Influence of the Composition and Testing Modes on the Electrochemical Performance of Li-Rich Cathode Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical and Structural Analyses
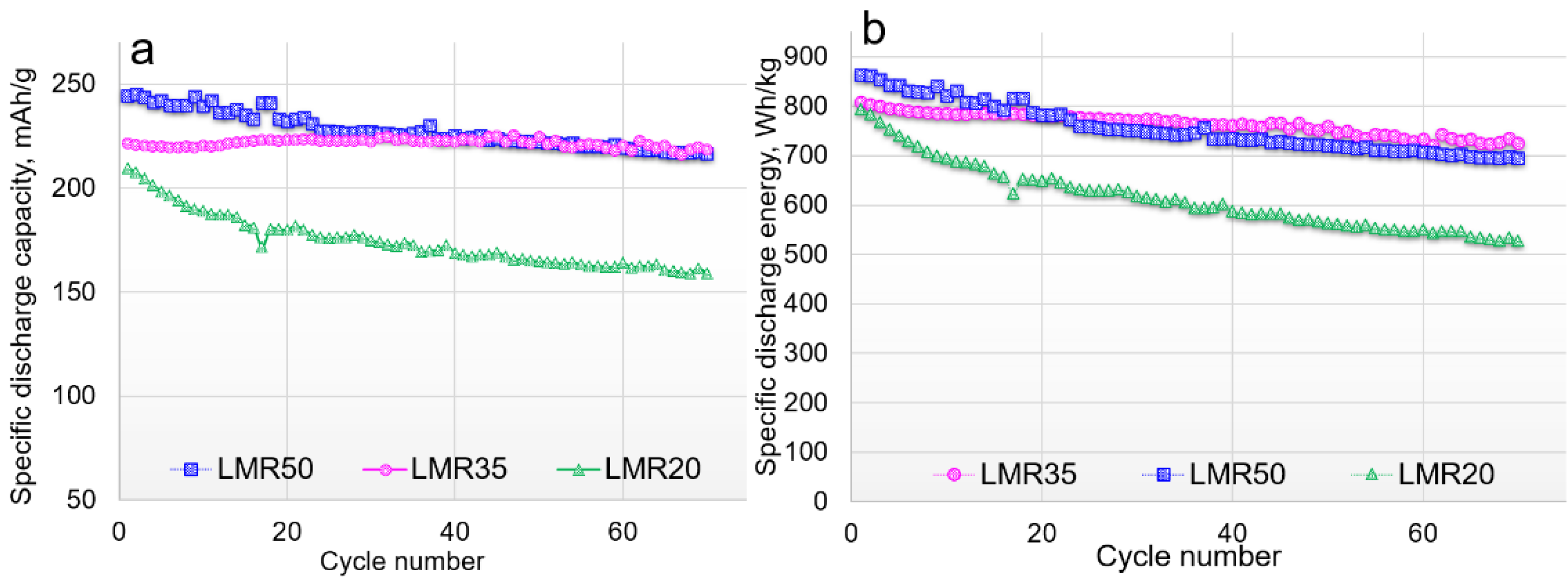
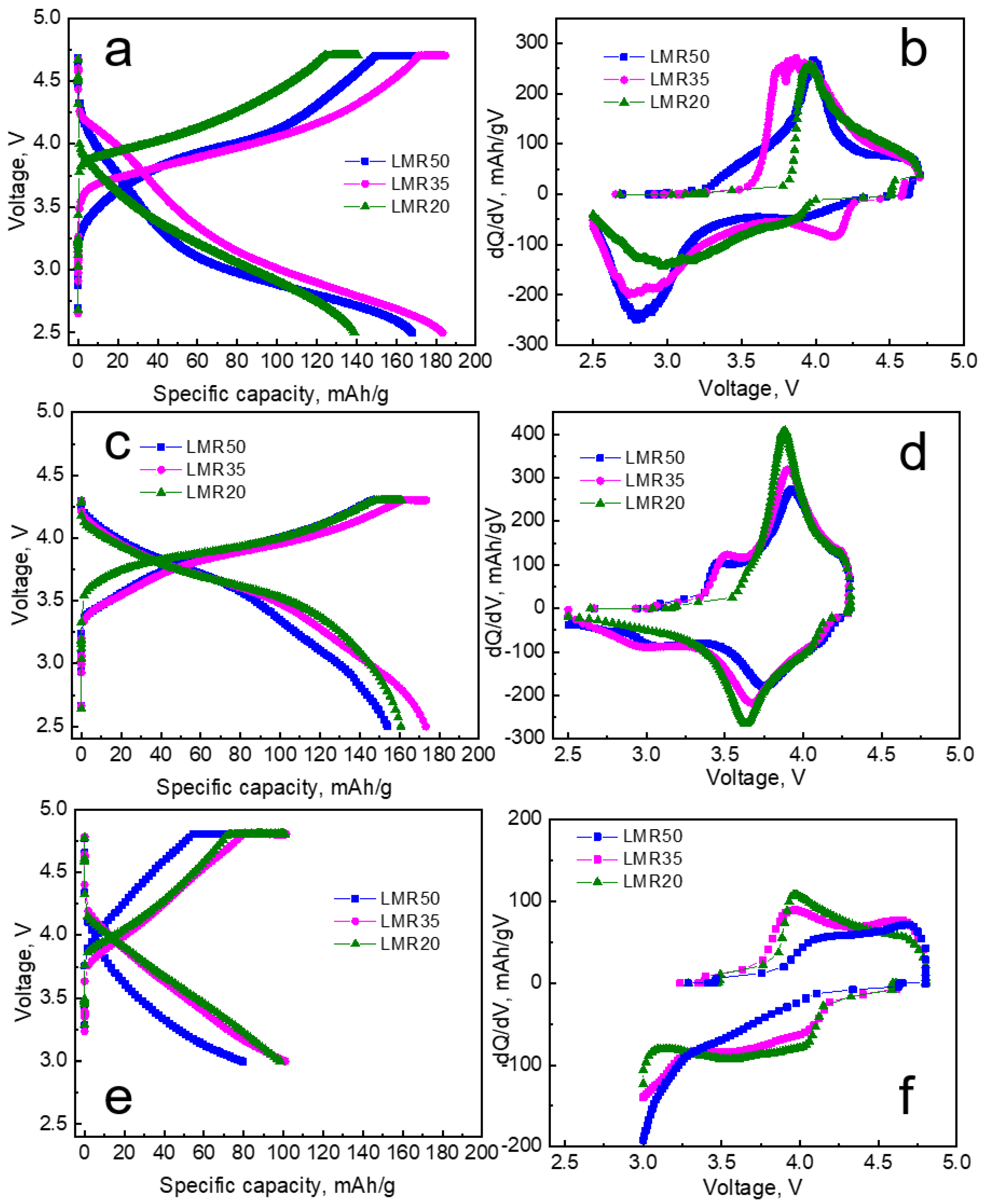
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poudel, M.B.; Kim, A.R.; Ramakrishan, S.; Logeshwaran, N.; Ramasamy, S.K.; Kim, H.J.; Yoo, D.J. Integrating the Essence of Metal Organic Framework-Derived ZnCoTe–N–C/MoS2 Cathode and ZnCo-NPS-N-CNT as Anode for High-Energy Density Hybrid Supercapacitors. Compos. B Eng. 2022, 247, 110339. [Google Scholar] [CrossRef]
- Zhang, M.; Kitchaev, D.A.; Lebens-Higgins, Z.; Vinckeviciute, J.; Zuba, M.; Reeves, P.J.; Grey, C.P.; Whittingham, M.S.; Piper, L.F.J.; van der Ven, A.; et al. Pushing the Limit of 3d Transition Metal-Based Layered Oxides That Use Both Cation and Anion Redox for Energy Storage. Nat. Rev. Mater. 2022, 7, 522–540. [Google Scholar] [CrossRef]
- House, R.A.; Marie, J.-J.; Pérez-Osorio, M.A.; Rees, G.J.; Boivin, E.; Bruce, P.G. The Role of O2 in O-Redox Cathodes for Li-Ion Batteries. Nat. Energy 2021, 6, 781–789. [Google Scholar] [CrossRef]
- Assat, G.; Iadecola, A.; Foix, D.; Dedryvère, R.; Tarascon, J.-M. Direct Quantification of Anionic Redox over Long Cycling of Li-Rich NMC via Hard X-Ray Photoemission Spectroscopy. ACS Energy Lett. 2018, 3, 2721–2728. [Google Scholar] [CrossRef]
- Li, Q.; Ning, D.; Wong, D.; An, K.; Tang, Y.; Zhou, D.; Schuck, G.; Chen, Z.; Zhang, N.; Liu, X. Improving the Oxygen Redox Reversibility of Li-Rich Battery Cathode Materials via Coulombic Repulsive Interactions Strategy. Nat. Commun. 2022, 13, 1123. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, F.; Chou, S.; Wang, J.; Gu, L.; Wang, H.; Yoshikawa, H.; Lu, Y.; Chen, J. Tuning Oxygen Redox Chemistry in Li-Rich Mn-Based Layered Oxide Cathodes by Modulating Cation Arrangement. Adv. Mater. 2019, 31, 1901808. [Google Scholar] [CrossRef]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The Structural and Chemical Origin of the Oxygen Redox Activity in Layered and Cation-Disordered Li-Excess Cathode Materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Guo, S.; Xu, Z.; Zhu, H.; Zhang, X.; Yuan, Y.; He, P.; Ishida, M.; Zhou, H. Direct Visualization of the Reversible O 2− /O − Redox Process in Li-Rich Cathode Materials. Adv. Mater. 2018, 30, 1705197. [Google Scholar] [CrossRef]
- Assat, G.; Tarascon, J.-M. Fundamental Understanding and Practical Challenges of Anionic Redox Activity in Li-Ion Batteries. Nat. Energy 2018, 3, 373–386. [Google Scholar] [CrossRef]
- Merz, M.; Ying, B.; Nagel, P.; Schuppler, S.; Kleiner, K. Reversible and Irreversible Redox Processes in Li-Rich Layered Oxides. Chem. Mater. 2021, 33, 9534–9545. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Piper, L.F.J.; Whittingham, M.S.; Zhou, G. Oxygen Loss in Layered Oxide Cathodes for Li-Ion Batteries: Mechanisms, Effects, and Mitigation. Chem. Rev. 2022, 122, 5641–5681. [Google Scholar] [CrossRef] [PubMed]
- Teufl, T.; Strehle, B.; Müller, P.; Gasteiger, H.A.; Mendez, M.A. Oxygen Release and Surface Degradation of Li- and Mn-Rich Layered Oxides in Variation of the Li 2 MnO 3 Content. J. Electrochem. Soc. 2018, 165, A2718–A2731. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, W.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.