Low-Temperature Processed Brookite Interfacial Modification for Perovskite Solar Cells with Improved Performance
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Shi, J.; Tian, C.; Wu, J.; Li, H.; Li, Y.; Yu, B.; Luo, Y.; Wu, H.; Xie, Z.; et al. CdS induced passivation toward high efficiency and stable planar perovskite solar cells. ACS Appl. Mater. Interfaces 2021, 13, 9771–9780. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, X.; Liang, L.; Bao, J.; Li, S.; Yang, W.; Xie, Y. High-performance flexible broadband photodetector based on organolead halide perovskite. Adv. Funct. Mater. 2014, 24, 7373–7380. [Google Scholar] [CrossRef]
- Geng, W.; Zhang, L.; Zhang, Y.-N.; Lau, W.-M.; Liu, L.-M. First-principles study of lead iodide perovskite tetragonal and orthorhombic phases for photovoltaics. J. Phys. Chem. C 2014, 118, 19565–19571. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graetzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Jeng, J.-Y.; Chiang, Y.-F.; Lee, M.-H.; Peng, S.-R.; Guo, T.-F.; Chen, P.; Wen, T.-C. CH3NH3PbI3 Perovskite/fullerene planar-heterojunction hybrid solar cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, T.J.; Correa-Baena, J.-P.; Pazoki, M.; Saliba, M.; Schenk, K.; Gratzel, M.; Hagfeldt, A. Exploration of the compositional space for mixed lead halogen perovskites for high efficiency solar cells. Energy Environ. Sci. 2016, 9, 1706–1724. [Google Scholar] [CrossRef]
