Abstract
Long-term stable secondary batteries are highly required. Here, we report a unique microcapsule encapsulated with metal organic frameworks (MOFs)-derived Co3O4 nanocages for a Li-S battery, which displays good lithium-storage properties. ZIF-67 dodecahedra are prepared at room temperature then converted to porous Co3O4 nanocages, which are infilled into microcapsules through a microfluidic technique. After loading sulfur, the Co3O4/S-infilled microcapsules are obtained, which display a specific capacity of 935 mAh g−1 after 200 cycles at 0.5C in Li-S batteries. A Coulombic efficiency of about 100% is achieved. The constructed Li-S battery possesses a high rate-performance during three rounds of cycling. Moreover, stable performance is verified under both high and low temperatures of 50 °C and −10 °C. Density functional theory calculations show that the Co3O4 dodecahedra display large binding energies with polysulfides, which are able to suppress shuttle effect of polysulfides and enable a stable electrochemical performance.
1. Introduction
Recently, the demands for electric vehicles and portable electronics have been rapidly increasing. Considering this, investigations on secondary batteries are considered to be a significant direction. People are expecting higher capacity, longer cycling life, and faster charging of secondary batteries. As a promising next-generation secondary battery, the Li-S battery possesses a high theoretical energy density (2600 Wh kg−1) and low cost of sulfur [1,2,3,4]. Recently, the research on Li-S batteries has been considered to be a significant field [5,6]. There are many problems for currently available Li-S batteries, such as the volumetric change of sulfur and shuttle effect reducing the electrochemical performance, which represent obstacles to commercialization [7].
In order to address those issues, many studies have been reported, in which the synthesis of yolk-shell structure hosts is considered to be a potential strategy [8,9,10]. Zhang et al. reported a yolk-shell ZnO by using a hydrothermal method, which provided a specific capacity of 1406 mAh g−1 at 0.1C [11]. Jiang et al. synthesized a yolk-shell nanomaterial consisting of SiO2 core and carbon shell [12]. The cathode based on the yolk-shell SiO2-carbon delivered 1200 mAh g−1 at a rate of 0.2C. Those achievements indicate that several yolk-shell structures exhibit enhanced adsorption towards polysulfides [13,14]. Reasonable engineering yolk-shell structure as sulfur host could promisingly improve the energy-storage properties [15,16]. However, general and simple preparation approaches for large-scale yolk-shell materials are still highly required. In addition, several reports indicated that typical semiconductor Co3O4 is a promising sulfur host because of its strong interaction with sulfur and polysulfides [17,18,19,20]. With this in mind, developing creative Co3O4-based composite for Li-S batteries is attractive and would be of great significance [21,22,23].
Here, we present a microcapsule infilling by a metal–organic framework (MOF)-derived cobalt oxide nanocage as the sulfur host, which displays a high electrochemical performance. By using ZIF-67 as a precursor which was prepared through a hydrothermal approach, a porous dodecahedral Co3O4 nanocage was obtained (Figure 1). The experimental procedures are presented in the Supplementary Material. Then, Co3O4 nanocages were encapsulated into microcapsules through a microfluidic strategy. The real-time process of the cone and the formation of drops are displayed by Movie S1 and S2, respectively. The prepared microcapsules were carbonized for use as a sulfur host, which showed a long life of 200 cycles, along with a stable specific capacity of 935 mAh g−1 and a 100% Coulombic efficiency. After repeated tests, it displays a stable rate-performance, and the battery remains stable at −10 °C and 50 °C. Furthermore, density functional theory (DFT) calculations show that Co3O4 possesses large binding energies towards polysulfides, which are important for reducing the shuttle effect and enabling a stable electrochemical performance.
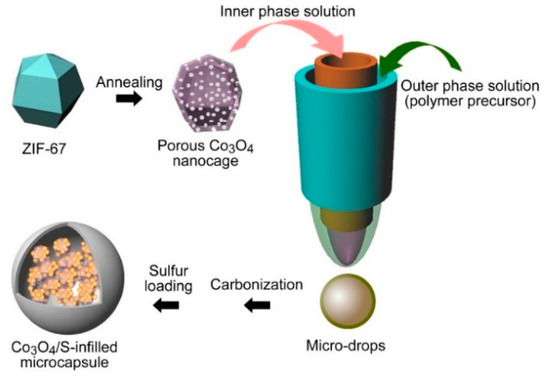
Figure 1.
Microfluidic preparation of Co3O4/S-infilled microcapsules.
2. Results and Discussion
2.1. Structural and Microstructural Characterization
SEM image (Figure 2a) of ZIF-67 precursor shows a dodecahedron morphology with a size of 500 nm. Figure 2b shows the SEM image of porous dodecahedral Co3O4 obtained after annealing the precursor. The TEM image (Figure 2c) displays the porous Co3O4 nanocage clearly. The dodecahedral Co3O4 was encapsulated in microcapsule by using a coaxial focusing method. Figure 2d shows the SEM image of microcapsules with a size of about 50 μm. The microcapsule was observed by using an optical microscope (Figure 2e), and it is verified the Co3O4 uniformly distributes in the microcapsule. An SEM image of Co3O4-infilled microcapsules after annealing is shown in Figure 2f. A TEM image (Figure 2g) presents the edge of the microcapsule. It is observed that the shell of the microcapsule is very thin after calcination. After the microcapsules were broken manually, dodecahedral Co3O4 nanocages inside the microcapsule were observed clearly in Figure 2h,i. Figure 2j displays the microcapsules after loading sulfur, forming a Co3O4/S-infilled microcapsule structure. The surface become rough, which indicates that some of the sulfur was coated on microcapsules.