-G. Electrochemical Kinetics and Performance of Layered Composite Cathode Material Li[Li 0.2 Ni 0.2 Mn 0.6 ]O 2. J. Electrochem. Soc. 2013, 160, A2212–A2219. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese Oxides for Lithium Batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Chai, K.; Zhang, J.; Li, Q.; Wong, D.; Zheng, L.; Schulz, C.; Bartkowiak, M.; Smirnov, D.; Liu, X. Facilitating Reversible Cation Migration and Suppressing O 2 Escape for High Performance Li-Rich Oxide Cathodes. Small 2022, 18, 2201014. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Li, S.; Shen, X.; Zhou, L.; Huang, Q.; Liang, C.; Wang, Z.; Wang, X.; Wei, W. Regulating Anion Redox and Cation Migration to Enhance the Structural Stability of Li-Rich Layered Oxides. ACS Appl. Mater Interfaces 2021, 13, 12159–12168. [Google Scholar] [CrossRef]
- Viji, M.; Budumuru, A.K.; Hebbar, V.; Gautam, S.; Chae, K.H.; Sudakar, C. Influence of Morphology and Compositional Mixing on the Electrochemical Performance of Li-Rich Layered Oxides Derived from Nanoplatelet-Shaped Transition Metal Oxide–Hydroxide Precursors. Energy Fuels 2021, 35, 4533–4549. [Google Scholar] [CrossRef]
- Guo, L.; Tan, X.; Mao, D.; Zhao, T.; Song, L.; Liu, Y.; Kang, X.; Wang, H.; Sun, L.; Chu, W. Improved Electrochemical Activity of the Li2MnO3-like Superstructure in High-Nickel Li-Rich Layered Oxide Li1.2Ni0.4Mn0.4O2 and Its Enhanced Performances via Tungsten Doping. Electrochim. Acta 2021, 370, 137808. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, R.; Yang, X. Effects of Structure and Magnetism on the Electrochemistry of the Layered Li1+x(Ni0.5Mn0.5)1–XO2 Cathode Material. Inorg. Chem. 2020, 59, 17535–17543. [Google Scholar] [CrossRef]
