Recent Advances in g-C3N4 for the Application of Perovskite Solar Cells
Abstract
1. Introduction
2. g-C3N4 as an Additive in Perovskite Films
2.1. Pure g–C3N4 Nanosheets as an Additive
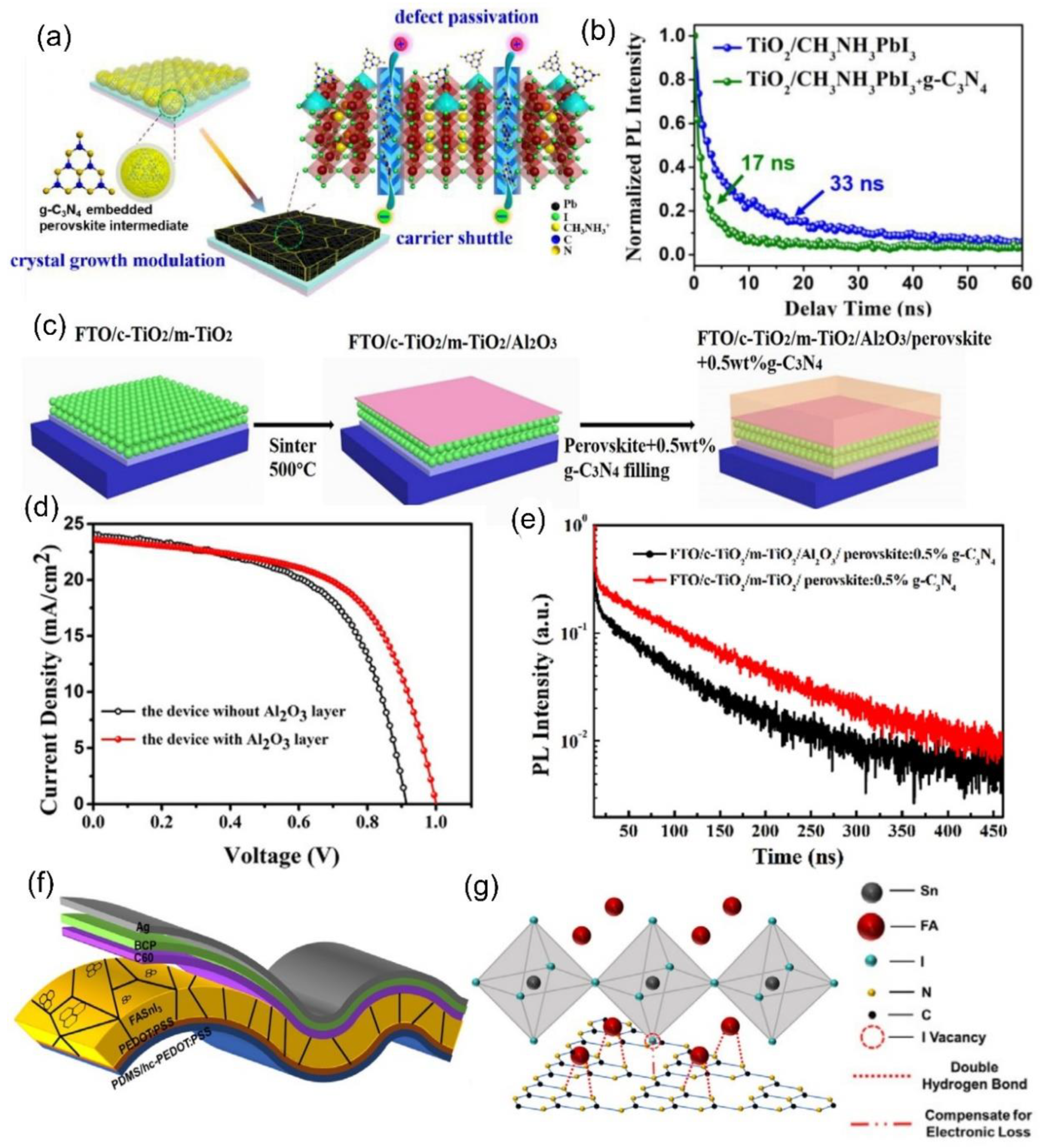
2.2. Functionalized g–C3N4 Nanosheets as an Additive
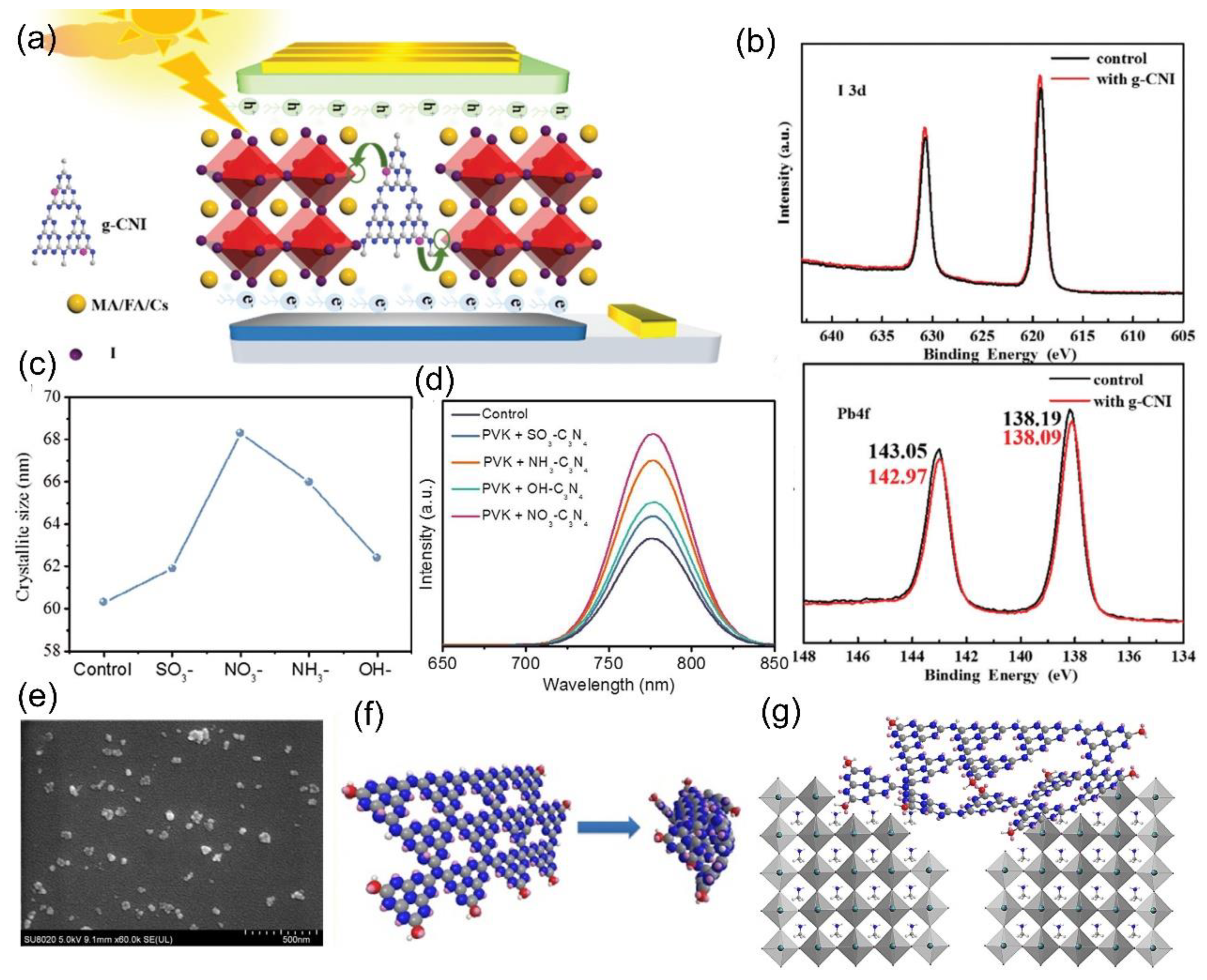
2.3. Ultrafine g–C3N4 Nanoparticles as an Additive
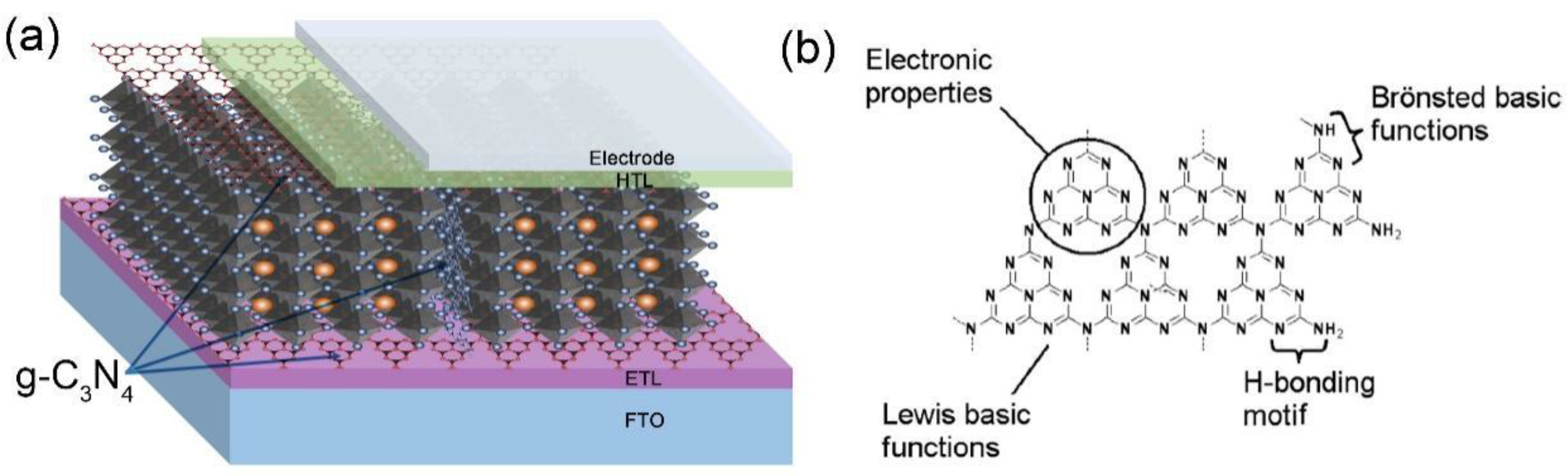
3. g–C3N4 as a Surface Modifier Layer
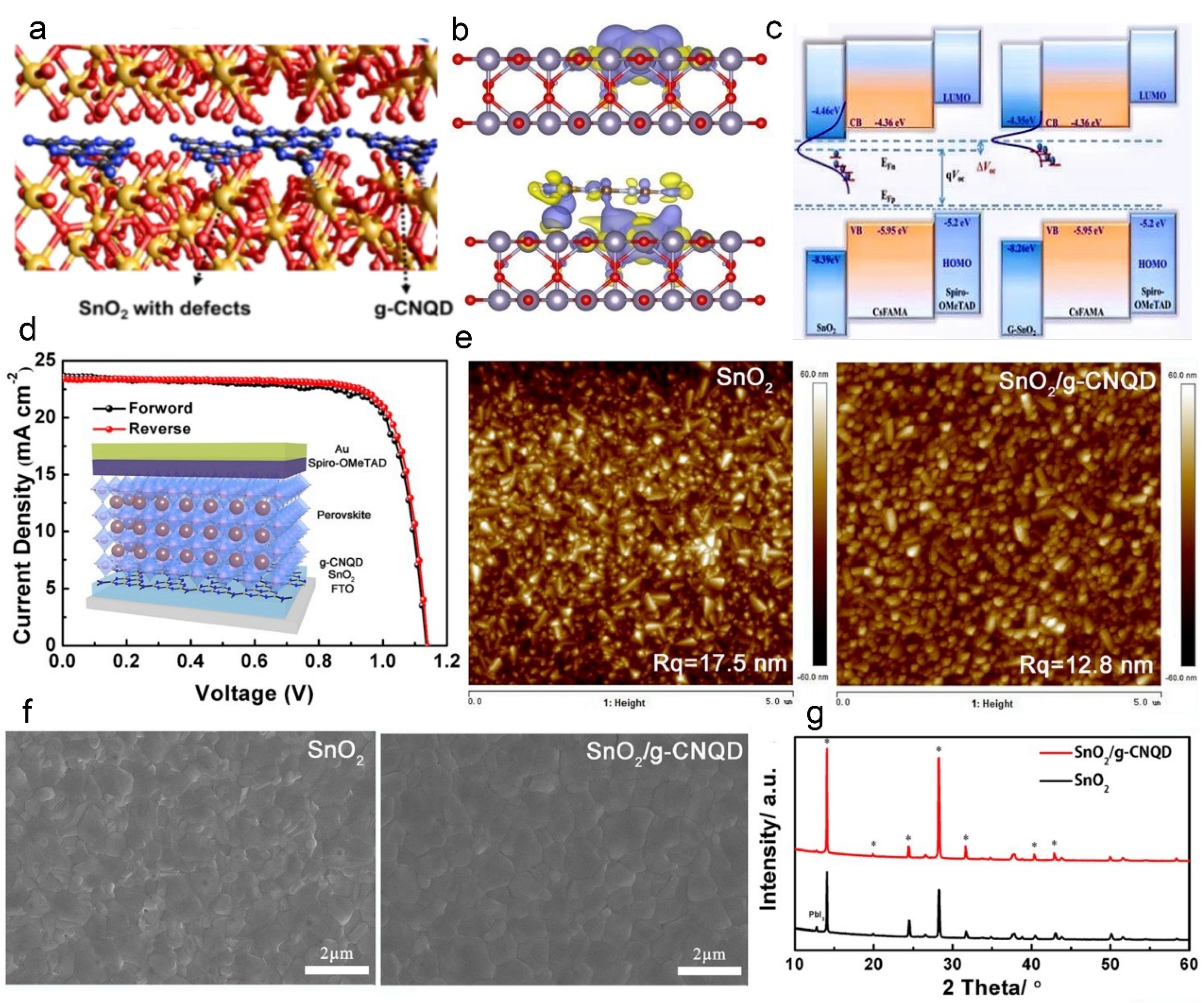
3.1. g–C3N4 Quantum Dots (g–CNQD) as Modifier Layer
3.2. g–C3N4 Nanosheets as a Modified Layer
3.3. Functionalized g–C3N4 as a Modified Layer
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Lu, Y.; Han, W.; Zhu, J.; Zhang, Y.; Huang, W. Solar energy conversion and utilization: Towards the emerging photo-electrochemical devices based on perovskite photovoltaics. Chem. Eng. J. 2020, 393, 124766. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, M.; Zhang, Y.; Du, J.; Han, D.; Yang, L.; Fan, L.; Sui, Y.; Sun, Y.; Meng, X.; et al. Constructing m-TiO2/a-WOx hybrid electron transport layer to boost interfacial charge transfer for efficient perovskite solar cells. Chem. Eng. J. 2020, 402, 126303. [Google Scholar] [CrossRef]
- Chen, H.; Ye, F.; Tang, W.; He, J.; Yin, M.; Wang, Y.; Xie, F.; Bi, E.; Yang, X.; Grätzel, M.; et al. A solvent- and vacuum-free route to large-area perovskite films for efficient solar modules. Nature 2017, 550, 92–95. [Google Scholar] [CrossRef]
- Li, H.; Chen, C.; Jin, J.; Bi, W.; Zhang, B.; Chen, X.; Xu, L.; Liu, D.; Dai, Q.; Song, H. Near-infrared and ultraviolet to visible photon conversion for full spectrum response perovskite solar cells. Nano Energy 2018, 50, 699–709. [Google Scholar] [CrossRef]
- Han, G.S.; Jung, H.S.; Park, N.-G. Recent cutting-edge strategies for flexible perovskite solar cells toward commercialization. Chem. Commun. 2021, 57, 11604–11612. [Google Scholar] [CrossRef]
- Tang, G.; Yan, F. Recent progress of flexible perovskite solar cells. Nano Today 2021, 39, 101155. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.-B.; et al. Conformal quantum dot–SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef]
- Cheng, Y.; Ding, L. Pushing commercialization of perovskite solar cells by improving their intrinsic stability. Energy Environ. Sci. 2021, 14, 3233–3255. [Google Scholar] [CrossRef]
- Rong, Y.G.; Hu, Y.; Mei, A.Y.; Tan, H.R.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H.W. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The Main Progress of Perovskite Solar Cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Kim, M.-c.; Ham, S.-Y.; Cheng, D.; Wynn, T.A.; Jung, H.S.; Meng, Y.S. Advanced Characterization Techniques for Overcoming Challenges of Perovskite Solar Cell Materials. Adv. Energy Mater. 2021, 11, 2001753. [Google Scholar] [CrossRef]
- Fan, R.; Huang, Y.; Wang, L.; Li, L.; Zheng, G.; Zhou, H. The Progress of Interface Design in Perovskite-Based Solar Cells. Adv. Energy Mater. 2016, 6, 1600460. [Google Scholar] [CrossRef]
