Graphitic Carbon Nitride as Visible-Light Photocatalyst Boosting Ozonation in Wastewater Treatment
Abstract
1. Introduction
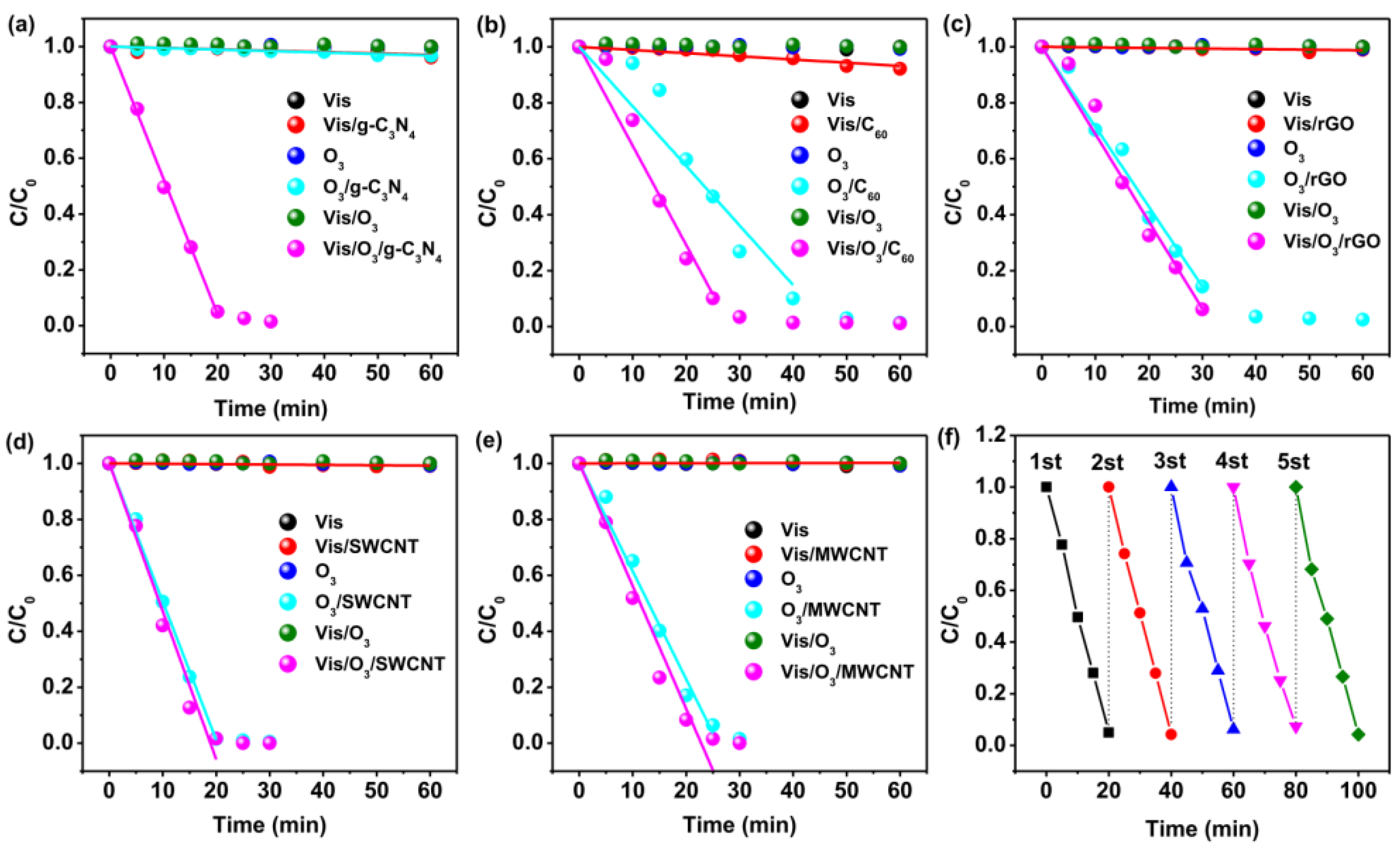
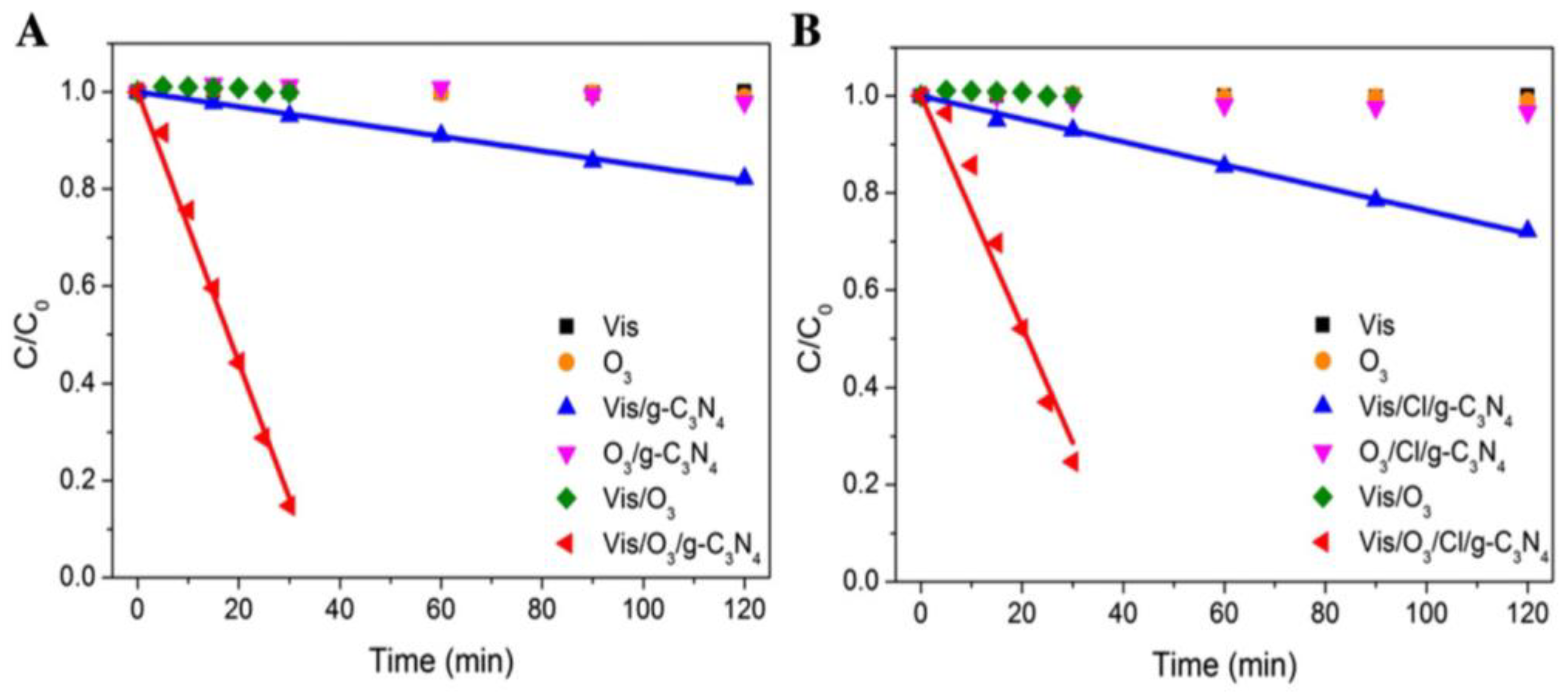
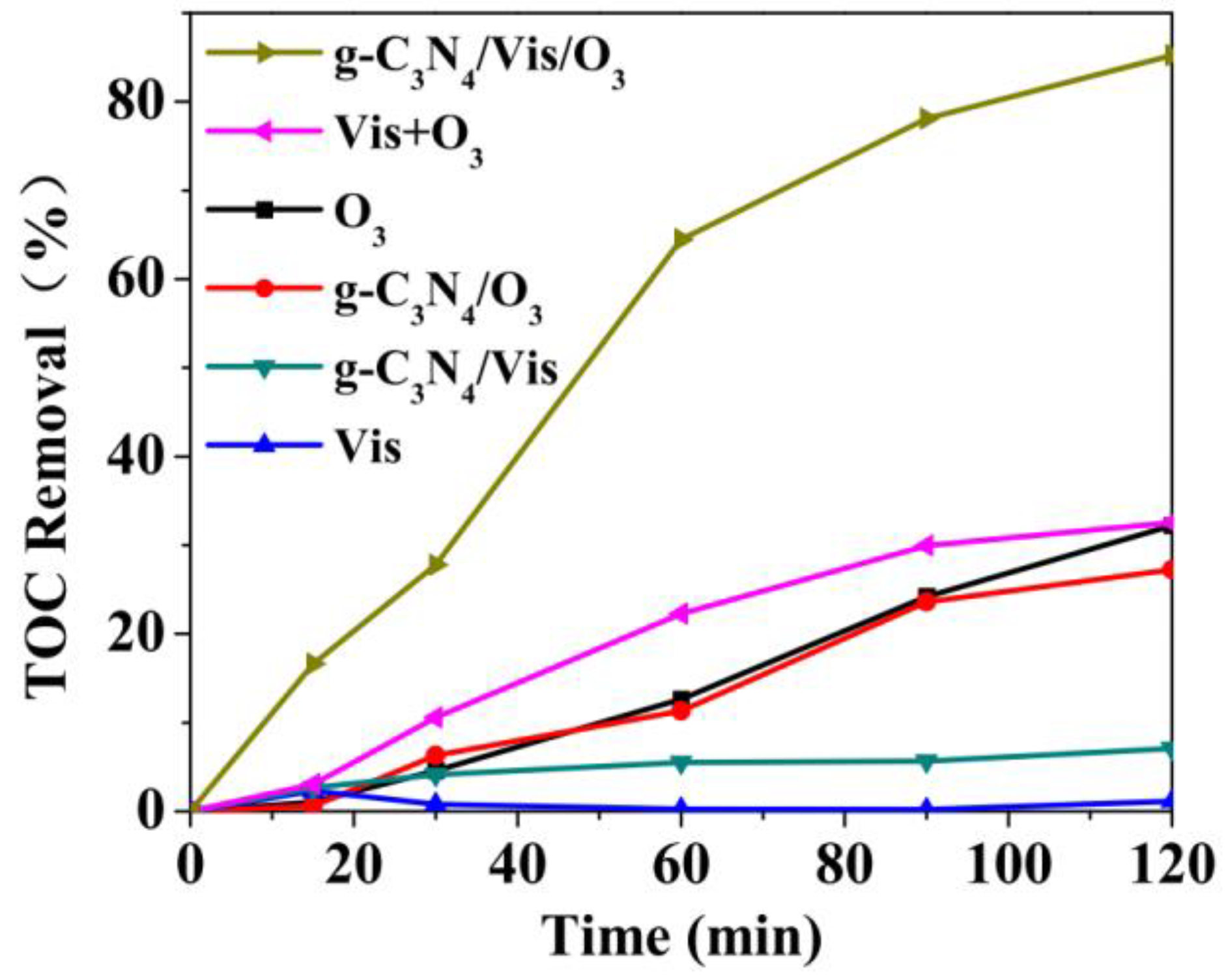
2. Oxalic Acid (OA)
4. Ciprofloxacin
5. Oxamic Acid (OMA)
6. Parabens
7. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. ACC Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. Photochemical advanced oxidation processes for water and wastewater treatment. Recent Pat. Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Buffle, M.-O.; Schumacher, J.; Meylan, S.; Jekel, M.; Von Gunten, U. Ozonation and advanced oxidation of wastewater: Effect of O3 dose, pH, DOM and HO.-scavengers on ozone decomposition and HO. generation. Ozone Sci. Eng. 2006, 28, 247–259. [Google Scholar] [CrossRef]
- Jans, U.; Hoigné, J. Activated carbon and carbon black catalyzed transformation of aqueous ozone into OH-radicals. Ozone Sci. Eng. 1998, 20, 67–90. [Google Scholar] [CrossRef]
- Wu, J.J.; Wu, C.-C.; Ma, H.-W.; Chang, C.-C. Treatment of landfill leachate by ozone-based advanced oxidation processes. Chemosphere 2004, 54, 997–1003. [Google Scholar] [CrossRef]
- Chin, A.; Bérubé, P.R. Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res. 2005, 39, 2136–2144. [Google Scholar] [CrossRef]