- Wei, Z.; Shi, Z.; Wen, X.; Li, X.; Qiu, B.; Gu, Q.; Sun, J.; Han, Y.; Luo, H.; Guo, H.; et al. Eliminating Oxygen Releasing of Li-Rich Layered Cathodes by Tuning the Distribution of Superlattice Domain. Mater. Today Energy 2022, 27, 101039. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Duncan, H.; Hage, F.; Chen, G. Unravelling Structural Ambiguities in Lithium- and Manganese-Rich Transition Metal Oxides. Nat. Commun. 2015, 6, 8711. [Google Scholar] [CrossRef] [PubMed]
- Genevois, C.; Koga, H.; Croguennec, L.; Ménétrier, M.; Delmas, C.; Weill, F. Insight into the Atomic Structure of Cycled Lithium-Rich Layered Oxide Li1.20Mn0.54Co0.13Ni0.13O2 Using HAADF STEM and Electron Nanodiffraction. J. Phys. Chem. C 2015, 119, 75–83. [Google Scholar] [CrossRef]
- Yin, W.; Grimaud, A.; Rousse, G.; Abakumov, A.M.; Senyshyn, A.; Zhang, L.; Trabesinger, S.; Iadecola, A.; Foix, D.; Giaume, D.; et al. Structural Evolution at the Oxidative and Reductive Limits in the First Electrochemical Cycle of Li1.2Ni0.13Mn0.54Co0.13O2. Nat. Commun. 2020, 11, 1252. [Google Scholar] [CrossRef]
- Chen, C.-J.; Pang, W.K.; Mori, T.; Peterson, V.K.; Sharma, N.; Lee, P.-H.; Wu, S.; Wang, C.-C.; Song, Y.-F.; Liu, R.-S. The Origin of Capacity Fade in the Li 2 MnO 3 ·Li M O 2 ( M = Li, Ni, Co, Mn) Microsphere Positive Electrode: An Operando Neutron Diffraction and Transmission X-Ray Microscopy Study. J. Am. Chem. Soc. 2016, 138, 8824–8833. [Google Scholar] [CrossRef]
- Xie, H.; Cui, J.; Yao, Z.; Ding, X.; Zhang, Z.; Luo, D.; Lin, Z. Revealing the Role of Spinel Phase on Li-Rich Layered Oxides: A Review. Chem. Eng. J. 2022, 427, 131978. [Google Scholar] [CrossRef]
