Remediation of Cr(VI)-Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and the Consequences for Indigenous Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Soil Used in the Experiment
2.3. Soil Incubation Experiment
2.4. Analysis of Soil Cr
2.5. Soil Physicochemical Analysis
2.6. Soil Microbial Analysis
2.7. Statistical Analysis
3. Results
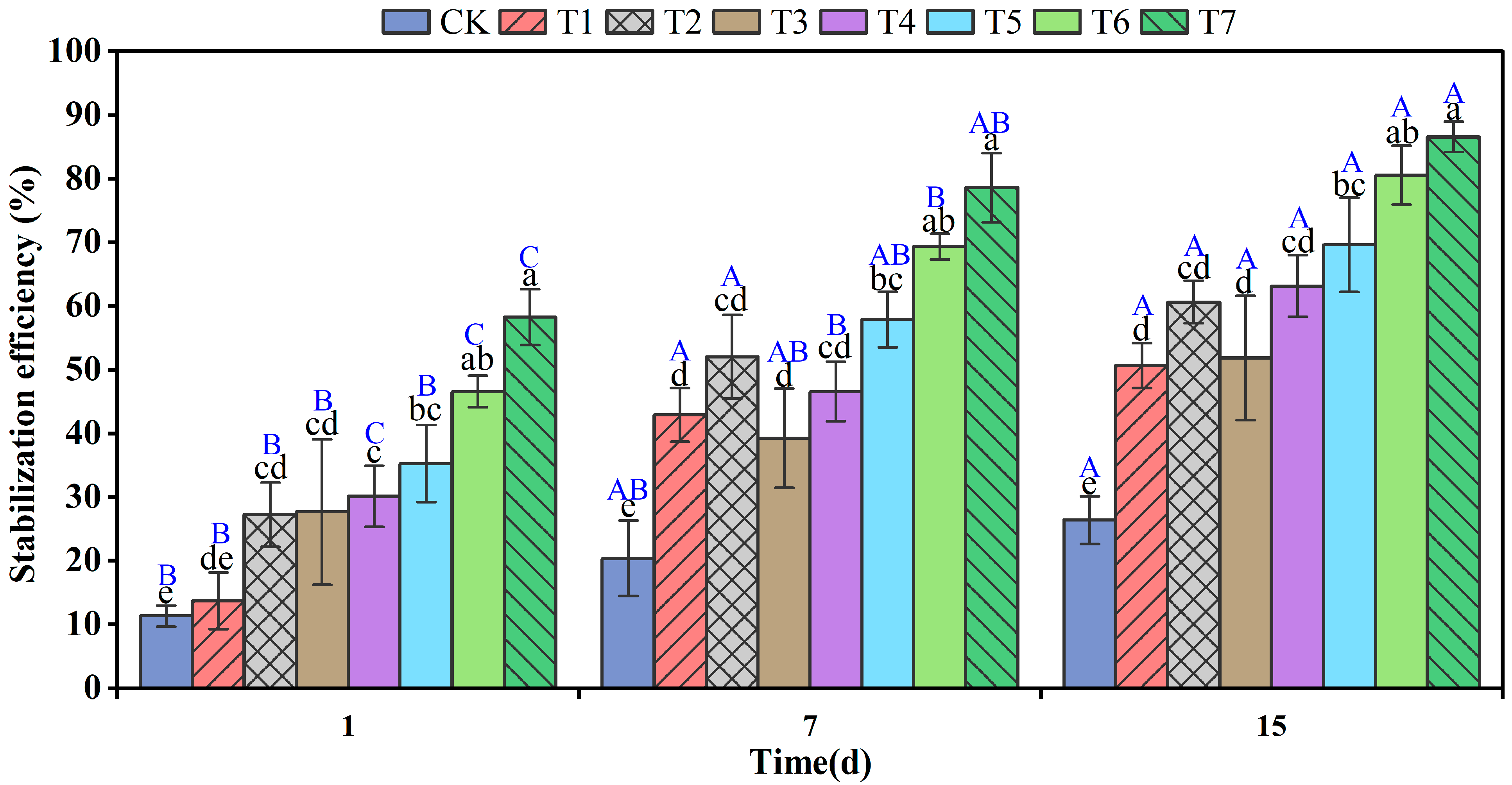
3.1. Remediation Effect of Cr(VI) in Soil
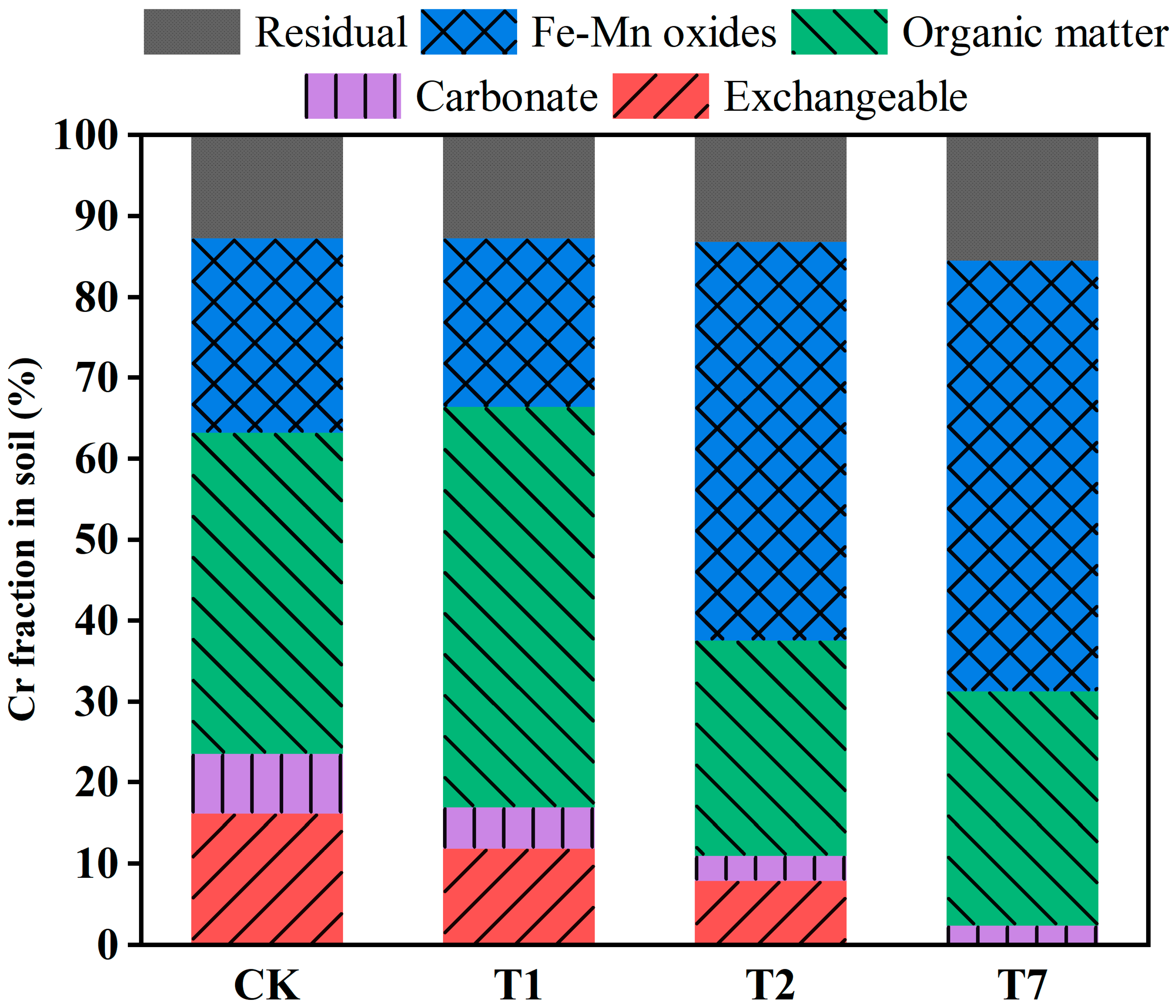
3.2. The Speciation of Cr in the Soil
3.3. Effect of BC-nZVI on Cr-Contaminated Soil Microorganisms
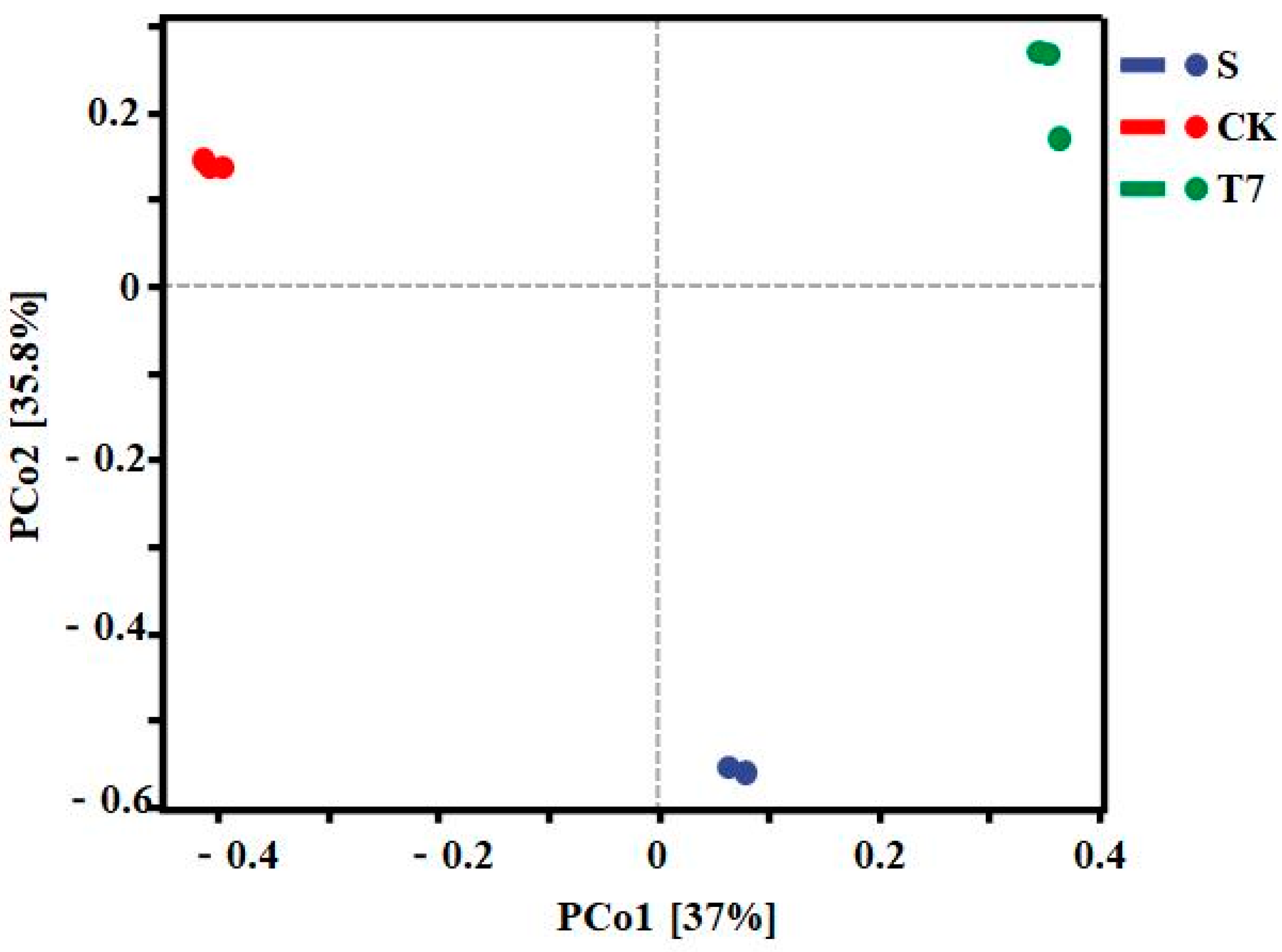
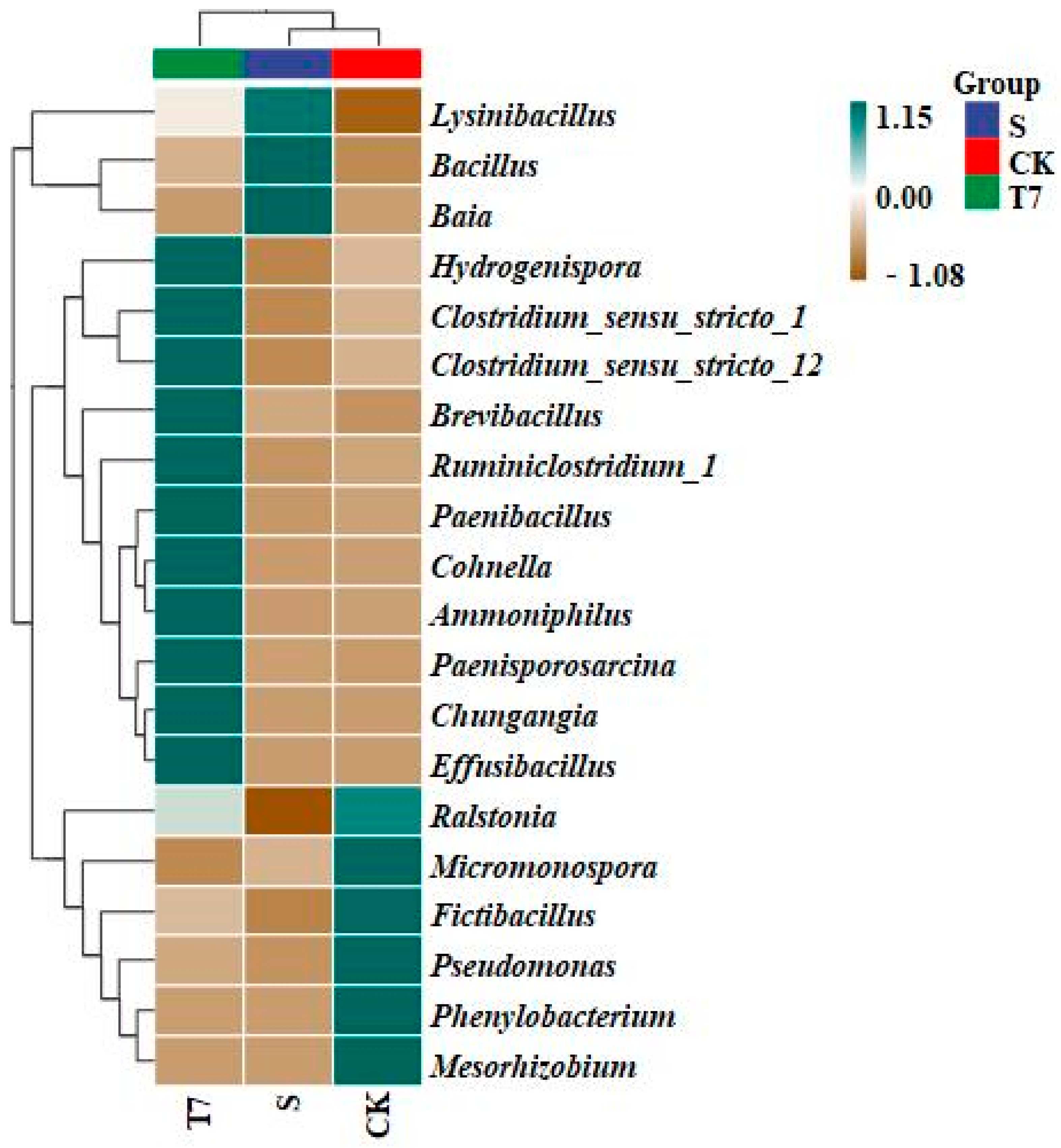
3.3.1. Soil Bacterial Community Diversity Analysis
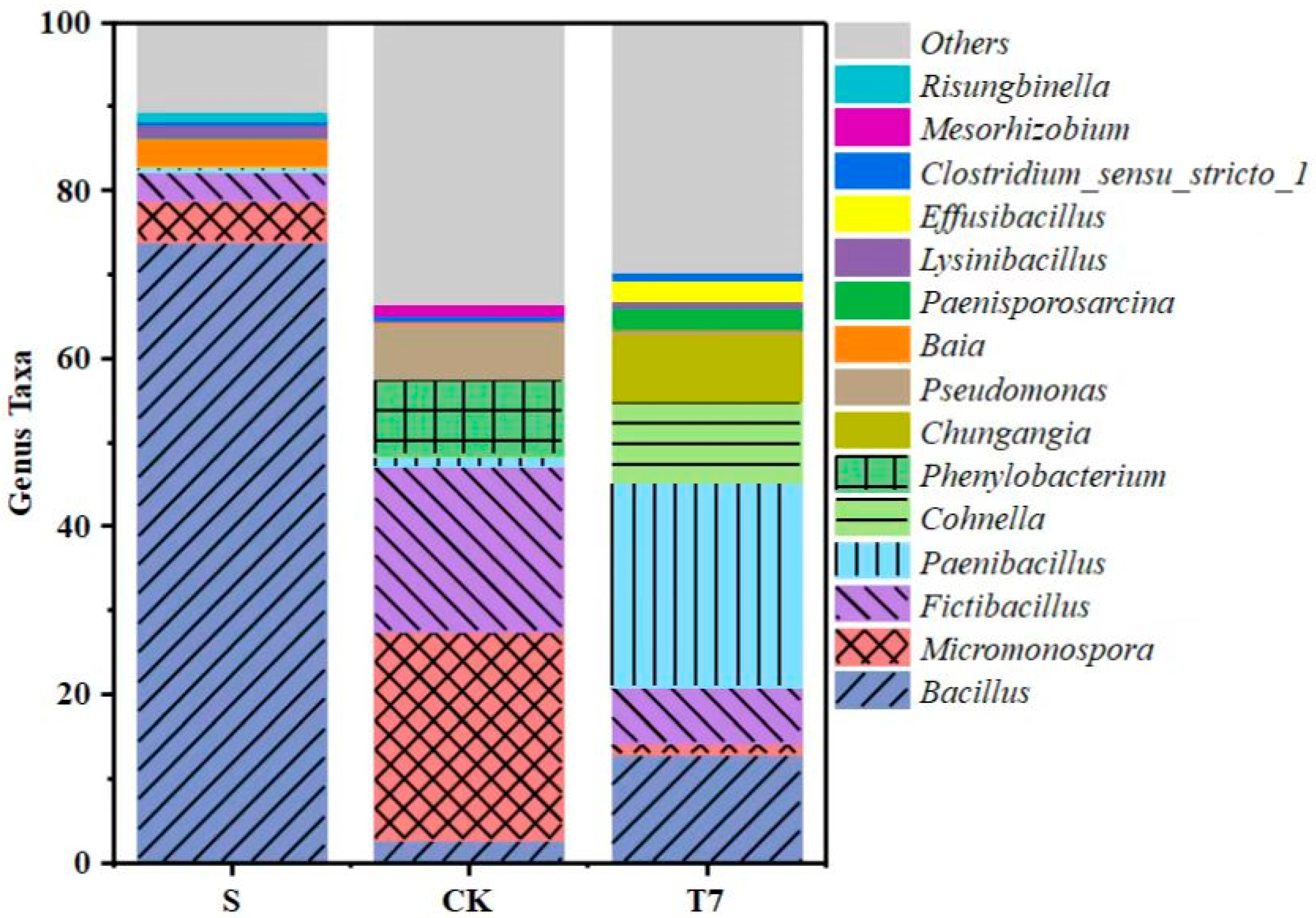
3.3.2. Soil Bacterial Community Structure Analysis
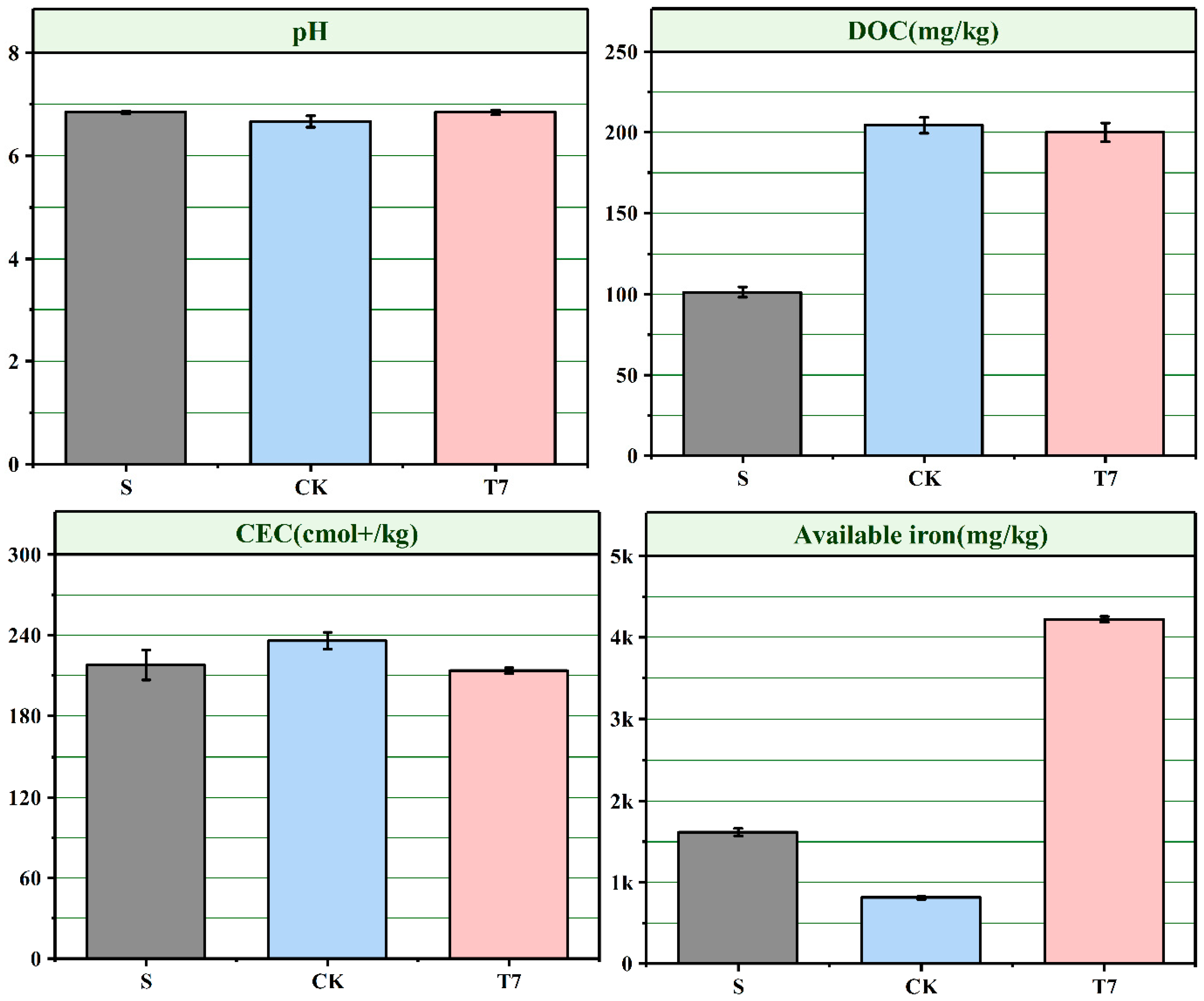
3.4. Effects of BC-nZVI on Soil Chemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.; Li, P. Surface Water Pollution in the Middle Chinese Loess Plateau with Special Focus on Hexavalent Chromium (Cr6+): Occurrence, Sources and Health Risks. Expo. Health 2020, 12, 385–401. [Google Scholar] [CrossRef]
- Gan, Y.; Huang, X.; Li, S.; Liu, N.; Li, Y.C.; Freidenreich, A.; Wang, W.; Wang, R.; Dai, J. Source quantification and potential risk of mercury, cadmium, arsenic, lead, and chromium in farmland soils of Yellow River Delta. J. Clean. Prod. 2019, 221, 98–107. [Google Scholar] [CrossRef]
- Deb, A.K.; Biswas, B.; Naidu, R.; Rahman, M.M. Mechanistic insights of hexavalent chromium remediation by halloysite-supported copper nanoclusters. J. Hazard. Mater. 2021, 421, 126812. [Google Scholar] [CrossRef]