- Sahli, F.; Werner, J.; Kamino, B.A.; Braeuninger, M.; Monnard, R.; Paviet-Salomon, B.; Barraud, L.; Ding, L.; Leon, J.J.D.; Sacchetto, D.; et al. Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 2018, 17, 820–826. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 2016, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, W.; van Reenen, S.; Sutton, R.J.; Fan, J.; Haghighirad, A.A.; Johnston, M.B.; Wang, L.; Snaith, H.J. Enhanced UV-light stability of planar heterojunction perovskite solar cells with caesium bromide interface modification. Energy Environ. Sci. 2016, 9, 490–498. [Google Scholar] [CrossRef]
- Adnan, M.; Lee, J.K. Highly efficient planar heterojunction perovskite solar cells with sequentially dip-coated deposited perovskite layers from a non-halide aqueous lead precursor. RSC Adv. 2020, 10, 5454–5461. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Yazdi, M.-R.; Gholampour, N.; Eslamian, M. Interface Engineering by Employing zeolitic imidazolate framework-8 (ZIF-8) as the only scaffold in the architecture of perovskite solar cells. Acs Appl. Energy Mater. 2020, 3, 3134–3143. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photon. 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Bao, S.; Wu, J.; He, X.; Tu, Y.; Wang, S.; Huang, M.; Lan, Z. Mesoporous Zn2SnO4 as effective electron transport materials for high-performance perovskite solar cells. Electrochim. Acta 2017, 251, 307–315. [Google Scholar] [CrossRef]
