Strategic Electrochemical Determination of Nitrate over Polyaniline/Multi-Walled Carbon Nanotubes-Gum Arabic Architecture
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of GA@PAni-CNT Nanocomposite
2.3. Characterization Details
2.4. Preparation of GA@PAni-CNT Electrode
2.5. Electrochemical Studies
2.6. Practical Application
3. Results and Discussion
3.1. UV–Visible Spectra
3.2. Raman and FTIR Analysis
3.3. XRD Pattern
3.4. XPS Study
3.5. FESEM Surface Morphology
3.6. EIS Performance
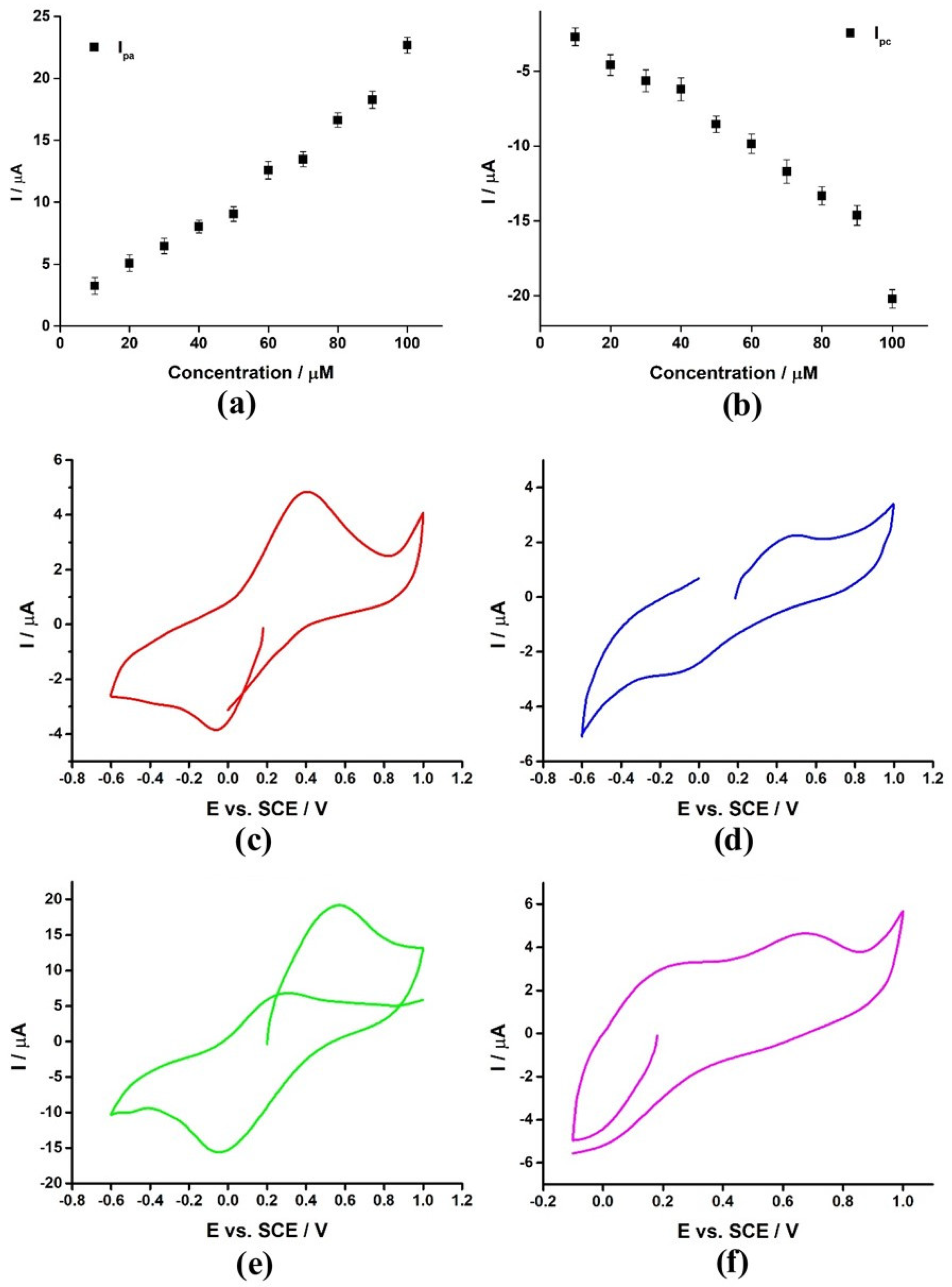
3.7. CV Behavior
3.8. GA@PAni-CNT as a Suitable Electrode
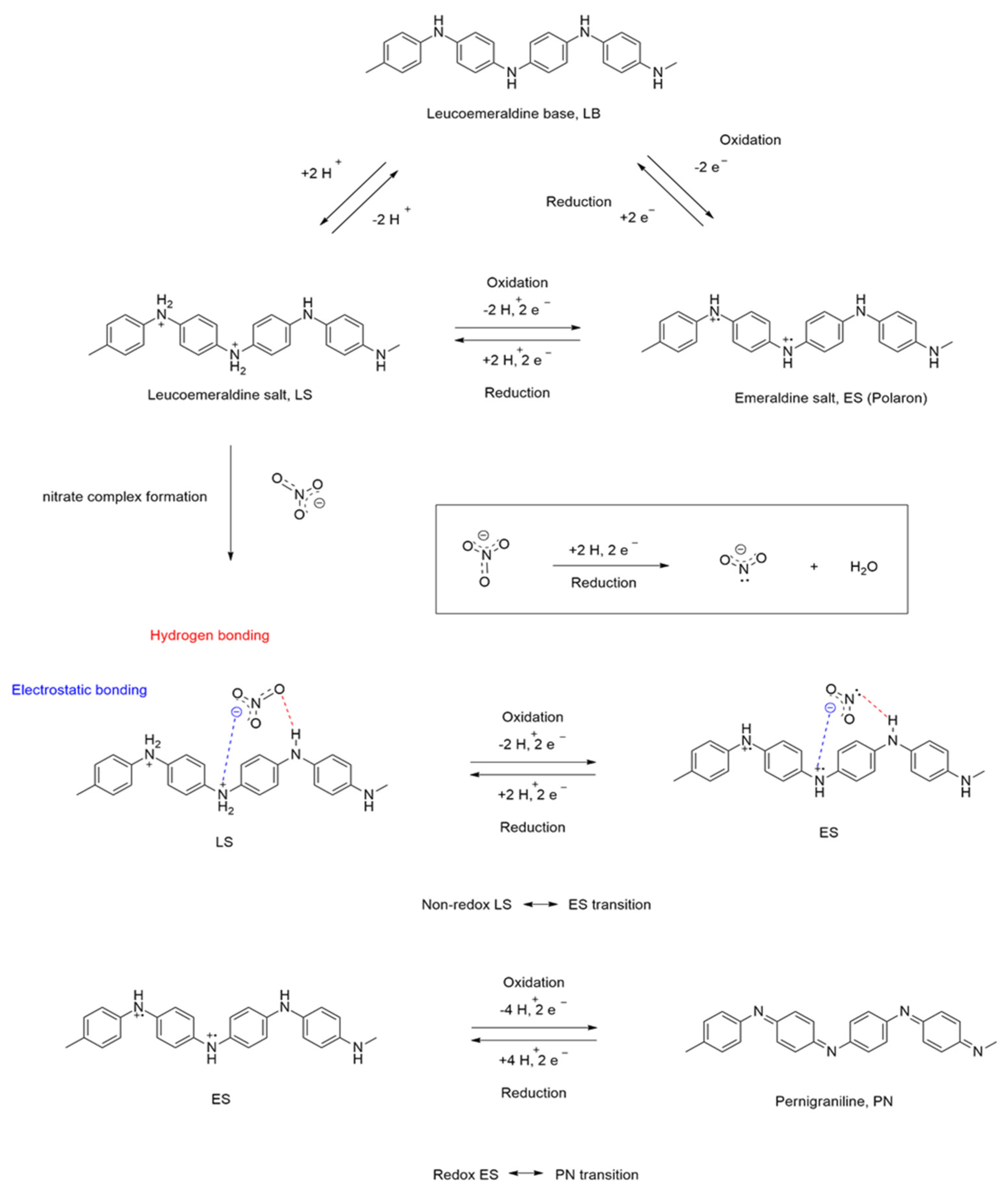
3.9. On the Oxidation Mechanism of GA@PAni-CNT Electrode
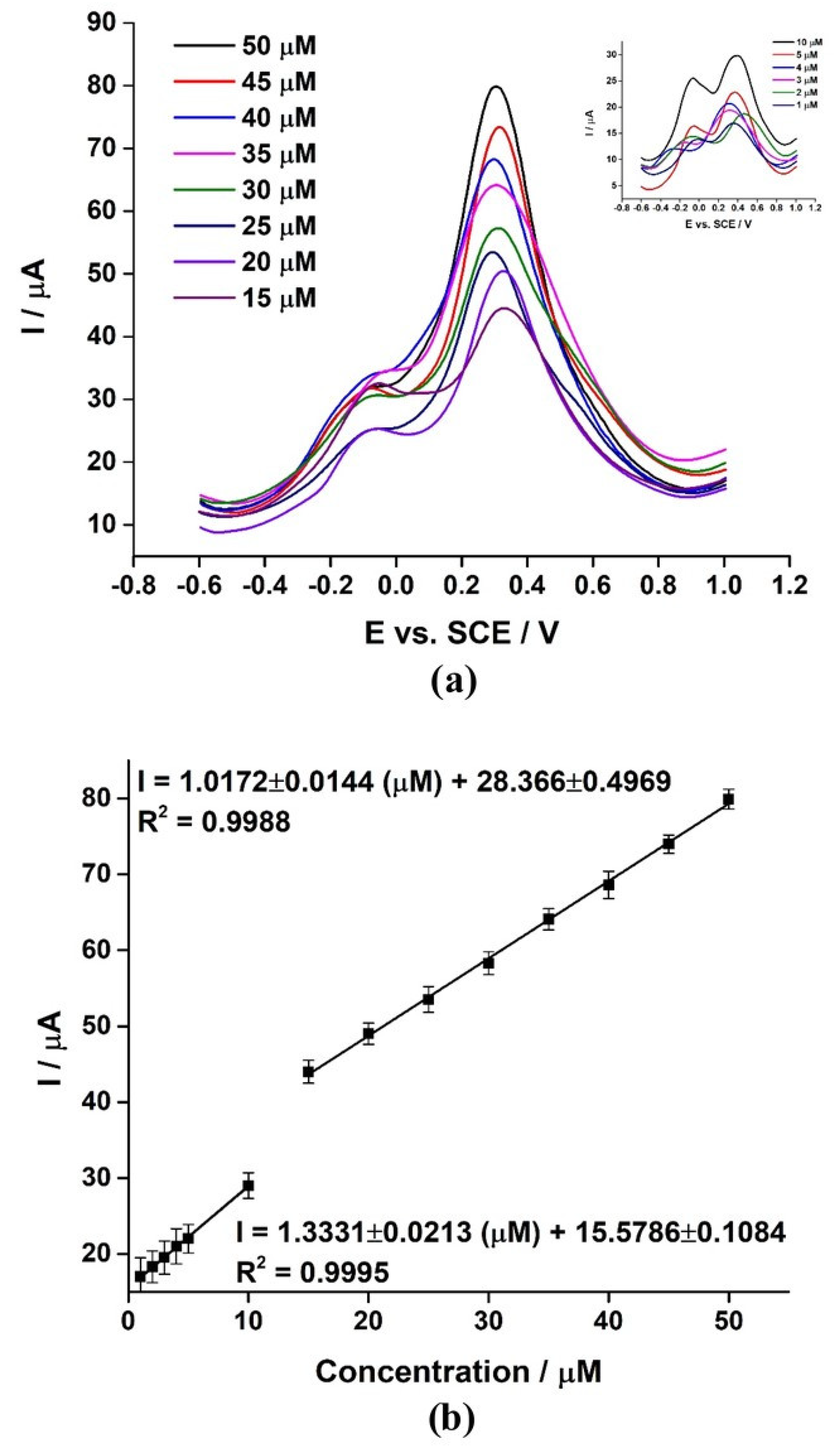
3.10. Selection and Optimization of SWV Response
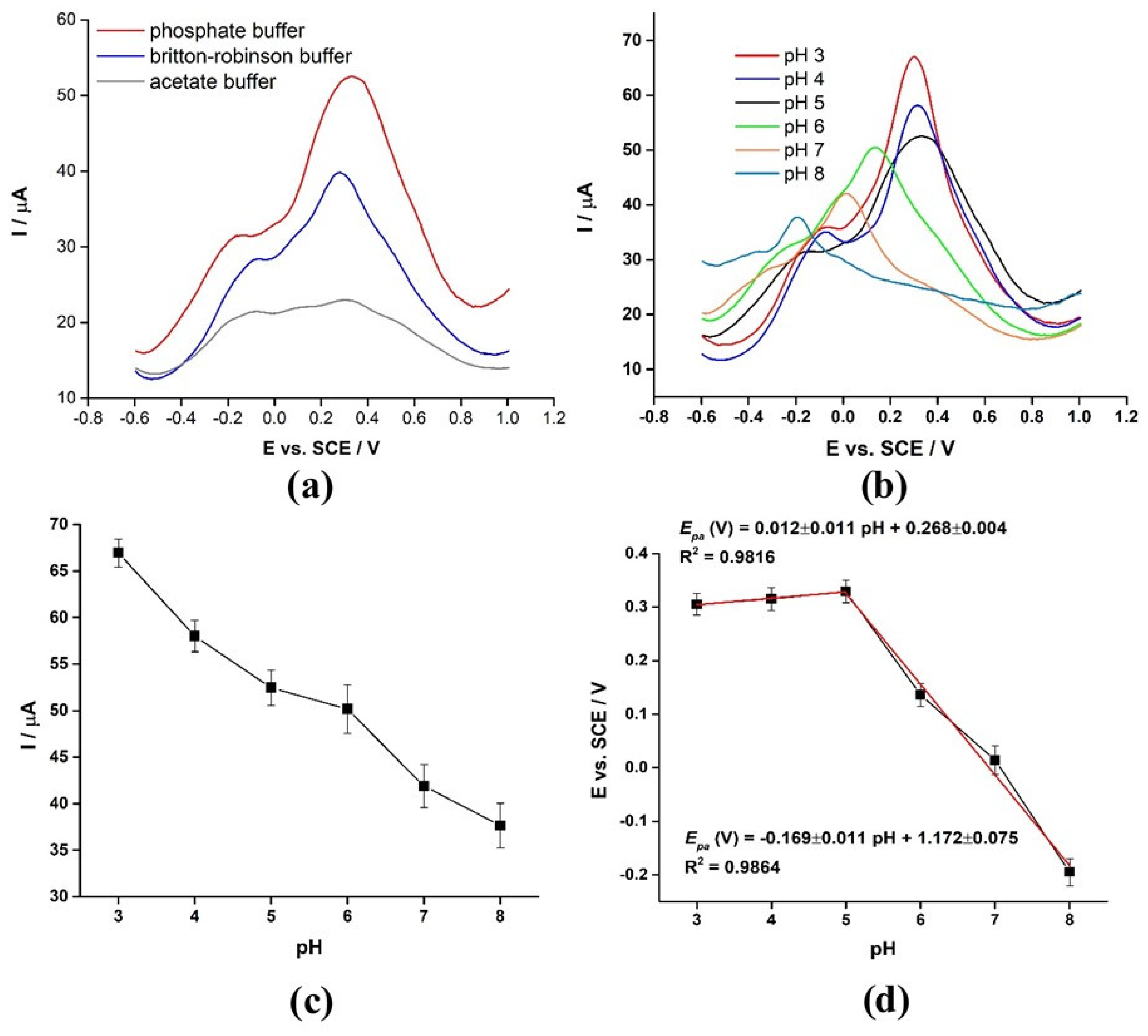
3.11. Buffer Electrolyte Selection and pH Study
3.12. Analytical and Interference Concerns
3.13. NO3− Recovery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Hu, S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Hopkins, J.; Fidanovski, K.; Lauto, A.; Mawad, D. All-organic semiconductors for electrochemical biosensors: An overview of recent progress in material design. Front. Bioeng. Biotechnol. 2019, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, K.; Ahmad, Z.; Fakir, M.S.; Wahab, F.A.; Abdullah, S.M.; Rahman, Z.A. Organic semiconductors: Applications in solar photovoltaic and sensor devices. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; Volume 737, pp. 126–132. [Google Scholar]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.-X. Carbon nanotube–polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Mishra, S.; Solanki, P.R.; Mayandi, V.; Beuerman, R.W.; Ramakrishna, S.; Lakshminarayanan, R.; Malhotra, B.D. Polyaniline-based biosensors. Nanobiosensors Dis. 2015, 4, 25. [Google Scholar]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Dutta, R.; Thangapandi, K.; Mondal, S.; Nanda, A.; Bose, S.; Sanyal, S.; Jana, S.K.; Ghorai, S. Polyaniline based electrochemical sensor for the detection of dengue virus infection. Avicenna J. Med. Biotechnol. 2020, 2, 77. [Google Scholar]
- Fayemi, O.E.; Adekunle, A.S.; Swamy, B.E.K.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine in real samples using polyaniline/NiO, ZnO, and Fe3O4 nanocomposites on glassy carbon electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Deng, J.; Duan, X.; Guo, J.; Liu, P. Improving cyclic stability of polyaniline by thermal crosslinking as electrode material for supercapacitors. RSC Adv. 2015, 5, 78545–78552. [Google Scholar] [CrossRef]
- Mažeikien, R.; Malinauskas, A. Electrochemical stability of polyaniline. Eur. Polym. J. 2002, 38, 1947–1952. [Google Scholar] [CrossRef]
