Hollow TiO2 Nanoparticles Capped with Polarizability-Tunable Conducting Polymers for Improved Electrorheological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
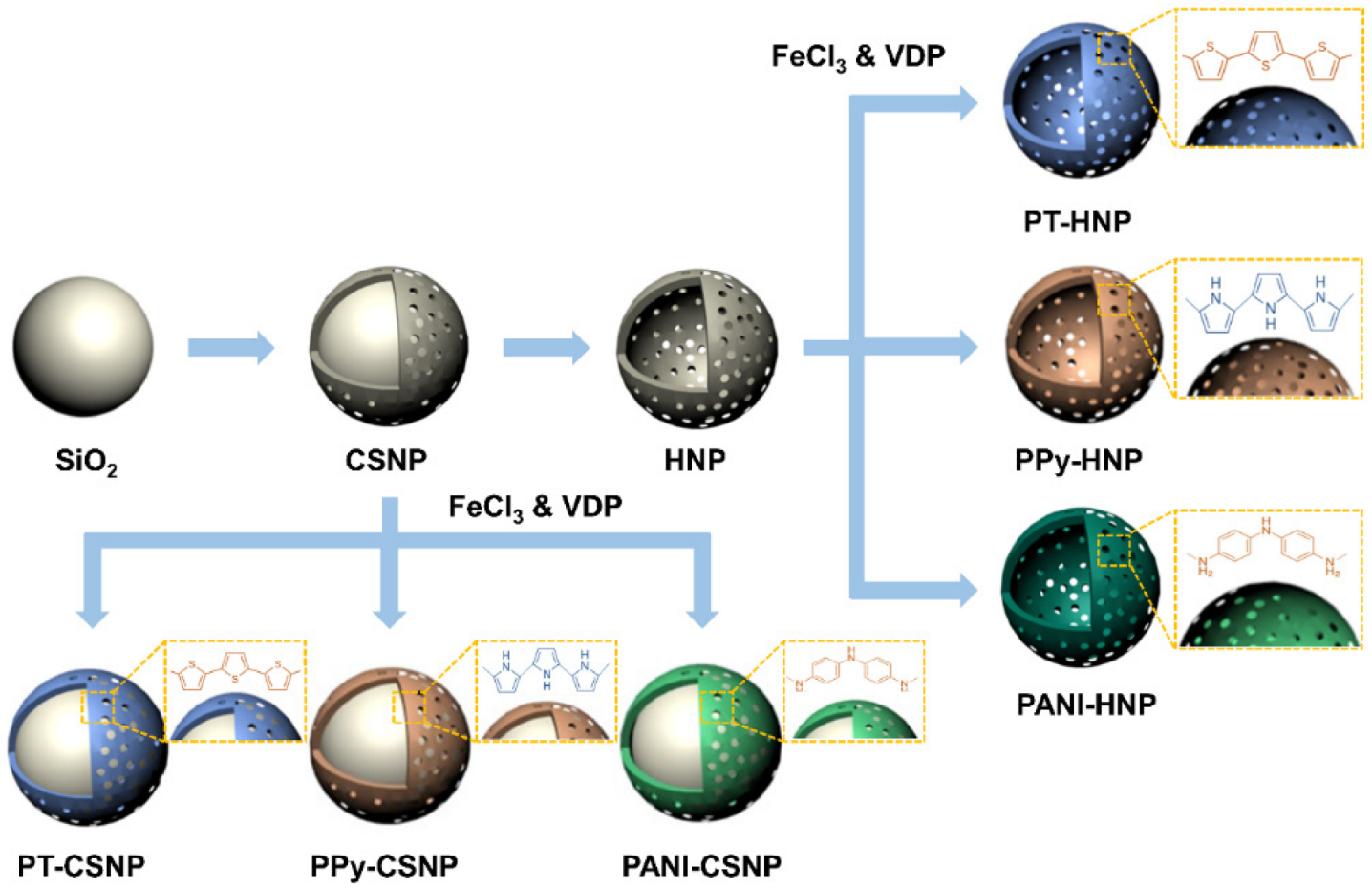
2.2. Synthesis of SiO2/TiO2 CSNPs
2.3. Fabrication of HNPs
2.4. Surface Modification with Conducting Polymers
2.5. Characterization
2.6. Investigation of ER Properties
3. Results and Discussion
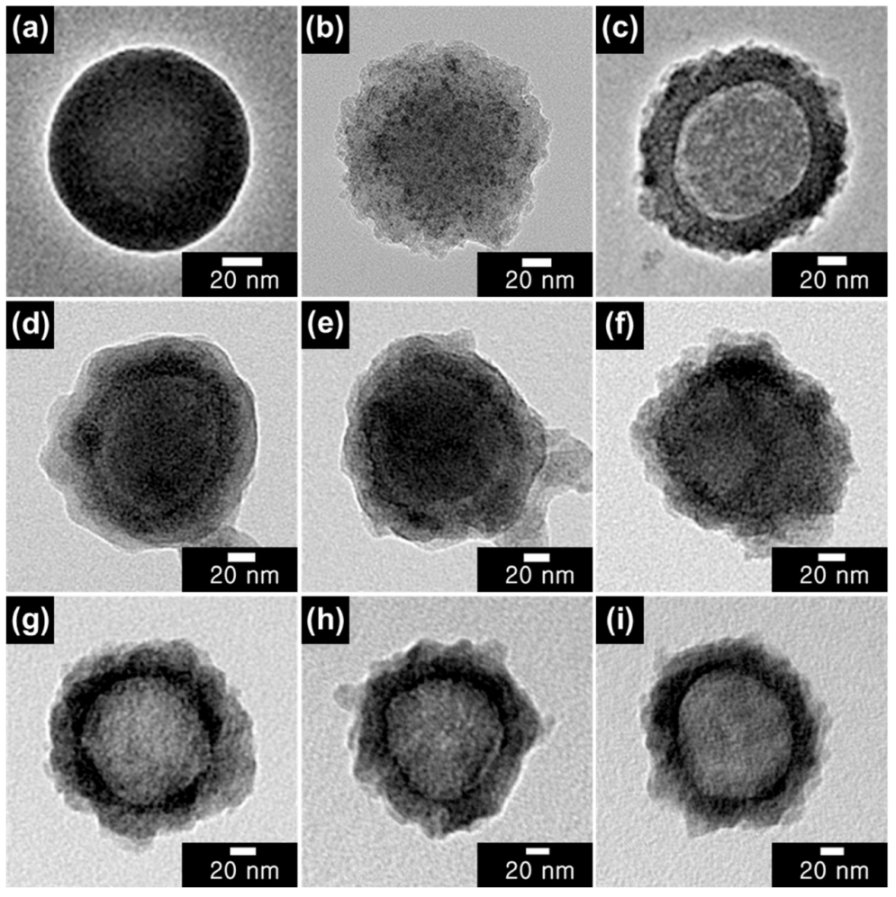
3.1. Fabrication of Various Conducting Polymer-Capped CSNPs and HNPs
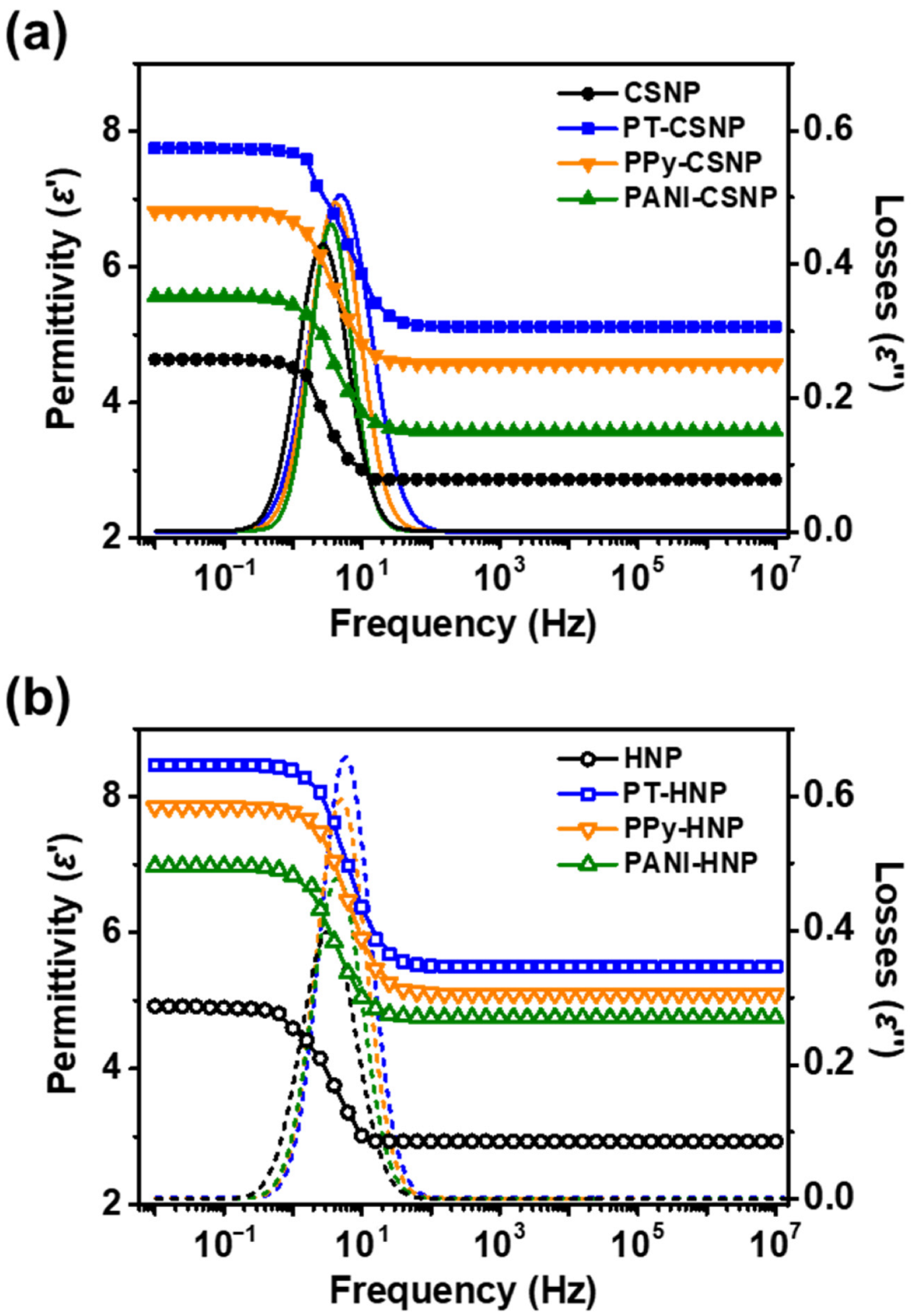
3.2. Investigation of ER Properties of Various Conducting Polymer-Capped CSNPs and HNPs
3.3. Effect of Physical and Electrical Parameters on ER Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSNPs | SiO2/TiO2 Core/Shell Nanoparticles |
| ELS | Electrophoretic Light Scattering |
| ER | Electrorheology |
| FTIR | Fourier-Transform Infrared |
| HNPs | Hollow TiO2 Nanoparticles |
| NPs | Nanoparticles |
| PANI | Polyaniline |
| PANI-CSNPs | Polyaniline-capped SiO2/TiO2 Core/Shell Nanoparticles |
| PANI-HNPs | Polyaniline-capped Hollow TiO2 Nanoparticles |
| PPy | Polypyrrole |
| PPy-CSNPs | Polypyrrole-capped SiO2/TiO2 Core/Shell Nanoparticles |
| PPy-HNPs | Polypyrrole-capped Hollow TiO2 Nanoparticles |
| PT | Polythiophene |
| PT-CSNPs | Polythiophene-capped SiO2/TiO2 Core/shell Nanoparticles |
| PT-HNPs | Polythiophene-capped Hollow TiO2 Nanoparticles |
| SEM | Scanning Electron Microscopy |
| SMER | Sonication-Mediated Etching and Redeposition |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate |
| TTIP | Titanium Isopropoxide |
| VDP | Vapor Deposition Polymerization |
References
- Halsey, T.C. Electrorheological Fluids. Science 1992, 258, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Hao, T. Electrorheological Fluids. Adv. Mater. 2001, 13, 1847–1857. [Google Scholar] [CrossRef]