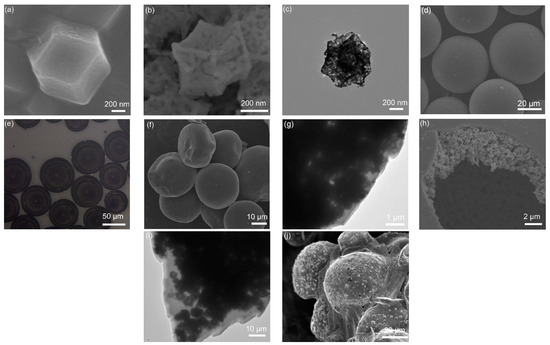
Figure 2.
SEM photographs of (a) ZIF-67 and (b) dodecahedral Co3O4 nanocage. (c) TEM image of Co3O4. (d) SEM and (e) optical photographs of Co3O4-infilled microcapsules before carbonization. (f) SEM and (g) TEM images of carbonized microcapsules. (h) SEM and (i) TEM photographs of Co3O4-infilled microcapsule after breaking manually. (j) SEM image of microcapsules after loading sulfur.
The XRD pattern (Figure 3) is assigned to Co3O4 in terms of JCPDS card No. 42-1467, while the other peaks are attributed to sulfur (JCPDS card No. 99-0066). The signal of Co3O4 becomes unobvious after loading sulfur, which would be ascribed to the cover of sulfur signals. Figure S1 displays the HRTEM image of the porous Co3O4. The Co3O4 obtained after annealing ZIF-67 precursor exhibits a good crystallinity. The 0.28 nm lattice spacing matches the (220) crystalline plane, while the SAED pattern displays several diffraction rings, indicating a polycrystalline structure [24].
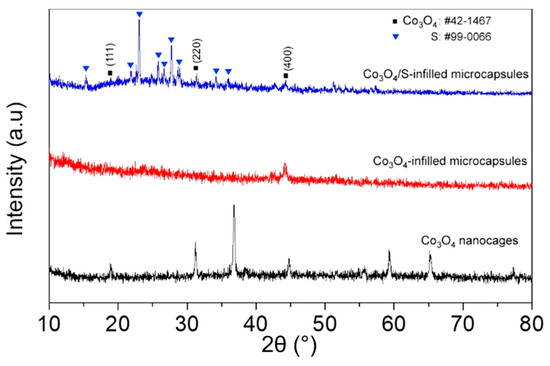
Figure 3.
XRD patterns of the pristine Co3O4 nanocages and the capsules infilled with Co3O4 or Co3O4/S composite.
The composition and chemical states of the microcapsules are presented in the XPS spectra (Figure 4). The peak at 285 eV is from C1s, and the ones at 530 and 790 eV are ascribed to the O1s and Co2p, respectively, as shown in Figure 4a. The C 1s spectrum (Figure 4b) shows two peaks, where the one at 284.6 eV is indexed to graphite carbon. Moreover, peak at 285.9 eV represents C=O [17]. O 1s spectrum (Figure 4c) exhibits three peaks at 530 eV, 532.2, and 533.5 eV, corresponding to lattice oxygen, ‒OH and H2O molecules, respectively [25,26,27]. The Co 2P spectrum (Figure 4d) shows 781.0 and 799 eV of Co2+ [28], and 778.7 and 796.6 eV peaks are from Co3+ [29,30].
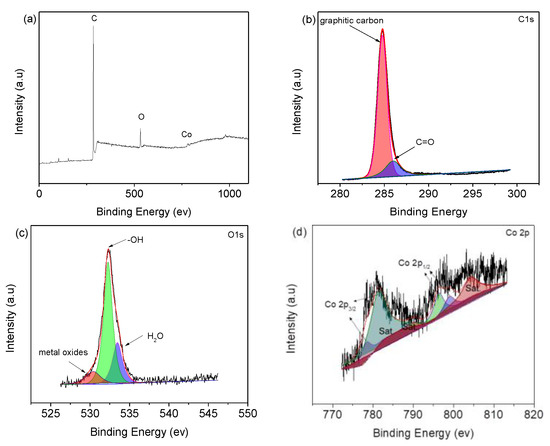
Figure 4.
(a) XPS survey spectrum of Co3O4-infilled microcapsules. XPS spectra of (b) C 1s, (c) O 1s, and (d) Co 2p.
The elemental distribution of Co3O4-infilled microcapsules is shown in Figure 5. The elements C, Co, and O evenly distribute. In Figure 5e, the EDS spectrum shows that the composition of Co3O4 includes C, Co, and O [31], which has a high purity. Figure 6a presents the TGA curves of Co3O4-infilled microcapsules measured in air. The mass loss from 350 °C to 480 °C is caused by the decomposition of the carbon shell. The drop from 250 to 350 °C is attributed to sulfur evaporation [32,33]. The sulfur in the microcapsules is about 85 wt%, which is significant for a high-sulfur loading. Moreover, pure Co3O4-infilled microcapsules show that the content of Co3O4 nanocages is about 35 wt%. Figure 6b shows the Raman spectra of the Co3O4-infilled microcapsules with and without loading sulfur. The D- and G-bands of carbon locate at 1370 and 1670 cm−1, respectively. In sulfur-loaded microcapsules, the peaks ranging from 150 to 475 cm−1 are assigned to sulfur.
