Synthesis of Silver, Gold, and Platinum Doped Zinc Oxide Nanoparticles by Pulsed Laser Ablation in Water
Abstract
1. Introduction
2. Experimental Procedure
2.1. Material Preparation
2.2. Material Characterization
3. Results and Discussion
3.1. Mass and Atomic Fractions
3.2. Optical Analysis
3.3. Structural Analysis
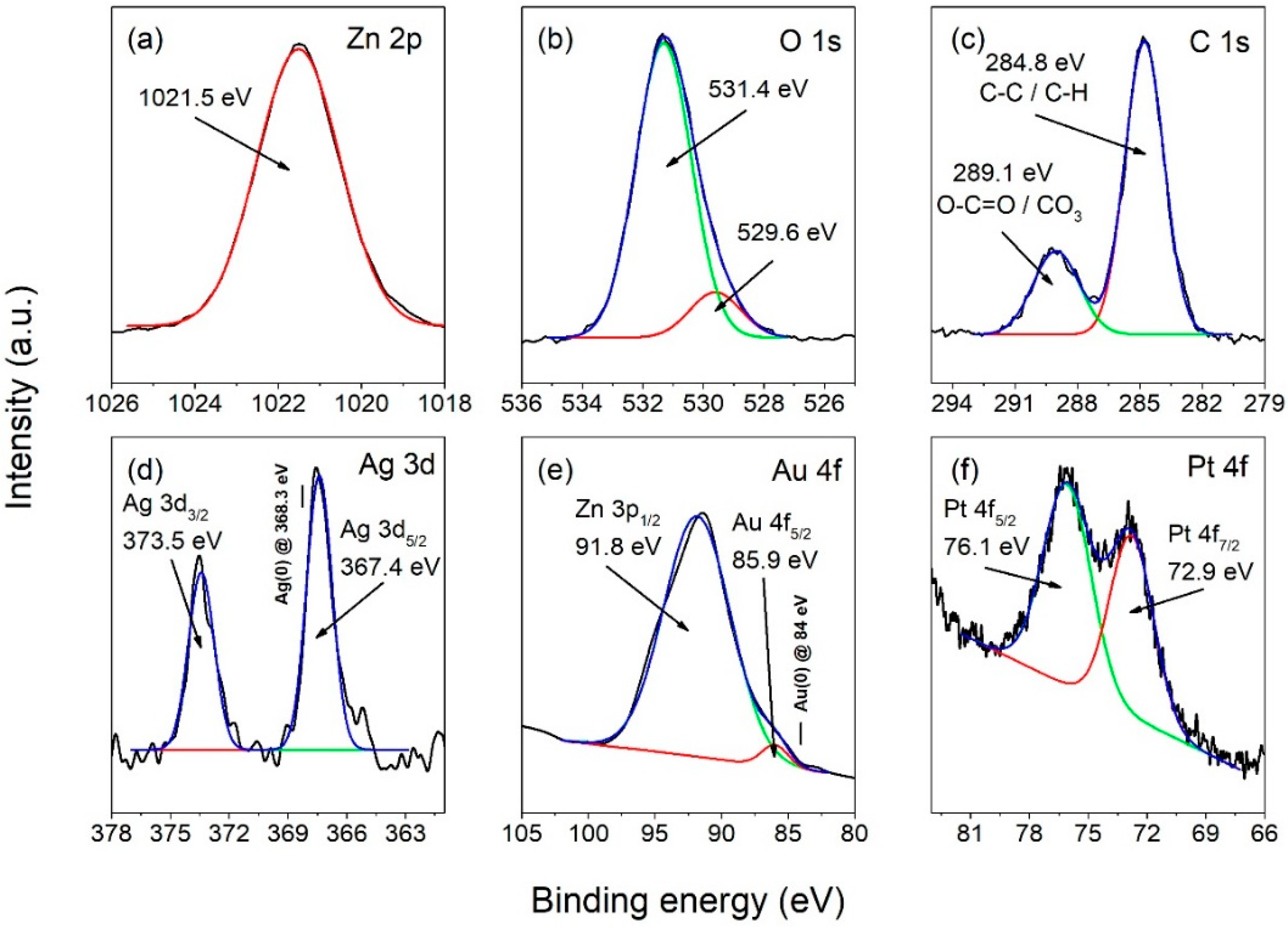
3.4. Chemical Composition Analysis
3.5. Morphological Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Chen, X.; Xue, Z.; Wang, T. Effect of structure: A new insight into nanoparticle assemblies from inanimate to animate. Sci. Adv. 2020, 6, eaba1321. [Google Scholar] [CrossRef] [PubMed]
- Fei Guo, C.; Sun, T.; Cao, F.; Liu, Q.; Ren, Z. Metallic nanostructures for light trapping in energy-harvesting devices. Light Sci Appl. 2014, 3, e161. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Bhowmik, B. Micro/nanostructured gas sensors: The physics behind the nanostructure growth, sensing and selectivity mechanisms. Nanoscale Adv. 2021, 3, 73–93. [Google Scholar] [CrossRef]
- Han, J.; Freyman, M.C.; Feigenbaum, E.; Yong-Jin Han, T. Electro-Optical Device with Tunable Transparency Using Colloidal Core/Shell Nanoparticles. ACS Photonics 2018, 5, 1343–1350. [Google Scholar] [CrossRef]
- Blažeka, D.; Car, J.; Klobučar, N.; Jurov, A.; Zavašnik, J.; Jagodar, A.; Kovačević, E.; Krstulović, N. Photodegradation of Methylene Blue and Rhodamine B Using Laser-Synthesized ZnO Nanoparticles. Materials 2020, 13, 4357. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Toxicol. Chem. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Zhang, J.; Chaker, M.; Ma, D. Pulsed Laser Ablation based Synthesis of Colloidal Metal Nanoparticles for Catalytic Applications. J. Coll. Interface Sci. 2017, 489, 138–149. [Google Scholar] [CrossRef]