- Kutalkova, E.; Mrlik, M.; IIcikova, M.; Osicka, J.; Sedlacik, M.; Mosnacek, J. Enhanced and Tunable Electrorheological Capability using Surface Initiated Atom Transfer Radical Polymerization Modification with Simultaneous Reduction of the Graphene Oxide by Silyl-Based Polymer Grafting. Nanomaterials 2019, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Chotpattananont, D.; Sirivat, A.; Jamieson, A.M. Creep and Recovery Behaviors of a Polythiophene-Based Electrorheological Fluid. Polymer 2006, 47, 3568–3575. [Google Scholar] [CrossRef]
- Noh, J.; Hong, S.; Yoon, C.-M.; Lee, S.; Jang, J. Dual External Field-Responsive Polyaniline-Coated Magnetite/Silica Nanoparticles for Smart Fluid Applications. Chem. Commun. 2017, 53, 6645–6648. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Z.; Dong, X.; Wang, Q.; Niu, C.; Han, B. Dynamic viscoelasticity and phenomenological model of electrorheological elastomers. J. Appl. Polym. Sci. 2017, 134, 45407. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, K.; Noh, J.; Lee, S.; Jang, J. Electrorheological performance of multigram-scale mesoporous silica particles with different aspect ratios. J. Mater. Chem. C 2016, 4, 1713–1719. [Google Scholar] [CrossRef]
- Jiang, J.; Tian, Y.; Meng, Y. Structure Parameter of Electrorheological Fluids in Shear Flow. Langmuir 2011, 27, 5814–5823. [Google Scholar] [CrossRef]
- Lee, S.; Noh, J.; Hong, S.; Kim, Y.K.; Jang, J. Dual Stimuli-Responsive Smart Fluid of Graphene Oxide-Coated Iron Oxide/Silica Core/Shell Nanoparticles. Chem. Mater. 2016, 28, 2624–2633. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Lee, K.; Jang, J. Enhanced Electrorheological Performance of Mixed Silica Nanomaterial Geometry. ACS Appl. Mater. Interfaces 2017, 9, 36358–36367. [Google Scholar] [CrossRef]
- Cho, M.S.; Cho, Y.H.; Choi, H.J.; Jhon, M.S. Synthesis and Electrorheological Characteristics of Polyaniline-Coated Poly(Methyl Methacrylate) Microsphere: Size Effect. Langmuir 2003, 19, 5875–5881. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Kwon, E.; Jang, J. Fabrication of Silica/Polythiophene Core/Shell Nanospheres and Their Electrorheological Fluid Application. Soft Matter 2009, 5, 951–953. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, C.-M.; Hong, J.-Y.; Jang, J. Enhanced electrorheological performance of a graphene oxide-wrapped silica rod with a high aspect ratio. J. Mater. Chem. C 2014, 2, 6010–6016. [Google Scholar] [CrossRef]