- Chavan, R.D.; Yadav, P.; Nimbalkar, A.; Bhoite, S.P.; Bhosale, P.N.; Hong, C.K. Ruthenium doped mesoporous titanium dioxide for highly efficient, hysteresis-free and stable perovskite solar cells. Sol. Energy 2019, 186, 156–165. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, J.; Zheng, L.; Yan, X.; Lin, H.; Zhang, F. Crack-free CH3NH3PbI3 layer via continuous dripping method for high-performance mesoporous perovskite solar cells. Appl. Surf. Sci. 2017, 392, 960–965. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Zhao, X.; Zhang, J.; Wang, H.; Zhu, Z.; Liu, Q. Band alignment strategy for printable triple mesoscopic perovskite solar cells with enhanced photovoltage. Acs Appl. Energy Mater. 2019, 2, 2034–2042. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Zhang, J.; Yang, Z.; Liu, S.; Li, C. High efficiency flexible perovskite solar cells using superior low temperature TiO2. Energy. Environ. Sci. 2015, 8, 3208–3214. [Google Scholar] [CrossRef]
- Lan, Z.; Xu, X.; Zhang, X.; Tang, J.; Zhang, L.; He, X.; Wu, J. Low-temperature solution-processed efficient electron-transporting layers based on BF4−-capped TiO2 nanorods for high-performance planar perovskite solar cells. J. Mater. Chem. C 2018, 6, 334–341. [Google Scholar] [CrossRef]
