Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions
Abstract
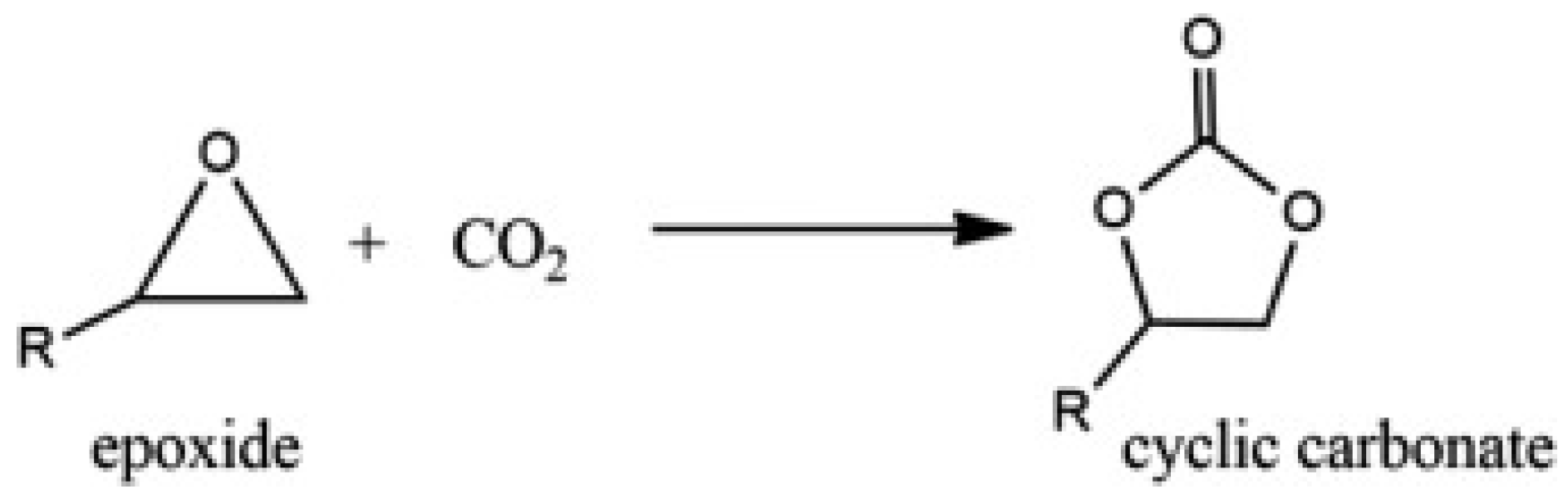
1. Introduction
2. Experimental
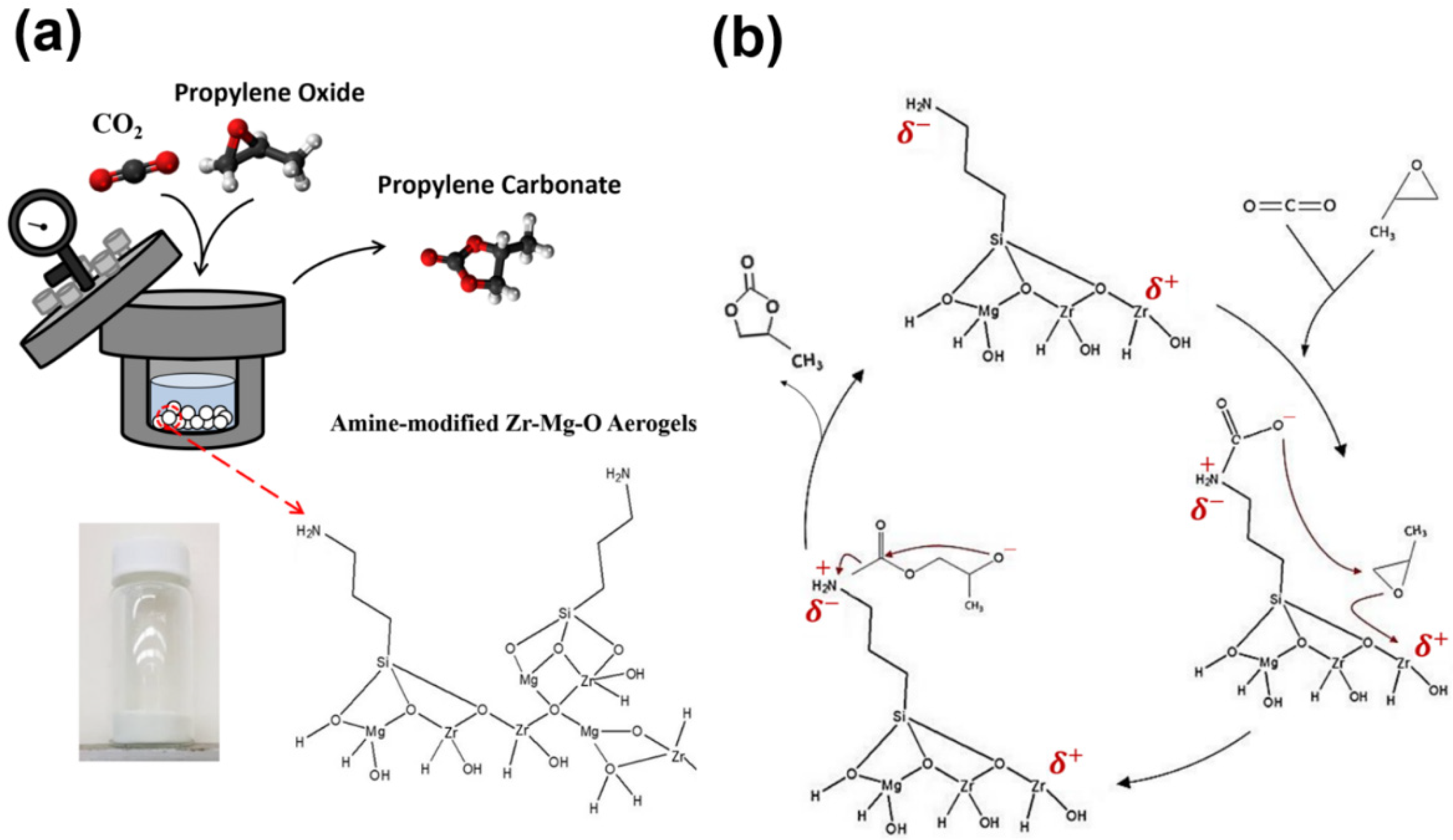
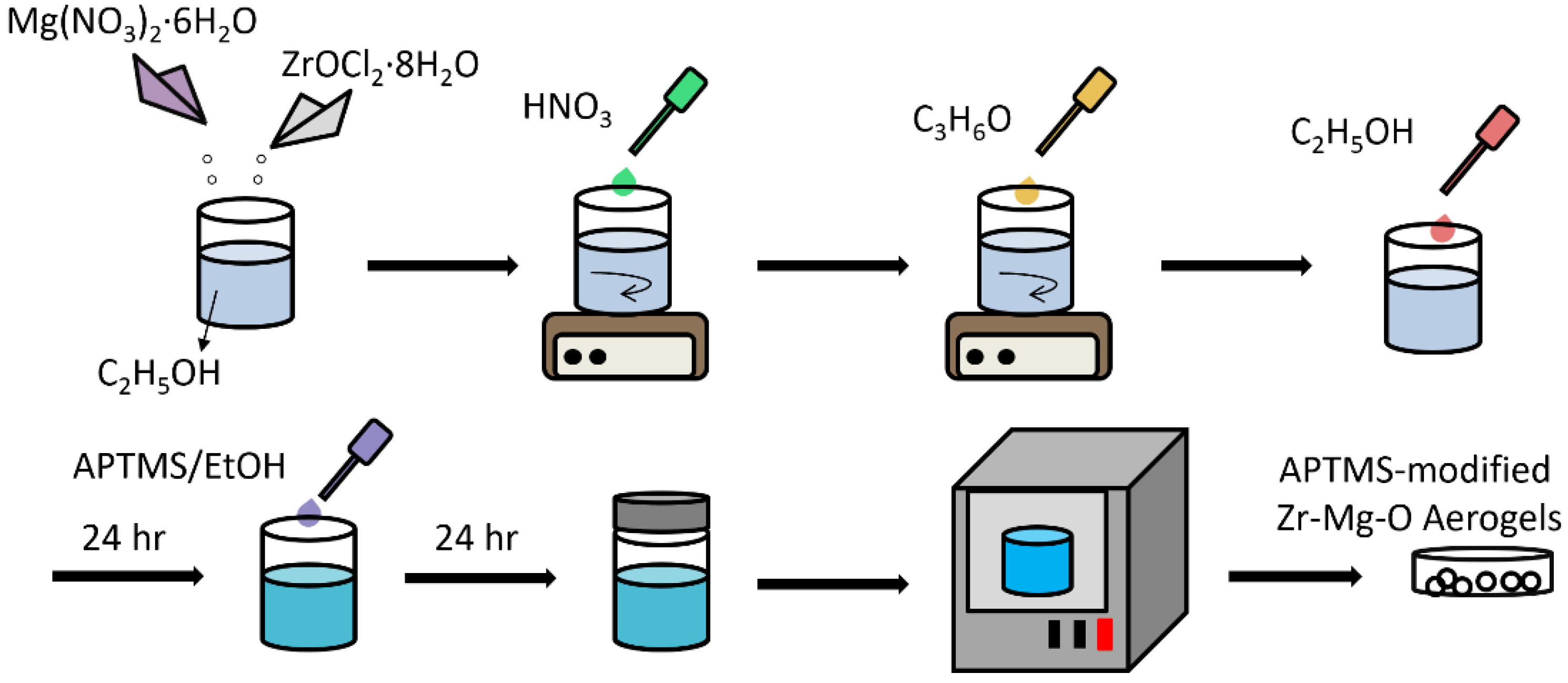
2.1. Synthesis of Zr–Mg Mixed Oxide Aerogels
2.2. Carbon Dioxide/Epoxide Cycloaddition Catalytic Reactions
2.3. Characterization
3. Results and Discussion
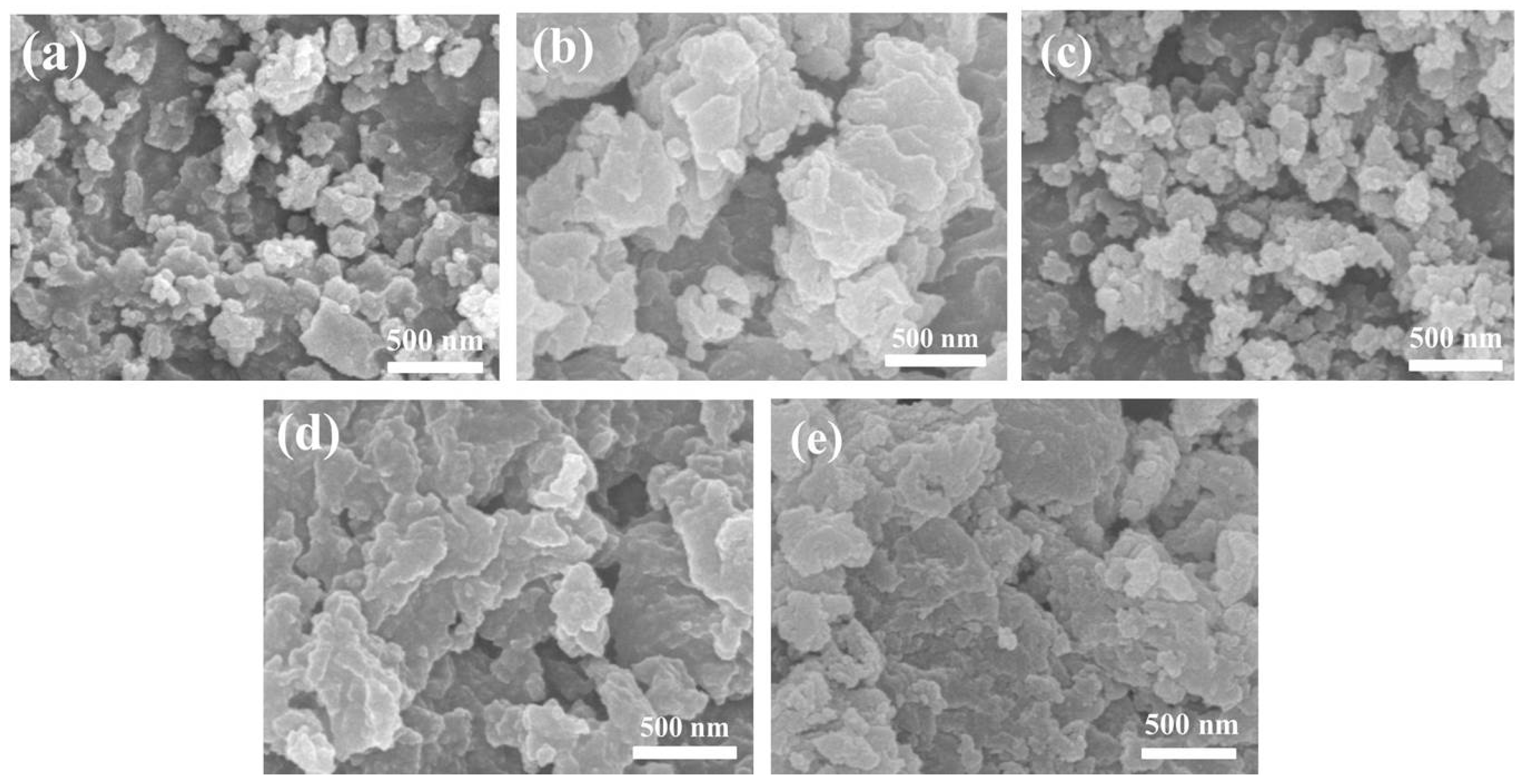
3.1. The Synthesis of Zr–Mg Mixed Oxide Aerogels
3.2. Mg–Zr Mixed Oxide Aerogels with Amino Functional Group Modification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Calvo, L.M.; Domingo, R. Influence of process operating parameters on CO2 emissions in continuous industrial plants. J. Clean. Prod. 2015, 96, 253–262. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. Sustain. Chem. Green Chem. 2017, 10, 1303–1317. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Anthony, E.J.; Jiang, X.; Duan, L.; Wu, Y.; Dong, W. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Prog. Energy Combust. Sci. 2013, 39, 515–534. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Arnette, A.N. Renewable energy and carbon capture and sequestration for a reduced carbon energy plan: An optimization model. Renew. Sustain. Energy Rev. 2017, 70, 254–265. [Google Scholar] [CrossRef]
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 1482–1497. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Roshan, K.R.; Mathai, G.; Kim, J.; Tharun, J.; Park, G.A.; Park, D.W. A biopolymer mediated efficient synthesis of cyclic carbonates from epoxides and carbon dioxide. Green Chem. 2012, 14, 2933–2940. [Google Scholar] [CrossRef]
- Dai, W.L.; Luo, S.L.; Yin, S.F.; Au, C.T. The direct transformation of carbon dioxide to organic carbonates over heterogeneous catalysts. Appl. Catal. A-Gen. 2009, 366, 2–12. [Google Scholar] [CrossRef]