- Arjun, V.; Muthukumaran, K.P.; Ramachandran, K.; Nithya, A.; Karuppuchamy, S. Fabrication of efficient and stable planar perovskite solar cell using copper oxide as hole transport material. J. Alloys Compd. 2022, 923, 166285. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Xue, X.; Wu, Y.; Hao, Y.; Zhang, C.; Liu, Y.; Dai, Z.; Sun, Q.; Hao, Y. Importance of tin (II) acetate additives in sequential deposited fabrication of Sn-Pb-based perovskite solar cells. J. Alloys Compd. 2022, 904, 164050. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, W.; Li, Y.; Ye, S.; Rao, H.; Gu, F.; Liu, Z.; Bian, Z.; Huang, C. Simplification of device structures for low-cost, high-efficiency perovskite solar cells. J. Mater. Chem. A 2017, 5, 4756–4773. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, K.; Gu, B.; Cao, F.; Lu, H.; Zhang, Y.; Li, L. Boosting Efficiency and Stability of Perovskite Solar Cells with CdS Inserted at TiO2/Perovskite Interface. Adv. Mater. Interfaces 2016, 3, 1600729. [Google Scholar] [CrossRef]
- Taheri, S.; Ahmadkhan kordbacheh, A.; Minbashi, M.; Hajjiah, A. Effect of defects on high efficient perovskite solar cells. Opt. Mater. 2021, 111, 110601. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wan, J.; Tao, H.; Liu, Q.; Xiong, L.; Qin, P.; Wang, J.; Lei, H.; Yang, G.; et al. Efficient hole-blocking layer-free planar halide perovskite thin-film solar cells. Nat. Commun. 2015, 6, 6700. [Google Scholar] [CrossRef]
- Li, B.; Ferguson, V.; Silva, S.R.P.; Zhang, W. Defect Engineering toward Highly Efficient and Stable Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800326. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Tavakoli, R.; Kong, J. Surface Engineering of TiO2 ETL for Highly Efficient and Hysteresis-Less Planar Perovskite Solar Cell (21.4%) with Enhanced Open-Circuit Voltage and Stability. Adv. Energy Mater. 2018, 8, 1800794. [Google Scholar] [CrossRef]
- Montoya, D.M.; Pérez-Gutiérrez, E.; Barbosa-Garcia, O.; Bernal, W.; Maldonado, J.-L.; Percino, M.J.; Meneses, M.-A.; Cerón, M. Defects at the interface electron transport layer and alternative counter electrode, their impact on perovskite solar cells performance. Sol. Energy 2020, 195, 610–617. [Google Scholar] [CrossRef]
- Dong, Y.; Li, W.; Zhang, X.; Xu, Q.; Liu, Q.; Li, C.; Bo, Z. Highly Efficient Planar Perovskite Solar Cells Via Interfacial Modification with Fullerene Derivatives. Small 2016, 12, 1098–1104. [Google Scholar] [CrossRef]
- Jain, S.M.; Phuyal, D.; Davies, M.L.; Li, M.; Philippe, B.; De Castro, C.; Qiu, Z.; Kim, J.; Watson, T.; Tsoi, W.C.; et al. An effective approach of vapour assisted morphological tailoring for reducing metal defect sites in lead-free, (CH3NH3)3Bi2I9 bismuth-based perovskite solar cells for improved performance and long-term stability. Nano Energy 2018, 49, 614–624. [Google Scholar] [CrossRef]
- Ali, J.; Li, Y.; Gao, P.; Hao, T.; Song, J.; Zhang, Q.; Zhu, L.; Wang, J.; Feng, W.; Hu, H.; et al. Interfacial and structural modifications in perovskite solar cells. Nanoscale 2020, 12, 5719–5745. [Google Scholar] [CrossRef]
- Han, T.H.; Tan, S.; Xue, J.; Meng, L.; Lee, J.W.; Yang, Y. Interface and Defect Engineering for Metal Halide Perovskite Optoelectronic Devices. Adv. Mater. 2019, 31, 1803515. [Google Scholar] [CrossRef]
