Microstructure and Antibacterial Properties of Chitosan-Fe3O4-AgNP Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4 Nanoparticles
2.2. Synthesis of Fe3O4@Chitosan
2.3. Synthesis of AgNPs
2.4. Synthesis of Fe3O4@Chitosan-AgNPs Nanocomposites
2.5. Characterization of Fe3O4@Chitosan-AgNPs Nanocomposite
2.6. Antibacterial Activity
3. Results
3.1. Characterization of Fe3O4@Chitosan-AgNPs
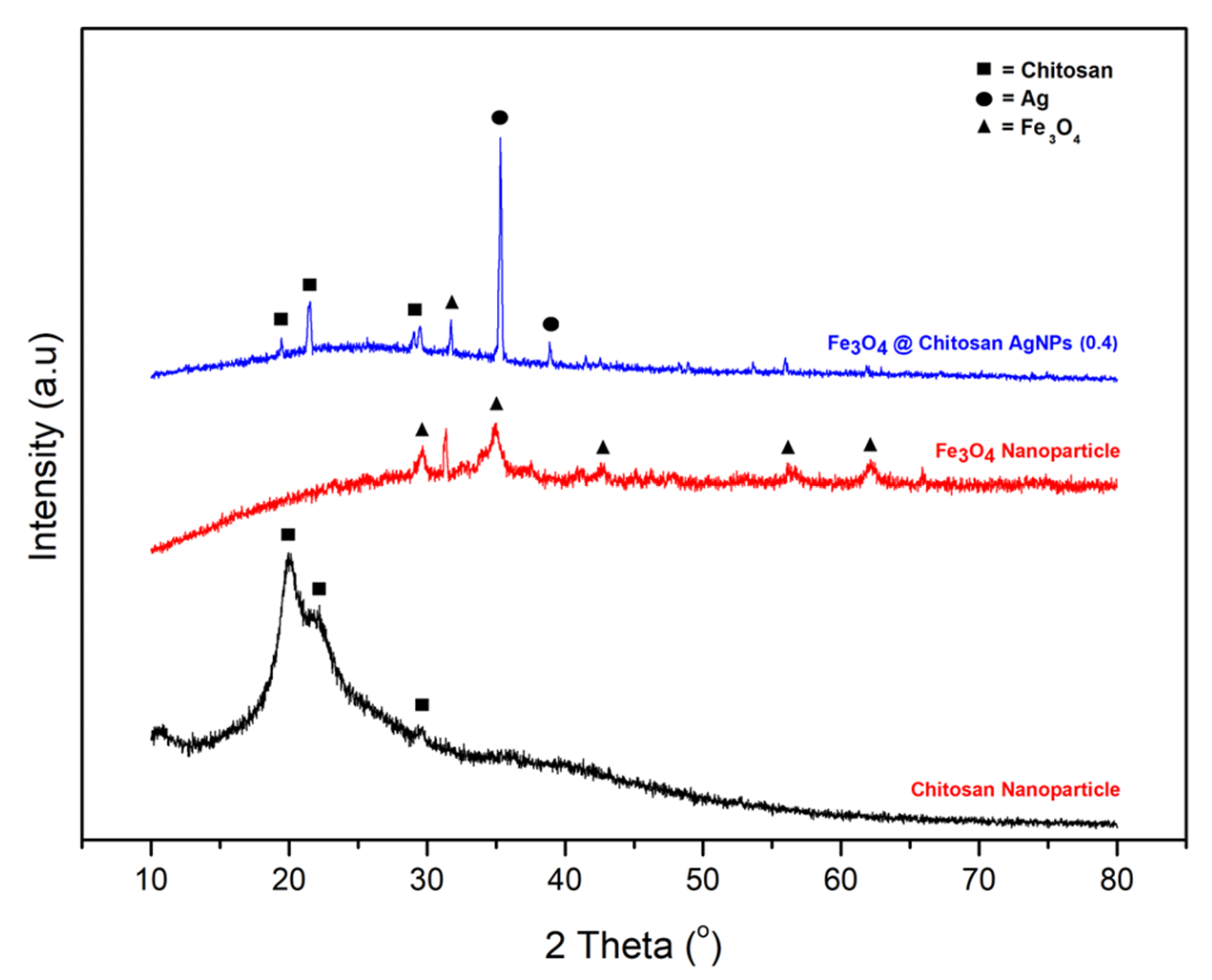
3.1.1. XRD Characterization (X-ray Diffraction)
3.1.2. SEM Characterization (Scanning Electron Microscope)
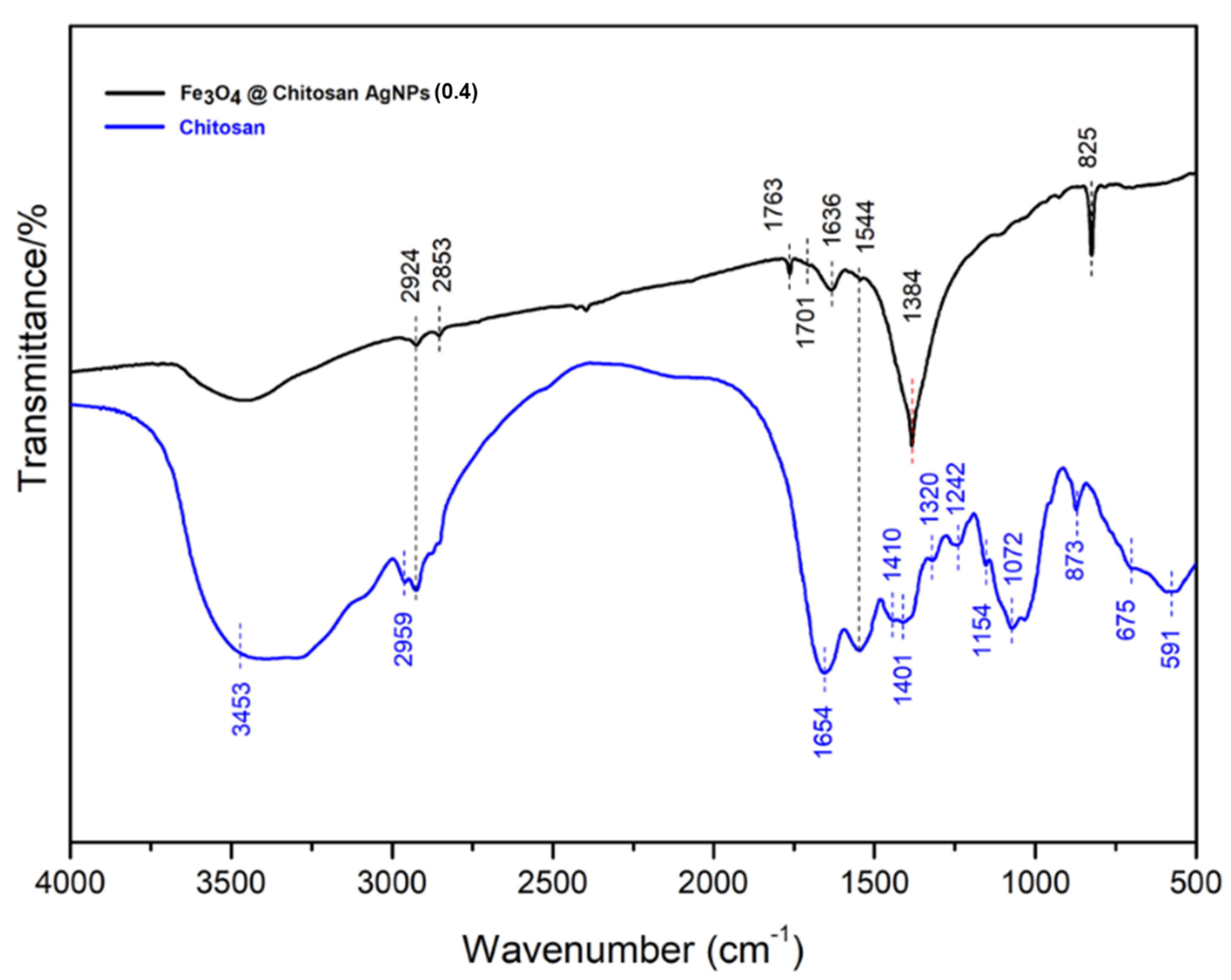
3.1.3. Characterization of FT-IR (Fourier Transform Infrared)
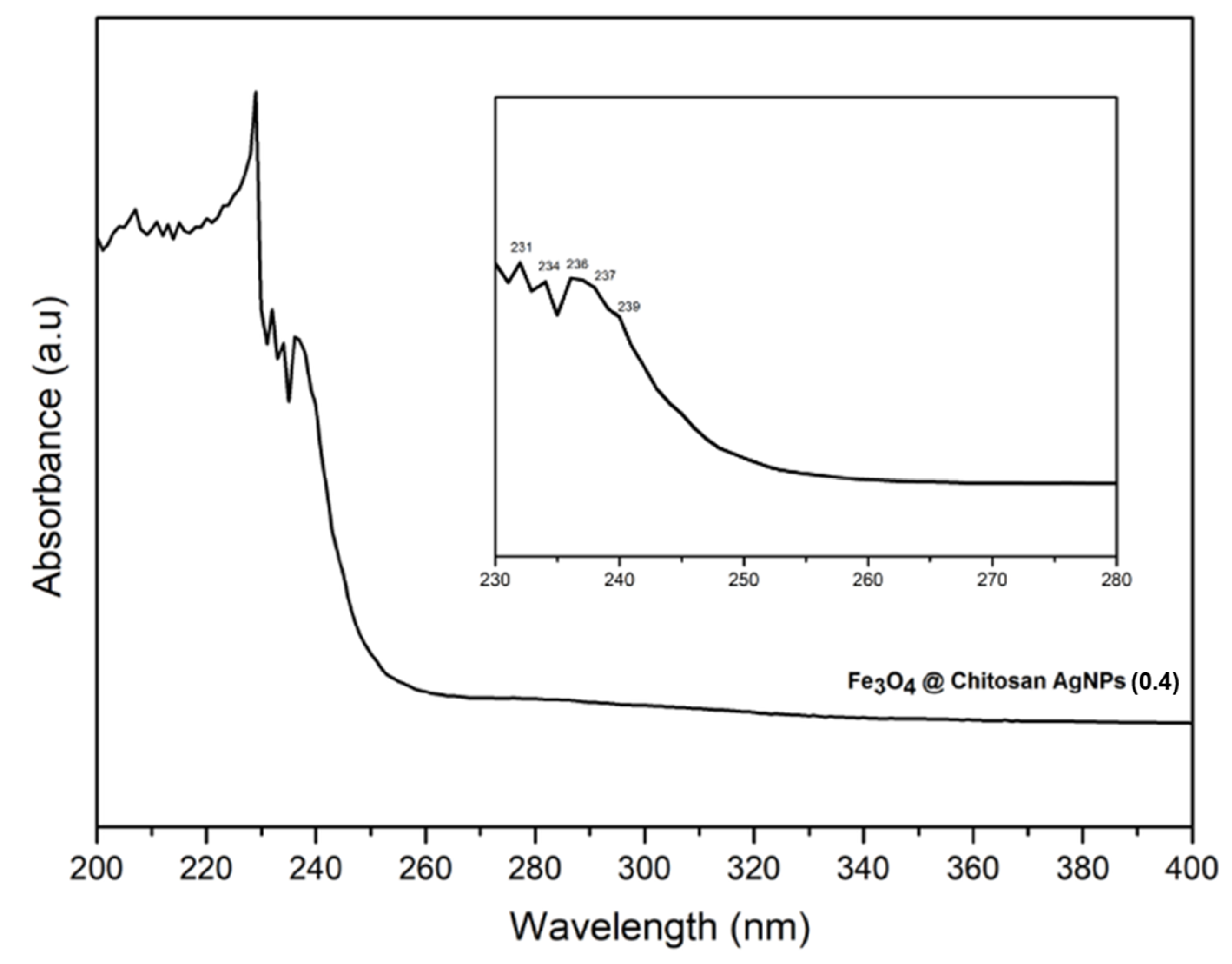
3.1.4. Characterization of UV-Vis
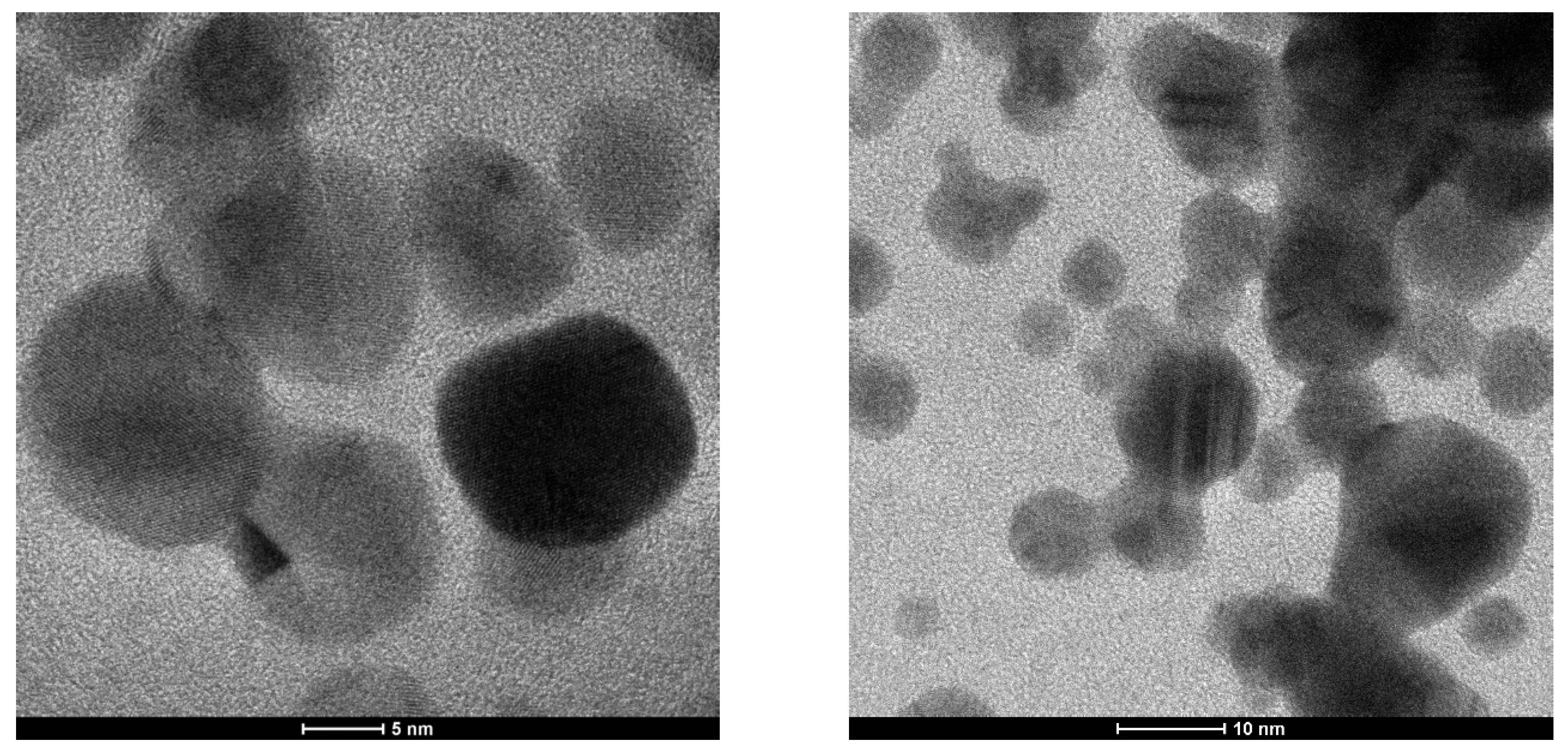
3.1.5. TEM (Transmission Electron Microscopy) Characterization
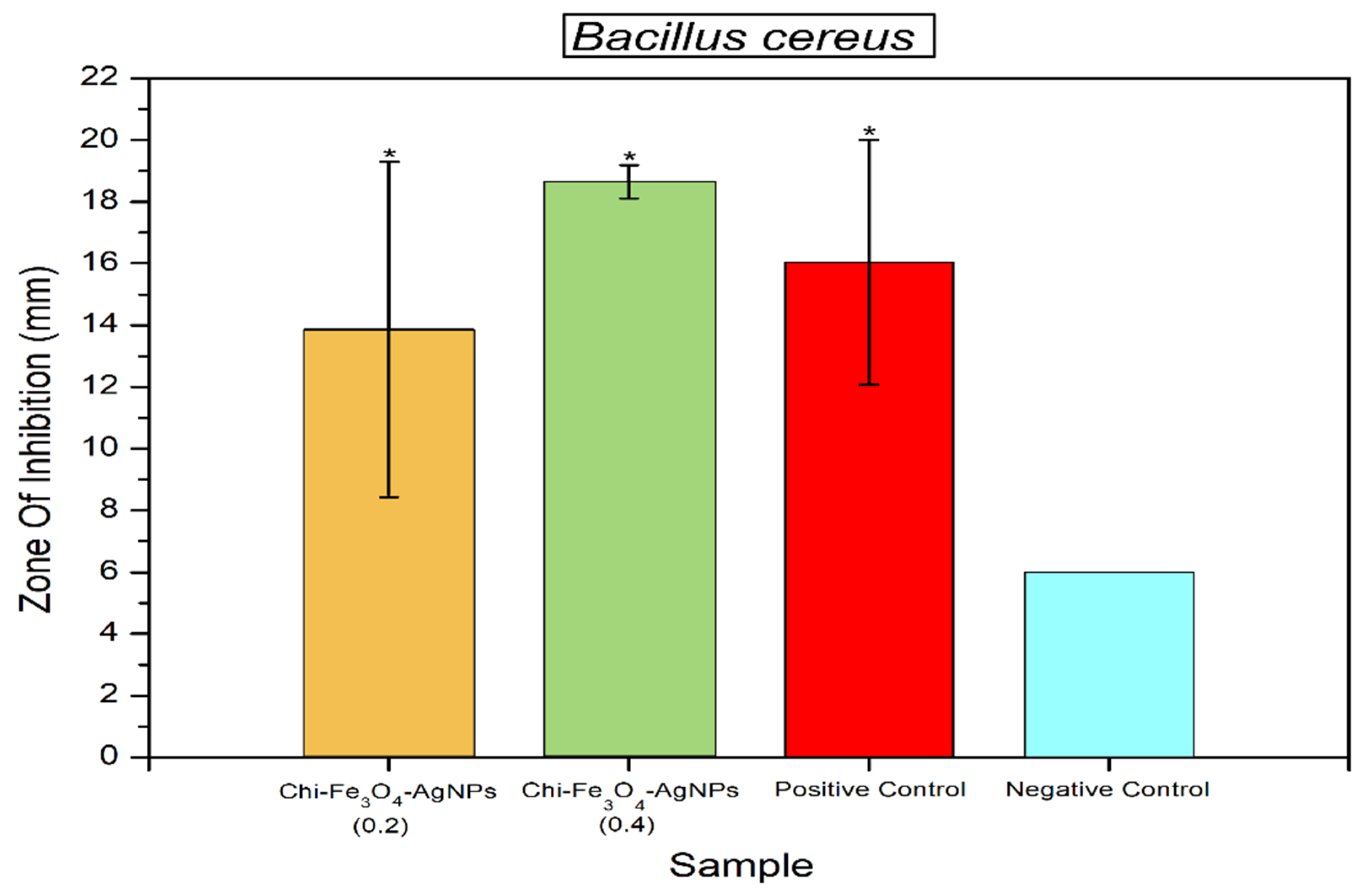
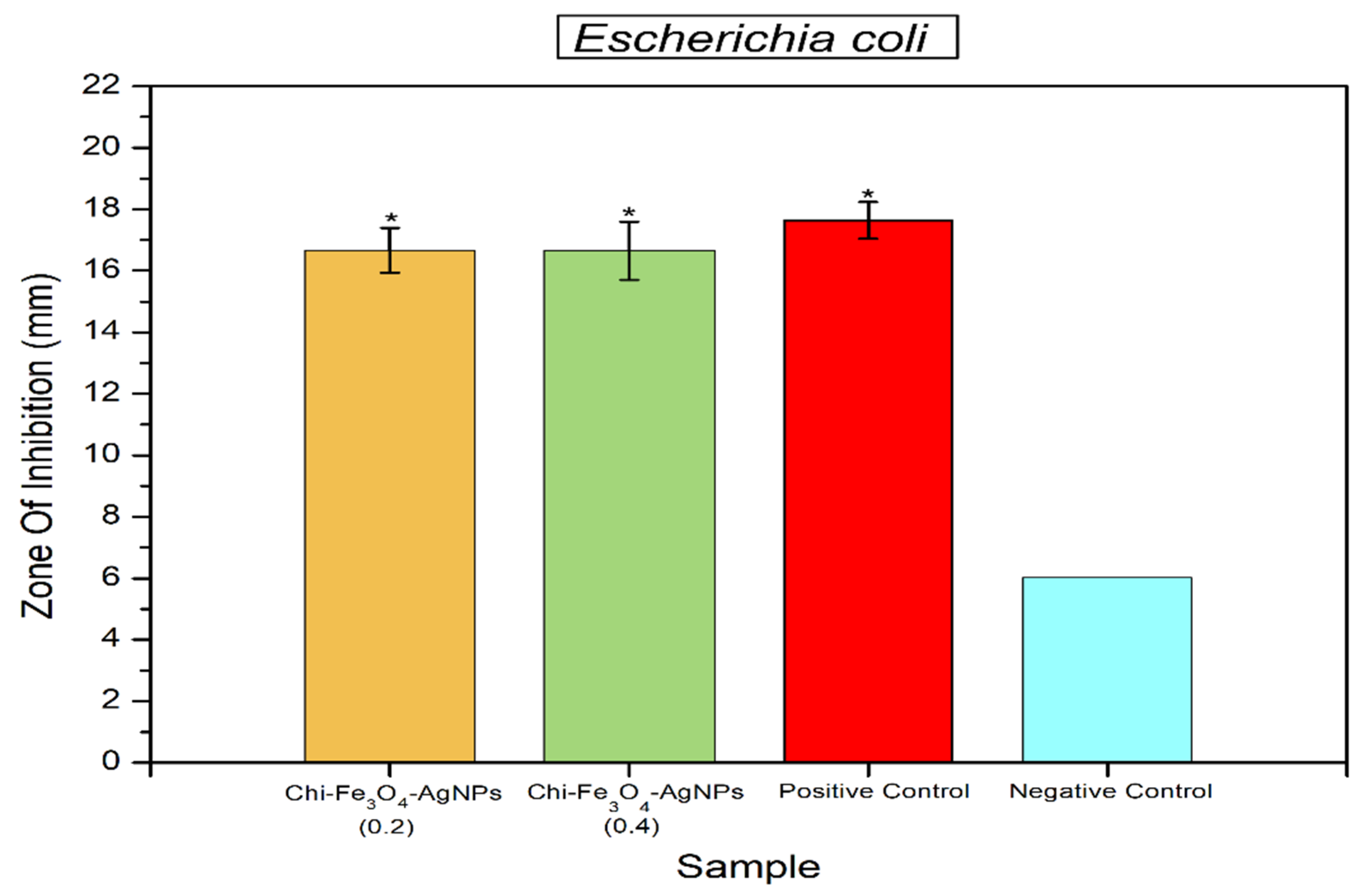