- Bettge, M.; Li, Y.; Gallagher, K.; Zhu, Y.; Wu, Q.; Lu, W.; Bloom, I.; Abraham, D.P. Voltage Fade of Layered Oxides: Its Measurement and Impact on Energy Density. J. Electrochem. Soc. 2013, 160, A2046–A2055. [Google Scholar] [CrossRef]
- Croy, J.R.; Gallagher, K.G.; Balasubramanian, M.; Long, B.R.; Thackeray, M.M. Quantifying Hysteresis and Voltage Fade in XLi2MnO3●(1-x)LiMn0.5Ni0.5O 2 Electrodes as a Function of Li2MnO3 Content. J. Electrochem. Soc. 2014, 161, A318–A325. [Google Scholar] [CrossRef]
- Pechen, L.; Makhonina, E.; Volkov, V.; Rumyantsev, A.; Koshtyal, Y.; Politov, Y.; Pervov, V.; Eremenko, I. Investigation of Capacity Fade and Voltage Decay in Li-Rich Cathode Materials with Different Phase Composition. In Proceedings of the 11th International Conference on Nanomaterials - Research & Application, Brno, Czech Republic, 16–18 October 2019; pp. 144–149. [Google Scholar]
- Makhonina, E.V.; Pechen, L.S.; Volkov, V.V.; Rumyantsev, A.M.; Koshtyal, Y.M.; Dmitrienko, A.O.; Politov, Y.A.; Pervov, V.S.; Eremenko, I.L. Synthesis, Microstructure, and Electrochemical Performance of Li-Rich Layered Oxide Cathode Materials for Li-Ion Batteries. Russ. Chem. Bull. 2019, 68, 301–312. [Google Scholar] [CrossRef]
- Fu, F.; Yao, Y.; Wang, H.; Xu, G.-L.; Amine, K.; Sun, S.-G.; Shao, M. Structure Dependent Electrochemical Performance of Li-Rich Layered Oxides in Lithium-Ion Batteries. Nano Energy 2017, 35, 370–378. [Google Scholar] [CrossRef]
- Riekehr, L.; Liu, J.; Schwarz, B.; Sigel, F.; Kerkamm, I.; Xia, Y.; Ehrenberg, H. Effect of Pristine Nanostructure on First Cycle Electrochemical Characteristics of Lithium-Rich Lithium–Nickel–Cobalt–Manganese-Oxide Cathode Ceramics for Lithium Ion Batteries. J. Power Sources 2016, 306, 135–147. [Google Scholar] [CrossRef]