- Qiu, L.; Ono, L.K.; Jiang, Y.; Leyden, M.R.; Raga, S.R.; Wang, S.; Qi, Y. Engineering Interface Structure to Improve Efficiency and Stability of Organometal Halide Perovskite Solar Cells. J. Phys. Chem. B 2018, 122, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, X.; Yang, S. Interface Engineering for Highly Efficient and Stable Planar p-i-n Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1701883. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Fakharuddin, A.; Coutsolelos, A.G.; Falaras, P.; Argitis, P.; Yusoff, A.; Nazeeruddin, M.K. Molecular materials as interfacial layers and additives in perovskite solar cells. Chem. Soc. Rev. 2020, 49, 4496–4526. [Google Scholar] [CrossRef]
- Boopathi, K.M.; Mohan, R.; Huang, T.-Y.; Budiawan, W.; Lin, M.-Y.; Lee, C.-H.; Ho, K.-C.; Chu, C.-W. Synergistic improvements in stability and performance of lead iodide perovskite solar cells incorporating salt additives. J. Mater. Chem. A 2016, 4, 1591–1597. [Google Scholar] [CrossRef]
- Gong, X.; Li, M.; Shi, X.-B.; Ma, H.; Wang, Z.-K.; Liao, L.-S. Controllable Perovskite Crystallization by Water Additive for High-Performance Solar Cells. Adv. Funct. Mater. 2015, 25, 6671–6678. [Google Scholar] [CrossRef]
- Kang, Y.F.; Wang, A.R.; Li, R.; Song, Y.L.; Dong, Q.F. A Review: Flexible Perovskite Solar Cells towards High Mechanical Stability. Acta Polym. Sin. 2021, 52, 920–937. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, L.; Li, H.; Huang, W.; Sun, P.; Liu, J.; Liu, S.; Sun, Q.; Cui, Z.; Sun, L.; et al. Efficient Planar Perovskite Solar Cells with Improved Fill Factor via Interface Engineering with Graphene. Nano Lett. 2018, 18, 2442–2449. [Google Scholar] [CrossRef]
- Ioakeimidis, A.; Choulis, S.A. Nitrobenzene as Additive to Improve Reproducibility and Degradation Resistance of Highly Efficient Methylammonium-Free Inverted Perovskite Solar Cells. Materials 2020, 13, 3289. [Google Scholar] [CrossRef]
- Chu, L.; Ahmad, W.; Liu, W.; Yang, J.; Zhang, R.; Sun, Y.; Yang, J.; Li, X.A. Lead-Free Halide Double Perovskite Materials: A New Superstar Toward Green and Stable Optoelectronic Applications. Nano-Micro Lett. 2019, 11, 16. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Yang, L.; Yin, S. Preparation and performance of perovskite solar cells with two dimensional MXene as active layer additive. J. Alloys Compd. 2022, 904, 163742. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zhang, J.; Yuan, Y.; Wang, Z.; Zhu, Z. Improving Photovoltaic Performance Using Perovskite/Surface-Modified Graphitic Carbon Nitride Heterojunction. Sol. RRL 2019, 4, 1900413. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.-C.; Fan, H.-C.; Liu, T.-W.; Chen, J.-W. Methylamine lead bromide perovskite/protonated graphitic carbon nitride nanocomposites: Interfacial charge carrier dynamics and photocatalysis. J. Mater. Chem. A 2017, 5, 25438–25449. [Google Scholar] [CrossRef]
- Jia, C.; Yang, L.; Zhang, Y.; Zhang, X.; Xiao, K.; Xu, J.; Liu, J. Graphitic Carbon Nitride Films: Emerging Paradigm for Versatile Applications. ACS Appl. Mater. Interfaces 2020, 12, 53571–53591. [Google Scholar] [CrossRef]