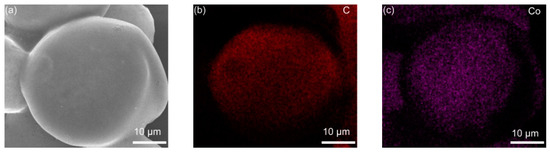
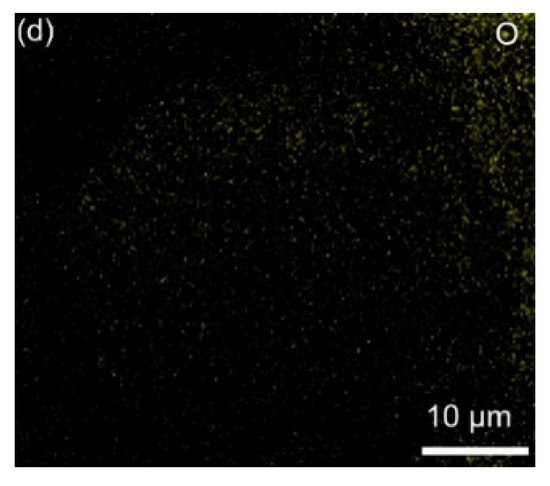
Figure 5.
(a) SEM and (b–d) mapping images of the Co3O4-infilled microcapsules. (e) Related EDS spectrum.
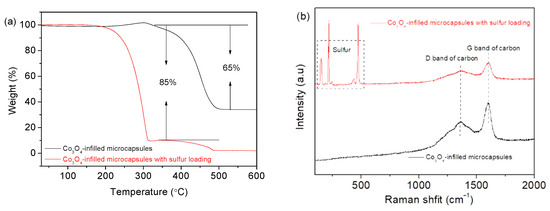
Figure 6.
(a) TGA profiles and (b) Raman spectra of Co3O4-infilled microcapsules with and without loading sulfur.
2.2. Electrochemical Characterization
Figure 7a displays the CV curves. During the discharge, two reduction peaks at 2.25 and 1.95 V are attributed to the conversion of high-order polysulfide to Li2S4 and reduction of Li2S4 to Li2S2/Li2S [34,35]. In the charging process, the oxidation at 2.4 V is ascribed to the conversion of Li2S2/Li2S to Li2Sn [36]. In Figure 7b, two discharge plateaus at 2.2 and 1.9 V are verified. The platform at 2.4 V in charge corresponds to oxidation peak [37]. It is noted that the overpotentials are observed, which may be attributed to the non-completed carbonization and the resulting limited conductivity. Figure 7c shows that the specific capacity remains 935 mAh g−1 after cycling 200 times at 0.5C (1C equates to fully charging or discharging the theoretical capacity in 1 h). The Coulombic efficiency is close to 100%. Compared to the performance of the Co3O4/S-infilled microcapsules, the capacity of pure sulfur powders is very low. In particular, the capacity decays rapidly after 150 cycles. In addition, the electrochemical performance of the microcapsules is also competitive compared to some other composites, as displayed in Table 1. Figure 7d shows the rate performance after repeated tests. In the second round, specific capacities are 1250, 1150, 860, and 500 mAh g−1 at rates of 0.1C, 0.2C, 0.5C, and 1C, respectively. It recovers to 1190 mAh g−1 once the rate is returned to 0.1C. Microcapsules exhibit a better reversibility and higher capacities than sulfur powders. It is attributed to the improved conductivity by the carbon shell and the reduced polysulfide loss by Co3O4 adsorption, which will be demonstrated by DFT calculations.
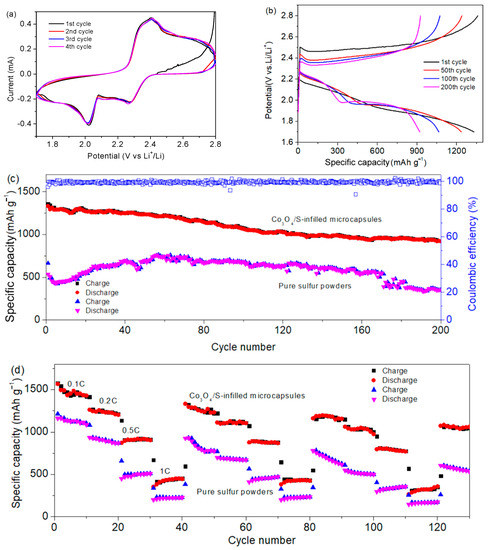
Figure 7.
(a) CV curves of Co3O4/S-infilled microcapsules-based Li-S battery scanning at a rate of 0.1 mV s−1. (b) Cycling curves at 0.5C. (c) Cycling performance at a rate of 0.5C. (d) Rate-performance of different samples.

Table 1.
Comparison on the electrochemical performance of some composite-based cathodes.