- Conroy, K.G.; Breslin, C.B. The electrochemical deposition of polyaniline at pure aluminium: Electrochemical activity and corrosion protection properties. Electrochim. Acta 2003, 48, 721–732. [Google Scholar] [CrossRef][Green Version]
- Song, E.; Choi, J.-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Rasmussen, S.C. The early history of polyaniline: Discovery and origins. Substantia 2017, 1, 99–109. [Google Scholar]
- Huerta, F.; Quijada, C.; Montilla, F.; Morallón, E. Revisiting the redox transitions of Polyaniline. Semiquantitative interpretation of electrochemically induced IR bands. J. Electroanal. Chem. 2021, 897, 115593. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Thanasamy, D.; Jesuraj, D.; Avadhanam, V.; Chinnadurai, K.; Kannan, S.K.K. Microstructural effect of various polyaniline-carbon nanotube core-shell nanocomposites on electrochemical supercapacitor electrode performance. J. Energy Storage 2022, 53, 105087. [Google Scholar] [CrossRef]
- Das, H.T.; Dutta, S.; Balaji, T.E.; Das, N.; Das, P.; Dheer, N.; Kanojia, R.; Ahuja, P.; Ujjain, S.K. Recent Trends in Carbon Nanotube Electrodes for Flexible Supercapacitors: A Review of Smart Energy Storage Device Assembly and Performance. Chemosensors 2022, 10, 223. [Google Scholar] [CrossRef]
- Al-Qasmi, N.; Soomro, M.T.; Ismail, I.M.I.; Danish, E.Y.; Al-Ghamdi, A.A. An enhanced electrocatalytic oxidation and determination of 2, 4-dichlorophenol on multilayer deposited functionalized multi-walled carbon nanotube/Nafion composite film electrode. Arabian J. Chem. 2019, 12, 946–956. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Awasthi, K. Polyaniline–carbon nanotube composites: Preparation methods, properties, and applications. Polym. Plast. Technol. Eng. 2018, 57, 70–97. [Google Scholar] [CrossRef]
- Gajendran, P.; Saraswathi, R. Polyaniline-carbon nanotube composites. Pure Appl. Chem. 2008, 80, 2377–2395. [Google Scholar] [CrossRef]
- Ebrahim, S.; El-Raey, R.; Hefnawy, A.; Ibrahim, H.; Soliman, M.; Abdel-Fattah, T.M. Electrochemical sensor based on polyaniline nanofibers/single wall carbon nanotubes composite for detection of malathion. Synth. Met. 2014, 190, 13–19. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, D.; Wang, X. Electrochemical preparation and application of PANI/MWNT and PPy/MWNT composite anodes for anaerobic fluidized bed microbial fuel cell. 3 Biotech 2020, 10, 3. [Google Scholar] [CrossRef]
- Che, B.; Li, H.; Zhou, D.; Zhang, Y.; Zeng, Z.; Zhao, C.; He, C.; Liu, E.; Lu, X. Porous polyaniline/carbon nanotube composite electrode for supercapacitors with outstanding rate capability and cyclic stability. Compos. B Eng. 2019, 165, 671–678. [Google Scholar] [CrossRef]