- Javad, S.; Guoxiu, W. Progress and prospects of two-dimensional materials for membrane-based osmotic power generation. Nano Res. Energy 2022, 1, e9120008. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Cao, Q.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Graphitic carbon nitride and polymers: A mutual combination for advanced properties. Mater. Horiz. 2020, 7, 762–786. [Google Scholar] [CrossRef]
- Niu, X.; Yi, Y.; Bai, X.; Zhang, J.; Zhou, Z.; Chu, L.; Yang, J.; Li, X. Photocatalytic performance of few-layer graphitic g-C3N4: Enhanced by interlayer coupling. Nanoscale 2019, 11, 4101–4107. [Google Scholar] [CrossRef]
- Lu, D.; Fang, P.; Wu, W.; Ding, J.; Jiang, L.; Zhao, X.; Li, C.; Yang, M.; Li, Y.; Wang, D. Solvothermal-assisted synthesis of self-assembling TiO2 nanorods on large graphitic carbon nitride sheets with their anti-recombination in the photocatalytic removal of Cr(vi) and rhodamine B under visible light irradiation. Nanoscale 2017, 9, 3231–3245. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Lu, D.; Wang, H.; Zhao, X.; Kondamareddy, K.K.; Ding, J.; Li, C.; Fang, P. Highly Efficient Visible-Light-Induced Photoactivity of Z-Scheme g-C3N4/Ag/MoS2 Ternary Photocatalysts for Organic Pollutant Degradation and Production of Hydrogen. ACS Sustain. Chem. Eng. 2017, 5, 1436–1445. [Google Scholar] [CrossRef]
- Ou, M.; Tu, W.; Yin, S.; Xing, W.; Wu, S.; Wang, H.; Wan, S.; Zhong, Q.; Xu, R. Amino-Assisted Anchoring of CsPbBr3 Perovskite Quantum Dots on Porous g-C3N4 for Enhanced Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 13570–13574. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B-environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Ansari, M.S.; Banik, A.; Qureshi, M. Morphological tuning of photo-booster g-C3N4 with higher surface area and better charge transfers for enhanced power conversion efficiency of quantum dot sensitized solar cells. Carbon 2017, 121, 90–105. [Google Scholar] [CrossRef]
- Yuan, Z.; Tang, R.; Zhang, Y.; Yin, L. Enhanced photovoltaic performance of dye-sensitized solar cells based on Co9S8 nanotube array counter electrode and TiO2/g-C3N4 heterostructure nanosheet photoanode. J. Alloys Compd. 2017, 691, 983–991. [Google Scholar] [CrossRef]
- Xie, F.; Dong, G.; Wu, K.; Li, Y.; Wei, M.; Du, S. In situ synthesis of g-C3N4 by glass-assisted annealing route to boost the efficiency of perovskite solar cells. J. Colloid Interface Sci. 2021, 591, 326–333. [Google Scholar] [CrossRef]
- Gao, Q.; Sun, S.; Li, X.; Zhang, X.; Duan, L.; Lu, W. Enhancing Performance of CdS Quantum Dot-Sensitized Solar Cells by Two-Dimensional g-C3N4 Modified TiO2 Nanorods. Nanoscale Res. Lett. 2016, 11, 463. [Google Scholar] [CrossRef]