- Tymoczko, A.; Kamp, M.; Rehbock, C.; Kienle, L.; Cattaruzza, E.; Barcikowski, S.; Amendola, V. One-step synthesis of Fe–Au core–shell magnetic-plasmonic nanoparticles driven by interface energy minimization. Nanoscale Horiz. 2019, 4, 1326–1332. [Google Scholar] [CrossRef]
- Oko, D.N.; Zhang, J.; Garbarino, S.; Chaker, M.; Ma, D.; Tavares, A.C.; Guay, D. Formic acid electro-oxidation at PtAu alloyed nanoparticles synthesized by pulsed laser ablation in liquids. J. Power Sources 2014, 248, 273–282. [Google Scholar] [CrossRef]
- Marzun, G.; Streich, C.; Jendrzej, S.; Barcikowski, S.; Wagener, P. Adsorption of colloidal platinum nanoparticles to supports: Charge transfer and effects of electrostatic and steric interactions. Langmuir 2014, 30, 11928–11936. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B Environ. 2013, 132–133, 107–115. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Özgür, Ü.; Hofstetter, D.; Morkoç, H. ZnO Devices and Applications: A Review of Current Status and Future Prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO nanostructured materials for emerging solar cell applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.K.; Peter, R.; Badovinac, I.J.; Šarić, I.; Perčić, M.; Radičić, R.; Marković, D.; Knez, M.; Ambrožić, G. ‘Sandwich’-like hybrid ZnO thin films produced by a combination of atomic layer deposition and wet-chemistry using a mercapto silane as single organic precursor. Nanotechnology 2020, 31, 185603. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Sharma, H.; Meena, R.S.; Palkar, V.R. A Novel ZnO Piezoelectric Microcantilever Energy Scavenger: Fabrication and Characterization. Sens. Bio-Sens. Res. 2016, 9, 45–52. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, R.; Pan, C.; Li, Z.; Yang, J.; Yi, F.; Wang, Z.L. Light-Induced Pyroelectric Effect as an Effective Approach for Ultrafast Ultraviolet Nanosensing. Nat. Commun. 