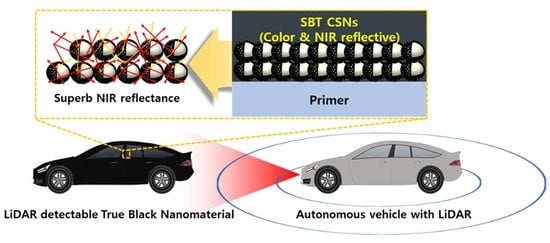
Synthesis of LiDAR-Detectable True Black Core/Shell Nanomaterial and Its Practical Use in LiDAR Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
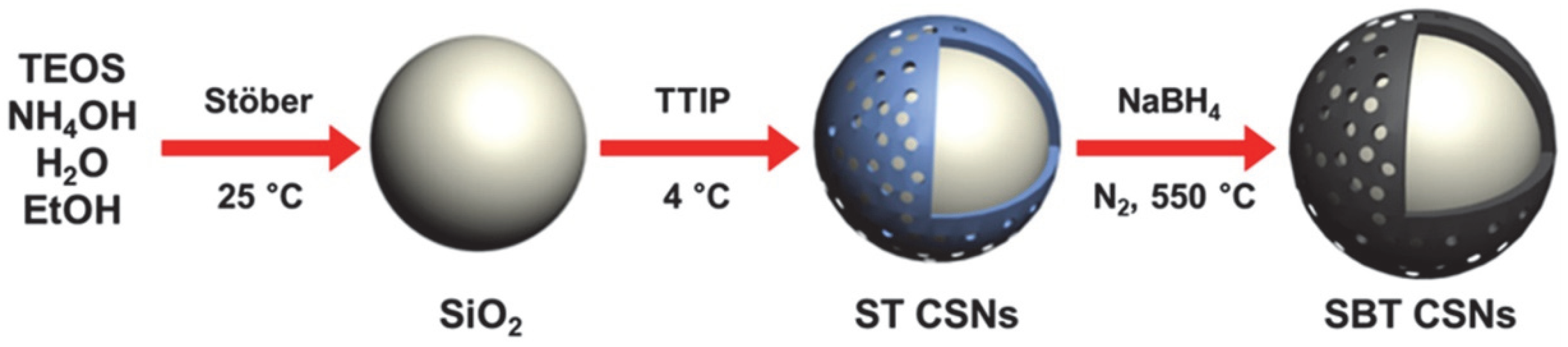
2.2. Synthesis of SiO2/TiO2 Core/Shell Nanoparticles (ST CSNs)
2.3. Fabrication of SiO2/Black TiO2 Core/Shell Black Nanoparticles (LiDAR-Detectable SBT CSNs)
2.4. Characterization
2.5. Measurement of Blackness of SBT CSN-Based Paint
2.6. Measurement of NIR Reflectance and Evaluation of LiDAR Detectability of SBT CSN-Based Paint
3. Results and Discussion
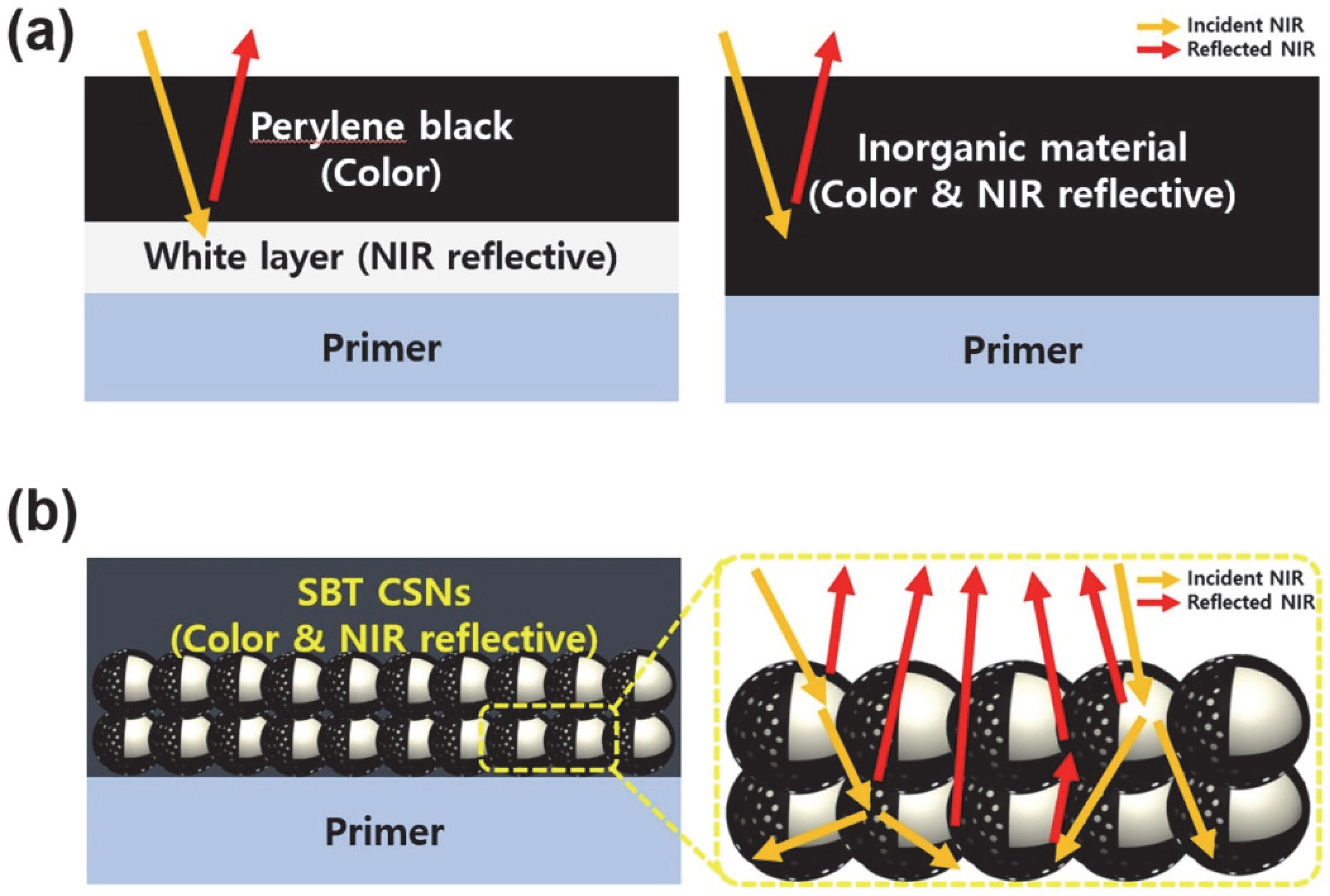
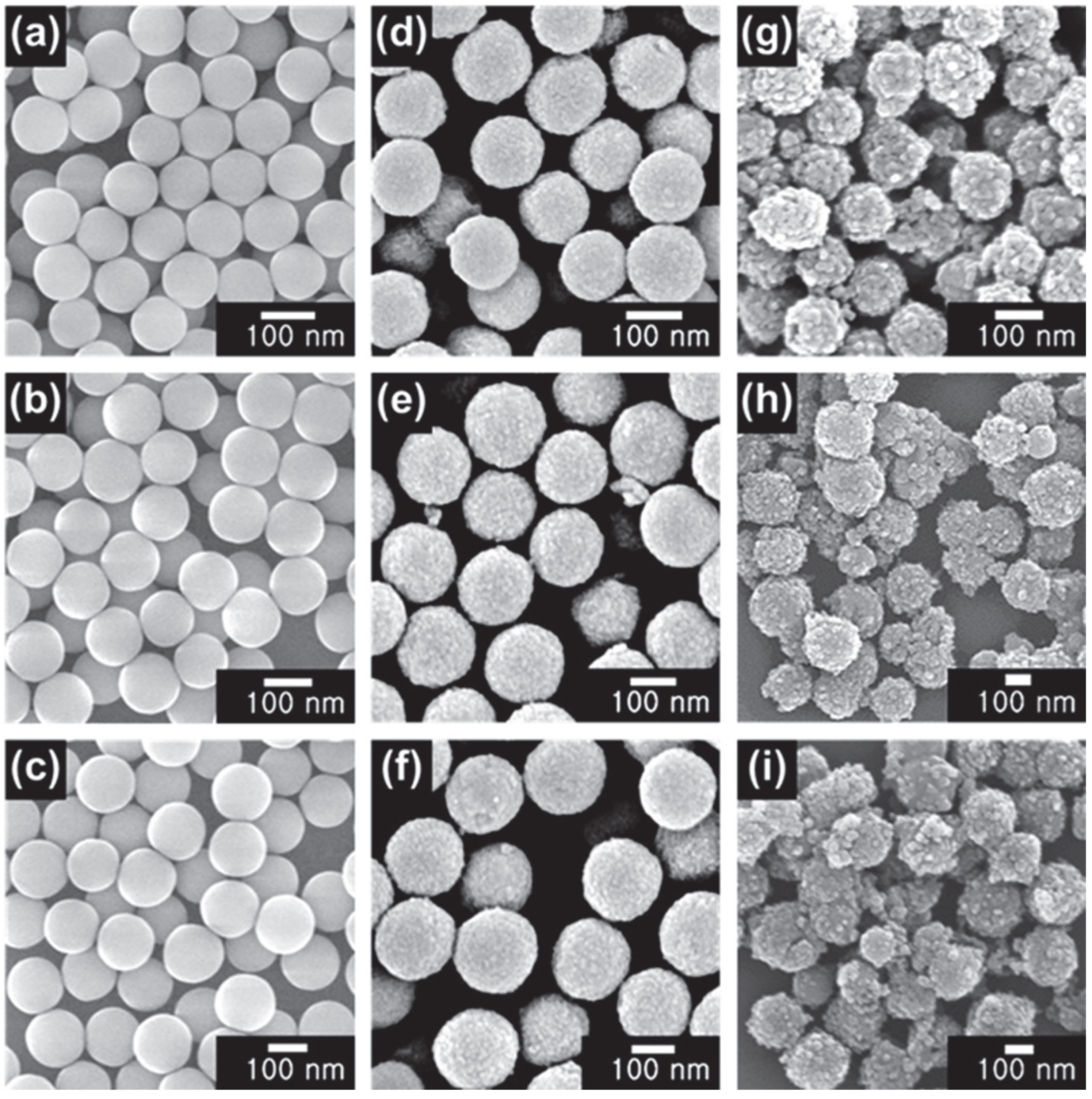
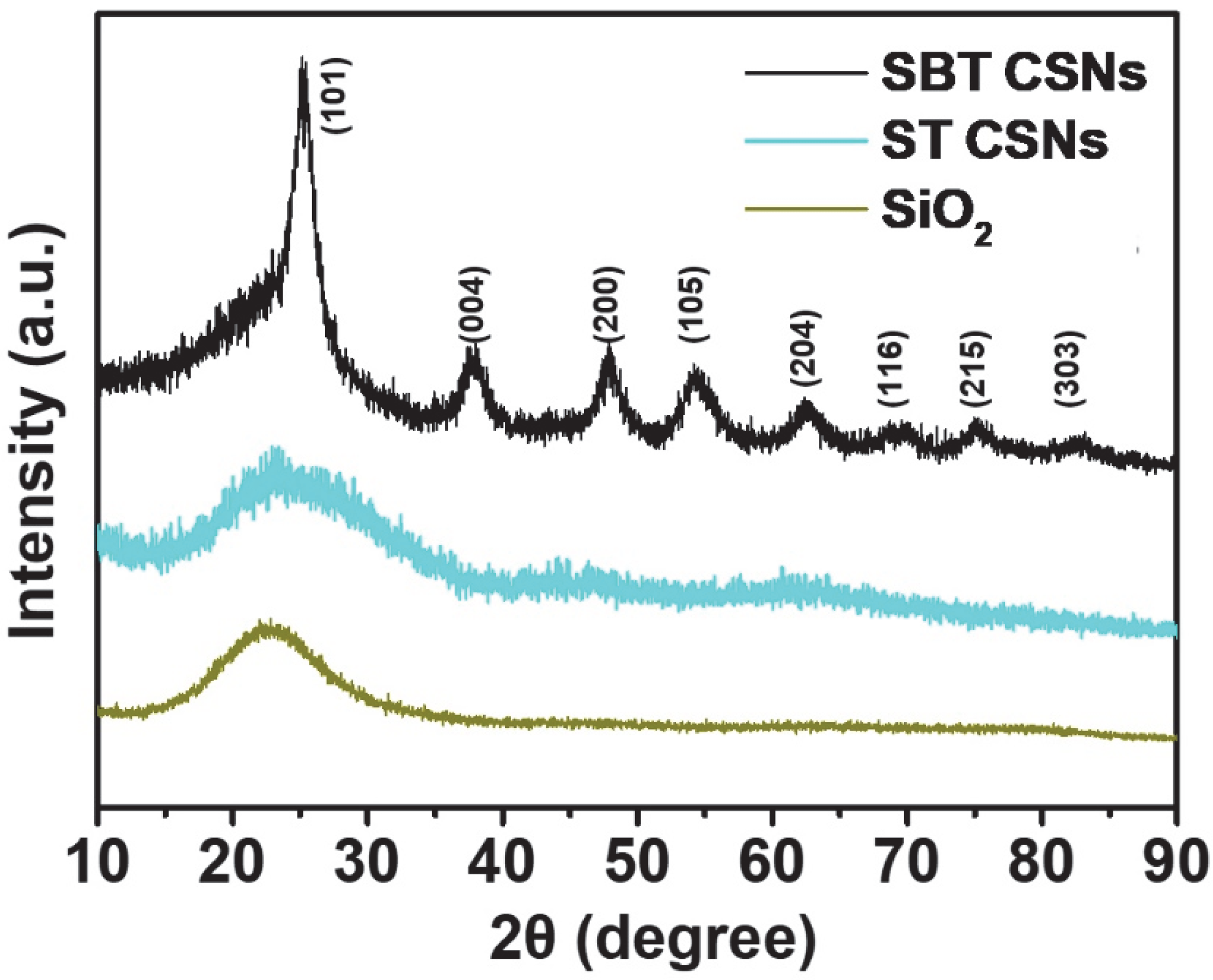
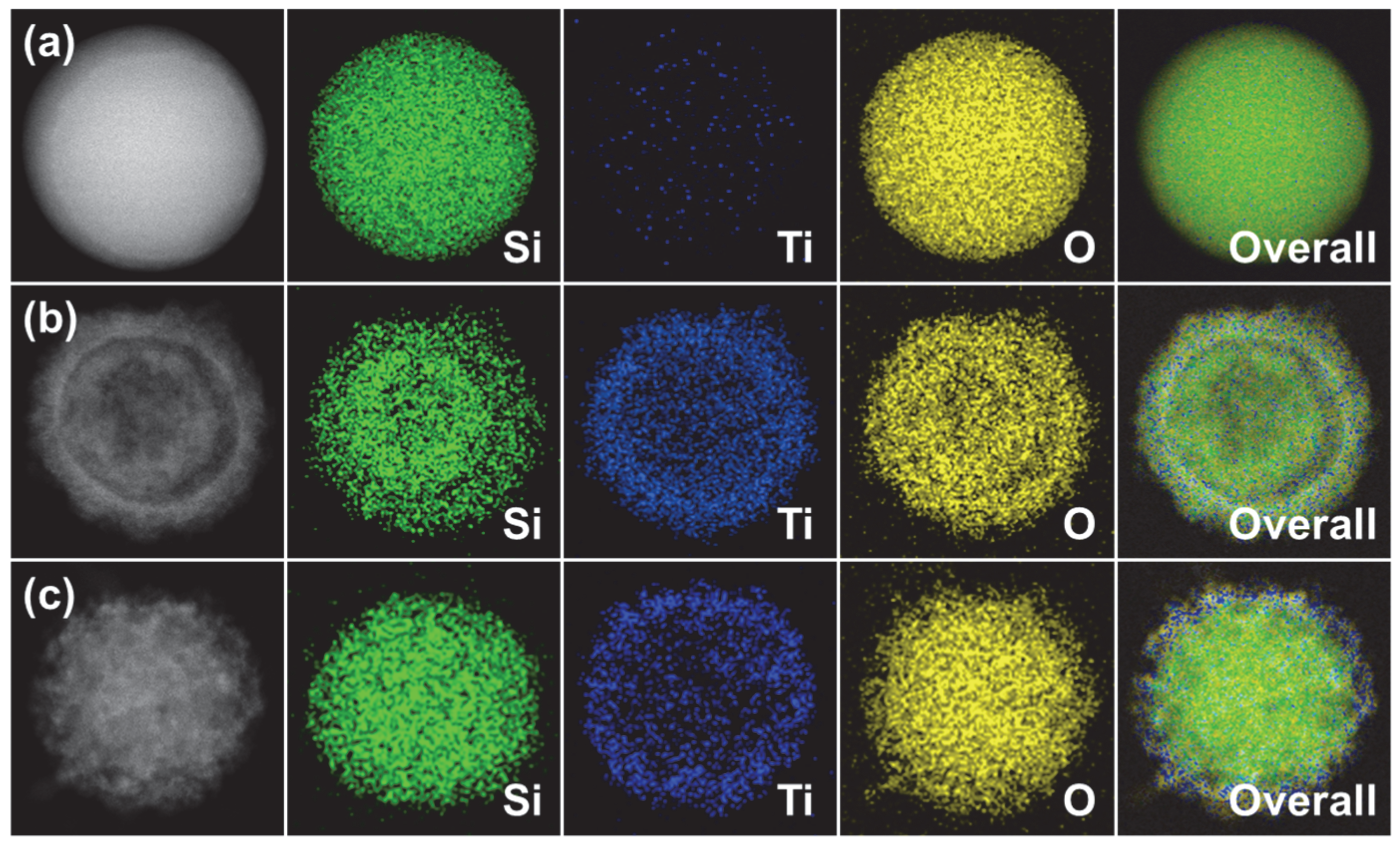
3.1. Characterization of SBT CSNs
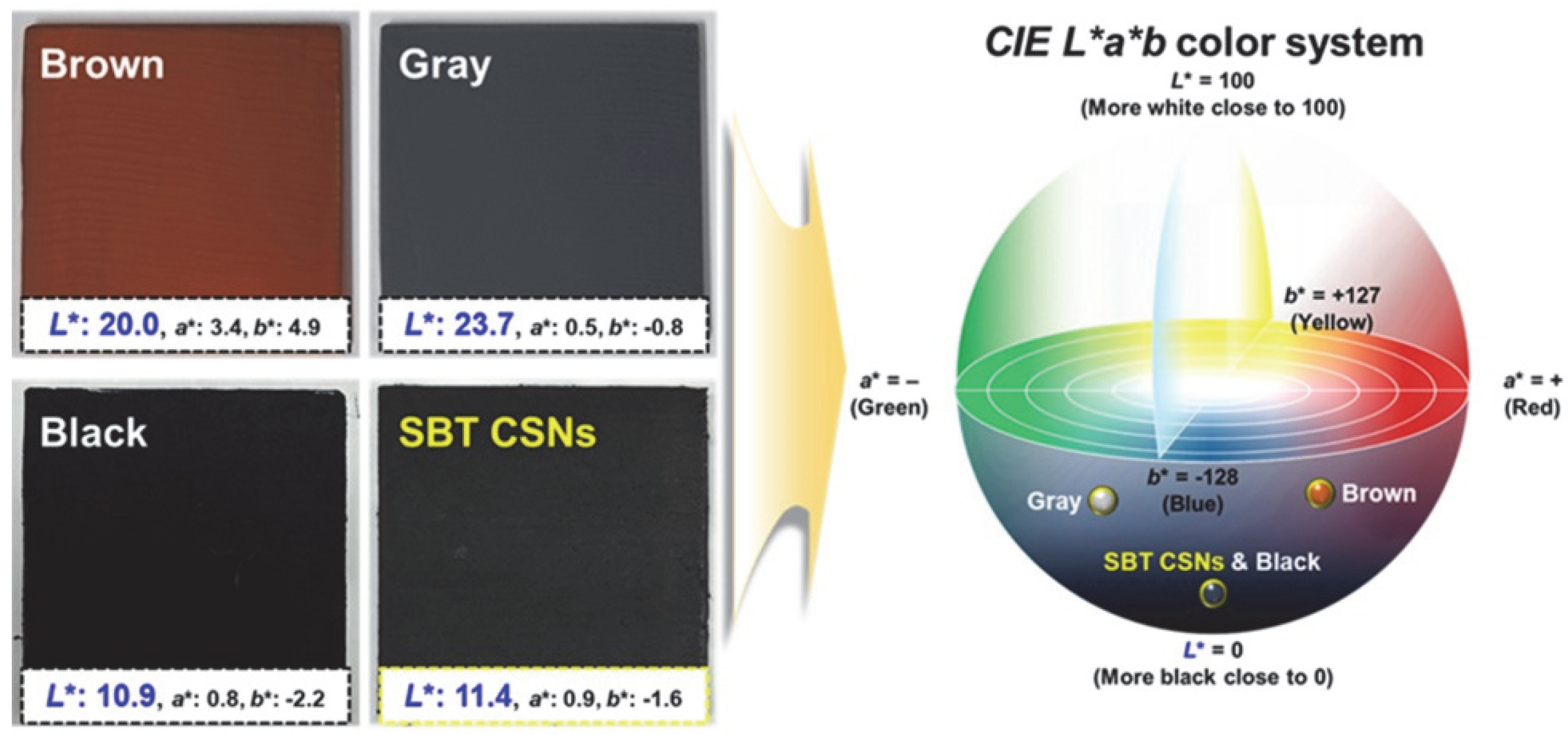
3.2. Blackness and Color of LiDAR-Detectable SBT CSNs
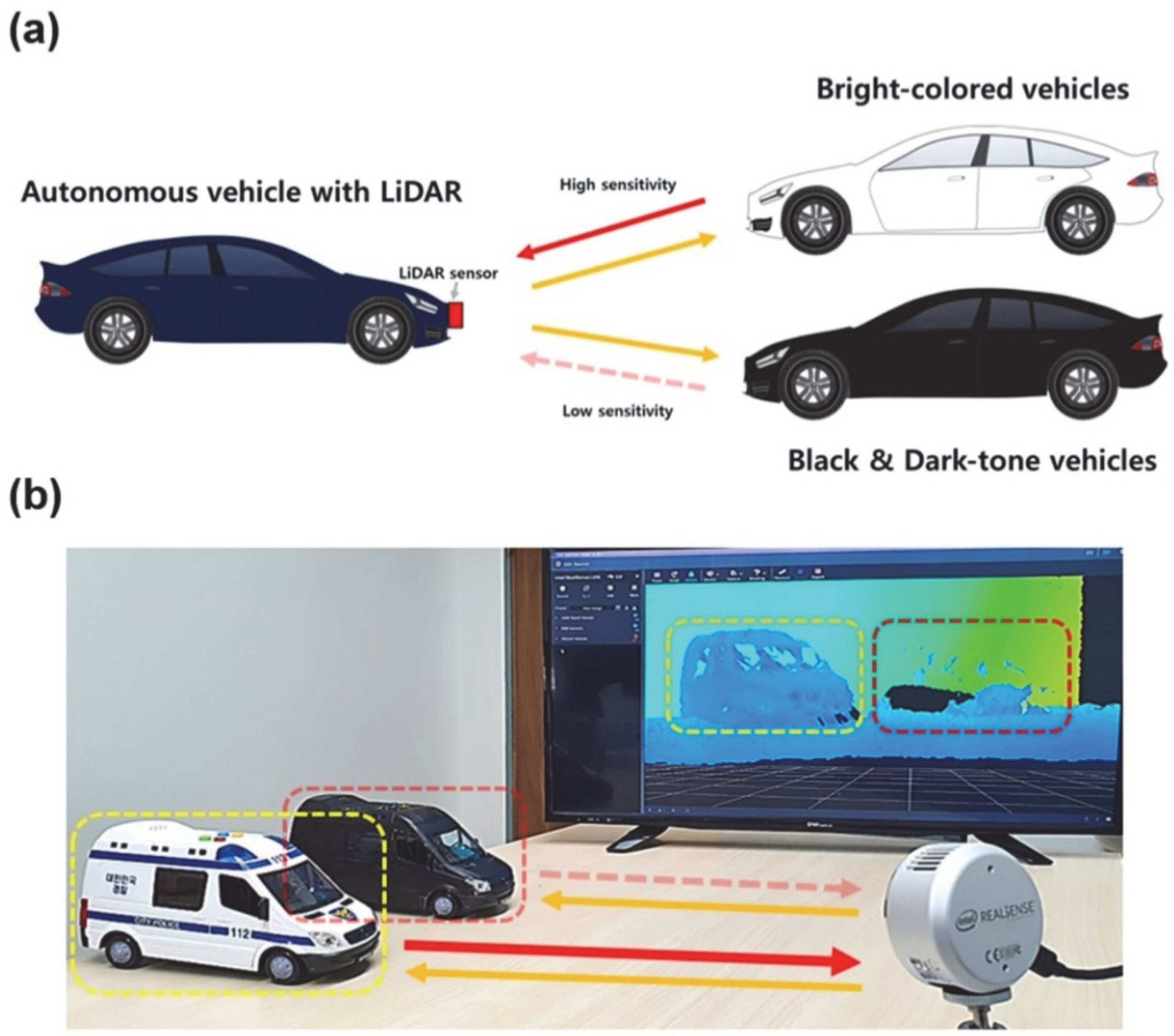
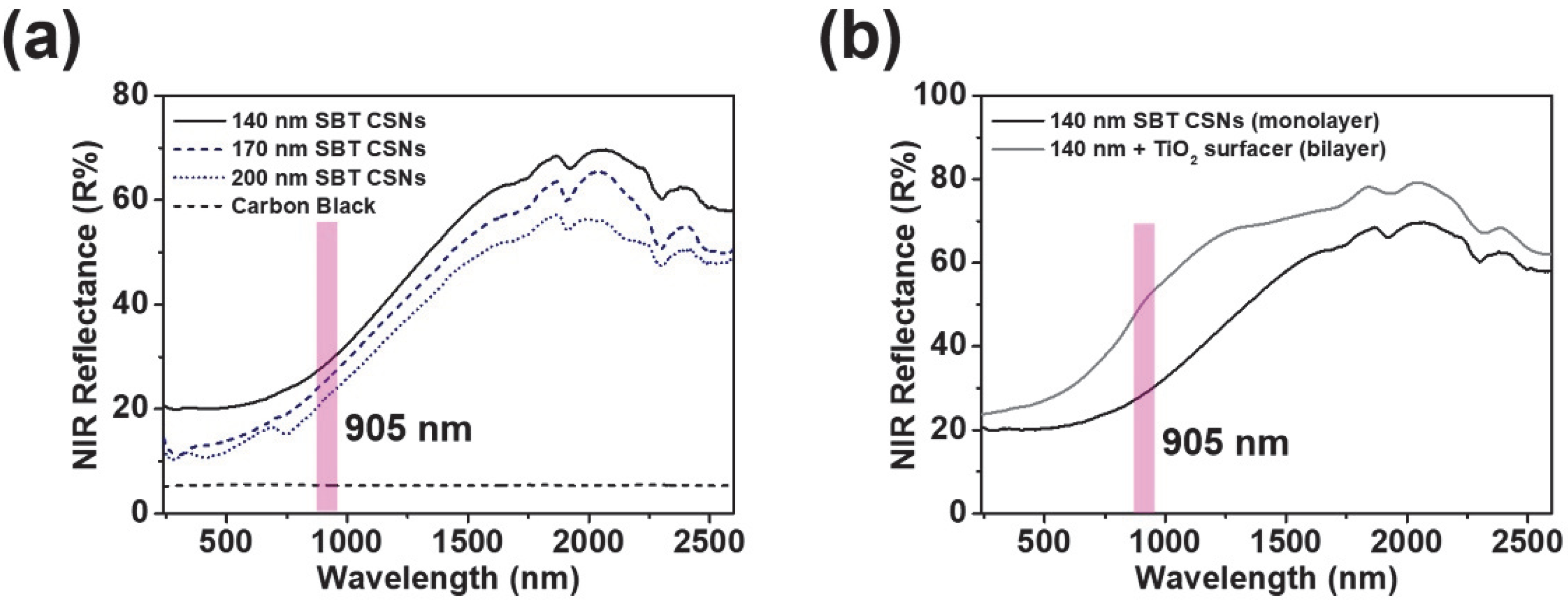
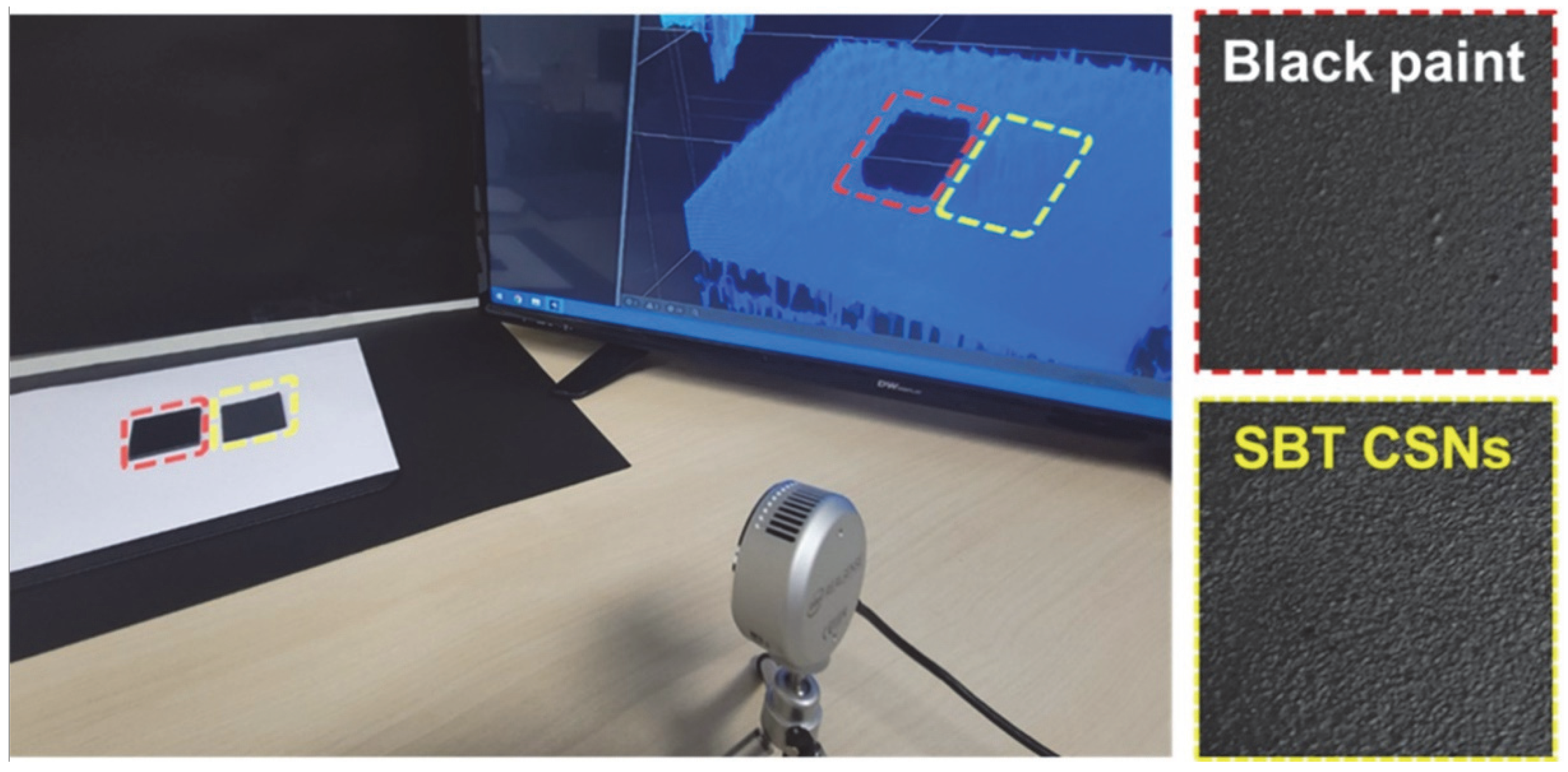
3.3. NIR Reflectance and Practical Use of SBT CSNs in LiDAR Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Brummelen, J.; O’Brien, M.; Gruyer, D.; Najjaran, H. Autonomous vehicle perception: The technology of today and tomorrow. Transp. Res. Part C Emerg. Technol. 2018, 89, 384–406. [Google Scholar] [CrossRef]
- Fayyad, J.; Jaradat, M.A.; Gruyer, D.; Najjaran, H. Deep Learning Sensor Fusion for Autonomous Vehicle Perception and Localization: A Review. Sensors 2020, 20, 4220. [Google Scholar] [CrossRef]
- Royo, S.; Ballesta-Garcia, M. An Overview of Lidar Imaging Systems for Autonomous Vehicles. Appl. Sci. 2019, 9, 4093. [Google Scholar] [CrossRef]