- Ding, K.; Zhou, X.; Hadiatullah, H.; Lu, Y.; Zhao, G.; Jia, S.; Zhang, R.; Yao, Y. Removal performance and mechanisms of toxic hexavalent chromium (Cr(VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J. Hazard. Mater. 2021, 420, 126551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gai, X.; Zhong, Z.; Bian, F.; Yang, C.; Li, Y.; Wen, X. Understanding variations in soil properties and microbial communities in bamboo plantation soils along a chromium pollution gradient. Ecotoxicol. Environ. Saf. 2021, 222, 112507. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Zhang, X.M.; Jiang, Z.; Li, Q.; Huang, P.C.; Zheng, C.J.; Liao, Q.; Yang, W.C. Reductive materials for remediation of hexavalent chromium contaminated soil—A review. Sci. Total Environ. 2021, 773, 145654. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Misra, V.; Singh, R.P. Removal of Cr(VI) by Nanoscale Zero-valent Iron (nZVI) From Soil Contaminated with Tannery Wastes. Bull. Environ. Contam. Toxicol. 2012, 88, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Su, H.J.; Fang, Z.Q.; Tsang, P.E.; Fang, J.Z.; Zhao, D.Y. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. 2016, 214, 94–100. [Google Scholar] [CrossRef]
- Su, H.J.; Fang, Z.Q.; Tsang, P.E.; Zheng, L.C.; Cheng, W.; Fang, J.Z.; Zhao, D.Y. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Wang, H.X.; Zhang, M.L.; Li, H.Y. Synthesis of Nanoscale Zerovalent Iron (nZVI) Supported on Biochar for Chromium Remediation from Aqueous Solution and Soil. Int. J. Environ. Res. Public Health 2019, 16, 4430. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Forján, R.; Álvarez, N.; Gallego, J.R.; González, A. Zero Valent Iron Nanoparticles and Organic Fertilizer Assisted Phytoremediation in A Mining Soil: Arsenic and Mercury Accumulation and Effects on the Antioxidative System of Medicago sativa L. J. Hazard. Mater. 2022, 433, 128748. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Xu, M.; Tang, S.; Zhou, J.; Pan, W.; Ma, Q.; Wu, L. Nano zero-valent iron-induced changes in soil iron species and soil bacterial communities contribute to the fate of Cd. J. Hazard. Mater. 2022, 424, 127343. [Google Scholar] [CrossRef]
- Xi, B.; Yu, H.; Li, Y.; Dang, Q.; Tan, W.; Wang, Y.; Cui, D. Insights into the effects of heavy metal pressure driven by long-term treated wastewater irrigation on bacterial communities and nitrogen-transforming genes along vertical soil profiles. J. Hazard. Mater. 2021, 403, 123853. [Google Scholar] [CrossRef]
- Jiang, D.N.; Zeng, G.M.; Huang, D.L.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Liu, N.; Gong, Y.; Peng, X.; Li, S.; Zhang, W.X. A win-win solution to chromate removal by sulfidated nanoscale zero-valent iron in sludge. J. Hazard. Mater. 2022, 432, 10. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2017, 232, 123–136. [Google Scholar] [CrossRef]
- Hui, C.; Liu, B.; Du, L.; Xu, L.; Zhao, Y.; Shen, D.; Long, Y. Transformation of sulfidized nanoscale zero-valent iron particles and its effects on microbial communities in soil ecosystems. Environ. Pollut. 2022, 306, 119363. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Koltowski, M. Effect of co-application of nano-zero valent iron and biochar on the total and freely dissolved polycyclic aromatic hydrocarbons removal and toxicity of contaminated soils. Chemosphere 2017, 168, 1467–1476. [Google Scholar] [CrossRef]
