Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.3. Preparation of Electrodes
2.4. Characterization
2.5. Electrochemical Measurements
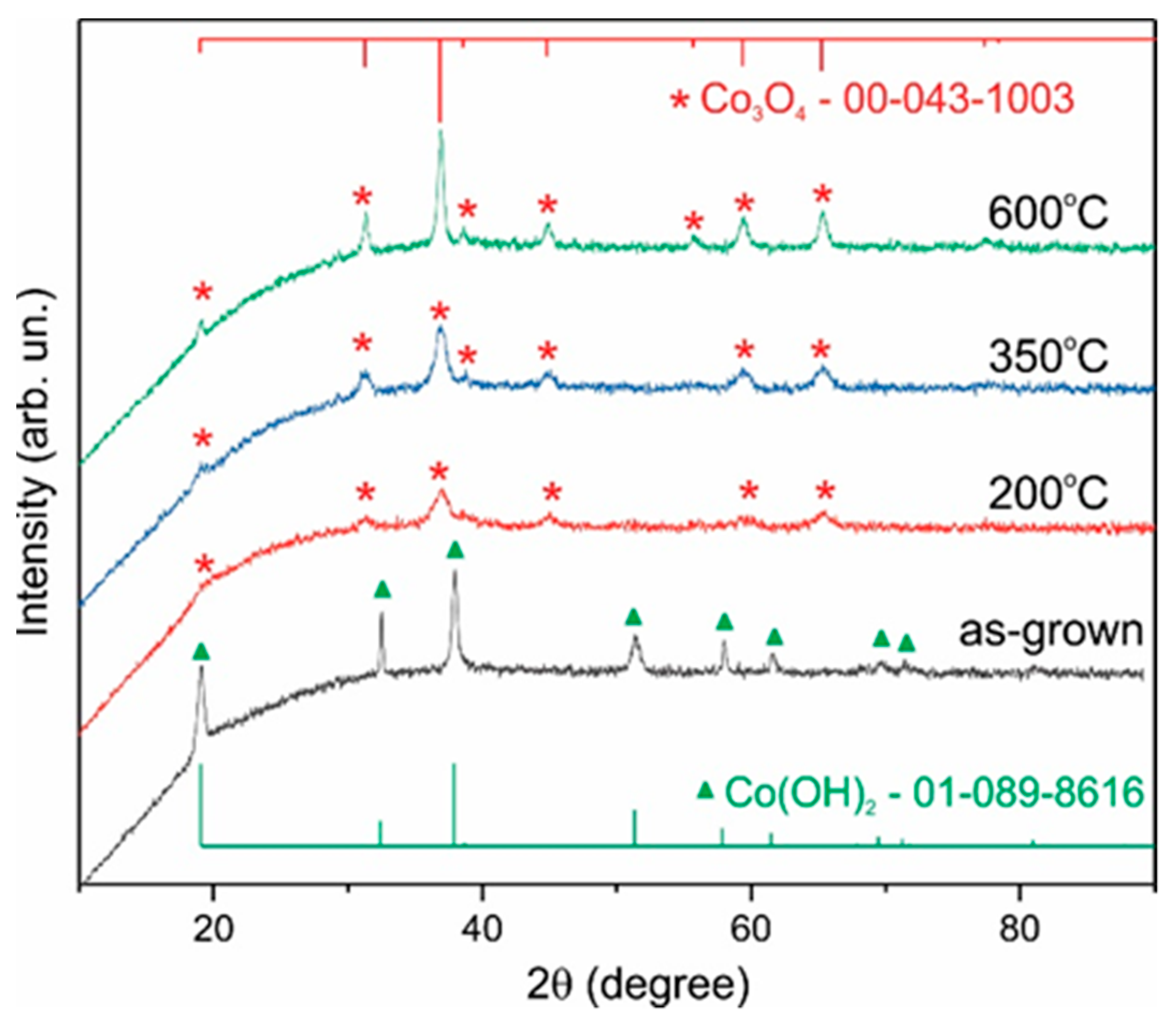
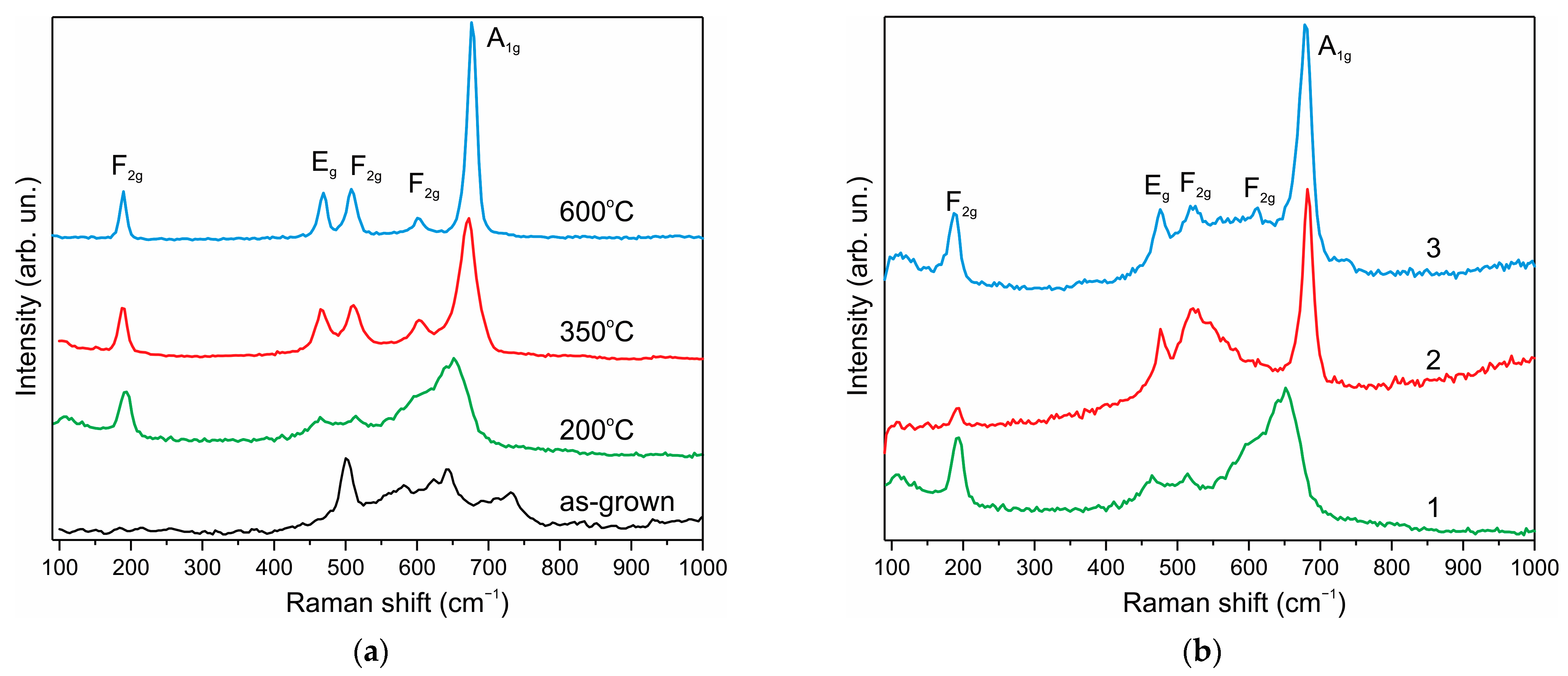
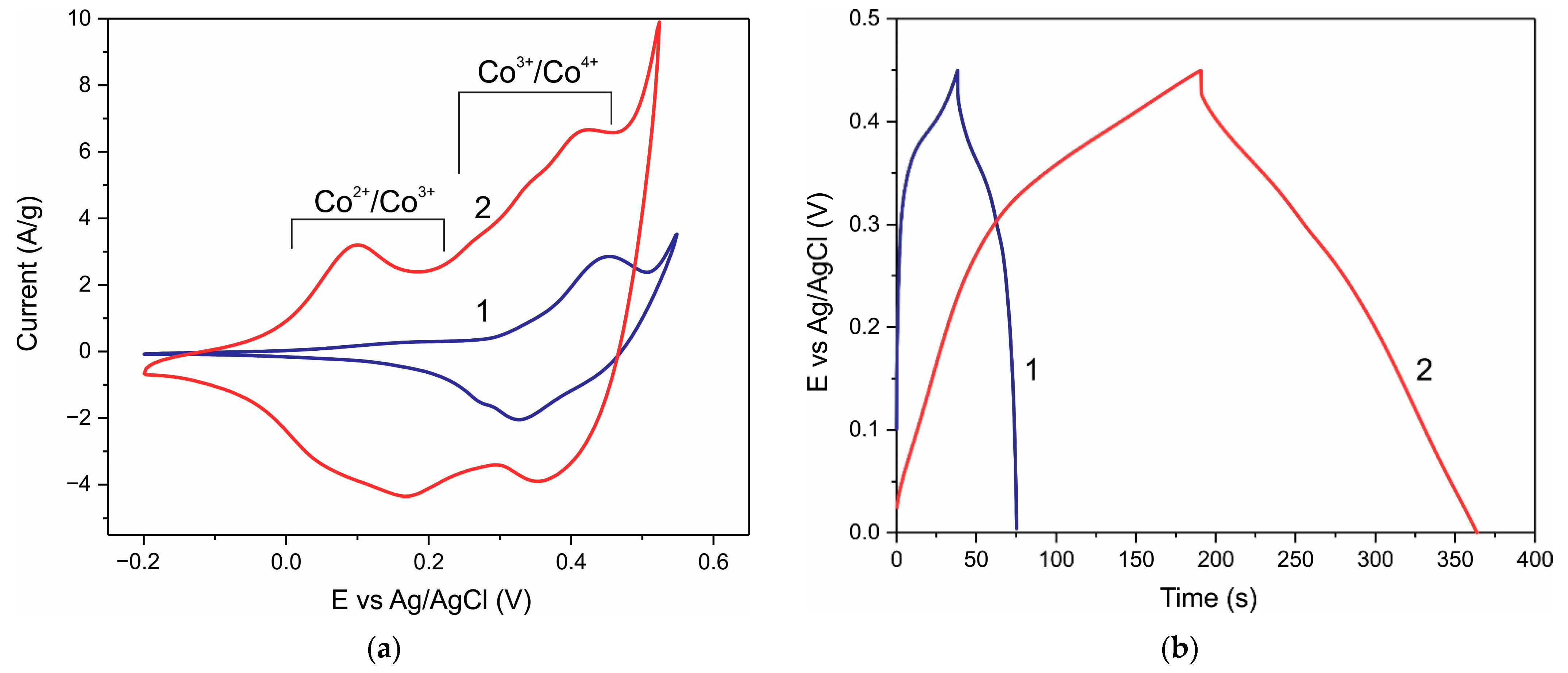
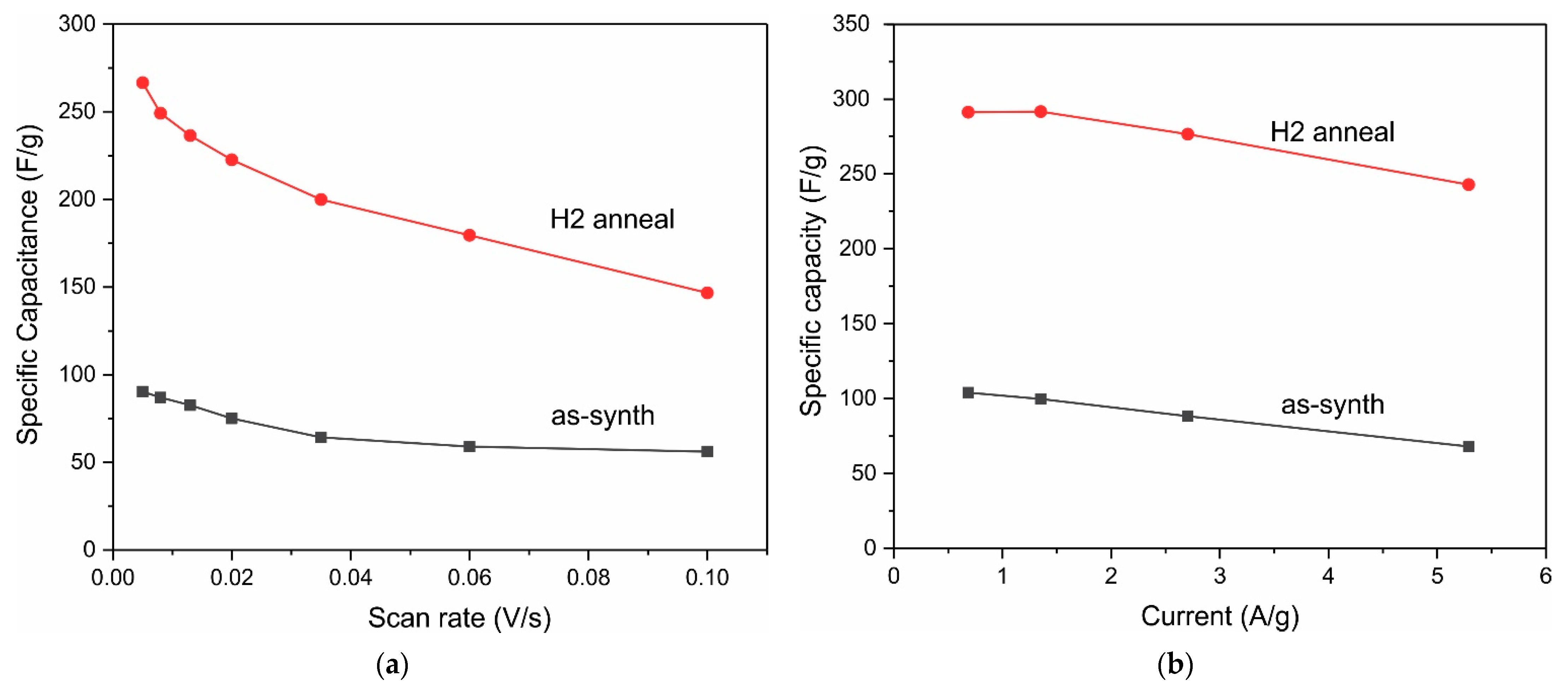
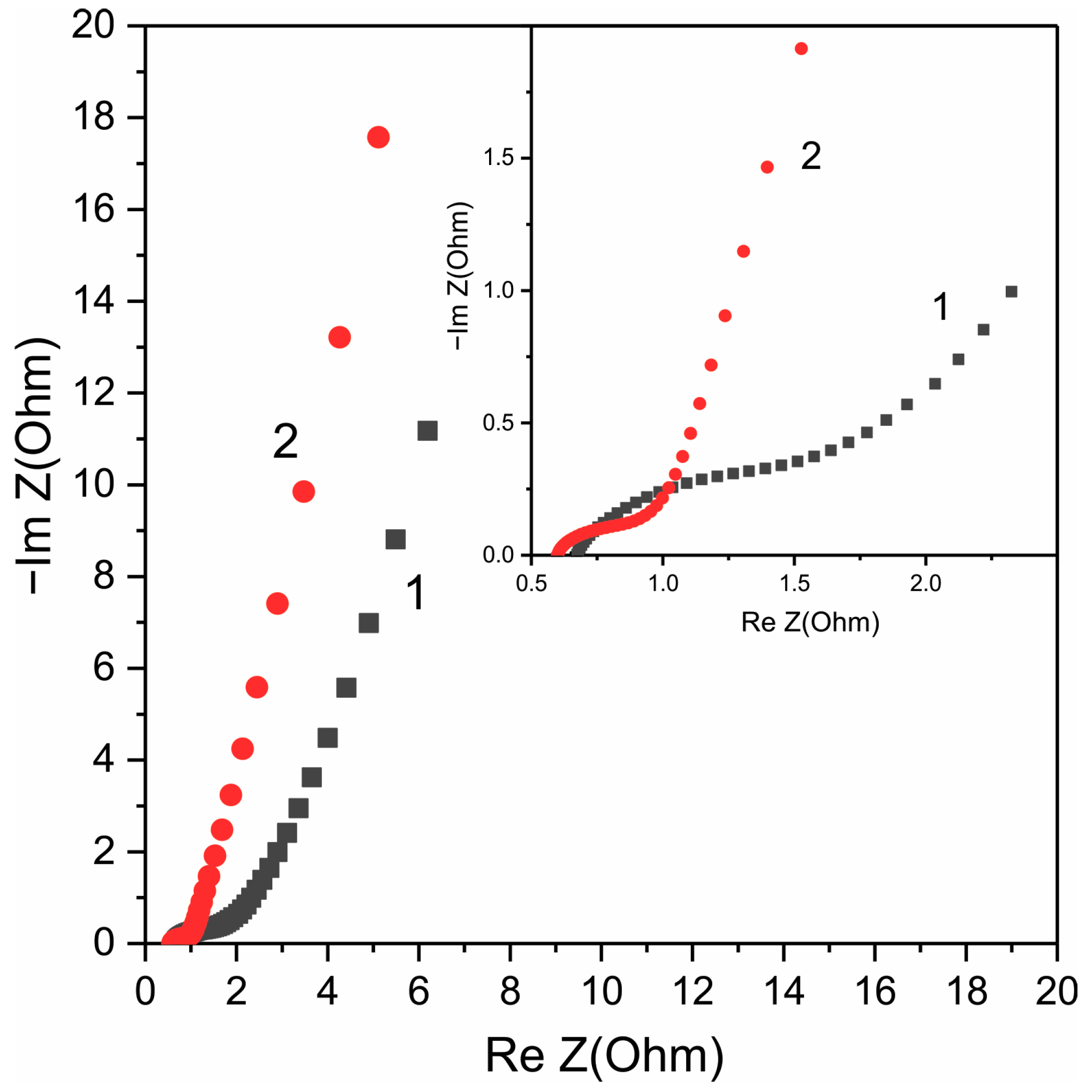
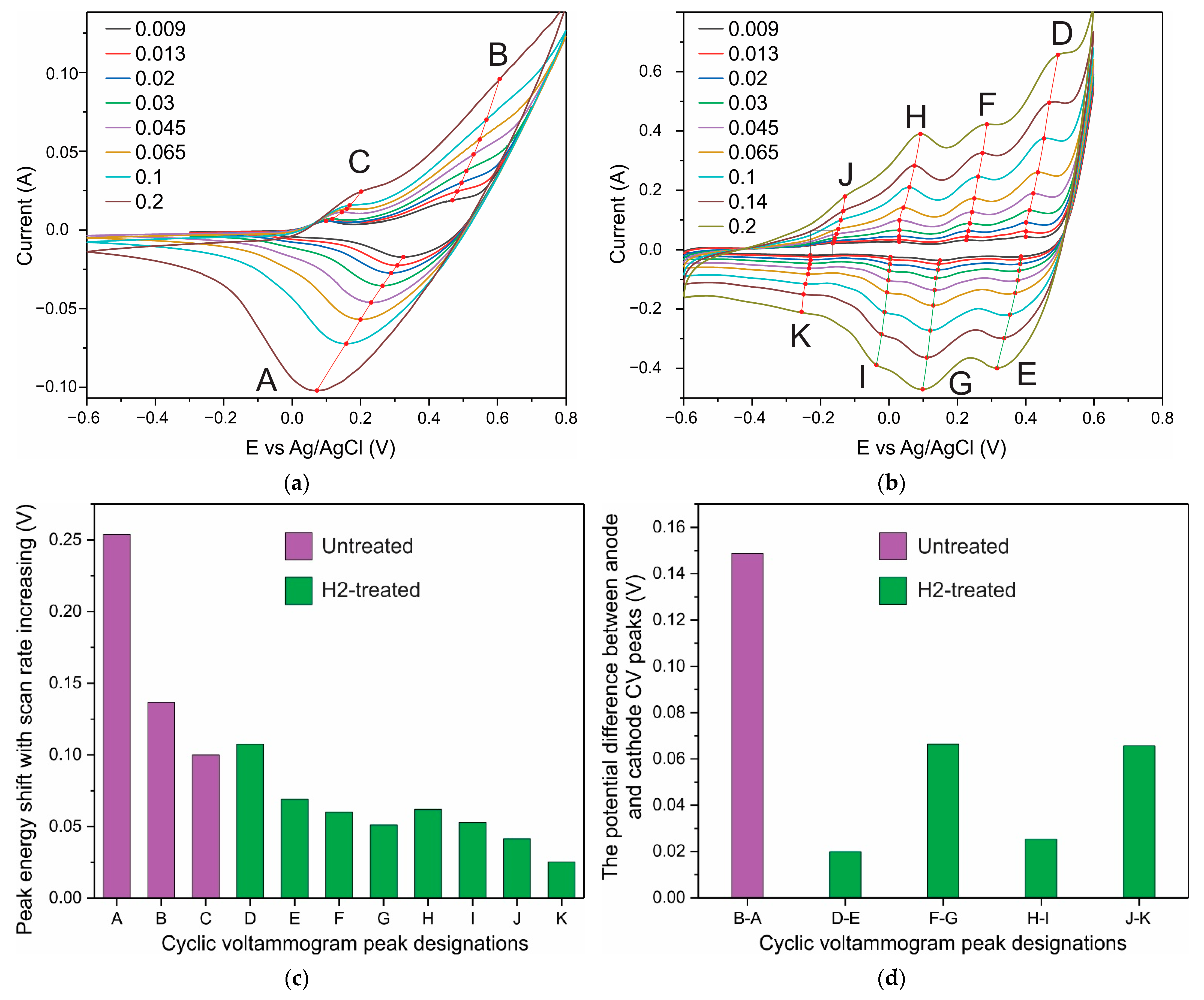
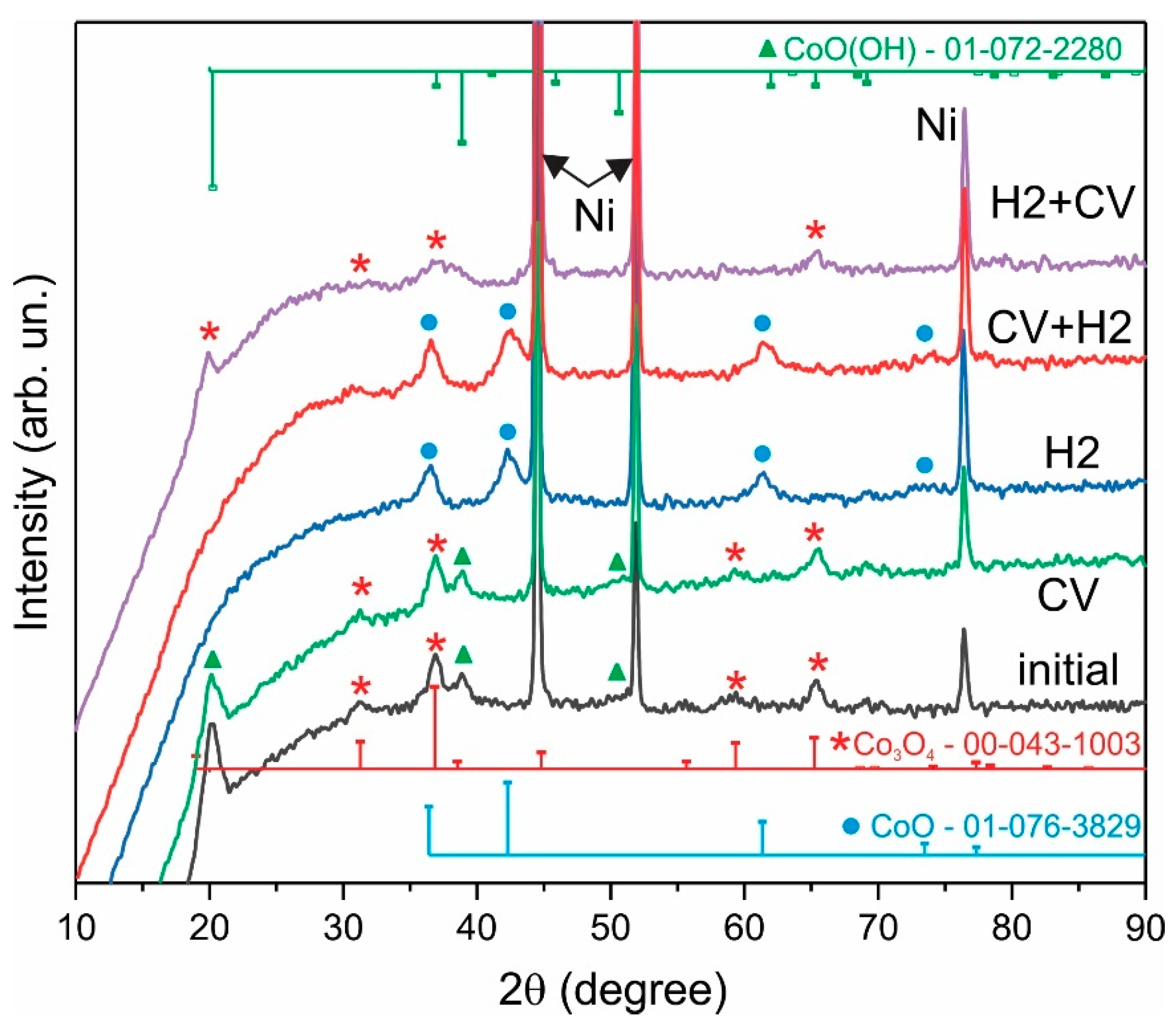
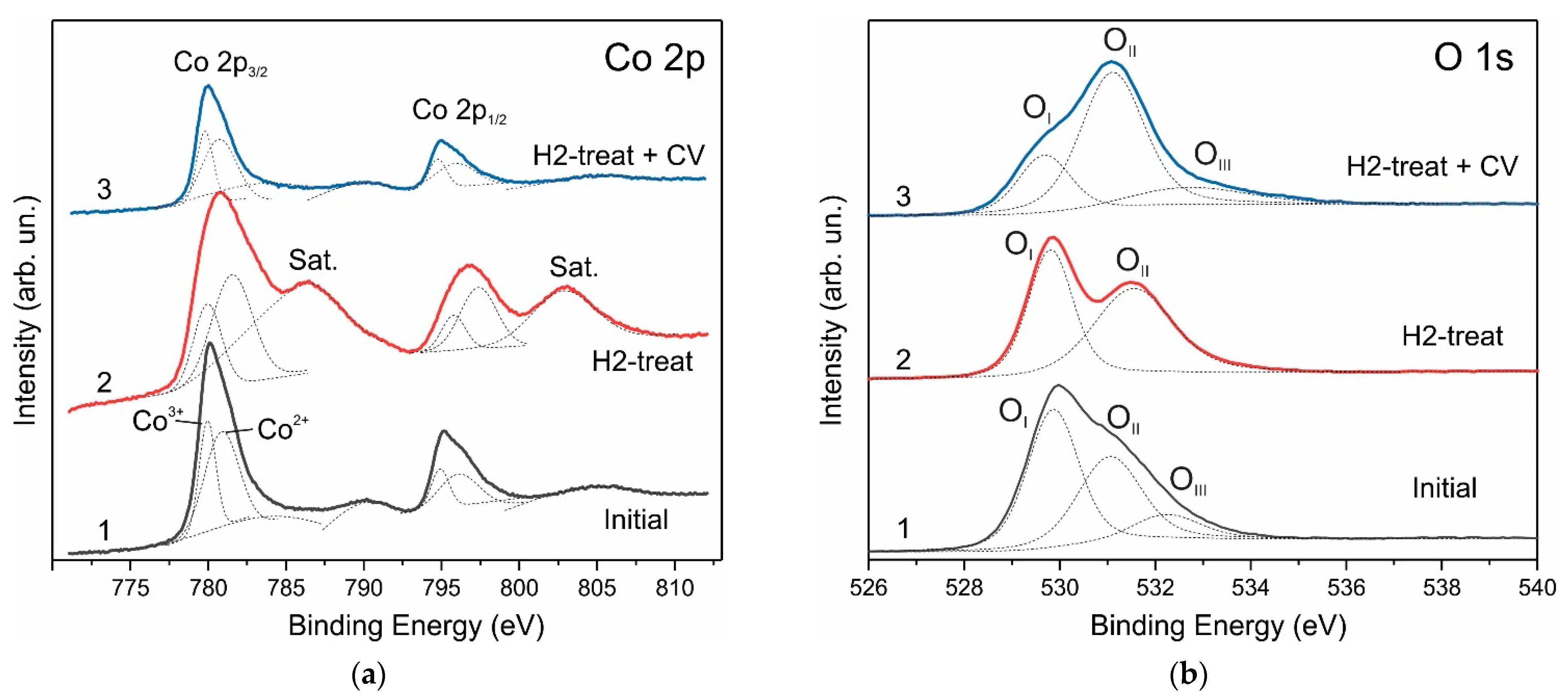
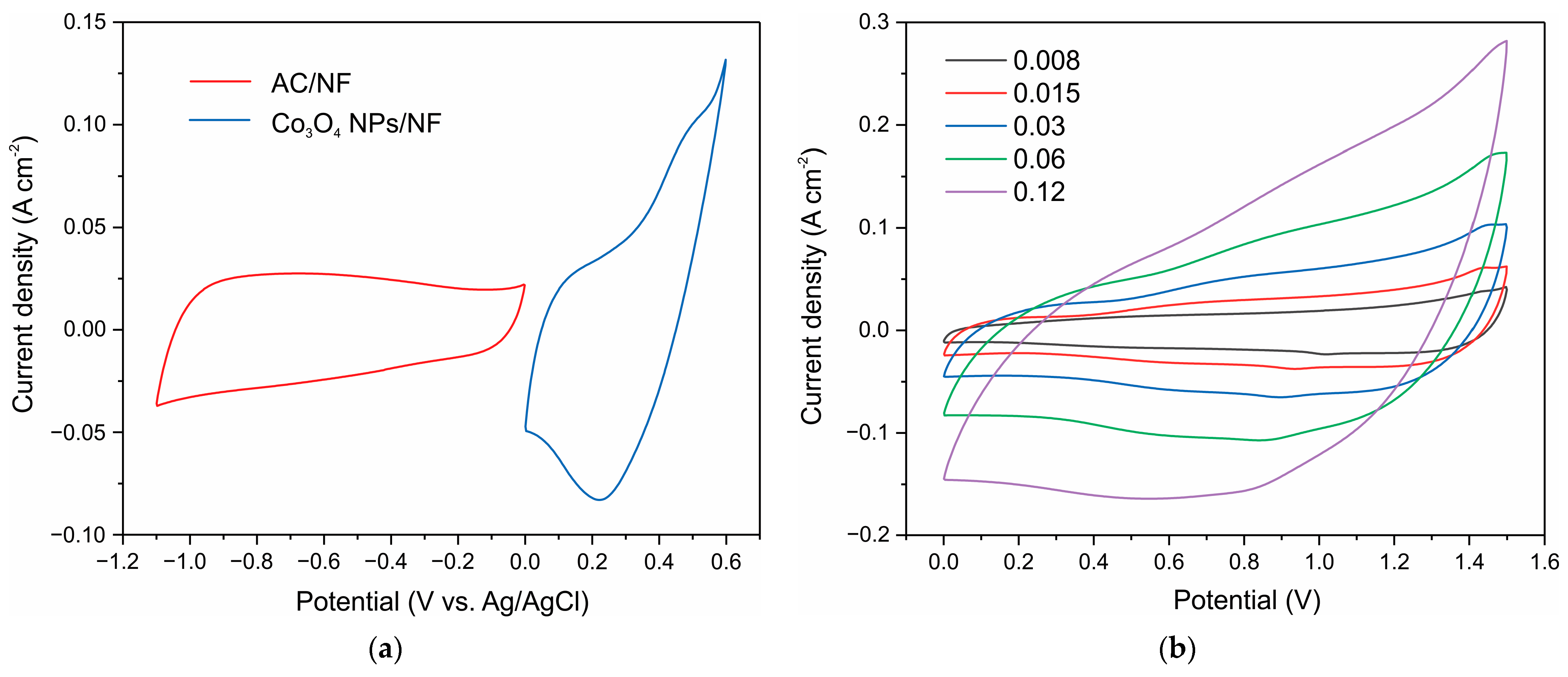
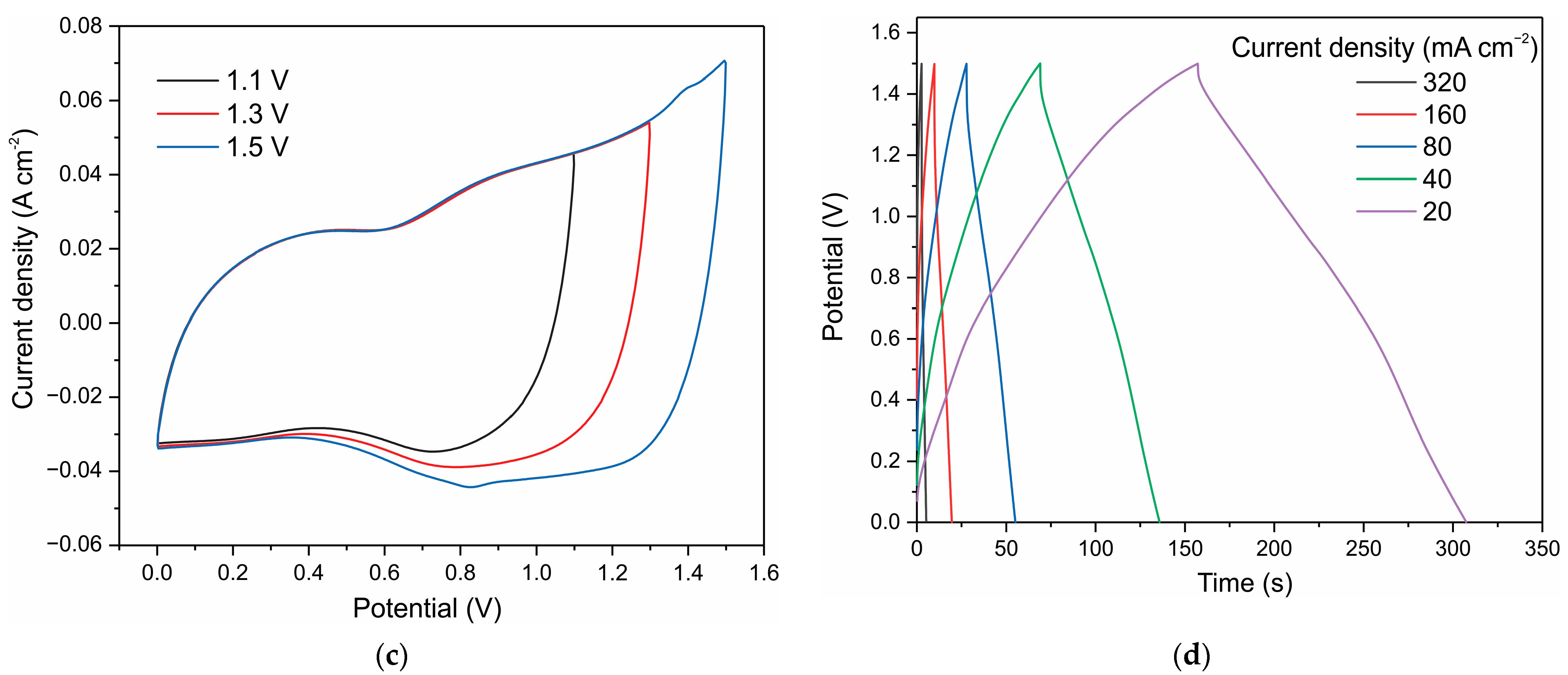
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Burke, A. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2020, 59, 276–291. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, N.; Qu, L. The Advance and Perspective on Electrode Materials for Metal Ion Hybrid Capacitors. Adv. Energy Sustain. Res. 2021, 2, 2100022. [Google Scholar] [CrossRef]
- Naskar, P.; Kundu, D.; Maiti, A.; Chakraborty, P.; Biswas, B.; Banerjee, A. Frontiers in Hybrid Ion Capacitors—A Review on Advanced Materials and Emerging Devices. ChemElectroChem 2021, 8, 1393–1429. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Zhang, Y.; Li, G.-Y.; Lv, Y.; Wang, L.-Z.; Zhang, A.-Q.; Song, Y.-H.; Huang, B.-L. Electrochemical investigation of MnO2 electrode material for supercapacitors. Int. J. Hydrogen Energy 2011, 36, 11760–11766. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.-E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Rajagopal, S.; Pulapparambil Vallikkattil, R.; Mohamed Ibrahim, M.; Velev, D.G. Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress. Condens. Matter 2022, 7, 6. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Jin, J.; Geng, X.; Chen, Q.; Ren, T.-L. A Better Zn-Ion Storage Device: Recent Progress for Zn-Ion Hybrid Supercapacitors. Nano-Micro. Lett. 2022, 14, 64. [Google Scholar] [CrossRef]
- Fang, H.; Li, D.; Zhao, M.; Zhang, Y.; Yang, J.; Wang, K. Research progress and prospect of hybrid supercapacitors as boosting the performance. Energy Sources Part A Recovery Util. Environ. Eff. 2022. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Elkodous, M.A.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced materials and technologies for supercapacitors used in energy conversion and storage: A review. Env. Chem. Lett. 2021, 19, 375–439. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.H.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef]
- Rehman, J.; Eid, K.; Ali, R.; Fan, X.; Murtaza, G.; Faizan, M.; Laref, A.; Zheng, W.; Varma, R.S. Engineering of Transition Metal Sulfide Nanostructures as Efficient Electrodes for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6481–6498. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430 Pt 2, 132745. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Bi, Q.; Ma, Q.; Han, L.; Tao, K. Recent advances in metal–organic framework-based electrode materials for supercapacitors. Dalton Trans. 2021, 50, 11701–11710. [Google Scholar] [CrossRef]
- Luo, W.; Ma, Y.; Li, T.; Thabet, H.K.; Hou, C.; Ibrahim, M.M.; El-Bahy, S.M.; Xu, B.B.; Guo, Z. Overview of MXene/conducting polymer composites for supercapacitors. J. Energy Storage 2022, 52 Pt B, 105008. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.; Cao, Y.; Yan, X.; Yan, J.; Gao, H.; Luo, H.; Wang, S.; Jia, X.; Kachalova, L.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Coord. Chem. Rev. 2021, 438, 213910. [Google Scholar] [CrossRef]
- Sun, Z.; Qi, H.; Chen, M.; Guo, S.; Huang, Z.; Maganti, S.; Murugadoss, V.; Huang, M.; Guo, Z. Progress in Cellulose/Carbon Nanotube Composite Flexible Electrodes for Supercapacitors. Eng. Sci. 2022, 18, 59–74. [Google Scholar] [CrossRef]
- Patil, S.S.; Bhat, T.S.; Teli, A.M.; Beknalkar, S.A.; Dhavale, S.B.; Faras, M.M.; Karanjkar, M.M.; Patil, P.S. Hybrid Solid State Supercapacitors (HSSC’s) for High Energy & Power Density: An Overview. Eng. Sci. 2020, 12, 38–51. [Google Scholar] [CrossRef]
- Nagarajarao, S.H.; Nandagudi, A.; Viswanatha, R.; Basavaraja, B.M.; Santosh, M.S.; Praveen, B.M.; Pandith, A. Recent Developments in Supercapacitor Electrodes: A Mini Review. ChemEngineering 2022, 6, 5. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, S.; Liu, H.-K.; Li, L. Transition Metal based Battery-Type Electrodes in Hybrid Supercapacitors A Review. Energy Storage Mater. 2020, 28, 122–145. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Liu, L.; Mu, Y.-P.; Zhang, H.-J.; Wang, Y. Designed synthesis of cobalt-oxide-based nanomaterials for superior electrochemical energy storage devices. Nano Res. 2014, 8, 321–339. [Google Scholar] [CrossRef]