The Co3O4/S-infilled microcapsules-based Li-S battery also displays good cycling stability under different temperatures. Figure 8a shows that the capacity remains at 647 mAh g−1 after cycling 200 times under −10 °C. Besides cycling at a low temperature, the electrochemical performance at 50 °C is presented in Figure 8b, showing a specific capacity of 713 mAh g−1 after 200 cycles. Stable performance indicates that microcapsules can be used in different conditions, which are significant for practical applications.
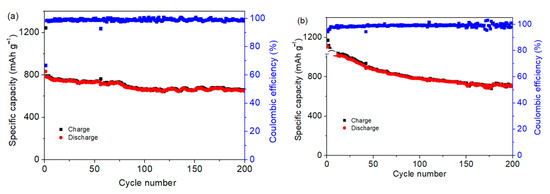
Figure 8.
Specific capacities of Co3O4/S-infilled microcapsules when cycling at different temperatures of (a) −10 °C and (b) 50 °C at 0.5C.
Figure 9a displays CV curves of Co3O4/S-infilled microcapsules at 0.6 to 1 mV s−1; Figure 9b displays a logarithmic relationship according to i = avb, where i and v stand for the peak current and rate, respectively [49]. The b value of 0.5 represents a diffusive-controlled process, and b = 1 indicates a capacitive behavior. In this investigation, b values suggest mainly diffusion-controlled processes. Figure 9c shows the diffusion contribution ratios calculated on the basis of i(v) = k1v + k2v1/2, where k1v and k2v1/2 stand for capacitive and diffusion-controlled contributions, respectively. The results were fitted (Figure 9d) based on IP = 2.69 × 105 × n3/2AD1/2Cv1/2 [50], where Ip is the peak current; n is the number of electrons transferred during the reaction, which is 2 for Li-S batteries; A is the active electrode area (1.13 cm2); D is the diffusion coefficient of lithium ion in unit of cm2 s−1; C is the concentration of Li ions in electrolyte in unit of mol mL−1 [51,52]; and n is the scanning rate in the unit of V s−1. On the basis of the obtained slopes, the Li ion diffusion coefficients are calculated to be 3.8 × 10−9 and 1.8 × 10−9 cm2 s−1, which are close to some reports [53,54]. The good diffusion property is ascribed to the specific microcapsule structure, which enables a good environment for contact with electrolytes; the porous scaffold of Co3O4 nanocages assembled by nanoparticles shortens the transfer pathway of ions.
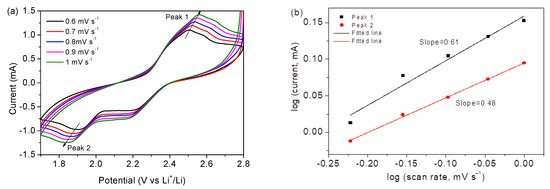
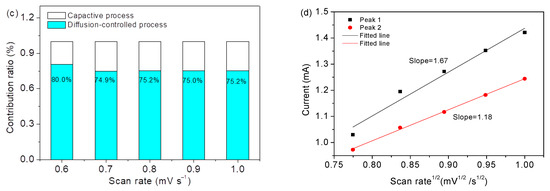
Figure 9.
(a) CV profiles of the Co3O4/S-infilled microcapsules. (b) Relationship of log(v) vs log(i). (c) Contribution ratios. (d) Relationship of peak current vs. rate.
2.3. DFT Calculations
The binding of the Co3O4 with polysulfides was investigated by using DFT calculation on the adsorption energies. All calculations were conducted by using a Vienna Ab initio Simulation Package. In Figure 10, a series of surface adsorptions of Co3O4 towards the polysulfides (Li2S, Li2S2, Li2S4, Li2S6, Li2S8) are presented. According to previous research [55,56,57], the (110) lattice plane in the Co3O4 is more prone to exposure due to the presence of Co3+ on the surface. Therefore, we focused on the adsorption energy of the polysulfides on the Co3O4 (110) surface. The side and top views of the geometric configurations of Co3O4 (110) surface are shown in Figure 10a. Then, the adsorption models were built up for the calculation of adsorption energy, which is shown in Figure 11. Figure 10b shows the surface adsorption energies toward the polysulfides, where the surface adsorption energies of the Co3O4 toward Li2S, Li2S2, Li2S4, Li2S6, Li2S8 are 3.8, 4.0, 1.7, 3.1, and 3.5 eV, respectively, indicating a good adsorption capability of Co3O4 towards polysulfides. Figure 10c displays the charge density difference of the adsorption models. The distribution of the charge density connects polysulfides and Co3O4, indicating an electron transfer between Co3O4 (110) surface and polysulfides. The charge density between polysulfides and Co3O4 illustrates the formation of bonds and further verifies the adsorption stability.
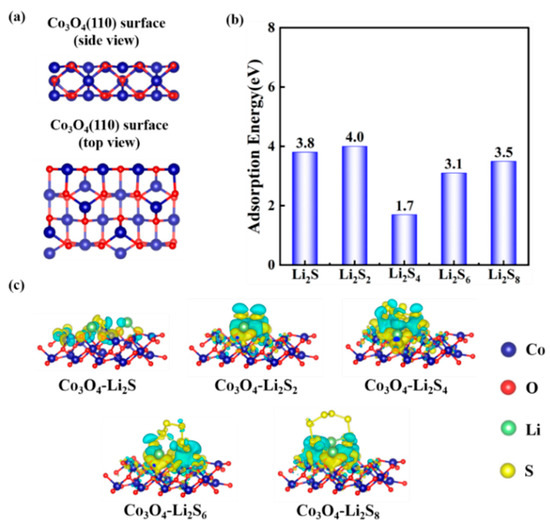
Figure 10.