- Jeong, J.; Yoon, T.S.; Park, J.B. Towards a Meaningful 3D Map Using a 3D Lidar and a Camera. Sensors 2018, 18, 2571. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jung, K.Y.; Kim, Y.-S. Nanostructured Fe2O3/TiO2 composite particles with enhanced NIR reflectance for application to LiDAR detectable cool pigments. RSC Adv. 2021, 11, 16834–16840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ibanez-Guzman, J. Lidar for Autonomous Driving: The Principles, Challenges, and Trends for Automotive Lidar and Perception Systems. IEEE Signal Process. Mag. 2020, 37, 50–61. [Google Scholar] [CrossRef]
- Wu, S.; Reddy, G.K.; Banerjee, D. Pitch-Black Nanostructured Copper Oxide as an Alternative to Carbon Black for Autonomous Environments. Adv. Intell. Syst. 2021, 3, 2100049. [Google Scholar] [CrossRef]
- Bakker, I.; Van Der Voordt, T.; Vink, P.; De Boon, J.; Bazley, C. Color Preferences for Different Topics in Connection to Personal Characteristics. Color Res. Appl. 2015, 40, 62–71. [Google Scholar] [CrossRef]
- Liu, L.; Han, A.; Ye, M.; Feng, W. The Evaluation of Thermal Performance of Cool Coatings Colored with High Near-Infrared Reflective Nano-brown Inorganic Pigments: Magnesium Doped ZnFe2O4 Compounds. Sol. Energy 2015, 113, 48–56. [Google Scholar] [CrossRef]
- Sangwong, N.; Suwan, M.; Supothina, S. Effect of Calcination Temperature and Dolomite or Al2O3 Doping on Properties of NIR–Reflective CoFe2O4 Black Pigment. Mater. Today Proc. 2019, 17, 1595–1601. [Google Scholar] [CrossRef]
- Moriomoto, T.; Oka, R.; Minagawa, K.; Masui, T. Novel Near-Infrared Reflective Black Inorganic Pigment Based on Cerium Vanadate. RSC Adv. 2022, 12, 16570–16575. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Bae, S.H.; Yu, S.H.; Baek, K.-Y.; Cho, S. Near-Infrared Reflective Dark-Tone Bilayer System for LiDAR-Based Autonomous Vehicles. Macromol. Res. 2022, 30, 342–347. [Google Scholar] [CrossRef]