- Jones, E.W.; Holliman, P.J.; Bowen, L.; Connell, A.; Kershaw, C.; Meza-Rojas, D.E. Hybrid Al2O3-CH3NH3PbI3 perovskites towards avoiding toxic solvents. Materials 2020, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Boschloo, G.; Schwarzmueller, S.; Yang, L.; Johansson, E.M.J.; Hagfeldt, A. Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cells. Nanoscale 2013, 5, 11686–11691. [Google Scholar] [CrossRef] [PubMed]
- Che, M.; Zhu, L.; Zhao, Y.; Yao, D.; Gu, X.; Song, J.; Qiang, Y. Enhancing current density of perovskite solar cells using TiO2-ZrO2 composite scaffold layer. Mater. Sci. Semicond. Proc. 2016, 56, 29–36. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, M.C.; Ke, W.J.; Zheng, X.L.; Chen, Z.; Qin, P.L.; Xiong, L.B.; Lei, H.W.; Wan, J.W.; Wen, J.; et al. Enhanced stability of perovskite solar cells with low-temperature hydrothermally grown SnO2 electron transport layers. Adv. Funct. Mater. 2016, 26, 6069–6075. [Google Scholar] [CrossRef]
- Huh, D.; Oh, K.; Kim, M.; Choi, H.-J.; Kim, D.S.; Lee, H. Selectively patterned TiO2 nanorods as electron transport pathway for high performance perovskite solar cells. Nano Res. 2019, 12, 601–606. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Graetzel, M.; Park, N.-G. High efficiency solid-state sensitized solar cell-based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. Nano. Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef]
- Bhoomanee, C.; Sanglao, J.; Kumnorkaew, P.; Wang, T.; Lohawet, K.; Ruankham, P.; Gardchareon, A.; Wongratanaphisan, D. Hydrothermally treated TiO2 nanorods as electron transport layer in planar perovskite solar cells. Phys. Status Solidi A 2021, 218, 2000238. [Google Scholar] [CrossRef]
