Lithographic SERS Aptasensor for Ultrasensitive Detection of SARS-CoV-2 in Biological Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Lithographic SERS-Substrate
- Si/SiO2 substrates were cleaned with acetone and isopropyl alcohol in an ultrasonic bath FB15047 (Fisher Scientific, Loughborough, UK).
- A single layer of AZ5214E photoresist was applied using a spin coater Delta 6RC (SUSS MicroTec SE, Garching, Germany) with a spin rate of 6000 rpm. After a 4 min prebaking on a hotplate (Wenesco, Addison, IL, USA) at 90 °C, 1.13 µm thickness of the resist film was achieved. Positive photolithography is accomplished by using optical focusing throughout the process. The development process was carried out in AZ 726 MIF Developer, with deionized water as a stop solution.
- Inside the chamber, aluminum was thermally evaporated onto the samples at the pressure of more than 8 × 10−6 Torr at the deposition rate of 1 A/s to obtain a 50 nm layer.
- The substrates with the metal on the top surface were immersed in acetone for the lift-off. As a result, a metal mask in the form of future pillars was created on the samples for the subsequent reactive-ion etching. After the etching process at the pressure of more than 2 × 10−5 Torr, the aluminum was rinsed off in AZ 726 MIF Developer.
- The substrate was washed with acetone and isopropyl alcohol in an ultrasonic bath to remove the residues of the developer.