- Rauhala, T.; Davodi, F.; Sainio, J.; Sorsa, O.; Kallio, T. On the stability of polyaniline/carbon nanotube composites as binder-free positive electrodes for electrochemical energy storage. Electrochim. Acta 2020, 336, 135735. [Google Scholar] [CrossRef]
- Xu, L.; Sitinamaluwa, H.; Li, H.; Qiu, J.; Wang, Y.; Yan, C.; Li, H.; Yuan, S.; Zhang, S. Low cost and green preparation process for α-Fe2O3@gum arabic electrode for high performance sodium ion batteries. J. Mater. Chem. A 2017, 5, 2102–2109. [Google Scholar] [CrossRef]
- Foukmeniok, S.M.; Ilboudo, O.; Njanja, E.; Tapsoba, I.; Pontie, M.; Kenfack, I.T. New electrochemical carbon paste electrode (CPE) based on Arabic Gum modifier and dedicated to 4-aminophenol. J. Appl. Electrochem. 2019, 49, 575–584. [Google Scholar] [CrossRef]
- Kim, M.T.; Park, H.S.; Hui, D.; Rhee, K.Y. Carbon nanotube modification using gum arabic and its effect on the dispersion and tensile properties of carbon nanotubes/epoxy nanocomposites. J. Nanosci. Nanotechnol. 2011, 11, 7369–7373. [Google Scholar] [CrossRef]
- Quintanilha, R.C.; Orth, E.S.; Grein-Iankovski, A.; Riegel-Vidotti, I.C.; Vidotti, M. The use of gum Arabic as “Green” stabilizer of poly(aniline)nanocomposites: A comprehensive study of spectroscopic, morphological and electrochemical properties. J. Colloid Interface Sci. 2014, 434, 18–27. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Othman, A.A.; Al-Rawashdeh, N.A.F. Exudate gum from acacia trees as green corrosion inhibitor for mild steel in acidic media. Int. J. Electrochem. Sci 2012, 7, 9303–9324. [Google Scholar]
- Rudd, S.; Dalton, M.; Buss, P.; Treijs, A.; Portmann, M.; Ktoris, N.; Evans, D. Selective uptake and sensing of nitrate in poly (3, 4-ethylenedioxythiophene). Sci. Rep. 2017, 7, 16581. [Google Scholar] [CrossRef] [PubMed]
- Manea, F.; Remes, A.; Radovan, C.; Pode, R.; Picken, S.; Schoonman, J. Simultaneous electrochemical determination of nitrate and nitrite in aqueous solution using Ag-doped zeolite-expanded graphite-epoxy electrode. Talanta 2010, 83, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Ruzybayev, I.; Shah, I.; Huang, C.P. The electrochemical reduction of nitrate over micro-architectured metal electrodes with stainless steel scaffold. Appl. Catal. B Environ. 2016, 180, 199–209. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.; Zhu, J.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Zainal, Z.; Ahmad, S.A.A. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Li, G.; Yuan, H.; Mou, J.; Dai, E.; Zhang, H.; Li, Z.; Zhao, Y.; Dai, Y.; Zhang, X. Electrochemical detection of nitrate with carbon nanofibers and copper co-modified carbon fiber electrodes. Compos. Commun. 2022, 29, 101043. [Google Scholar] [CrossRef]
- Tan, J.F.; Anastasi, A.; Chandra, S. Electrochemical Detection of Nitrate, Nitrite and Ammonium for on-Site Water Quality Monitoring. Curr. Opin. Electrochem. 2021, 32, 100926. [Google Scholar] [CrossRef]
