bacteria, such as E. coli, S. aureus, E. faecalis, P. aeruginosa, and Candida albicans [22,139,145,146,147,148,149]. In addition, Di Giulio et al. also reported significant antibiofilm efficacy against biofilms produced by S. aureus, P. aeruginosa, and C. albicans [150]. Recently, attention has also been given to GO-based combination antibacterial therapies. The ternary nanocomposites obtained by combining GO with hydroxyapatite and copper oxide have inhibitory effects on Gram-negative E. coli and Gram-positive S. aureus [151]. Innovative bionanomaterials composed of GO, agarose, and hydroxyapatite have also shown the ability to significantly reduce S. aureus [152].
The strong antibacterial activity of GO is associated with both physical and chemical damages. The physical interactions of GO with bacteria that are reported to date include interactions via direct contact of its sharp edges, lipid extraction, bacteria isolation from their nutrient environment by wrapping, and photothermal/photocatalytic effects owing to the semiconductor properties of graphene [139,153,154,155,156]. Interestingly, the mechanism of action of GO on Gram-positive and Gram-negative bacteria appears to be distinct. Pulingam et al. reported that cell entrapment via mechanical wrapping was mainly observed for the Gram-positive bacteria S. aureus and E. faecalis, whereas with Gram-negative bacteria E. coli and P. aeruginosa it was observed that membrane rupture due to physical contact [22] was the predominant mechanism. Chemical damages are additionally caused via oxidative stress and generation of reactive oxygen species (ROS) and charge transfer, thereby inhibiting bacterial metabolism, disrupting cellular functions, causing inactivation of intracellular and subcellular proteins, and inducing lipid peroxidation, leading to cellular inactivation [147,157,158,159]. Zhang et al. recently highlighted electrical conductivity as a key property of GO that may be underestimated in terms of its antibacterial activity role [160]. Research by Chong et al. proposed that sunlight irradiation could increase the antibacterial activity of GO due to enhancing the electron transportation of antioxidants [161]. GO’s diverse physicochemical properties including sheet size, shape, number of layers, surface charge, defect density, and the presence of surface functional groups and oxygen content have a strong impact on its antibacterial activity and biological performance [116,162]. However, the physicochemical properties related to antibacterial activity are not fully elucidated yet. Deepening the understanding of the physicochemical properties related to antibacterial activity is a crucial step in designing GO-based nanomaterials for optimized antibacterial activity. Together, these studies provide important insights into the way forwards.
3. Antibacterial Inorganic Nanomaterials
Inorganic nanomaterials do not contain either carbon or hydrogen atoms that are associated with biological matter. As an alternative, inorganic nanomaterials comprise metallic and non-metallic elemental compounds that have weak intermolecular interactions which form nanostructures with higher dimensionality. Among two classes of the inorganic nanomaterials, metallic inorganic nanomaterials (Ag, Au, Zn, Cu, Bi) have attracted significant attention over non-metallic nanomaterials (S, Si, B, Te and Se). This is particularly due to the inherent water insolubility of the non-metallic inorganic nanomaterials that restricted their use in antibacterial applications. Therefore, this review primarily focuses on the development of inorganic metallic nanomaterials in antibacterial applications. Other inorganic nanomaterials such as fluoride in oral treatment has also been researched for a long time. For instance, in the prevention of dental caries, the addition of fluoride has a significant antibacterial effect on Streptococcus mutans, Lactobacillus acidophilus, E. faecalis, Actinomyces naeslundii, and Parvimonas micra [163,164,165,166].
Inorganic metallic nanomaterials do not readily self-assemble into 1D nanowires, nanotubes, nanoribbons, and 2D nanowalls and nanofilms [167]. Therefore, complicated synthesis methodologies are required to promote the “growth” of inorganic metallic nanomaterials into nanostructures with higher dimensionality [168,169]. Zero-dimensional metal and metal oxide nanoparticles are the most popular candidates that can readily be synthesized for antibacterial applications, which are discussed in Section 3.1 and Section 3.2 below.
3.1. Metal Nanoparticles
Metal nanoparticles are the most promising candidate in this class of materials with inherently strong antibacterial activities amongst the nanomaterials. A summary of the possible bactericidal effects of metal nanoparticles on different bacteria is shown in Table 3. Researchers reviewed a variety of metal nanoparticles including silver, gold, copper, zinc, and super-paramagnetic iron which demonstrated promising antibacterial effects, with silver nanoparticles (AgNPs) being the most effective against bacteria [169,170,171,172]. AgNPs exhibit bactericidal activity at concentrations well below their cytotoxicity and exhibit synergistic antibacterial efficacy with conventional antibiotics when used against MDR bacteria [173,174,175,176].

Table 3.
Antibacterial activity of metal nanoparticles against different bacteria.
The antibacterial mechanisms of AgNPs are still poorly understood despite extensive studies [171,176]. Currently accepted antibacterial mechanisms include cell wall penetration and membrane damage, toxicity associated with metal ion release, and induction of oxidative stress [177,178,179,180]. AgNPs have already been used in various biomedical and antibacterial applications and products, including surface coatings on medical devices, topical treatments, wound dressings, dental fillings, personal care products with sanitizing effects, disinfectants, and detergents [180,181]. A recent study demonstrated the potential of AgNP-containing disinfectants as active ingredients for disinfecting surgical masks, effectively improving mask protection by inhibiting the growth of E. coli, K. pneumoniae, and S. aureus [23]. Over the past few decades, the AgNP market has been growing steadily, with an estimated annual production of more than 500 tons of nanoparticles, which also reflects the widespread interest of AgNPs [182].
Despite the promising antibacterial effects and the wide use of metal nanoparticles in different applications, metal nanoparticles suffer from several drawbacks. The potential toxicity of metal nanoparticles affects the basic functioning of mammalian cells as metal nanoparticles or released metal ions via direct uptake from mammalian cells [24]. Colloidal metal nanoparticles tend to aggregate over time [25]. The aggregation along with increased particle size reduces their peculiar properties at the nanoscale, including their antibacterial activities. Bacteria have the ability to develop resistance to metal nanoparticles by using adhesive flagellin [183]. Phenotypic changes of adhesive flagellin production triggers the aggregation of metal nanoparticles, thereby reducing their antibacterial activity. Metal nanoparticles are potential environmental hazards and difficult to recover or deactivate in solid-waste incineration plants or wastewater treatment systems [184,185,186]. To overcome the above limitations, metal nanoparticles could be incorporated into other nanomaterials to form nanocomposites, which show a greater dispersion of metal nanoparticles, improved antibacterial activity, and reduced toxicity [187,188,189]. This will be further discussed in Section 4 below.
3.2. Metal Oxide Nanoparticles
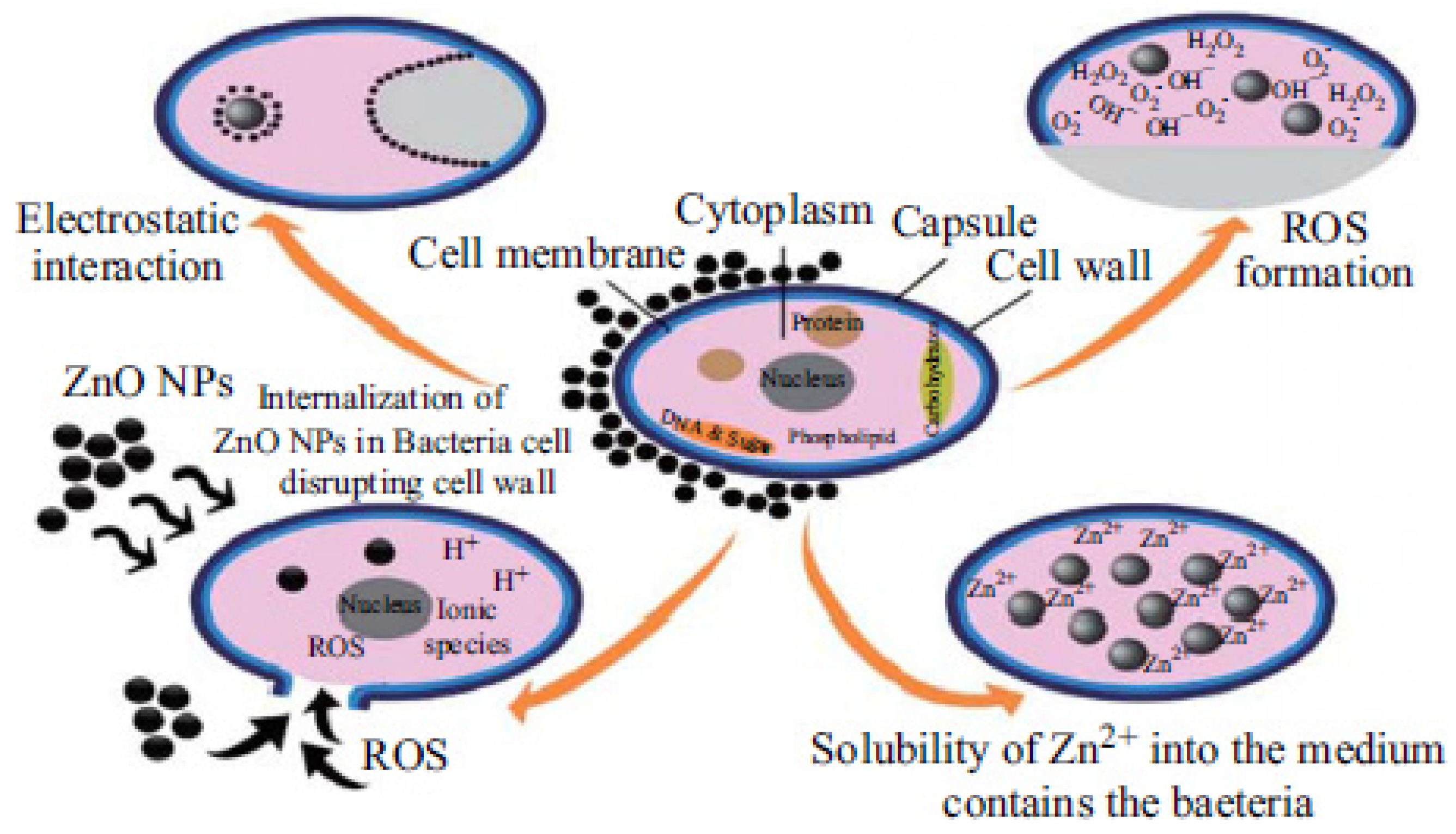
Metal oxide nanoparticles offer another alternative promising solution against MDR bacteria. A variety of metal oxide nanoparticles including titanium dioxide, zinc oxide, magnesium oxide, copper oxide, and aluminium oxide have been demonstrated to exhibit antibacterial effects [215], which will not be discussed in detail here. Zinc oxide (ZnO) nanoparticles are the most-established candidate of these metal oxide nanoparticles due to the following reasons: ZnO is one of the most important metal oxide nanoparticles with widespread applications [26] and worldwide production is up to 1 million tons per year [216]. ZnO can be easily biodegraded and absorbed in the body and has been listed as a Generally Recognized as Safe (GRAS) material by the FDA. ZnO nanoparticles have a higher biocompatibility and lower toxicity than other metal oxide nanoparticles [217,218]. The semiconductor properties of ZnO nanoparticles with a wide band-gap energy readily absorb ultraviolet (UV) light. This allows them to act as potential photosensitizing agents for various antibacterial applications [27]. ZnO nanoparticles exhibit multiple antibacterial mechanisms, as described by Figure 6. Current postulated mechanisms include photo-triggered production of ROS [219] and Zn2+ ions mediating a poisoning effect [220]. In a study by Azam et al., the potential of ZnO as an antibacterial agent was demonstrated against Gram-positive bacteria (B. subtilis, S. aureus) and Gram-negative bacteria (P. aeruginosa, Campylobacter jejuni, E. coli) [213]. In another study, ZnO nanoparticles were also shown to be significantly inhibitory against Gram-positive (S. aureus) and Gram-negative (E. coli and P. aeruginosa) strains [221].
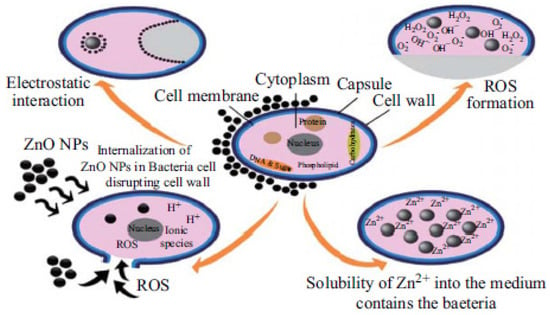
Figure 6.
Multiple antibacterial mechanisms that are associated with ZnO nanoparticles. (Adapted with permission from Ref. [222]. 2015, Sirelkhatim et al. More details on “Copyright and Licensing” are available via the following link: https://www.mdpi.com/ethics#10 (accessed on 10 October 2022).)
The aggregation of metal oxide nanoparticles over time remains a problem, which limits their use in vivo applications [223]. One approach to this problem is to disperse metal oxide nanoparticles into a polymer matrix, forming polymer–metal oxide nanocomposites. Polymer–metal oxide nanocomposites have been widely investigated and applied in the textile and polymer industries for various antibacterial applications, which will be discussed in the following section. [224].
4. Nanocomposite/Nanohybrid Antibacterial Materials
Nanocomposites or nanohybrids are a novel class of multiphase materials that exhibit a hierarchical structure, where one phase of the material has at least one dimension in the nanometer range [225]. They have attracted significant attention due to their unprecedented properties compared with their mono-constituent parts, largely attributed to strong reinforcing effects of additional materials. Currently, synthesizing a variety of nanocomposites with unprecedented physical properties and enhanced antibacterial activities is the main focus in the field over the last 10 years. Despite the large volume of studies on nanocomposites, the understanding of the structure–property–activity changes remains in its infancy [226]. It is important to understand the mechanisms behind the property’s changes within nanocomposites in order to design materials with enhanced improvements in the desired properties for a specific purpose. Section 4.1, Section 4.2, Section 4.3, Section 4.4 and Section 4.5 discusses the development and potential applications of the nanocomposite antibacterial materials. Of note, graphene oxide is especially emphasized in the section below due to its versatility to form nanocomposites with organic and inorganic metallic nanomaterials, respectively.
4.1. Polymer–Metal Nanocomposite Nanoparticles
Polymer–metal composite nanoparticles are another promising solution to achieve a greater dispersion of metal nanoparticles and prevent metal nanoparticle aggregation. Polymer–metal composite nanoparticles comprise a metal nanoparticle core surrounded by a polymer shell with the alkyl tail arranged toward the surrounding environment. Polymer–metal composites are essentially insoluble in water and the colloidal stability of the polymer–metal composite nanoparticles in aqueous environments has a huge potential for in vivo antibacterial therapies [227]. The polymer macromolecular matrix acts as a reaction chamber for metal nanoparticle synthesis, a capping agent to prevent nanoparticle aggregation and as a scaffold for nanoparticle immobilization [228]. Moreover, synergies between the polymer and the metal nanoparticles confer the nanocomposite with unprecedented performance and improved antibacterial properties [229]. Tamayo et al. summarized the synthesis, properties, and recent applications of polymer composites with metal nanoparticles [230]. The incorporated metal nanoparticles are focused on the use of gold (AuNPs) and silver (AgNPs) due to their antimicrobial properties, catalytic activity, and conductivity properties enabling a wide range of applications.
4.1.1. Development of Synthesis Approaches for Polymer–Metal Nanocomposites
Both in situ and ex situ approaches can be employed to synthesize polymer–metal composite nanoparticles and polymer-matrix metal nanocomposites. In the last decade, several studies have been conducted to develop and improve synthetic methods at higher efficiencies resulting in improved antimicrobial outcomes.
By using in situ methods, the precursor of the nanoparticle is required to be dispersed in a monomeric solution before polymerization. The metal ions can be reduced into the polymer matrix or simultaneous metal ion reduction and polymerization can occur [230]. In general, these in situ reduction methods need a relatively long time to produce nanocomposite films. Kazuhiko et al. developed a rapid and scalable synthetic method exploiting use of a mid-infrared laser, CO2 laser, at 10.6 µm without the use of reducing agents [231]. The polymer film (polyvinyl alcohol (PVA) or polyethylene glycol (PEG)) containing Ag ions were coated on a glass substrate and then the CO2 laser was used to heat the substrate. Subsequently, the thermal energy was absorbed by the polymer film, causing Ag ion reduction. Eventually, Ag-PVA or Ag-PEG nanocomposite films were formed in several seconds. This process is industrially scalable by increasing the power of the CO2 laser.
