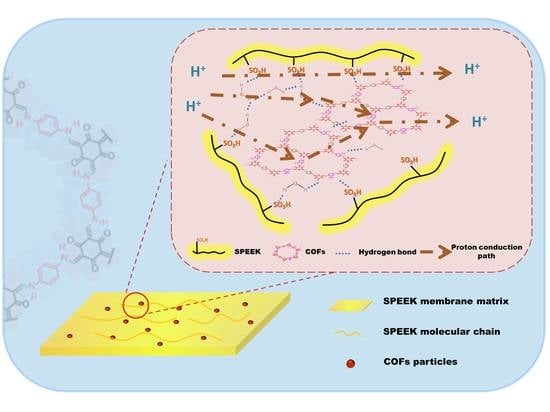
Effect of Covalent Organic Frameworks Containing Different Groups on Properties of Sulfonated Poly(ether ether ketone) Matrix Proton Exchange Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of SPEEK
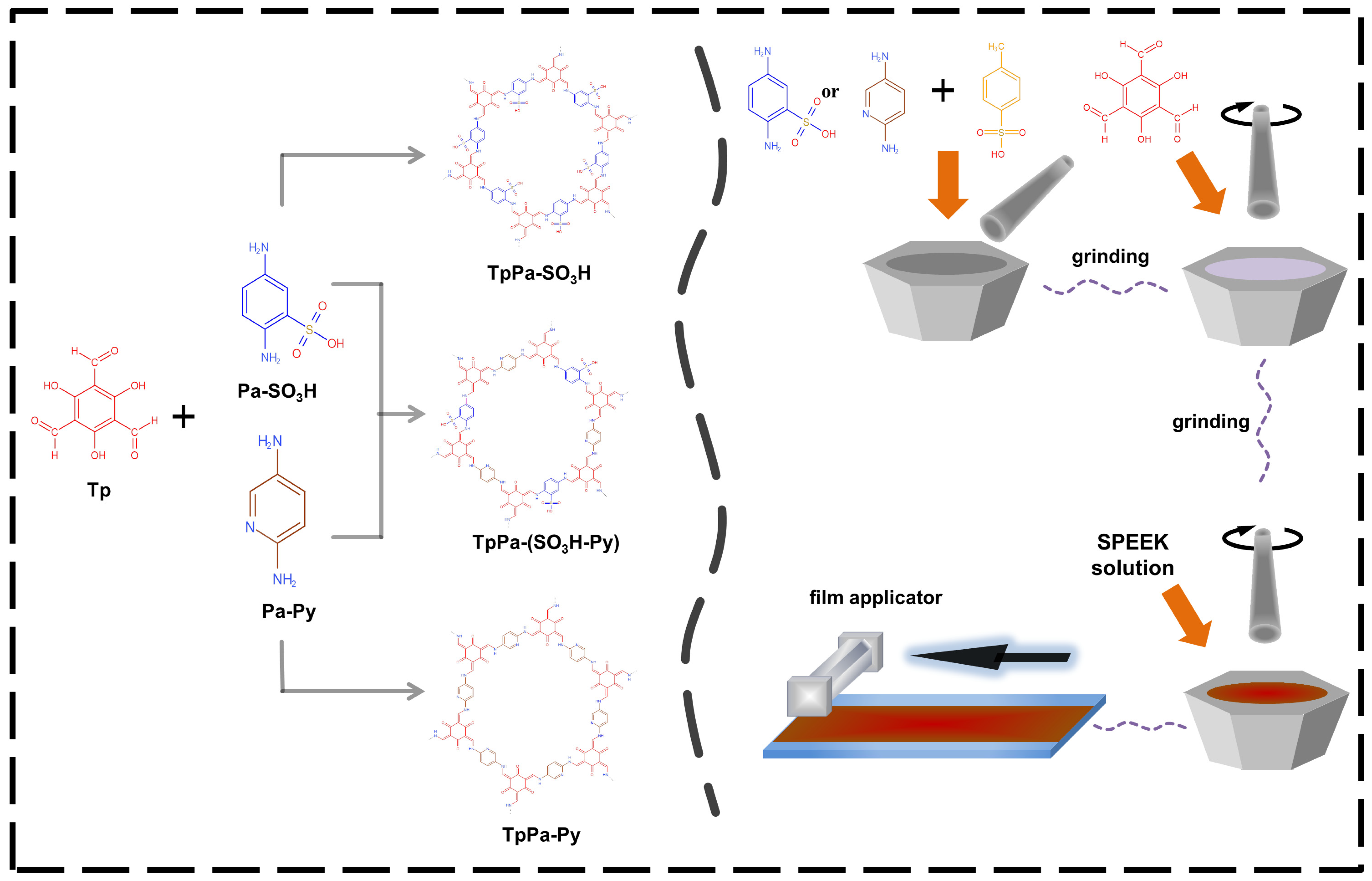
2.3. Preparation of SPEEK/COF Composite Membranes
2.4. Characterizations
2.5. Water Uptaking and Swelling Ratio
2.6. Proton Conductivity
2.7. Ion Exchange Capacity (IEC) and Degree of Sulfonation (DS)
3. Results and Discussion
3.1. SPEEK/TpPa−SO3H and SPEEK/TpPa−Py Composite Membranes
3.1.1. Characterization of TpPa−SO3H and TpPa−Py
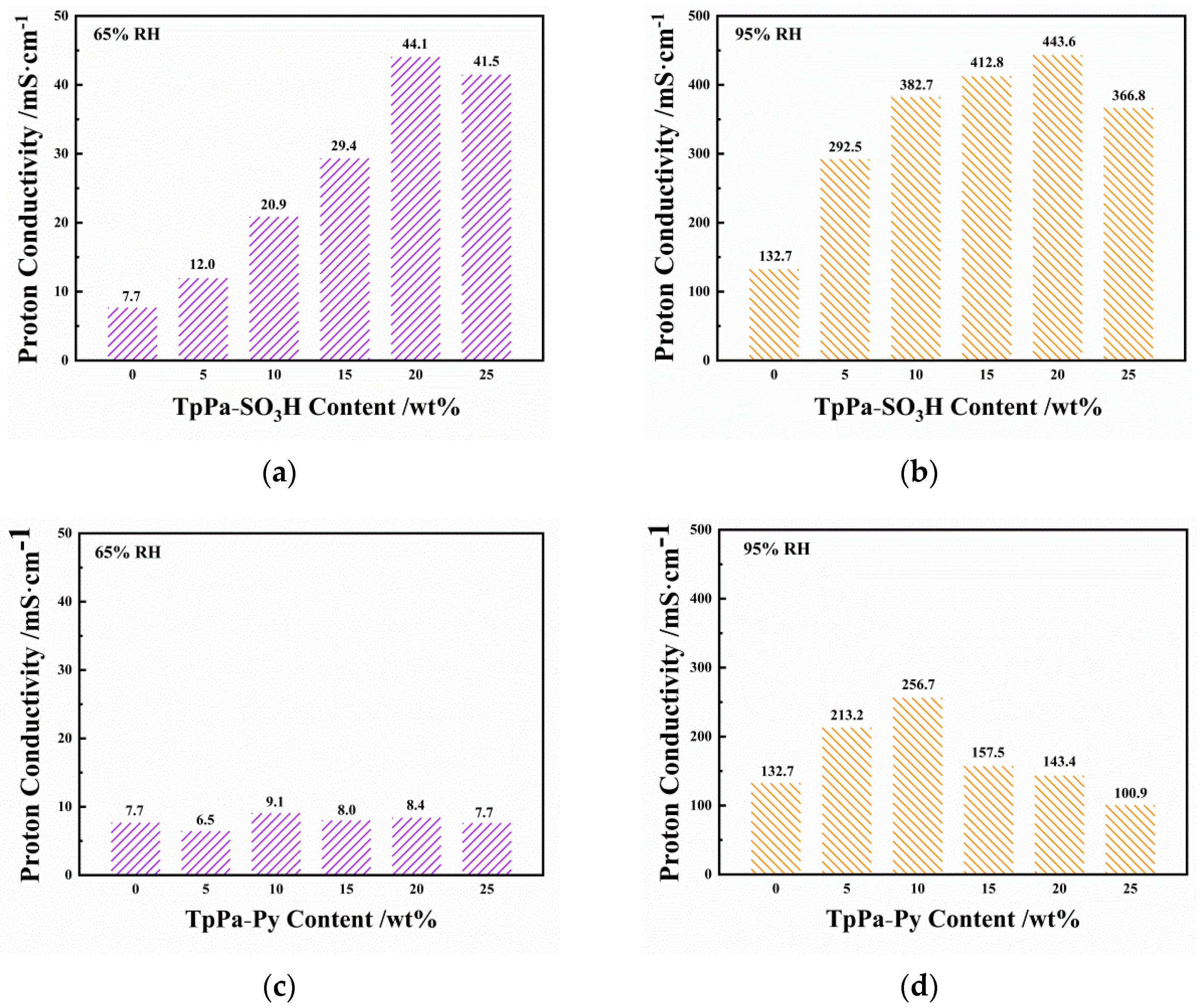
3.1.2. Proton Conductivities of SPEEK/TpPa−SO3H and SPEEK/TpPa−Py Composite Membranes
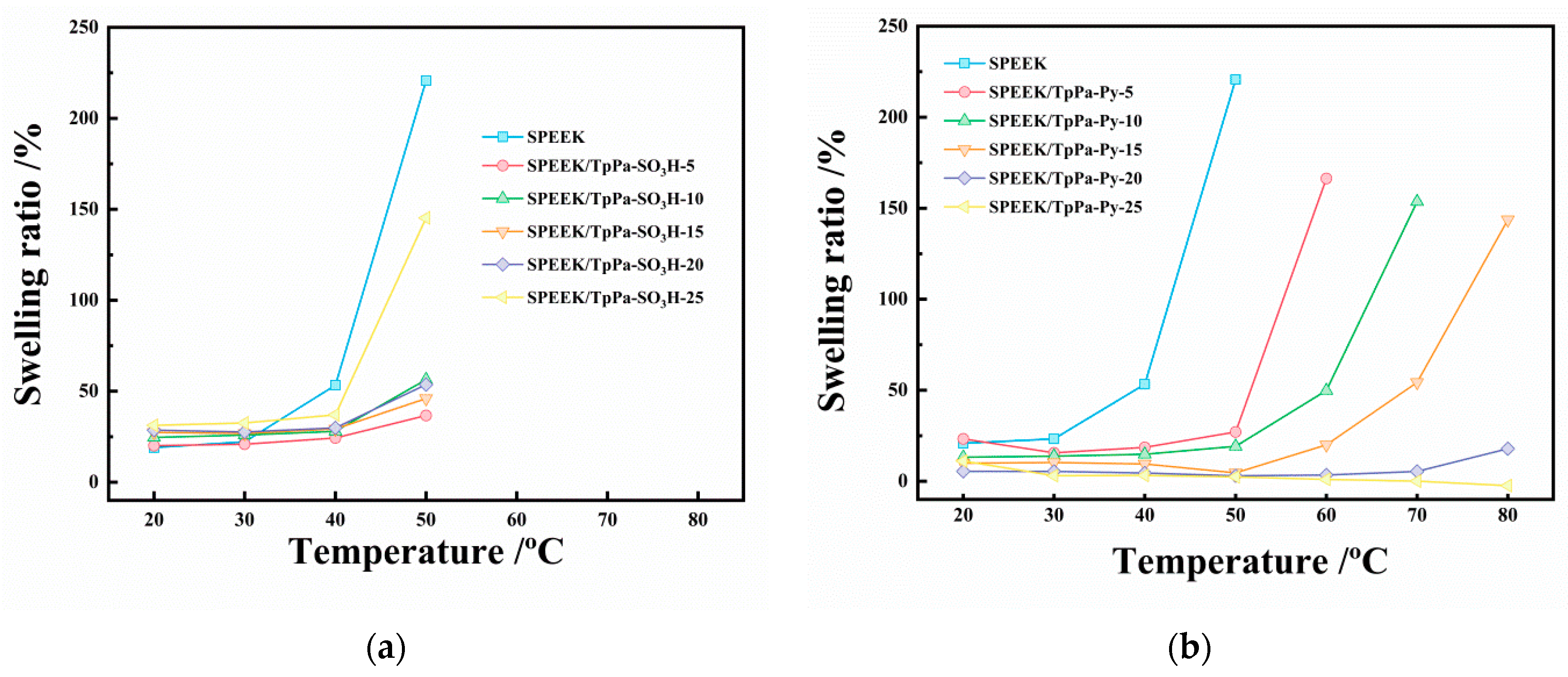
3.1.3. Dimensional Stability
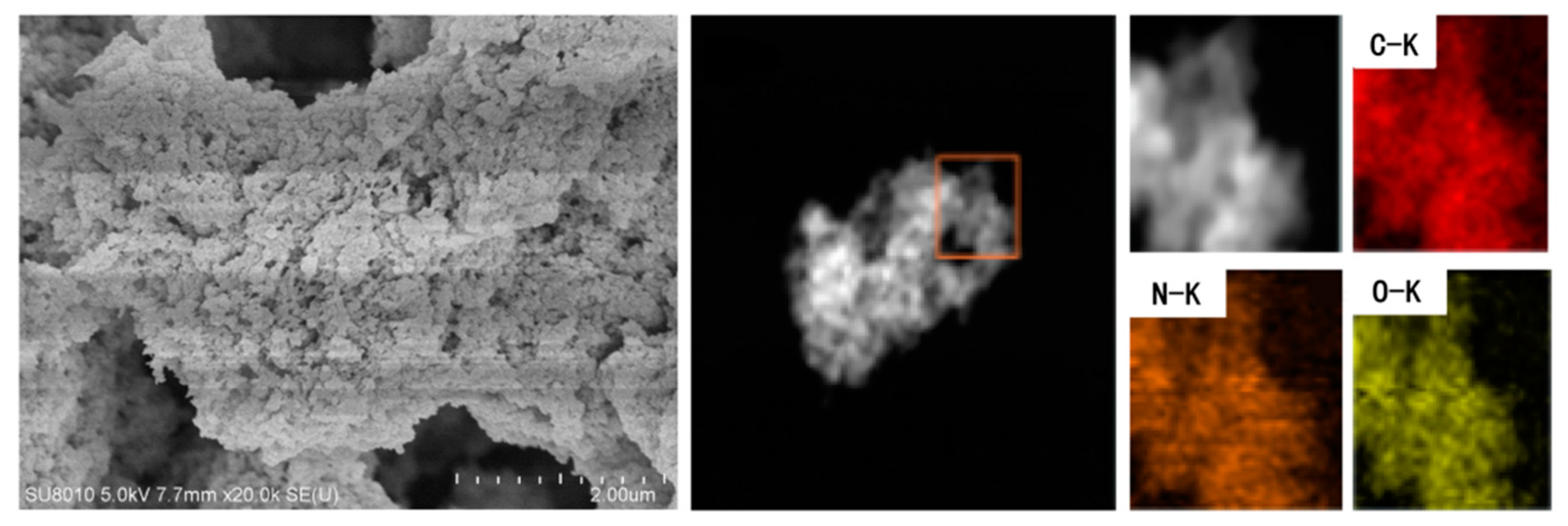
3.1.4. Structure and Morphology Characterization of SPEEK/TpPa−SO3H and SPEEK/TpPa−Py Composite Membranes
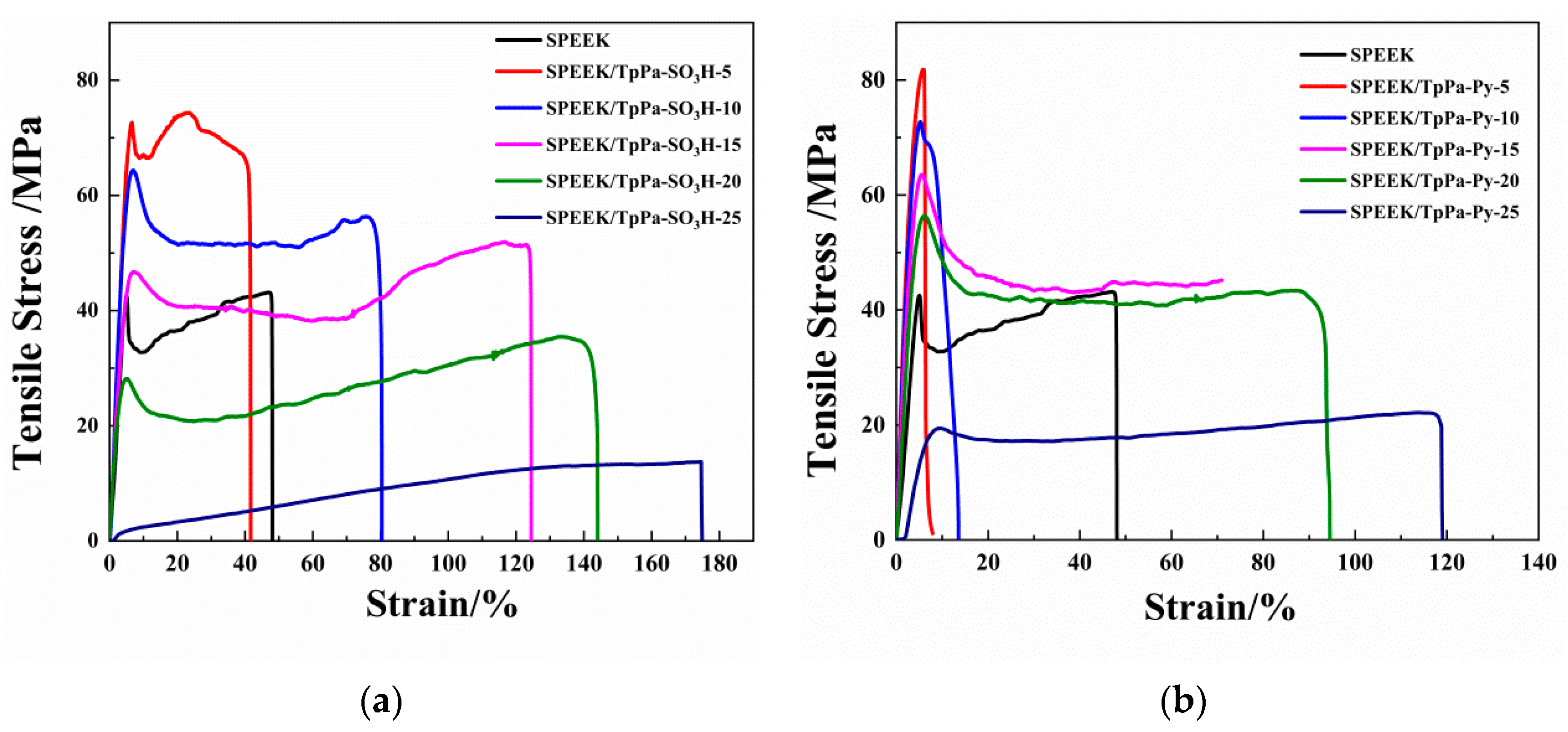
3.1.5. Mechanical Properties
3.2. SPEEK/TpPa−(SO3H-Py) Composite Membrane
3.2.1. Characterization of SPEEK/TpPa−(SO3H-Py)
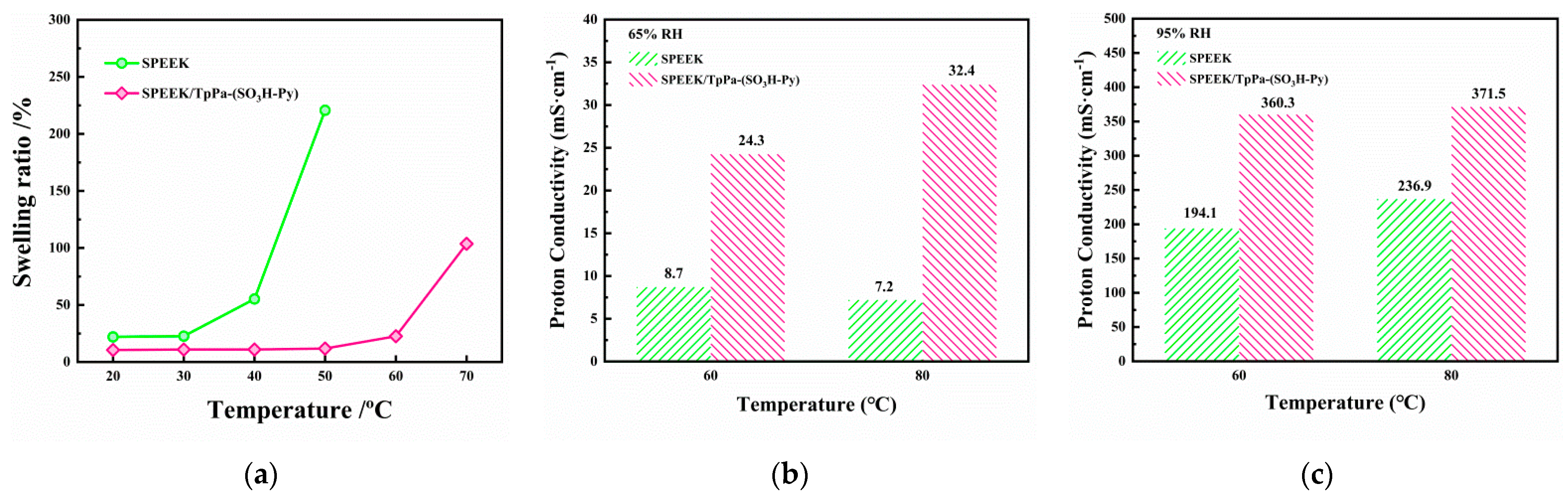