- Wereley, N.M.; Lindler, J.; Rosenfeld, N.; Choi, Y.-T. Biviscous Damping Behavior in Electrorheological Shock Absorbers. Smart Mater. Struct. 2004, 13, 743–752. [Google Scholar] [CrossRef]
- Djokoto, S.S.; Dragasius, E.; Jurenas, V.; Agelin-Chaab, M. Controlling of Vibrations in Micro-Cantilever Beam Using a Layer of Active Electrorheological Fluid Support. IEEE Sens. J. 2020, 20, 4072–4079. [Google Scholar] [CrossRef]
- Han, Y.-M.; Kang, P.-S.; Sung, K.-G.; Choi, S.-B. Force Feedback Control of a Medical Haptic Master Using an Electrorheological Fluid. J. Intell. Mater. Syst. Struct 2007, 18, 1149–1154. [Google Scholar] [CrossRef]
- Wen, W.; Huang, X.; Sheng, P. Electrorheological Fluids: Structures and Mechanisms. Soft Matter 2008, 4, 200–210. [Google Scholar] [CrossRef]
- Liu, Y.D.; Choi, H.J. Electrorheological Fluids: Smart Soft Matter and Characteristics. Soft Matter 2012, 8, 11961–11978. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.-M.; Jang, J. Enhanced Electrorheological Activity of Polyaniline Coated Mesoporous Silica with High Aspect Ratio. J. Colloid Interface Sci. 2016, 470, 237–244. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Noh, J.; Jang, Y.; Jang, J. Fabrication of a Silica/Titania Hollow Nanorod and Its Electroresponsive Activity. RSC Adv. 2017, 7, 19754–19763. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, G.; Noh, J.; Lee, C.; Cheong, O.J.; Jang, J. A Comparative Study of the Electrorheological Properties of Various N-Doped Nanomaterials Using Ammonia Plasma Treatment. Chem. Commun. 2016, 52, 4808–4811. [Google Scholar] [CrossRef] [PubMed]
- Zygo, M.; Mrlik, M.; IIcikova, M.; Hrabalikova, M.; Osicka, J.; Cvek, M.; Sedlacik, M.; Hanulikova, B.; Munster, L.; Skoda, D.; et al. Effect of Structure of Polymers Grafted from Graphene Oxide on the Compatibility of Particles with a Silicone-Based Environment and the Stimuli-Responsive Capabilities of Their Composites. Nanomaterials 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Jang, J. Smart Fluid System Dually Responsive to Light and Electric Fields: An Electrophotorheological Fluid. ACS Nano 2017, 11, 9789–9801. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Mrlík, M.; Plachý, T.; Trchová, M.; Kovářová, J.; Li, Y. Molybdenum and Tungsten Disulfides Surface-Modified with a Conducting Polymer, Polyaniline, for Application in Electrorheology. React. Funct. Polym. 2017, 120, 30–37. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Choi, K.; Kwon, S.H.; Nam, J.-D.; Choi, H.J. Nanoparticles Functionalized by Conducting Polymers and Their Electrorheological and Magnetorheological Applications. Polymers 2020, 12, 204. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Lee, S.; Jang, J. Dual electric and magnetic responsivity of multilayered magnetite-embedded core/shell silica/titania nanoparticles with outermost silica shell. J. Mater. Chem. C 2018, 6, 10241–10249. [Google Scholar] [CrossRef]
- Fang, F.F.; Kim, J.H.; Choi, H.J.; Seo, Y. Organic/inorganic hybrid of polyaniline/BaTiO3 composites and their electrorheological and dielectric characteristics. J. Appl. Polym. Sci. 2007, 105, 1853–1860. [Google Scholar] [CrossRef]
- Bessergenev, V.G.; Mariano, J.F.; Mateus, M.C.; Lourenço, J.P.; Ahmed, A.; Hantusch, M.; Burkel, E.; Botelho Do Rego, A.M. Dielectric Properties and Spectral Characteristics of Photocatalytic Constant of TiO2 Nanoparticles Doped with Cobalt. Nanomaterials 2021, 11, 2519. [Google Scholar] [CrossRef]
- Wang, B.-X.; Zhao, Y.; Zhao, X.-P. The Wettability, Size Effect and Electrorheological Activity of Modified Titanium Oxide Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 27–33. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Preparation and Enhanced Electrorheological Activity of TiO2 Doped with Chromium Ion. Chem. Mater. 2004, 16, 321–328. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, S.; Cheong, O.J.; Jang, J. Enhanced Electroresponse of Alkaline Earth Metal-Doped Silica/Titania Spheres by Synergetic Effect of Dispersion Stability and Dielectric Property. ACS Appl. Mater. Interfaces 2015, 7, 18977–18984. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Effect of Dispersion Stability on Electrorheology of Water-Based ZnO Nanofluids. Energy Fuels 2016, 30, 6169–6177. [Google Scholar] [CrossRef]