- Hou, C.; Wang, B.; Murugadoss, V.; Vupputuri, S.; Chao, Y.; Guo, Z.; Wang, C.; Du, W. Recent Advances in Co3O4 as Anode Materials for High-Performance Lithium-Ion Batteries. Eng. Sci. 2020, 11, 19–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Zhu, K.; Maganti, S.; Zhao, Z.; Bai, P. Three-dimensional printing of the copper sulfate hybrid composites for supercapacitor electrodes with ultra-high areal and volumetric capacitances. Adv. Compos. Hybrid Mater. 2022, 5, 1537–1547. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597. [Google Scholar] [CrossRef]
- Gaire, M.; Khatoon, N.; Chrisey, D. Preparation of Cobalt Oxide–Reduced Graphitic Oxide Supercapacitor Electrode by Photothermal Processing. Nanomaterials 2021, 11, 717. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Ji, X.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Nickel/cobalt bimetallic metal-organic frameworks ultrathin nanosheets with enhanced performance for supercapacitors. J. Alloys Compd. 2020, 825, 154069. [Google Scholar] [CrossRef]
- Priyadharsini, C.I.; Marimuthu, G.; Pazhanivel, T.; Anbarasan, P.M.; Aroulmoji, V.; Siva, V.; Mohana, L. Sol–Gel synthesis of Co3O4 nanoparticles as an electrode material for supercapacitor applications. J. Sol-Gel Sci. Technol. 2020, 96, 416–422. [Google Scholar] [CrossRef]
- Jadhav, S.; Kalubarme, R.S.; Suzuki, N.; Terashima, C.; Kale, B.; Gosavi, S.W.; Ashokkumar, M.; … Fujishima, A. Probing electrochemical charge storage of 3D porous hierarchical cobalt oxide decorated rGO in ultra-high-performance supercapacitor. Surf. Coat. Technol. 2021, 419, 127287. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Haider, S.S.; Zakar, S.; Numan, A.; Afzal, A.M.; Kamran, M.A. Cobalt-oxide/carbon composites for asymmetric solid-state supercapacitors. Mater. Res. Bull. 2020, 131, 110974. [Google Scholar] [CrossRef]