- Javier Benitez, F.; Acero, J.L.; Real, F.J. Degradation of carbofuran by using ozone, UV radiation and advanced oxidation processes. J. Hazard. Mater. 2002, 89, 51–65. [Google Scholar] [CrossRef]
- Kusic, H.; Koprivanac, N.; Bozic, A.L. Minimization of organic pollutant content in aqueous solution by means of AOPs: UV- and ozone-based technologies. Chem. Eng. J. 2006, 123, 127–137. [Google Scholar] [CrossRef]
- Agustina, T.E.; Ang, H.M.; Vareek, V.K. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C 2005, 6, 264–273. [Google Scholar] [CrossRef]
- Chávez, A.M.; Rey, A.; Beltrán, F.J.; Álvarez, P.M. Solar photo-ozonation: A novel treatment method for the degradation of water pollutants. J. Hazard. Mater. 2016, 317, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xie, Y.; Rabeah, J.; Brückner, A.; Cao, H. Visible-Light Photocatalytic Ozonation Using Graphitic C3N4 Catalysts: A Hydroxyl Radical Manufacturer for Wastewater Treatment. Acc. Chem. Res. 2020, 53, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Rey, A.; Mena, E.; Beltrán, F.J. Application of solar photocatalytic ozonation in water treatment using supported TiO2. Appl. Catal. B Environ. 2019, 254, 237–245. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Mueller, J.; Schloegl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Komatsu, T. Attempted chemical synthesis of graphite-like carbon nitride. J. Mater. Chem. 2001, 11, 799–801. [Google Scholar] [CrossRef]