3.2.2. Dimensional Stability and Proton Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colicchio, I.; Wen, F.; Keul, H.; Simon, U.; Moeller, M. Sulfonated poly (ether ether ketone)–silica membranes doped with phosphotungstic acid. Morphology and proton conductivity. J. Membr. Sci. 2009, 326, 45–57. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Tan, A.R.; de Carvalho, L.M.; de Ramos Filho, F.G.; de Souza Gomes, A. Nanocomposite membranes based on sulfonated poly (etheretherketone) structured with modified silica for direct ethanol fuel cell. Macromol. Symp. 2006, 245–246, 470–475. [Google Scholar] [CrossRef]
- Byun, G.H.; Kim, J.A.; Kim, N.Y.; Cho, Y.S.; Park, C.R. Molecular engineering of hydrocarbon membrane to substitute perfluorinated sulfonic acid membrane for proton exchange membrane fuel cell operation. Mater. Today Energy 2020, 17, 100483. [Google Scholar] [CrossRef]
- Wong, C.; Wong, W.; Ramya, K.; Khalid, M.; Loh, K.; Daud, W.; Lim, K.; Walvekar, R.; Kadhum, A. Additives in proton exchange membranes for low-and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Ramakrishnan, S.; Kim, A.R.; Lee, H.K.; Yoo, D.J. Ceria Stabilized by Titanium Carbide as a Sustainable Filler in the Nafion Matrix Improves the Mechanical Integrity, Electrochemical Durability, and Hydrogen Impermeability of Proton-Exchange Membrane Fuel Cells: Effects of the Filler Content. ACS Appl. Mater. Interfaces 2020, 12, 5704–5716. [Google Scholar] [CrossRef] [PubMed]