- Davis, J.; Moorcroft, M.J.; Wilkins, S.J.; Compton, R.G.; Cardosi, M.F. Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 2000, 125, 737–742. [Google Scholar] [CrossRef]
- Remes, A.; Manea, F.; Sonea, D.; Burtica, G.; Picken, S.; Schoonman, J. Electrochemical determination of nitrate from water sample using Ag-doped zeolite-modified expanded graphite composite electrode. Ovidius Univ. Ann. Chem. 2009, 20, 61–65. [Google Scholar]
- Nosuhi, M.; Nezamzadeh-Ejhieh, A. A sensitive and simple modified zeolitic carbon paste electrode for indirect voltammetric determination of nitrate. Ionics 2018, 24, 2135–2145. [Google Scholar] [CrossRef]
- Bláha, M.; Riesová, M.; Zedník, J.; Anžlovar, A.; Žigon, M.; Vohlídal, J. Polyaniline synthesis with iron (III) chloride–hydrogen peroxide catalyst system: Reaction course and polymer structure study. Synth. Met. 2011, 161, 1217–1225. [Google Scholar] [CrossRef]
- Dhivya, C.; Vandarkuzhali, S.A.A.; Radha, N. Antimicrobial activities of nanostructured polyanilines doped with aromatic nitro compounds. Arabian J. Chem. 2019, 12, 3785–3798. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Mohammad, F.; Ahmad, A. Synthesis, electrical conductivity, spectral and thermal stability studies on poly (aniline-co-o-nitroaniline). J. Macromol. Sci. A 2011, 48, 952–961. [Google Scholar] [CrossRef]
- Rasheed, H.K.; Kareem, A.A. Effect of multiwalled carbon nanotube reinforcement on the opto-electronic properties of polyaniline/c-Si heterojunction. J. Opt. Commun. 2021, 42, 25–29. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Ghosh, A.; Mondal, A.; Banerjee, D. Polyaniline–single walled carbon nanotube composite–a photocatalyst to degrade rose bengal and methyl orange dyes under visible-light illumination. RSC Adv. 2017, 7, 36403–36415. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Abril-Milan, D.; Amalraj, J. Synthesis of gum acacia capped polyaniline-based nanocomposite hydrogel for the removal of methylene blue dye. J. Polym. Environ. 2021, 29, 2447–2462. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. A comparative multiwavelength Raman spectroelectrochemical study of polyaniline: A review. J. Solid State Electrochem. 2019, 23, 1631–1640. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Wang, Y.; Sun, J.; Zeng, H.; Jin, Z.; Huang, X.; Chen, L. The synergic regulation of conductivity and Seebeck coefficient in pure polyaniline by chemically changing the ordered degree of molecular chains. J. Mater. Chem. A 2014, 2, 2634–2640. [Google Scholar] [CrossRef]
- Trchová, M.; Konyushenko, E.N.; Stejskal, J.; Kovářová, J.; Ćirić-Marjanović, G. The conversion of polyaniline nanotubes to nitrogen-containing carbon nanotubes and their comparison with multi-walled carbon nanotubes. Polym. Degrad. Stab. 2009, 94, 929–938. [Google Scholar] [CrossRef]