- Qin, J.; Qu, J.; Song, J.; Song, Z.; Zhang, W.; Shi, Y.; Zhang, T.; Xue, X.; Zhang, R.; Zhang, H.; et al. The Optical Properties of Black Coatings and Their Estimated Cooling Effect and Cooling Energy Savings Potential. J. Power Energy Eng. 2014, 2, 68–75. [Google Scholar] [CrossRef]
- Jose, S.; Prakash, A.; Laha, S.; Natarajan, S.; Reddy, M.L. Green colored nano-pigments derived from Y2BaCuO5: NIR reflective coatings. Dyes Pigm. 2014, 107, 118–126. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Ryu, J.; Yun, J.; Kim, Y.K.; Jang, J. Synthesis of Hierarchical Silica/Titania Hollow Nanoparticles and Their Enhanced Electroresponsive Activity. ACS Appl. Mater. Interfaces 2018, 10, 6570–6579. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Cho, K.H.; Jang, Y.; Kim, J.; Lee, K.; Yu, H.; Lee, S.; Jang, J. Synthesis and Electroresponse Activity of Porous Polypyrrole/Silica−Titania Core/Shell Nanoparticles. Langmuir 2018, 34, 15773–15782. [Google Scholar] [CrossRef]
- Sun, S.; Deng, T.; Ding, H.; Chen, Y.; Chen, W. Preparation of Nano-TiO2-Coated SiO2 Microsphere Composite Material and Evaluation of Its Self-Cleaning Property. Nanomaterials 2017, 7, 367. [Google Scholar] [CrossRef]
- Son, S.; Hwang, S.H.; Kim, C.; Yun, J.Y.; Jang, J. Designed Synthesis of SiO2/TiO2 Core/Shell Structure as Light Scattering Material for Highly Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 4815–4820. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.; Lee, S.; Yoon, C.