(a) Side and top views of the geometric configurations of Co3O4 (110) surface. (b) Surface adsorption energies towards polysulfides. (c) Electron density differences of the polysulfides (Li2S, Li2S2, Li2S4, Li2S6, Li2S8).
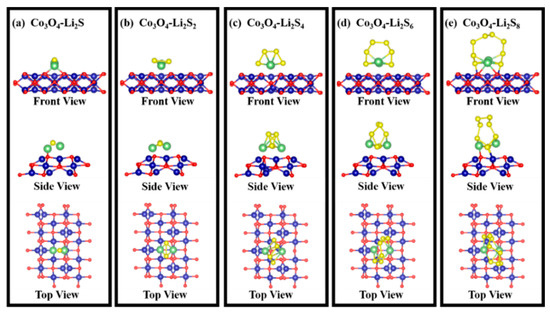
Figure 11.
Adsorption models between polysulfides and Co3O4 (110) surface: (a) Co3O4-Li2S; (b) Co3O4-Li2S2; (c) Co3O4-Li2S4; (d) Co3O4-Li2S6; (e) Co3O4-Li2S8.
3. Conclusions
In summary, a novel microcapsule system encapsulated with MOFs-derived Co3O4/S nanocages is developed, which displays a good electrochemical performance as a Li-S battery cathode. Dodecahedral ZIF-67 was synthesized, then it was converted to a porous Co3O4 nanocage which was infilled into a microcapsule through a microfluidic strategy. After 200 cycles at 0.5C, the specific capacity of Co3O4/S-infilled microcapsules remains 935 mAh g−1. The Coulombic efficiency is about 100%. The constructed battery also shows a stable rate-performance, while good capacities are also achieved under both high and low temperatures of 50 °C and −10 °C. In addition, DFT calculations verify that the Co3O4 displays large binding energies towards all polysulfides including Li2S, Li2S2, Li2S4, Li2S6, and Li2S8, reducing the loss of polysulfides. It is expected that the developed microcapsule system and the high performance will be applicable for engineering other emerging Li-storage nanomaterials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12020236/s1, Figure S1: (a) HRTEM image and (b) selective area electron diffraction (SAED) pattern of the porous Co3O4. Movie S1: Real-time process of the cone. Movie S2: Formation of drops.
Author Contributions
Conceptualization, J.L. (Jinyun Liu); methodology, J.L. (Jinyun Liu); software, J.C. and J.L. (Jinjin Li); validation, Y.Z. (Yajun Zhu), Y.Z. (Yan Zhong) and T.H.; formal analysis, J.L. (Jinyun Liu), Y.Z. (Yan Zhong) and Z.C.; investigation, Y.Z. (Yajun Zhu); resources, J.C. and Z.C.; data curation, Y.Z. (Yan Zhong); writing—original draft preparation, J.L. (Jinyun Liu) and Y.Z. (Yajun Zhu); writing—review and editing, J.L. (Jinyun Liu), Z.C. and J.L. (Jinjin Li); visualization, Y.Z. (Yan Zhong); supervision, J.L. (Jinyun Liu) and J.L. (Jinjin Li); project administration, J.L. (Jinyun Liu); funding acquisition, J.L. (Jinyun Liu) and J.L. (Jinjin Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key Research and Development Program of Wuhu, grant number 2019YF07; Natural Science Research Project for Universities in Anhui Province, grant numbers KJ2018ZD034 and KJ2019A0502; National Key R&D Program of China, grant number 2021YFC2100100, National Natural Science Foundation of China, grant number 21901157, Shanghai Science and Technology Project of China, grant number 21JC1403400, University Synergy Innovation Program of Anhui Province, grant number GXXT-2020-073; and Foundation of Anhui Laboratory of Molecule-Based Materials, grant number FZJ21012. The APC was funded by Natural Science Research Project for Universities in Anhui Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Wang, J.; Dong, X.; Zhu, L.; Hou, D.; Zeng, W.; Wang, J. The potential application of VS2 as an electrode material for Mg ion battery: A DFT study. Appl. Surf. Sci. 2021, 544, 148775. [Google Scholar] [CrossRef]
- Bella, F.; De Luca, S.; Fagiolari, L.; Versaci, D.; Amici, J.; Francia, C.; Bodoardo, S. An overview on anodes for magnesium batteries: Challenges towards a promising storage solution for renewables. Nanomaterials 2021, 11, 810. [Google Scholar] [CrossRef]
- Narumoto, N.; Okamoto, N.; Saito, T. Surface structure control and charge/discharge characteristics of bismuth anode materials by electrodeposition for magnesium-ion batteries. J. Mater. Sci. 2021, 32, 9990–9997. [Google Scholar]
- Shah, R.; Mittal, V.; Matsil, E.; Rosenkranz, A. Magnesium-ion batteries for electric vehicles: Current trends and future perspectives. Adv. Mech. Eng. 2021, 13, 16878140211003398. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Kim, C.; Zaghib, K.; Mauger, A.; Julien, C. Advances in lithium-sulfur batteries. Sci. Eng. R 2017, 121, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, Y.; Lv, T.; Liu, W.; Luo, Y.; Guo, R.; Pei, H.; Lai, C.; Xie, J. Ti3C2/CNTs Macroporous conductive network boosts Li4Ti5O12-TiO2 anode performance for practical Li ion and Mg ion batteries. J. Electrochem. Soc. 2021, 168, 030505. [Google Scholar] [CrossRef]