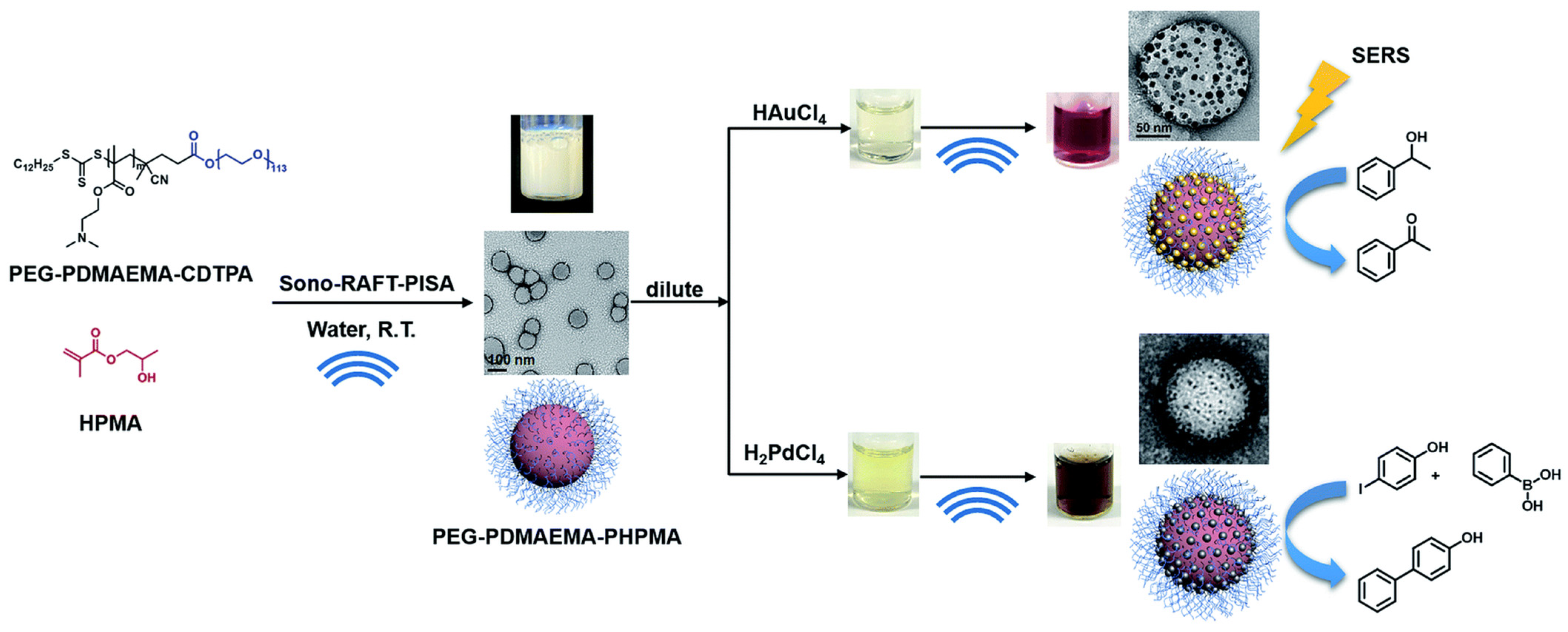
Ultrasound is a promising tool to be applied in for the in situ production of polymer–metal nanocomposites. Ultrasound radiation was employed as a homogenizing tool to fabricate composites with homogeneously dispersed metal nanoparticles [232]. The ultrasound was used to disperse organic liquids of polymerizing monomer (pyrrole) in the aqueous solution of the oxidizer (Ag+ or AuCl−). The aqueous solution was placed in an ultrasonic chamber and droplets of the organic solution were added continuously until achieving a volume ratio of 4:1. After polymerization, the nanocomposites could be obtained at the liquid–liquid interface [232]. Wan et al. used ultrasound as both an initiating and reducing agent in the nanocomposite preparation process, shown in Figure 7 [233]. Tertiary amine-containing polymeric nanoparticles were produced by ultrasound-initiated polymerization-induced self-assembly (sono-PISA), following which, the metal ions (Au and Pd) were reduced in situ by radicals generated via the sonolysis of water, forming polymer–metal composites.
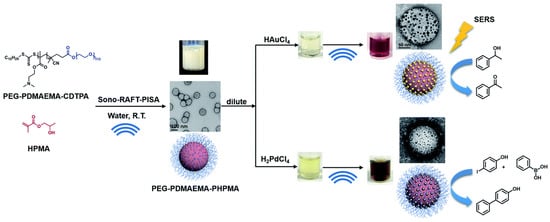
Figure 7.
Synthesis of tertiary amine-containing polymeric nanoparticles, and in situ formation of the Au and Pd nanocomposite by ultrasound. (Adapted with permission from Ref. [233]. 2021, Wan et al. More details on “Copyright and Licensing” are available via the following link: https://www.mdpi.com/ethics#10 (accessed on 10 October 2022).)
In contrast, metal nanoparticles are synthesized before they are incorporated into the polymer via an ex situ method. The subsequent deposition of the nanoparticles into the polymer can exploit processes such as melt compounding or solution blending [230]. However, a significant drawback exists when using ex situ methods, which is the fact that the nanoparticles are not optimally distributed in the polymer.
4.1.2. Synergistic or Combined Antibacterial Effects When Using More Than Just a Metal Nanoparticle Agent
A combination of metal nanoparticles and cationic polymers also facilitates the enhanced antibacterial activity of a composite nanoparticle, possibly due to the synergism between the antibacterial mechanisms from two different nanomaterials [234,235,236,237]. Nanoparticle formation was also expected to increase the local density of cationic polymer, leading to stronger binding on the negatively charged bacterial membranes [234]. This polyvalent interaction between cationic polymers and bacterial membranes is followed by the synergistic antibacterial mechanisms, including bacterial membrane disruption, internalization of composite nanoparticles, inhibition of intracellular enzymatic activity, and eventual cell death [234]. Imidazole-capped chitosan–gold nanocomposites exhibited enhanced antimicrobial activity to eradicate staphylococcal biofilms in a rabbit wound infection model [238]. The antibacterial mechanism of the composite nanoparticles also involved the binding of cationic polymer to the bacterial surface, and the subsequent synergistic effects from the gold nanoparticles, imidazole, and the chitosan polymer to strongly eradicate the biofilm [238].
Polymer–metal composite nanoparticles could potentially solve the environmental hazards associated with many metal nanoparticles. Richter et al. postulated that a metallic core is not necessary for the antimicrobial action [187]. Instead of synthesizing the entire nanoparticle of metal, the composite nanoparticle can be produced by infusion with a minimum amount of silver ions to the biodegradable lignin core, followed by surface functionalization with a layer of cationic polyelectrolyte [187]. The resulting composite nanoparticles exhibited broad-spectrum bactericidal activity against E. coli, P. aeruginosa, and a quaternary-amine-resistant Ralstonia. This is attributed to the enhanced binding to bacterial membranes by the polyelectrolyte shell and the synergistic antibacterial activity between the silver ions and the polyelectrolyte. The required silver ions were ten times lesser than when using conventional silver nanoparticles. The gradual diffusion of silver ions from the silver-infused lignin core composite nanoparticles into water will rapidly lose their post-utilization activity and be biodegradable in the environment after disposal [187].
4.1.3. On the Potential Clinical Use of Antibacterial Polymer-Matrix Metal Nanocomposites
Biocompatible and safe antibacterial materials are constantly sought to avoid inflammatory syndromes in patients. The formulation of polymer–metal composite nanoparticles generally improves their biocompatibility and reduces the toxicity associated with metal nanoparticles owing to protection by the polymeric shell. For instance, the viability of NIH3T3 cells was not affected at a dosage exceeding 20 times that of the minimum inhibitory concentration (MIC) of the polymer–silver composite nanoparticle, showing the low toxicity of these materials to mammalian cells [234]. Lu et al. further demonstrated that imidazole-capped chitosan–gold nanocomposites did not display hemolytic activity and significant toxicity towards L929 cell line [238]. Pryjmaková et al. modified the surface of polyethylene naphthalate (PEN) by a 248 nm KrF excimer laser and subsequently, Ag and Au nanowires were incorporated onto the modified PEN surface by vacuum evaporation [239]. The resulted nanocomposites displayed antibacterial effects against Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. epidermidis) via a 24-hr incubation drop plate test and were suggested as a non-toxic material by a WST-1 cytotoxicity test. In addition to the complete eradication of the biofilm, accelerated wound healing by a composite nanoparticle was demonstrated in a rabbit model [238]. These studies demonstrate the great potential of composite nanoparticles as novel antibacterial agents against bacterial infections.
4.2. Polymer-Matrix Metal Oxide Nanocomposites
Fine dispersions of metal oxide nanoparticles can be achieved by forming polymer-matrix metal oxide nanocomposites in a manufacturing process similar to polymer-matrix metal nanocomposites. There is an increasing interest in using metal oxide nanoparticles to replace metal nanoparticles for synthesizing polymer-matrix metal oxide nanocomposites [240,241,242,243]. Metal oxide nanoparticles, especially zinc oxide nanoparticles, are more desirable than metal nanoparticles as nanofillers in forming polymer-matrix nanocomposites [244,245,246,247]. Zinc oxide nanoparticles feature new UV-absorption and photosensitizer characteristics, with higher biocompatibilities and lower toxicities than metal nanoparticles [218,248]. Furthermore, the low cost of production and high stability of zinc oxide nanoparticles present advantages over conventional metal nanoparticles, even in extreme synthesis conditions [249]. The advantages of metal oxide nanoparticles as nanofillers in forming polymer-matrix metal oxide nanocomposites has open a new avenue for research into novel bio-nanocomposites for use as antimicrobial surfaces in various antibacterial applications, which will be depicted in following sections.
Polymer-matrix metal nanocomposites exhibit several advantages. Polymer flexibility allows the final product to be fabricated into complex structures or forms for various antibacterial applications, of which the details are shown in Table 4. The polymeric matrix immobilizes metal nanoparticles and prevents their aggregation, thus extending the antibacterial activity of the metal nanoparticles [228]. Localized release of metal nanoparticles to the desired application site can also be achieved, reducing in vivo toxicity and the environmental hazards caused by undesirable release of metal nanoparticles [250]. Synergistic antibacterial activity between metal nanoparticles and polymers is obtained with inherent antibacterial activities [229]. The strong interfacial binding and intermolecular interactions between the well dispersed metal nanoparticles and the polymer matrix further enhance the mechanical properties of the polymer, including the tensile strength, Young’s modulus, yield stress, and ductility [251].

Table 4.
Versatility of polymer-matrix metal nanocomposites for various antibacterial applications.
4.2.1. Development of Synthesis Approaches for the Industrial Production of Polymer-Matrix Metal Nanocomposites
A prerequisite to the aforementioned advantages exhibited by polymer-matrix metal nanocomposites is the formation of homogenous dispersions of metal nanoparticles in the polymer matrix without metal nanoparticle aggregation [262]. The delicate synthesis conditions to meet this prerequisite is often time-consuming, laborious, and difficult to be industrialized [263]. Therefore, recent studies have focused on developing facile and convenient synthesis approaches, aiming to produce polymer-matrix metal nanocomposites on industrial scales [228,250,264]. Other recent review papers have summarized developments, so we will not discuss these in detail [240,241,242].
For instance, Tran et al., developed a simple one-pot synthesis method in producing polymer matrix silver nanocomposites [228]. The ionic liquid medium, butylmethylimmidazolium chloride, was utilized as the only reaction medium for dissolving the biopolymer keratin and cellulose, and reduction of a silver ion precursor in the polymeric matrix. The synthesized polymer-matrix silver nanocomposite was found to retain the enhanced mechanical strength by cellulose and controlled release of silver nanoparticles by keratin, with a homogenous dispersion of silver nanoparticles. At 0.48 mmol of silver content, the nanocomposite demonstrated good biocompatibility and excellent antibacterial activity against E. coli, P. aeruginosa, MRSA, and vancomycin-resistant E. faecalis (VRE). An in vitro release assay demonstrated that less than 0.02% of the silver nanoparticles were released from the nanocomposite even after 7 days of soaking in solution, indicating good immobilization of silver nanoparticles using this simple one-pot synthesis method [228].
A scalable approach was recently developed to produce a silver-nanoparticles-doped nanoclay–polylactic acid composite nanocomposite, which involved doped nanoclay with minimal alteration to the fabrication processes and industry standard equipment [264]. Loading the nanoclay can significantly reduce the affinity of the nanocomposites for bacterial adhesion. With this synthesis method, only a 0.1 wt % of silver loading content was required to have satisfactory antibacterial activity. S. aureus and E. coli numbers were reduced by 91.3% and 90.7% after 48 h of incubation. The material costs associated with the silver-loading content is dramatically reduced compared with other studies which utilize at least 1 wt % of silver nanoparticles in the polymer-matrix metal nanocomposite to achieve a 90% reduction in bacterial numbers [265,266]. This is a great advantage for industrial production, in which the high costs associated with higher loading amounts of metal nanoparticles is a considerable problem. In addition, 3D printing is a promising method to produce metal oxide nanocomposites, with the advantages of keeping the integrity and functionality of the materials and reduce waste from traditional manufacturing methods [267].
4.2.2. The Application of Polymer-Matrix Metal Oxide Nanocomposites as Self-Sterilizing Antimicrobial Surfaces in Healthcare Environments
There are some recent literature reviews on the application of polymer-matrix metal oxide nanocomposites as self-sterilizing antimicrobial surfaces in healthcare environments which will not be repeated here [268,269,270]. The antibacterial and photosensitizing activity of ZnO nanoparticles has been well exploited in the absence or presence of light irradiation [271]. With the exploitation of their light absorption characteristic, ROS are produced from ZnO nanoparticles to act on bacteria, leading to a self-cleaning or self-sterilizing effect of the polymer-matrix zinc oxide nanocomposites [272]. For instance, Sehmi et al. and Ozkan et al. have developed self-sterilizing surfaces that was coupled with light-activated photodynamic therapy in killing bacteria [272,273]. Both studies showed the polymer matrix zinc oxide nanocomposites demonstrated lethal photosensitization of E. coli and S. aureus under white light irradiation that has a similar light intensity to that in a clinical setting [272,273]. This could potentially lower the rates of healthcare-associated infections by eliminating bacterial transfer in healthcare environments.
4.2.3. Wound Healing Applications of Polymer-Matrix Metal Oxide Nanocomposites
Polymer-matrix metal oxide nanocomposites are an attracting candidate in wound healing applications. Gobi et al. summarized the recent applications of nanocomposites in wound dressings [274]. A novel polymer-matrix metal oxide nanocomposite comprising a castor oil polymeric matrix reinforced with a chitosan-modified ZnO nanocomposite was recently developed [275]. This novel bio-nanocomposite showed enhanced mechanical properties, porosity, water absorption, hydrophilicity, water vapor transmission rate, and oxygen permeability [275]. These enhanced properties are important for wound healing, which provides porosity to absorb wound exudates and water, enables a moist wound healing environment, a cooling effect for pain alleviation, and gases to exchange for ventilation. ZnO nanofillers also enhanced the antibacterial activity and keratinocyte migration of a polymer-matrix zinc oxide nanocomposite, leading to stronger antibacterial activity that prevented the reoccurrence of a bacterial infection and promoted healing [276]. Recent research has reported a prepared nanocomposite consisting of a Lawsone-loaded o-carboxymethyl chitosan and ZnO which was evaluated against bacterial strains such as Salmonella, S. aureus, P. aeruginosa, and E. coli [277]. This prepared nanocomposite tended to prevent the evolution of these harmful bacteria compared to an o-carboxymethyl chitosan or nano-zinc oxide alone, further supporting this advantageous strategy of using a polymer-matrix ZnO nanocomposite in wound dressings. Finally, a polymer-matrix ZnO nanocomposite demonstrated promising in vivo efficacy, biodegradability, cytocompatibility, and promoted cell attachment on the material [278,279]. Taken as a whole, polymer-matrix metal oxide nanocomposites could possibly satisfy all the required standards as wound materials, highlighting their huge potential in wound healing applications.
4.2.4. Food Packaging Applications of Polymer-Matrix Metal Oxide Nanocomposites
For food packaging applications, the addition of ZnO nanoparticles as nanofillers to biodegradable polymeric materials greatly enhances the physiochemical properties and antibacterial activities of the resulting bio-nanocomposites to protect the environment [270,280,281]. ZnO nanoparticles create a barrier effect to hinder the diffusion of the decomposition products from the polymer matrix to the gas phase, which further improve polymer thermal stability and avoid thermal degradation under the wide polymer melt processing window [282]. In addition, ZnO nanoparticles act as a nucleating agent in raising the crystallinity level of the polymer matrix [283]. The combined effect of the increased crystallinity of the polymer along with the barrier effect of ZnO nanoparticles creates a highly tortuous path for the gases, water vapors, and organic compounds [284].
The barrier properties to gases, water vapors, and organic compounds subsequently improve the product quality and shelf life by blocking the diffusion of moisture and oxygen. Mechanical performance such as stiffness, glass transition temperature, tensile strength, and toughness is also enhanced because of the strong polymer matrix–ZnO nanofiller interactions [282,285]. The antibacterial and UV-absorption properties of ZnO nanoparticles inhibit the growth of food-borne pathogens and prevent the photo-oxidative degradation of food, respectively [286]. Taken together, polymer matrix–zinc oxide nanocomposites are promising materials to be used as cutlery, overwrap films, and containers in preventing growth of food-borne pathogens and achieving good quality packaged food with extended shelf-life.
4.3. Graphene Oxide–Metal Nanocomposites
As mentioned in Section 3, the shortcomings of metal nanoparticles limit their potential for medical applications. To overcome these issues, many nanocomposites composed of metal nanoparticles and graphene have been prepared experimentally and studied against various bacterial strains [287,288,289,290,291,292]. Some of the available literature reviewed the development of graphene–metal matrix nanocomposites which will not be repeated here [293,294,295,296]. Among them, a combination of graphene oxide and silver nanoparticles to form nanocomposites has attracted a lot of attention as antibacterial agents in antibacterial therapies, since Ag and its compounds have been used since the time of the ancient Egyptians. The antibacterial and antiviral properties of Ag, Ag ions, and Ag-based compounds have been thoroughly researched [202,297,298]. With the incorporation of GO as the supporting matrix, silver nanoparticles could be dispersed in aqueous solution while minimizing the aggregation problem that would otherwise greatly affect the antibacterial activity of the silver nanoparticles [299]. The large surface area and abundant functional groups on the basal plane of GO allows GO to interact with silver ions or silver nanoparticles through electrostatic interactions, charge–transfer interactions and physical absorption [300]. This allows GO–Ag nanocomposites to be synthesized through loading of pre-synthesized silver nanoparticles into GO (ex situ approach) or via reduction of silver ions in a graphene matrix to form silver nanoparticles in situ [301,302,303,304,305].