- Raja Sulaiman, R.R.; Rashmi, W.; Khalid, M.; Wong, W.; Priyanka, J. Recent progress in the development of aromatic polymer-based proton exchange membranes for fuel cell applications. Polymers 2020, 12, 1061. [Google Scholar] [CrossRef]
- Maier, G.; Meier-Haack, J. Sulfonated aromatic polymers for fuel cell membranes. In Fuel Cells II; Scherer, G.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–62. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; He, G.; Benziger, J. Differences in water sorption and proton conductivity between Nafion and SPEEK. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1437–1445. [Google Scholar] [CrossRef]
- Xie, J.; Wood, D.L., III; Wayne, D.M.; Zawodzinski, T.A.; Atanassov, P.; Borup, R.L. Durability of PEFCs at high humidity conditions. J. Electrochem. Soc. 2004, 152, A104. [Google Scholar] [CrossRef]
- Kreuer, K. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and characterization of sulfonated poly (ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tang, Y.; Aguila, B.; Wang, S.; Xiao, F.S.; Thallapally, P.K.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Reaction environment modification in covalent organic frameworks for catalytic performance enhancement. Angew. Chem. Int. Ed. 2019, 58, 8670–8675. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Xiao, L.; Ling, Y.; Ching, C.; Matsumoto, M.; Bisbey, R.P.; Helbling, D.E.; Dichtel, W.R. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 12677–12681. [Google Scholar] [CrossRef]
- Cui, D.; Perepichka, D.F.; MacLeod, J.M.; Rosei, F. Surface-confined single-layer covalent organic frameworks: Design, synthesis and application. Chem. Soc. Rev. 2020, 49, 2020–2038. [Google Scholar] [CrossRef] [PubMed]
- Merí-Bofí, L.; Royuela, S.; Zamora, F.; Ruiz-González, M.L.; Segura, J.L.; Muñoz-Olivas, R.; Mancheño, M.J. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg (II). J. Mater. Chem. A 2017, 5, 17973–17981. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An azine-linked covalent organic framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef]
- Bhunia, S.; Das, S.K.; Jana, R.; Peter, S.C.; Bhattacharya, S.; Addicoat, M.; Bhaumik, A.; Pradhan, A. Electrochemical stimuli-driven facile metal-free hydrogen evolution from pyrene-porphyrin-based crystalline covalent organic framework. ACS Appl. Mater. Interfaces 2017, 9, 23843–23851. [Google Scholar] [CrossRef]