- Lin, X.; Liu, J.; Zhang, H.; Zhong, Y.; Zhu, M.; Zhou, T.; Qiao, X.; Zhang, H.; Han, T.; Li, J. General liquid-driven coaxial flow focusing preparation of novel microcapsules for rechargeable magnesium batteries. Adv. Sci. 2021, 8, 2002298. [Google Scholar] [CrossRef]
- Zuo, C.; Tang, W.; Lan, B.; Xiong, F.; Tang, H.; Dong, S.; Zhang, W.; Tang, C.; Li, J.; Ruan, Y.; et al. Unexpected discovery of magnesium-vanadium spinel oxide containing extractable Mg2+ as a high-capacity cathode material for magnesium ion batteries. Chem. Eng. J. 2021, 405, 127005. [Google Scholar] [CrossRef]
- Du, C.; Younas, W.; Wang, Z.; Yang, X.; Meng, E.; Wang, L.; Huang, J.; Ma, X.; Zhu, Y.; Cao, C. Constructing sheet-assembled hollow CuSe nanocubes to boost the rate capability of rechargeable magnesium batteries. J. Mater. Chem. A 2021, 9, 3648–3656. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Song, X.; Tao, A.; Yan, W.; Kong, W.; Jin, Z. Electrochemical Mg2+ displacement driven reversible copper extrusion/intrusion reactions for high-rate rechargeable magnesium batteries. Adv. Funct. Mater. 2021, 31, 2009394. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, Z.; Wang, S.; Ru, Q.; Zhao, L.; Sun, L.; Hou, X.; Chen, F. Cucumber-shaped construction combining bismuth nanoparticles with carbon nanofiber networks as a binder-free and freestanding anode for Li-ion batteries. Energy Fuels 2020, 34, 8987–8992. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Du, J.; Xiao, H.; Zhang, W.; Gan, Y. Bio-inspired fabrication of carbon nanotiles for high performance cathode of Li–S batteries. J. Mater. Chem. A 2014, 2, 2290–2296. [Google Scholar] [CrossRef]
- Zuo, C.; Xiao, Y.; Pan, X.; Xiong, F.; Zhang, W.; Long, J.; Dong, S.; An, Q.; Luo, P. Organic-inorganic superlattices of vanadium oxide@polyaniline for high-performance magnesium-ion batteries. ChemSusChem 2021, 14, 2093–2099. [Google Scholar] [CrossRef]
- Sopha, H.; Tesfaye, A.T.; Zazpe, R.; Michalicka, J.; Dvorak, F.; Hromadko, L.; Krbal, M.; Prikryl, J.; Djenizian, T.; Macak, J.M. ALD growth of MoS2 nanosheets on TiO2 nanotube supports. FlatChem 2019, 17, 100130. [Google Scholar] [CrossRef]
- Panwar, V.; Jain, S.L. Ternary hybrid TiO2-PANI-AuNPs for photocatalytic A3-coupling of aldehydes, amines and alkynes: First photochemical synthesis of propargyl amines. Mater. Sci. Eng. C 2019, 99, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, F.; Gao, C.; Wan, T.; Wang, L.; Wang, L.; Wang, L. Enhancement of the thermoelectric property of nanostructured polyaniline/carbon nanotube composites by introducing pyrrole unit onto polyaniline backbone via a sustainable method. Chem. Eng. J. 2019, 370, 322–329. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, X. Bamboo-like Co3O4 nanofiber as host materials for enhanced lithium-sulfur battery performance. J. Alloys Compd. 2019, 777, 688–692. [Google Scholar] [CrossRef]
- Bosubabu, D.; Sivaraj, J.; Gurunathan, P.; Ramesha, K. Hollow Co3O4 microspheres grafted with nitrogen-doped carbon nanotubes as efficient sulfur host for high performing lithium-sulfur batteries. Energy Fuels 2020, 34, 16810–16818. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, R.; You, L.; Tang, H.; Zhong, J.; Wang, S.; Yang, T. Graphene-like matrix composites with Fe2O3 and Co3O4 as cathode materials for lithium-sulfur batteries. ACS Appl. Nano Mater. 2020, 3, 1382–1390. [Google Scholar] [CrossRef]
- Saroha, R.; Oh, J.H.; Lee, J.S.; Kang, Y.C.; Jeong, S.M.; Kang, D.W.; Cho, C.; Cho, J.S. Hierarchically porous nanofibers comprising multiple core-shell Co3O4@graphitic carbon nanoparticles grafted within N-doped CNTs as functional interlayers for excellent Li-S batteries. Chem. Eng. J. 2021, 426, 130805. [Google Scholar] [CrossRef]
- Wang, S.; Hou, X.; Zhong, Z.; Shen, K.; Zhang, G.; Yao, L.; Chen, F. Co3O4-NP embedded mesoporous carbon rod with enhanced electrocatalytic conversion in lithium-sulfur battery. Sci. Rep. 2018, 8, 16133. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Su, D.; Wang, G. Co3O4-carbon cloth free standing cathode for lithium sulfur battery. IOP Conf. Ser. Mater. Sci. Eng. 2017, 222, 012013. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Wang, S.; Tao, D.; Wang, M. Sulfur/Co3O4 nanotube composite with high performances as cathode materials for lithium sulfur batteries. Funct. Mater. Lett. 2014, 7, 1450020. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Lu, Y.; Zhu, Y.; Qian, Y.; Wang, D.; Zhou, J.; Lin, N.; Qian, Y. A graphene oxide-wrapped bipyramidal sulfur@polyaniline core–shell structure as a cathode for Li–S batteries with enhanced electrochemical performance. J. Mater. Chem. A 2016, 4, 6404–6410. [Google Scholar] [CrossRef]
