The pH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Manufacturing of the Working Electrodes
2.3. Electrochemical Experiments
2.4. Electron Microscopy Characterisation
3. Results and Discussions
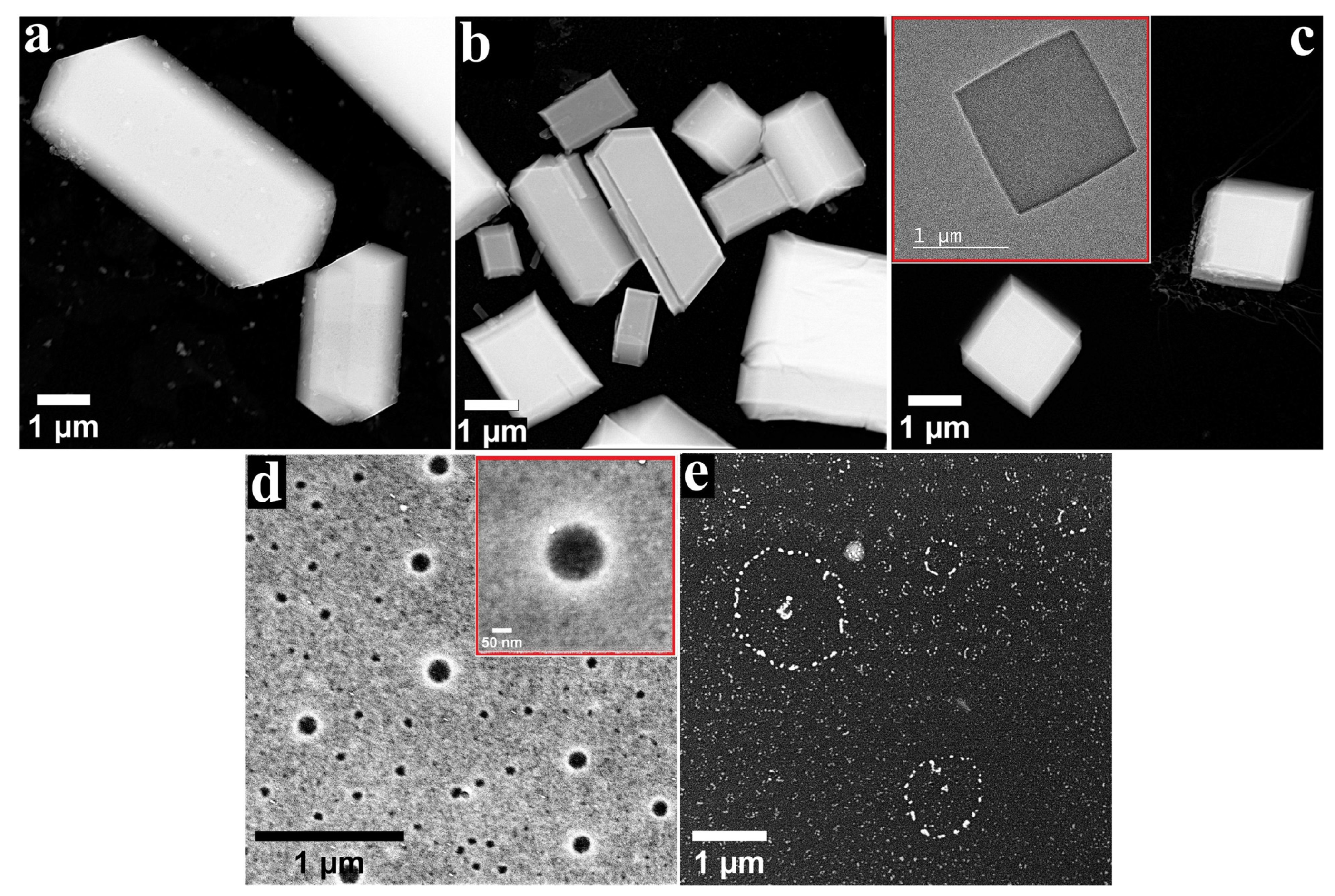
3.1. TEM/STEM Characterisation of Metalloporphyrin Specimens
3.2. The OER and HER Electrocatalytic Activities of the ZnP- and CoP-Modified Electrodes
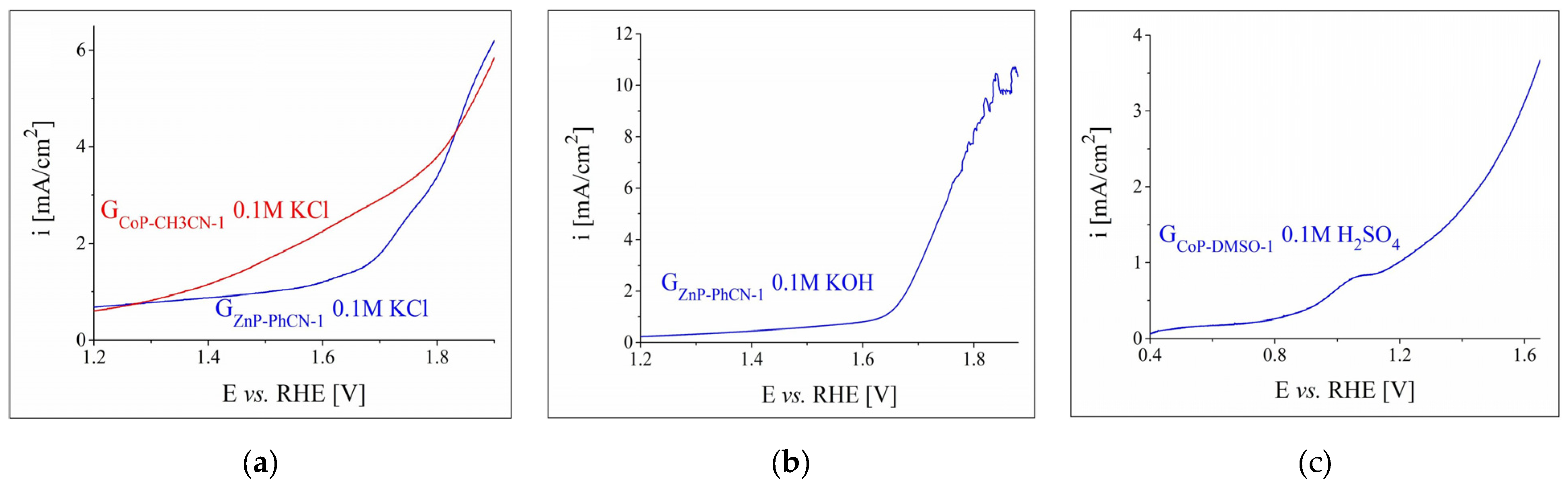
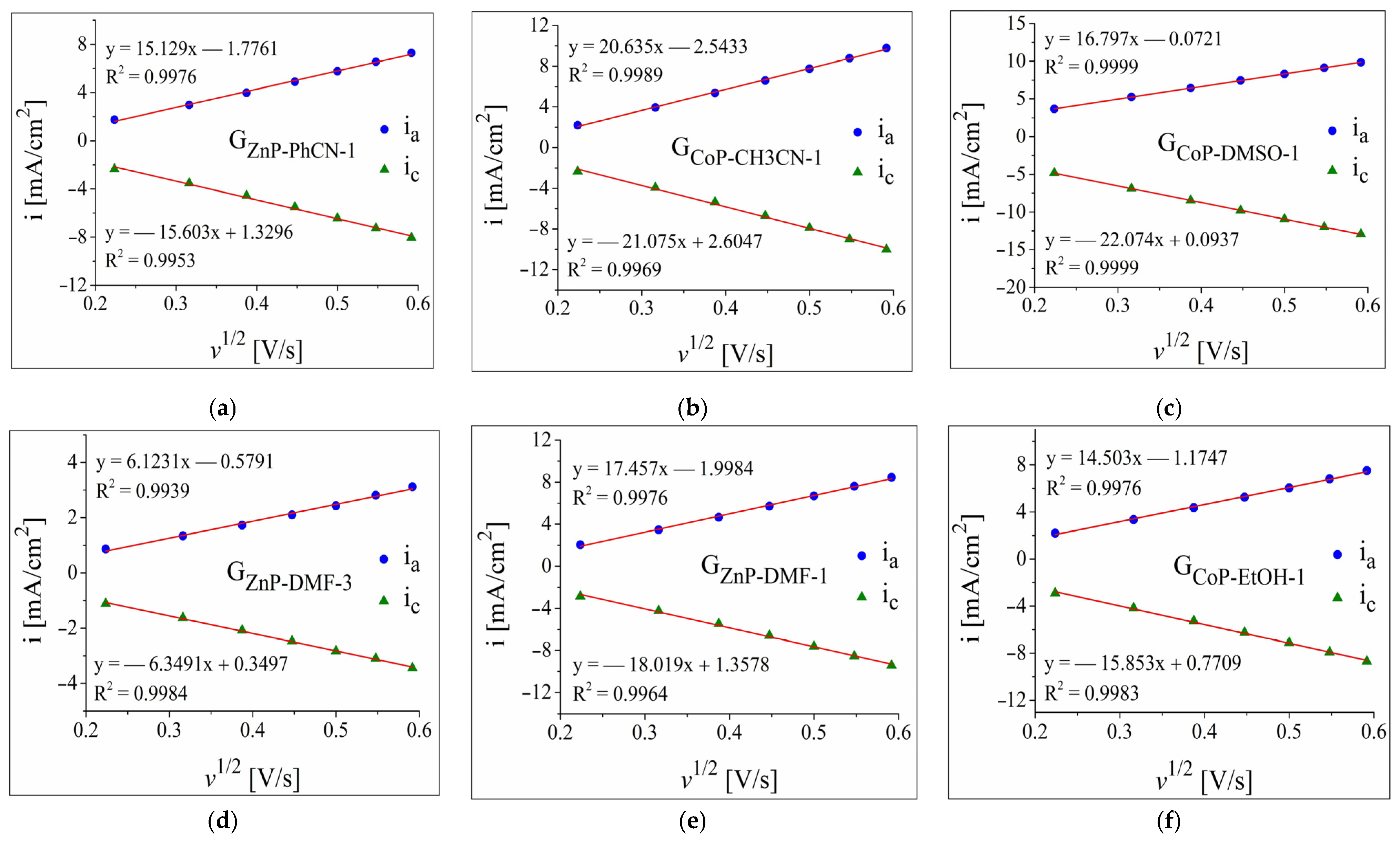
3.2.1. OER and HER Investigations in 0.1 m Electrolyte Solutions
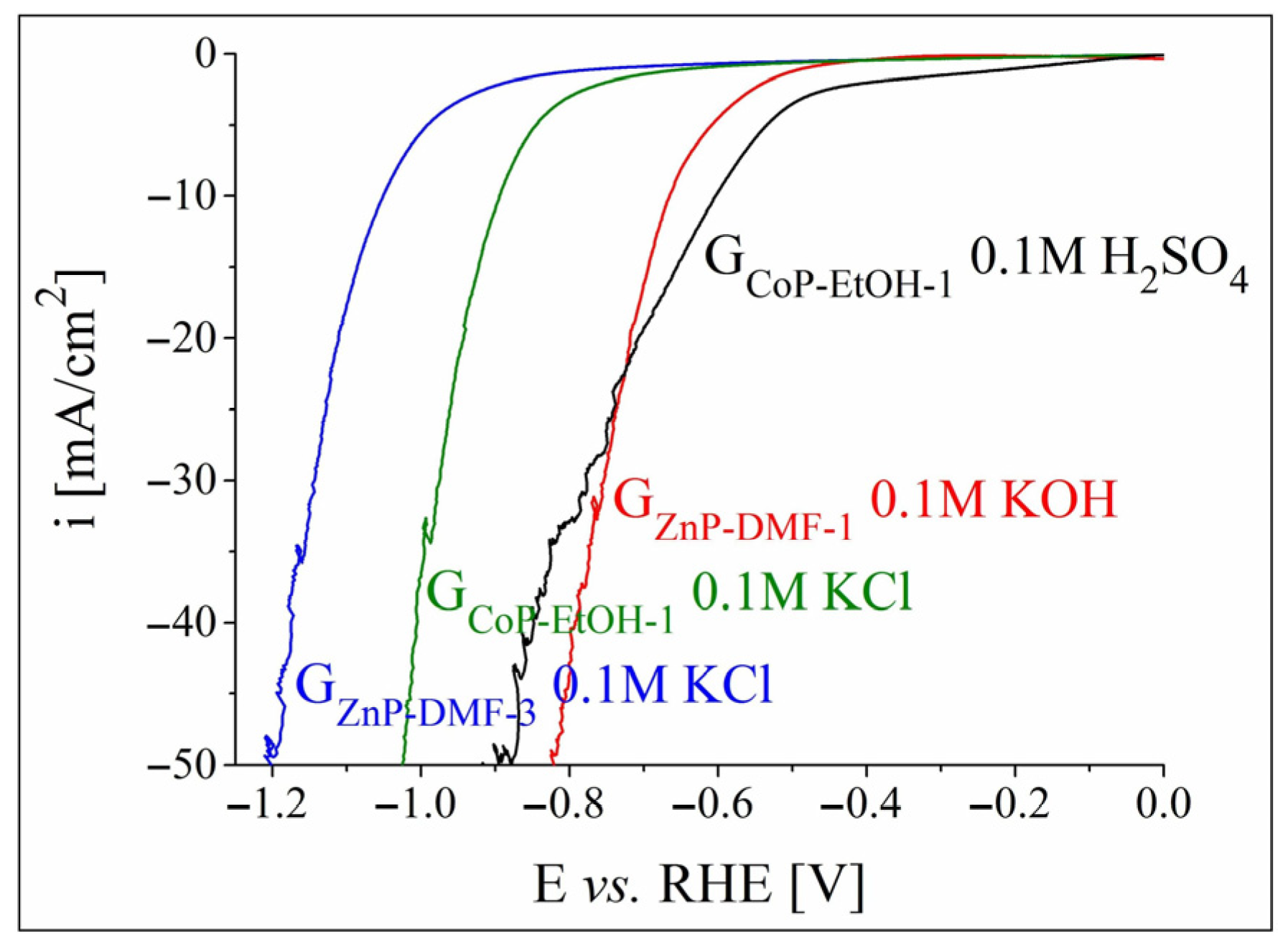
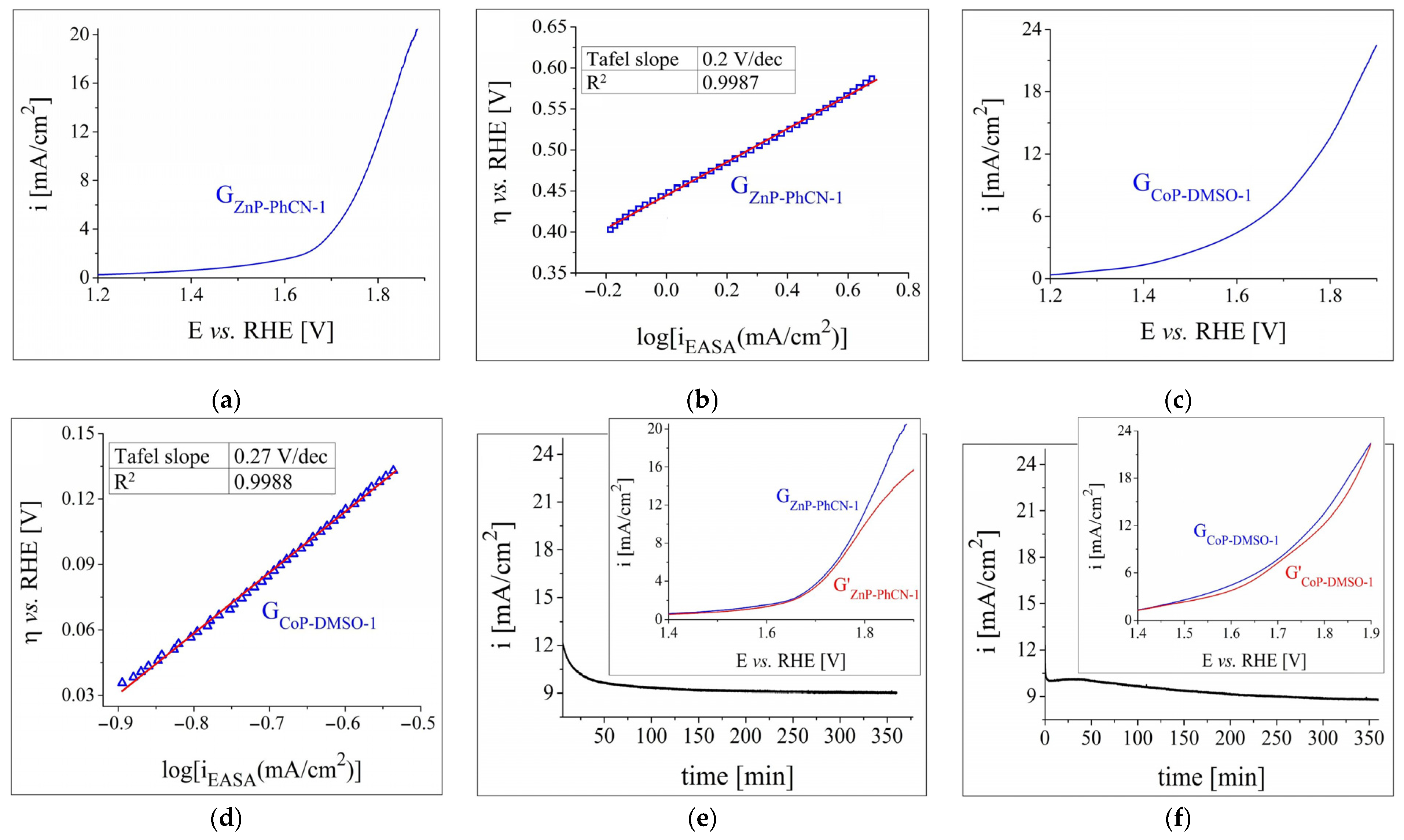
3.2.2. OER and HER Investigations in Strong Alkaline and Acidic Electrolyte Solutions
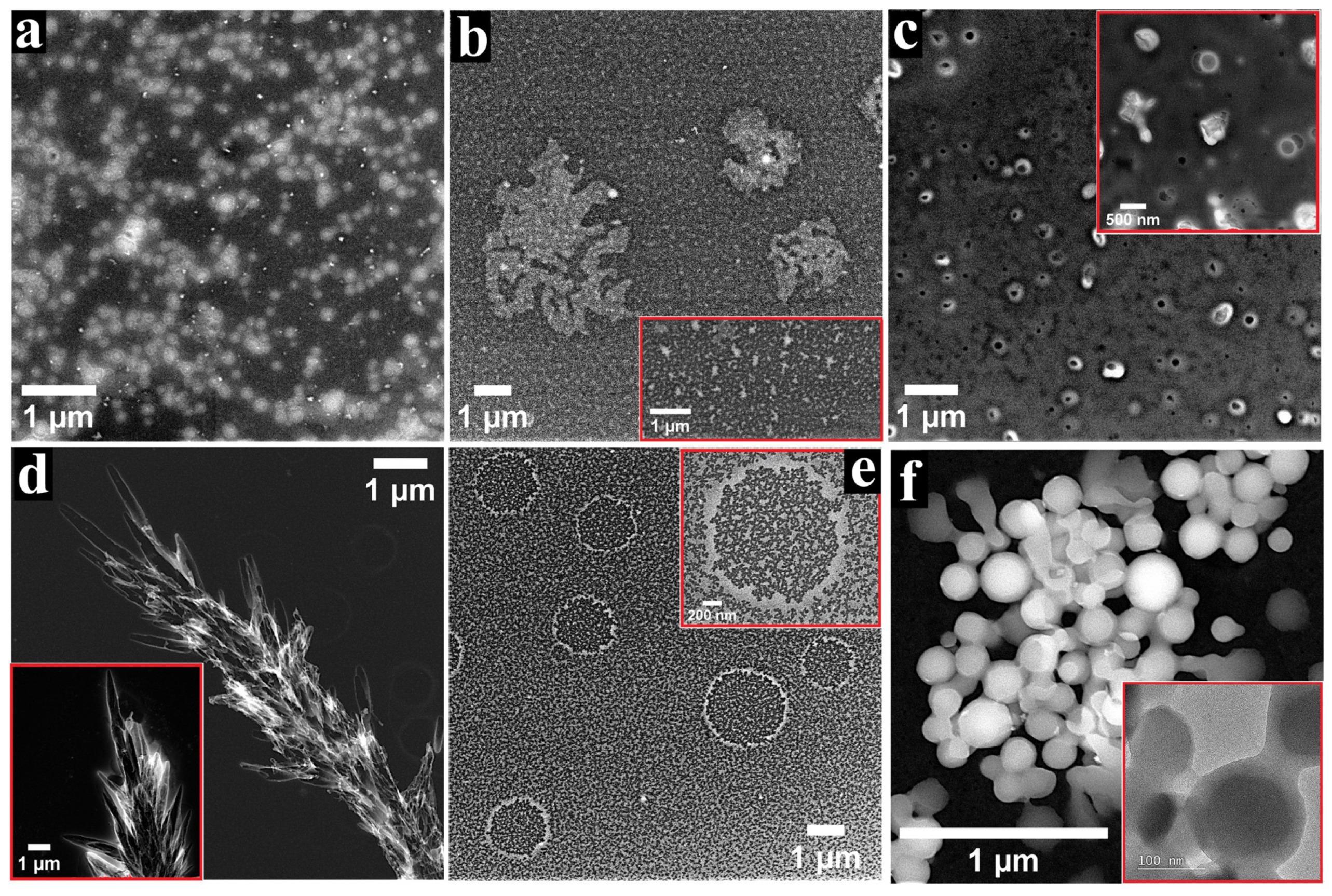
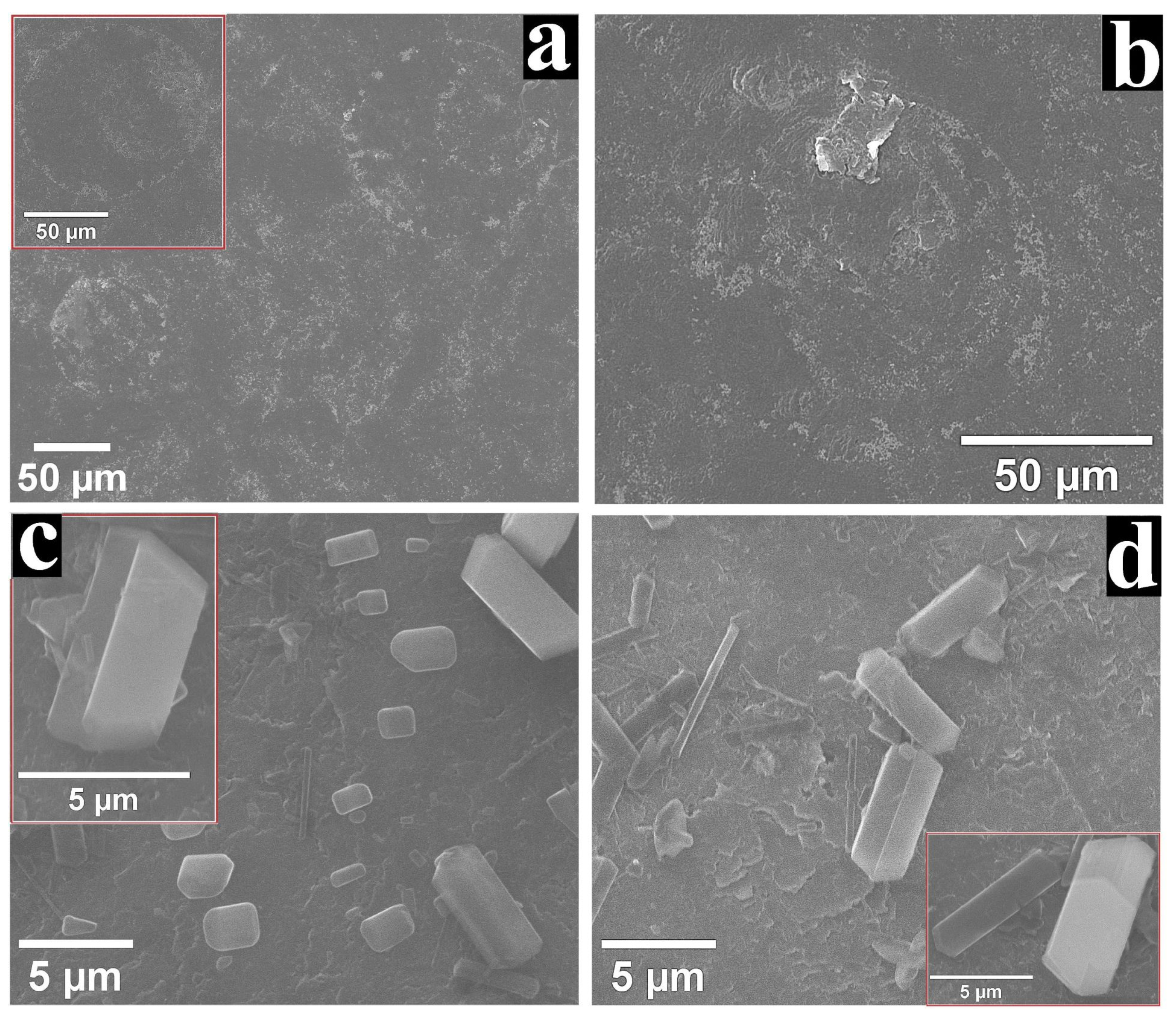
3.3. SEM Characterisation of the GCoP-DMSO-1 and GZnP-DMF-1 Electrodes
3.4. Additional Observations Concerning the GCoP-DMSO-1 and GZnP-DMF-1 Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shandilya, P.; Sharma, R.; Arya, R.K.; Kumar, A.; Vo, D.V.N.; Sharma, G. Recent Progress and Challenges in Photocatalytic Water Splitting Using Layered Double Hydroxides (LDH) Based Nanocomposites. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, K. Impact of Industrialization and Non-Renewable Energy on Environmental Pollution in Australia: Do Renewable Energy and Financial Development Play a Mitigating Role? Renew. Energy 2022, 195, 203–213. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen Sensors—A Review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Beswick, R.R.; Oliveira, A.M.; Yan, Y. Does the Green Hydrogen Economy Have a Water Problem? ACS Energy Lett. 2021, 6, 3167–3169. [Google Scholar] [CrossRef]
- Naimi, Y.; Antar, A. Hydrogen Generation by Electrolysis. In Advances in Hydrogen Generation Technologies; Eyvaz, M., Ed.; IntechOpen Limited: London, UK, 2018; pp. 1–18. ISBN 978-1-78923-535-7. [Google Scholar]
- Hausmann, J.N.; Schlögl, R.; Menezes, P.W.; Driess, M. Is Direct Seawater Splitting Economically Meaningful? Energy Environ. Sci. 2021, 14, 3679–3685. [Google Scholar] [CrossRef]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-Abundant Transition-Metal-Based Electrocatalysts for Water Electrolysis to Produce Renewable Hydrogen. Chem.-A Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, L.; Mishra, I.K.; Yu, Y.; Ren, Z.F.; Zhou, H.Q. Recent Developments in Earth-Abundant and Non-Noble Electrocatalysts for Water Electrolysis. Mater. Today Phys. 2018, 7, 121–138. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A Review on Fundamentals for Designing Oxygen Evolution Electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Yu, J.; Guan, D.; She, S.; Zhou, W.; Shao, Z. Self-Catalyzed Formation of Strongly Interconnected Multiphase Molybdenum-Based Composites for Efficient Hydrogen Evolution. Carbon Energy 2022, 4, 77–87. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lu, Y.X.; Li, B.; Huang, G.F.; Zeng, F.; Li, Y.Y.; Pan, A.; Chai, Y.F.; Huang, W.Q. Acid-Induced Topological Morphology Modulation of Graphitic Carbon Nitride Homojunctions as Advanced Metal-Free Catalysts for OER and Pollutant Degradation. J. Mater. Sci. Technol. 2021, 86, 210–218. [Google Scholar] [CrossRef]
- Huynh, M.; Ozel, T.; Liu, C.; Lau, E.C.; Nocera, D.G. Design of Template-Stabilized Active and Earth-Abundant Oxygen Evolution Catalysts in Acid. Chem. Sci. 2017, 8, 4779–4794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, L.; Guo, S. Noble Metal-Free Electrocatalytic Materials for Water Splitting in Alkaline Electrolyte. EnergyChem 2021, 3, 100053. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; Xu, S.; Duan, J.; Chen, S. Metallic Two-Dimensional Metal-Organic Framework Arrays for Ultrafast Water Splitting. J. Power Sources 2021, 494, 229733. [Google Scholar] [CrossRef]
- Ibn Shamsah, S.M. Earth-Abundant Electrocatalysts for Water Splitting: Current and Future Directions. Catalysts 2021, 11, 429. [Google Scholar] [CrossRef]
- Li, Q.; Bao, Y.; Bai, F. Porphyrin and Macrocycle Derivatives for Electrochemical Water Splitting. MRS Bull. 2020, 45, 569–573. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G. Porfirinele de La Sinteză La Aplicații; Eurostampa: Timisoara, Romania, 2008; ISBN 978-973-687-680-6. [Google Scholar]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Kellett, R.M.; Spiro, T.G. Cobalt(I) Porphyrin Catalysis of Hydrogen Production from Water. Inorg. Chem. 1985, 24, 2373–2377. [Google Scholar] [CrossRef]
- Naruta, Y.; Sasayama, M.-A.; Sasaki, T. Oxygen Evolution by Oxidation of Water with Manganese Porphyrin Dimers. Angew. Chem. Int. Ed. Engl. 1994, 33, 1839–1841. [Google Scholar] [CrossRef]
- Yao, B.; He, Y.; Wang, S.; Sun, H.; Liu, X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. Int. J. Mol. Sci. 2022, 23, 6036. [Google Scholar] [CrossRef]
- Cui, S.; Qian, M.; Liu, X.; Sun, Z.; Du, P. A Copper Porphyrin-Based Conjugated Mesoporous Polymer-Derived Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution. ChemSusChem 2016, 9, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-cosma, E.; Enache, C.; Armeanu, I.; Fagadar-cosma, G. Comparative Investigations of the Absorption and Fluorescence Spectra of Tetrapyridylporphyrine and Zn(II) Tetrapyridylporphyrine. Dig. J. Nanomater. Biostructures 2007, 2, 175–183. [Google Scholar]
- Cristescu, R.; Popescu, C.; Popescu, A.C.; Mihailescu, I.N.; Ciucu, A.A.; Andronie, A.; Iordache, S.; Stamatin, I.; Fagadar-Cosma, E.; Chrisey, D.B. Functional Porphyrin Thin Films Deposited by Matrix Assisted Pulsed Laser Evaporation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 169, 106–110. [Google Scholar] [CrossRef]