- Feng, S.; Runa, A.; Liu, L.; Wang, J.; Su, P.; Liu, T.; Su, S.; Zhu, G.; Fu, W.; Yang, H. Fabrication of TiO2 nanorods/nanoparticles mixed phase structure via a simple dip-coating method and its application in perovskite solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 16903–16910. [Google Scholar] [CrossRef]
- Shahvaranfard, F.; Altomare, M.; Hou, Y.; Hejazi, S.; Meng, W.; Osuagwu, B.; Li, N.; Brabec, C.J.; Schmuki, P. Engineering of the electron transport layer/perovskite interface in solar cells designed on TiO2 rutile nanorods. Adv. Funct. Mater. 2020, 30, 1909738. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Yan, K.; Yi, Y.; Wang, J.; Yang, S. Liquid phase deposition of TiO2 nanolayer affords CH3NH3PbI3/nanocarbon solar cells with high open-circuit voltage. Faraday Discuss. 2014, 176, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.S.; Shim, C.S.; Park, H.K.; Heo, J.; Patil, P.S.; Hong, C.K. Ultrathin Atomic layer deposited TiO2 for surface passivation of hydrothermally grown 1D TiO2 nanorod arrays for efficient solid-state perovskite solar cells. Chem. Mater. 2015, 27, 1541–1551. [Google Scholar] [CrossRef]
- Yang, J.S.; Liao, W.P.; Wu, J.J. Morphology and interfacial energetics controls for hierarchical anatase/rutile TiO2 nanostructured array for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2013, 5, 7425–7431. [Google Scholar] [CrossRef] [PubMed]