- Tan, X.; Shaaban, M.; Yang, J.; Cai, Y.; Wang, B.; Peng, Q.-A. Efficient Removal of Hexavalent Chromium from an Aquatic System Using Nanoscale Zero-Valent Iron Supported by Ramie Biochar. Nanomaterials 2021, 11, 2698. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Li, H.; Peng, D.; Zeng, C.; Xu, H. Remediation of hexavalent chromium contaminated soil by nano-FeS coated humic acid complex in combination with Cr-resistant microflora. Chemosphere 2020, 242, 125251. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Shaaban, M.; Abid, M.; Qi-An, P. Short term influence of gypsum, farm manure and commercial humic acid on physical properties of salt affected soil in rice paddy system. J. Chem. Soc. 2013, 34, 1034–1040. [Google Scholar]
- Shaaban, M.; Peng, Q.; Lin, S.; Wu, Y.; Zhao, J.; Hu, R. Nitrous oxide emission from two acidic soils as affected by dolomite application. Soil Res. 2014, 52, 841–848. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ. Pollut. 2021, 291, 118129. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; Zhang, C.; Zeng, G.; Liu, Y.; Zhou, C.; Xu, W.; Wu, Y.; Tang, X.; Liu, W.; et al. Enhanced biological stabilization of heavy metals in sediment using immobilized sulfate reducing bacteria beads with inner cohesive nutrient. J. Hazard. Mater. 2017, 324, 340–347. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; la Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef]