- Enache, C.; Fagadar-Cosma, E.; Armeanu, I.; Dudas, Z.; Ianasi, C.; Vasile, M.; Dascalu, D. Hybrid Silica-Metalloporphyrin Nanomaterials Exhibiting Intensive Absorption of Light in the Red-Region. Dig. J. Nanomater. Biostructures 2010, 5, 683–689. [Google Scholar]
- Bratosin, D.; Fagadar-Cosma, E.; Gheorghe, A.M.; Rugina, A.; Ardelean, A.; Montreuil, J.; Marinescu, A.G. In Vitro Toxi- and Ecotoxicological Assessment of Porphyrine Nanomaterials by Flow Cytometry Using Nucleated Erythrocytes. Carpathian J. Earth Environ. Sci. 2011, 6, 225–234. [Google Scholar]
- Birdeanu, M.; Fagadar-Cosma, E. The Self-Assembly of Porphyrin Derivatives into 2D and 3D Architectures. In Quantum Nanosystems: Structure, Properties and Interactions; Putz, M.V., Ed.; Apple Academic Press: Toronto, ON, Canada, 2015; pp. 174–206. ISBN 9781774633144. [Google Scholar]
- Birdeanu, M.; Birdeanu, A. –V.; Vaida, M.; Milovanovic, D.; Lascu, A.; Fagadar-Cosma, E. Corrosion Behaviour of ZnTa2O6 Pseudo-Binary Oxide, Zinc Meso-Tetra(4-Pyridyl)Porphyrin (ZnTPyP) and Hybrid ZnTa2O6/ZnTPyP Layers Deposited by PLD. Phys. Scr. 2019, 94, 075702. [Google Scholar] [CrossRef]
- Salageanu, L.; Muntean, D.; George, H.F.; Lascu, A.; Anghel, D.; Bagiu, I.C.; Fagadar-Cosma, E. Antimicrobial Activity of Different Substituted Meso-Porphyrin Derivatives. Rev. Rom. Med. Lab. 2020, 28, 205–216. [Google Scholar] [CrossRef]
- Salageanu, L.; Muntean, D.; Licker, M.; Lascu, A.; Anghel, D.; Fagadar-Cosma, E. Symmetrical and Asymmetrical Meso-Substituted Porphyrins and Zn-Metalloporphyrins in Gold Colloid Environment. Optical Properties and Evaluation of Antibacterial Activity. Farmacia 2020, 68, 288–298. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Sebarchievici, I.; Lascu, A.; Creanga, I.; Palade, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, G. Optical and Electrochemical Behavior of New Nano-Sized Complexes Based on Gold-Colloid and Co-Porphyrin Derivative in the Presence of H2O2. J. Alloys Compd. 2016, 686, 896–904. [Google Scholar] [CrossRef]
- Lascu, A.; Palade, A.; Birdeanu, M.; Fagadar-Cosma, E. Procaine Detection Using Hybrids of Cobalt-Metalloporphyrin with Gold and Silver Nanoparticles. J. Chem. Soc. Pak. 2019, 41, 43–51. [Google Scholar] [CrossRef]
- Giannoudis, E.; Benazzi, E.; Karlsson, J.; Copley, G.; Panagiotakis, S.; Landrou, G.; Angaridis, P.; Nikolaou, V.; Matthaiaki, C.; Charalambidis, G.; et al. Photosensitizers for H2 Evolution Based on Charged or Neutral Zn and Sn Porphyrins. Inorg. Chem. 2020, 59, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T.; Sakai, H.; Mase, K.; Ohkubo, K.; Fukuzumi, S. Remarkable Enhancement of Photocatalytic Hydrogen Evolution Efficiency Utilizing an Internal Cavity of Supramolecular Porphyrin Hexagonal Nanocylinders under Visible-Light Irradiation. J. Phys. Chem. C 2013, 117, 4441–4449. [Google Scholar] [CrossRef]