- Kumar, R.; Soam, A.; Sahajwalla, V. Carbon coated cobalt oxide (CC-Co3O4) as electrode material for supercapacitor applications. Mater. Adv. 2021, 2, 2918. [Google Scholar] [CrossRef]
- Rabani, I.; Yoo, J.; Kim, H.; Lam, D.V.; Hussain, S.; Karuppasamy, K.; Seo, Y. Highly dispersive Co3O4 nanoparticles incorporated in a cellulose nanofiber for a high-performance flexible supercapacitor. Nanoscale 2021, 13, 355. [Google Scholar] [CrossRef]
- Gaire, M.; Subedi, B.; Adireddy, S.; Chrisey, D. Ultra-long cycle life and binder-free manganese-cobalt oxide supercapacitor electrodes through photonic nanostructuring. RSC Adv. 2020, 10, 40234. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, J.; Lin, C.; Xie, A.; Lin, B.; He, L.; Sun, D. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro/mesoporous hierarchy in Co3O4/C composite for asymmetric supercapacitors. Microporous Mesoporous Mater. 2020, 291, 109709. [Google Scholar] [CrossRef]
- Lia, S.; Yang, K.; Ye, P.; Ma, K.; Zhang, Z.; Huang, Q. Three-dimensional Porous Carbon/Co3O4 Composites Derived from Graphene/Co-MOF for High Performance Supercapacitor electrodes. Appl. Surf. Sci. 2020, 503, 144090. [Google Scholar] [CrossRef]
- Sun, J.; Man, P.; Zhang, Q.; He, B.; Zhou, Z.; Li, C.; Wang, X.; Guo, J.; Zhao, J.; Xie, L.; et al. Hierarchically-Structured Co3O4 Nanowire Arrays Grown on Carbon Nanotube Fibers as Novel Cathodes for High-Performance Wearable Fiber-Shaped Asymmetric Supercapacitors. Appl. Surf. Sci. 2018, 447, 795–801. [Google Scholar] [CrossRef]