- Jin, L.; Jiang, Y.; Zhang, M.; Li, H.; Xiao, L.; Li, M.; Ao, Y. Oriented polyaniline nanowire arrays grown on dendrimer (PAMAM) functionalized multiwalled carbon nanotubes as supercapacitor electrode materials. Sci. Rep. 2018, 8, 6268. [Google Scholar] [CrossRef]
- Chen, X.; Meng, F.; Zhou, Z.; Tian, X.; Shan, L.; Zhu, S.; Xu, X.; Jiang, M.; Wang, L.; Hui, D.; et al. One-step synthesis of graphene/polyaniline hybrids by in situ intercalation polymerization and their electromagnetic properties. Nanoscale 2014, 6, 8140–8148. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Meng, Q.; Wang, T.; Guo, W.; Wu, G.; You, L. Preparation of high antistatic HDPE/polyaniline encapsulated graphene nanoplatelet composites by solution blending. RSC Adv. 2017, 7, 2796–2803. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Karve, M.; Aiyer, R.; Haram, S. Agarose–guar gum assisted synthesis of processable polyaniline composite: Morphology and electro-responsive characteristics. RSC Adv. 2014, 4, 59716–59725. [Google Scholar] [CrossRef]
- Ali, A.M.; Rashid, K.T.; Yahya, A.A.; Majdi, H.S.; Salih, I.K.; Yusoh, K.; Alsalhy, Q.F.; AbdulRazak, A.A.; Figoli, A. Fabrication of Gum arabic-graphene (GGA) modified polyphenylsulfone (PPSU) mixed matrix membranes: A systematic evaluation study for ultrafiltration (UF) applications. Membranes 2021, 11, 542. [Google Scholar] [CrossRef]
- Mariano, L.C.; Souza, V.H.R.; Kowalski, E.L.; Rocco, M.L.M.; Zarbin, A.J.G.; Koehler, M.; Roman, L.S. Electrical and morphological study of carbon nanotubes/polyaniline composite films: A model to explain different tunneling regimes induced by a vertical electric field. Thin Solid Films 2017, 636, 314–324. [Google Scholar] [CrossRef]
- Sulaiman, M.G.M.; Ammar, A.-F. Synthesis of Gum Arabic-g-polyaniline using diode laser. Int. J. Biol. Macromol. 2020, 161, 848–853. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, H.; Yu, P.; Wang, H.; Liu, J. Enhancement of adhesion, mechanical strength and anti-corrosion by multilayer superhydrophobic coating embedded electroactive PANI/CNF nanocomposite. J. Polym. Res. 2018, 25, 151. [Google Scholar] [CrossRef]
- Qin, T.; Deng, L.; Zhang, P.; Tang, M.; Li, C.; Xie, H.; Huang, S.; Gao, X. Enhancement of Electrochromic Properties of Polyaniline Induced by Copper Ions. Nanoscale Res. Lett. 2022, 17, 51. [Google Scholar] [CrossRef]
- Liu, F.; Luo, S.; Liu, D.; Chen, W.; Huang, Y.; Dong, L.L.; Wang, L. Facile processing of free-standing polyaniline/SWCNT film as an integrated electrode for flexible supercapacitor application. ACS Appl. Mater. Interfaces 2017, 9, 33791–33801. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Gomaa, M.H.; Rehim, S.S.A.; Hamid, M.A.; Ibrahim, A. Synthesis and characterization of nanostructured polyaniline thin films with superhydrophobic properties. Coatings 2019, 9, 748. [Google Scholar] [CrossRef]