- Kuriki, R.; Sekizawa, K.; Ishitani, O.; Maeda, K. Visible-light-driven CO2 reduction with carbon nitride: Enhancing the activity of ruthenium catalysts. Angew. Chem. Int. Ed. 2015, 54, 2406–2409. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Yin, S.; Han, J.; Zhou, T.; Xu, R. Recent progress in g-C3N4 based low cost photocatalytic system: Activity enhancement and emerging applications. Catal. Sci. Technol. 2015, 5, 5048–5061. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, F. Recent development in exfoliated two-dimensional g-C3N4 nanosheets for photocatalytic applications. J. Mater. Chem. 2015, 3, 23642–23652. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.; Wang, X. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, W.; Wang, S.; Cai, J.; Zhang, L.; Dong, L.; Zhao, L.; He, Y. Synthesis and photocatalytic activity of SiO2/g-C3N4 composite photocatalyst. Mater. Lett. 2014, 115, 53–56. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Chen, X.; Lin, S.; Moehlmann, L.; Dolega, G.; Lipner, G.; Antonietti, M.; Blechert, S.; Wang, X. Co-monomer control of carbon nitride semiconductors to optimize hydrogen evolution with visible light. Angew. Chem. Int. Ed. 2012, 51, 3183–3187. [Google Scholar] [CrossRef]
- Gu, Y.L.; Chen, L.Y.; Shi, L.; Ma, J.H.; Yang, Z.H.; Qian, Y.T. Synthesis of C3N4 and graphite by reacting cyanuric chloride with calcium cyanamide. Carbon 2003, 41, 2674–2676. [Google Scholar] [CrossRef]