- Ramos-Tejada, M.M.; Rodríguez, J.M.; Delgado, Á.V. Electrorheology of Clay Particle Suspensions. Effects of Shape and Surface Treatment. Rheol. Acta 2018, 57, 405–413. [Google Scholar] [CrossRef]
- Kwon, S.H.; Piao, S.H.; Choi, H.J. Electric Field-Responsive Mesoporous Suspensions: A Review. Nanomaterials 2015, 5, 2249–2267. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, L.; Zhao, Y.; Xing, L.; Yu, X.; Chen, G.; Zhang, J.; Che, R. Boosted Interfacial Polarization from Multishell TiO2@Fe3O4@PPy Heterojunction for Enhanced Microwave Absorption. Small 2019, 15, 1902885. [Google Scholar] [CrossRef]
- Mikie, T.; Hayakawa, M.; Okamoto, K.; Iguchi, K.; Yashiro, S.; Koganezawa, T.; Sumiya, M.; Ishii, H.; Yamaguchi, S.; Fukazawa, A.; et al. Extended π-Electron Delocalization in Quinoid-Based Conjugated Polymers Boosts Intrachain Charge Carrier Transport. Chem. Mater. 2021, 33, 8183–8193. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C.; Dissado, L.A. Electrical Aging and Life Models: The Role of Space Charge. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 876–890. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, H.J. Core-shell structured semiconducting poly(diphenylamine)-coated polystyrene microspheres and their electrorheology. Polymer 2017, 131, 120–131. [Google Scholar] [CrossRef]
- Choi, M.; Kim, C.; Jeon, S.O.; Yook, K.S.; Lee, J.Y.; Jang, J. Synthesis of titania embedded silica hollow nanospheres via sonication mediated etching and re-deposition. Chem. Commun. 2011, 47, 7092–7094. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, S.H.; Yun, J.; Jang, J. Fabrication of SiO2/TiO2 Double-Shelled Hollow Nanospheres with Controllable Size via Sol–Gel Reaction and Sonication-Mediated Etching. ACS Appl. Mater. Interfaces 2014, 6, 15420–15426. [Google Scholar] [CrossRef]