- Zou, J.; Liao, G.; Wang, H.; Ding, Y.; Wu, P.; Hsu, J.-P.; Jiang, J. Controllable interface engineering of g-C3N4/CuS nanocomposite photocatalysts. J. Alloys Compd. 2022, 911, 165020. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, A.; Yu, L.; Yuan, S.; Di, Y.; Liu, C.; Dong, L.; Gan, Z. Highly Efficient Charge Transfer between Perovskite Nanocrystals and g-C3N4 Nanosheets. Phys. Status Solidi (B) 2020, 257, 2000198. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Yin, J.; Aydin, E.; Harrison, G.T.; Liu, W.; Chen, S.; Mohammed, O.F.; De Wolf, S. Defect Passivation in Perovskite Solar Cells by Cyano-Based π-Conjugated Molecules for Improved Performance and Stability. Adv. Funct. Mater. 2020, 30, 2002861. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Wang, Z.-K.; Li, M.; Zhang, C.-C.; Ye, Q.-Q.; Hu, K.-H.; Lu, D.-Z.; Fang, P.-F.; Liao, L.-S. Passivated Perovskite Crystallization via g-C3N4 for High-Performance Solar Cells. Adv. Funct. Mater. 2018, 28, 1705875. [Google Scholar] [CrossRef]
- Liao, J.-F.; Wu, W.-Q.; Zhong, J.-X.; Jiang, Y.; Wang, L.; Kuang, D.-B. Enhanced efficacy of defect passivation and charge extraction for efficient perovskite photovoltaics with a small open circuit voltage loss. J. Mater. Chem. A 2019, 7, 9025–9033. [Google Scholar] [CrossRef]
- Li, X.; Bi, D.; Yi, C.; Décoppet, J.-D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef]
- Liu, W.W.; Liu, Y.C.; Cui, C.Y.; Niu, S.T.; Niu, W.J.; Liu, M.C.; Liu, M.J.; Gu, B.; Zhang, L.Y.; Zhao, K.; et al. All-inorganic CsPbBr3 perovskite solar cells with enhanced efficiency by exploiting lone pair electrons via passivation of crystal boundary using carbon nitride (g-C3N4) nanosheets. Mater. Today Energy 2021, 21, 100782. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, S.; Luo, X.; Gao, Y.; Li, S.; Zhu, J.; Tan, H. Simultaneous Contact and Grain-Boundary Passivation in Planar Perovskite Solar Cells Using SnO2-KCl Composite Electron Transport Layer. Adv. Energy Mater. 2019, 10, 1903083. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Zhang, Z.-Y.; Fan, W.-L.; Hu, C.-s.; Zhang, L.; Qi, J.-J. High-performance g-C3N4 added carbon-based perovskite solar cells insulated by Al2O3 layer. Sol. Energy 2019, 193, 859–865. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, X.; Mei, A.; Qin, F.; Liu, S.; Zhang, S.; Jiang, Y.; Zhou, Y.; Han, H. Bifunctional Al2O3 Interlayer Leads to Enhanced Open-Circuit Voltage for Hole-Conductor-Free Carbon-Based Perovskite Solar Cells. Sol. RRL 2018, 2, 1800002. [Google Scholar] [CrossRef]
- Han, G.S.; Chung, H.S.; Kim, B.J.; Kim, D.H.; Lee, J.W.; Swain, B.S.; Mahmood, K.; Yoo, J.S.; Park, N.-G.; Lee, J.H.; et al. Retarding charge recombination in perovskite solar cells using ultrathin MgO-coated TiO2 nanoparticulate films. J. Mater. Chem. A 2015, 3, 9160–9164. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, C.; Feng, Z.; Wu, Z.; Wang, Z.; Chen, X.; Huang, S. Synergetic Effect of Plasmonic Gold Nanorods and MgO for Perovskite Solar Cells. Nanomaterials 2020, 10, 1830. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Meng, X.; Xiao, S.; Xing, Z.; Fu, Q.; Wang, H.; Gong, C.; Hu, T.; Hu, X.; Guo, R.; et al. Wearable Tin-Based Perovskite Solar Cells Achieved by a Crystallographic Size Effect. Angew. Chem. Int. Ed. Engl. 2021, 60, 14693–14700. [Google Scholar] [CrossRef] [PubMed]