4.3.1. Development of Synthetic Approaches for Improving the In Vivo Performance of Graphene Oxide–Metal Nanocomposites
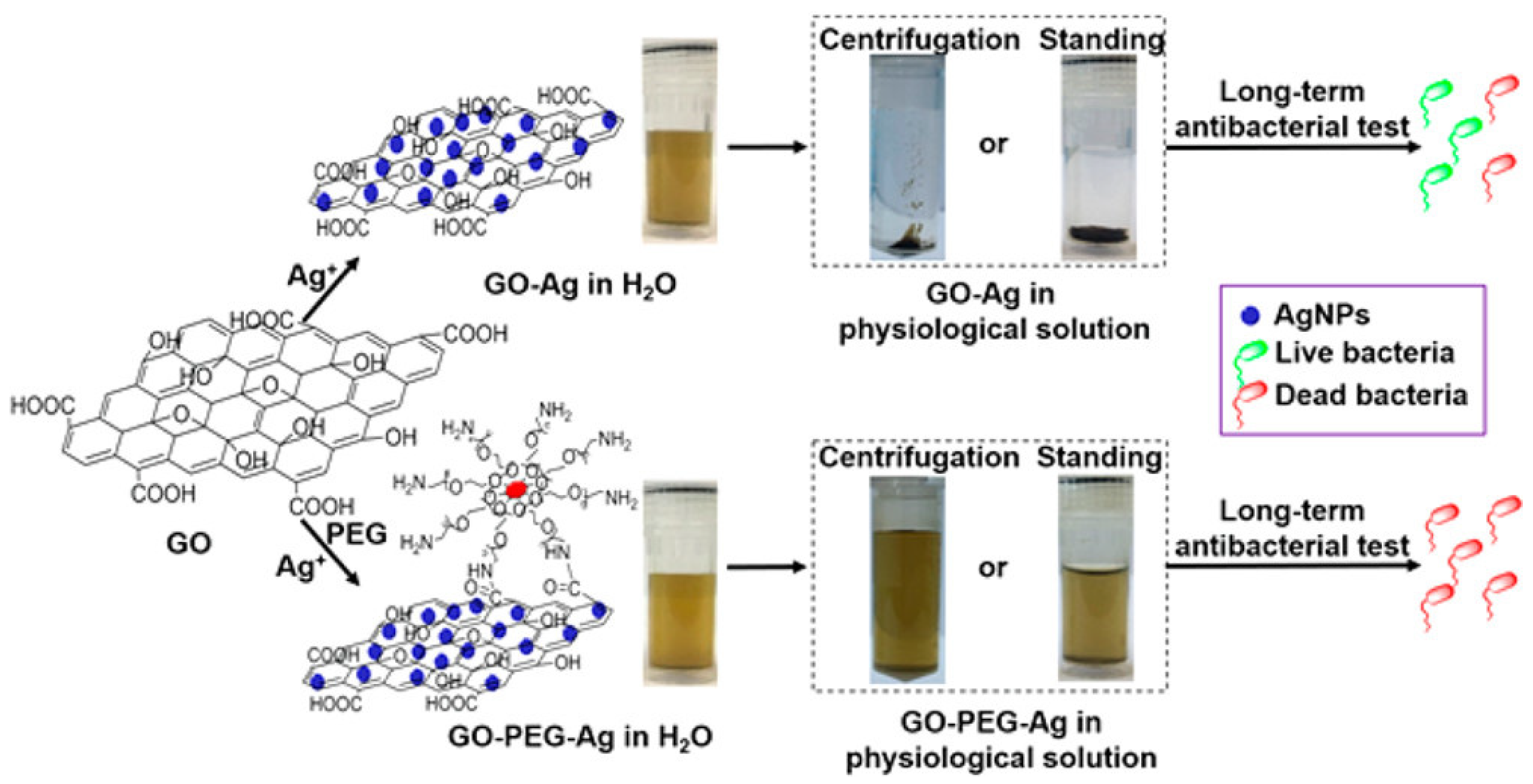
The synthesis process of GO/reduced GO (rGO)–Ag nanocomposites involves harsh conditions, as well as highly toxic reducing agents and organic solvents, which minimizes their use in biomedical applications, which is an unmet area of need that requires more exploration [306,307]. Despite the good dispersity of GO–Ag in aqueous solution, it has been discovered that GO aggregates irreversibly in physiological solutions over time [308]. This greatly affect its bioavailability, significantly weakening its antibacterial efficiency and long-term effectiveness. In an effort to solve the problem, the (polyethylene glycol) PEGylation of GO was carried out for long term antibacterial activity and stability of GO–Ag in physiological solution [309]. The PEGylated GO–Ag nanocomposite remained stable in a series of complex media over one month and resisted centrifugation (Figure 8). In contrast, non-PEGylated GO–Ag aggregated to varying degrees in the media after 1 h, and complete precipitation was observed after 1 week of equilibration. GO−PEG−Ag nanocomposites displayed remarkable long-term antibacterial activity after 1 week of storage in physiological saline, preserving >99% antibacterial activity against S. aureus and >95% antibacterial activity against E. coli. GO−PEG−Ag inhibited bacterial growth in nutrient rich Luria–Bertani (LB) broth for at least one week, and the repeated usage of GO−PEG−Ag up to three times did not reduce the antibacterial efficacy. In contrast, unmodified GO−Ag exhibited a >60% decline in antibacterial activity after 1 week of storage in physiological saline. This study provides a direct solution for the synthesis of homogenously dispersed and stable GO–Ag nanocomposites under physiological conditions. This result was also confirmed by a subsequent study, in which ternary hybrids of PEG-functionalized GO with Ag nanoparticles exhibited excellent bactericidal effects against E. coli, and it was found that those with smaller Ag nanoparticles (8 nm) showed better antibacterial activity than those with larger nanoparticles (50 nm) [310]. Furthermore, modification of GO with polyethyleneimine polymers dramatically enhanced the long-term antibacterial activity and stability of the GO–Ag nanocomposite [311]. In addition to this, Parandhaman and his colleagues recently designed GO–Ag nanocomposites functionalized with the natural antimicrobial peptide poly-L-lysine with remarkably improved stability and adhesion to S. aureus biofilms [312]. Notably, poly-L-lysine functionalization prevented the leaching of anions, thereby reducing the cytotoxicity of the graphene–silver nanocomposites. In order to obtain nanomaterials with long-term and stable antibacterial activity, a facile and green method has also been proposed to prepare AgNPs/polymer/GO composites with catalytic and antibacterial activities via the incorporation of furan-functional poly(styrene-alt-maleic anhydride) [313].
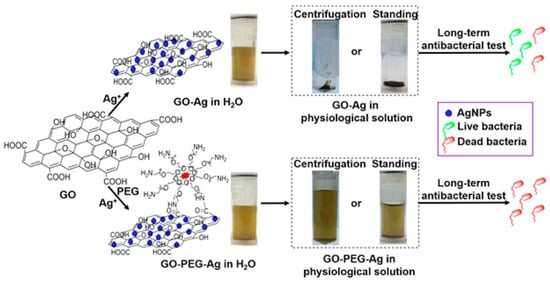
Figure 8.
Long-term stability and antibacterial effectiveness of a PEGylated GO–Ag nanocomposite. (Adapted with permission from Ref. [309]. 2017, Zhao et al. More details on “Copyright and Licensing” are available via the following link: https://www.mdpi.com/ethics#10 (accessed on 10 October 2022).)
4.3.2. Potential of Graphene Oxide–Metal Nanocomposites for In Vivo Therapies
In terms of biological activity, GO–Ag nanocomposites have been shown to demonstrate synergistic antibacterial activities against planktonic bacteria and biofilms, with low cytotoxicity and good biocompatibilities [314,315,316,317]. The enhanced antibacterial activity of GO–Ag nanocomposites is often ascribed to the synergistic activity of GO and Ag; however, the full antibacterial mechanism remains to be elucidated [299]. Malik et al. have also demonstrated that GO–Ag nanocomposites exhibit significantly enhanced growth inhibition of E. coli, S. aureus, and P. aeruginosa relative to silver nanoparticles alone [291]. Recent studies have accomplished the surface functionalization of GO and Ag nanoparticles by using lantana plant extract, and the results also affirmed the potential of GO–Ag nanocomposites as antibacterial agents against biological pollutants [290]. The negatively charged oxygen-containing groups of graphene oxide can absorb Ag ions through electron absorption, which can improve the confinement of Ag nanoparticle agglomeration and burst release, and synergistically enhance their antibacterial properties [292]. Intriguingly, GO–Ag nanocomposites exhibit species-specific bactericidal mechanisms, with cell wall disruption being observed against E. coli and inhibition of cell division against S. aureus [318].
The promising physical properties of GO, along with its synergistic activity with Ag nanoparticles, hold great potential for a targeted nanocomposite system [319]. A photothermal nanocomposite was produced which was composed of hyaluronic-acid-coated Ag nanoparticles that were integrated with GO [319]. Hyaluronic-acid-coated Ag nanoparticles confer additional protection by preventing the release of metal ions to surrounding mammalian cells. Upon encountering bacteria that secrete hyaluronidase, such as S. aureus, hyaluronic acid is degraded, followed by interaction of the GO–Ag nanocomposite and bacteria to further enhance the antibacterial action. Together with the photocatalytic characteristic of GO, local photothermal therapy under light irradiation could be achieved to further enhance the antibacterial activity of the GO–Ag nanocomposite [291].
4.3.3. Potential of Graphene Oxide–Metal Nanocomposites to Reduce Membrane Biofouling Issues for Water Decontamination and Filtration
The intrinsic characteristics of GO, including its availability as single-atomic-thick sheets, high hydrophilicity, extraordinary electrical, thermal, mechanical, structural properties, and low systemic toxicity could potentially reduce membrane biofouling issues for water decontamination or filtration [320,321]. GO–Ag nanocomposites are also a promising membrane surface modifier that contributed to enhanced membrane hydrophilicity, wettability and permeability, and good water influx [322,323,324,325]. Surface modification of membranes using GO/Ag nanocomposites exhibited stronger antimicrobial activities than AgNP-modified membranes and GO-modified membranes, without significantly altering the membrane transport properties [326]. The feasibility of using GO–Ag nanocomposites in membrane regeneration for a long-term anti-biofouling effect was demonstrated by conducting, in situ, the Ag-formation procedure to regenerate AgNPs on GO–Ag-modified membranes [327]. This potentially solves the problem of weakening biofouling properties of the functionalized GO–Ag nanocomposite membranes over time due to constant leaching of silver ions throughout the process. More complex membranes containing GO, Ag, and metal-organic frameworks (MOFs) in PES were prepared for water treatments, exploiting the synergistic effects of graphene oxide and silver to enhance the anti-biofouling properties of the membranes [328]. In conclusion, the metal oxide/graphene nanocomposites exhibit enhanced antibacterial properties under visible light irradiation and have great potential as photocatalysts in the field of water purification [321]. Nevertheless, more studies are required to examine the long-term usage, membrane reusability, and regeneration potential of functionalized GO–Ag nanocomposite membranes.
4.4. Graphene Oxide–Polymer Nanocomposites
Graphene oxide–polymer nanocomposites exhibit enhanced antibacterial activity, biocompatibility, hemocompatibility, hydrophilicity, and stability, compared with the polymeric based nanomaterials [329,330,331]. In addition, GO can greatly reinforce the mechanical properties of GO–polymer nanocomposites, including their breaking strength, Young’s modulus, compressive strength, flexural strength, and tensile strength [332,333,334]. The reinforcing effect is usually explained via strong interactions and bonding between the homogenously dispersed GO and polymeric components [332,333].
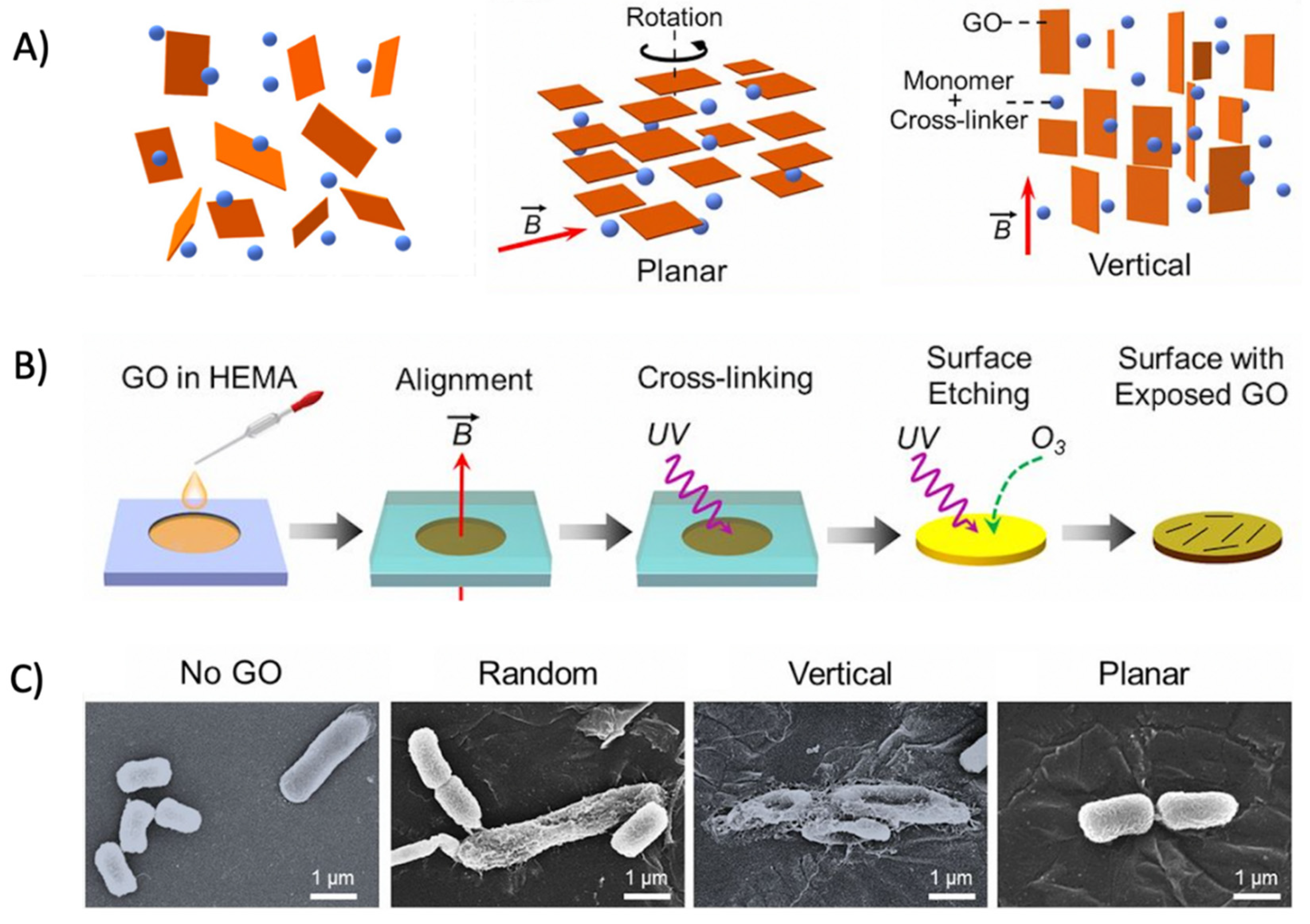
The alignment of GO sheets on the polymer film has been suggested to greatly affect the antibacterial activity of the resulting graphene oxide-based polymer nanocomposite [335]. Lu et al. synthesized graphene oxide–polymer nanocomposites by aligning GO in planar, vertical, and random orientations with the aid of a magnetic field (Figure 9A). GO was then immobilized by cross-linking with the surrounding polymer matrix, followed by oxidative etching to expose GO on the surface (Figure 9B). The vertically aligned GO nanosheets on the polymer film exhibited enhanced antibacterial activity compared with the random and horizontal orientations. Mechanistic examinations revealed that direct, edge-mediated contact with bacteria was the major mechanism in causing a greater physical disruption of the bacteria membranes (Figure 9C) [335]. Subsequently, greater levels of intracellular electron donors, for instance, glutathione, would release into the external environment upon membrane disruption, favoring GO to induce antibacterial activity via an oxidative stress mechanism [335]. This study highlights the importance of GO alignment and provides direct implications for the designing of GO–polymer nanocomposite films with enhanced antibacterial activities.
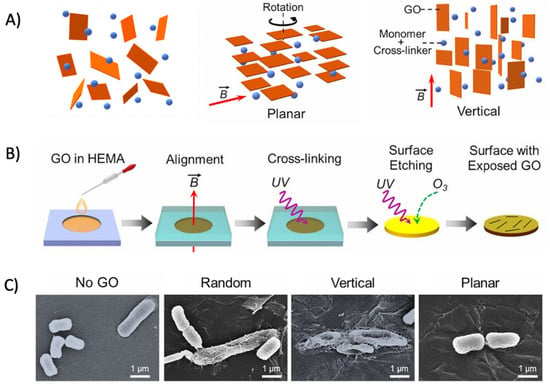
Figure 9.
(A) Schematic illustration of the GO film with different orientations. From left to right: random, planar, and vertical orientations. (B) Schematic illustration of the film fabrication procedure. A magnetic field is applied to control the orientation of dispersed GO nanosheets, with the orientation preserved by photo-cross-linking the dispersing agents. (C) SEM micrographs pictured the intact E. coli morphology treated by no-GO film, retained E. coli morphological integrity treated by randomly aligned- or planar aligned-GO film and flattened and wrinkled E. coli morphologies after being treated by a vertically aligned-GO film. (Adapted with permission from Ref. [335]. 2017, Lu et al. More details on “Copyright and Licensing” are available via the following link: https://www.mdpi.com/ethics#10 (accessed on 10 October 2022)).