2015, 6, 8401. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.; Huang, S.; Yue, S.; Liu, K.; Lu, S.; Wang, Z.; Qu, S.; Wang, Z. Ultra-Thin ZnO Film as an Electron Transport Layer for Realizing the High Efficiency of Organic Solar Cells. RSC Adv. 2017, 7, 14694–14700. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Ahmed, W.; Elhissi, A.; Khalid, N.R. Photocatalytic, Sonocatalytic and Sonophotocatalytic Degradation of Rhodamine B Using ZnO/CNTs Composites Photocatalysts. Ultrason. Sonochem. 2014, 21, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef] [PubMed]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Sathasivam, S.; Carmalt, C.J. N-Type Conducting P Doped ZnO Thin Films via Chemical Vapor Deposition. RSC Adv. 2020, 10, 34527–34533. [Google Scholar] [CrossRef]
- Munawaroh, H.; Wahyuningsih, S.; Ramelan, A.H. Synthesis and Characterization of Al doped ZnO (AZO) by Sol-gel Method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012049. [Google Scholar] [CrossRef]
- Lee, D.-J.; Kim, K.-J.; Kim, S.-H.; Kwon, J.-Y.; Xu, J.; Kim, K.-B. Atomic Layer Deposition of Ti-Doped ZnO Films with Enhanced Electron Mobility. J. Mater. Chem. C 2013, 1, 4761. [Google Scholar] [CrossRef]
- Kaur, G.; Mitra, A.; Yadav, K.L. Pulsed laser deposited Al-doped ZnO thin films for optical applications. Prog. Nat. Sci. 2015, 25, 12–21. [Google Scholar] [CrossRef]
- Rana, A.K.; Bankar, P.; Kumar, Y.; More, M.A.; Late, D.J.; Shirage, P.M. Synthesis of Ni-Doped ZnO Nanostructures by Low-Temperature Wet Chemical Method and Their Enhanced Field Emission Properties. RSC Adv. 2016, 6, 104318–104324. [Google Scholar] [CrossRef]