- The layers of Cr, Ag, and Au, with respective thicknesses of 7, 40, and 15 nm, were successively evaporated onto the samples at the pressure of more than 10−7 Torr at the rate of 1 A/s. This final step was performed just before taking the measurements.
2.3. Viruses
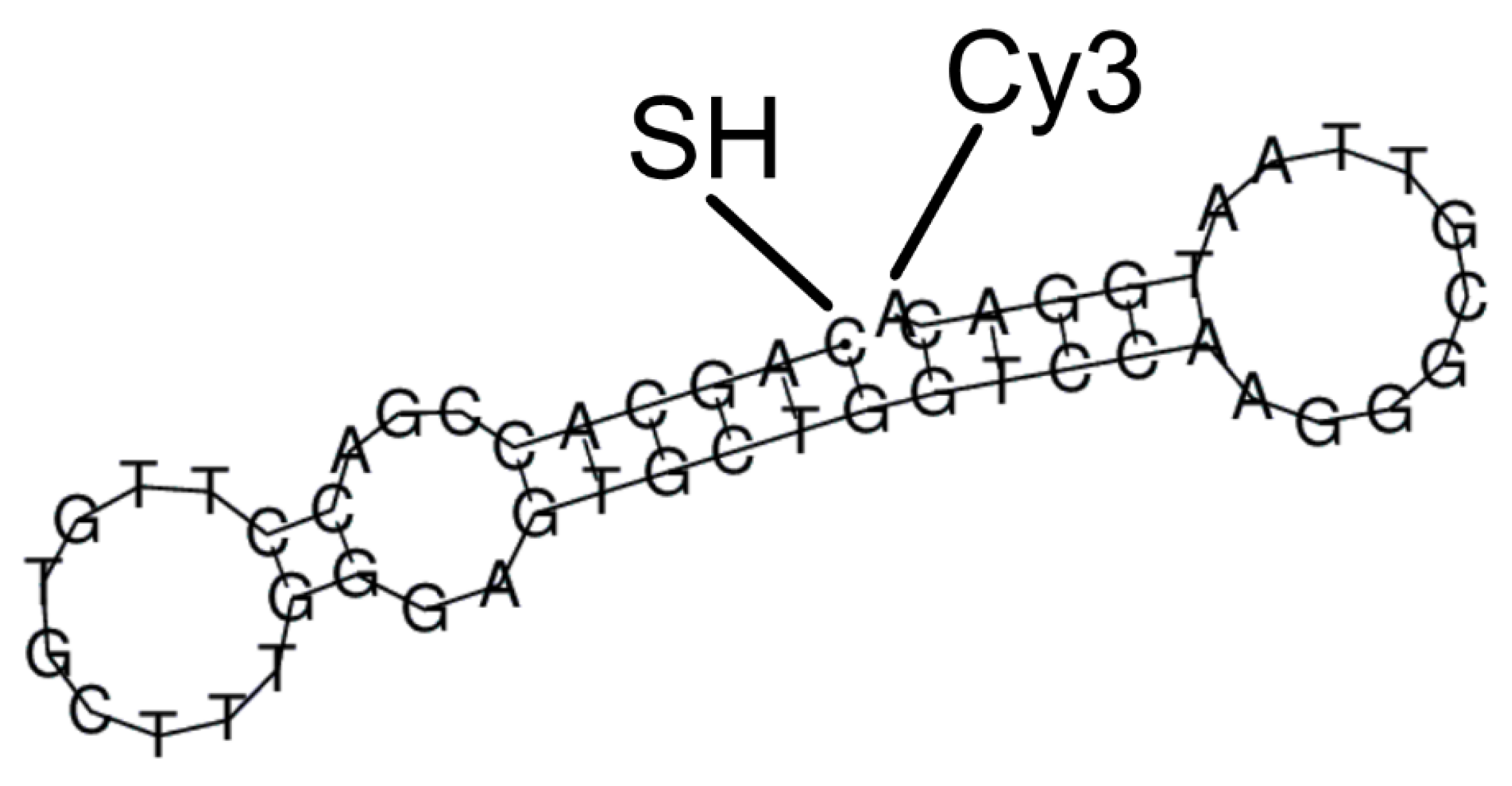
2.4. Aptamers and Their Assembly
2.5. SERS Measurements
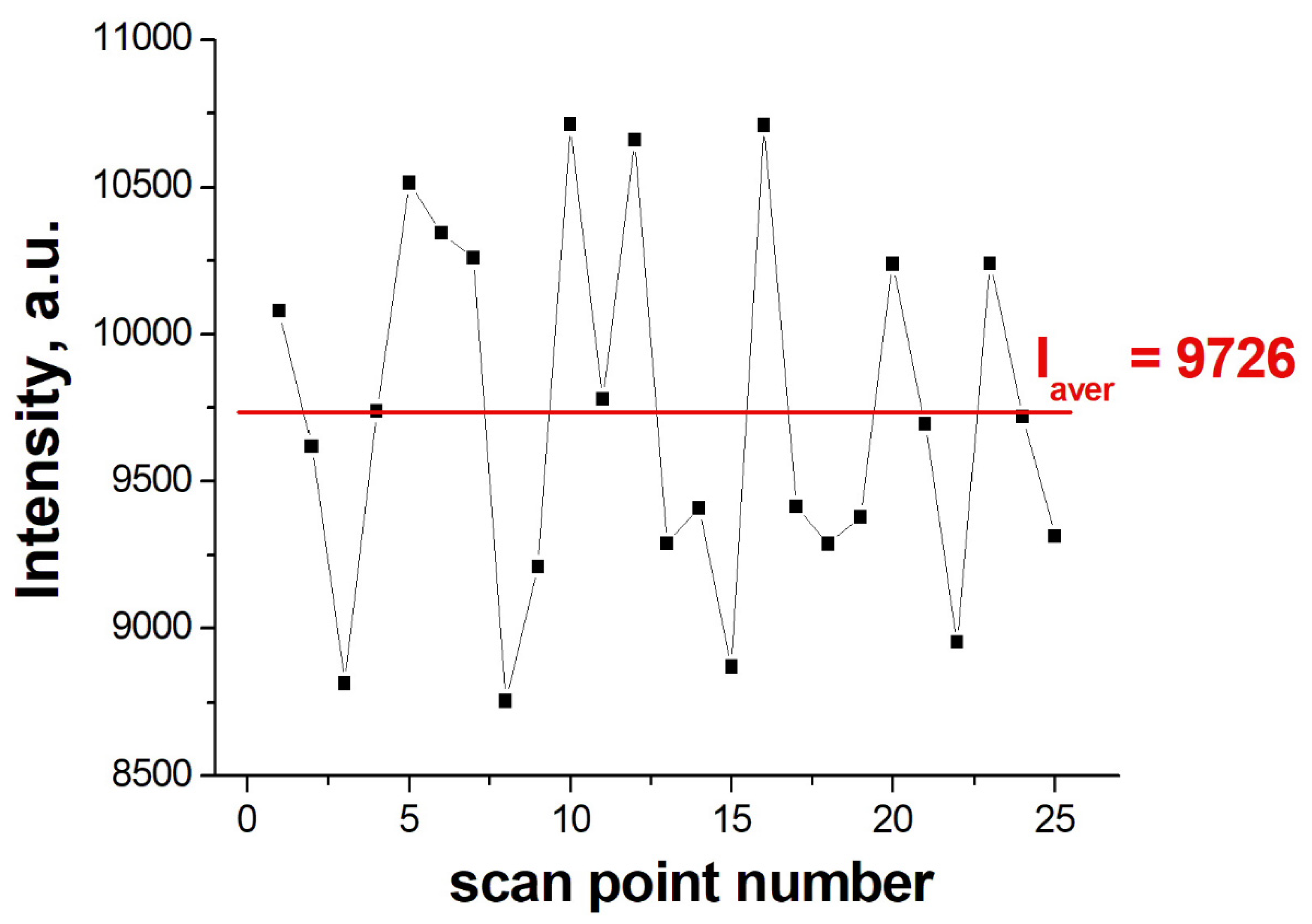
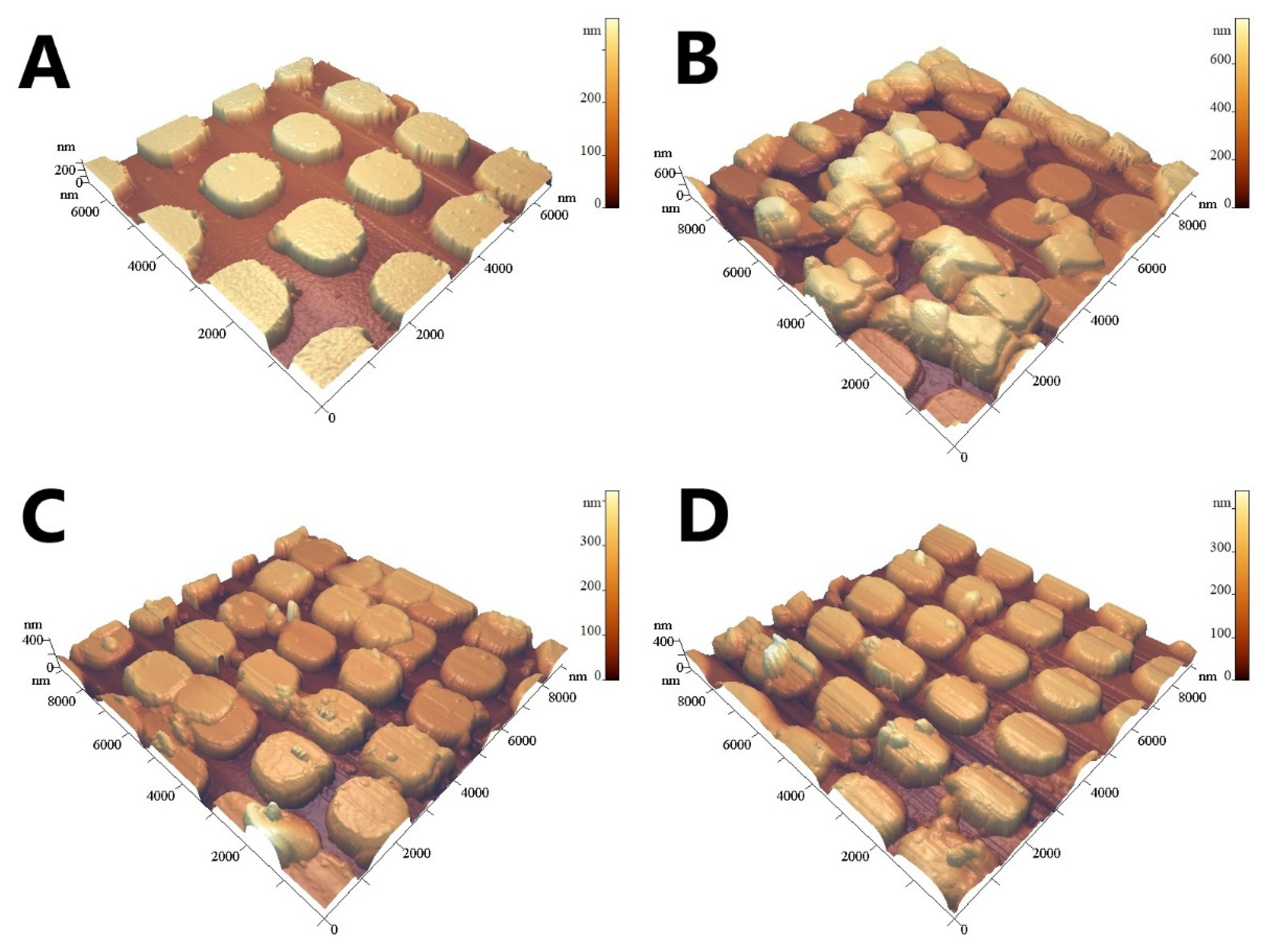
2.6. Study of the Surface Topology
3. Results
3.1. Aptamer as a Reporter Molecule
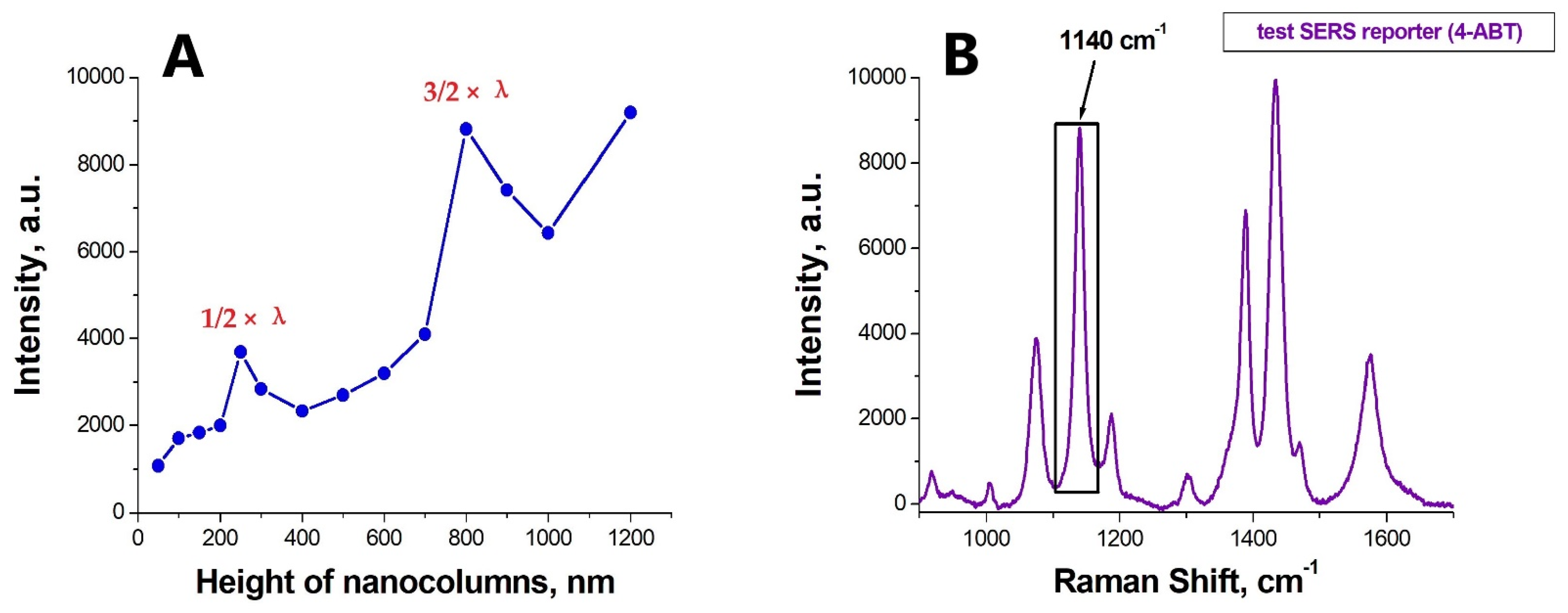
3.2. Optimization of the SERS-Substrate
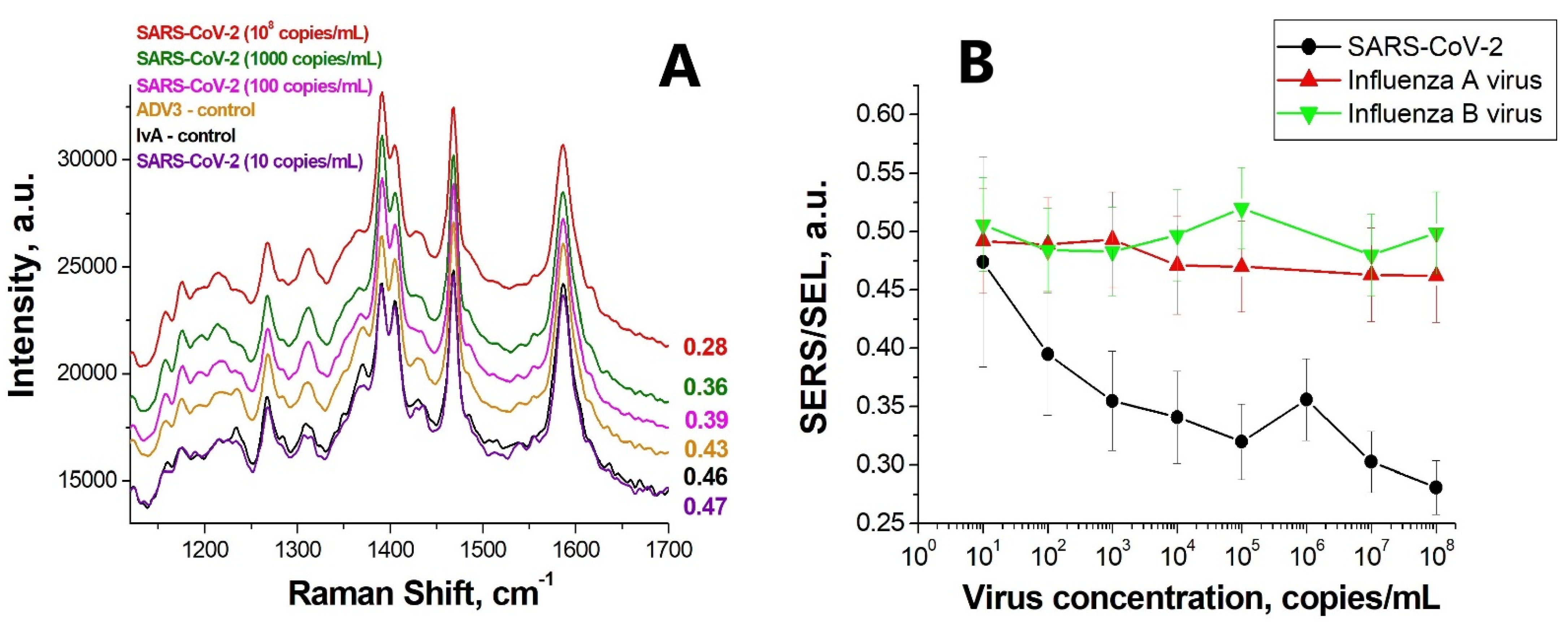
3.3. Lithographic SERS-Aptasensor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Munne, K.; Bhanothu, V.; Bhor, V.; Patel, V.; Mahale, S.D.; Pande, S. Detection of SARS-CoV-2 infection by RT-PCR test: Factors influencing interpretation of results. Virus Dis. 2021, 32, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.F.; So, P.K.; Yao, Z.P. Advances in rapid detection of SARS-CoV-2 by mass spectrometry. Trends Anal. Chem. 2022, 157, 116759. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.; Carvalho, V.; Faria, B.; Miranda, I.; Catarino, S.; Teixeira, S.; Lima, R.; Minas, G.; Ribeiro, J. Diagnosis Methods for COVID-19: A Systematic Review. Micromachines 2022, 13, 1349. [Google Scholar] [CrossRef] [PubMed]
- Filchakova, O.; Dossym, D.; Ilyas, A.; Kuanysheva, T.; Abdizhamil, A.; Bukasov, R. Review of COVID-19 testing and diagnostic methods. Talanta 2022, 244, 123409. [Google Scholar] [CrossRef]