The abundant functional groups present on GO–polymer nanocomposites provide various interactions with nanoparticles, creating GO–polymer-based metal nanocomposites with superior characteristics. For instance, GO–chitosan nanocomposites have been demonstrated to act as both nucleation sites for calcium phosphates mineralization and absorption sites for nanoparticles [336]. The resulting GO/chitosan nanocomposites comprise micro- and nanohierarchical porous structures that allow cell attachment and proliferation after biomineralization [336]. In addition, the immobilization of the AgNPs and growth-factor-encapsulated nanoparticles on the GO–chitosan nanocomposites greatly enhances the antibacterial activity and osteo-inductivity, respectively [336]. Taken together, GO–polymer nanocomposites have great potential for use as multifunctional nanocomposite materials in various antibacterial applications. This is due to the substantial property enhancements and their ability to interact with various metal or metal oxide nanoparticles. Díez-Pascual and Luceño-Sánchez described several antibacterial applications of GO–polymer nanocomposites [325].
4.4.1. Development of Synthetic Approaches for the Production and Use of Graphene Oxide–Polymer Nanocomposites
Various synthesis routes can be applied to prepare graphene oxide–polymer nanocomposite with covalent or non-covalent interactions [337]. Shahryari et al. summarized a range of synthesis routes in a thorough review [338]. Generally, three common methods are utilized: solution blending, melt blending, and in situ polymerization [338,339]. The solution blending approach is the most commonly used method to synthesize graphene oxide–polymer nanocomposites in a dispersion form, due to its convenience and ease of implementation. With this method, facile synthesis is achieved by simply blending the polymer with GO in solution, followed by sonication, or magnetic stirring, or shear mixing to obtain homogenous dispersions of the nanocomposite products as depicted in Figure 10 [340,341]. In Rusakova et al.’s study, an ultrasonic bath was used to disperse GO in a solution of styrene or a polyester resin in styrene, adding toluene and benzoyl peroxide and then, the mixture was placed into a special mold for polymerizing the nanocomposite [342].
Figure 10.
Formation of a well-dispersed graphene oxide–polymer nanocomposite with graphene being embedded in polymeric matrix.
Melt blending is applied commonly in industry due to its low cost and scalability. Unlike solution blending, melt blending eliminates the use of any toxic solvents for the dispersion. Following melting the polymer at high temperature, the GO nanofillers are dispersed into a polymer matrix by mechanical shear forces. However, it is likely to induce particle aggregation by thermal heating and local mechanical stresses during melt blending. A study employed wet phase inversion to prepare a sponge-like structure of a polymer/GO/solvent mixture as an exfoliating agent. It was subsequently ground into powder form and mixed with a polymer using melt blending [343]. The hybrid method was exemplified with two polymers, polyamide 6 (PA6) and poly (ethylene-co-vinyl acetate) (EVA). The produced nanocomposites exhibited enhanced dispersions with improved mechanical and dynamic–mechanical properties, compared with the nanocomposites prepared via melt blending [343].
In situ polymerization can lead to a more homogeneous dispersion of the graphene derivatives within the polymer matrix than the two methods mentioned above. In this technique, GO was initially mixed with the monomers, followed by polymerization. The polymerization can be initiated by heat or radiation. Microwave heating has been demonstrated to be a particularly efficient method to produce well-dispersed rGO polymer nanocomposites [344,345]. Hou et al. exploited microwave heating to simultaneously reduce GO and conducted a nitroxide-mediated polymerization of styrene, forming rGO-polystyrene nanocomposites [346].
The resulting nanocomposite could be further modified into various forms and shapes for different antibacterial applications [336,347,348].
4.4.2. Application of Graphene Oxide–Polymer-Based Metal Nanocomposites in Wound Healing
Graphene oxide–polymer-based metal nanocomposites have a potential emerging role in wound healing application as in situ-forming hydrogels. Yan et al. have synthesized a novel GO–polymer-based metal nanocomposite (PEP-Ag@GO) for a wound healing application [349]. PEP-Ag@GO comprises a poly(Nisopropylacrylamide166-co-n-butyl acrylate9)-poly (ethyleneglycol)-poly(N-isopropylacrylamide166-co-n-butyl acrylate9) copolymer, denoted as PEP and AgNPs decorated on reduced GO nanosheets, denoted as Ag@GO. An aqueous mixture of the PEP-Ag@GO could transit to a hydrogel immediately in situ upon contact with the skin area that has a higher temperature than the transition temperature of PEP-Ag@GO (30 °C). The in situ formation of the hydrogel allows the treatment of wound areas that are difficult to access and minimizes tissue damage that is associated with changing of the wound dressing material [350]. More importantly, the strong interactions and bonding that arises from the PEP-GO give rise to good stability of the composite network. Therefore, the PEP-Ag@GO hydrogel resisted a transition back to liquid form at lower temperatures, even at 5 °C. This is particularly advantageous as commonly synthesized thermo-responsive hydrogels would phase transition back to a liquid at lower temperatures [351]. In vitro and in vivo experiments have also demonstrated good biocompatibility and enhanced antibacterial activity of a PEP-Ag@GO against MRSA (Mu50), leading to a much faster wound healing rate of an MRSA-infected skin defect [349].
4.4.3. Application of Graphene Oxide–Polymer-Based Nanocomposites in Water Treatment
Membrane technologies have been widely applied in water treatments, but the growth of biofilms causes deterioration of water filtration membranes. Therefore, GO–polymer-based nanocomposites are promising materials for use in water treatments due to their antibacterial and antifouling properties. A recent study discussed newly developed GO-based nanocomposites in water treatments and identified limitations for future improvements [352]. Zeng et al. modified poly(vinylidene fluoride) (PVDF) membranes by covalently immobilizing graphene oxide quantum dots (GOQDs) to exert the antibiofouling and antibacterial properties and maintain excellent permeation properties of PVDF membranes [353]. It was found that the GOQDs–PVDF membrane inhibited the growth of E. coli and S. aureus more effectively than two-dimensional GO sheets. Cheng et al. evaluated the performances of GO-coated and GO-blended polysulfone ultrafiltration membranes and GO-coated membranes presented lower declines in water flux and higher flux recoveries than GO-blended membranes [354]. They showed strong antibacterial activity and biofouling resistance against E. coli. To enhance the antibacterial activity, metal nanoparticles can also be incorporated into GO–polymer-based nanocomposites. Mahmoudi et al. incorporated Ag-decorated GO nanoplates into polysulfone membranes, which demonstrated superior antibacterial properties and inhibited the formation of biofouling [355].
4.4.4. Application of Graphene Oxide–Polymer-Based Nanocomposites in Food Packaging
Graphene oxide–polymer-based membranes are attractive candidates to be applied in food packaging due to the fact that their incorporation increases the permeability, selectivity, barrier, and antibacterial activities of packaging and prolongs the durability of the material. For example, GO was cross-linked with chitosan at 120 °C to form nanocomposite films which improved the tensile strength and thermal stability and exhibited antimicrobial properties against E. coli and B. subtilis [356]. As a result of these enhancements, the films are suitable for use in food packaging. Multiple reviews have been conducted on the development and applications of graphene oxide nanocomposites in food packaging [357,358,359,360]. However, the current state of GO–polymer composite membranes in food packaging applications are yet to be commercialized to the best of our knowledge.
4.5. Lipid Polymer Hybrid Nanoparticles
Lipid polymer hybrid nanoparticles (LPHNPs) have emerged as a potentially superior nanocomposite delivery system by combining the advantages and mitigating the limitations associated with liposomes and polymeric nanoparticles alone [361,362,363,364,365,366]. LPHNPs comprise a biodegradable polymeric core for drug encapsulation, an inner lipid layer surrounding the polymeric core, and an outer polymeric stealth layer (Figure 11). The polymeric core provides high structural integrity, mechanical stability, a narrow size distribution, and higher lipophilic drug loading capacities of the LPHNPs [367]. The inner lipid layer confers the biocompatibility and delays the polymeric degradation of LPHNPs by limiting inward water diffusion, contributing to the sustained release of the composite system [368]. Finally, the outer polymeric stealth layer provides steric stabilization of the nanoparticles, acting as a stealth coating that enhances the in vivo circulation time and protect the composite system from immune recognition [369].
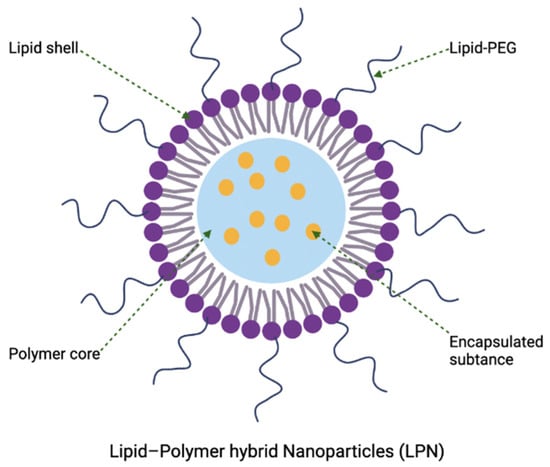
Figure 11.
Structure of a lipid–polymer hybrid nanoparticle that comprises a polymeric core, an inner lipid layer, and an outer lipid–polyethylene glycol (PEG) shell (polymeric stealth layer).
4.5.1. Development of Lipid Polymer Hybrid Nanoparticles Using a Quality-by-Design Approach
LPHNPs are still in their early developmental stage for antibacterial applications despite the advances witnessed in cancer therapeutics [370]. The synthesis conditions of antibiotic loaded LPHNPs with desired particle characteristics is highly challenging due to the strong interplay between process variables. Therefore, a quality-by-design approach is often utilized to customize the LPHNPs in meeting the requirements or desired particle characteristics [371,372,373,374,375]. A quality-by-design approach is a statistical method that applies multiple factorial concepts and modelling to determine the interactions between two or more process variables and the desired and observed response conditions [374,376,377,378]. For instance, Dave et al. developed and optimized norfloxacin loaded LPHNPs for a topical drug delivery application [372]. They utilized a Box–Behnken design to determine the effect of process conditions including the concentration of soya lecithin (lipid) and the concentration of a polylactic acid (polymer) on the response conditions. This included parameters such as the entrapment efficiency, particle size, and cumulative drug release. It was found that the optimal norfloxacin-loaded LPHNPs have a high drug encapsulation efficiency, desired particle size with a narrow distribution range, and an improved drug release profile and stability [372]. In a study aimed at optimizing LPHNPs, Thanki et al. customized LPHNPs composed of lipidoid 5 (cationic lipid-like molecule) and poly(DL-lactic-co-glycolic acid) (PLGA) for loading antisense oligonucleotide-mediated luciferase gene (Luc-ASO) transcripts and they achieved efficient cellular delivery by using a quality-by-design approach [374]. In their study, they determined the effect of process conditions including the lipidoid 5 content and the lipidoid 5: Luc-ASO ratio against the response conditions (intensity-weighted average hydrodynamic diameter, polydispersity index, zeta potential, Luc-ASO encapsulation efficiency, Luc-ASO loading, in vitro splice-correction efficiency, and in vitro cell viability) to achieve efficient cell delivery [374]. A recent study by Ma et al. involved efficient delivery of hydroxycamptothecin (HCPT) via PEGylated LPHNPs [375]. A quality-by-design strategy was used to optimize HCPT-loaded LPHNPs with desired properties, among them, the factors representing key process conditions were the lipid/polymer mass ratio, polymer concentration, medium chain triglyceride volume, water-solvent ratio, and poly(D,L-lactide-co-glycolide) molecular weight, and the response conditions are particle size, particle size distribution, and drug-loading capacity [375]. The experimental results showed that the optimal LPHNPs had greater controlled release behavior and good stability in plasma, and effectively increased the loading of HCPT [375].
4.5.2. Potential of Lipid Polymer Hybrid Nanoparticles as Antibacterial Delivery Vehicles
Recently, LPHNPs have received great attention in antibacterial applications as efficient drug delivery systems due to their combined advantages of liposomes and polymer nanoparticles [361,363,364,365,366,379]. Cai et al. have investigated the application of LPHNPs to act as promising antibacterial delivery vehicles for biofilm eradication [379]. In this study, the lipid layer was designed to contain mixed lipids of phospholipids and rhamnolipids, which acted as anti-adhesive and disrupting agents against the biofilms. The inner polymer core comprises multidrug regimens including antibiotic amoxicillin to exert antibacterial activity and the amoxicillin potentiator pectin sulfate that prevent the re-adherence of H. pylori. As a result, a complete eradication of H. pylori biofilm with impaired antibacterial resistance was observed under in vitro conditions. The performance of vancomycin-loaded LPHNPs was enhanced via the synthesis using multiple lipid excipients, including glyceryl triplamitate, oleic acid, polymer excipients Eudragit RS100, chitosan, and sodium alginate [361]. Compared to LPHNPs using mono-constituents of the lipid and polymer, the LPHNPs with multiple co-excipients demonstrated higher drug loading capacities and enhanced antibacterial activity against both sensitive strains of S. aureus and MRSA [361]. In one recent experiment, Jaglal et al. designed a pH-responsive LPHNP system for co-delivery of vancomycin and 18β-glycyrrhetinic acid, showing its ability to eliminate 75% of MRSA in less than 12 h with the advantage of sustained and rapid release of vancomycin in acidic conditions [363]. LPHNPs consisting of a poly(lactic-co-glycolic acid) core and a dioleoyl-3-trimethylpropane lipid shell were developed for loading vancomycin and were shown to have prominent antibacterial effect against planktonic S. aureus cells [364]. In this study, enhanced interactions with bacterial cells and penetration into biofilms was due to the presence of lipid shells. These studies indicate that LPHNPs could be a useful strategy to deliver and enhance the antibacterial activity of the loaded drugs against planktonic cells and biofilms of diverse species. However, more studies are needed to accelerate the clinical translation of the LPHNPs in antibacterial application.
5. Conclusion, Bottlenecks, and Future Perspective of Nanotechnologies Being Developed for Antibacterial Applications
This review mainly focuses on the progress and development of the prospects of nanomaterials in antibacterial applications. As described, the use of nanomaterials to combat bacterial infections has great potential for human health and medical development. Over the past few decades, significant progress has been made in understanding the antibacterial activity and potential of different classes of nanomaterials as drug carriers, leading to the discovery of a number of particularly promising candidate nanoparticle systems. Moreover, Chieruzzi et al. discussed that nanomodification such as incorporating fluoroapatite nanobioceramics into traditional clinical treatment materials, such as dental restorative glass ionomer cement, can lead to significant changes in the mechanical properties of materials, which is a very noteworthy direction for the development of new nano antibacterial materials [380]. Given their vast therapeutic potential and wide range of antibacterial applications of these nanomaterials and nanocomposites, we anticipate that more studies with emphasis on aiding the clinical translation and subsequent clinical trials of these nanotechnology-associated products will increase rapidly in the next decade. Several challenges remain to be addressed. Despite the importance of these nanomaterials as therapeutics and use in the field of biomedicine, their current limitations on human health cannot be ignored. The high-efficiency properties of nanomaterials as antibacterial agents or drug delivery vehicles allow their diffusion in different bodily organs, and sometimes the accumulation of these nanomaterials in various cells and tissues may cause negative health effects. It should be noted that the establishment of the consensus of nanomaterials physiochemical properties leading to maximum antibacterial activity and minimum toxicity is of utmost importance. With the understanding of the structure–property–activity relationship, researchers are able to reduce off-target effects of nanomaterials and effectively deliver nanotherapeutics to a desired infected tissue. For example, the use of single nanoparticles such as metal particles have many drawbacks due to their inherent toxicity. Therefore, combining a variety of nanomaterials to develop a new type of nanocomposite or nanohybrid material to obtain enhanced antibacterial activity has become a major research trend. In addition, exploring the mechanisms behind the antibacterial and physical properties of nanomaterials and nanocomposites should not be neglected. The more we learn, the better we as a community can devise new strategies to combat antimicrobial resistance. More studies in assessing the dose calibration and identification of the appropriate routes of administration for a wide range of nanomaterials are also needed. This will greatly speed up the progress towards clinical trial progression and commercialization of nanotechnology-associated end products.