- Streubel, R.; Barcikowski, S.; Gökce, B. Continuous Multigram Nanoparticle Synthesis by High-Power, High-Repetition-Rate Ultrafast Laser Ablation in Liquids. Opt. Lett. 2016, 41, 1486. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Miryala, M.; Dixit, T.; Klimkowicz, A.; Francis, B.; Murakami, M.; Rao, M.S.R.; Krishnan, S. Femtosecond Pulse Ablation Assisted Mg-ZnO Nanoparticles for UV-Only Emission. Nanomaterials 2020, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Chelnokov, E.; Rivoal, M.; Colignon, Y.; Gachet, D.; Bekere, L.; Thibaudau, F.; Giorgio, S.; Khodorkovsky, V.; Marine, W. Band Gap Tuning of ZnO Nanoparticles via Mg Doping by Femtosecond Laser Ablation in Liquid Environment. Appl. Surf. Sci. 2012, 23, 9408–9411. [Google Scholar] [CrossRef]
- Qin, W.J.; Sun, J.; Yang, J.; Du, X.W. Control of Cu-doping and optical properties of ZnO quantum dots by laser ablation of composite targets. Mater. Chem. Phys. 2011, 130, 425–430. [Google Scholar] [CrossRef]
- Yudasari, N.; Suliyanti, M.M.; Imawan, C. Antibacterial Activity of Fe-Doped ZnO Nanoparticles Synthesised via Pulsed Laser Ablation in Liquid against Staphylococcus Aureus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025003. [Google Scholar] [CrossRef]
- Anugrahwidya, R.; Yudasari, N.; Tahir, D. Optical and Structural Investigation of Synthesis ZnO/Ag Nanoparticles Prepared by Laser Ablation in Liquid. Mater. Sci. Semicond. Process. 2020, 105, 104712. [Google Scholar] [CrossRef]
- Khashan, K.S.; Mahdi, M. Preparation of Indium-Doped Zinc Oxide Nanoparticles by Pulsed Laser Ablation in Liquid Technique and Their Characterization. Appl. Nanosci. 2017, 7, 589–596. [Google Scholar] [CrossRef]
- Krstulović, N.; Umek, P.; Salamon, K.; Capan, I. Synthesis of Al-Doped ZnO Nanoparticles by Laser Ablation of ZnO:Al2O3 Target in Water. Mater. Res. Express 2017, 4, 105003. [Google Scholar] [CrossRef]