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kühn, F.E. Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide by Using Organocatalysts. Sustain. Chem. Green Chem. 2015, 8, 2436–2454. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Fiorani, G.; Guo, W.; Kleij, A.W. Sustainable conversion of carbon dioxide: The advent of organocatalysis. Green Chem. 2015, 17, 1375–1389. [Google Scholar] [CrossRef]
- Büttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent Developments in the Synthesis of Cyclic Carbonates from Epoxides and CO2. Top. Curr. Chem. 2017, 375, 89–144. [Google Scholar]
- Spinner, N.S.; Vega, J.A.; Mustain, W.E. Recent progress in the electrochemical conversion and utilization of CO2. Catal. Sci. Technol. 2012, 2, 19–28. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Tsuda, T.; Kondo, K.; Tomioka, T.; Takahashi, Y.; Matsumoto, H.; Kuwabata, S.; Hussey, C.L. Design, Synthesis, and Electrochemistry of Room-Temperature Ionic Liquids Functionalized with Propylene Carbonate. Angew. Chem. Int. Ed. 2011, 50, 1310–1313. [Google Scholar] [CrossRef]
- Sakakura, T.; Kohno, K. The synthesis of organic carbonates from carbon dioxide. Chem. Commun. 2009, 11, 1312–1330. [Google Scholar] [CrossRef]
- Lu, X.B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, N.; Furusho, Y.; Endo, T. Convenient Synthesis of Cyclic Carbonates from CO2 and Epoxides by Simple Secondary and Primary Ammonium Iodides as Metal-Free Catalysts under Mild Conditions and Its Application to Synthesis of Polymer Bearing Cyclic Carbonate Moiety. J. Polym. Sci. 2013, 51, 1230–1242. [Google Scholar] [CrossRef]
- Liu, S.; Suematsu, N.; Maruoka, K.; Shirakawa, S. Design of bifunctional quaternary phosphonium salt catalysts for CO2 fixation reaction with epoxides under mild conditions. Green Chem. 2016, 18, 4611–4615. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, F.; Xing, H.; Yang, Q.; Bao, Z.; Ren, Q. Efficient synthesis of cyclic carbonates from atmospheric CO2 using a positive charge delocalized ionic liquid catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2841–2846. [Google Scholar] [CrossRef]