- Seo, S.; Lee, K.; Min, M.; Cho, Y.; Kim, M.; Lee, H. A Molecular Approach to an Electrocatalytic Hydrogen Evolution Reaction on Single-Layer Graphene. Nanoscale 2017, 9, 3969–3979. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, H.; Liu, P.; Wang, Y.; Yao, X.; Tang, Z.; Zhao, H. Molecular Engineering of Ni-/Co-Porphyrin Multilayers on Reduced Graphene Oxide Sheets as Bifunctional Catalysts for Oxygen Evolution and Oxygen Reduction Reactions. Chem. Sci. 2016, 7, 5640–5646. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shen, X.; Ren, J.; Wang, Q.; Zhao, W.; Zhu, W.; Shang, D. Regulating the Type of Cobalt Porphyrins for Synergistic Promotion of Photoelectrochemical Water Splitting of BiVO4. Dye. Pigment. 2021, 192, 109468. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; Volume 3, ISBN 978-0-471-00703-6. [Google Scholar]
- Szabadai, Z.; Sbarcea, L.; Udrescu, L. Analiza Fizică Și Chimică a Medicamentului; Victor Babes Publishing House: Timisoara, Romania, 2016; ISBN 978-606-786-020-7. [Google Scholar]
- Menezes, P.W.; Panda, C.; Loos, S.; Bunschei-Bruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A Structurally Versatile Nickel Phosphite Acting as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2018, 11, 1287–1298. [Google Scholar] [CrossRef]
- Fratilescu, I.; Lascu, A.; Taranu, B.O.; Epuran, C.; Birdeanu, M.; Macsim, A.-M.; Tanasa, E.; Vasile, E.; Fagadar-Cosma, E. One A3B Porphyrin Structure—Three Successful Applications. Nanomaterials 2022, 12, 1930. [Google Scholar] [CrossRef]
- Taranu, B.O.; Ivanovici, M.G.; Svera, P.; Vlazan, P.; Sfirloaga, P.; Poienar, M. Ni11□(HPO3)8(OH)6 Multifunctional Materials: Electrodes for Oxygen Evolution Reaction and Potential Visible-Light Active Photocatalysts. J. Alloys Compd. 2020, 848, 156595. [Google Scholar] [CrossRef]
- Poienar, M.; Taranu, B.O.; Svera, P.; Sfirloaga, P.; Vlazan, P. Disclosing the Thermal Behaviour, Electrochemical and Optical Properties of Synthetic Fe3(PO4)2(OH)2 Materials. J. Therm. Anal. Calorim. 2022, 147, 11839–11855. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Auwärter, W.; Écija, D.; Klappenberger, F.; Barth, J.V. Porphyrins at Interfaces. Nat. Chem. 2015, 7, 105–120. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Kearney, L.; Brandon, M.P.; Pryce, M.T. Design Components of Porphyrin-Based Photocatalytic Hydrogen Evolution Systems: A Review. Coord. Chem. Rev. 2022, 467, 214599. [Google Scholar] [CrossRef]
- Cosma, E.F.; Lascu, A.; Shova, S.; Zaltariov, M.F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-Ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive Aunps-Plasmonic Hybrids Capable of Detecting Triiodide Anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef]