- Gillan, E.G. Synthesis of Nitrogen-Rich Carbon Nitride Networks from an Energetic Molecular Azide Precursor. Chem. Mater. 2000, 12, 3906–3912. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Hsu, Y.F.; Wang, S.F.; George, M.; Veerakumar, P.; Lin, K.C. Electrochemical sensor-based barium zirconate on sulphur-doped graphitic carbon nitride for the simultaneous determination of nitrofurantoin (antibacterial agent) and nilutamide (anticancer drug). J. Electroanal. Chem. 2021, 901, 115782. [Google Scholar] [CrossRef]
- Rakibuddin, M.; Kim, H.; Khan, M.E. Graphite-like carbon nitride (C3N4) modified N-doped LaTiO3 nanocomposite for higher visible light photocatalytic and photo-electrochemical performance. Appl. Surf. Sci. 2018, 452, 400–412. [Google Scholar] [CrossRef]
- Cao, W.; Lin, K.; Li, J.; Qiu, L.; Dong, Y.; Wang, J.; Xia, D.; Fan, R.; Yang, Y. Iodine-doped graphite carbon nitride for enhancing photovoltaic device performance via passivation trap states of triple cation perovskite films. J. Mater. Chem. C 2019, 7, 12717–12724. [Google Scholar] [CrossRef]
- Niu, T.; Lu, J.; Munir, R.; Li, J.; Barrit, D.; Zhang, X.; Hu, H.; Yang, Z.; Amassian, A.; Zhao, K.; et al. Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation. Adv. Mater. 2018, 30, e1706576. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Q.; Li, J.; Zhao, C.; Guo, X.; Zhao, Y.; Jiu, T. Grain boundary passivation with triazine-graphdiyne to improve perovskite solar cell performance. Sci. China Mater. 2020, 63, 2465–2476. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Sun, X.; Yang, Z.; Moreels, I.; Xu, K.; Li, H. Polymer assisted deposition of high-quality CsPbI2Br film with enhanced film thickness and stability. Nano Res. 2020, 13, 684–690. [Google Scholar] [CrossRef]
- Zeng, J.; Bi, L.; Cheng, Y.; Xu, B.; Jen, A.K.Y. Self-assembled monolayer enabling improved buried interfaces in blade-coated perovskite solar cells for high efficiency and stability. Nano Res. Energy 2022, 1, e9120004. [Google Scholar] [CrossRef]
- Ye, S.; Rao, H.; Zhao, Z.; Zhang, L.; Bao, H.; Sun, W.; Li, Y.; Gu, F.; Wang, J.; Liu, Z.; et al. A Breakthrough Efficiency of 19.9% Obtained in Inverted Perovskite Solar Cells by Using an Efficient Trap State Passivator Cu(thiourea)I. J. Am. Chem. Soc. 2017, 139, 7504–7512. [Google Scholar] [CrossRef]
- Wei, X.; Liu, X.; Liu, H.; Yang, S.; Zeng, H.; Meng, F.; Lei, X.; Liu, J. Exfoliated graphitic carbon nitride self-recognizing CH3NH3PbI3 grain boundaries by hydrogen bonding interaction for improved perovskite solar cells. Sol. Energy 2019, 181, 161–168. [Google Scholar] [CrossRef]
- Kang, B.; Biswas, K. Preferential CH3NH3+ Alignment and Octahedral Tilting Affect Charge Localization in Cubic Phase CH3NH3PbI3. J. Phys. Chem. C 2017, 121, 8319–8326. [Google Scholar] [CrossRef]
- Hao, Q.; Jia, G.; Wei, W.; Vinu, A.; Wang, Y.; Arandiyan, H.; Ni, B.-J. Graphitic carbon nitride with different dimensionalities for energy and environmental applications. Nano Res. 2019, 13, 18–37. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Wu, Q.; Du, P.; Zhu, J.; Dai, S.; Yang, S. Incorporating Graphitic Carbon Nitride (g-C3N4) Quantum Dots into Bulk-Heterojunction Polymer Solar Cells Leads to Efficiency Enhancement. Adv. Funct. Mater. 2016, 26, 1719–1728. [Google Scholar] [CrossRef]