- Laing, S.; Gracie, K.; Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918. [Google Scholar] [CrossRef]
- Zhan, W.; Tang, S.; Jin, Y.; Yang, C.; He, L.; Wang, J.; Chen, Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J. Hazard. Mater. 2020, 393, 122348. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.K.; Joung, Y.; Kang, T.; Lee, M.K.; Choo, J. SERS-based dual-mode DNA aptasensors for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection. Sens. Actuators B Chem. 2022, 355, 131324. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M. Human ACE2-functionalized gold “virus-trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano Micro Lett. 2021, 13, 109. [Google Scholar] [CrossRef]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Ray, P.C. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Wang, X.; Wang, Y.; Lin, J.; Jiang, S.; Wu, Z.; He, Y.; Gao, X.; Zhu, Z.; et al. Rapid detection of viruses: Based on silver nanoparticles modified with bromine ions and acetonitrile. Chem. Eng. J. 2022, 438, 135589. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Ambartsumyan, O.; Zhdanov, G.; Gribanyov, D.; Gushchin, V.; Tkachuk, A.; Rudakova, E.; Nikiforova, M.; Kuznetsova, N.; Popova, L.; et al. SERS-Based Aptasensor for Rapid Quantitative Detection of SARS-CoV-2. Nanomaterials 2021, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Filonov, A.; Yaminsky, I.; Akhmetova, A.; Meshkov, G. FemtoScan