- Krstulović, N.; Blažeka, D.; Car, J.; Maletić, D.; Rakić, M. Method of Production of Two-Component Nanoparticles Using Laser. Croatian Patent P20211098A, 9 July 2021. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chastain, J., Ed.; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-Ablated ZnO Nanoparticles and Their Photocatalytic Activity Toward Organic Pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band Gap Narrowing and Widening of ZnO Nanostructures and Doped Materials. Nanoscale Res. Lett. 2015, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.J.; Kokila, M.K.; Nagabhushana, H.; Rao, J.L.; Shivakumara, C.; Nagabhushana, B.M.; Chakradhar, R.P.S. Combustion Synthesis, Characterization and Raman Studies of ZnO Nanopowders. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 53–58. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Chen, C.; Liao, J.; Li, Z. Enhanced Visible Light Photocatalytic Activity of ZnO Nanowires Doped with Mn2+ and Co2+ Ions. Nanomaterials 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ahmad, M.; Rahmanuddin Khan, S.; Aziz, U.; Ali, Z.; Khan, A.; Mahmood, A. The Role of Al Doping on ZnO Nanowire Evolution and Optical Band Gap Tuning. Appl. Phys. A 2019, 125, 713. [Google Scholar] [CrossRef]
- Khokhra, R.; Bharti, B.; Lee, H.-N.; Kumar, R. Visible and UV Photo-Detection in ZnO Nanostructured Thin Films via Simple Tuning of Solution Method. Sci. Rep. 2017, 7, 15032. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, G.H.; Shingange, K.; Tshabalala, Z.P.; Dhonge, B.P.; Mahmoud, F.A.; Mwakikunga, B.W.; Motaung, D.E. Room temperature ferromagnetism and gas sensing in ZnOnanostructures: Influence of intrinsic defects and Mn, Co, Cu doping. Appl. Surf. Sci. 2016, 390, 804–815. [Google Scholar] [CrossRef]
- Ahmad, I.; Shoaib Akhtar, M.; Ahmed, E.; Ahmad, M.; Keller, V.; Qamar Khan, W.; Khalid, N.R. Rare Earth Co-Doped ZnO Photocatalysts: Solution Combustion Synthesis and Environmental Applications. Sep. Purif. Technol. 2020, 237, 116328. [Google Scholar] [CrossRef]