-M.; Lee, I.; Jang, J. Graphene Oxide Wrapped SiO2/TiO2 Hollow Nanoparticles Loaded with Photosensitizer for Photothermal and Photodynamic Combination Therapy. Chem. Eur. J. 2017, 23, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Choi, M.; Jang, J. Nitrogen-Doped SiO2/TiO2 Core/Shell Nanoparticles as Highly Efficient Visible Light Photocatalyst. Catal. Commun. 2010, 11, 378–382. [Google Scholar] [CrossRef]
- Liao, S.; Lin, L.; Huang, J.; Jing, X.; Chen, S.; Li, Q. Microorganism-Templated Nanoarchitectonics of Hollow TiO2-SiO2 Microspheres with Enhanced Photocatalytic Activity for Degradation of Methyl Orange. Nanomaterials 2022, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Delgado, J.J.; Gil, M.L.A.; Mosquera, M.J. TiO2-SiO2 Coatings with a Low Content of AuNPs for Producing Self-Cleaning Building Materials. Nanomaterials 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.; Serrano-Garcia, R.; Govan, J.; Gun’ko, Y.K. Synthesis Characterization and Photocatalytic Studies of Cobalt Ferrite-Silica-Titania Nanocomposites. Nanomaterials 2014, 4, 331–343. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Lee, S.; Jang, J. Dual Electric and Magnetic Responsivity of Multilayered Magnetite-Embedded Core/Shell Silica/Titania Nanoparticles with Outermost Silica Shell. J. Mater. Chem. C 2018, 6, 10241–10249. [Google Scholar] [CrossRef]
- Rahulan, K.M.; Ganesan, S.; Aruna, P. Synthesis and Optical Limiting Studies of Au-Doped TiO2 Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 025012. [Google Scholar] [CrossRef]
- Redina, E.; Greish, A.; Novikov, R.; Strelkova, A.; Kirichenko, O.; Tkachenko, O.; Kapustin, G.; Sinev, I.; Kustov, L. Au/Pt/TiO2 Catalysts Prepared by Redox Method for the Chemoselective 1,2-Propanediol Oxidation to Lactic Acid and an NMR Spectroscopy Approach for Analyzing the Product Mixture. Appl. Catal. A Gen. 2015, 491, 170–183. [Google Scholar] [CrossRef]