- Peng, J.-f.; Song, Y.-h.; Yuan, P.; Cui, X.-y.; Qiu, G.-l. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Wang, F.-H.; Zhao, B.; Zhang, F.; Gao, J.; Hao, H.-T.; Zhang, S. A novel heavy metal chelating agent sixthio guanidine acid for in situ remediation of soils contaminated with multielements: Its synthesis, solidification, biodegradability, and leachability. J. Soils Sediments 2016, 16, 371–381. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.Q.; Kang, Y.; Tsang, E.P. Immobilization and phytotoxicity of chromium in contaminated soil remediated by CMC-stabilized nZVI. J. Hazard. Mater. 2014, 275, 230–237. [Google Scholar] [CrossRef]
- Wu, M.; Li, G.; Li, P.; Jiang, N.; Wei, S.; Petropoulos, E.; Li, Z. Assessing the ecological risk of pesticides should not ignore the impact of their transformation byproducts—The case of chlorantraniliprole. J. Hazard. Mater. 2021, 418, 126270. [Google Scholar] [CrossRef]
- Ma, W.; Sun, T.; Xu, Y.; Zheng, S.; Sun, Y. In–situ immobilization remediation, soil aggregate distribution, and microbial community composition in weakly alkaline Cd–contaminated soils: A field study. Environ. Pollut. 2022, 292, 118327. [Google Scholar] [CrossRef]
- Li, D.; Li, G.; Zhang, D. Field-scale studies on the change of soil microbial community structure and functions after stabilization at a chromium-contaminated site. J. Hazard. Mater. 2021, 415, 125727. [Google Scholar] [CrossRef]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Brookes, P.C.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Liu, S.; Pu, S. Impact of ZnO nanoparticles on soil lead bioavailability and microbial properties. Sci. Total Environ. 2022, 806, 150299. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. ACM Sigmobile Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Peng, Q.