- Hen, Y.; Tao, Q.; Fu, W.; Yang, H.; Zhou, X.; Su, S.; Ding, D.; Mu, Y.; Li, X.; Li, M. Enhanced photoelectric performance of PbS/CdS quantum dot co-sensitized solar cells via hydrogenated TiO2 nanorod arrays. Chem. Commun. 2014, 50, 9509–9512. [Google Scholar]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(Nh2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 16177. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-structure perovskite solar cells with efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Chen, D.; Cheng, Y.-B.; Caruso, R.A. Three-dimensional titanium oxide nanoarrays for perovskite photovoltaics: Surface engineering for cascade charge extraction and beneficial surface passivation. Sustain. Energy Fuels 2017, 1, 1960–1967. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, T.; Tang, Z.; Sun, B.; Liao, G. Using a low-temperature carbon electrode for preparing hole-conductor-free perovskite heterojunction solar cells under high relative humidity. Nanoscale 2016, 8, 7017–7023. [Google Scholar] [CrossRef]
- Makuła, P.; Michał, P.; Wojciech, M. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Žerjav, G.; Žižek, K.; Zavašnik, J.A.; Pinter, A. Brookite vs. rutile vs. anatase: What’s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Paola, A.D.; Bellardita, M.; Brookite, L.P. The least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Nardes, A.M.; Zhu, K. Mesoporous perovskite solar cells: Material composition, charge-carrier dynamics, and device characteristics. Faraday Discuss. 