Online! Why? Nanoindustry 2018, 5, 336–342. [Google Scholar] [CrossRef]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal Chem. 2020, 92, 9895–9900. [Google Scholar] [CrossRef]
- Fedotova, Y.V.; Kukushkin, V.I.; Solovyev, V.V.; Kukushkin, I.V. Spoof plasmons enable giant Raman scattering enhancement in Near-Infrared region. Opt. Express 2019, 27, 32578–32586. [Google Scholar] [CrossRef]
- Grabovenko, F.; Nikiforova, L.; Yanenko, B.; Ulitin, A.; Loktyushov, E.; Zatsepin, T.; Zavyalova, E. and Zvereva, M. Glycosylation of receptor binding domain of SARS-CoV-2 S-protein influences on binding to immobilized DNA aptamers. Int. J. Mol. Sci. 2022, 23, 557. [Google Scholar] [CrossRef]
- Le Ru, E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy; Cambridge University Press: Cambridge, UK, 2009; Volume 1. [Google Scholar]
- Goryachev, N.S.; Kukushkin, V.I.; Belik, A.Y.; Rybkin, A.Y.; Kraevaya, O.A.; Troshin, P.A.; Kotelnikov, A.I. Using SERS and SEF Spectroscopy to Detect Fullerene-Dye Dyads in Water and Biological Structures. Bull. Russ. Acad. Sci. Phys. 2022, 86, 418–422. [Google Scholar] [CrossRef]
- Kim, H.N.; Yoon, S.Y.; Lim, C.S.; Yoon, J. Comparison of Three Molecular Diagnostic Assays for SARS-CoV-2 Detection: Evaluation of Analytical Sensitivity and Clinical Performance. J. Clin. Lab. Anal. 2022, 36, e24242. [Google Scholar] [CrossRef]