3.2. Antibacterial Properties of Fe3O4@Chitosan-AgNPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sathishkumar, G.; Logeshwaran, V.; Sarathbabu, S.; Jha, P.K.; Jeyaraj, M.; Rajkuberan, C.; Senthilkumar, N.; Sivaramakrishnan, S. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 589–598. [Google Scholar] [CrossRef]
- Arivizhivendhan, V.; Mahesh, M.; Boopathy, R.; Karthikeyan, S.; Mary, R.R.; Sekaran, G. Functioned silver nanoparticle loaded activated carbon for the recovery of bioactive molecule from bacterial fermenter for its bactericidal activity. Appl. Surf. Sci. 2018, 427, 813–824. [Google Scholar] [CrossRef]
- Ghiută, I.; Cristea, D.; Croitoru, C.; Kost, J.; Wenkert, R.; Vyrides, I.; Anayiotos, A.; Munteanu, D. Characterization and antimicrobial activity of silver nanoparticles, biosynthesized using Bacillus species. Appl. Surf. Sci. 2018, 438, 66–73. [Google Scholar] [CrossRef]
- Mitra, C.; Gummadidala, P.M.; Afshinnia, K.; Merrifield, R.C.; Baalousha, M.; Lead, J.R.; Chanda, A. Citrate-coated silver nanoparticles growth-independently inhibit aflatoxin synthesis in Aspergillus parasiticus. Environ. Sci. Technol. 2017, 51, 8085–8093. [Google Scholar] [CrossRef]