- Zimmerman, J.L.; Williams, R.; Khabashesku, V.N.; Margrave, J.L. Synthesis of spherical carbon nitride nanostructures. Nano Lett. 2001, 1, 731–734. [Google Scholar] [CrossRef]
- Xie, M.; Wei, W.; Xu, Y.; Jiang, Z.; Xie, J. Carbon nitride nanowires/nanofibers: A novel template-free synthesis from a cyanuric chloride-melamine precursor towards enhanced adsorption and visible-light photocatalytic performance. Ceram. Int. 2016, 42, 4158–4170. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Lu, D.; Nishihara, Y.; Antonietti, M.; Domen, K. Photocatalytic activities of graphitic carbon nitride powder for water reduction and oxidation under visible light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Ji, H.; Hu, X.; Chang, F.; Qin, W.; Shen, J. Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation. Chem. Eng. J. 2013, 218, 183–190. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. Mater. Sci. Process. 2009, 94, 387–392. [Google Scholar] [CrossRef]
- Cao, L.; Wang, R.; Wang, D. Synthesis and characterization of sulfur self-doped gC3N4 with efficient visible-light photocatalytic activity. Mater. Lett. 2015, 149, 50–53. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric gC3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Luo, H.; Fu, H.; Yin, H.; Lin, Q. Carbon materials in persulfate-based advanced oxidation processes: The roles and construction of active sites. J. Hazard. Mater. 