- Mohtasebi, A.; Chowdhury, T.; Hsu, L.H.H.; Biesinger, M.C.; Kruse, P. Interfacial charge transfer between phenyl-capped aniline tetramer films and iron oxide surfaces. J. Phys. Chem. C 2016, 120, 29248–29263. [Google Scholar] [CrossRef]
- Abdul Bashid, H.A.; Lim, H.N.; Kamaruzaman, S.; Abdul Rashid, S.; Yunus, R.; Huang, N.M.; Yin, C.Y.; Rahman, M.M.; Altarawneh, M.; Jiang, Z.T.; et al. Electrodeposition of polypyrrole and reduced graphene oxide onto carbon bundle fibre as electrode for supercapacitor. Nanoscale Res. Lett. 2017, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yao, L.; Huang, Q.-A.; Su, Q.; Zhang, J.; Zhang, F.; Du, G. Facile assembly of a S@ carbon nanotubes/polyaniline/graphene composite for lithium–sulfur batteries. RSC Adv. 2017, 7, 9819–9825. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Sonawane, S.S.; Shimpi, N.G. Polyaniline/zinc oxide nanocomposite as room-temperature sensing layer for methane. Polym. Eng. Sci. 2018, 58, 1438–1445. [Google Scholar] [CrossRef]
- Wang, S.; Liu, F.; Gao, C.; Wan, T.; Wang, L.; Wang, L.; Wang, L. Enhancement of the thermoelectric property of nanostructured polyaniline/carbon nanotube composites by introducing pyrrole unit onto polyaniline backbone via a sustainable method. Chem. Eng. J. 2019, 370, 322–329. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Khattak, R.; Khan, M.; Khan, M.S.; Ali, O.M. Preparation of electrochemical supercapacitor based on polypyrrole/gum arabic composites. Polymers 2022, 14, 242. [Google Scholar] [CrossRef]
- Brzyski, P. The Influence of Gum Arabic Admixture on the Mechanical Properties of Lime-Metakaolin Paste Used as Binder in Hemp Concrete. Materials 2021, 14, 6775. [Google Scholar] [CrossRef]
- Bashami, R.M.; Hameed, A.; Aslam, M.; Ismail, I.M.I.; Soomro, M.T. The suitability of ZnO film-coated glassy carbon electrode for the sensitive detection of 4-nitrophenol in aqueous medium. Anal. Methods 2015, 7, 1794–1801. [Google Scholar] [CrossRef]
- Bashami, R.M.; Soomro, M.T.; Khan, A.N.; Aazam, E.S.; Ismail, I.M.I.; El-Shahawi, M.S. A highly conductive thin film composite based on silver nanoparticles and malic acid for selective electrochemical sensing of trichloroacetic acid. Anal. Chim. Acta 2018, 1036, 33–48. [Google Scholar] [CrossRef]