- Bonardd, S.; Saldiás, C.; Ramiŕez, O.; Radić, D.; Recio, F.J.; Urzúa, M.; Leiva, A. A novelen-vironmentally friendly method in solid phase for in situ synthesis of chitosan-gold bionanocomposites with catalytic applications. Carbohydr. Polym. 2019, 207, 533–541. [Google Scholar] [CrossRef]
- Pounraj, S.; Somu, P.; Paul, S. Chitosan and graphene oxide hybrid nanocomposite film doped with silver nanoparticles efficiently prevents biofouling. Appl. Surf. Sci. 2018, 452, 487–497. [Google Scholar] [CrossRef]
- Siegel, J.; Staszek, M.; Polívková, M.; Řezníčková, A. Green synthesized noble metals for biological applications. Mater. Today Proc. 2016, 3, 608–616. [Google Scholar] [CrossRef]
- Pramanik, A.; Laha, D.; Chattopahyay, S.; Dash, S.K.; Roy, S.; Pramanik, P.; Karmakar, P. Targeted delivery of “copper carbonate” nanoparticles to cancer cells in vivo. Toxicol. Res. 2015, 4, 1604–1612. [Google Scholar] [CrossRef]
- Ghosh, D.; Pramanik, A.; Sikdar, N.; Pramanik, P. Synthesis of low molecular weight alginic acid nanoparticle through persulfate treatment as effective drug delivery system to manage drug resisten bacteria. Biotechnol. Bioprocess Eng. 2011, 16, 383–392. [Google Scholar] [CrossRef]
- Mingyue, H.; Li, Y.; Yu, L.; Peng, S.; Gu, N.; Li, L.; Jia, J.; Li, B. Doubel-sided nano-ZnO superior Antibacterial propreties and Induced Hepatotoxicity in Zebrafish embryos. Toxics 2022, 10, 144. [Google Scholar]
- Jingmao, Q.; Liu, G.; Wong, Y.; Hong, R. Preparation of Fe3O4-chitosan nanoparticles used for hyperthermia. Adv. Powder Technol. 2010, 21, 461–467. [Google Scholar]
- Ritthichai, T.; Pimpan, V. Ammonia sensing of silver nanoparticles synthesized using tannic acid combined with UV radiation: Effect of UV exposure time. J. King Saud Univ.-Sci. 2019, 31, 277–284. [Google Scholar] [CrossRef]