2022, 426, 128044. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Q.; Xia, Y.; Wang, J.; Chen, H.; Xu, Q.; Liu, J.; Feng, W.; Chen, S. Preparation and characterization of Cu-doped TiO2 nanomaterials with anatase/rutile/brookite triphasic structure and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation on alumina and zeolites in water: Formation of hydroxyl radicals. Appl. Catal. B Environ. 2012, 123–124, 94–106. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y.; Lee, J.H. Remediation of wastewater: Role of hydroxyl radicals. J. Photochem. Photobiol. B Biol. 2014, 141, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xie, Y.; Nawaz, F.; Jin, S.; Duan, F.; Li, M.; Cao, H. Super synergy between photocatalysis and ozonation using bulk g-C3N4 as catalyst: A potential sunlight/O3/g-C3N4 method for efficient water decontamination. Appl. Catal. B Environ. 2016, 181, 420–428. [Google Scholar] [CrossRef]
- Xiao, J.; Rabeah, J.; Yang, J.; Xie, Y.; Cao, H.; Brückner, A. Fast Electron Transfer and •OH Formation: Key Features for High Activity in Visible-Light-Driven Ozonation with C3N4 Catalysts. ACS Catal. 2017, 7, 6198–6206. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Cao, H.; Wang, Y.; Guo, Z.; Chen, Y. Towards effective design of active nanocarbon materials for integrating visible-light photocatalysis with ozonation. Carbon 2016, 107, 658–666. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Cao, H.; Wang, Y.; Zhao, Z. g-C3N4-triggered super synergy between photocatalysis and ozonation attributed to promoted •OH generation. Catal. Commun. 2015, 66, 10–14. [Google Scholar] [CrossRef]
- Liao, G.; Zhu, D.; Li, L.; Lan, B. Enhanced photocatalytic ozonation of organics by g-C3N4 under visible light irradiation. J. Hazard. Mater. 2014, 280, 531–535. [Google Scholar] [CrossRef]
- Xiao, J.; Han, Q.; Xie, Y.; Yang, J.; Su, Q.; Chen, Y.; Cao, H. Is C3N4 Chemically Stable toward Reactive Oxygen Species in Sunlight-Driven Water Treatment? Environ. Sci. Technol. 2017, 51, 13380–13387. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Ran, J.; Ma, T.Y.; Gao, G.; Du, X.-W.; Qiao, S.Z. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Nawaz, F.; Wang, Y.; Du, P.; Cao, H. Dramatic coupling of visible light with ozone on honeycomb-like porous g-C3N4 towards superior oxidation of water pollutants. Appl. Catal. B Environ. 2016, 183, 417–425. [Google Scholar] [CrossRef]
- Jimenez-Salcedo, M.; Monge, M.; Teresa Tena, M. Study of intermediate by-products and mechanism of the photocatalytic degradation of ciprofloxacin in water using graphitized carbon nitride nanosheets. Chemosphere 2020, 247, 125910. [Google Scholar] [CrossRef] [PubMed]