- Ali, M.; Guzman, R.C.; Cojocari, O.; Nellen, S.; Santamaría, G.; García-Munoz, L.E.; Segovia-Vargas, D.; Globisch, B.; Carpintero, G. Quasi-optic transmitter and receiver modules enabling next-Generation ultra-broadband wireless links at carrier-wave frequencies ranging from 60 to 180 GHz. J. Infrared Millim. Terahertz Waves 2019, 40, 688–695. [Google Scholar] [CrossRef]
- Lv, C.; Li, S.; Che, Y.; Chen, H.; Shu, Y.; He, J.; Song, J. Study on the molybdenum electro-extraction from MoS2 in the molten salt. Sep. Purif. Technol. 2021, 258, 118048. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, M.; Pan, Y.; Du, X.; Wang, Q. MoS2 as highly efficient co-catalyst enhancing the performance of Fe0 based electro-fenton process in degradation of sulfamethazine: Approach and mechanism. Chem. Eng. J. 2021, 403, 126361. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wen, T.; Liu, X.; Wang, Y.; Yang, H.; Sun, J.; Feng, J.; Dong, S.; Sun, J. Highly effective remediation of Pb(II) and Hg(II) contaminated wastewater and soil by flower-like magnetic MoS2 nanohybrid. Sci. Total Environ. 2020, 699, 134341. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Chen, S.S.; Yu, T.-C.; Wu, K.C.W.; Hou, C.H. Effective electrochemically controlled removal of fluoride ions using electrodeposited polyaniline-carbon nanotube composite electrodes. Sep. Purif. Technol. 2021, 254, 117561. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, L.; Muhammad, Y.; Li, L. Effect of ionic liquid assisted hydrothermal carbonization on the properties and gasification reactivity of hydrochar derived from eucalyptus. J. Colloid Interface Sci. 2021, 586, 423–432. [Google Scholar] [CrossRef]
- Xu, K.; Liao, N.; Zhang, M.; Xue, W. Atomic-scale investigation of enhanced lithium, sodium and magnesium storage performance from defects in MoS2/graphene heterostructures. Nanoscale 2020, 12, 7098–7108. [Google Scholar] [CrossRef] [PubMed]
- Parra-Arciniega, S.M.; González-Juárez, E.; Hernández-Carrillo, R.A.; Briones-Martínez, R.; Jiménez-Barrera, R.M.; Garcia-Gómez, N.A.; Sánchez, E.M. A Mg2+/Li+ hybrid-ion battery based on MoS2 prepared by solvothermal synthesis with ionic liquid assistance. J. Mater. Sci. Mater. Electron. 2020, 31, 14702–14713. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, L.; Kang, Y.M.; An, Q. Recent advances in the rational design and synthesis of two-dimensional materials for multivalent ion batteries. ChemSusChem 2020, 13, 1071–1092. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.H.; Zhao, W.W.; Chen, K.X.; Wang, Y.F.; Liu, S.B.; Zhou, X.X.; Chen, L.; Li, Y. MOF-71 derived layered Co-CoP/C for advanced Li-S batteries. J. Alloy. Compd. 2021, 886, 161203. [Google Scholar] [CrossRef]
- Pu, X.; Song, T.; Tang, L.; Tao, Y.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Rose-like vanadium disulfide coated by hydrophilic hydroxyvanadium oxide with improved electrochemical performance as cathode material for aqueous zinc-ion batteries. J. Power Sources 2019, 437, 226917. [Google Scholar] [CrossRef]
- Sihag, A.; Xie, Z.L.; Thang, H.V.; Kuo, C.L.; Tseng, F.G.; Dyer, M.S.; Chen, H.Y.T. DFT Insights into Comparative Hydrogen Adsorption and Hydrogen Spillover Mechanisms of Pt4/Graphene and Pt4/Anatase (101) Surfaces. J. Phys. Chem. C 2019, 123, 25618–25627. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Zhang, D.; Wei, Y.; Zhang, R.; Guo, Y. Study on MnO2/MXene–Ti3C2 composite materials as cathode materials for magnesium batteries. RSC Adv. 2019, 9, 33572–33577. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, B.; Guo, P.; Shang, X.; Lv, M.; Liu, D.; He, D. Enhanced electrochemical kinetics in lithium-sulfur batteries by using carbon nanofibers/manganese dioxide composite as a bifunctional coating on sulfur cathode. Electrochim. Acta 2018, 28, 180–187. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Tang, C. Advanced Co3O4 interlayer as an efficient polysulfide barrier for high-performance Li-S batteries. Int. J. Electrochem. Sci. 2019, 14, 3245–3252. [Google Scholar]