- Panigrahy, B.; Aslam, M.; Misra, D.S.; Ghosh, M.; Bahadur, D. Defect-Related Emissions and Magnetization Properties of ZnO Nanorods. Adv. Funct. Mater. 2010, 20, 1161–1165. [Google Scholar] [CrossRef]
- Blažeka, D.; Radičić, R.; Maletić, D.; Živković, S.; Momčilović, M.; Krstulović, N. Enhancement of Methylene Blue Photodegradation Rate Using Laser Synthesized Ag-Doped ZnO Nanoparticles. Nanomaterials 2022, 12, 2677. [Google Scholar] [CrossRef]
- Singh, A.; Vishwakarma, H.L. Study of structural, morphological, optical and electroluminescent properties of undoped ZnO nanorods grown by a simple chemical precipitation. Mater. Sci.-Pol. 2015, 33, 751–759. [Google Scholar] [CrossRef]
- Ekambaram, S.; Iikubo, Y.; Kudo, A. Combustion Synthesis and Photocatalytic Properties of Transition Metal-Incorporated ZnO. J. Alloy Compd. 2007, 433, 237–240. [Google Scholar] [CrossRef]
- Edinger, S.; Bansal, N.; Bauch, M.; Wibowo, R.A.; Újvári, G.; Hamid, R.; Trimmel, G.; Dimopoulos, T. Highly Transparent and Conductive Indium-Doped Zinc Oxide Films Deposited at Low Substrate Temperature by Spray Pyrolysis from Water-Based Solutions. J. Mater. Sci. 2017, 52, 8591–8602. [Google Scholar] [CrossRef]
- Narath, S.; Koroth, S.K.; Shankar, S.S.; George, B.; Mutta, V.; Wacławek, S.; Černík, M.; Padil, V.V.T.; Varma, R.S. Tamala, Cinnamomum Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation. Nanomaterials 2021, 11, 1558. [Google Scholar] [CrossRef]
- Gruzintsev, A.N.; Volkov, V.T.; Yakimov, E.E. Photoelectric properties of ZnO films doped with Cu and Ag acceptor impurities. Semiconductors 2003, 37, 259–262. [Google Scholar] [CrossRef]
- Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Beyond Chemical Bonding Interaction: An Insight into the Growth Process of 1D ZnO on Few-Layer Graphene for Excellent Photocatalytic and Room Temperature Gas Sensing Applications. Chem. Sel. 2018, 3, 7302–7309. [Google Scholar] [CrossRef]