Author Contributions
Conceptualization, R.R., C.L. and S.L; writing—original draft preparation, R.R., C.L. and S.L.; writing—review and editing, Y.W., J.S., T.-W.L., B.W.M., H.-Y.H. and H.-H.S.; supervision, H.-H.S.; funding acquisition, H.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the NHMRC Career Development Fellowship GNT1106798.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| MDR | Multidrug-resistant |
| WHO | World Health Organization |
| SARS-CoV-2 | Severe acute respiratory coronavirus 2 |
| mRNA | Messenger RNA |
| FDA | Food and Drug Administration |
| LLA | Liposomal linolenic acid |
| S. aureus | Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| H. pylori | Helicobacter pylori |
| P. acnes | Propionibacterium acnes |
| DHA | Docosahexaenoic acid |
| IR | Infrared radiation |
| P. aeruginosa | Pseudomonas aeruginosa |
| K. pneumoniae | Klebsiella pneumoniae |
| MDR-PA | Multidrug-resistant Pseudomonas aeruginosa |
| CNM | Carbon-based nanomaterial |
| GO | Graphene oxide |
| E. coli | Escherichia coli |
| NGO–HA | Nanographene oxide–hyaluronic acid |
| E. faecalis | Enterococcus faecalis |
| C. albicans | Candida albicans |
| ROS | Reactive oxygen species |
| NPs | Nanoparticles |
| B. subtilis | Bacillus subtilis |
| S. epidermidis | Staphylococcus epidermidis |
| L. monocytogenes | Listeria monocytogenes |
| GRAS | Generally Recognized as Safe |
| UV | Ultraviolet |
| PVA | Polyvinyl alcohol |
| PEG | Polyethylene glycol |
| MIC | Minimum inhibitory concentration |
| PEN | Polyethylene naphthalate |
| KrF | Krypton fluoride |
| VRE | Vancomycin-resistant E. faecalis |
| rGO | Reduced graphene oxide |
| PEG | Polyethylene glycol |
| LB | Luria–Bertani |
| MOFs | Metal-organic frameworks |
| PES | Polyethersulfone |
| SEM | Scanning electron microscope |
| PA6 | Polyamide 6 |
| EVA | Ethylene-co-vinyl acetate |
| PEP | Poly(Nisopropylacrylamide166-co-n-butyl acrylate9)-poly(ethyleneglycol)-poly (N-isopropylacrylamide166-co-n-butyl acrylate9) |
| PVDF | Poly(vinylidene fluoride) |
| GOQDs | Graphene oxide quantum dots |
| LPHNPs | Lipid polymer hybrid nanoparticles |
| PLGA | Poly(DL-lactic-co-glycolic acid) |
| Luc | Luciferase gene |
| ASO | Antisense oligonucleotide |
| HCPT | Hydroxycamptothecin |
References
- Davis, M.; Whittaker, A.; Lindgren, M.; Djerf-Pierre, M.; Manderson, L.; Flowers, P. Understanding media publics and the antimicrobial resistance crisis. Glob. Public Health 2018, 13, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019; ECDC: Solna kommun, Stockholm, 2020. [Google Scholar]
- Kwon, J.H.; Powderly, W.G. The post-antibiotic era is here. Science 2021, 373, 471. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-I.; The Korean Network for Study of Infectious Diseases (KONSID); Chung, D.R.; Ko, K.S.; Peck, K.R.; Song, J.-H. Risk factors for infection and treatment outcome of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in patients with hematologic malignancy. Ann. Hematol. 2012, 91, 115–121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Genet. 2021, 19, 5–6. [Google Scholar] [CrossRef]
- Blackman, L.D.; Sutherland, T.D.; De Barro, P.J.; Thissen, H.; Locock, K.E.S. Addressing a future pandemic: How can non-biological complex drugs prepare us for antimicrobial resistance threats? Mater. Horiz. 2022, 9, 2076–2096. [Google Scholar] [CrossRef]
- Lardani, L.; Derchi, G.; Marchio, V.; Carli, E. One-Year Clinical Performance of Activa™ Bioactive-Restorative Composite in Primary Molars. Children 2022, 9, 433. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, W.; Zhang, J.; Xia, X. Antimicrobial lipids in nano-carriers for antibacterial delivery. J. Drug Target. 2020, 28, 271–281. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Hochvaldová, L.; Večeřová, R.; Kolář, M.; Prucek, R.; Kvítek, L.; Lapčík, L.; Panáček, A. Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis. Nanotechnol. Rev. 2022, 11, 1115–1142. [Google Scholar] [CrossRef]
- Kankala, R.K. Organic- or Inorganic-based Nanomaterials: Opportunities and Challenges in the Selection for Biomedicine. Curr. Pharm. Des. 2022, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Singh, R.; RamaRao, P. Accumulated Polymer Degradation Products as Effector Molecules in Cytotoxicity of Polymeric Nanoparticles. Toxicol. Sci. 2013, 136, 131–143. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surfaces B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltran-Partida, E.; Cheng, N.; Salvador-Carlos, J.; Valdez-Salas, E.A.; Curiel-Alvarez, M.; Ibarra-Wiley, R. Promotion of Surgical Masks Antimicrobial Activity by Disinfection and Impregnation with Disinfectant Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 2689–2702. [Google Scholar] [CrossRef]
- Milić, M.; Leitinger, G.; Pavicic, I.; Avdičević, M.Z.; Dobrović, S.; Goessler, W.; Vrček, I.V. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015, 35, 581–592. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2009, 4, 15–41. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Yan, Y.; Xue, L.; Zhang, C.; Li, G.; Zheng, Q.; Zhao, Y.S.; Jiang, H.; Yao, J. Controlling the Structures and Photonic Properties of Organic Nanomaterials by Molecular Design. Angew. Chem. Int. Ed. 2013, 52, 8713–8717. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2023–2050. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Romano, G.L.; Platania, C.B.M.; Drago, F.; Puglisi, G.; Pignatello, R. Influence of different surfactants on the technological properties and in vivo ocular tolerability of lipid nanoparticles. Int. J. Pharm. 2014, 470, 133–140. [Google Scholar] [CrossRef]
- Yang, D.; Pornpattananangkul, D.; Nakatsuji, T.; Chan, M.; Carson, D.; Huang, C.-M.; Zhang, L. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials 2009, 30, 6035–6040. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Fu, V.; Thamphiwatana, S.; Zhang, L.; Chen, M.; Vecchio, J.; Gao, W.; Huang, C.-M.; Zhang, L. In Vivo Treatment of Propionibacterium acnes Infection with Liposomal Lauric Acids. Adv. Health Mater. 2013, 2, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Nehate, C.; Barman, T.K.; Rathore, A.S.; Koul, V. Design, preparation, and evaluation of liposomal gel formulations for treatment of acne: In vitro and in vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Shi, S.; Rong, L.; Feng, M.-Q.; Zhong, L. The impact of liposomal linolenic acid on gastrointestinal microbiota in mice. Int. J. Nanomed. 2018, 13, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Tran, T.-Q.; Hsieh, M.-F.; Chang, K.-L.; Pho, Q.-H.; Nguyen, V.-C.; Cheng, C.-Y.; Huang, C.-M. Bactericidal Effect of Lauric Acid-Loaded PCL-PEG-PCL Nano-Sized Micelles on Skin Commensal Propionibacterium acnes. Polymers 2016, 8, 321. [Google Scholar] [CrossRef]
- Huynh, N.; Passirani, C.; Saulnier, P.; Benoit, J. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef]
- Umerska, A.; Cassisa, V.; Matougui, N.; Joly-Guillou, M.-L.; Eveillard, M.; Saulnier, P. Antibacterial action of lipid nanocapsules containing fatty acids or monoglycerides as co-surfactants. Eur. J. Pharm. Biopharm. 2016, 108, 100–110. [Google Scholar] [CrossRef]
- Umerska, A.; Cassisa, V.; Bastiat, G.; Matougui, N.; Nehme, H.; Manero, F.; Eveillard, M.; Saulnier, P. Synergistic interactions between antimicrobial peptides derived from plectasin and lipid nanocapsules containing monolaurin as a cosurfactant against Staphylococcus aureus. Int. J. Nanomed. 2017, 12, 5687–5699. [Google Scholar] [CrossRef]
- Rozenbaum, R.T.; Su, L.; Umerska, A.; Eveillard, M.; Håkansson, J.; Mahlapuu, M.; Huang, F.; Liu, J.; Zhang, Z.; Shi, L.; et al. Antimicrobial synergy of monolaurin lipid nanocapsules with adsorbed antimicrobial peptides against Staphylococcus aureus biofilms in vitro is absent in vivo. J. Control. Release 2019, 293, 73–83. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, S.; Imran, M.; Habib, H.; Shabbir, S.; Ihsan, A.; Zafar, Y.; Hafeez, F.Y. Potential of monolaurin based food-grade nano-micelles loaded with nisin Z for synergistic antimicrobial action against Staphylococcus aureus. LWT Food Sci. Technol. 2016, 71, 227–233. [Google Scholar] [CrossRef]
- Taylor, E.N.; Kummer, K.M.; Dyondi, D.; Webster, T.J.; Banerjee, R. Multi-scale strategy to eradicate Pseudomonas aeruginosa on surfaces using solid lipid nanoparticles loaded with free fatty acids. Nanoscale 2014, 6, 825–832. [Google Scholar] [CrossRef]
- Hallaj-Nezhadi, S.; Hassan, M. Nanoliposome-based antibacterial drug delivery. Drug Deliv. 2015, 22, 581–589. [Google Scholar] [CrossRef] [PubMed]
- El-Nesr, O.H.; Yahiya, S.A.; El-Gazayerly, O.N. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm. J. 2010, 18, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Orizondo, R.A.; Fabiilli, M.L.; Morales, M.A.; Cook, K.E. Effects of Emulsion Composition on Pulmonary Tobramycin Delivery During Antibacterial Perfluorocarbon Ventilation. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 251–259. [Google Scholar] [CrossRef]
- Severino, P.; Silveira, E.F.; Loureiro, K.; Chaud, M.V.; Antonini, D.; Lancellotti, M.; Sarmento, V.H.; da Silva, C.F.; Santana, M.H.A.; Souto, E.B. Antimicrobial activity of polymyxin-loaded solid lipid nanoparticles (PLX-SLN): Characterization of physicochemical properties and in vitro efficacy. Eur. J. Pharm. Sci. 2017, 106, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Magalhães, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomedicine 2017, 12, 2721–2736. [Google Scholar] [CrossRef]
- Walduck, A.; Sangwan, P.; Vo, Q.A.; Ratcliffe, J.; White, J.; Muir, B.W.; Tran, N. Treatment of Staphylococcus aureus skin infection in vivo using rifampicin loaded lipid nanoparticles. RSC Adv. 2020, 10, 33608–33619. [Google Scholar] [CrossRef]
- Tran, N.; Mulet, X.; Hawley, A.M.; Fong, C.; Zhai, J.; Le, T.C.; Ratcliffe, J.; Drummond, C.J. Manipulating the Ordered Nanostructure of Self-Assembled Monoolein and Phytantriol Nanoparticles with Unsaturated Fatty Acids. Langmuir 2018, 34, 2764–2773. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Ding, Y.; Wu, C.-M.; Chen, X.; Jiang, J.-H.; Hsu, H.-Y.; Wang, Y.; Le Brun, A.P.; Song, J.; Han, M.-L.; et al. Phytantriol-Based Cubosome Formulation as an Antimicrobial against Lipopolysaccharide-Deficient Gram-Negative Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 44485–44498. [Google Scholar] [CrossRef]
- Lai, X.; Han, M.-L.; Ding, Y.; Chow, S.H.; Le Brun, A.P.; Wu, C.-M.; Bergen, P.J.; Jiang, J.-H.; Hsu, H.-Y.; Muir, B.W.; et al. A polytherapy based approach to combat antimicrobial resistance using cubosomes. Nat. Commun. 2022, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Meikle, T.G.; Dyett, B.P.; Strachan, J.B.; White, J.; Drummond, C.J.; Conn, C.E. Preparation, Characterization, and Antimicrobial Activity of Cubosome Encapsulated Metal Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 6944–6954. [Google Scholar] [CrossRef]
- Boge, L.; Umerska, A.; Matougui, N.; Bysell, H.; Ringstad, L.; Davoudi, M.; Eriksson, J.; Edwards, K.; Andersson, M. Cubosomes post-loaded with antimicrobial peptides: Characterization, bactericidal effect and proteolytic stability. Int. J. Pharm. 2017, 526, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Meikle, T.G.; Dharmadana, D.; Hoffmann, S.V.; Jones, N.C.; Drummond, C.J.; Conn, C.E. Analysis of the structure, loading and activity of six antimicrobial peptides encapsulated in cubic phase lipid nanoparticles. J. Colloid Interface Sci. 2021, 587, 90–100. [Google Scholar] [CrossRef]
- Dyett, B.P.; Yu, H.; Sarkar, S.; Strachan, J.B.; Drummond, C.J.; Conn, C.E. Uptake Dynamics of Cubosome Nanocarriers at Bacterial Surfaces and the Routes for Cargo Internalization. ACS Appl. Mater. Interfaces 2021, 13, 53530–53540. [Google Scholar] [CrossRef]
- Jackman, J.A.; Yoon, B.K.; Li, D.; Cho, N.-J. Nanotechnology Formulations for Antibacterial Free Fatty Acids and Monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Thorn, C.R.; Thomas, N.; Boyd, B.J.; Prestidge, C.A. Nano-fats for bugs: The benefits of lipid nanoparticles for antimicrobial therapy. Drug Deliv. Transl. Res. 2021, 11, 1598–1624. [Google Scholar] [CrossRef] [PubMed]
- Boushehri, M.A.S.; Dietrich, D.; Lamprecht, A. Nanotechnology as a Platform for the Development of Injectable Parenteral Formulations: A Comprehensive Review of the Know-Hows and State of the Art. Pharmaceutics 2020, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation strategies for improving intestinal permeability of drugs: A more precise look at permeability assessment methods and pharmacokinetic properties changes. J. Control. Release 2020, 321, 669–709. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.B.; Yoon, B.K.; Cho, N.-J.; Lovrić, J.; Jug, M.; Jackman, J.A. Lipid Nanoparticle Technology for Delivering Biologically Active Fatty Acids and Monoglycerides. Int. J. Mol. Sci. 2021, 22, 9664. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-M.; Chen, C.-H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.-F.; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar] [CrossRef]
- Obonyo, M.; Zhang, L.; Thamphiwatana, S.; Pornpattananangkul, D.; Fu, V.; Zhang, L. Antibacterial Activities of Liposomal Linolenic Acids against Antibiotic-Resistant Helicobacter pylori. Mol. Pharm. 2012, 9, 2677–2685. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Gao, W.; Obonyo, M.; Zhang, L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 17600–17605. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Transforming linoleic acid into a nanoemulsion for enhanced activity against methicillin susceptible and resistant Staphylococcus aureus. RSC Adv. 2015, 5, 90482–90492. [Google Scholar] [CrossRef]
- Silva, E.L.; Carneiro, G.; De Araújo, L.A.; Trindade, M.D.J.V.; Yoshida, M.I.; Oréfice, R.L.; Farias, L.D.M.; De Carvalho, M.A.R.; Dos Santos, S.G.; Goulart, G.A.C.; et al. Solid Lipid Nanoparticles Loaded with Retinoic Acid and Lauric Acid as an Alternative for Topical Treatment of Acne Vulgaris. J. Nanosci. Nanotechnol. 2015, 15, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Carbone, C.; Souto, E. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Da Silva Santos, V.; Ribeiro, A.P.B.; Santana, M.H.A. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef]
- Brandelli, A.; Pola, C.C.; Gomes, C.L. Antimicrobial delivery systems. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 665–694. [Google Scholar]
- Pinilla, C.; Lopes, N.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef]
- Seabra, C.L.; Nunes, C.; Gomez-Lazaro, M.; Correia, M.; Machado, J.C.; Gonçalves, I.C.; Reis, C.A.; Reis, S.; Martins, M.C.L. Docosahexaenoic acid loaded lipid nanoparticles with bactericidal activity against Helicobacter pylori. Int. J. Pharm. 2017, 519, 128–137. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H. Polymeric Nanomaterials for Efficient Delivery of Antimicrobial Agents. Pharmaceutics 2021, 13, 2108. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Román-García, M.F.; Ramírez-Díaz, C.A.; Ulloa-Ramírez, M.; Morones-Ramírez, J.R. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics 2022, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Ding, X.; Wang, A.; Tong, W.; Xu, F.J. Biodegradable Antibacterial Polymeric Nanosystems: A New Hope to Cope with Multidrug-Resistant Bacteria. Small 2019, 15, e1900999. [Google Scholar] [CrossRef]
- Iyisan, B.; Landfester, K. Modular Approach for the Design of Smart Polymeric Nanocapsules. Macromol. Rapid Commun. 2019, 40, e1800577. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym. Technol. Mater. 2021, 60, 1996–2024. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic Lipid Polymer Nanoparticles for Drug Delivery. In Nanoparticles in Biology and Medicine: Methods and Protocols; Ferrari, E., Soloviev, M., Eds.; Springer US: New York, NY, USA, 2020; pp. 45–60. [Google Scholar]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef]
- Saini, R.K.; Bagri, L.P.; Bajpai, A.K.; Mishra, A. Responsive polymer nanoparticles for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 289–320. [Google Scholar]
- Dararatana, N.; Seidi, F.; Hamela, J.; Crespy, D. Controlling release kinetics of pH-responsive polymer nanoparticles. Polym. Chem. 2020, 11, 1752–1762. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, D.; Sui, G.; Wang, D.; Wu, M.; Han, L.; Mu, H.; Duan, J. Gentamicin decorated phosphatidylcholine-chitosan nanoparticles against biofilms and intracellular bacteria. Int. J. Biol. Macromol. 2020, 156, 640–647. [Google Scholar] [CrossRef]
- Walvekar, P.; Gannimani, R.; Salih, M.; Makhathini, S.; Mocktar, C.; Govender, T. Self-assembled oleylamine grafted hyaluronic acid polymersomes for delivery of vancomycin against methicillin resistant Staphylococcus aureus (MRSA). Colloids Surfaces B Biointerfaces 2019, 182, 110388. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Lin, M.; Sun, J. Antimicrobial peptide-inspired antibacterial polymeric materials for biosafety. Biosaf. Health 2022, 4, 269–279. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Zheng, W.; Prananty, D.; Li, J.; Koh, C.H.; Kang, E.-T.; Pethe, K.; Chan-Park, M.B. Polymers as advanced antibacterial and antibiofilm agents for direct and combination therapies. Chem. Sci. 2022, 13, 345–364. [Google Scholar] [CrossRef]