2014, 176, 301–312. [Google Scholar] [CrossRef]
- Kogo, A.; Sanehira, Y.; Numata, Y.; Ikegami, M.; Miyasaka, T. Amorphous metal oxide blocking layers for highly efficient low-temperature brookite TiO2-based perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Visal, S.; Kuniyoshi, M.; Kaneko, T.; Umezu, S.; Katsumata, T.; Iwamori, S.; Kakihana, M.; Taima, T.; Isomura, M.; et al. Low-temperature-processed brookite-based TiO2 heterophase junction enhances performance of planar perovskite solar cells. Nano Lett. 2018, 19, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wan, L.; Kong, M.; Hu, H.; Gan, Y.; Wang, J.; Chen, F.; Guo, Z.; Eder, D.; Wang, S. Influence of Rutile-TiO2 nanorod arrays on Pb-free (CH3NH3)3Bi2I9-based hybrid perovskite solar cells fabricated through two-step sequential solution process. J. Alloy. Compd. 2018, 738, 422–431. [Google Scholar] [CrossRef]

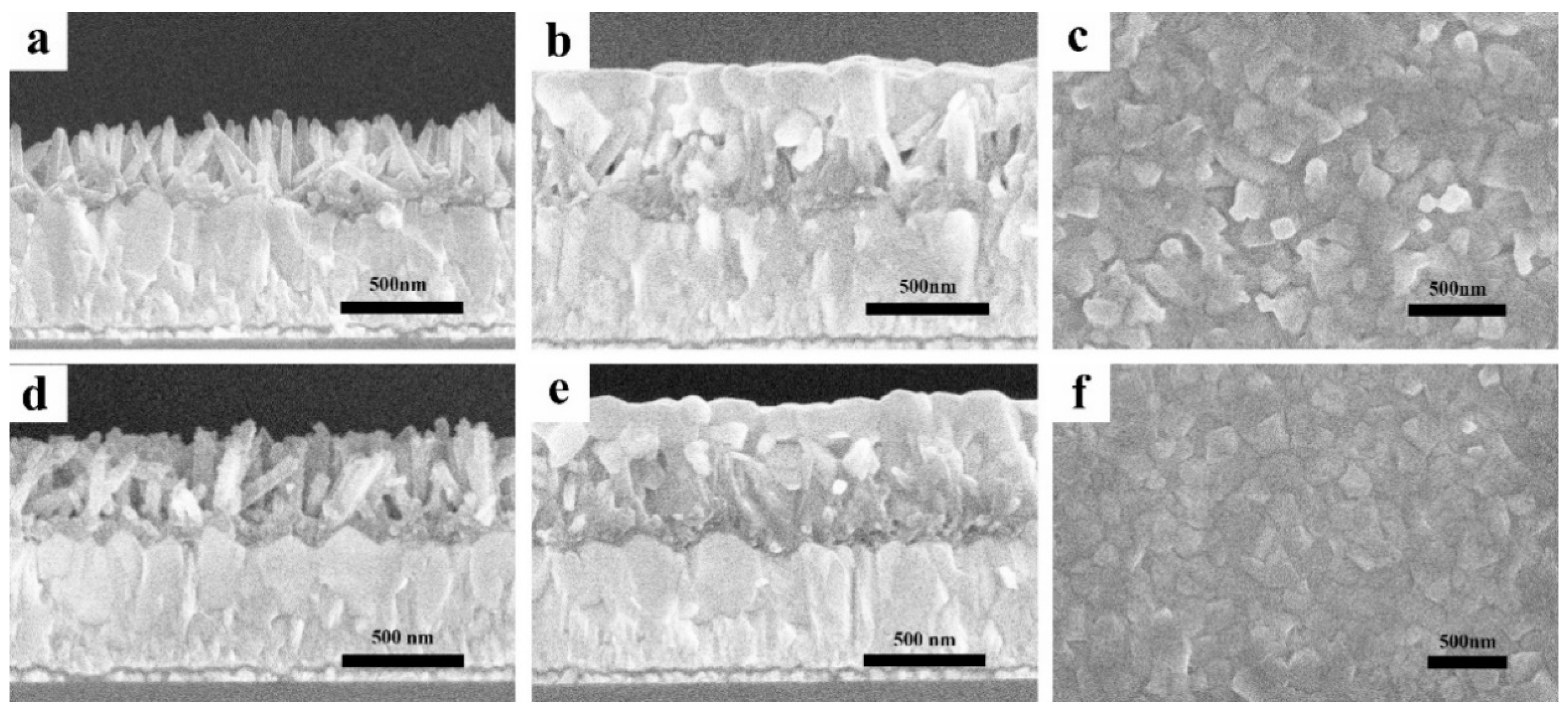
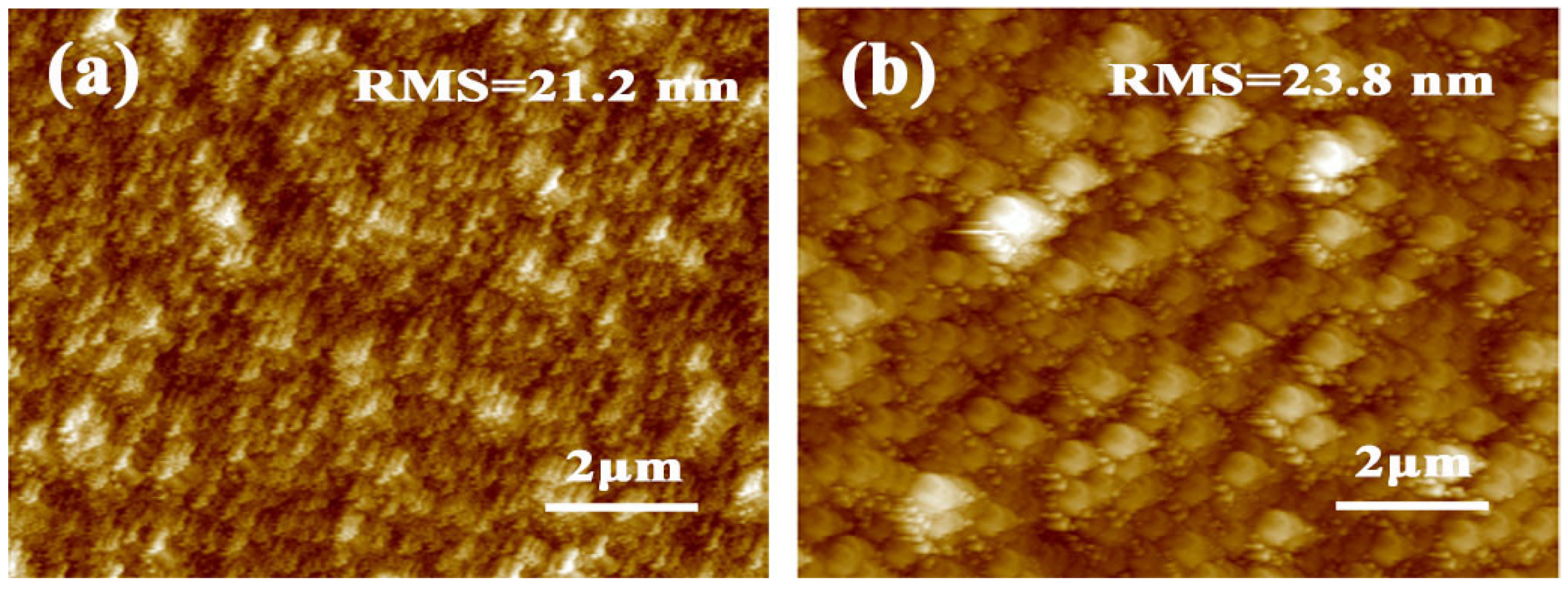
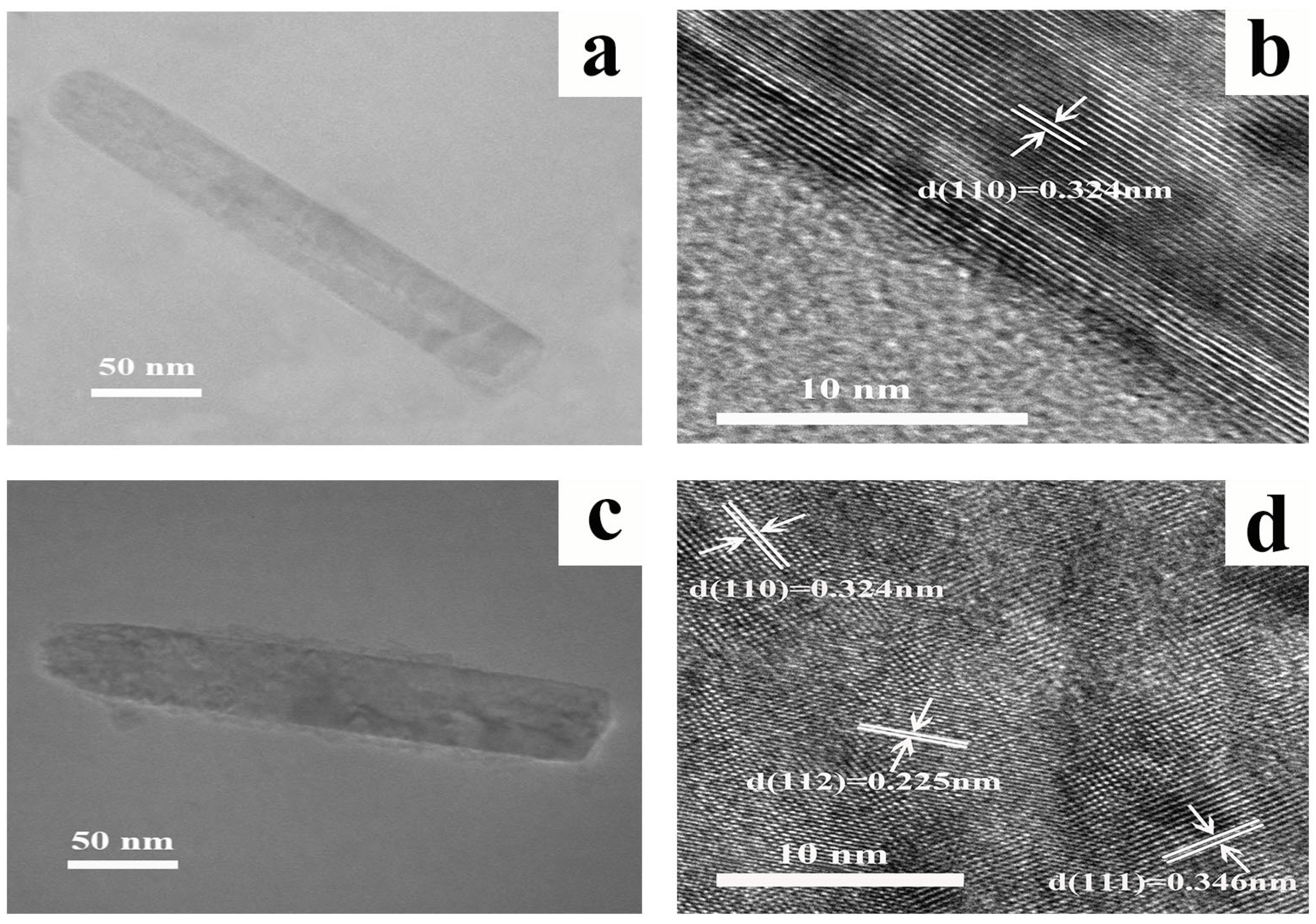
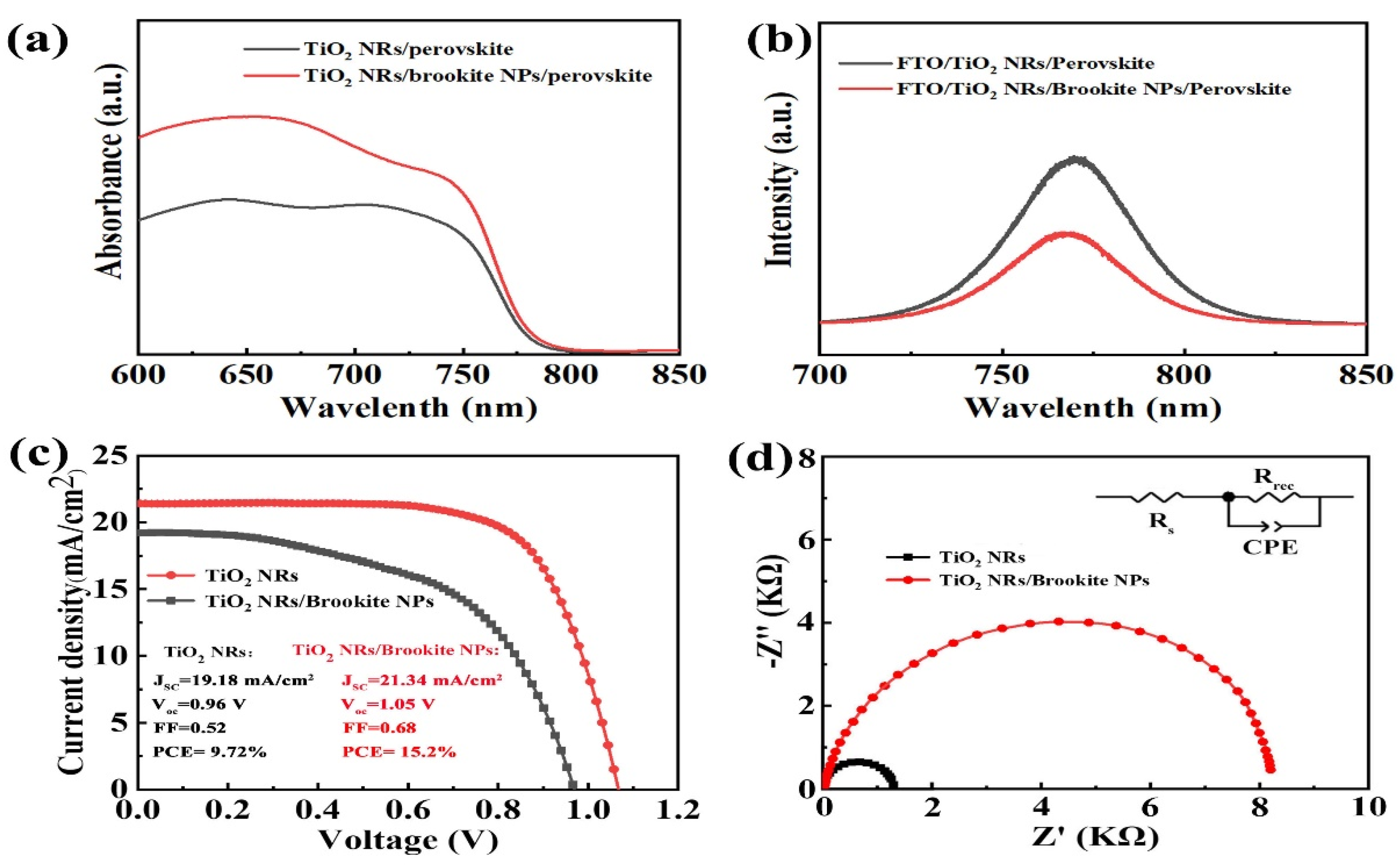
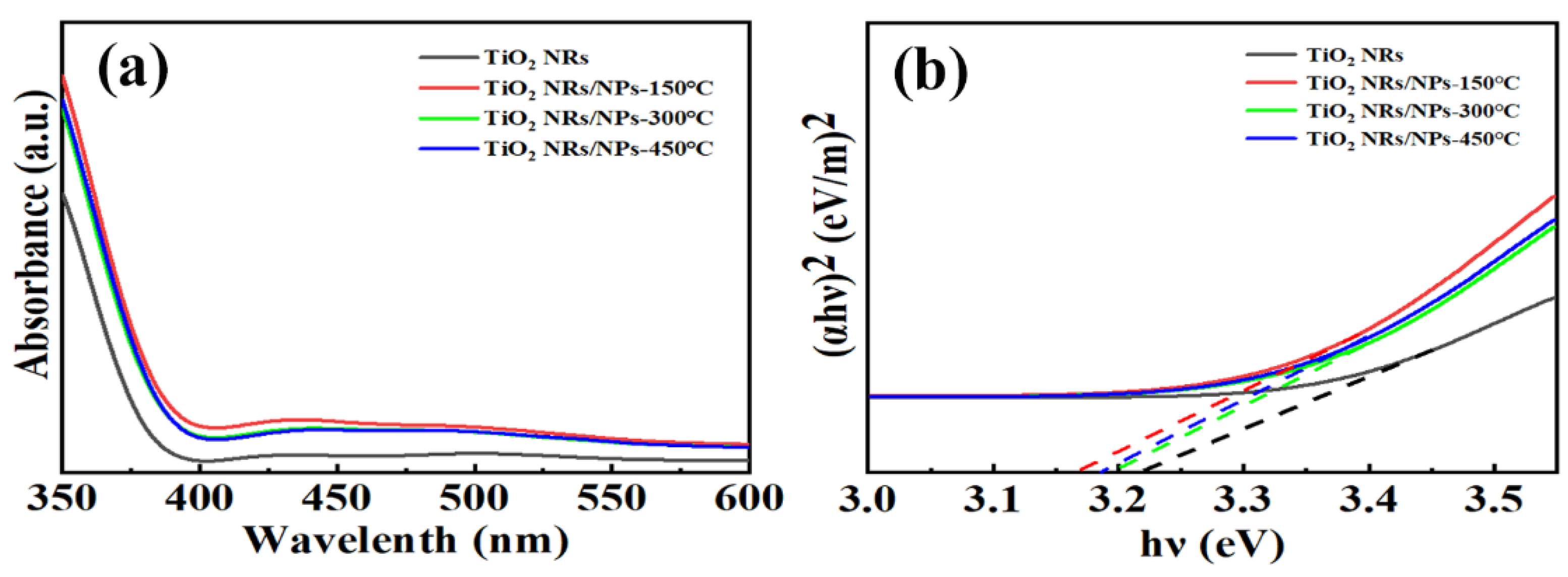
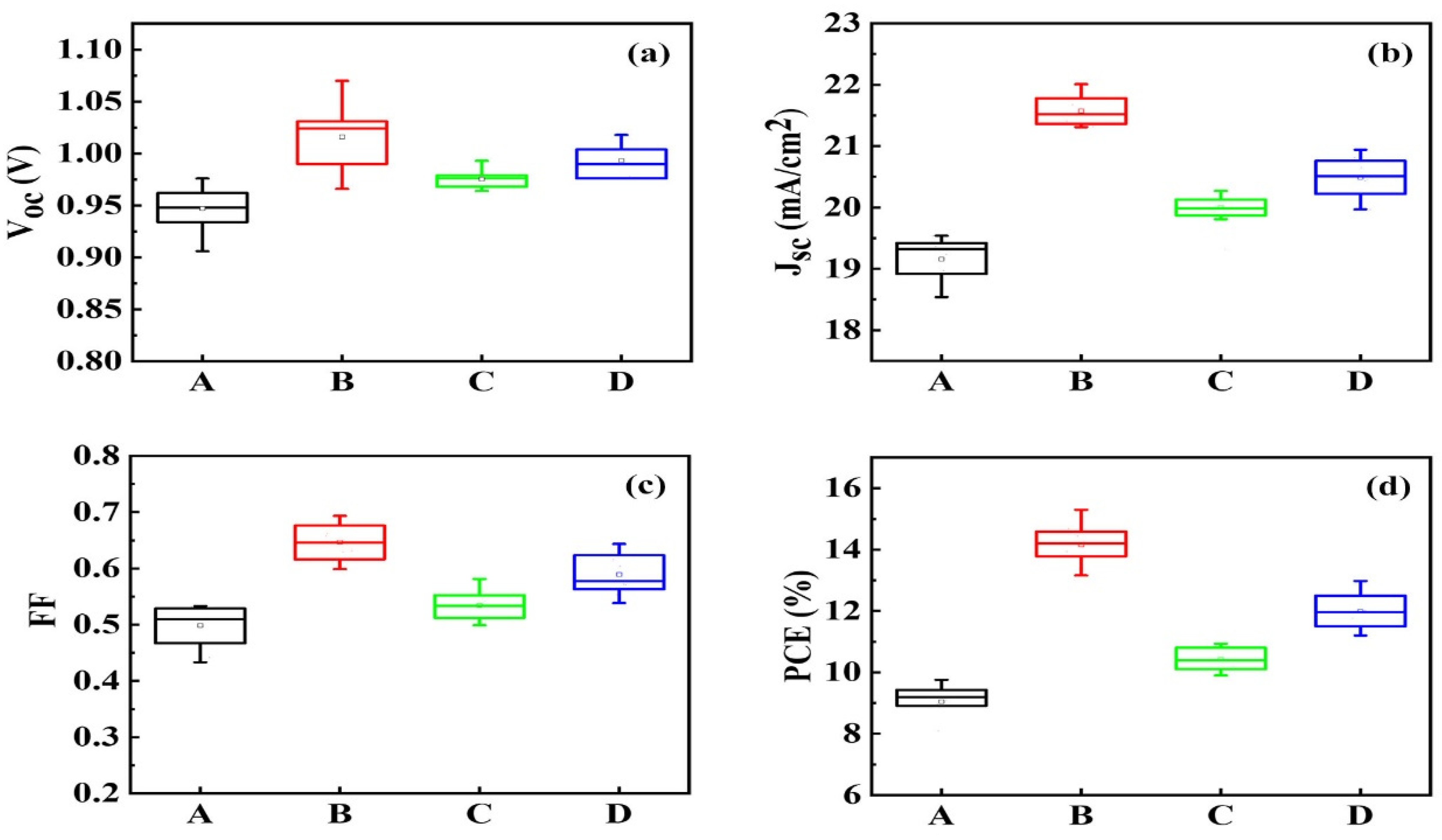
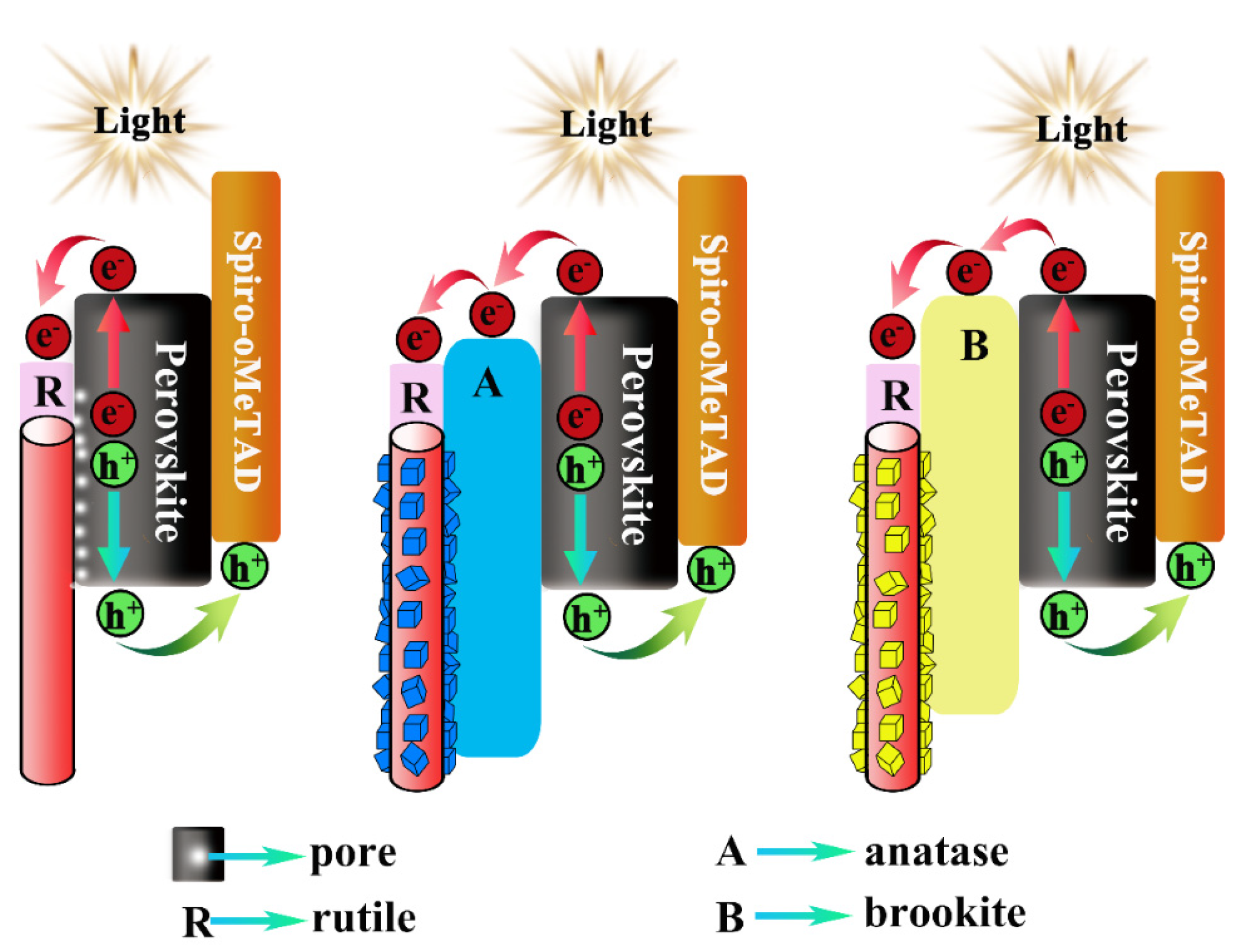
| Sample | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) | PCEbest (%) |