- Jang, Y.; Yoon, C.-M.; Kim, S.; Lee, I.; Jang, J. Single/Dual Alkaline Earth Metal-Doped Hollow Nanoparticles as Nanocarrier for Accelerating Neurite Development by Activating PERK and PJNK. Part. Part. Syst. Charact. 2018, 35, 1800132. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, S.; Cheong, O.J.; Jang, J. Enhanced Electroresponse of Alkaline Earth Metal-Doped Silica/Titania Spheres by Synergetic Effect of Dispersion Stability and Dielectric Property. ACS Appl. Mater. Inter. 2015, 7, 18977–18984. [Google Scholar] [CrossRef]
- Bhambhani, M.R.; Cutting, P.A.; Sing, K.S.W.; Turk, D.H. Analysis of Nitrogen Adsorption Isotherms on Porous and Nonporous Silicas by the BET and As Methods. J. Colloid Interface Sci. 1972, 38, 109–117. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Z.; Yue, W.; Mine, S.; Toyao, T.; Matsuoka, M.; Xi, X.; Wang, L.; Zhang, J. Single-Atom High-Valent Fe(IV) for Promoted Photocatalytic Nitrogen Hydrogenation on Porous TiO2-SiO2. ACS Catal. 2021, 11, 4362–4371. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2011, 6, 27. [Google Scholar] [CrossRef]
- Rossmanith, R.; Weiss, C.K.; Geserick, J.; Hüsing, N.; Hörmann, U.; Kaiser, U.; Landfester, K. Porous Anatase Nanoparticles with High Specific Surface Area Prepared by Miniemulsion Technique. Chem. Mater. 2008, 20, 5768–5780. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-Ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.-S.; Mun, H.; Jae, H.; Moon, K.-S.; Roh, D.K. Nanosheet coated dual-shell TiO2 sphere with high solar reflectance for thermal-sheild materials. Compos. Commun. 2020, 22, 100432. [Google Scholar] [CrossRef]