- Chandra, S.; Kundu, T.; Kandambeth, S.; BabaRao, R.; Marathe, Y.; Kunjir, S.M.; Banerjee, R. Phosphoric Acid Loaded Azo (−N=N−) Based Covalent Organic Framework for Proton Conduction. J. Am. Chem. Soc. 2014, 136, 6570–6573. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, G.; Hu, Z.; Cheng, Y.; Chi, C.; Yuan, D.; Cheng, H.; Zhao, D. Mechanoassisted synthesis of sulfonated covalent organic frameworks with high intrinsic proton conductivity. ACS Appl. Mater. Interfaces 2016, 8, 18505–18512. [Google Scholar] [CrossRef]
- Mohammed, O.F.; Pines, D.; Nibbering, E.T.J.; Pines, E. Base-induced solvent switches in acid-base reactions. Angew. Chem. Int. Ed. 2007, 46, 1458–1461. [Google Scholar] [CrossRef]
- Chandra, S.; Kundu, T.; Dey, K.; Addicoat, M.; Heine, T.; Banerjee, R. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 2016, 28, 1489–1494. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Yang, X.; Cao, L.; Wang, C.; Zhang, B.; Wu, H.; Jiang, Z. Enhanced proton conductivity of Nafion composite membrane by incorporating phosphoric acid-loaded covalent organic framework. J. Power Sources 2016, 332, 265–273. [Google Scholar] [CrossRef]
- Fan, C.; Wu, H.; Li, Y.; Shi, B.; He, X.; Qiu, M.; Mao, X.; Jiang, Z. Incorporating self-anchored phosphotungstic acid@triazole-functionalized covalent organic framework into sulfonated poly (ether ether ketone) for enhanced proton conductivity. Solid State Ion. 2020, 349, 115316. [Google Scholar] [CrossRef]