- Orge, C.A.; Sampaio, M.J.; Faria, J.L.; Fernando, M.; Pereira, R.; Silva, C.G. Efficiency and stability of metal-free carbon nitride in the photocatalytic ozonation of oxamic acid under visible light. J. Environ. Chem. Eng. 2020, 8, 104172. [Google Scholar] [CrossRef]
- Fernandes, E.; Drosopoulou, S.; Mazierski, P.; Miodynska, M.; Gołaszewska, D.; Zaleska-Medynska, A.; Martins, R.C.; Gomes, J. Carbon nitride photoactivation evaluation and degradation of a mixture of parabens by ozone assistance. J. Water Proc. Eng. 2022, 49, 103018. [Google Scholar] [CrossRef]
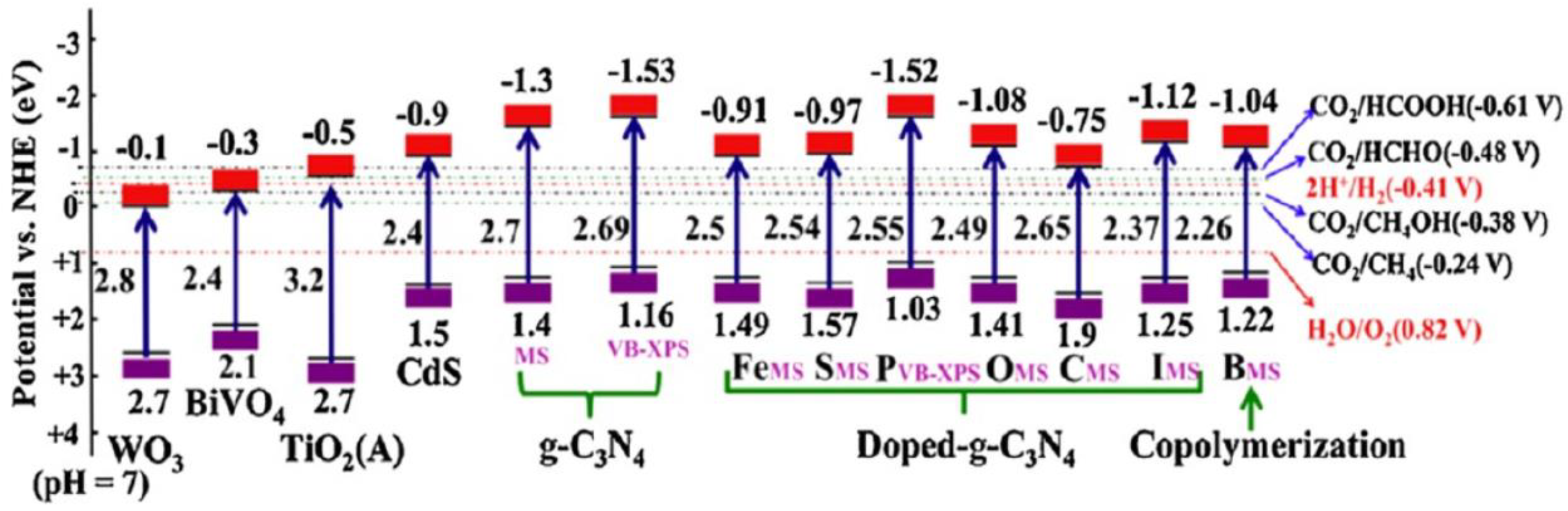
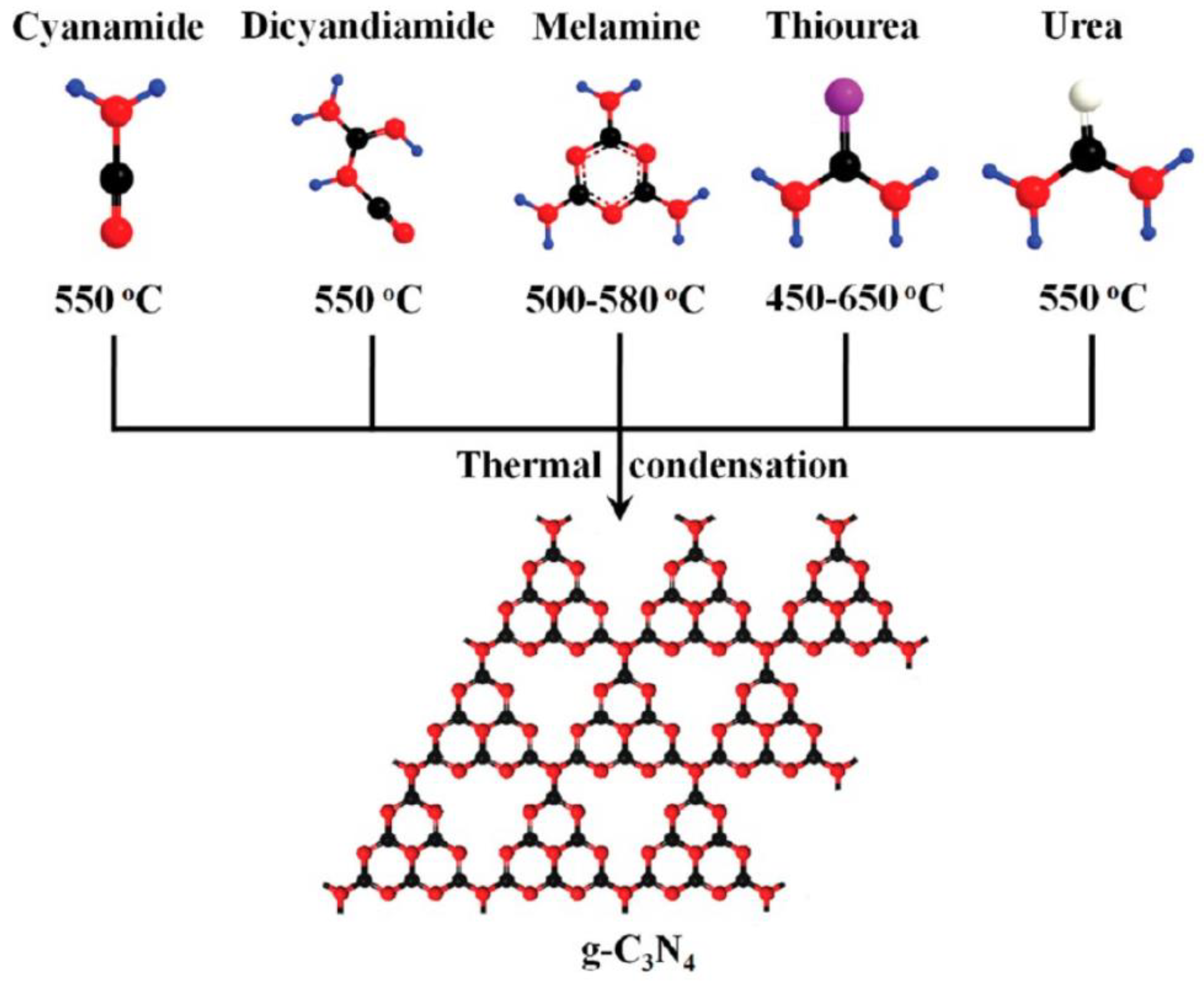

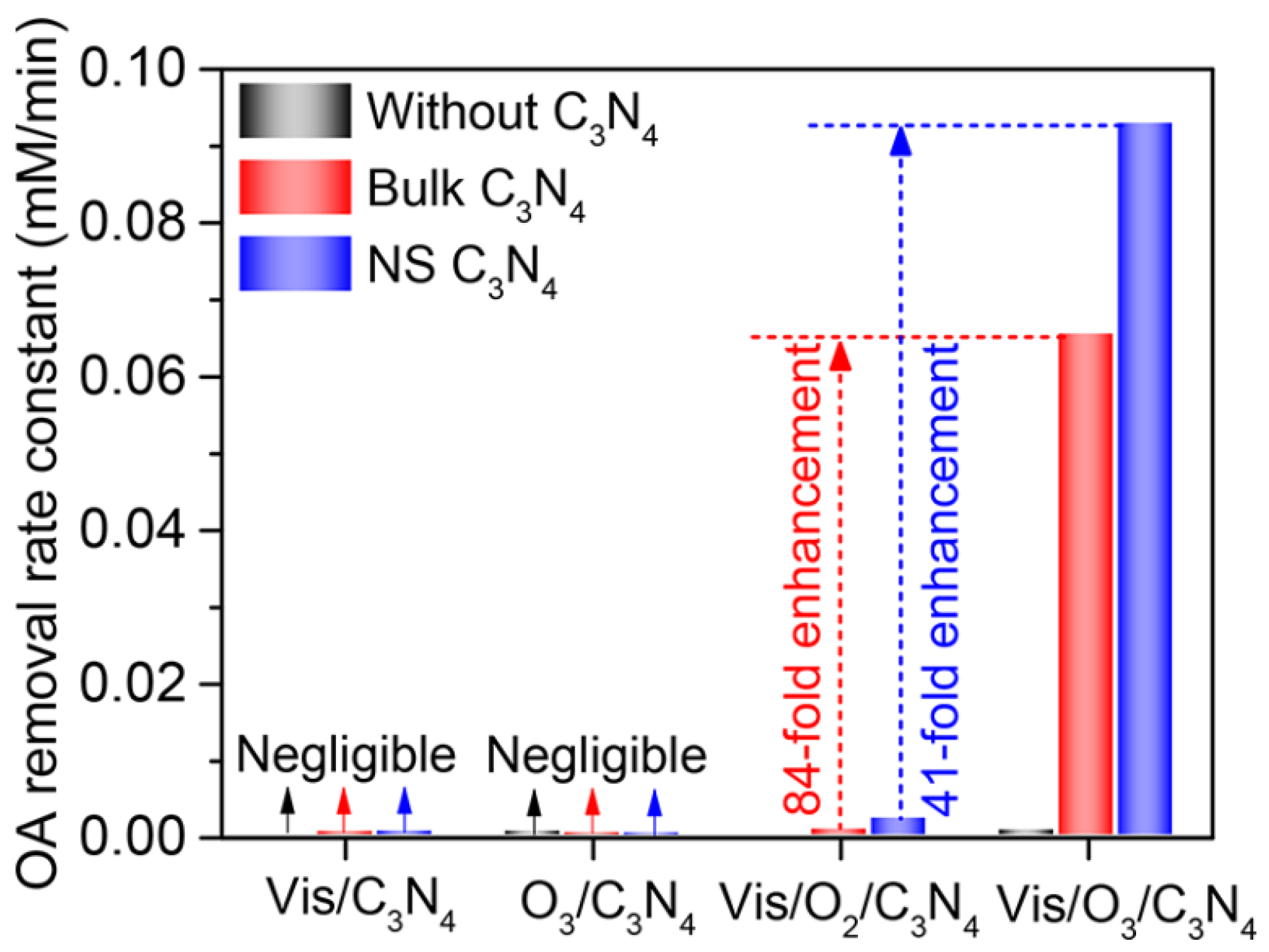


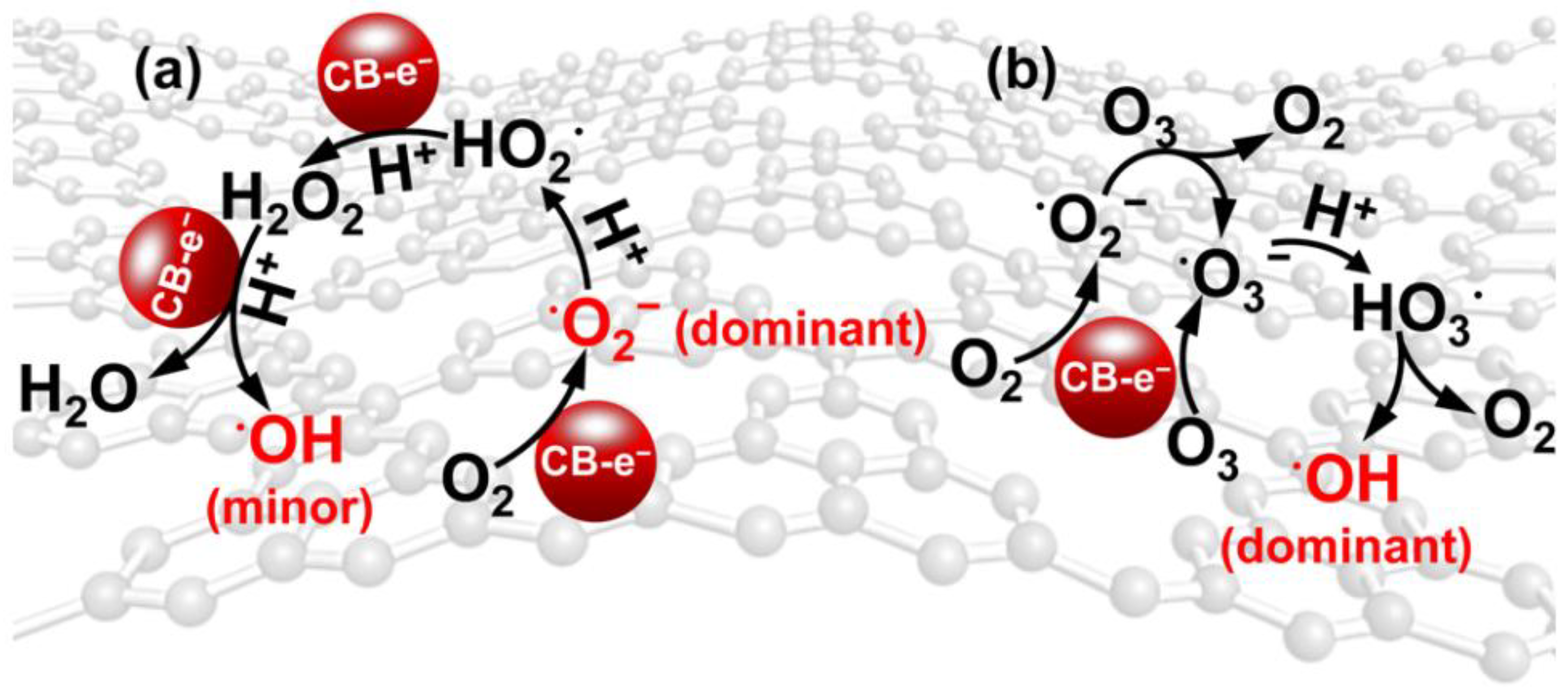

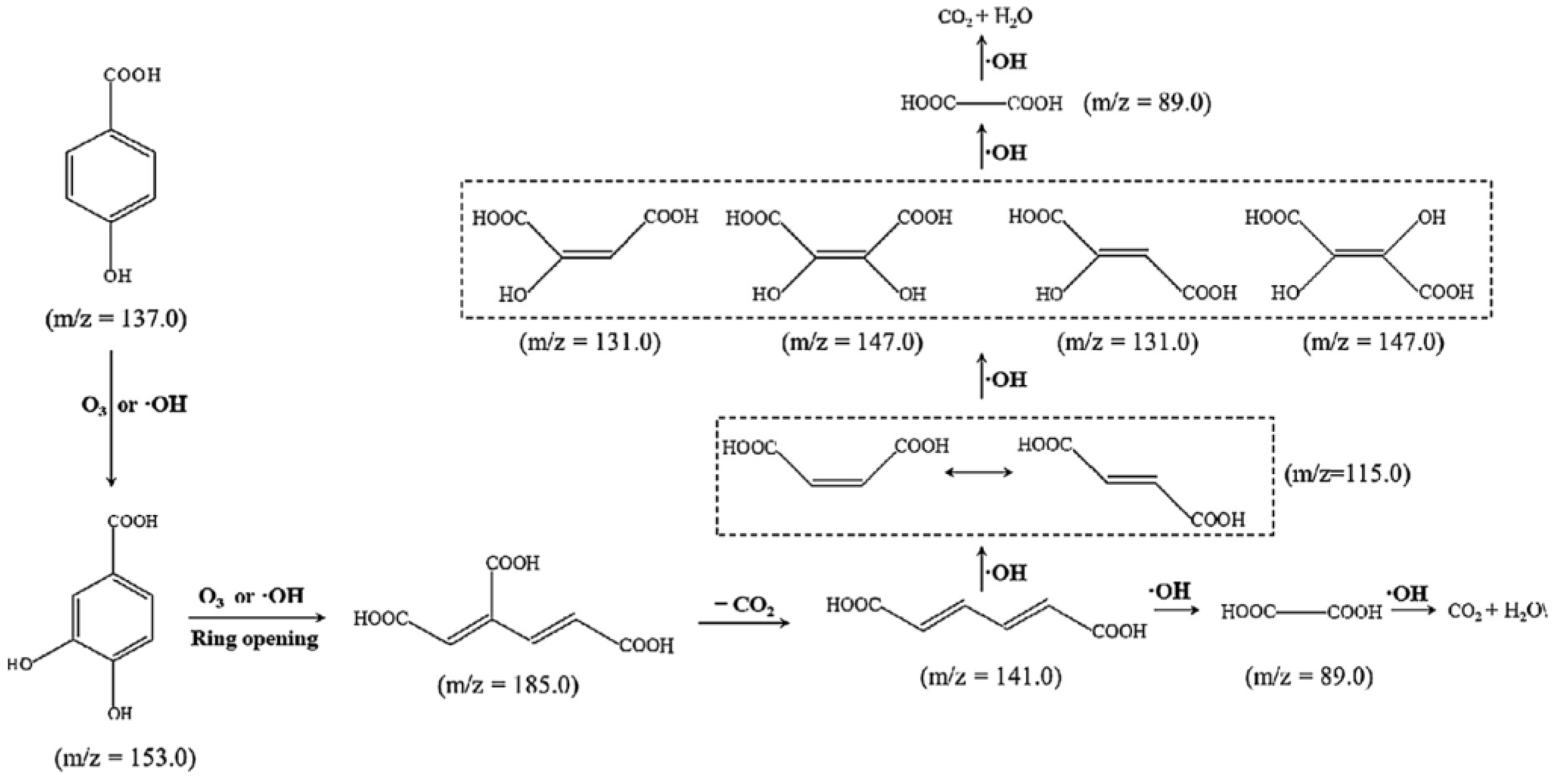

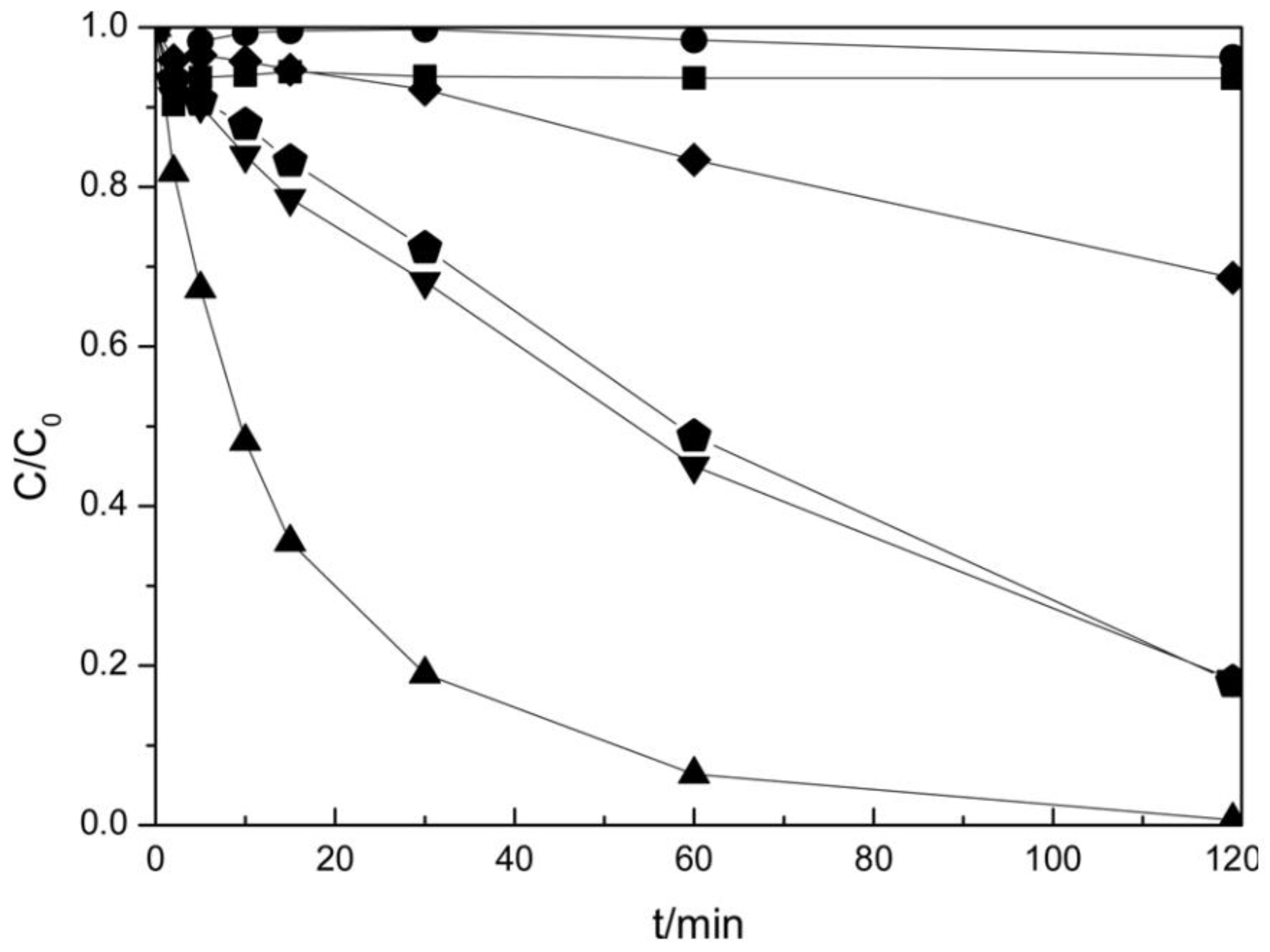
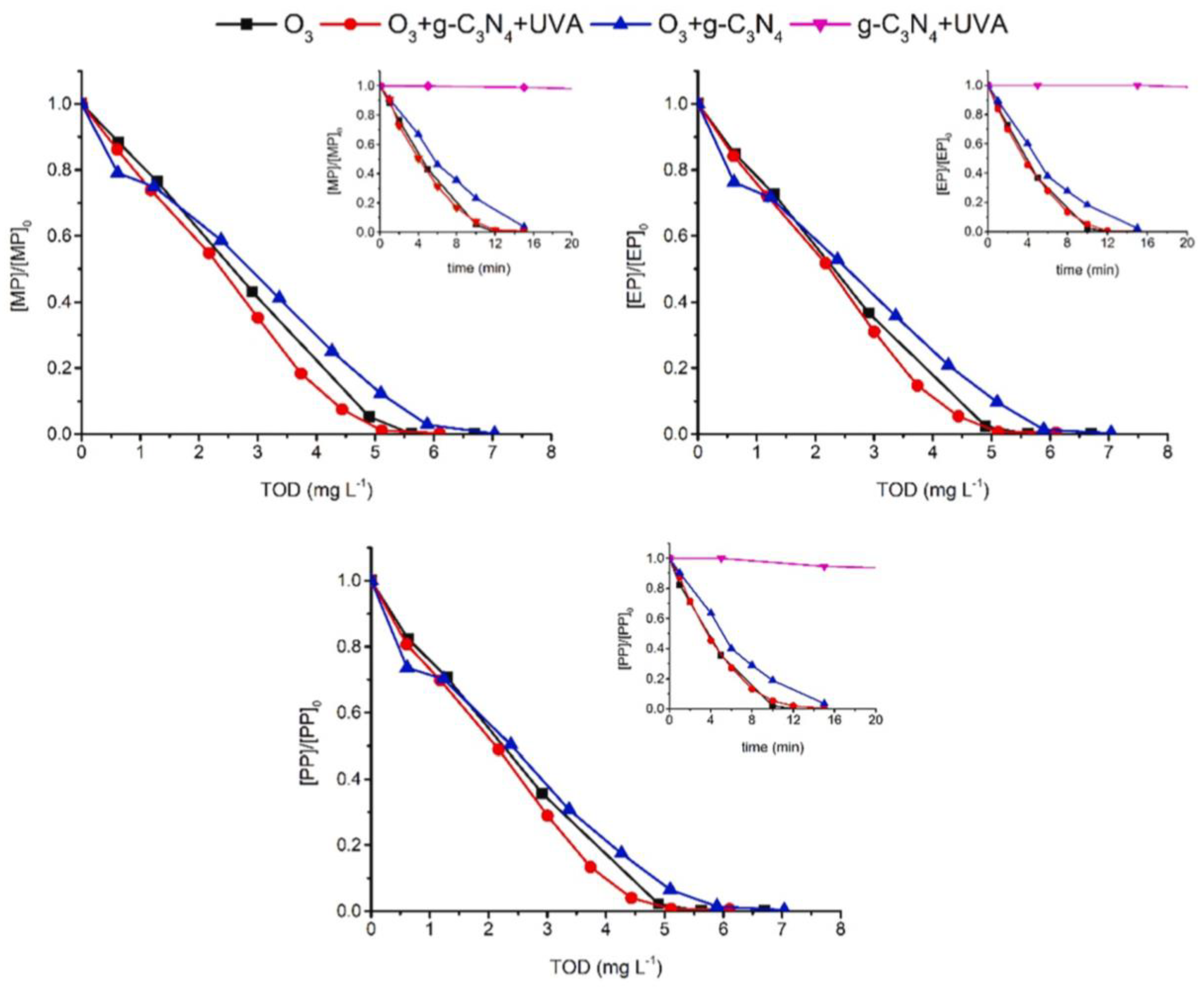
| Catalyst | Detection Method | Quantification | Ref. |
|---|---|---|---|
| Vis/O3/GCN-D | Trapping experiments with N2, t-butanol and p-benzoquinone | - | [54] |
| Vis/O2/C3N4 | DMPO-OH signal evidenced by EPR | 17-fold enhancement of •OH formation | [55] |
| Vis/O3/g-C3N4 | Trapping experiment with t-butanol and the detection of DMPO-OH by EPR | - | [56] |
| Vis/O3/Cl/g-C3N4 | Trapping experiments with N2, t-butanol and p-benzoquinone | - | [57] |
| g-C3N4/Vis/O3 | Trapping experiments with t-butanol and triethanolamine | - | [58] |