- Mowery, B.P.; Lindner, A.H.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Structure−activity Relationships among Random Nylon-3 Copolymers That Mimic Antibacterial Host-Defense Peptides. J. Am. Chem. Soc. 2009, 131, 9735–9745. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Takahashi, H.; Caputo, G.A.; Vemparala, S.; Kuroda, K. Synthetic Random Copolymers as a Molecular Platform To Mimic Host-Defense Antimicrobial Peptides. Bioconjug. Chem. 2017, 28, 1340–1350. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bam, M.; Luat, E.; Jui, M.S.; Ganewatta, M.S.; Shokfai, T.; Nagarkatti, M.; Decho, A.W.; Tang, C. Macromolecular-clustered facial amphiphilic antimicrobials. Nat. Commun. 2018, 9, 5231. [Google Scholar] [CrossRef]
- Corti, M.B.; Campagno, L.P.; Romero, V.L.; Gutierrez, S.; Alovero, F.L. Cationic polymer contributes to broaden the spectrum of vancomycin activity achieving eradication of Pseudomonas aeruginosa. Arch. Microbiol. 2022, 204, 507. [Google Scholar] [CrossRef]
- Nederberg, F.; Zhang, Y.; Tan, J.P.K.; Xu, K.; Wang, H.; Yang, C.; Gao, S.; Guo, X.D.; Fukushima, K.; Li, L.; et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef] [PubMed]
- Hisey, B.; Ragogna, P.J.; Gillies, E.R. Phosphonium-Functionalized Polymer Micelles with Intrinsic Antibacterial Activity. Biomacromolecules 2017, 18, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yuan, Y.; Zhou, P.; Wang, F.; Hong, Y.; Wang, N.; Xu, S.; Du, J. Highly Effective Antibacterial Vesicles Based on Peptide-Mimetic Alternating Copolymers for Bone Repair. Biomacromolecules 2017, 18, 4154–4162. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, Self-Assembly, and Biomedical Applications of Antimicrobial Peptide-Polymer Conjugates. Biomacromolecules 2018, 19, 1701–1720. [Google Scholar] [CrossRef]
- Chin, W.; Zhong, G.; Pu, Q.; Yang, C.; Lou, W.; De Sessions, P.F.; Periaswamy, B.; Lee, A.; Liang, Z.C.; Ding, X.; et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 2018, 9, 917. [Google Scholar] [CrossRef]
- Yang, C.; Lou, W.; Zhong, G.; Lee, A.; Leong, E.; Chin, W.; Ding, B.; Bao, C.; Tan, J.P.; Pu, Q.; et al. Degradable antimicrobial polycarbonates with unexpected activity and selectivity for treating multidrug-resistant Klebsiella pneumoniae lung infection in mice. Acta Biomater. 2019, 94, 268–280. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Singh, R.; Jha, D.; Dhawan, U.; Gautam, H.K.; Kumar, P. Therapeutic Applications of Self-assembled Indole-3-butanoyl-polyethylenimine Nanostructures. Indian J. Microbiol. 2022, 62, 411–418. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, e1804838. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. State of the Art in the Antibacterial and Antiviral Applications of Carbon-Based Polymeric Nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, T.; Wang, D.; Yi, S.; Li, J.; Li, X. Recent advances in carbon-based nanomaterials for combating bacterial biofilm-associated infections. J. Hazard. Mater. 2022, 431, 128597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zou, F.; Koh, K.; Lee, J. Antibacterial Activity of Graphene-Based Nanomaterials. In Multifaceted Biomedical Applications of Graphene; Han, D.-W., Hong, S.W., Eds.; Springer Singapore: Singapore, 2022; pp. 233–250. [Google Scholar]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Aqueous Solubilization of Fullerenes Using Poly(amidoamine) Dendrimers Bearing Cyclodextrin and Poly(ethylene Glycol). Bioconjugate Chem. 2008, 19, 2280–2284. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Wei, X. Lidocaine-loaded reduced graphene oxide hydrogel for prolongation of effects of local anesthesia: In vitro and in vivo analyses. J. Biomater. Appl. 2021, 35, 1034–1042. [Google Scholar] [CrossRef]
- Luo, S.; Jin, S.; Yang, T.; Wu, B.; Xu, C.; Luo, L.; Chen, Y. Sustained release of tulobuterol from graphene oxide laden hydrogel to manage asthma. J. Biomater. Sci. Polym. Ed. 2021, 32, 524–535. [Google Scholar] [CrossRef]
- Choi, M.; Chung, J.-H.; Cho, Y.; Hong, B.Y.; Hong, J. Nano-film modification of collagen hydrogels for controlled growth factor release. Chem. Eng. Sci. 2015, 137, 626–630. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; Achaby, M.E.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, M.-Y.; Kong, W.H.; Do, I.H.; Hahn, S.K. Nano graphene oxide–hyaluronic acid conjugate for target specific cancer drug delivery. RSC Adv. 2014, 4, 14197–14200. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Wang, J.; Ouyang, Z.; Li, J.; Wei, G.; Su, Z. Interactive Oxidation–Reduction Reaction for the in Situ Synthesis of Graphene–Phenol Formaldehyde Composites with Enhanced Properties. ACS Appl. Mater. Interfaces 2014, 6, 4254–4263. [Google Scholar] [CrossRef]
- Singh, V.; Sagar, P.; Kaul, S.; Sandhir, R.; Singhal, N.K. Liver Phosphoenolpyruvate Carboxykinase-1 Downregulation via siRNA-Functionalized Graphene Oxide Nanosheets Restores Glucose Homeostasis in a Type 2 Diabetes Mellitus In Vivo Model. Bioconjugate Chem. 2021, 32, 259–278. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Shen, Y. Plasmonic-Enhanced Graphene Oxide-Based Aquatic Robot for Target Cargo Delivery. ACS Appl. Mater. Interfaces 2021, 13, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Deng, X.; Liang, H.; Yang, W.; Shao, Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111913. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Saebfar, H.; Gholami, M.H.; Hushmandi, K.; Zabolian, A.; Bikarannejad, P.; Hashemi, M.; Daneshi, S.; Mirzaei, S.; Sharifi, E.; et al. Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: Stimuli-responsive carriers, co-delivery and suppressing resistance. Expert Opin. Drug Deliv. 2022, 19, 355–382. [Google Scholar] [CrossRef]
- Barrera, C.C.; Groot, H.; Vargas, W.L.; Narváez, D.M. Efficacy and Molecular Effects of a Reduced Graphene Oxide/Fe(3)O(4) Nanocomposite in Photothermal Therapy against Cancer. Int. J. Nanomed. 2020, 15, 6421–6432. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Moger, J.; Wu, T.; Lourenco, C.; Chen, B.; Ciric, L.; Parkin, I.P.; et al. Microstructure and antibacterial efficacy of graphene oxide nanocomposite fibres. J. Colloid Interface Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Zhuo, N.; Tian, Z.; Xu, P.; Yang, Z.; Yang, W. Interactions between Antibiotics and Graphene-Based Materials in Water: A Comparative Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2016, 8, 24273–24280. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Jihad, M.; Noori, F.; Jabir, M.; Albukhaty, S.; AlMalki, F.; Alyamani, A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wang, Y.; Ren, X.; Huang, T.-S.; Kim, I.S. Graphene oxide as a polymeric N-halamine carrier and release platform: Highly-efficient, sustained-release antibacterial property and great storage stability. Mater. Sci. Eng. C 2019, 103, 109877. [Google Scholar] [CrossRef]
- Chen, H.; Leng, S. Rapid synthesis of hollow nano-structured hydroxyapatite microspheres via microwave transformation method using hollow CaCO3 precursor microspheres. Ceram. Int. 2015, 41, 2209–2213. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Patan, N.K.; Al-Maadeed, S. Graphene oxide as antimicrobial against two gram-positive and two gram-negative bacteria in addition to one fungus. Online J. Biol. Sci. 2014, 14, 230–239. [Google Scholar] [CrossRef]
- Hu, M.; Cui, Z.; Li, J.; Zhang, L.; Mo, Y.; Dlamini, D.S.; Wang, H.; He, B.; Li, J.; Matsuyama, H. Ultra-low graphene oxide loading for water permeability, antifouling and antibacterial improvement of polyethersulfone/sulfonated polysulfone ultrafiltration membranes. J. Colloid Interface Sci. 2019, 552, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; DI Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef]
- Afifi, M.; Ahmed, M.; Ibrahium, H.A.; Awwad, N.S.; Abdel-Fattah, E.; Alshahrani, M.Y. Improvement of physicochemical properties of ternary nanocomposites based on hydroxyapatite/CuO/graphene oxide for biomedical usages. Ceram. Int. 2022, 48, 3993–4004. [Google Scholar] [CrossRef]
- Khosalim, I.P.; Zhang, Y.Y.; Yiu, C.K.Y.; Wong, H.M. Synthesis of a graphene oxide/agarose/hydroxyapatite biomaterial with the evaluation of antibacterial activity and initial cell attachment. Sci. Rep. 2022, 12, 1971. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by Near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Wu, M.-C.; Deokar, A.R.; Liao, J.-H.; Shih, P.-Y.; Ling, Y.-C. Graphene-Based Photothermal Agent for Rapid and Effective Killing of Bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, P.; Guo, Z.; Li, X.; Pang, Q.; Zheng, K.; He, X.; Ma, Y.; Zhang, Z.; Lynch, I. Elucidating the origin of the surface functionalization—dependent bacterial toxicity of graphene nanomaterials: Oxidative damage, physical disruption, and cell autolysis. Sci. Total Environ. 2020, 747, 141546. [Google Scholar] [CrossRef]
- Wang, Y.; Basdogan, Y.; Zhang, T.; Lankone, R.S.; Wallace, A.N.; Fairbrother, D.H.; Keith, J.A.; Gilbertson, L.M. Unveiling the Synergistic Role of Oxygen Functional Groups in the Graphene-Mediated Oxidation of Glutathione. ACS Appl. Mater. Interfaces 2020, 12, 45753–45762. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, F.; Wang, A.; Chen, X.; Li, H.; Zhang, X.; Zheng, H.; Ji, R.; Li, B.; Yu, X.; et al. Defect-Rich Adhesive Molybdenum Disulfide/rGO Vertical Heterostructures with Enhanced Nanozyme Activity for Smart Bacterial Killing Application. Adv. Mater. 2020, 32, 2005423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Z.; Chen, C.; Lynch, I. Uncertainties in the antibacterial mechanisms of graphene family materials. Nano Today 2022, 43, 101436. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Wu, R.; Zhang, H.; Chai, Z.; Chen, C.; Yin, J.-J. Light-Enhanced Antibacterial Activity of Graphene Oxide, Mainly via Accelerated Electron Transfer. Environ. Sci. Technol. 2017, 51, 10154–10161. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Wassel, M.O.; Khattab, M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef]
- Soleimani, B.; Goli, H.; Naranjian, M.; Mousavi, S.J.; Nahvi, A. Comparison of Antimicrobial Activity of Fluoride Varnishes against Streptococcusmutans and Lactobacillus acidophilus: An In Vitro Study. Iran. J. Pediatr. 2021, in press. [Google Scholar]
- Briseño-Marroquín, B.; Ismael, Y.; Callaway, A.; Tennert, C.; Wolf, T.G. Antibacterial effect of silver diamine fluoride and potassium iodide against E. faecalis, A. naeslundii and P. micra. BMC Oral Health 2021, 21, 175. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Mahanti, M.; Koga, K.; Shitomi, K.; Miyaji, H. Rapid and area-specific coating of fluoride-incorporated apatite layers by a laser-assisted biomimetic process for tooth surface functionalization. Acta Biomater. 2018, 79, 148–157. [Google Scholar] [CrossRef]
- Rao, B.C.N.R.; Govindaraj, A.; Vivekchand, S.R.C. Inorganic nanomaterials: Current status and future prospects. Annu. Rep. Sect. A Inorganic Chem. 2006, 102, 20–45. [Google Scholar] [CrossRef]
- Gasparotto, A.; Barreca, D.; Maccato, C.; Tondello, E. Manufacturing of inorganic nanomaterials: Concepts and perspectives. Nanoscale 2012, 4, 2813–2825. [Google Scholar] [CrossRef]
- Kannan, P.K.; Late, D.J.; Morgan, H.; Rout, C.S. Recent developments in 2D layered inorganic nanomaterials for sensing. Nanoscale 2015, 7, 13293–13312. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti-infective Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Smékalová, M.; Kilianová, M.; Prucek, R.; Bogdanová, K.; Večeřová, R.; Kolář, M.; Havrdová, M.; Płaza, G.A.; Chojniak, J.; et al. Strong and Nonspecific Synergistic Antibacterial Efficiency of Antibiotics Combined with Silver Nanoparticles at Very Low Concentrations Showing No Cytotoxic Effect. Molecules 2015, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Mukha, I.P.; Eremenko, A.M.; Smirnova, N.P.; Mikhienkova, A.I.; Korchak, G.I.; Gorchev, V.F.; Chunikhin, A.Y. Antimicrobial activity of stable silver nanoparticles of a certain size. Appl. Biochem. Microbiol. 2013, 49, 199–206. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size Dependent and Reactive Oxygen Species Related Nanosilver Toxicity to Nitrifying Bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver Nanoparticles (AgNP) in the Environment: A Review of Potential Risks on Human and Environmental Health. Water Air Soil Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Qamar, S.U.R.; Ahmad, J.N. Nanoparticles: Mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J. Mol. Liq. 2021, 334, 116040. [Google Scholar] [CrossRef]
- Ramzan, U.; Majeed, W.; Hussain, A.A.; Qurashi, F.; Qamar, S.U.R.; Naeem, M.; Uddin, J.; Khan, A.; Al-Harrasi, A.; Razak, S.I.A.; et al. New Insights for Exploring the Risks of Bioaccumulation, Molecular Mechanisms, and Cellular Toxicities of AgNPs in Aquatic Ecosystem. Water 2022, 14, 2192. [Google Scholar] [CrossRef]
- Shumbula, N.P.; Nkabinde, S.S.; Ndala, Z.B.; Mpelane, S.; Shumbula, M.P.; Mdluli, P.S.; Njengele-Tetyana, Z.; Tetyana, P.; Hlatshwayo, T.; Mlambo, M.; et al. Evaluating the antimicrobial activity and cytotoxicity of polydopamine capped silver and silver/polydopamine core-shell nanocomposites. Arab. J. Chem. 2022, 15, 103798. [Google Scholar] [CrossRef]
- Peña-Juarez, M.G.; Sanchez-Vargas, L.O.; Flores-Gonzalez, L.A.; Almendarez-Camarillo, A.; Gutierrez-Castañeda, E.J.; Navarrete-Damian, J.; Pérez, E.; Gonzalez-Calderon, J.A. Mechanical, antibacterial, and non-cytotoxic performance of polypropylene nanocomposites reinforced with sTiO2 deposited with AgNPs mediated by quercetin biomolecule. Polym. Bull. 2022, 1–27. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Hubal, E.A.C.; Paunov, V.; Stoyanov, S.; Velev, O. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- El-Azizi, M.M.; El Din, S.N.; El-Tayeb, T.A.; Aisha, K.A. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int. J. Nanomed. 2016, 11, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Lanh, L.T.; Hoa, T.T.; Cuong, N.D.; Khieu, D.Q.; Quang, D.T.; Van Duy, N.; Hoa, N.D.; Van Hieu, N. Shape and size controlled synthesis of Au nanorods: H 2 S gas-sensing characterizations and antibacterial application. J. Alloy. Compd. 2015, 635, 265–271. [Google Scholar] [CrossRef]
- Lee, K.; Nagajyothi, P.; Sreekanth, T.; Park, S. Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J. Ind. Eng. Chem. 2015, 26, 67–72. [Google Scholar] [CrossRef]
- Vanaraj, S.; Jabastin, J.; Sathiskumar, S.; Preethi, K. Production and Characterization of Bio-AuNPs to Induce Synergistic Effect against Multidrug Resistant Bacterial Biofilm. J. Clust. Sci. 2017, 28, 227–244. [Google Scholar] [CrossRef]
- Boomi, P.; Poorani, G.P.; Selvam, S.; Palanisamy, S.; Jegatheeswaran, S.; Anand, K.; Balakumar, C.; Premkumar, K.; Prabu, H.G. Green biosynthesis of gold nanoparticles using Croton sparsiflorus leaves extract and evaluation of UV protection, antibacterial and anticancer applications. Appl. Organomet. Chem. 2020, 34, e5574. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Esparza-González, S.; Sánchez-Valdés, S.; Ramírez-Barrón, S.; Loera-Arias, M.; Bernal, J.; Meléndez-Ortiz, H.I.; Betancourt-Galindo, R. Effects of different surface modifying agents on the cytotoxic and antimicrobial properties of ZnO nanoparticles. Toxicol. Vitr. 2016, 37, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Byeon, J.H.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology 2012, 23, 85103. [Google Scholar] [CrossRef]
- Cabral-Romero, C.; Hernandez-Delgadillo, R.; Velasco-Arias, D.; Diaz, D.; Arevalo-Niño, K.; A De la Garza-Ramos, M. Zerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilm. Int. J. Nanomed. 2012, 7, 2109–2113. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Arellano-Jimenez, M.J.; Lopez-Ribot, J.L. Bismuth nanoparticles obtained by a facile synthesis method exhibit antimicrobial activity against Staphylococcus aureus and Candida albicans. BMC Biomed. Eng. 2020, 2, 11. [Google Scholar] [CrossRef]
- Azad, A.; Rostamifar, S.; Modaresi, F.; Bazrafkan, A.; Rezaie, Z. Assessment of the Antibacterial Effects of Bismuth Nanoparticles against Enterococcus faecalis. BioMed Res. Int. 2020, 2020, 5465439. [Google Scholar] [CrossRef]
- Jawad, K.H.; Marzoog, T.R.; Hasoon, B.A.; Sulaiman, G.M.; Jabir, M.S.; Ahmed, E.M.; Khalil, K.A.A. Antibacterial Activity of Bismuth Oxide Nanoparticles Compared to Amikacin against Acinetobacter baumannii and Staphylococcus aureus. J. Nanomater. 2022, 2022, 8511601. [Google Scholar] [CrossRef]
- Ashfaq, M.; Verma, N.; Khan, S. Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: A novel potential antibiotic material. Mater. Sci. Eng. C 2016, 59, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Cruces, E.; Arancibia-Miranda, N.; Manquián-Cerda, K.; Perreault, F.; Bolan, N.; Azócar, M.I.; Cubillos, V.; Montory, J.; Rubio, M.A.; Sarkar, B. Copper/Silver Bimetallic Nanoparticles Supported on Aluminosilicate Geomaterials as Antibacterial Agents. ACS Appl. Nano Mater. 2022, 5, 1472–1483. [Google Scholar] [CrossRef]