- Meng, X.Y.; Song, K.; Lv, Y.A.; Cong, C.A.B.; Ye, H.M.; Dong, Y.H.; Zhou, Q. SPEEK proton exchange membrane with enhanced proton conductivity stability from phosphotungstic acid-encapsulated silica nanorods. Mater. Chem. Phys. 2021, 272, 125045. [Google Scholar] [CrossRef]
- Zheng, Y.; Shen, J.L.; Yuan, J.Q.; Khan, N.A.; You, X.D.; Yang, C.; Zhang, S.Y.; El-Gendi, A.; Wu, H.; Zhang, R.N.; et al. 2D nanosheets seeding layer modulated covalent organic framework membranes for efficient desalination. Desalination 2022, 532, 115753. [Google Scholar] [CrossRef]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu H, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Yao, J.; Xu, G.; Zhao, Z.; Guo, J.; Li, S.; Cai, W.; Zhang, S. An enhanced proton conductivity and reduced methanol permeability composite membrane prepared by sulfonated covalent organic nanosheets/Nafion. Int. J. Hydrogen Energy 2019, 44, 24985–24996. [Google Scholar] [CrossRef]
- Jiang, W.; Cui, W.-R.; Liang, R.-P.; Qiu, J.-D. Difunctional covalent organic framework hybrid material for synergistic adsorption and selective removal of fluoroquinolone antibiotics. J. Hazard. Mater. 2021, 413, 125302. [Google Scholar] [CrossRef]