- Nare, R.K.; Ramesh, S.; Basavi, P.K.; Kakani, V.; Bathula, C.; Yadav, H.M.; Dhanapal, P.B.; Reddy Kotanka, R.K.; Pasupuleti, V. Rao. Sonication supported synthesis of cobalt oxide assembled on an N-MWCNT composite for electrochemical supercapacitors. Sci. Rep. 2022, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- Zallouz, S.; Réty, B.; Vidal, L.; Le Meins, J.-M.; Ghimbeu, C.M. Co3O4 Nanoparticles Embedded in Mesoporous Carbon for Supercapacitor Applications. ACS Appl. Nano Mater. 2021, 4, 5022–5037. [Google Scholar] [CrossRef]
- Mousaabadi, K.Z.; Ensafi, A.A.; Rezaei, B. Co3O4/MoCo/Layered Double Hydroxide Nanosheets for Asymmetric Supercapacitor. ACS Appl. Nano Mater. 2022, 5, 8097–8104. [Google Scholar] [CrossRef]
- Liang, H.; Lin, T.; Wang, S.; Jia, H.; Li, C.; Cao, J.; Feng, J.; Fei, W.; Qi, J. A free-standing manganese cobalt sulfide-cobalt nickel layered double hydroxide core–shell heterostructure for an asymmetric supercapacitor. Dalton Trans. 2020, 49, 196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gaur, A. Cu Doped Zinc Cobalt Oxide Based Solid-State Symmetric Supercapacitors: A Promising Key for High Energy Density. J. Phys. Chem. C 2020, 124, 9–16. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khalid, N.R. Facile synthesis and properties of chromium-doped cobalt oxide (Cr-doped Co3O4) nanostructures for supercapacitor applications. Appl. Nanosci. 2020, 10, 1481–1488. [Google Scholar] [CrossRef]