- Balachandran, K.; Venckatesh, R.; Sivaraj, R.; Rajiv, P. TiO2 nanoparticles versus TiO2–SiO2 nanocomposites: A comparative study of photo catalysis on acid red 88. Spectrochim. Acta A 2014, 128, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Hessel, C.M.; Henderson, E.J.; Veinot, J.G.C. Hydrogen Silsesquioxane: A Molecular Precursor for Nanocrystalline Si−SiO2 Composites and Freestanding Hydride-Surface-Terminated Silicon Nanoparticles. Chem. Mater. 2006, 18, 6139–6146. [Google Scholar]
- Mishra, A.K.; Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. FT-IR and XPS studies of polyurethane-urea-imide coatings. Prog. Org. Coat. 2006, 55, 231–243. [Google Scholar] [CrossRef]
- Park, I.H.; Kwon, S.H.; Choi, H.J. Emulsion-polymerized polyindole nanoparticles and their electrorheology. J. Appl. Polym. Sci. 2018, 135, 46384. [Google Scholar]
- Zhang, K.; Liu, Y.D.; Choi, H.J. Carbon nanotube coated snowman-like particles and their electro-responsive characteristics. Chem. Commun. 2012, 48, 136–138. [Google Scholar] [CrossRef]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Core−Shell Structured Semiconducting PMMA/Polyaniline Snowman-like Anisotropic Microparticles and Their Electrorheology. Langmuir 2010, 26, 12849–12854. [Google Scholar] [PubMed]
- Li, Z.; Du, B.; Han, C.; Xu, H. Trap Modulated Charge Carrier Transport in Polyethylene/Graphene Nanocomposites. Sci. Rep. 2017, 7, 4015. [Google Scholar] [CrossRef]
- Hao, T.; Kawai, A.; Ikazaki, F. Mechanism of the Electrorheological Effect: Evidence from the Conductive, Dielectric, and Surface Characteristics of Water-Free Electrorheological Fluids. Langmuir 1998, 14, 1256–1262. [Google Scholar] [CrossRef]
- Starczewska, A.; Mistewicz, K.; Kozioł, M.; Zubko, M.; Stróż, D.; Dec, J. Interfacial Polarization Phenomena in Compressed Nanowires of SbSI. Materials 2022, 15, 1543. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, S.; Hong, S.H.; Jang, J. Fabrication of density-controlled graphene oxide-coated mesoporous silica spheres and their electrorheological activity. J. Colloid Interface Sci. 2015, 438, 14–21. [Google Scholar] [CrossRef]