- Shi, X.; Ma, D.; Xu, F.; Zhang, Z.; Wang, Y. Table-salt enabled interface-confined synthesis of covalent organic framework (COF) nanosheets. Chem. Sci. 2020, 11, 989–996. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu H, S.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes. Adv. Mater. 2017, 29, 1603945. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yang, L.; Luo, X.; Wang, K.; Chang, G. Novel phosphoric acid (PA)-poly (ether ketone sulfone) with flexible benzotriazole side chains for high-temperature proton exchange membranes. Polym. J. 2019, 51, 69–75. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Zhou, L.; Che, Q.; He, R.; Li, Q. Hydroxyl pyridine containing polybenzimidazole membranes for proton exchange membrane fuel cells. J. Membr. Sci. 2013, 446, 318–325. [Google Scholar] [CrossRef]
- Lakshmi, R.M.; Choudhary, V.; Varma, I. Sulphonated poly (ether ether ketone): Synthesis and characterisation. J. Mater. Sci. 2005, 40, 629–636. [Google Scholar] [CrossRef]




| Membranes | IEC (mmol·g−1) | DS (%) |
|---|---|---|
| SPEEK | 1.41 | 47.3 |
| SPEEK/TpPa−SO3H-5 | 2.04 | - |
| SPEEK/TpPa−SO3H-10 | 2.11 | - |
| SPEEK/TpPa−SO3H-15 | 2.16 | - |
| SPEEK/TpPa−SO3H-20 | 2.26 | - |
| SPEEK/TpPa−SO3H-25 | 2.11 | - |
| SPEEK/TpPa−Py-5 | 1.50 | - |
| SPEEK/TpPa−Py-10 | 1.61 | - |
| SPEEK/TpPa−Py-15 | 1.45 | - |
| SPEEK/TpPa−Py-20 | 1.43 | - |
| SPEEK/TpPa−Py-25 | 1.34 | - |