- Bahrami, M.; Kraft, S.; Becker, J.; Hartmann, H.; Vogler, B.; Wardelmann, K.; Behle, H.; Elemans, J.A.A.W.; Barke, I.; Speller, S. Correlative Microscopy of Morphology and Luminescence of Cu Porphyrin Aggregates. J. Phys. B At. Mol. Opt. Phys. 2018, 51, 144002. [Google Scholar] [CrossRef]
- van Hameren, R. Functional Materials from Self-Assembled Compounds. Ph.D. Thesis, Radboud University, Nijmegen, The Netherlands, 2010. [Google Scholar]
- Lensen, M.C.; Takazawa, K.; Elemans, J.A.A.W.; Jeukens, C.R.L.P.N.; Christianen, P.C.M.; Maan, J.C.; Rowan, A.E.; Nolte, R.J. Aided Self-Assembly of Porphyrin Nanoaggregates into Ring-Shaped Architectures. Chem.-A Eur. J. 2004, 10, 831–839. [Google Scholar] [CrossRef]
- Taranu, B.-O. Contribuții La Caracterizarea Fizico-Chimică a Porfirinelor. Aplicații În Senzoristică Și Coroziune. Ph.D. Thesis, Institute of Chemistry Timisoara of Romanian Academy, Timisoara, Romania, 2016. [Google Scholar]
- Taranu, B.O.; Fagadar-Cosma, E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes 2022, 10, 611. [Google Scholar] [CrossRef]
- Taranu, B.O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New Functional Hybrid Materials Based on Clay Minerals for Enhanced Electrocatalytic Activity. J. Alloys Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Taranu, B.O.; Vlazan, P.; Racu, A. Water Splitting Studies in Alkaline Medium Using Graphite Electrodes Modified with Transition Metal Oxides and Compositions Containing Them. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 79–95. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Sebarchievici, I.; Taranu, B.O.; Birdeanu, M.; Rus, S.F.; Fagadar-Cosma, E. Electrocatalytic Behaviour and Application of Manganese Porphyrin/Gold Nanoparticle- Surface Modified Glassy Carbon Electrodes. Appl. Surf. Sci. 2016, 390, 131–140. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion Coefficients of Ferri- and Ferrocyanide Ions in Aqueous Media, Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Sun, B.; Su, D.; Huang, X.; Liu, H.; Yan, Y.; Sun, K.; Wang, G. Graphene-Co3O4 Nanocomposite as Electrocatalyst with High Performance for Oxygen Evolution Reaction. Sci. Rep. 2015, 5, 7629. [Google Scholar] [CrossRef] [PubMed]
- Babar, P.; Patil, K.; Mahmood, J.; Kim, S.j.; Kim, J.H.; Yavuz, C.T. Low-Overpotential Overall Water Splitting by a Cooperative Interface of Cobalt-Iron Hydroxide and Iron Oxyhydroxide. Cell Rep. Phys. Sci. 2022, 3, 100762. [Google Scholar] [CrossRef]
- Mani, V.; Anantharaj, S.; Mishra, S.; Kalaiselvi, N.; Kundu, S. Iron Hydroxyphosphate and Sn-Incorporated Iron Hydroxyphosphate: Efficient and Stable Electrocatalysts for Oxygen Evolution Reaction. Catal. Sci. Technol. 2017, 7, 5092–5104. [Google Scholar] [CrossRef]