- He, Q.; O′Brien, J.W.; Kitselman, K.A.; Tompkins, L.E.; Curtis, G.C.; Kerton, F.M. Synthesis of cyclic carbonates from CO2 and epoxides using ionic liquids and related catalysts including choline chloride–metal halide mixtures. Catal. Sci. Technol. 2014, 4, 1513–1528. [Google Scholar] [CrossRef]
- Kim, D.; Moon, Y.; Ji, D.; Kim, H.; Cho, D. Metal-Containing Ionic Liquids as Synergistic Catalysts for the Cycloaddition CO2: A Density Functional Theory and Response Surface Methodology Corroborated Study. ACS Sustain. Chem. Eng. 2016, 4, 4591–4600. [Google Scholar] [CrossRef]
- Kumar, P.; Varyani, M.; Khatri, P.K.; Paul, S.; Jain, S.L. Post combustion capture and conversion of carbon dioxide using histidine derived ionic liquid at ambient conditions. J. Ind. Eng. Chem. 2017, 49, 152–157. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) Complex Catalyzed Cyclic Carbonate Synthesis at Ambient Temperature and Pressure. ACS Catal. 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- Librando, I.L.; Mahmoud, A.G.; Carabineiro, S.A.C.; Silva, M.F.C.G.; Geraldes, C.F.G.C.; Pombeiro, A.J.L. Synthesis of a novel series of Cu(I) complexes bearing alkylated% 1,3,5-Triaza-7-phosphaadamantane as homogenerous and carbon-supported catalysts for the synthesis of 1- and 2-substituted-1,2,3-triazoles. Nanomaterials 2021, 11, 2702. [Google Scholar] [CrossRef]
- Lang, X.D.; Yu, Y.C.; He, L.N. Zn-salen complexes with multiple hydrogen bonding donor and protic ammonium bromide: Bifunctional catalysts for CO2 fixation with epoxides at atmospheric pressure. J. Mol. Catal. A-Chem. 2016, 420, 208–215. [Google Scholar] [CrossRef]