-A.; Shaaban, M.; Wu, Y.; Liu, F.; Hu, R.; Wang, B. Nitrate dependent Fe-oxidizing bacterial diversity in subtropical soils of China. Catena 2019, 176, 181–188. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Wu, J.; Huang, L.; Brookes, P.C.; Rodrigues, J.L.M.; Xu, J.; Liu, X. Effects of magnetic biochar-microbe composite on Cd remediation and microbial responses in paddy soil. J. Hazard. Mater. 2021, 414, 125494. [Google Scholar] [CrossRef]
- Katsaveli, K.; Vayenas, D.; Tsiamis, G.; Bourtzis, K. Bacterial diversity in Cr(VI) and Cr(III)-contaminated industrial wastewaters. Extremophiles 2012, 16, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Garcia-Cantalejo, J.; Botias, P.; Cost, G.; Nande, M.; Martin, M. New insights into the impact of nZVI on soil microbial biodiversity and functionality. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2019, 54, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent Chromium Reduction with Zero-Valent Iron (ZVI) in Aquatic Systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Fan, J.; Chen, X.; Xu, Z.B.; Xu, X.Y.; Zhao, L.; Qiu, H.; Cao, X.D. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J. Hazard. Mater. 2020, 398, 122901. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.-R.; Tang, J.-F.; Cotner, J.B.; Xu, Y.-Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef]
- Wu, C.; Shi, L.; Xue, S.; Li, W.; Jiang, X.; Rajendran, M.; Qian, Z. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Q.; Li, T.; Zhan, W.; Zheng, K.; Liu, Y.; Chen, R. Influences of modified biochar on metal bioavailability, metal uptake by wheat seedlings (Triticum aestivum L.) and the soil bacterial community. Ecotoxicol. Environ. Saf. 2021, 220, 112370. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2020, 19, 1413–1431. [Google Scholar] [CrossRef]