- Van De Krol, R.; Goossens, A.; Meulenkamp, E.A. In Situ X-ray Diffraction of Lithium Intercalation in Nanostructured and Thin Film Anatase TiO2. J. Electrochem. Soc. 1999, 146, 3150–3154. [Google Scholar] [CrossRef]
- Larina, T.V.; Dovlitova, L.S.; Kaichev, V.V.; Malakhov, V.V.; Glazneva, T.S.; Paukshtis, E.A.; Bal’zhinimaev, B.S. Influence of the Surface Layer of Hydrated Silicon on the Stabilization of Co2+ Cations in Zr–Si Fiberglass Materials according to XPS, UV-Vis DRS, and Differential Dissolution Phase Analysis. RSC Adv. 2015, 5, 79898–79905. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-Free Rutile and Anatase TiO2 Nanocrystals as Electron Extraction Layers for High Performance Inverted Polymer Solar Cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Junior, A.G.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Cai, Z.; Huang, H.; Huang, T.; Wang, P.; Wang, X. Facile and Large-Scale Synthesis of Defective Black TiO2−x(B) Nanosheets for Efficient Visible-Light-driven Photocatalytic Hydrogen Evolution. Catalysts 2019, 9, 1048. [Google Scholar] [CrossRef]
- Taheri, M.; Jahanfar, M.; Ogino, K. Self-Cleaning Traffic Marking Paint. Surf. Interfaces 2017, 9, 13–20. [Google Scholar] [CrossRef]
- Von Pidoll, U.; Krämer, H. Flammability Characteristics of Sprays of Water-based Paints. Fire Saf. J. 1997, 29, 27–39. [Google Scholar] [CrossRef]
- Marquez, A.; Serratosa, M.P.; Merida, J. Anthocyanin evolution and color changes in red grapes during their chamber drying. J. Agr. Food. Chem. 2013, 61, 9908–9914. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, F.; Luo, M.R.; Cui, G.; Morovic, J. Color-Difference Formula for Evaluating Color Pairs with No Separation: ΔENS. J. Opt. Soc. Am. A 2019, 36, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Erfanzadeh, M.; Zhu, Q. Photoacoustic Imaging with Low-Cost Sources; A review. Photoacoustics 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.H.; Lee, J.B.; Lee, J.H. Highly Scattering Hierarchical Porous Polymer Microspheres with a High-Refractive Index Inorganic Surface for a Soft-Focus Effect. Polymers 2020, 12, 2418. [Google Scholar] [CrossRef] [PubMed]



| BET Specific Surface Area (m2∙g−1) a | Pore Size (nm) | Pore Volume (cm3∙g−1) b | |
|---|---|---|---|
| 100 nm SiO2 | 74.1 m2∙g−1 | - | 0.28 cm3∙g−1 |
| 120 nm SiO2 | 63.0 m2∙g−1 | - | 0.25 cm3∙g−1 |
| 140 nm SiO2 | 45.6 m2∙g−1 | - | 0.23 cm3∙g−1 |
| 130 nm ST CSNs | 262.2 m2∙g−1 | 2.8 nm | 0.56 cm3∙g−1 |
| 160 nm ST CSNs | 210.7 m2∙g−1 | 2.9 nm | 0.48 cm3∙g−1 |
| 190 nm ST CSNs | 198.2 m2∙g−1 | 2.8 nm | 0.43 cm3∙g−1 |
| 140 nm SBT CSNs | 208.8 m2∙g−1 | 2.5 and 6.7 nm | 0.38 cm3∙g−1 |
| 170 nm SBT CSNs | 178.2 m2∙g−1 | 2.6 and 7.0 nm | 0.32 cm3∙g−1 |
| 200 nm SBT CSNs | 150.3 m2∙g−1 | 2.6 and 7.1 nm | 0.29 cm3∙g−1 |
| Material | Ratio of Ti Species a | |
|---|---|---|
| Ti4+ | Ti3+ | |
| 130 nm ST CSNs | 95 | 5 |
| 160 nm ST CSNs | 96 | 4 |
| 190 nm ST CSNs | 94 | 6 |
| 140 nm SBT CSNs | 83 | 17 |
| 170 nm SBT CSNs | 81 | 19 |
| 200 nm SBT CSNs | 77 | 23 |
| Base Material (Layer System) | Reflectance at 905 nm (R%) |
|---|---|
| 140 nm SBT CSNs (monolayer) | 28.2 |
| 170 nm SBT CSNs (monolayer) | 25.2 |
| 200 nm SBT CSNs (monolayer) | 22.1 |
| 140 nm SBT CSNs/TiO2 surfacer (bilayer) | 50.2 |
| Commercial carbon black (black, monolayer) | 5.4 |
| Materials | NIR Reflectance | CIE L*a*b* System a | Layer System | Drawbacks | Ref | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| SBT CSNs b | 28.2 R% (905 nm) | 11.4 | 0.9 | −1.6 | Monolayer | – | Present work |