| SPEEK/TpPa−(SO3H-Py) | 2.09 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Lv, Y.; Ding, L.; Peng, L.; Peng, Q.; Cong, C.; Ye, H.; Zhou, Q. Effect of Covalent Organic Frameworks Containing Different Groups on Properties of Sulfonated Poly(ether ether ketone) Matrix Proton Exchange Membranes. Nanomaterials 2022, 12, 3518. https://doi.org/10.3390/nano12193518
Meng X, Lv Y, Ding L, Peng L, Peng Q, Cong C, Ye H, Zhou Q. Effect of Covalent Organic Frameworks Containing Different Groups on Properties of Sulfonated Poly(ether ether ketone) Matrix Proton Exchange Membranes. Nanomaterials. 2022; 12(19):3518. https://doi.org/10.3390/nano12193518
Chicago/Turabian StyleMeng, Xiaoyu, Yinan Lv, Lei Ding, Luman Peng, Qiwang Peng, Chuanbo Cong, Haimu Ye, and Qiong Zhou. 2022. "Effect of Covalent Organic Frameworks Containing Different Groups on Properties of Sulfonated Poly(ether ether ketone) Matrix Proton Exchange Membranes" Nanomaterials 12, no. 19: 3518. https://doi.org/10.3390/nano12193518
APA StyleMeng, X., Lv, Y., Ding, L., Peng, L., Peng, Q., Cong, C., Ye, H., & Zhou, Q. (2022). Effect of Covalent Organic Frameworks Containing Different Groups on Properties of Sulfonated Poly(ether ether ketone) Matrix Proton Exchange Membranes. Nanomaterials, 12(19), 3518. https://doi.org/10.3390/nano12193518