- Liu, C.; Ma, H.; Yuan, M.; Yu, Z.; Li, J.; Shi, K.; Liang, Z.; Yang, Y.; Zhu, T.; Sun, G.; et al. (NiFe)S2 Nanoparticles Grown on Graphene as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Electrochim. Acta 2018, 286, 195–204. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, L.; Zhao, W.; Shen, X.; Zhu, W. Electrochemical Hydrogen and Oxygen Evolution Reactions from a Cobalt-Porphyrin-Based Covalent Organic Polymer. J. Colloid Interface Sci. 2020, 579, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, H.W.; Deng, Y.; Lu, Z.; Hsu, P.C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional Non-Noble Metal Oxide Nanoparticle Electrocatalysts through Lithium-Induced Conversion for Overall Water Splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Zhou, Z.; Zaman, W.Q.; Sun, W.; Cao, L.M.; Tariq, M.; Yang, J. Cultivating Crystal Lattice Distortion in IrO2: Via Coupling with MnO2 to Boost the Oxygen Evolution Reaction with High Intrinsic Activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef]
- Han, Y.; Fang, H.; Jing, H.; Sun, H.; Lei, H.; Lai, W.; Cao, R. Singly versus Doubly Reduced Nickel Porphyrins for Proton Reduction: Experimental and Theoretical Evidence for a Homolytic Hydrogen-Evolution Reaction. Angew. Chem.-Int. Ed. 2016, 55, 5457–5462. [Google Scholar] [CrossRef] [PubMed]
- Shahamirian, M.; Szatylowicz, H.; Krygowski, T.M. How OH and O− Groups Affect Electronic Structure of Meta-Substituted and Para-Substituted Phenols and Phenolates. Struct. Chem. 2017, 28, 1563–1572. [Google Scholar] [CrossRef]
- Kano, K.; Minamizono, H.; Kitae, T.; Negi, S. Self-Aggregation of Cationic Porphyrins in Water. Can π-π Stacking Interaction Overcome Electrostatic Repulsive Force? J. Phys. Chem. A 1997, 101, 6118–6124. [Google Scholar] [CrossRef]
- Seidel, R.W.; Goddard, R.; Hoch, C.; Breidung, J.; Oppel, I.M. On the Structure of Unsolvated Free-Base 5,10,15,20-Tetra(3-Pyridyl) Porphyrin. J. Mol. Struct. 2011, 985, 307–315. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.H.; Wang, L.N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, Q.; Wang, Y.; Gao, Y.; Wang, Y.; Liang, Z.; Zhang, W.; Cao, R. Significantly Boosted Oxygen Electrocatalysis with Cooperation between Cobalt and Iron Porphyrins. Dalt. Trans. 2021, 50, 5120–5123. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Guo, H.; Guo, K.; Lei, H.; Zhang, W.; Zheng, H.; Liang, Z.; Cao, R. Substituent Position Effect of Co Porphyrin on Oxygen Electrocatalysis. Chin. Chem. Lett. 2021, 32, 2841–2845. [Google Scholar] [CrossRef]
- Qin, H.; Wang, Y.; Wang, B.; Duan, X.; Lei, H.; Zhang, X.; Zheng, H.; Zhang, W.; Cao, R. Cobalt Porphyrins Supported on Carbon Nanotubes as Model Catalysts of Metal-N4/C Sites for Oxygen Electrocatalysis. J. Energy Chem. 2020, 53, 77–81. [Google Scholar] [CrossRef]