- Chen, J.; Dong, H.; Zhang, L.; Li, J.; Jia, F.; Jiao, B.; Xu, J.; Hou, X.; Liu, J.; Wu, Z. Graphitic carbon nitride doped SnO2 enabling efficient perovskite solar cells with PCEs exceeding 22%. J. Mater. Chem. A 2020, 8, 2644–2653. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Wang, S.; Sakurai, T.; Wen, W.; Qi, Y. Energy Level Alignment at Interfaces in Metal Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800260. [Google Scholar] [CrossRef]
- Jena, A.K.; Ishii, A.; Guo, Z.; Kamarudin, M.A.; Hayase, S.; Miyasaka, T. Cesium Acetate-Induced Interfacial Compositional Change and Graded Band Level in MAPbI3 Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 33631–33637. [Google Scholar] [CrossRef]
- Singh, A.N.; Kajal, S.; Kim, J.; Jana, A.; Kim, J.Y.; Kim, K.S. Interface Engineering Driven Stabilization of Halide Perovskites against Moisture, Heat, and Light for Optoelectronic Applications. Adv. Energy Mater. 2020, 10, 2000768. [Google Scholar] [CrossRef]
- Liu, P.; Sun, Y.; Wang, S.; Zhang, H.; Gong, Y.; Li, F.; Shi, Y.; Du, Y.; Li, X.; Guo, S.-s.; et al. Two dimensional graphitic carbon nitride quantum dots modified perovskite solar cells and photodetectors with high performances. J. Power Sources 2020, 451, 227825. [Google Scholar] [CrossRef]
- Ameri, M.; Ghaffarkani, M.; Ghahrizjani, R.T.; Safari, N.; Mohajerani, E. Phenomenological morphology design of hybrid organic-inorganic perovskite solar cell for high efficiency and less hysteresis. Sol. Energy Mater. Sol. Cells 2020, 205, 110251. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, X.; Guo, X.; Niu, Q.; Yi, J.; Xia, R.; Min, Y. Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells. Molecules 2017, 22, 520. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, D.; Ma, Y.; Lu, Z.; Chen, Z.; Wang, S.; Xiao, L.; Gong, Q. Morphology control of the perovskite films for efficient solar cells. Dalton Trans. 2015, 44, 10582–10593. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, S.; Yang, X.; Zhou, Y.; Jin, J.; Sun, J.; Zhao, L.; Wang, S. The dual interfacial modification of 2D g-C3N4 for high-efficiency and stable planar perovskite solar cells. Nanoscale Adv. 2020, 2, 5396–5402. [Google Scholar] [CrossRef]
- Cao, J.; Tang, G.; You, P.; Wang, T.; Zheng, F.; Zhao, J.; Yan, F. Enhanced Performance of Planar Perovskite Solar Cells Induced by Van Der Waals Epitaxial Growth of Mixed Perovskite Films on WS2 Flakes. Adv. Funct. Mater. 2020, 30, 2002358. [Google Scholar] [CrossRef]
- Jin, J.; Wu, S.; Yang, X.; Zhou, Y.; Li, Z.; Cao, Q.; Chi, B.; Li, J.; Zhao, L.; Wang, S. Improve the efficiency of perovskite solar cells through the interface modification of g-C3N4 nanosheets. Mater. Lett. 2021, 304, 130685. [Google Scholar] [CrossRef]
- Yang, J.; Chu, L.; Hu, R.; Liu, W.; Liu, N.; Ma, Y.; Ahmad, W.; Li, X.a. Work function engineering to enhance open-circuit voltage in planar perovskite solar cells by g-C3N4 nanosheets. Nano Res. 2021, 14, 2139–2144. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Wu, J.; Hu, Q.; Wang, Y.; Russell, T.P.; Tu, Y.; Zhu, R. Optimizing Vertical Crystallization for Efficient Perovskite Solar Cells by Buried Composite Layers. Sol. RRL 2021, 5, 2100457. [Google Scholar] [CrossRef]