| Carbon black | 5.4 R% (905 nm) | 7.2 | 0.96 | 1.12 | – | – | – |
| Mg-doped ZnFeO4 | 51 R% (700–2500 nm) | 49.01 | 14.58 | 9.68 | Monolayer | High L* value | [9] |
| Al2O3-doped CoFe2O4 | 24.5 R% (700–2500 nm) | 23.09 | 0.66 | −0.88 | Monolayer | Low NIR reflectance | [10] |
| Ce1−xGdxVO4 | 66.3 R% (700–2500 nm) | 22.2 | 5.71 | 2.77 | Monolayer | Heavy metal included | [11] |
| Perylene-derived | 67.71 R% (905 nm) | 28.88 | 4.6 | −1.1 | Bilayer | Low productivity | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jekal, S.; Kim, J.; Kim, D.-H.; Noh, J.; Kim, M.-J.; Kim, H.-Y.; Kim, M.-S.; Oh, W.-C.; Yoon, C.-M. Synthesis of LiDAR-Detectable True Black Core/Shell Nanomaterial and Its Practical Use in LiDAR Applications. Nanomaterials 2022, 12, 3689. https://doi.org/10.3390/nano12203689
Jekal S, Kim J, Kim D-H, Noh J, Kim M-J, Kim H-Y, Kim M-S, Oh W-C, Yoon C-M. Synthesis of LiDAR-Detectable True Black Core/Shell Nanomaterial and Its Practical Use in LiDAR Applications. Nanomaterials. 2022; 12(20):3689. https://doi.org/10.3390/nano12203689
Chicago/Turabian StyleJekal, Suk, Jiwon Kim, Dong-Hyun Kim, Jungchul Noh, Min-Jeong Kim, Ha-Yeong Kim, Min-Sang Kim, Won-Chun Oh, and Chang-Min Yoon. 2022. "Synthesis of LiDAR-Detectable True Black Core/Shell Nanomaterial and Its Practical Use in LiDAR Applications" Nanomaterials 12, no. 20: 3689. https://doi.org/10.3390/nano12203689
APA StyleJekal, S., Kim, J., Kim, D.-H., Noh, J., Kim, M.-J., Kim, H.-Y., Kim, M.-S., Oh, W.-C., & Yoon, C.-M. (2022). Synthesis of LiDAR-Detectable True Black Core/Shell Nanomaterial and Its Practical Use in LiDAR Applications. Nanomaterials, 12(20), 3689. https://doi.org/10.3390/nano12203689