- Wang, C.; Deng, H.; Zhao, F. The Remediation of Chromium (VI)-Contaminated Soils Using Microbial Fuel Cells. Soil Sediment Contam. 2016, 25, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Gao, P.; Li, D.; Liu, J.; Yang, N.; Gu, W.; He, X.; Tang, W. Risk assessment for and microbial community changes in Farmland soil contaminated with heavy metals and metalloids. Ecotoxicol. Environ. Saf. 2019, 185, 109685. [Google Scholar] [CrossRef]
- Branco, R.; Chung, A.-P.; Veríssimo, A.; Morais, P.V. Impact of chromium-contaminated wastewaters on the microbial community of a river. FEMS Microbiol. Ecol. 2005, 54, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Camargo, F.A.; Okeke, B.C.; Bento, F.M.; Frankenberger, W.T. Diversity of chromium-resistant bacteria isolated from soils contaminated with dichromate. Appl. Soil Ecol. 2005, 29, 193–202. [Google Scholar] [CrossRef]
- Fajardo, C.; Sacca, M.L.; Martinez-Gomariz, M.; Costa, G.; Nande, M.; Martin, M. Transcriptional and proteomic stress responses of a soil bacterium Bacillus cereus to nanosized zero-valent iron (nZVI) particles. Chemosphere 2013, 93, 1077–1083. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Meléndez, G.; Korre, A.; Parry, S.J. Influence of soil pH on the fractionation of Cr, Cu and Zn in solid phases from a landfill site. Environ. Pollut. 2000, 110, 497–504. [Google Scholar] [CrossRef]