- Yang, J.; Han, Y.; Zhang, R.; Zhang, R.; Li, J. Comparison of Analytical Sensitivity of SARS-CoV-2 Molecular Detection Kits. Int. J. Infect. Dis. 2021, 111, 233–241. [Google Scholar] [CrossRef]
- Shidlovskaya, E.V.; Kuznetsova, N.A.; Divisenko, E.V.; Nikiforova, M.A.; Siniavin, A.E.; Ogarkova, D.A.; Shagaev, A.V.; Semashko, M.A.; Tkachuk, A.P.; Burgasova, O.A.; et al. The Value of Rapid Antigen Tests for Identifying Carriers of Viable SARS-CoV-2. Viruses 2021, 13, 2012. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Tikhonova, D.; Zhdanov, G.; Rudakova, E.; Alferova, V.; Moiseenko, A.; Kamzeeva, P.; Khrulev, A.; Zalevsky, A.; Arutyunyan, A.; et al. SERS-based biosensor with Raman-active external responsive element for rapid determination of adenosine monophosphate. Anal. Chim. Acta 2022, 1221, 340140. [Google Scholar] [CrossRef] [PubMed]
- Zengin, A.; Tamer, U.; Caykara, U. Fabrication of a SERS based aptasensor for detection of ricin B toxin. J. Mater. Chem. B 2015, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering (SERS) biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta. 2019, 1077, 288–296. [Google Scholar] [CrossRef]
- Zhdanov, G.; Nyhrikova, E.; Meshcheryakova, N.; Kristavchuk, O.; Akhmetova, A.; Andreev, E.; Rudakova, E.; Gambaryan, A.; Yaminsky, I.; Aralov, A.; et al. A Combination of Membrane Filtration and Raman-Active DNA Ligand Greatly Enhances Sensitivity of SERS-Based Aptasensors for Influenza A Virus. Front. Chem. 2022, 10, 