- Biao, L.; Tan, S.; Wang, Y.; Guo, X.; Fu, Y.; Xu, F.; Zu, Y.; Liu, Z. Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag Nanoparticles, Mater. Sci. Eng. C 2017, 76, 73–80. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F.; Qi, Y.; Yan, J. Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater. Sci. Eng. C 2015, 48, 487–498. [Google Scholar] [CrossRef]
- Tomke, P.D.; Rothod, K.V. Facile fabrication of silver on magnetic nanocomposite (Fe3O4@Chitosan-AgNPs nanocomposite) for catalytic reduction of anthropogenic pollutant and agricultural pathogens. Int. J. Biolog. Macromol. 2020, 149, 989–999. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Khan, H.K.; Pathak, B.; Fulekar, M.H. Spherical surfaced magnetic (Fe3O4) Nanoparticles as nano adsorbent material for Treatment of Industrial Dye Effluents. Int. J. Nanosci. Nanotechnol. 2017, 13, 169–175. [Google Scholar]
- Zhang, J.; Xu, Q.; Li, H.; Zhang, S.; Hong, A.; Jiang, Y.; Hu, N.; Chen, G.; Fu, H.; Yuan, M.; et al. Self-powered electrodeposition system for sub-10-nm silver nanoparticles with High efficiency antibacterial activity. J. Phys. Chem. Lett. 2022, 13, 6721–6730. [Google Scholar] [CrossRef]