- Attatsi, I.K.; Zhu, W.; Liang, X. Noncovalent Immobilization of Co(Ii)Porphyrin through Axial Coordination as an Enhanced Electrocatalyst on Carbon Electrodes for Oxygen Reduction and Evolution. New J. Chem. 2020, 44, 4340–4345. [Google Scholar] [CrossRef]
- Wu, Y.; Veleta, J.M.; Tang, D.; Price, A.D.; Botez, C.E.; Villagrán, D. Efficient Electrocatalytic Hydrogen Gas Evolution by a Cobalt-Porphyrin-Based Crystalline Polymer. Dalt. Trans. 2018, 47, 8801–8806. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Gu, Z.G.; Zhang, W.; Kang, Y.; Zhang, J. Epitaxial Encapsulation of Homodispersed CeO2 in a Cobalt-Porphyrin Network Derived Thin Film for the Highly Efficient Oxygen Evolution Reaction. J. Mater. Chem. A 2017, 5, 20126–20130. [Google Scholar] [CrossRef]
- Ma, J.; Liu, L.; Chen, Q.; Yang, M.; Wang, D.; Tong, Z.; Chen, Z. A Facile Approach to Prepare Crumpled CoTMPyP/Electrochemically Reduced Graphene Oxide Nanohybrid as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2017, 399, 535–541. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhunia, K.; Patra, B.C.; Das, S.K.; Pradhan, D.; Bhaumik, A.; Pradhan, A.; Bhattacharya, S. Efficacious Electrochemical Oxygen Evolution from a Novel Co(II) Porphyrin/Pyrene-Based Conjugated Microporous Polymer. ACS Appl. Mater. Interfaces 2019, 11, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Sahabudeen, H.; Qi, H.; Glatz, B.A.; Tranca, D.; Dong, R.; Hou, Y.; Zhang, T.; Kuttner, C.; Lehnert, T.; Seifert, G.; et al. Wafer-Sized Multifunctional Polyimine-Based Two-Dimensional Conjugated Polymers with High Mechanical Stiffness. Nat. Commun. 2016, 7, 13461. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yao, Y.; Gao, Y.; Lu, D.; Du, P. Pyrolyzed Cobalt Porphyrin-Based Conjugated Mesoporous Polymers as Bifunctional Catalysts for Hydrogen Production and Oxygen Evolution in Water. Chem. Commun. 2016, 52, 13483–13486. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Sun, Z.; Jiang, D.; Du, P. Covalent Cobalt Porphyrin Framework on Multiwalled Carbon Nanotubes for Efficient Water Oxidation at Low Overpotential. Chem. Mater. 2015, 27, 4586–4593. [Google Scholar] [CrossRef]
- Huang, D.; Lu, J.; Li, S.; Luo, Y.; Zhao, C.; Hu, B.; Wang, M.; Shen, Y. Fabrication of Cobalt Porphyrin. Electrochemically Reduced Graphene Oxide Hybrid Films for Electrocatalytic Hydrogen Evolution in Aqueous Solution. Langmuir 2014, 30, 6990–6998. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced Electrochemical Response of Diclofenac at a Fullerene–Carbon Nanofiber Paste Electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum Nanoparticles-Doped Sol-Gel/Carbon Nanotubes Composite Electrochemical Sensors and Biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Zeng, L.; He, L.; Sun, S.; Tong, Y.; Zhang, J. Imine Gels Based on Ferrocene and Porphyrin and Their Electrocatalytic Property. Chem.-An Asian J. 2020, 15, 1963–1969. [Google Scholar] [CrossRef]