|---|---|---|---|---|---|
| A | 19.16 ± 0.61 | 0.947 ± 0.029 | 0.4987 ± 0.03 | 9.09 ± 0.62 | 9.71 |
| B | 21.57 ± 0.44 | 1.016 ± 0.054 | 0.6465 ± 0.04 | 14.16 ± 1.15 | 15.2 |
| C | 19.99 ± 0.65 | 0.975 ± 0.018 | 0.5345 ± 0.04 | 10.42 ± 0.51 | 10.93 |
| D | 20.49 ± 0.32 | 0.99 ± 0.019 | 0.5891 ± 0.04 | 11.98 ± 1.00 | 12.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, J.; Yang, W.; Zhu, Y.; Feng, S.; Su, P.; Fu, W. Low-Temperature Processed Brookite Interfacial Modification for Perovskite Solar Cells with Improved Performance. Nanomaterials 2022, 12, 3653. https://doi.org/10.3390/nano12203653
Yang J, Wang J, Yang W, Zhu Y, Feng S, Su P, Fu W. Low-Temperature Processed Brookite Interfacial Modification for Perovskite Solar Cells with Improved Performance. Nanomaterials. 2022; 12(20):3653. https://doi.org/10.3390/nano12203653
Chicago/Turabian StyleYang, Jiandong, Jun Wang, Wenshu Yang, Ying Zhu, Shuang Feng, Pengyu Su, and Wuyou Fu. 2022. "Low-Temperature Processed Brookite Interfacial Modification for Perovskite Solar Cells with Improved Performance" Nanomaterials 12, no. 20: 3653. https://doi.org/10.3390/nano12203653
APA StyleYang, J., Wang, J., Yang, W., Zhu, Y., Feng, S., Su, P., & Fu, W. (2022). Low-Temperature Processed Brookite Interfacial Modification for Perovskite Solar Cells with Improved Performance. Nanomaterials, 12(20), 3653. https://doi.org/10.3390/nano12203653