- Guragain, D.; Karna, S.; Choi, J.; Bhattarai, R.; Poudel, T.P.; Gupta, R.K.; Shen, X.; Mishra, S.R. Electrochemical Performance of Iron-Doped Cobalt Oxide Hierarchical Nanostructure. Processes 2021, 9, 2176. [Google Scholar] [CrossRef]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-Solid-State Symmetric Supercapacitor Based on Co3O4 Nanoparticles on Vertically Aligned Graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Hao, Y.; Yang, X.; Goddard, W.A.; Zhang, X.L.; Cao, B. Oxygen-Vacancy Abundant Ultrafine Co3O4/Graphene Composites for High-Rate Supercapacitor Electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef]
- Fan, X.; Ohlckers, P.; Chen, X. Tunable Synthesis of Hollow Co3O4 Nanoboxes and Their Application in Supercapacitors. Appl. Sci. 2020, 10, 1208. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Srikesh, G.; Mohan, A.; Arivazhagan, V. In-situ synthesis of Co3O4/graphite nanocomposite for high-performance supercapacitor electrode applications. Appl. Surf. Sci. 2017, 403, 578–583. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, Y.-W.; Bang, J.H. Supercapacitor electrode with an ultrahigh Co3O4 loading for a high areal capacitance. Mater. Lett. 2013, 110, 237–240. [Google Scholar] [CrossRef]
- Meng, F.; Fang, Z.; Li, Z.; Xu, W.; Wang, M.; Liu, Y.; Zhang, J.; Wang, W.; Zhao, D.; Guo, X. Porous Co3O4 materials prepared by solid-state thermolysis of a novel Co-MOF crystal and their superior energy storage performances for supercapacitors. J. Mater. Chem. A 2013, 1, 7235. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeian, M.; Akhanova, N.; Gabdullin, M.; Kalkozova, Z.; Tulegenova, A.; Nurbolat, S.; Abdullin, K. Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors. Energies 2020, 13, 5228. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Koza, J.A.; Switzer, J.A. Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim. Acta 2014, 140, 359–365. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO– Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Ravindra, A.V.; Behera, B.C.; Padhan, P.; Lebedev, O.I.; Prellier, W. Tailoring of crystal phase and Nel temperature of cobalt monoxides nanocrystals with synthetic approach conditions. J. Appl. Phys. 2014, 116, 033912. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.B.; Yang, Z.; Zheng, G. Reduced Mesoporous Co3O4 Nanowires as Efficient Water Oxidation Electrocatalysts and Supercapacitor Electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullin, K.A.; Gabdullin, M.T.; Kalkozova, Z.K.; Nurbolat, S.T.; Mirzaeian, M. Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications. Nanomaterials 2022, 12, 3669. https://doi.org/10.3390/nano12203669
Abdullin KA, Gabdullin MT, Kalkozova ZK, Nurbolat ST, Mirzaeian M. Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications. Nanomaterials. 2022; 12(20):3669. https://doi.org/10.3390/nano12203669
Chicago/Turabian StyleAbdullin, Khabibulla A., Maratbek T. Gabdullin, Zhanar K. Kalkozova, Shyryn T. Nurbolat, and Mojtaba Mirzaeian. 2022. "Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications" Nanomaterials 12, no. 20: 3669. https://doi.org/10.3390/nano12203669
APA StyleAbdullin, K. A., Gabdullin, M. T., Kalkozova, Z. K., Nurbolat, S. T., & Mirzaeian, M. (2022). Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications. Nanomaterials, 12(20), 3669. https://doi.org/10.3390/nano12203669