- Merugu, R.; Gothalwal, R.; Deshpande, P.K.; De Mandal, S.; Padala, G.; Chitturi, K.L. Synthesis of Ag/Cu and Cu/Zn bimetallic nanoparticles using toddy palm: Investigations of their antitumor, antioxidant and antibacterial activities. Mater. Today Proc. 2021, 44, 99–105. [Google Scholar] [CrossRef]
- Khatak, S.; Wadhwa, N.; Jain, P. Monometallic Zinc and Bimetallic Cu-Zn Nanoparticles Synthesis Using Stem Extracts of Cissusquadrangularis (Haddjod) and Proneness as Alternative Antimicrobial Agents. Biosci. Biotechnol. Res. Asia 2021, 17, 763–774. [Google Scholar] [CrossRef]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu Monometallic and Ag/Cu Bimetallic Nanoparticle–Graphene Composites with Enhanced Antibacterial Performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite Nanostructures as Novel Strategies for Anti-Infectious Therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Stoeger, T.; van den Brule, S.; Saber, A.T.; Beyerle, A.; Vietti, G.; Mortensen, A.; Szarek, J.; Budtz, H.C.; Kermanizadeh, A.; et al. Acute and subacute pulmonary toxicity and mortality in mice after intratracheal instillation of ZnO nanoparticles in three laboratories. Food Chem. Toxicol. 2015, 85, 84–95. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 35004. [Google Scholar] [CrossRef]
- Asture, A.; Rawat, V.; Srivastava, C.; Vaya, D. Investigation of properties and applications of ZnO polymer nanocomposites. Polym. Bull. 2022, 1–39. [Google Scholar] [CrossRef]
- Dutta, R.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surfaces B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Dadi, R.; Azouani, R.; Traore, M.; Mielcarek, C.; Kanaev, A. Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains. Mater. Sci. Eng. C 2019, 104, 109968. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Tso, C.-P.; Zhung, C.-M.; Shih, Y.-H.; Tseng, Y.-M.; Wu, S.-C.; Doong, R.-A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef]
- Kumar, R.; Reddy, P.; Shankar, K.; Rambabu, D.; Venkateswarulu, M.; Kumbam, L.R.; Sagara, P.; Nakka, N.; Yogesh, M. 9—Surface coating and functionalization of metal and metal oxide nanoparticles for biomedical applications. In Metal Oxides for Biomedical and Biosensor Applications; Mondal, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 205–231. [Google Scholar]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Martino, S.; Torre, L. Nanocomposites Based on Biodegradable Polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.; Starr, F.; Kumar, S.; Cassagnau, P.; Lesser, A.; Sternstein, S.; Buehler, M. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Tran, C.D.; Prosenc, F.; Franko, M.; Benzi, G. One-Pot Synthesis of Biocompatible Silver Nanoparticle Composites from Cellulose and Keratin: Characterization and Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 34791–34801. [Google Scholar] [CrossRef]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Palza, H.; Bejarano, J.; Zapata, P.A. 8—Polymer Composites With Metal Nanoparticles: Synthesis, Properties, and Applications. In Polymer Composites with Functionalized Nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–286. [Google Scholar]
- Kashihara, K.; Uto, Y.; Nakajima, T. Rapid in situ synthesis of polymer-metal nanocomposite films in several seconds using a CO2 laser. Sci. Rep. 2018, 8, 14719. [Google Scholar] [CrossRef] [PubMed]
- Gniadek, M.; Malinowska, S.; Rapecki, T.; Stojek, Z.; Donten, M. Synthesis of polymer–metal nanocomposites at liquid–liquid interface supported by ultrasonic irradiation. Synth. Met. 2014, 187, 193–200. [Google Scholar] [CrossRef]
- Wan, J.; Fan, B.; Thang, S.H. Sonochemical preparation of polymer–metal nanocomposites with catalytic and plasmonic properties. Nanoscale Adv. 2021, 3, 3306–3315. [Google Scholar] [CrossRef]
- Mei, L.; Lu, Z.; Zhang, X.; Li, C.; Jia, Y. Polymer-Ag Nanocomposites with Enhanced Antimicrobial Activity against Bacterial Infection. ACS Appl. Mater. Interfaces 2014, 6, 15813–15821. [Google Scholar] [CrossRef]
- Dai, X.; Chen, X.; Zhao, J.; Zhao, Y.; Guo, Q.; Zhang, T.; Chu, C.; Zhang, X.; Li, C. Structure–Activity Relationship of Membrane-Targeting Cationic Ligands on a Silver Nanoparticle Surface in an Antibiotic-Resistant Antibacterial and Antibiofilm Activity Assay. ACS Appl. Mater. Interfaces 2017, 9, 13837–13848. [Google Scholar] [CrossRef]
- Manoswini, M.; Bhattacharya, D.; Sen, P.; Ganguly, N.; Mohanty, P.S. Antibacterial and cytotoxic activity of polymer-metal hybrid nanoparticle. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 25003. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Lu, B.; Lu, F.; Ran, L.; Yu, K.; Xiao, Y.; Li, Z.; Dai, F.; Wu, D.; Lan, G. Imidazole-molecule-capped chitosan–gold nanocomposites with enhanced antimicrobial activity for treating biofilm-related infections. J. Colloid Interface Sci. 2018, 531, 269–281. [Google Scholar] [CrossRef]
- Pryjmaková, J.; Kaimlová, M.; Vokatá, B.; Hubáček, T.; Slepička, P.; Švorčík, V.; Siegel, J. Bimetallic Nanowires on Laser-Patterned PEN as Promising Biomaterials. Nanomaterials 2021, 11, 2285. [Google Scholar] [CrossRef]
- Prasanna, S.R.V.S.; Balaji, K.; Pandey, S.; Rana, S. Chapter 4—Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. [Google Scholar]
- Soytaş, S.H.; Oğuz, O.; Menceloğlu, Y.Z. 9—Polymer Nanocomposites With Decorated Metal Oxides. In Polymer Composites with Functionalized Nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–323. [Google Scholar]
- Shameem, M.M.; Sasikanth, S.; Annamalai, R.; Raman, R.G. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- Aktitiz, I.; Aydın, K.; Darıcık, F.; Topcu, A. Production of different metal oxide nanoparticle embedded polymer matrix composite structures by the additive manufacturing technology and investigation of their properties. Polym. Compos. 2022. [Google Scholar] [CrossRef]
- Li, L.; Yu, T. Curing comparison and performance investigation of polyurethane concrete with retarders. Constr. Build. Mater. 2022, 326, 126883. [Google Scholar] [CrossRef]
- Pholnak, C.; Sirisathitkul, C.; Soontaranon, S.; Rugmai, S. UV–Vis Absorption and Small Angle X-ray Scattering Spectra of Commercial Polyurethane Coating Filled with Zinc Oxide. Natl. Acad. Sci. Lett. 2016, 39, 125–128. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Bahadur, A.; Iqbal, S.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A. Thermal degradation study of polymethylmethacrylate with AlI 3 nanoadditive. Microsc. Res. Tech. 2022, 85, 1494–1501. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.S.; Wang, Z.L. Dissolving Behavior and Stability of ZnO Wires in Biofluids: A Study on Biodegradability and Biocompatibility of ZnO Nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Rai, P.; Kwak, W.-K.; Yu, Y.-T. Solvothermal Synthesis of ZnO Nanostructures and Their Morphology-Dependent Gas-Sensing Properties. ACS Appl. Mater. Interfaces 2013, 5, 3026–3032. [Google Scholar] [CrossRef]
- Poyraz, S.; Cerkez, I.; Huang, T.S.; Liu, Z.; Kang, L.; Luo, J.; Zhang, X. One-Step Synthesis and Characterization of Polyaniline Nanofiber/Silver Nanoparticle Composite Networks as Antibacterial Agents. ACS Appl. Mater. Interfaces 2014, 6, 20025–20034. [Google Scholar] [CrossRef]
- Hashemi, A.; Jouault, N.; Williams, G.A.; Zhao, D.; Cheng, K.J.; Kysar, J.W.; Guan, Z.; Kumar, S.K. Enhanced Glassy State Mechanical Properties of Polymer Nanocomposites via Supramolecular Interactions. Nano Lett. 2015, 15, 5465–5471. [Google Scholar] [CrossRef]
- Nikitin, D.; Madkour, S.; Pleskunov, P.; Tafiichuk, R.; Shelemin, A.; Hanuš, J.; Gordeev, I.; Sysolyatina, E.; Lavrikova, A.; Ermolaeva, S.; et al. Cu nanoparticles constrain segmental dynamics of cross-linked polyethers: A trade-off between non-fouling and antibacterial properties. Soft Matter 2019, 15, 2884–2896. [Google Scholar] [CrossRef]
- Kostic, D.; Sekulic, M.V.; Armentano, I.; Torre, L.; Obradovic, B. Multifunctional ternary composite films based on PLA and Ag/alginate microbeads: Physical characterization and silver release kinetics. Mater. Sci. Eng. C 2019, 98, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rangel, A.; Silva-Bermudez, P.; España-Sánchez, B.; Luna-Hernández, E.; Almaguer-Flores, A.; Ibarra, C.; Garcia-Perez, V.; Velasquillo, C.; Luna-Barcenas, G. Fabrication and in vitro behavior of dual-function chitosan/silver nanocomposites for potential wound dressing applications. Mater. Sci. Eng. C 2019, 94, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Mehramouz, B.; Rahimi, M.; Kafil, H.S. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int. J. Biol. Macromol. 2018, 114, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Díez, B.; Santiago-Morales, J.; Martínez-Bueno, M.J.; Fernández-Alba, A.R.; Rosal, R. Antimicrobial organic–inorganic composite membranes including sepiolite-supported nanometals. RSC Adv. 2017, 7, 2323–2332. [Google Scholar] [CrossRef]
- Huang, X.; Bao, X.; Wang, Z.; Hu, Q. A novel silver-loaded chitosan composite sponge with sustained silver release as a long-lasting antimicrobial dressing. RSC Adv. 2017, 7, 34655–34663. [Google Scholar] [CrossRef]
- Baek, K.; Liang, J.; Lim, W.T.; Zhao, H.; Kim, D.H.; Kong, H. In Situ Assembly of Antifouling/Bacterial Silver Nanoparticle-Hydrogel Composites with Controlled Particle Release and Matrix Softening. ACS Appl. Mater. Interfaces 2015, 7, 15359–15367. [Google Scholar] [CrossRef]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible Hydrogel Nanocomposite with Covalently Embedded Silver Nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Cao, D.; Zhang, L.; Guo, Z. Nanoparticle Dispersion and Aggregation in Polymer Nanocomposites: Insights from Molecular Dynamics Simulation. Langmuir 2011, 27, 7926–7933. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Pourdeyhimi, B.; Loboa, E.G. High-Throughput Fabrication Method for Producing a Silver-Nanoparticles-Doped Nanoclay Polymer Composite with Novel Synergistic Antibacterial Effects at the Material Interface. ACS Appl. Mater. Interfaces 2017, 9, 21105–21115. [Google Scholar] [CrossRef] [PubMed]
- Mohiti-Asli, M.; Pourdeyhimi, B.; Loboa, E.G. Skin Tissue Engineering for the Infected Wound Site: Biodegradable PLA Nanofibers and a Novel Approach for Silver Ion Release Evaluated in a 3D Coculture System of Keratinocytes and Staphylococcus aureus. Tissue Eng. Part C Methods 2014, 20, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Butola, B.S.; Thakur, S. Development and performance optimization of knitted antibacterial materials using polyester–silver nanocomposite fibres. Mater. Sci. Eng. C 2015, 54, 26–31. [Google Scholar] [CrossRef]
- Abudula, T.; Qurban, R.O.; Bolarinwa, S.O.; Mirza, A.A.; Pasovic, M.; Memic, A. 3D Printing of Metal/Metal Oxide Incorporated Thermoplastic Nanocomposites With Antimicrobial Properties. Front. Bioeng. Biotechnol. 2020, 8, 568186. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bauman, L.; Nogueira, C.L.; Aucoin, M.G.; Anderson, W.A.; Zhao, B. Antimicrobial polymeric composites for high-touch surfaces in healthcare applications. Curr. Opin. Biomed. Eng. 2022, 22, 100395. [Google Scholar] [CrossRef]
- Murthy, P.S.; Pandiyan, V.; Das, A. Potential of Metal Oxide Nanoparticles and Nanocomposites as Antibiofilm Agents: Leverages and Limitations. In Emerging Nanomaterials for Advanced Technologies; Krishnan, A., Ravindran, B., Balasubramanian, B., Swart, H.C., Panchu, S.J., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 163–209. [Google Scholar]
- Choudhury, M.; Bindra, H.S.; Singh, K.; Singh, A.K.; Nayak, R. Antimicrobial polymeric composites in consumer goods and healthcare sector: A healthier way to prevent infection. Polym. Adv. Technol. 2022, 33, 1997–2024. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Ozkan, E.; Allan, E.; Parkin, I.P. White-Light-Activated Antibacterial Surfaces Generated by Synergy between Zinc Oxide Nanoparticles and Crystal Violet. ACS Omega 2018, 3, 3190–3199. [Google Scholar] [CrossRef]
- Sehmi, S.K.; Noimark, S.D.; Pike, S.; Bear, J.C.; Peveler, W.J.; Williams, C.K.; Shaffer, M.; Allan, E.; Parkin, I.P.; MacRobert, A.J. Enhancing the Antibacterial Activity of Light-Activated Surfaces Containing Crystal Violet and ZnO Nanoparticles: Investigation of Nanoparticle Size, Capping Ligand, and Dopants. ACS Omega 2016, 1, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Gobi, R.; Ravichandiran, P.; Babu, R.; Yoo, D. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Wound Healing Bionanocomposites Based on Castor Oil Polymeric Films Reinforced with Chitosan-Modified ZnO Nanoparticles. Biomacromolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef]
- Mohandas, A.; Kumar P T, S.; Raja, B.; Lakshmanan, V.K.; Jayakumar, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10 (Suppl. S1), 53–66. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Preparation and In Vitro Biological Evaluation of Lawsone Loaded O-Carboxymethyl Chitosan/Zinc Oxide Nanocomposite for Wound-Healing Application. ChemistrySelect 2020, 5, 2710–2718. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Lakshmanan, V.-K.; Anilkumar, T.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.; Nair, S.V.; Jayakumar, R. Flexible and Microporous Chitosan Hydrogel/Nano ZnO Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Raafat, A.I.; El-Sawy, N.M.; Badawy, N.A.; Mousa, E.A.; Mohamed, A.M. Radiation fabrication of Xanthan-based wound dressing hydrogels embedded ZnO nanoparticles: In vitro evaluation. Int. J. Biol. Macromol. 2018, 118, 1892–1902. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W. Chapter 3—Applications of nanotechnology in food microbiology. In Methods in Microbiology; Gurtler, V., Ball, A.S., Soni, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 43–60. [Google Scholar]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Al Hazza, A.; Jacob, H.; Joseph, A. Comparative effects of untreated and 3-methacryloxypropyltrimethoxysilane treated ZnO nanoparticle reinforcement on properties of polylactide-based nanocomposite films. Int. J. Biol. Macromol. 2017, 101, 1041–1050. [Google Scholar] [CrossRef]
- Pantani, R.; Giuliana, G.; Vigliottab, G.; Murariuc, M.; Duboisc, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Zahedi, Y.; Fathi-Achachlouei, B.; Yousefi, A.R. Physical and mechanical properties of hybrid montmorillonite/zinc oxide reinforced carboxymethyl cellulose nanocomposites. Int. J. Biol. Macromol. 2018, 108, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Saikia, R.; Kale, V.S.; Shelke, M.V.; Sengupta, P. Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surfaces B Biointerfaces 2011, 83, 16–22. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef]
- Salunkhe, A.; Tandon, S.; Dudhwadkar, S. Surface Functionalization of Graphene Oxide with Silver Nanoparticles Using Phyto Extract and its Antimicrobial Properties against Biological Contaminants. Arab. J. Sci. Eng. 2022, 1–15. [Google Scholar] [CrossRef]
- Malik, S.B.; Saggu, J.I.; Gul, A.; Abbasi, B.A.; Iqbal, J.; Waris, S.; Bin Jardan, Y.A.; Chalgham, W. Synthesis and Characterization of Silver and Graphene Nanocomposites and Their Antimicrobial and Photocatalytic Potentials. Molecules 2022, 27, 5184. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Tian, X. Silver-modified graphene oxide nanosheets for antibacterial performance of bone scaffold. AIP Adv. 2022, 12, 15024. [Google Scholar] [CrossRef]
- Ranjan, R.; Bajpai, V. Graphene-based metal matrix nanocomposites: Recent development and challenges. J. Compos. Mater. 2021, 55, 2369–2413. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, H.; Yang, G.; Zhu, D.; Luan, X.; He, P.; Wei, G. Graphene-Based Functional Hybrid Membranes for Antimicrobial Applications: A Review. Appl. Sci. 2022, 12, 4834. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int J Nanomedicine 2014, 9, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Tomioka, T.; Tomita, K.; Nishino, A.; Ueda, S. Inactivation of Enveloped Viruses by a Silver-Thiosulfate Complex. Met. Drugs 1994, 1, 511. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Hui, K.; Dinh, D.; Tsang, C.; Cho, Y.; Zhou, W.; Hong, X.; Chun, H.-H. Green synthesis of dimension-controlled silver nanoparticle–graphene oxide with in situ ultrasonication. Acta Mater. 2014, 64, 326–332. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Wang, Y.; Yan, X.; Sun, D.D. Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity. New J. Chem. 2011, 35, 1418–1423. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Borah, S.C.; Kumari, R.; Saikia, R.; Deshmukh, A.B.; Shelke, M.V.; Sengupta, P.; Szunerits, S.; Boukherroub, R. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf. B Biointerfaces 2013, 105, 128–136. [Google Scholar] [CrossRef]