- Tjeng, L.H.; Meinders, M.B.J.; van Elp, J.; Ghijsen, J.; Sawatzky, G.A.; Johnson, R.L. Electronic structure of Ag2O. Phys. Rev. B 1990, 41, 3190. [Google Scholar] [CrossRef]
- Vitale, F.; Fratoddi, I.; Battocchio, C.; Piscopiello, E.; Tapfer, L.; Russo, M.V.; Polzonetti, G.; Giannini, C. Mono- and bi-functional arenethiols as surfactants for gold nanoparticles: Synthesis and characterization. Nanoscale Res. Lett. 2011, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Gogurla, N.; Kumar Sinha, A.; Santra, S.; Manna, S.; Kumar Ray, S. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.R.; Ke, S.-C. Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles. Nanomaterials 2021, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Le, T.; Peng, J.; Ravindra, A.V.; Xu, L. Pt quantum dots decorated nest-like 3D porous ZnO nanostructures for enhanced visible-light degradation of RhB. J. Porous Mater. 2020, 27, 1339–1348. [Google Scholar] [CrossRef]
- Naitabdi, A.; Boucly, A.; Rochet, F.; Fagiewicz, R.; Olivieri, G.; Bournel, F.; Benbalagh, R.; Sirotti, F.; Galleta, J.-J. CO oxidation activity of Pt, Zn and ZnPt nanocatalysts: A comparative study by in situ near-ambient pressure X-ray photoelectron spectroscopy. Nanoscale 2018, 10, 6566. [Google Scholar] [CrossRef] [PubMed]
- Massalski, T.B. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar] [CrossRef]
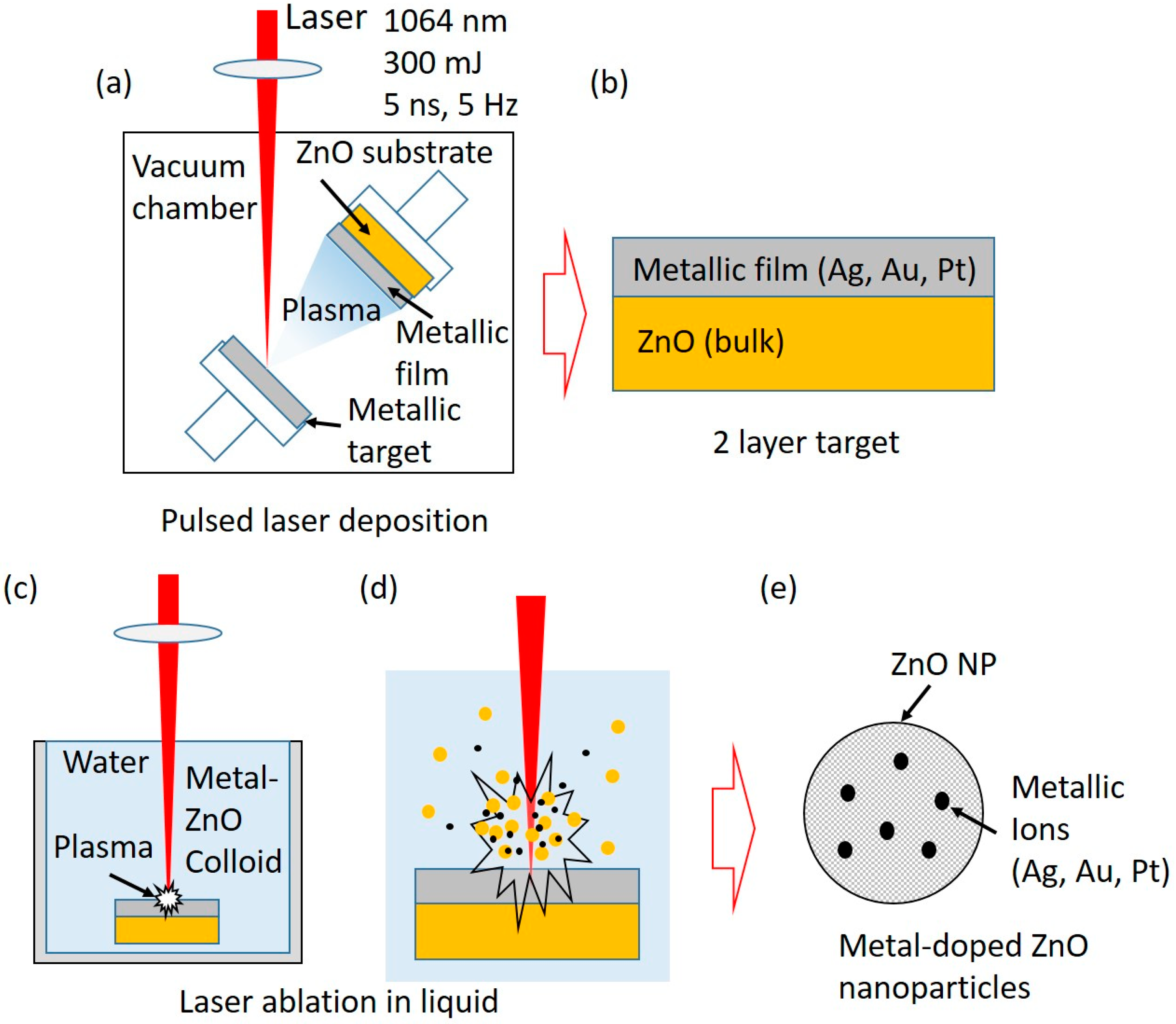
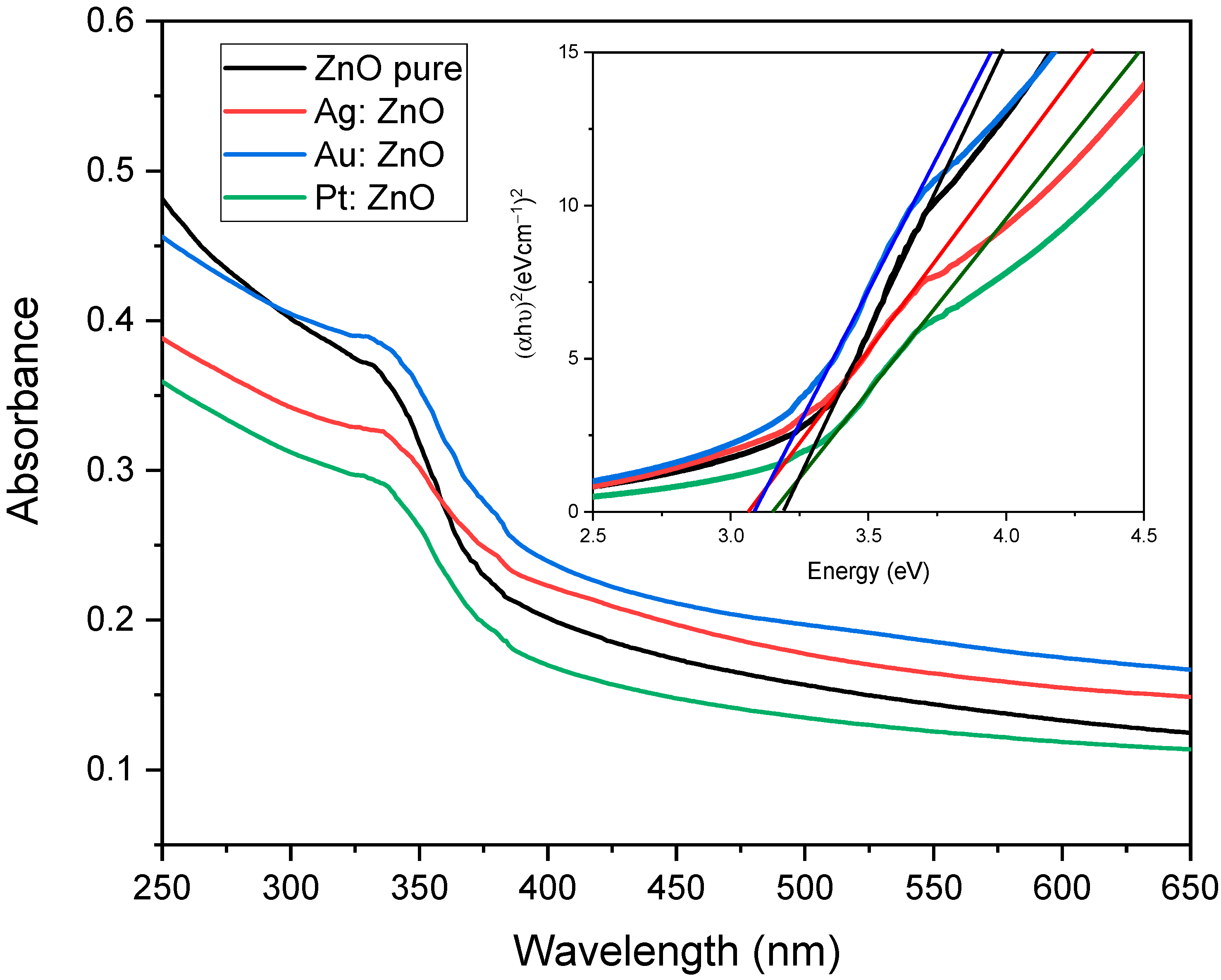
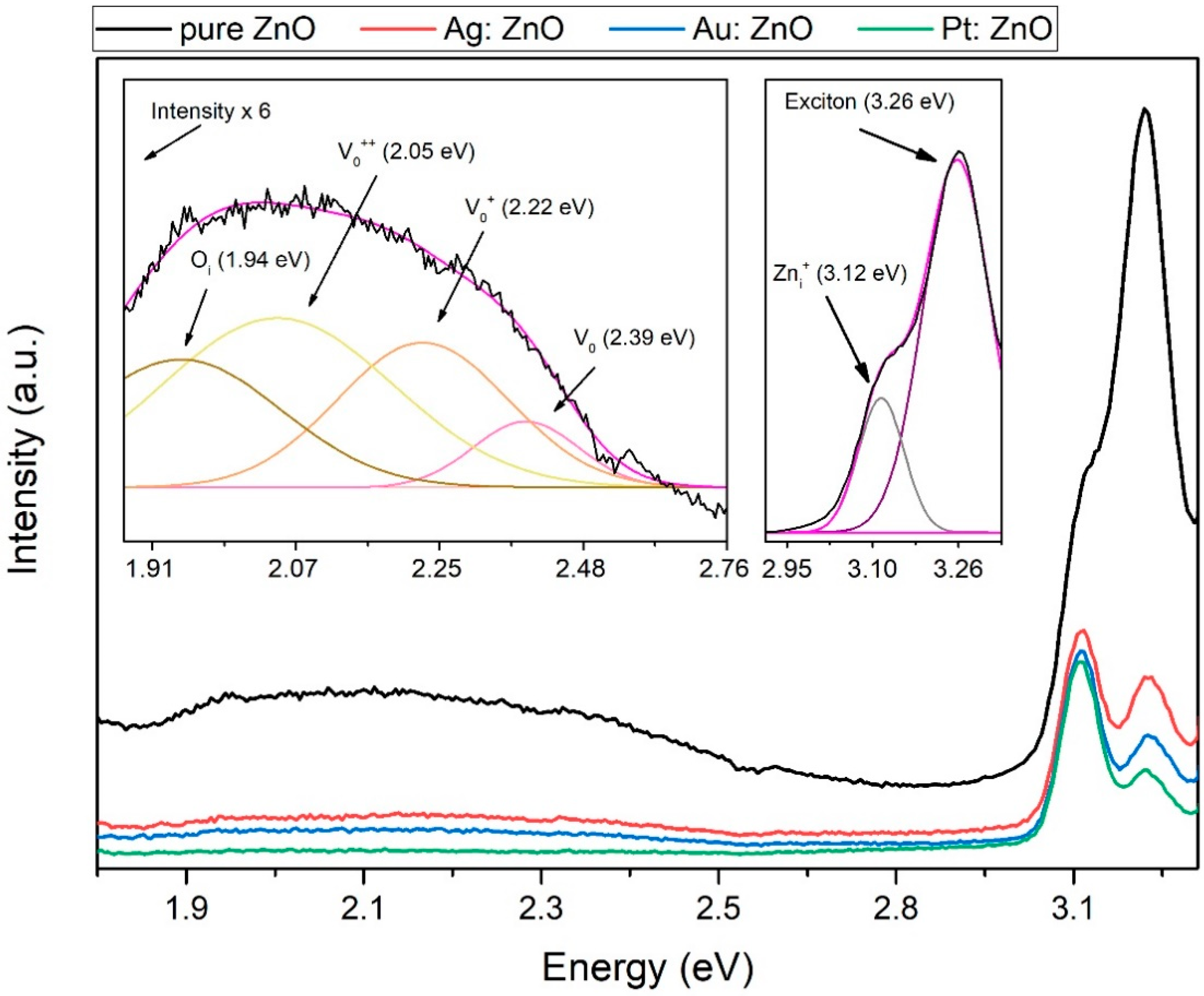

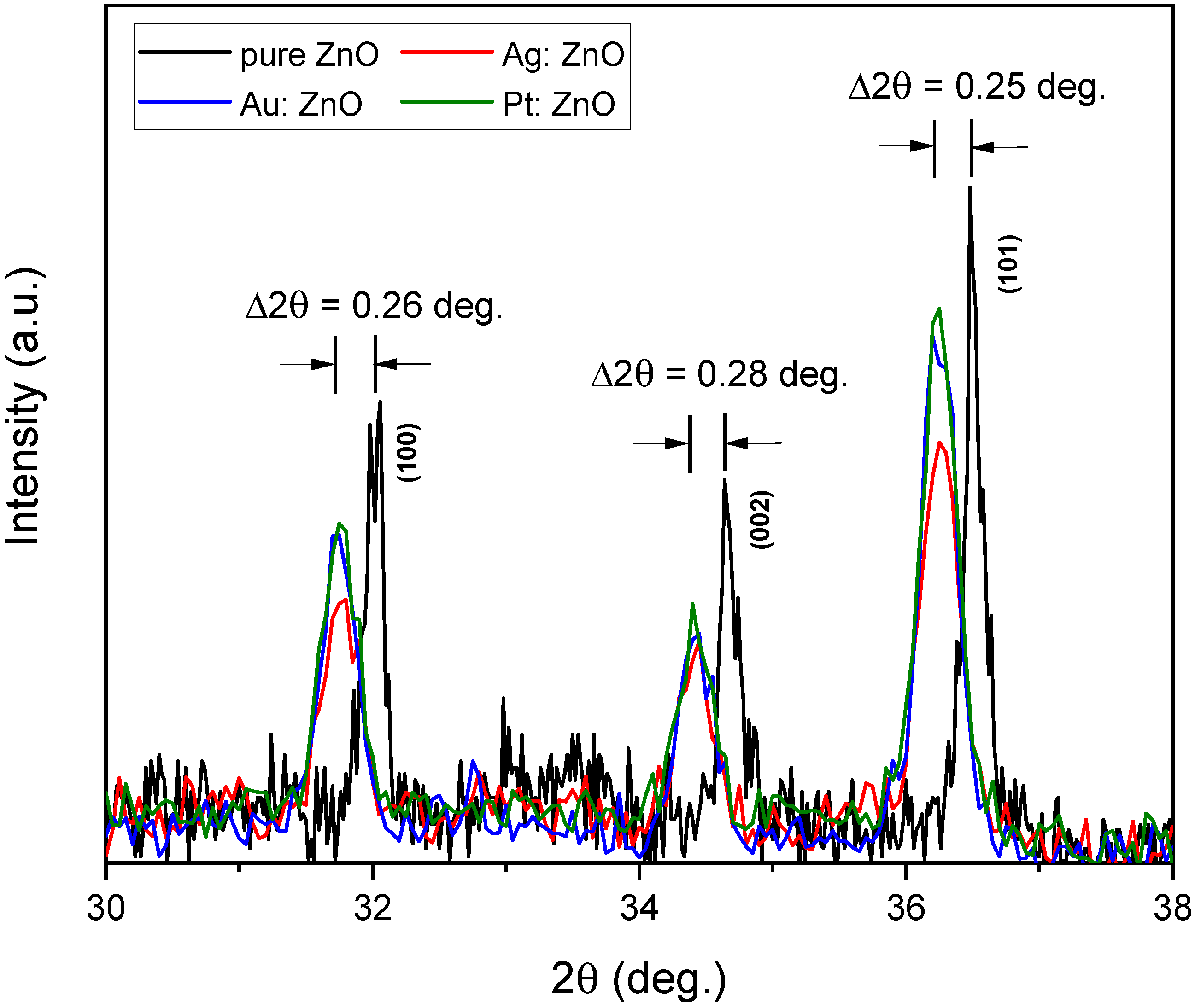




| PLD | PLAL | w (%) | at. (%) | |||
|---|---|---|---|---|---|---|
| m (Ag) | 0.036 mg | m (Ag: ZnO) | 1.171 mg | Ag | 3.37 ± 0.2 | 2.32 ± 0.2 |
| m (Au) | 0.024 mg | m (Au: ZnO) | 1.798 mg | Au | 1.33 ± 0.1 | 0.55 ± 0.1 |