- Makhonina, E.; Pechen, L.; Medvedeva, A.; Politov, Y.; Rumyantsev, A.; Koshtyal, Y.; Volkov, V.; Goloveshkin, A.; Eremenko, I. Effects of Mg Doping at Different Positions in Li-Rich Mn-Based Cathode Material on Electrochemical Performance. Nanomaterials 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Pechen, L.S.; Makhonina, E.V.; Medvedeva, A.E.; Rumyantsev, A.M.; Koshtyal, Y.M.; Politov, Y.A.; Goloveshkin, A.S.; Eremenko, I.L. Effect of Dopants on the Functional Properties of Lithium-Rich Cathode Materials for Lithium-Ion Batteries. Russ. J. Inorg. Chem. 2021, 66, 682–694. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zhao, S.; Lai, W.-H.; Sun, B.; Notten Peter, H.L.; Wang, G. Oxygen Redox Chemistry in Lithium-Rich Cathode Materials for Li-Ion Batteries: Understanding from Atomic Structure to Nano-Engineering. Nano Mater. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Yu, X.; Lyu, Y.; Gu, L.; Wu, H.; Bak, S.-M.; Zhou, Y.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.-W.; et al. Understanding the Rate Capability of High-Energy-Density Li-Rich Layered Li 1.2 Ni 0.15 Co 0.1 Mn 0.55 O 2 Cathode Materials. Adv. Energy Mater. 2014, 4, 1300950. [Google Scholar] [CrossRef]
- Shanmugam, V.; Natarajan, S.; Lobo, L.S.; Mathur, A.; Sahana, M.B.; Sundararajan, G.; Gopalan, R. Surface Oxygen Vacancy Engineering and Physical Protection by In-Situ Carbon Coating Process of Lithium Rich Layered Oxide. J. Power Sources 2021, 515, 230623. [Google Scholar] [CrossRef]