- Al-Qasmi, N.; Soomro, M.T.; Aslam, M.; Rehman, A.; Ali, S.; Danish, E.Y.; Ismail, I.M.I.; Hameed, A. The efficacy of the ZnO:α-Fe2O3 composites modified carbon paste electrode for the sensitive electrochemical detection of loperamide: A detailed investigation. J. Electroanal. Chem. 2016, 783, 112–124. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and structural analysis of polyaniline and poly (o-anisidine) layers generated in a DC glow discharge plasma by using an oblique angle electrode deposition configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Kumar, M.S.; Yasoda, K.Y.; Batabyal, S.K.; Kothurkar, N.K. Carbon-polyaniline nanocomposites as supercapacitor materials. Mater. Res. Express 2018, 5, 045505. [Google Scholar] [CrossRef]
- Chulkin, P.; Łapkowski, M. An insight into ionic conductivity of polyaniline thin films. Materials 2020, 13, 2877. [Google Scholar] [CrossRef]
- Yano, J.; Fukuoka, H.; Kitani, A. Electro-oxidation of polyaniline in the presence of electronic acceptors and the magnetic properties of the resulting polyaniline. Thin Solid Films 2016, 618, 165–171. [Google Scholar] [CrossRef]
- Herath, A.; Reid, C.; Perez, F.; Pittman, C.U.; Mlsna, T.E. Biochar-supported polyaniline hybrid for aqueous chromium and nitrate adsorption. J. Environ. Manage. 2021, 296, 113186. [Google Scholar] [CrossRef]
- Vujković, M.J.; Etinski, M.; Vasić, B.; Kuzmanović, B.; Bajuk-Bogdanović, D.; Dominko, R.; Mentus, S. Polyaniline as a charge storage material in an aqueous aluminum-based electrolyte: Can aluminum ions play the role of protons? J. Power Sources 2021, 482, 228937. [Google Scholar] [CrossRef]
- Scotto, J.; Marmisollé, W.A.; Posadas, D. About the capacitive currents in conducting polymers: The case of polyaniline. J. Solid State Electrochem. 2019, 23, 1947–1965. [Google Scholar] [CrossRef]
- Haki, M.A.; Laabd, H.M.; Chafai, H.; Kabli, H.; Ez-Zahery, M.; Bazzaoui, M.; Lakhmiri, R.; Albourine, A. Comparative adsorption of nitrate ions on the polypyrrole and polyaniline from aqueous solution. J. Dispers. Sci. Technol. 2017, 38, 598–603. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.H.; Azzem, M.A. Gum Arabic-capped silver nanoparticles for electrochemical amplification sensing of methylene blue in river water. Electrochim. Acta 2021, 394, 139152. [Google Scholar] [CrossRef]
- Hejazian, S.; Ahmadyari-Sharamin, M. Synergistic effect of Arabic and guar gums on corrosion behavior of steel reinfforced concrete in a simulator environment. Case Stud. Constr. Mater. 2022, 16, e01024. [Google Scholar]
- Al-Qasmi, N.; Hameed, A.; Khan, A.N.; Aslam, M.; Ismail, I.M.I.; Soomro, M.T. Mercury meniscus on solid silver amalgam electrode as a sensitive electrochemical sensor for tetrachlorvinphos. J. Saudi Chem. Soc. 2018, 22, 496–507. [Google Scholar] [CrossRef]