- Cozzolino, M.; Rosen, T.; Goldberg, I.; Mazzeo, M.; Lamberti, M. Selective Synthesis of Cyclic Carbonate by Salalen-Aluminum Complexes and Mechanistic Studies. Sustain. Chem. Green Chem. 2017, 10, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Shirakawa, S. Potassium Iodide−Tetraethylene Glycol Complex as a Practical Catalyst for CO2 Fixation Reactions with Epoxides under Mild Conditions. ACS Sustain. Chem. Eng. 2017, 5, 2836–2840. [Google Scholar] [CrossRef]
- Song, J.; Zhang, B.; Zhang, P.; Ma, J. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides catalyzed by KI/lecithin. Catal. Today 2012, 183, 130–135. [Google Scholar] [CrossRef]
- Tomishige, K.; Yasuda, H.; Yoshida, Y.; Nurunnabi, M.; Li, B.; Kunimori, K. Catalytic performance and properties of ceria based catalysts for cyclic carbonate synthesis from glycol and carbon dioxide. Green Chem. 2004, 6, 206–214. [Google Scholar] [CrossRef]
- Mousavi, B.; Chaemchuen, S.; Moosavi, B.; Luo, Z.; Gholampour, N.; Verpoort, F. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates. R. Soc. Chem. 2016, 40, 5170–5176. [Google Scholar] [CrossRef]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef] [PubMed]
- Kuruppathparambil, R.R.; Jose, T.; Babu, R.; Hwang, G.Y.; Kathalikkattil, A.C.; Kim, D.W.; Park, D.W. A room temperature synthesizable and environmental friendly heterogeneous ZIF-67 catalyst for the solvent less and co-catalyst free synthesis of cyclic carbonates. Appl. Catal. B-Environ. 2016, 182, 562–569. [Google Scholar] [CrossRef]
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal organic framework based catalysts for CO2 conversion. Mater. Horiz. 2017, 4, 345–361. [Google Scholar]
- Bhin, K.M.; Tharun, J.; Roshan, K.R.; Kim, D.W.; Chung, Y.; Park, D.W. Catalytic performance of zeolitic imidazolate framework ZIF-95 for the solventless synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2017, 17, 112–118. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Lukoyanov, I.A.; Panchenko, V.N.; Shefer, K.I.; Mel′gunov, M.S.; Bhadra, B.N.; Jhung, S.H. Tuning the catalytic properties for cycloaddition of CO2 to propylene oxide on zeolitic-imidazolate frameworks through variation of structure and chemical composition. Mol. Catal. 