- Jo, Y.N.; Prasanna, K.; Park, S.J.; Lee, C.W. Characterization of Li-Rich XLi2MnO3·(1−x)Li[MnyNizCo1−y−z]O2 as Cathode Active Materials for Li-Ion Batteries. Electrochim. Acta 2013, 108, 32–38. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, H.; Tang, T.; Du, W.; Gao, M.; Liu, Y.; Jian, D.; Pan, H. Insight into the Synergistic Effect Mechanism between the Li2MO3 Phase and the LiMO2 Phase (M = Ni, Co, and Mn) in Li- and Mn-Rich Layered Oxide Cathode Materials. Electrochim. Acta 2018, 266, 66–77. [Google Scholar] [CrossRef]
- Amalraj, F.; Kovacheva, D.; Talianker, M.; Zeiri, L.; Grinblat, J.; Leifer, N.; Goobes, G.; Markovsky, B.; Aurbach, D. Integrated Materials XLi[Sub 2]MnO[Sub 3]⋅(1−x)LiMn[Sub 1/3]Ni[Sub 1/3]Co[Sub 1/3]O[Sub 2] (X=0.3, 0.5, 0.7) Synthesized. J. Electrochem. Soc. 2010, 157, A1121. [Google Scholar] [CrossRef]
- Galkin, V.V.; Lanina, E.V.; Shel’deshov, N.V. Dependence of the Electrochemical Characteristics of Lithium-Ion Battery in the Initial State and after Degradation of the Structural Parameters of the Positive Electrode. Electrochem. Energetics 2013, 13, 103–112. [Google Scholar] [CrossRef]

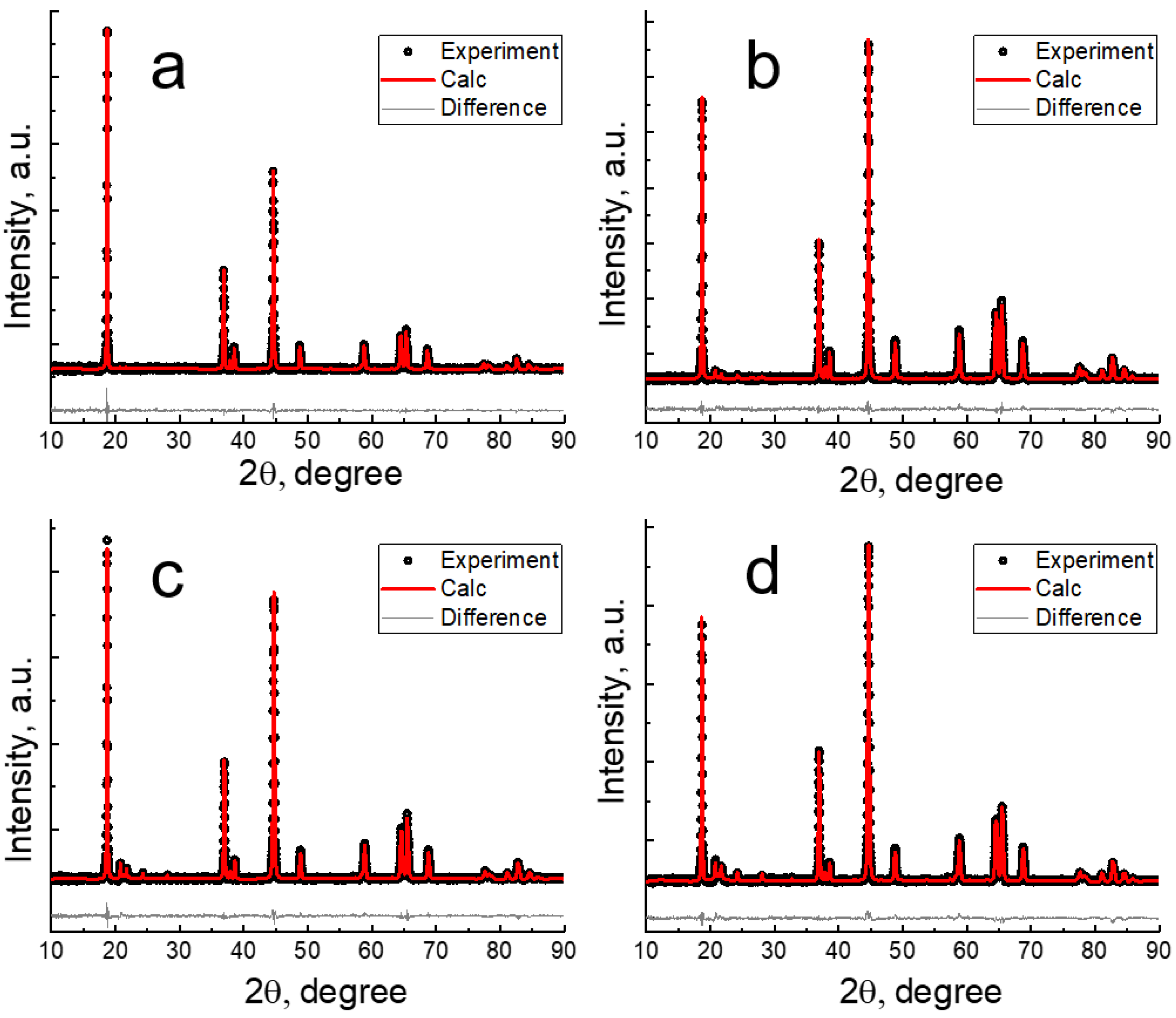
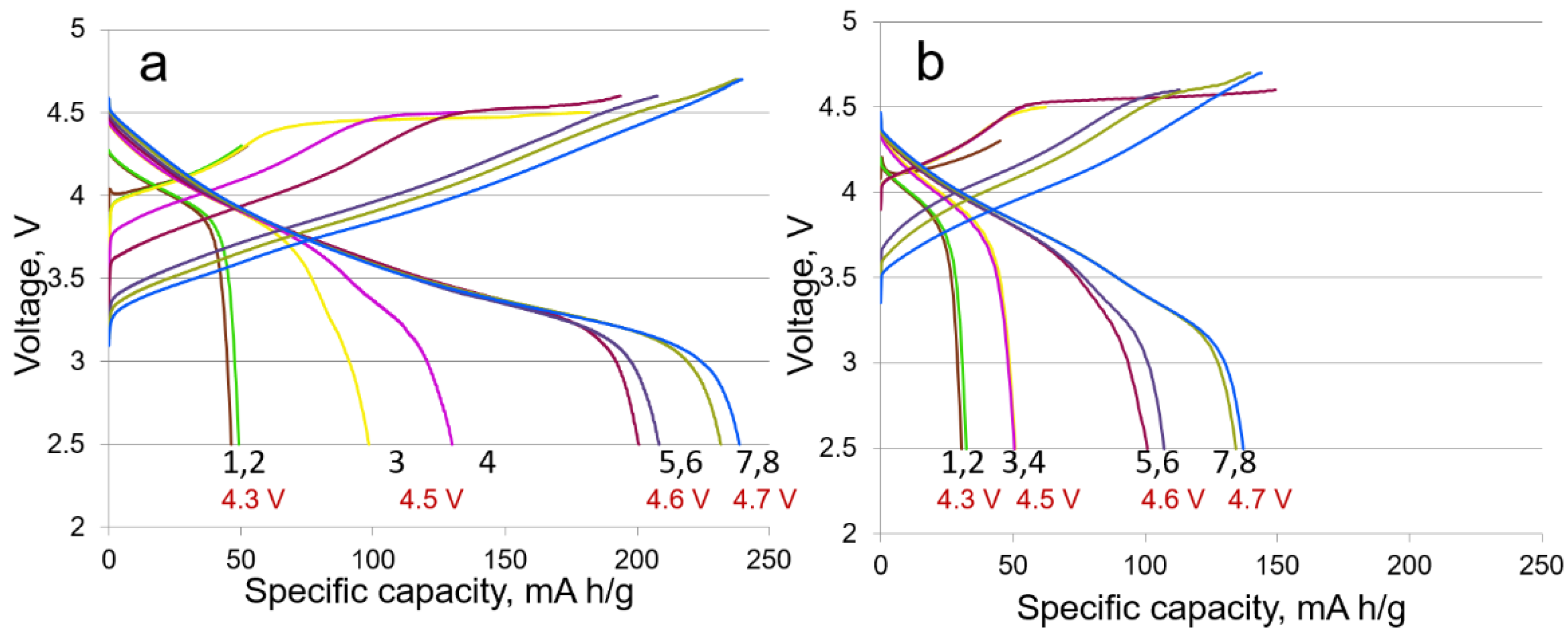
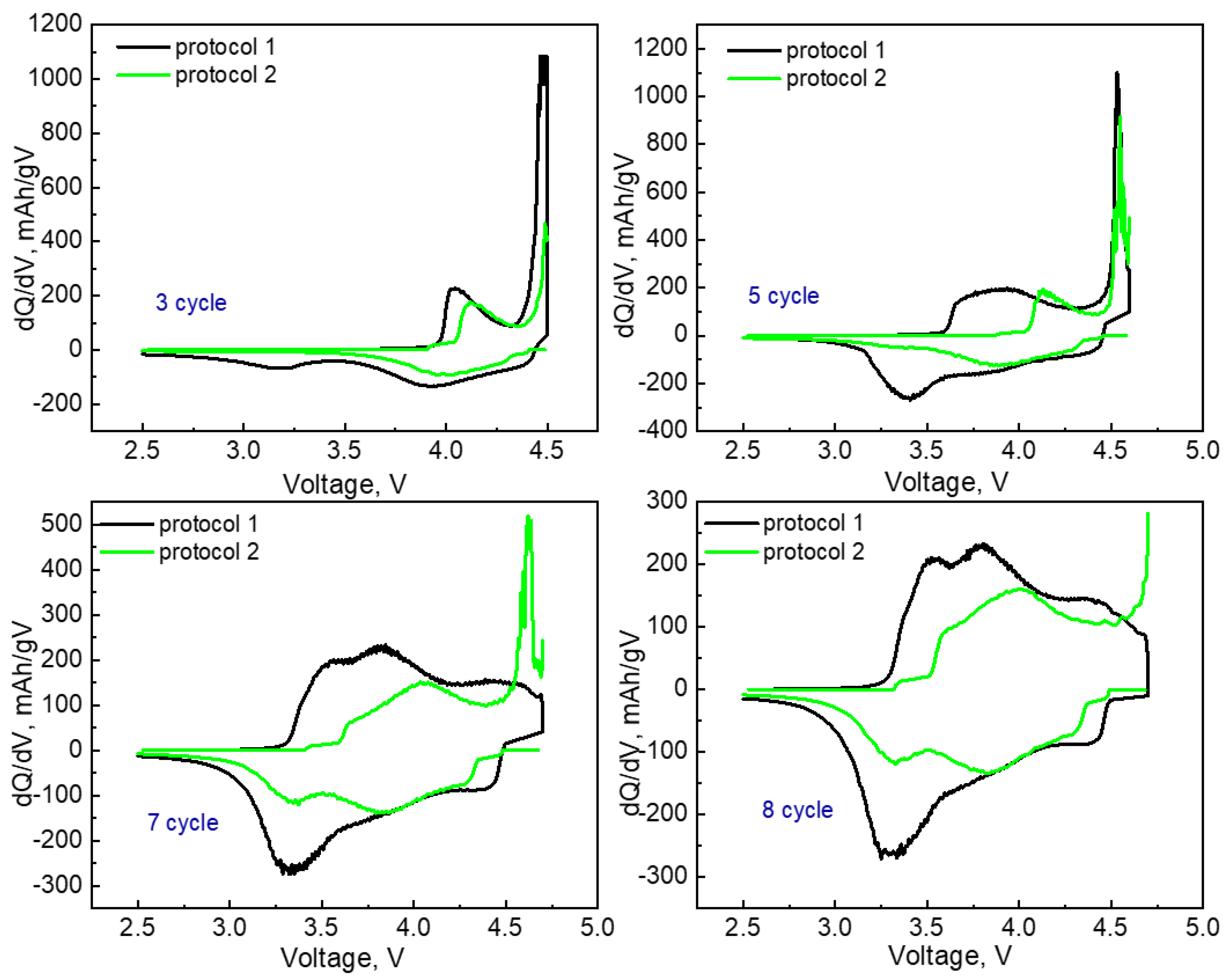
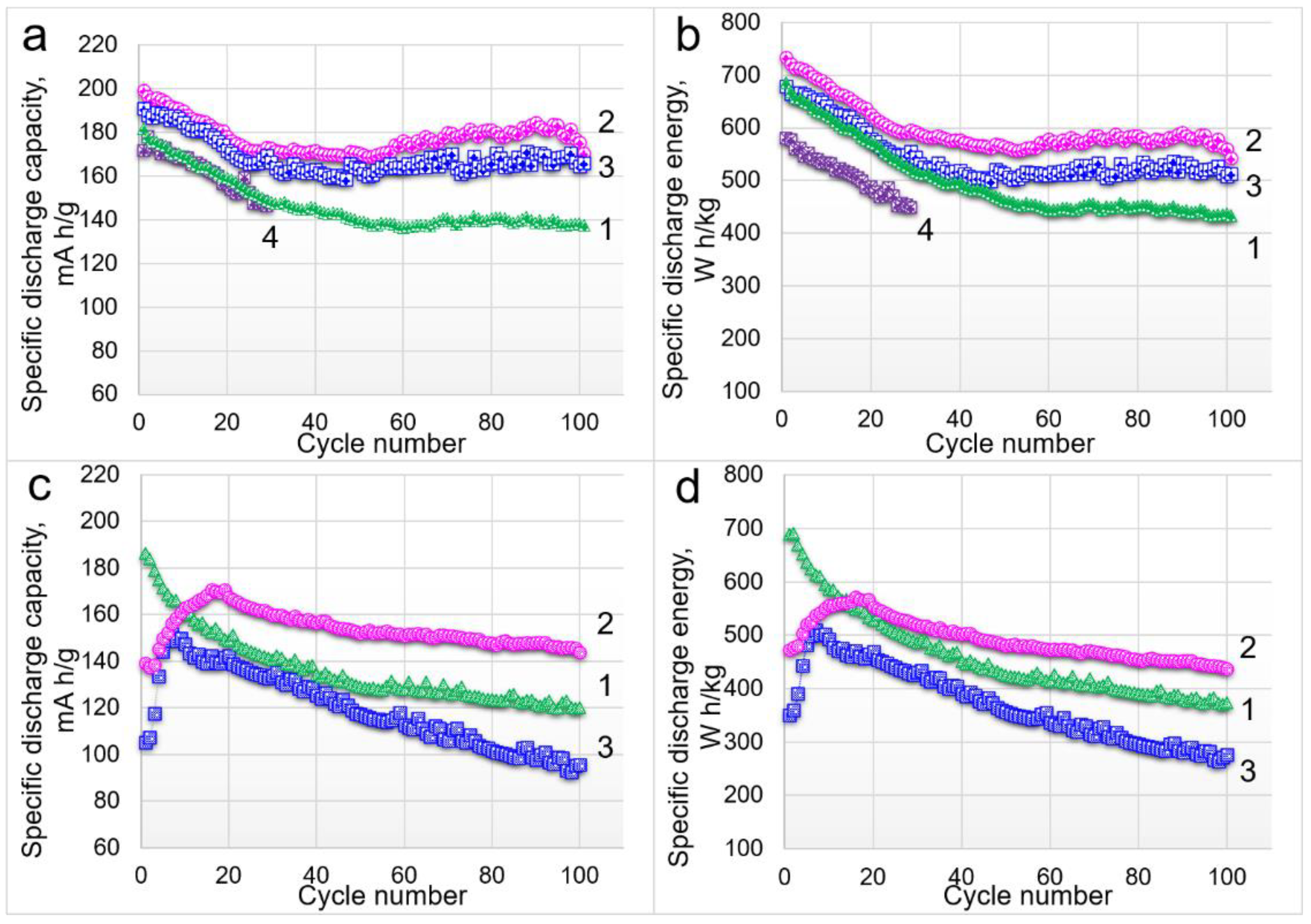



| Samples | Targeted Composition | Li/(Mn + Ni + Co) |
|---|---|---|
| LMR20 | Li1.091Ni0.242Mn0.424Co0.242O2 | 1.20 |
| LMR35 | Li1.149Ni0.184Mn0.482Co0.184O2 | 1.35 |
| LMR50 | Li1.200Ni0.134Mn0.534Co0.134O2 | 1.50 |
| LMR65 | Li1.245Ni0.088Mn0.579Co0.088O2 | 1.65 |
| LMR20 | LMR35 | LMR50 | LMR65 | |
|---|---|---|---|---|
| d10, μm | 3.50 | 4.56 | 6.23 | 8.75 |
| d50, μm | 8.11 | 8.54 | 10.88 | 16.97 |
| d90, μm | 15.97 | 15.26 | 17.64 | 27.15 |
| (d90-d10)/d50 | 1.54 | 1.25 | 1.05 | 1.08 |
| LMR20 | LMR35 | LMR50 | LMR65 | |
|---|---|---|---|---|