- Pasela, B.R.; Castillo, A.P.; Simon, R.; Pulido, M.T.; Mana-Ay, H.; Abiquibil, M.R.; Montecillo, R.; Thumanu, K.; Tumacder, D.; Taaca, K.L. Synthesis and characterization of acetic acid-doped polyaniline and polyaniline–chitosan composite. Biomimetics 2019, 4, 15. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R. Polyaniline Synthesized by Different Dopants for Fluorene Detection via Photoluminescence Spectroscopy. Materials 2021, 14, 7382. [Google Scholar] [CrossRef]
- Noufi, M.; Yarnitzky, C.; Ariel, M. Determination of nitrate with a flow-injection system combining square-wave polarographic detection with on-line deaeration. Anal. Chim. Acta 1990, 234, 475–478. [Google Scholar] [CrossRef]





| Electrode | Indirect/Direct Detection | Method | Linear Range (µM) | DL (µM) | Sensitivity (A/M cm2) | References |
|---|---|---|---|---|---|---|
| GA@PAni-CNT | Indirect | SWV | 1–10 15–50 | 0.42 | ~443 A/M cm2 | This work |
| Fe(II)-NClin/CPE | Indirect | SWV | 80–1100 | 5.4 | 90 µA (µM) | [42] |
| SMDE | Indirect | SWP | 0.6–5 | 2 | - | [88] |
| AgZEGE | Direct | MPA | 1000–10,000 | 80 | 0.0166 mA mM−1 | [33] |
| AgZEGE | Direct | DPV | 1000–10,000 | 500 | 2 × 10–3 mA mM−1 | [41] |
| Inferences | Concentration (µM) | EES↔PN Change (%) | IES↔PN Change (%) |
|---|---|---|---|
| Cl− | 40 | 1.36 | 0.96 |
| Br− | 40 | 1.07 | 1.72 |
| I− | 40 | 0.89 | 1.86 |
| SO4−2 | 40 | 1.10 | 0.72 |
| NO2− | 40 | −2.35 | 4.34 |
| CH3COO− | 40 | 5.97 | 25.76 |
| Analyte | Detected | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| NO3− | 0 | 20 | 19.43 ± 1.53 | 97.3 ± 2.61 |
| 0 | 40 | 42.60 ± 1.10 | 106.5 ± 3.53 | |
| 0 | 60 | 60.09 ± 1.37 | 100.2 ± 2.83 | |
| 0 | 80 | 80.18 ± 0.99 | 100.2 ± 2.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosa, S.A.M.; Khan, A.N.; Ahmed, S.; Aslam, M.; Bawazir, W.A.; Hameed, A.; Soomro, M.T. Strategic Electrochemical Determination of Nitrate over Polyaniline/Multi-Walled Carbon Nanotubes-Gum Arabic Architecture. Nanomaterials 2022, 12, 3542. https://doi.org/10.3390/nano12193542
Kosa SAM, Khan AN, Ahmed S, Aslam M, Bawazir WA, Hameed A, Soomro MT. Strategic Electrochemical Determination of Nitrate over Polyaniline/Multi-Walled Carbon Nanotubes-Gum Arabic Architecture. Nanomaterials. 2022; 12(19):3542. https://doi.org/10.3390/nano12193542
Chicago/Turabian StyleKosa, Samia Abdulhammed Mohamad, Amna Nisar Khan, Sana Ahmed, Mohammad Aslam, Wafa AbuBaker Bawazir, Abdul Hameed, and Muhammad Tahir Soomro. 2022. "Strategic Electrochemical Determination of Nitrate over Polyaniline/Multi-Walled Carbon Nanotubes-Gum Arabic Architecture" Nanomaterials 12, no. 19: 3542. https://doi.org/10.3390/nano12193542
APA StyleKosa, S. A. M., Khan, A. N., Ahmed, S., Aslam, M., Bawazir, W. A., Hameed, A., & Soomro, M. T. (2022). Strategic Electrochemical Determination of Nitrate over Polyaniline/Multi-Walled Carbon Nanotubes-Gum Arabic Architecture. Nanomaterials, 12(19), 3542. https://doi.org/10.3390/nano12193542