- Shakibaie, M.; Haghri, M.; Jafari, M.; Amirpour-Rostani, S.; Men, A.; Forootanfar, H.; Mehrabani, M. Preparation and Evaluation of the effect Fe3O4@piroctone olamine magnetic nanoparticles on matrix metalloproteinase-2; a preliminary in vitro study. Biotechnol. Appl. Biochem. 2014, 61, 676–682. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Shen, Y.; Xiao, Y.; Gao, L.; Shi, S. Cytotoxicity studies of Fe3O4 nanoparticle in Chicken Macrophage cells. R. Soc. Open Sci. 2020, 7, 191561. [Google Scholar] [CrossRef]
- Patel, S.; Jana, S.; Chetty, R.; Thalcore, S.; Singh, M.; Devkas, R. Toxicity evaluation of Magnetic iron Oxide nanoparticle reveals neuromal loss in chicken embryo. Drug Chem. Toxicol. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Gong, M.; Yong, H.; Zhang, S.; Yong, Y.; Zhag, D.; Qi, Y.; Zoy, L. Superparamagnetic core/shell Goldmag Nanoparticles; Size, concentration, and time-dependent celluler nanotoxicity on human umbilical vein endothelial cells and the suitable conditions for magnetic resonance imaging. J. Nanobiotechnol. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, L.; Badalyan, H.; Gevorgyan, V.; Trchunion, A. Comparable antibacterial effects and action mechanisms of silver an iron oxide nanoparticles on Escherichia coli and Salmonella typhimurim. Sci. Rep. 2020, 10, 13145. [Google Scholar] [CrossRef] [PubMed]
- Armijo, L.M.; Wawrzyniec, S.J.; Kopciuch, M.; Brandt, Y.I.; Rivera, A.C.; Withers, N.J.; Cook, N.C.; Huber, D.I.; Monson, T.C.; Smyth, H.D.; et al. Antibacterial activity of Iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilm. J. Nanobiotechnol. 2020, 18, 35. [Google Scholar] [CrossRef] [PubMed]
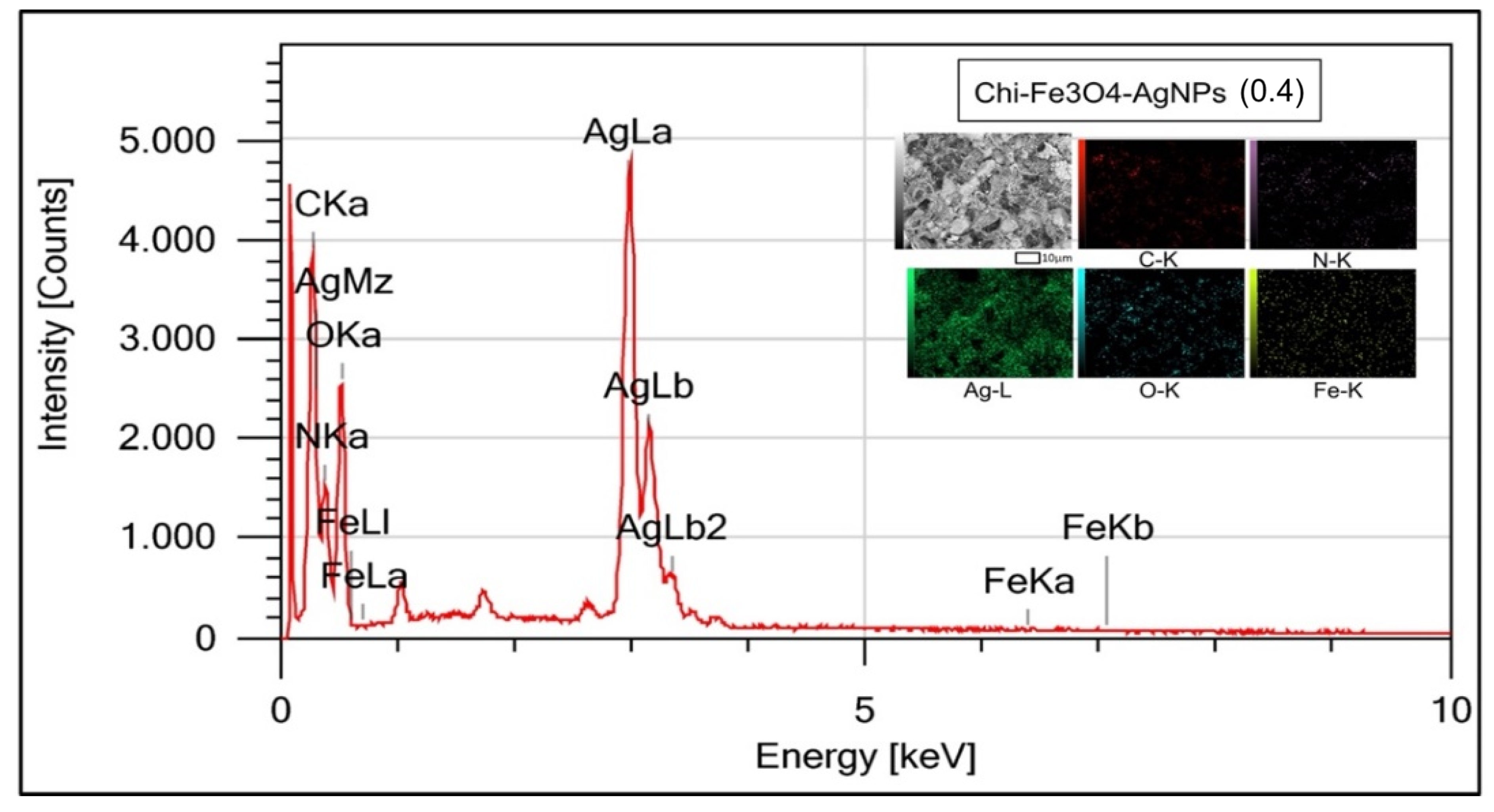

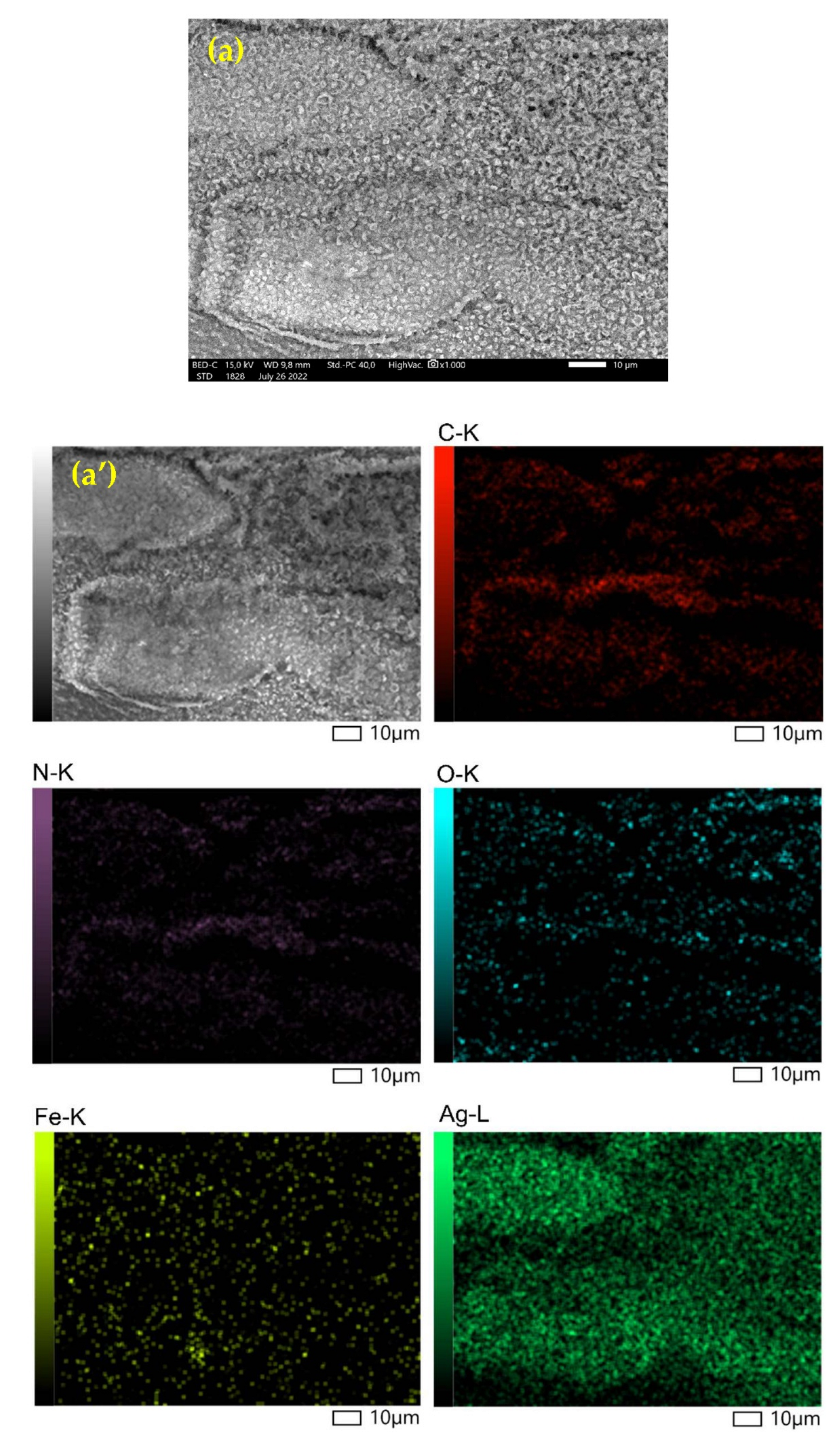
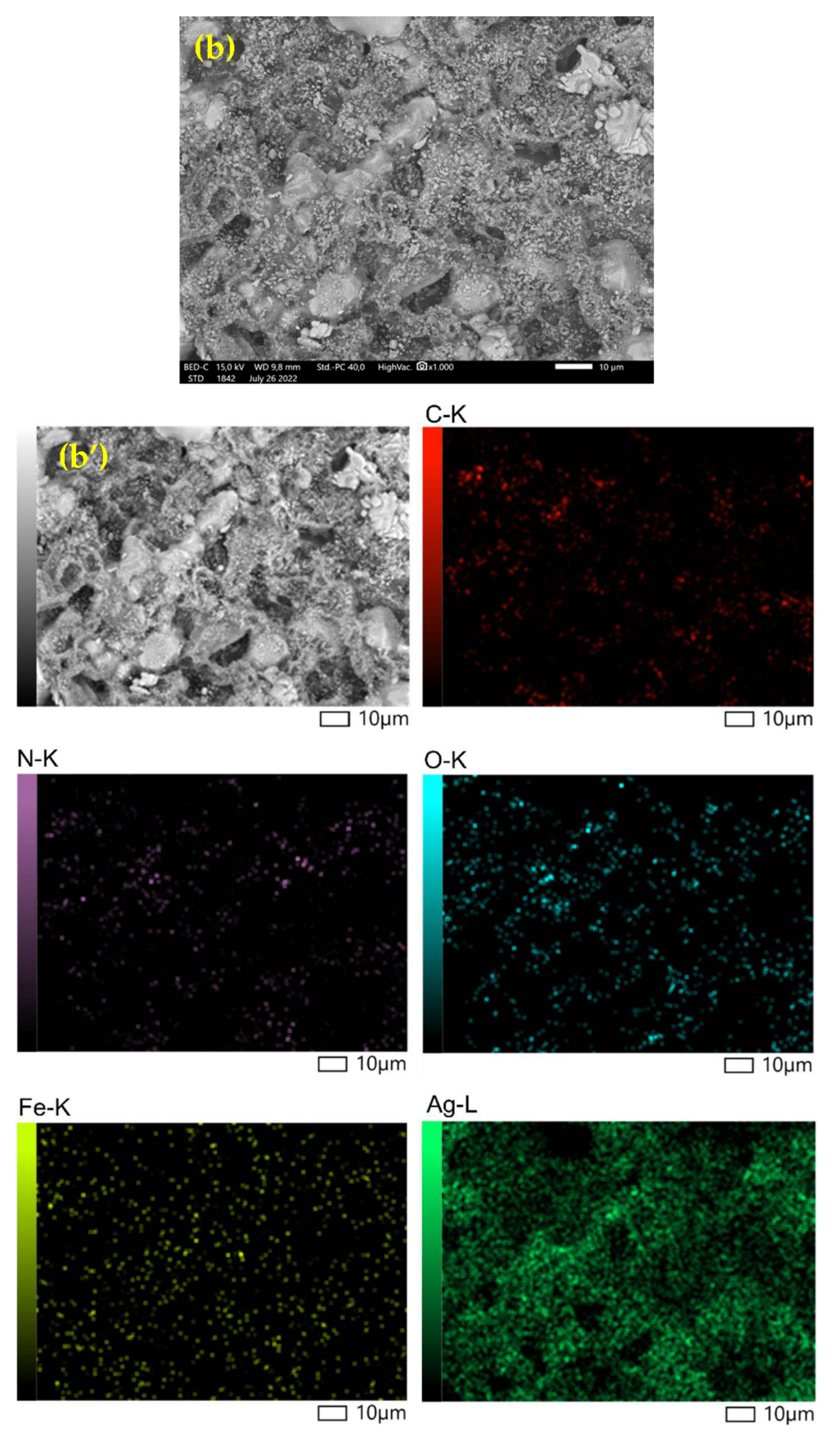
| Element | Mass (%) | Atom (%) |
|---|---|---|
| C | 20.30 ± 0.07 | 32.19 ± 0.11 |
| N | 17.01 ± 0.18 | 23.14 ± 0.24 |
| O | 33.13 ± 0.26 | 39.44 ± 0.31 |
| Fe | 0.06 ± 0.02 | 0.02 ± 0.01 |