| m (Pt) | 0.019 mg | m (Pt: ZnO) | 1.925 mg | Pt | 1.01 ± 0.1 | 0.42 ± 0.1 |
| a (Å) | c (Å) | c/a (Å) | |
|---|---|---|---|
| pure ZnO | 3.226 | 5.170 | 1.603 |
| Ag: ZnO | 3.250 | 5.207 | 1.602 |
| Au: ZnO | 3.252 | 5.206 | 1.601 |
| Pt: ZnO | 3.251 | 5.207 | 1.603 |
| DD-S (nm) | DW-H (nm) | DS-S (nm) | δ(D-S) × 10−3 (nm−2) | δ(W-H) × 10−4 (nm−2) | δ(S-S) × 10−4 (nm−2) | ε(W-H) × 10−4 | ε(S-S) × 10−4 | |
|---|---|---|---|---|---|---|---|---|
| ZnO | 50 | 44 ± 8 | 35 ± 6 | 0.04 | 0.05 | 0.08 | 1.4 ± 0.4 | 1.1 ± 0.7 |
| Ag: ZnO | 24 | 24 ± 5 | 25 ± 4 | 0.17 | 0.17 | 0.16 | 2.1 ± 0.1 | 0.2 ± 0.1 |
| Au: ZnO | 25 | 30 ± 7 | 24 ± 5 | 0.16 | 0.11 | 0.17 | 5.2 ± 2.3 | 0.8 ± 0.2 |
| Pt: ZnO | 24 | 39 ± 7 | 21 ± 6 | 0.17 | 0.06 | 0.23 | 1.5 ± 0.3 | 1.3 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radičić, R.; Maletić, D.; Blažeka, D.; Car, J.; Krstulović, N. Synthesis of Silver, Gold, and Platinum Doped Zinc Oxide Nanoparticles by Pulsed Laser Ablation in Water. Nanomaterials 2022, 12, 3484. https://doi.org/10.3390/nano12193484
Radičić R, Maletić D, Blažeka D, Car J, Krstulović N. Synthesis of Silver, Gold, and Platinum Doped Zinc Oxide Nanoparticles by Pulsed Laser Ablation in Water. Nanomaterials. 2022; 12(19):3484. https://doi.org/10.3390/nano12193484
Chicago/Turabian StyleRadičić, Rafaela, Dejan Maletić, Damjan Blažeka, Julio Car, and Nikša Krstulović. 2022. "Synthesis of Silver, Gold, and Platinum Doped Zinc Oxide Nanoparticles by Pulsed Laser Ablation in Water" Nanomaterials 12, no. 19: 3484. https://doi.org/10.3390/nano12193484
APA StyleRadičić, R., Maletić, D., Blažeka, D., Car, J., & Krstulović, N. (2022). Synthesis of Silver, Gold, and Platinum Doped Zinc Oxide Nanoparticles by Pulsed Laser Ablation in Water. Nanomaterials, 12(19), 3484. https://doi.org/10.3390/nano12193484