937180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Ji, X.; Wang, P.; Ding, C. SERS and fluorescence detection of circulating tumor cells (CTCs) with specific capture-release mode based on multifunctional gold nanomaterials and dual-selective recognition. Anal. Chim. Acta 2021, 1141, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, R.; Gao, M.; Zhang, X. A rapid and simple method for efficient capture and accurate discrimination of circulating tumor cells using aptamer conjugated magnetic beads and surface-enhanced Raman scattering imaging. Anal. Bioanal. Chem. 2015, 407, 8883–8892. [Google Scholar] [CrossRef]
- Kukushkin, V.I.; Kristavchuk, O.V.; Zhdanov, G.A.; Keshek, A.K.; Gambaryan, A.S.; Andreev, Y.V.; Nechaev, A.N.; Zavyalova, E.G. Aptasensors based on track-etched membranes coated with nanostructured silver layer for influenza A and B virus detection. Bull. Russ. Acad. Sci. Phys. 2023; 87, in press. [Google Scholar]


| Type 3 of Human Adenoviruses (ADV 3) | Human Respiratory Syncytial Virus A2 Strain (RSV A2) | SARS-CoV-2 | |||
|---|---|---|---|---|---|
| Concentration, TCID50/mL | SERS/SEL, a.u. | Concentration, TCID50/mL | SERS/SEL, a.u. | Concentration, TCID50/mL | SERS/SEL, a.u. |
| 2.9 | 0.43 ± 0.03 | 0.63 | 0.51 ± 0.05 | 1.2 | 0.34 ± 0.04 |
| 29 | 0.47 ± 0.04 | 6.3 | 0.44 ± 0.04 | 12 | 0.32 ± 0.03 |
| 290 | 0.52 ± 0.04 | 63 | 0.47 ± 0.04 | 120 | 0.36 ± 0.03 |