| Ag | 29.50 ± 0.16 | 5.21 ± 0.03 |
| Total | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartati, H.; Subaer, S.; Hasri, H.; Wibawa, T.; Hasriana, H. Microstructure and Antibacterial Properties of Chitosan-Fe3O4-AgNP Nanocomposite. Nanomaterials 2022, 12, 3652. https://doi.org/10.3390/nano12203652
Hartati H, Subaer S, Hasri H, Wibawa T, Hasriana H. Microstructure and Antibacterial Properties of Chitosan-Fe3O4-AgNP Nanocomposite. Nanomaterials. 2022; 12(20):3652. https://doi.org/10.3390/nano12203652
Chicago/Turabian StyleHartati, Hartati, Subaer Subaer, Hasri Hasri, Teguh Wibawa, and Hasriana Hasriana. 2022. "Microstructure and Antibacterial Properties of Chitosan-Fe3O4-AgNP Nanocomposite" Nanomaterials 12, no. 20: 3652. https://doi.org/10.3390/nano12203652
APA StyleHartati, H., Subaer, S., Hasri, H., Wibawa, T., & Hasriana, H. (2022). Microstructure and Antibacterial Properties of Chitosan-Fe3O4-AgNP Nanocomposite. Nanomaterials, 12(20), 3652. https://doi.org/10.3390/nano12203652