| Rwp, % | 1.76 | 2.84 | 2.10 | 2.40 |
| Rp, % | 1.28 | 2.22 | 1.60 | 1.81 |
| GOOF, % | 3.19 | 5.05 | 4.58 | 5.59 |
| a, Å | 4.9441(4) | 4.9363(4) | 4.9317(4) | 4.9305(3) |
| b, Å | 8.5637(8) | 8.5502(6) | 8.5422(8) | 8.5401(6) |
| c, Å | 5.0245(14) | 5.0218(4) | 5.0216(4) | 5.0233(4) |
| β, Å | 109.226(3) | 109.264(3) | 109.283(2) | 109.303(2) |
| V, Å3 | 200.87(7) | 200.08(3) | 199.68(4) | 199.63(3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pechen, L.; Makhonina, E.; Medvedeva, A.; Politov, Y.; Rumyantsev, A.; Koshtyal, Y.; Goloveshkin, A.; Eremenko, I. Influence of the Composition and Testing Modes on the Electrochemical Performance of Li-Rich Cathode Materials. Nanomaterials 2022, 12, 4054. https://doi.org/10.3390/nano12224054
Pechen L, Makhonina E, Medvedeva A, Politov Y, Rumyantsev A, Koshtyal Y, Goloveshkin A, Eremenko I. Influence of the Composition and Testing Modes on the Electrochemical Performance of Li-Rich Cathode Materials. Nanomaterials. 2022; 12(22):4054. https://doi.org/10.3390/nano12224054
Chicago/Turabian StylePechen, Lidia, Elena Makhonina, Anna Medvedeva, Yury Politov, Aleksander Rumyantsev, Yury Koshtyal, Alexander Goloveshkin, and Igor Eremenko. 2022. "Influence of the Composition and Testing Modes on the Electrochemical Performance of Li-Rich Cathode Materials" Nanomaterials 12, no. 22: 4054. https://doi.org/10.3390/nano12224054
APA StylePechen, L., Makhonina, E., Medvedeva, A., Politov, Y., Rumyantsev, A., Koshtyal, Y., Goloveshkin, A., & Eremenko, I. (2022). Influence of the Composition and Testing Modes on the Electrochemical Performance of Li-Rich Cathode Materials. Nanomaterials, 12(22), 4054. https://doi.org/10.3390/nano12224054