- Alvarez, I.B.; Wu, Y.; Sanchez, J.; Ge, Y.; Ramos-Garcés, M.V.; Chu, T.; Jaramillo, T.F.; Colón, J.L.; Villagrán, D. Cobalt Porphyrin Intercalation into Zirconium Phosphate Layers for Electrochemical Water Oxidation. Sustain. Energy Fuels 2021, 5, 430–437. [Google Scholar] [CrossRef]

| Electrode Label | Metallo- Porphyrin | Solvent | Applied Layers | Electrode Label | Metallo- Porphyrin | Solvent | Applied Layers |
|---|---|---|---|---|---|---|---|
| GZnP-DMSO-1 | ZnP | DMSO | 1 | GCoP-DMSO-1 | CoP | DMSO | 1 |
| GZnP-DMSO-2 | 2 | GCoP-DMSO-2 | 2 | ||||
| GZnP-DMSO-3 | 3 | GCoP-DMSO-3 | 3 | ||||
| GZnP-DMF-1 | DMF | 1 | GCoP-DMF-1 | DMF | 1 | ||
| GZnP-DMF-2 | 2 | GCoP-DMF-2 | 2 | ||||
| GZnP-DMF-3 | 3 | GCoP-DMF-3 | 3 | ||||
| GZnP-PhCN-1 | PhCN | 1 | GCoP-CH3CN-1 | CH3CN | 1 | ||
| GZnP-PhCN-2 | 2 | GCoP-CH3CN-2 | 2 | ||||
| GZnP-PhCN-3 | 3 | GCoP-CH3CN-3 | 3 | ||||
| GZnP-THF-1 | ZnP | THF | 1 | GCoP-PhCN-1 | CoP | PhCN | 1 |
| GZnP-THF-2 | 2 | GCoP-PhCN-2 | 2 | ||||
| GZnP-THF-3 | 3 | GCoP-PhCN-3 | 3 | ||||
| GZnP-DCM-1 | DCM | 1 | GCoP-EtOH-1 | EtOH | 1 | ||
| GZnP-DCM-2 | 2 | GCoP-EtOH-2 | 2 | ||||
| GZnP-DCM-3 | 3 | GCoP-EtOH-3 | 3 | ||||
| GCoP-THF-1 | THF | 1 | |||||
| GCoP-THF-2 | 2 | ||||||
| GCoP-THF-3 | 3 | ||||||
| Electrode Label | EASA [cm2] | Diffusion Coefficient [cm2/s] |
|---|---|---|
| G0 | 0.325 ± 0.007 | 9.35 × 10–6 ± 0.14 × 10–6 |
| GZnP-PhCN-1 | 0.8 ± 0.06 | 6.15 × 10–5 ± 0.24 × 10–5 |
| GZnP-DMF-3 | 0.422 ± 0.0014 | 1.525 × 10–5 ± 0.007 × 10–5 |
| GZnP-DMF-1 | 0.942 ± 0.015 | 7.7 × 10–5 ± 0.24 × 10–5 |
| GCoP-CH3CN-1 | 0.948 ± 0.07 | 8.375 × 10–5 ± 0.62 × 10–5 |
| GCoP-EtOH-1 | 1.19 ± 0.08 | 5.7 × 10–5 ± 0.35 × 10–5 |
| GCoP-DMSO-1 | 0.94 ± 0.04 | 7.62 × 10–5 ± 0.72 × 10–5 |
| Electrode Label | ηH2 at i = −10 mA/cm2 | Tafel Slope |
|---|---|---|
| GZnP-DMF-1 | 0.67 V, in 0.1 M KOH | 0.15 V/dec, in 1 M KOH |
| 0.52 V, in 1 M KOH | ||
| GCoP-EtOH-1 | 0.6 V, in 0.1 M H2SO4 | 0.31 V/dec, in 0.5 M H2SO4 |
| 0.57 V, in 0.5 M H2SO4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, B.-O.; Fagadar-Cosma, E. The pH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins. Nanomaterials 2022, 12, 3788. https://doi.org/10.3390/nano12213788
Taranu B-O, Fagadar-Cosma E. The pH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins. Nanomaterials. 2022; 12(21):3788. https://doi.org/10.3390/nano12213788
Chicago/Turabian StyleTaranu, Bogdan-Ovidiu, and Eugenia Fagadar-Cosma. 2022. "The pH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins" Nanomaterials 12, no. 21: 3788. https://doi.org/10.3390/nano12213788
APA StyleTaranu, B.-O., & Fagadar-Cosma, E. (2022). The pH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins. Nanomaterials, 12(21), 3788. https://doi.org/10.3390/nano12213788