- Wang, L.; Fu, L.; Li, B.; Li, H.; Pan, L.; Chang, B.; Yin, L. Thiazole-Modified C3N4 Interfacial Layer for Defect Passivation and Charge Transport Promotion in Perovskite Solar Cells. Sol. RRL 2021, 5, 2000720. [Google Scholar] [CrossRef]
- Cruz, D.; Garcia Cerrillo, J.; Kumru, B.; Li, N.; Dario Perea, J.; Schmidt, B.; Lauermann, I.; Brabec, C.J.; Antonietti, M. Influence of Thiazole-Modified Carbon Nitride Nanosheets with Feasible Electronic Properties on Inverted Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 12322–12328. [Google Scholar] [CrossRef]

| Structure | PCE (%) | Voc (V) | Jsc (mA·cm−2) | FF (%) | Ref. | Year |
|---|---|---|---|---|---|---|
| ITO/PTAA/NO3-g-C3N4:CsFAMAPbI3−xBrx/PCBM/BCP/Ag | 20.08 | 1.11 | 22.84 | 79.20 | [41] | 2019 |
| FTO/c-TiO2/g-C3N4:MAPbI3/spiro-OMeTAD /MoO3/Ag | 19.49 | 1.07 | 24.31 | 74.0 | [63] | 2018 |
| FTO/c-TiO2/g-C3N4:MAPbI3/spiro-OMeTAD /Au | 21.10 | 1.16 | 23.00 | 79.0 | [64] | 2019 |
| FTO/c-TiO2/m-TiO2/g-C3N4:CsPbBr3/carbon | 8.00 | 1.277 | 7.80 | 80.32 | [66] | 2021 |
| FTO/c-TiO2/m-TiO2/Al2O3/g-C3N4:MAPbI3/carbon | 14.34 | 1.00 | 23.80 | 60.1 | [68] | 2019 |
| PDMS/hc-PEDOT:PSS/PEDOT:PSS/g-C3N4:FASnI3/C60/BCP/Ag | 8.56 | 0.621 | 20.68 | 66.68 | [72] | 2021 |
| FTO/TiO2/G-CNI:CsFAMAPbI3−xBrx/spiro-OMeTAD/Au | 18.28 | 1.07 | 22.97 | 74.0 | [76] | 2019 |
| FTO/c-TiO2/U-g-C3N4:MAPbI3/spiro-OMeTAD/Au | 15.80 | 1.10 | 23.20 | 62.0 | [82] | 2019 |
| ITO/CNQDs:SnO2/CsFAMAPbI3−xBrx/Spiro-MeOTAD/Au | 22.13 | 1.18 | 24.03 | 78.3 | [86] | 2020 |
| FTO/SnO2/CNQDs/(FA/MA/Cs)PbI3−(x+y)BrxCly/spiro-OMeTAD/Au | 21.23 | 1.14 | 23.39 | 79.6 | [91] | 2020 |
| FTO/SnO2/g-C3N4/MAPbI3/g-C3N4/Sspiro-OMeTAD/Au | 19.67 | 1.14 | 21.45 | 80.7 | [95] | 2020 |
| FTO/c-TiO2/m-TiO2/g-C3N4 nanosheets/MAPbI3/ Carbon | 11.37 | 1.02 | 16.91 | 66 | [97] | 2021 |
| FTO/c-TiO2/g-C3N4/MAPbI3/spiro-OMeTAD/Ag | 19.55 | 1.11 | 23.69 | 74.0 | [98] | 2021 |
| ITO/g-C3N4:SnO2/FA0.85MA0.11Cs0.04PbI2.67Br0.33·xPbI2/spiro-MeOTAD/Au | 21.54 | 1.19 | 23.21 | 78 | [99] | 2021 |
| ITO/PTAA/MAPbI3/PC60BM/CMB-vTA/AZO/Ag | 17.15 | 1.09 | 20.17 | 78.03 | [100] | 2019 |
| FTO/TiO2/thiazole-C3N4/(FAPbI3)0.875(CsPbBr3)0.125/spiro-OMeTAD/Ag | 19.23 | 1.11 | 22.50 | 77 | [101] | 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ma, Y.; Yang, J.; Liu, W.; Li, X. Recent Advances in g-C3N4 for the Application of Perovskite Solar Cells. Nanomaterials 2022, 12, 3625. https://doi.org/10.3390/nano12203625
Yang J, Ma Y, Yang J, Liu W, Li X. Recent Advances in g-C3N4 for the Application of Perovskite Solar Cells. Nanomaterials. 2022; 12(20):3625. https://doi.org/10.3390/nano12203625
Chicago/Turabian StyleYang, Jian, Yuhui Ma, Jianping Yang, Wei Liu, and Xing’ao Li. 2022. "Recent Advances in g-C3N4 for the Application of Perovskite Solar Cells" Nanomaterials 12, no. 20: 3625. https://doi.org/10.3390/nano12203625
APA StyleYang, J., Ma, Y., Yang, J., Liu, W., & Li, X. (2022). Recent Advances in g-C3N4 for the Application of Perovskite Solar Cells. Nanomaterials, 12(20), 3625. https://doi.org/10.3390/nano12203625