- Wang, J.; Xiao, K.; Ouyang, B.; Zhang, L.; Yang, H. Simultaneous immobilization and conversion of polysulfides on Co3O4-CoN heterostructured mediators toward high-performance lithium–sulfur batteries. ACS Appl. Energy Mater. 2019, 2, 2570–2578. [Google Scholar] [CrossRef]
- Mussa, Y.; Arsalan, M.; Alsharaeh, E. Cobalt oxide/graphene nanosheets/hexagonal boron nitride (Co3O4/CoO/GNS/h-BN) catalyst for high sulfur utilization in Li−S batteries at elevated temperatures. Energy Fuels 2021, 35, 8365–8377. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Wu, X.; Zhang, Y.; Danilov, D.; Eichel, R.; Notten, P. Double-shelled Co3O4/C nanocages enabling polysulfides adsorption for high-performance lithium-sulfur batteries. ACS Appl. Energy Mater. 2019, 2, 8153–8162. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, C.; Wang, C.; Yang, P.; Wu, R.; Fei, L.; Zhang, Y.; Jiang, Y. An approach through steam to form sulfur nanoparticles for lithium sulfur batteries. Electorchem. Commun. 2021, 125, 107010. [Google Scholar] [CrossRef]
- Liu, B.; Huang, S.; Kong, D.; Hu, J.; Yang, H. Bifunctional NiCo2S4 catalysts supported on a carbon textile interlayer for ultra-stable Li-S battery. J. Mater. Chem. A 2019, 7, 7604. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, M.; Luo, J.; Liu, H.; Li, Y.; Peng, J.; Li, J.; Yu, H. Carbon-coated yttria hollow spheres as both sulfur immobilizer and catalyst of polysulfides conversion in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 11, 42104–42113. [Google Scholar] [CrossRef]
- Liu, Y.; Chatterjee, A.; Rusch, P.; Wu, C.; Nan, P.; Peng, M. Monodisperse Molybdenum Nanoparticles as Highly Efficient Electrocatalysts for Li-S Batteries. ACS Nano 2021, 15, 15047–15056. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, J.; Rao, D.; Zhu, L.; Niu, S.; Cai, J.; Fang, Y. Regulating the electron filling state of d orbitals in Ta-based compounds for tunable lithium-sulfur chemistry. Sustain Mater. Technol. 2021, 28, e00271. [Google Scholar] [CrossRef]
- Zhou, P.; Han, T.; Gu, C.; Li, J.; Shen, Z.; Zhang, H.; Niu, J. A novel wheel-confined composite as cathode in Li-S batteries with high capacity retention. J. Alloy Compd. 2019, 776, 504–510. [Google Scholar] [CrossRef]
- Du, C.; Zhu, Y.; Wang, Z.; Wang, L.; Younas, W.; Ma, X.; Cao, C. Cuprous self-doping regulated mesoporous CuS nanotube cathode materials for rechargeable magnesium batteries. ACS Appl. Mater. Interfaces 2020, 12, 35035–35042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Zhang, C.; Chi, Q.; Zhang, T.; Feng, Y.; Zhu, K.; Zhang, Y.; Chen, Q.; Cao, D. Low-cost MgFexMn2-xO4 cathode materials for high-performance aqueous rechargeable magnesium-ion batteries. Chem. Eng. J. 2020, 392, 123652. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.; Seh, Z.; Cai, Q.; Li, W.; Zhou, G.; et al. Balancing surface adsorption and diffusion of lithium polysulfides on nonconductive oxides for lithium-sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, J.; Zang, J.; Chen, C.; Lu, Y.; Jiang, M.; Yan, C.; Dirican, M.; Selvan, R.; Zhang, X. A novel bi-functional double-layer rGO-PVDF/PVDF composite nanofiber membrane separator with enhanced thermal stability and effective polysulfide inhibition for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 15096–15104. [Google Scholar] [CrossRef]
- Gao, G.; Zheng, F.; Wang, L. Solid 3D Li−S battery design via stacking 2D conductive microporous coordination polymers and amorphous Li−S layers. Chem. Mater. 2020, 32, 1974–1982. [Google Scholar] [CrossRef]
- Wang, K.; Guan, Y.; Jin, Z.; Wang, W.; Wang, A. Te0.045S0.955 PAN composite with high average discharge voltage for Li-S battery. J. Energy Chem. 2019, 39, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cao, D.; Bai, X. Ni-Doped magnesium manganese oxide as a cathode and its application in aqueous magnesium-ion batteries with high rate performance. Inorg. Chem. Front. 2020, 7, 2168–2177. [Google Scholar] [CrossRef]
- Harudin, N.; Osman, Z.; Majid, S.R.; Othman, L.; Hambali, D.; Silva, M.M. Improved electrochemical properties of MgMn2O4 cathode materials by Sr doping for Mg ion cells. Ionics 2020, 26, 3947–3958. [Google Scholar] [CrossRef]
- Asif, M.; Rashad, M.; Shah, J.H.; Zaidi, S.D.A. Surface modification of tin oxide through reduced graphene oxide as a highly efficient cathode material for magnesium-ion batteries. J. Colloid Interface Sci. 2020, 561, 818–828. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).