- Yang, D.; Yang, S.Y.; Yuan, H.H.; Wang, F.; Wang, H.L.; Xu, J.M.; Liu, X.M. Co-benefits of biochar-supported nanoscale zero-valent iron in simultaneously stabilizing soil heavy metals and reducing their bioaccessibility. J. Hazard. Mater. 2021, 418, 126292. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Wang, G.H.; Liu, L.; Bian, H.; Jiang, L.L.; Wang, H.D.; Zhang, Y.J.; Zhang, W.; Peng, C.; Wang, X.D. Immobilization of Ni (II) at three levels of contaminated soil by rhamnolipids modified nano zero valent iron (RL@nZVI): Effects and mechanisms. Chemosphere 2021, 276, 130139. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Tan, X.; Shaaban, M.; Cai, Y.; Wang, B.; Peng, Q. Remediation of Cr(VI)-Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and the Consequences for Indigenous Microbial Communities. Nanomaterials 2022, 12, 3541. https://doi.org/10.3390/nano12193541
Yang J, Tan X, Shaaban M, Cai Y, Wang B, Peng Q. Remediation of Cr(VI)-Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and the Consequences for Indigenous Microbial Communities. Nanomaterials. 2022; 12(19):3541. https://doi.org/10.3390/nano12193541
Chicago/Turabian StyleYang, Jianwei, Xiangpeng Tan, Muhammad Shaaban, Yajun Cai, Buyun Wang, and Qi’an Peng. 2022. "Remediation of Cr(VI)-Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and the Consequences for Indigenous Microbial Communities" Nanomaterials 12, no. 19: 3541. https://doi.org/10.3390/nano12193541
APA StyleYang, J., Tan, X., Shaaban, M., Cai, Y., Wang, B., & Peng, Q. (2022). Remediation of Cr(VI)-Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and the Consequences for Indigenous Microbial Communities. Nanomaterials, 12(19), 3541. https://doi.org/10.3390/nano12193541