- Asami, K. Characterization of heterogeneous systems by dielectric spectroscopy. Prog. Polym. Sci. 2002, 27, 1617–1659. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Cho, K.H.; Jang, Y.; Kim, J.; Lee, K.; Yu, H.; Lee, S.; Jang, J. Synthesis and Electroresponse Activity of Porous Polypyrrole/Silica−Titania Core/Shell Nanoparticles. Langmuir 2018, 34, 15773–15782. [Google Scholar] [CrossRef] [PubMed]
- Gwon, H.; Park, S.; Lee, S. Ecoresorbable smart fluids with controlled electroresponsive properties by various metal doping. J. Mater. Chem. C 2020, 8, 15751–15758. [Google Scholar] [CrossRef]
- Kurimoto, M.; Ozaki, H.; Sawada, T.; Kato, T.; Funabashi, T.; Suzuoki, Y. Filling Ratio Control of TiO2 and SiO2 in Epoxy Composites for Permittivity-Graded Insulator with Low Coefficient of Thermal Expansion. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1112–1120. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Ryu, J.; Yun, J.; Kim, Y.K.; Jang, J. Synthesis of Hierarchical Silica/Titania Hollow Nanoparticles and Their Enhanced Electroresponsive Activity. ACS Appl. Mater. Interfaces 2018, 10, 6570–6579. [Google Scholar] [CrossRef]
- Grimm, A.; Nowak, C.; Hoffmann, J.; Schartl, W. Electrophoretic Mobility of Gold Nanoparticles in Thermoresponsive Hydrogels. Macromolecules 2009, 42, 6231–6238. [Google Scholar] [CrossRef]
- Lu, Q.; Han, W.J.; Choi, H.J. Smart and Functional Conducting Polymers: Application to Electrorheological Fluids. Molecules 2018, 23, 2854. [Google Scholar] [CrossRef]


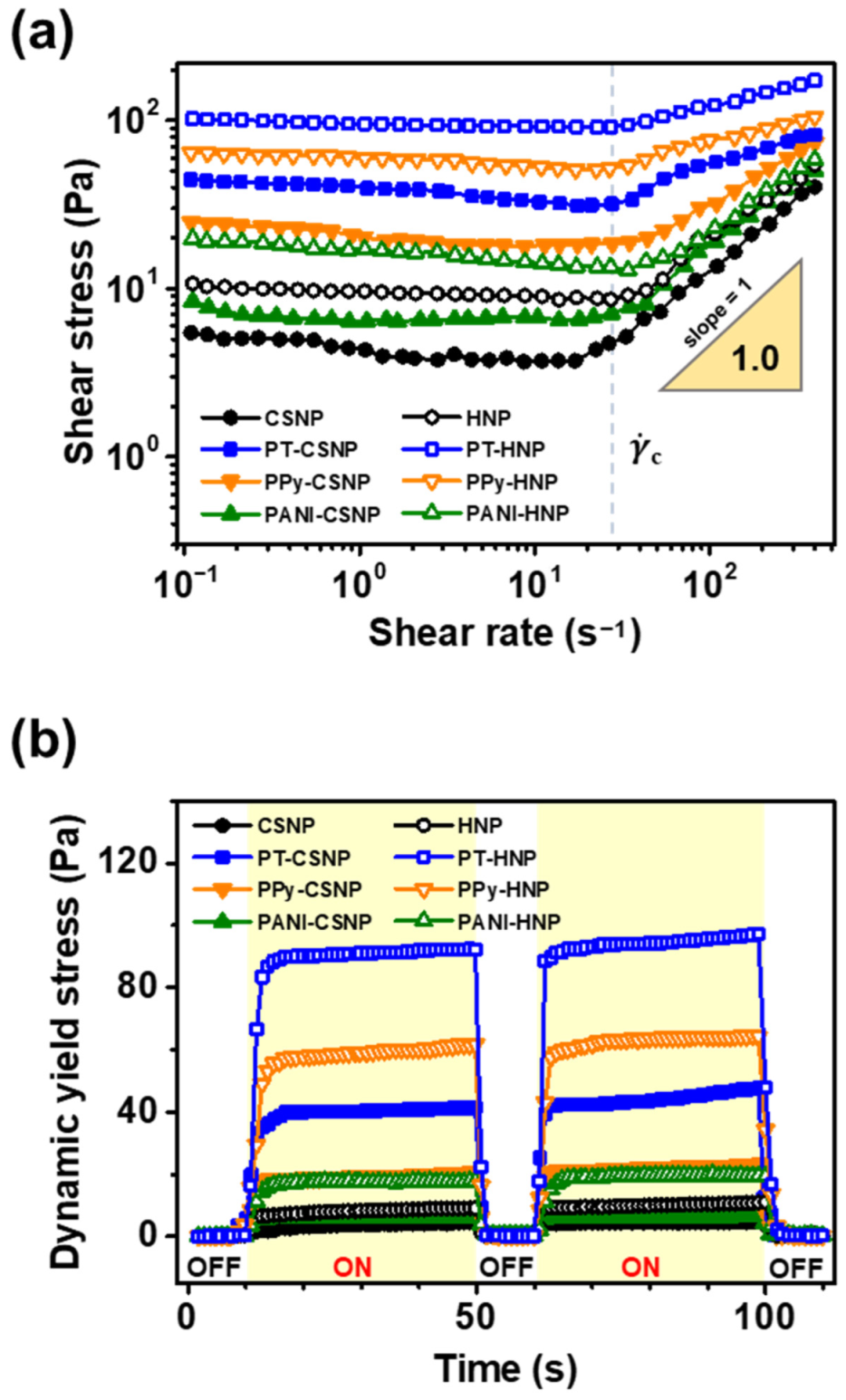
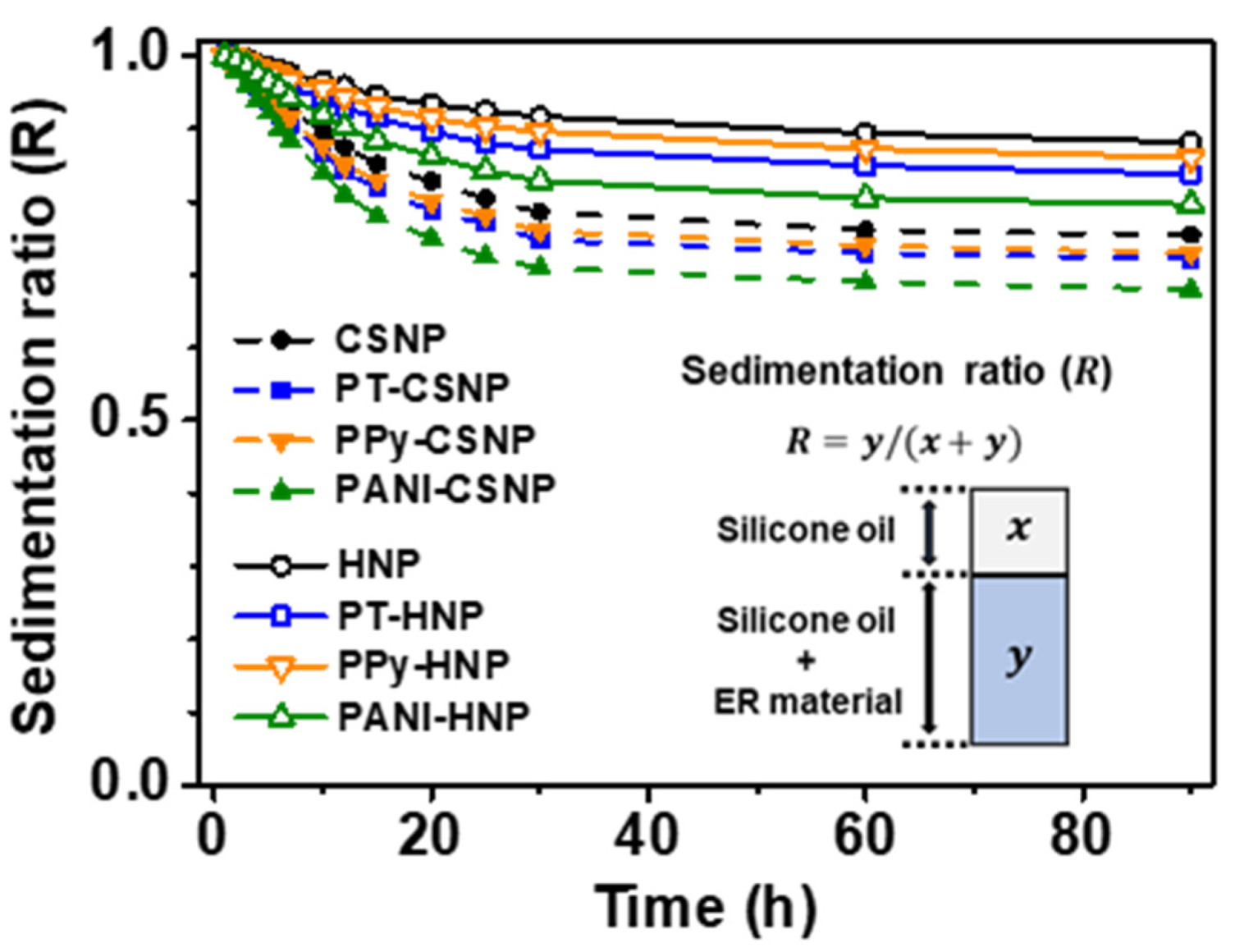
| Sample | Yield Stress (Pa) |
|---|---|
| SiO2/TiO2 CSNPs | 4.6 ± 0.1 |
| PT-CSNPs | 42.2 ± 0.6 |
| PPy-CSNPs | 21.0 ± 0.5 |
| PANI-CSNPs | 6.3 ± 0.1 |
| TiO2 HNPs | 9.8 ± 0.4 |
| PT-HNPs | 94.2 ± 0.9 |
| PPy-HNPs | 62.6 ± 0.7 |
| PANI-HNPs | 19.0 ± 0.4 |
| Sample | (Hz) | (ms) | |||
|---|---|---|---|---|---|
| SiO2/TiO2 CSNPs | 4.63 ± 0.05 | 2.85 ± 0.04 | 1.78 ± 0.04 | 2.44 ± 0.08 | 65.2 ± 2.2 |