| Vis/O3/C3N4 | DMPO-OH signal evidenced by EPR | Vis/O3/g-C3N4 generates 6–18 times more •OH | [59] |
| Vis/O3/PGCN | DMPO-OH signal evidenced by EPR | - | [62] |
| Vis/O3/C3N4 nanosheets | Trapping experiments with t-butanol and triethanolamine | - | [63] |
| Vis/O3/C3N4-500 | Trapping experiments with t-butanol and ethylenediaminetetraacetic acid | - | [64] |
| O3/g-C3N4/UV-A | - | - | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakshinamoorthy, A.; López-Francés, A.; Navalon, S.; Garcia, H. Graphitic Carbon Nitride as Visible-Light Photocatalyst Boosting Ozonation in Wastewater Treatment. Nanomaterials 2022, 12, 3494. https://doi.org/10.3390/nano12193494
Dhakshinamoorthy A, López-Francés A, Navalon S, Garcia H. Graphitic Carbon Nitride as Visible-Light Photocatalyst Boosting Ozonation in Wastewater Treatment. Nanomaterials. 2022; 12(19):3494. https://doi.org/10.3390/nano12193494
Chicago/Turabian StyleDhakshinamoorthy, Amarajothi, Antón López-Francés, Sergio Navalon, and Hermenegildo Garcia. 2022. "Graphitic Carbon Nitride as Visible-Light Photocatalyst Boosting Ozonation in Wastewater Treatment" Nanomaterials 12, no. 19: 3494. https://doi.org/10.3390/nano12193494
APA StyleDhakshinamoorthy, A., López-Francés, A., Navalon, S., & Garcia, H. (2022). Graphitic Carbon Nitride as Visible-Light Photocatalyst Boosting Ozonation in Wastewater Treatment. Nanomaterials, 12(19), 3494. https://doi.org/10.3390/nano12193494