- Ganjouzadeh, F.; Khorrami, S.; Gharbi, S. Controlled cytotoxicity of Ag-GO nanocomposite biosynthesized using black peel pomegranate extract against MCF-7 cell line. J. Drug Deliv. Sci. Technol. 2022, 71, 103340. [Google Scholar] [CrossRef]
- Dat, N.M.; Quan, T.H.; Nguyet, D.M.; Anh, T.N.M.; Thinh, D.B.; Diep, T.C.; Huy, L.A.; Tai, L.T.; Hai, N.D.; Khang, P.T.; et al. Hybrid graphene oxide-immobilized silver nanocomposite with optimal fabrication route and multifunctional application. Appl. Surf. Sci. 2021, 551, 149434. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, S.; Sharma, B.; Jain, P. Antibacterial efficacy of poly (vinyl alcohol) nanocomposites reinforced with graphene oxide and silver nanoparticles for packaging applications. Polym. Compos. 2021, 42, 2829–2837. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Ku, H.-C.; Tien, D.-C.; Stobinski, L. Novel Preparation of Reduced Graphene Oxide–Silver Complex using an Electrical Spark Discharge Method. Nanomaterials 2019, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Li, Y.; Han, F.; Su, T.; Li, J.; Zhang, R. Synthesis of Ag/rGO composite materials with antibacterial activities using facile and rapid microwave-assisted green route. J. Mater. Sci. Mater. Med. 2018, 29, 69. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mao, X.; Wang, Y.; Li, D.; Du, Z.; Wu, W.; Jiang, L.; Yang, J.; Li, J. Study on the interaction of graphene oxide-silver nanocomposites with bovine serum albumin and the formation of nanoparticle-protein corona. Int. J. Biol. Macromol. 2018, 116, 492–501. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable Nanocomposite Based on PEGylated and Silver Nanoparticles Loaded Graphene Oxide for Long-Term Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef]
- Han, F.; Lv, S.; Li, Z.; Jin, L.; Fan, B.; Zhang, J.; Zhang, R.; Zhang, X.; Han, L.; Li, J. Triple-synergistic 2D material-based dual-delivery antibiotic platform. NPG Asia Mater. 2020, 12, 15. [Google Scholar] [CrossRef]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, M.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly Stable Graphene-Based Nanocomposite (GO-PEI-Ag) with Broad-Spectrum, Long-Term Antimicrobial Activity and Antibiofilm Effects. ACS Appl Mater Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Parandhaman, T.; Choudhary, P.; Ramalingam, B.; Schmidt, M.; Janardhanam, S.; Das, S.K. Antibacterial and Antibiofouling Activities of Antimicrobial Peptide-Functionalized Graphene–Silver Nanocomposites for the Inhibition and Disruption of Staphylococcus aureus Biofilms. ACS Biomater. Sci. Eng. 2021, 7, 5899–5917. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; An, T.N.M.; Pham, S.Q.T.; Cao, X.T. Synthesis of silver nanoparticles stabilized polymer/graphene oxide for catalytic and antibacterial application. Mol. Cryst. Liq. Cryst. 2022, 742, 56–63. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Qayyum, S.; Khan, A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017, 103, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, Q.; Yu, N.; Wang, T.; Wang, C.; Yang, R. GO-AgCl/Ag nanocomposites with enhanced visible light-driven catalytic properties for antibacterial and biofilm-disrupting applications. Colloids Surf B Biointerfaces 2018, 162, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, H.; Xiao, N. Advancement of Near-infrared (NIR) laser interceded surface enactment of proline functionalized graphene oxide with silver nanoparticles for proficient antibacterial, antifungal and wound recuperating therapy in nursing care in hospitals. J. Photochem. Photobiol. B Biol. 2018, 187, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, S.; Guo, J.; Luo, L.; Zhou, Y.; Lin, C.; Shi, J.; Fan, C.; Lv, M.; Wang, L. Graphene oxide–silver nanocomposites modulate biofilm formation and extracellular polymeric substance (EPS) production. Nanoscale 2018, 10, 19603–19611. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene Oxide–Silver Nanocomposite As a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef]
- Ran, X.; Du, Y.; Wang, Z.; Wang, H.; Pu, F.; Ren, J.; Qu, X. Hyaluronic Acid-Templated Ag Nanoparticles/Graphene Oxide Composites for Synergistic Therapy of Bacteria Infection. ACS Appl. Mater. Interfaces 2017, 9, 19717–19724. [Google Scholar] [CrossRef]
- Chae, H.-R.; Lee, J.; Lee, C.-H.; Kim, I.-C.; Park, P.-K. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Singh, P.; Shandilya, P.; Raizada, P.; Sudhaik, A.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 2020, 13, 3498–3520. [Google Scholar] [CrossRef]
- Chen, G.-E.; Wu, Q.; Sun, W.-G.; Xu, Z.-L.; Xu, S.-J.; Zhu, W.-W.; Zheng, X.-P. Synergy of graphene oxide–silver nanocomposite and amphiphilic co-polymer F127 on antibacterial properties and permeability of PVDF membrane. RSC Adv. 2016, 6, 100334–100343. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Qureshi, R.B.; Muntha, S.T.; Farooq, M.; Siddiq, M. Facile synthesis of graphene oxide–silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J. Environ. Manag. 2019, 230, 199–211. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Li, Y.; Zhou, Q.; Xu, H.; Gao, B.; Wang, Z. Antibacterial Thin-Film Nanocomposite Membranes Incorporated with Graphene Oxide Quantum Dot-Mediated Silver Nanoparticles for Reverse Osmosis Application. ACS Sustain. Chem. Eng. 2019, 7, 8724–8734. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Luceño-Sánchez, J.A. Antibacterial Activity of Polymer Nanocomposites Incorporating Graphene and Its Derivatives: A State of Art. Polymers 2021, 13, 2105. [Google Scholar] [CrossRef] [PubMed]
- Soroush, A.; Ma, W.; Silvino, Y.; Rahaman, S. Surface modification of thin film composite forward osmosis membrane by silver-decorated graphene-oxide nanosheets. Environ. Sci. Nano 2015, 2, 395–405. [Google Scholar] [CrossRef]
- Soroush, A.; Ma, W.; Cyr, M.; Rahaman, S.; Asadishad, B.; Tufenkji, N. In Situ Silver Decoration on Graphene Oxide-Treated Thin Film Composite Forward Osmosis Membranes: Biocidal Properties and Regeneration Potential. Environ. Sci. Technol. Lett. 2016, 3, 13–18. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedpour, S.F.; Sharifian Gh., M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting Synergetic Effects of Graphene Oxide and a Silver-Based Metal–Organic Framework To Enhance Antifouling and Anti-Biofouling Properties of Thin-Film Nanocomposite Membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef]
- He, C.; Shi, Z.-Q.; Cheng, C.; Lu, H.-Q.; Zhou, M.; Sun, S.-D.; Zhao, C.-S. Graphene oxide and sulfonated polyanion co-doped hydrogel films for dual-layered membranes with superior hemocompatibility and antibacterial activity. Biomater. Sci. 2016, 4, 1431–1440. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef]
- Shams, E.; Yeganeh, H.; Naderi-Manesh, H.; Gharibi, R.; Hassan, Z.M. Polyurethane/siloxane membranes containing graphene oxide nanoplatelets as antimicrobial wound dressings: In vitro and in vivo evaluations. J. Mater. Sci. Mater. Med. 2017, 28, 75. [Google Scholar] [CrossRef]
- Yadav, S.K.; Jung, Y.C.; Kim, J.H.; Ko, Y.-I.; Ryu, H.J.; Yadav, M.K.; Kim, Y.A.; Cho, J.W. Mechanically Robust, Electrically Conductive Biocomposite Films Using Antimicrobial Chitosan-Functionalized Graphenes. Part. Part. Syst. Charact. 2013, 30, 721–727. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(propylene fumarate)/Polyethylene Glycol-Modified Graphene Oxide Nanocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef]
- Khan, Y.H.; Islam, A.; Sarwar, A.; Gull, N.; Khan, S.M.; Munawar, M.A.; Zia, S.; Sabir, A.; Shafiq, M.; Jamil, T. Novel green nano composites films fabricated by indigenously synthesized graphene oxide and chitosan. Carbohydr. Polym. 2016, 146, 131–138. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.-H.; Osuji, C.O.; Elimelech, M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. USA 2017, 114, E9793–E9801. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lu, X.; Han, L.; Xu, J.; Wang, Z.; Jiang, L.; Wang, K.; Zhang, H.; Ren, F.; Tang, Y. Biomimetic Mineralized Hierarchical Graphene Oxide/Chitosan Scaffolds with Adsorbability for Immobilization of Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Tegou, E.; Magana, M.; Katsogridaki, A.E.; Ioannidis, A.; Raptis, V.; Jordan, S.; Chatzipanagiotou, S.; Chatzandroulis, S.; Ornelas, C.; Tegos, G.P. Terms of endearment: Bacteria meet graphene nanosurfaces. Biomaterials 2016, 89, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Shahryari, Z.; Yeganeh, M.; Gheisari, K.; Ramezanzadeh, B. A brief review of the graphene oxide-based polymer nanocomposite coatings: Preparation, characterization, and properties. J. Coatings Technol. Res. 2021, 18, 945–969. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Rao, G.S.S.; Mathur, A.B.; Jasra, R. Polyolefin/graphene nanocomposites: A review. RSC Adv. 2017, 7, 23615–23632. [Google Scholar] [CrossRef]
- Mejias Carpio, I.E.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer-graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef]
- Li, P.; Gao, Y.; Sun, Z.; Chang, D.; Gao, G.; Dong, A. Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites. Molecules 2016, 22, 12. [Google Scholar] [CrossRef]
- Rusakova, H.V.; Fomenko, L.S.; Lubenets, S.V.; Dolbin, A.V.; Vinnikov, N.A.; Basnukaeva, R.M.; Khlistyuck, M.V.; Blyznyuk, A.V. Synthesis and micromechanical properties of graphene oxide-based polymer nanocomposites. Low Temp. Phys. 2020, 46, 276–284. [Google Scholar] [CrossRef]
- Maio, A.; Fucarino, R.; Khatibi, R.; Rosselli, S.; Bruno, M.; Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Compos. Sci. Technol. 2015, 119, 131–137. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Othman, A.A.; Alsharaeh, E.H. Synthesis and Characterization of the in Situ Bulk Polymerization of PMMA Containing Graphene Sheets Using Microwave Irradiation. Molecules 2013, 18, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Alsharaeh, E.H.; Othman, A.A.; Aldosari, M.A. Microwave Irradiation Effect on the Dispersion and Thermal Stability of RGO Nanosheets within a Polystyrene Matrix. Materials 2014, 7, 5212–5224. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Bostwick, J.E.; Shallenberger, J.R.; Zofchak, E.S.; Colby, R.H.; Liu, Q.; Hickey, R.J. Simultaneous Reduction and Polymerization of Graphene Oxide/Styrene Mixtures To Create Polymer Nanocomposites with Tunable Dielectric Constants. ACS Appl. Nano Mater. 2020, 3, 962–968. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, J.-K.; Kim, D.-A.; Patel, K.D.; Kim, H.-W.; Lee, H.-H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Konwar, A.; Kalita, S.; Kotoky, J.; Chowdhury, D. Chitosan–Iron Oxide Coated Graphene Oxide Nanocomposite Hydrogel: A Robust and Soft Antimicrobial Biofilm. ACS Appl. Mater. Interfaces 2016, 8, 20625–20634. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction. Angew. Chem. Int. Ed. 2016, 55, 9984–9987. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Ng, L.Y.; Chua, H.S.; Ng, C.Y. Incorporation of graphene oxide-based nanocomposite in the polymeric membrane for water and wastewater treatment: A review on recent development. J. Environ. Chem. Eng. 2021, 9, 105994. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.-X.; Zhang, Y.; Wang, R.; Bhattacharyya, D.; Tan, T. Graphene Oxide Quantum Dots Covalently Functionalized PVDF Membrane with Significantly-Enhanced Bactericidal and Antibiofouling Performances. Sci. Rep. 2016, 6, 20142. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, X.; Kaneda, M.; Zhang, W.; Bernstein, R.; Ma, J.; Elimelech, M. Graphene Oxide-Functionalized Membranes: The Importance of Nanosheet Surface Exposure for Biofouling Resistance. Environ. Sci. Technol. 2020, 54, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Ng, L.Y.; Ba-Abbad, M.M.; Mohammad, A. Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chem. Eng. J. 2015, 277, 1–10. [Google Scholar] [CrossRef]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Macromol. Symp. 2017, 374, 1600114. [Google Scholar] [CrossRef]
- Rossa, V.; Ferreira, L.E.M.; Vasconcelos, S.d.C.; Shimabukuro, E.T.T.; Madriaga, V.G.d.C.; Carvalho, A.P.; Pergher, S.B.C.; Silva, F.D.C.D.; Ferreira, V.F.; Junior, C.A.C.; et al. Nanocomposites based on the graphene family for food packaging: Historical perspective, preparation methods, and properties. RSC Adv. 2022, 12, 14084–14111. [Google Scholar] [CrossRef]
- Ahmed, J. Use of Graphene/Graphene Oxide in Food Packaging Materials: Thermomechanical, Structural and Barrier Properties. Reference Module in Food Science. 2019, 452–473. [Google Scholar] [CrossRef]
- Zeng, L.; Zhu, Z.; Sun, D.-W. Novel graphene oxide/polymer composite membranes for the food industry: Structures, mechanisms and recent applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 3705–3722. [Google Scholar] [CrossRef]
- Lyn, F.H.; Hanani, Z.A.N. Graphene-based polymer nanocomposites in food packaging and factors affecting the behaviour of graphene-based materials: A review. J. Nanoparticle Res. 2022, 24, 179. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Jaglal, Y.; Osman, N.; Omolo, C.A.; Mocktar, C.; Devnarain, N.; Govender, T. Formulation of pH-responsive lipid-polymer hybrid nanoparticles for co-delivery and enhancement of the antibacterial activity of vancomycin and 18β-glycyrrhetinic acid. J. Drug Deliv. Sci. Technol. 2021, 64, 102607. [Google Scholar] [CrossRef]
- Lee, H.W.; Kharel, S.; Loo, S.C.J. Lipid-Coated Hybrid Nanoparticles for Enhanced Bacterial Biofilm Penetration and Antibiofilm Efficacy. ACS Omega 2022. Research Square. [Google Scholar] [CrossRef]
- Mohanty, A.; Uthaman, S.; Park, I.-K. Chapter 12—Lipid–polymer hybrid nanoparticles as a smart drug delivery platform. In Stimuli-Responsive Nanocarriers; Gajbhiye, V., Gajbhiye, K.R., Hong, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 319–349. [Google Scholar]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Grabnar, P.A.; Kristl, J. The manufacturing techniques of drug-loaded polymeric nanoparticles from preformed polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Ravikumar, R.; Karuppagounder, V.; Bennet, D.; Rangasamy, S.; Thandavarayan, R.A. Lipid–polymer hybrid nanoparticle-mediated therapeutics delivery: Advances and challenges. Drug Discov. Today 2017, 22, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.I.; Zhang, L. Lipid–polymer hybrid nanoparticles: Synthesis, characterization and applications. Nano Life 2010, 1, 163–173. [Google Scholar] [CrossRef]
- Garg, N.K.; Tandel, N.; Jadon, R.S.; Tyagi, R.K.; Katare, O.P. Lipid–polymer hybrid nanocarrier-mediated cancer therapeutics: Current status and future directions. Drug Discov. Today 2018, 23, 1610–1621. [Google Scholar] [CrossRef]
- Bachhav, S.S.; Dighe, V.D.; Kotak, D.; Devarajan, P.V. Rifampicin Lipid-Polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int. J. Pharm. 2017, 532, 612–622. [Google Scholar] [CrossRef]
- Dave, V.; Yadav, R.B.; Kushwaha, K.; Yadav, S.; Sharma, S.; Agrawal, U. Lipid-polymer hybrid nanoparticles: Development & statistical optimization of norfloxacin for topical drug delivery system. Bioact. Mater. 2017, 2, 269–280. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Patil, A.; Katare, O.P. Chitosan-tailored lipidic nanoconstructs of Fusidic acid as promising vehicle for wound infections: An explorative study. Int. J. Biol. Macromol. 2018, 115, 1012–1025. [Google Scholar] [CrossRef]
- Thanki, K.; Papai, S.; Lokras, A.; Rose, F.; Falkenberg, E.; Franzyk, H.; Foged, C. Application of a Quality-By-Design Approach to Optimise Lipid-Polymer Hybrid Nanoparticles Loaded with a Splice-Correction Antisense Oligonucleotide: Maximising Loading and Intracellular Delivery. Pharm. Res. 2019, 36, 37. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, X.; Xu, Y.; Liu, D.; He, H.; Wang, Y.; Tang, X. Hydroxycamptothecin (HCPT)-loaded PEGlated lipid–polymer hybrid nanoparticles for effective delivery of HCPT: QbD-based development and evaluation. Drug Deliv. Transl. Res. 2022, 12, 306–324. [Google Scholar] [CrossRef]
- Cunha, S.; Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102206. [Google Scholar] [CrossRef] [PubMed]
- Soni, G.; Kale, K.; Shetty, S.; Gupta, M.; Yadav, K.S. Quality by design (QbD) approach in processing polymeric nanoparticles loading anticancer drugs by high pressure homogenizer. Heliyon 2020, 6, e03846. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Kohli, K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov. Today 2019, 24, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, H.; Song, W.; Hu, H.; Chen, J.; Zhang, L.; Li, P.; Wu, R.; Wu, C. Preparation and evaluation of lipid polymer nanoparticles for eradicating H. pylori biofilm and impairing antibacterial resistance in vitro. Int. J. Pharm. 2015, 495, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Lombardo, G.; Marinucci, L.; Kenny, J.M.; Torre, L.; Cianetti, S. Effect of nanohydroxyapatite, antibiotic, and mucosal defensive agent on the mechanical and thermal properties of glass ionomer cements for special needs patients. J. Mater. Res. 2018, 33, 638–649. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).