| PT-CSNPs | 7.71 ± 0.07 | 5.14 ± 0.06 | 2.57 ± 0.06 | 4.36 ± 0.13 | 36.5 ± 1.1 |
| PPy-CSNPs | 6.78 ± 0.08 | 4.56 ± 0.06 | 2.22 ± 0.07 | 3.69 ± 0.10 | 43.1 ± 1.2 |
| PANI-CSNPs | 5.60 ± 0.06 | 3.56 ± 0.06 | 2.04 ± 0.06 | 3.19 ± 0.11 | 49.9 ± 1.7 |
| TiO2 HNPs | 4.88 ± 0.08 | 2.97 ± 0.06 | 1.91 ± 0.07 | 2.94 ± 0.07 | 54.1 ± 1.3 |
| PT-HNPs | 8.45 ± 0.09 | 5.52 ± 0.07 | 2.93 ± 0.08 | 5.15 ± 0.15 | 30.1 ± 0.9 |
| PPy-HNPs | 7.85 ± 0.09 | 5.07 ± 0.07 | 2.78 ± 0.08 | 4.45 ± 0.12 | 35.8 ± 1.1 |
| PANI-HNPs | 6.97 ± 0.08 | 4.74 ± 0.07 | 2.23 ± 0.07 | 3.93 ± 0.10 | 40.5 ± 1.0 |
| Sample | Electrophoretic Mobility b (cm2/V∙s) | Electrical Conductivity c (S/m) |
|---|---|---|
| PT-CSNPs | 10−4 | 10−9 |
| PPy-CSNPs | 10−4 | 10−9 |
| PANI-CSNPs | 10−4 | 10−9 |
| PT-HNPs | 10−4 | 10−9 |
| PPy-HNPs | 10−4 | 10−9 |
| PANI-HNPs | 10−4 | 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Noh, J.; Jekal, S.; Kim, J.; Oh, W.-C.; Sim, H.-S.; Choi, H.-J.; Yi, H.; Yoon, C.-M. Hollow TiO2 Nanoparticles Capped with Polarizability-Tunable Conducting Polymers for Improved Electrorheological Activity. Nanomaterials 2022, 12, 3521. https://doi.org/10.3390/nano12193521
Lee S, Noh J, Jekal S, Kim J, Oh W-C, Sim H-S, Choi H-J, Yi H, Yoon C-M. Hollow TiO2 Nanoparticles Capped with Polarizability-Tunable Conducting Polymers for Improved Electrorheological Activity. Nanomaterials. 2022; 12(19):3521. https://doi.org/10.3390/nano12193521
Chicago/Turabian StyleLee, Seungae, Jungchul Noh, Suk Jekal, Jiwon Kim, Won-Chun Oh, Hyung-Sub Sim, Hyoung-Jin Choi, Hyeonseok Yi, and Chang-Min Yoon. 2022. "Hollow TiO2 Nanoparticles Capped with Polarizability-Tunable Conducting Polymers for Improved Electrorheological Activity" Nanomaterials 12, no. 19: 3521. https://doi.org/10.3390/nano12193521
APA StyleLee, S., Noh, J., Jekal, S., Kim, J., Oh, W.-C., Sim, H.-S., Choi, H.-J., Yi, H., & Yoon, C.-M. (2022). Hollow TiO2 Nanoparticles Capped with Polarizability-Tunable Conducting Polymers for Improved Electrorheological Activity. Nanomaterials, 12(19), 3521. https://doi.org/10.3390/nano12193521