| 2900 | 0.43 ± 0.03 | 630 | 0.48 ± 0.04 | 1200 | 0.30 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukushkin, V.; Ambartsumyan, O.; Astrakhantseva, A.; Gushchin, V.; Nikonova, A.; Dorofeeva, A.; Zverev, V.; Gambaryan, A.; Tikhonova, D.; Sovetnikov, T.; et al. Lithographic SERS Aptasensor for Ultrasensitive Detection of SARS-CoV-2 in Biological Fluids. Nanomaterials 2022, 12, 3854. https://doi.org/10.3390/nano12213854
Kukushkin V, Ambartsumyan O, Astrakhantseva A, Gushchin V, Nikonova A, Dorofeeva A, Zverev V, Gambaryan A, Tikhonova D, Sovetnikov T, et al. Lithographic SERS Aptasensor for Ultrasensitive Detection of SARS-CoV-2 in Biological Fluids. Nanomaterials. 2022; 12(21):3854. https://doi.org/10.3390/nano12213854
Chicago/Turabian StyleKukushkin, Vladimir, Oganes Ambartsumyan, Anna Astrakhantseva, Vladimir Gushchin, Alexandra Nikonova, Anastasia Dorofeeva, Vitaly Zverev, Alexandra Gambaryan, Daria Tikhonova, Timofei Sovetnikov, and et al. 2022. "Lithographic SERS Aptasensor for Ultrasensitive Detection of SARS-CoV-2 in Biological Fluids" Nanomaterials 12, no. 21: 3854. https://doi.org/10.3390/nano12213854
APA StyleKukushkin, V., Ambartsumyan, O., Astrakhantseva, A., Gushchin, V., Nikonova, A., Dorofeeva, A., Zverev, V., Gambaryan, A., Tikhonova, D., Sovetnikov, T., Akhmetova, A., Yaminsky, I., & Zavyalova, E. (2022). Lithographic SERS Aptasensor for Ultrasensitive Detection of SARS-CoV-2 in Biological Fluids. Nanomaterials, 12(21), 3854. https://doi.org/10.3390/nano12213854