2022, 529, 112530. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Lukoyanov, I.A.; Panchenko, V.N.; Bhadra, B.N.; Gerasimov, E.Y.; Jhung, S.H. Effect of MAF-6 crystal size on its physicochemical and catalytic properties in the cycloaddition of CO2 to propylene oxide. Catalysts 2021, 11, 1061. [Google Scholar] [CrossRef]
- Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S.W.; Harvey, A. Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides. J. Environ. Chem. Eng. 2021, 9, 105113. [Google Scholar] [CrossRef]
- Yano, T.; Matsui, H.; Koike, T.; Ishiguro, H.; Fujihara, H.; Yoshihara, M.; Maeshima, T. Magnesium oxide-catalysed reaction of carbon dioxide with an epoxide with retention of stereochemistry. Chem. Commun. 1997, 12, 1129–1130. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Fujita, S.I.; Ikushima, Y.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides, and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity. Appl. Catal. A Gen. 2001, 219, 259–266. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg−Al mixed oxides as highly active acid−base catalysts for cycloaddition of carbon dioxide to epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, G.; Kong, Y.; Shen, X.; Cui, S. Facile preparation of cross-linked polyimide aerogels with carboxylic functionalization for CO2 capture. Chem. Eng. J. 2017, 322, 1–9. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Krumm, M.; Pueyo, C.L.; Polarz, S. Monolithic Zinc Oxide Aerogels from Organometallic Sol-Gel Precursors. Chem. Mater. 2010, 22, 5129–5136. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P. Synthesis of Metal Oxide Nanostructures by Direct Sol−Gel Chemistry in Supercritical Fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Feinle, A.; Hüsing, N. Mixed Metal Oxide Aerogels from Tailor-Made Precursors. J. Supercrit. Fluids 2015, 106, 2–8. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H., Jr.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of Epoxides in the Sol-Gel Synthesis of Porous Iron(III) Oxide Monoliths from Fe(III) Salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Cui, H.; Zayat, M.; Levy, D. A general epoxide-assisted sol-gel route for the synthesis of metal oxide-silica composites and silicates ultrafine particles. J. Alloy. Compd. 2009, 474, 292–296. [Google Scholar] [CrossRef]
- Xiao, Z.; Ma, Z.; Wang, X.; Williams, C.T.; Liang, C. Effects of Synthetic Parameters on the Structure and Catalytic Performance of Cu-Cr Catalysts Prepared by a Non-Alkoxide Sol-Gel Route. Ind. Eng. Chem. Res. 2011, 50, 2031–2039. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, X.; Song, H.; Guo, K.; Hu, Z. Synthesis of monolithic zirconia aerogel via a nitric acid assisted epoxide addition method. RSC Adv. 2014, 4, 31666–31671. [Google Scholar] [CrossRef]
- Sádaba, I.; Ojeda, M.; Mariscal, R.; Richards, R.; López Granados, M. Preparation and Characterization of Mg-Zr Mixed Oxide Aerogels and Their Application as Aldol Condensation Catalysts. J. Chem. Phys. Phys. Chem. 2012, 13, 3282–3292. [Google Scholar] [CrossRef]
- Kongpanna, P.; Pavarajarn, V.; Gani, R.; Assabumrungrat, S. Techno-economic evaluation of different CO2-based processes for dimethyl carbonate production. Chem. Eng. Res. Des. 2015, 93, 496–510. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Z.; Hu, S.; Wu, T.; Jiang, T.; Han, B. MOF-5/n-Bu4NBr: An Efficient Catalyst System for the Synthesis of Cyclic Carbonates from Epoxide and CO2 under Mild Conditions. Green Chem. 2009, 11, 1031–1036. [Google Scholar] [CrossRef]
- Zhu, M.; Srinivas, D.; Bhogeswararao, S.; Ratnasamy, P.; Carreon, M.A. Catalytic Activity of ZIF-8 in the Synthesis of Styrene Carbonate from CO2 and Styrene Oxide. Catal. Commun. 2013, 32, 36–40. [Google Scholar] [CrossRef]
- Lin, Y.F.; Huang, K.W.; Ko, B.T.; Lin, K.Y.A. Bifunctioanl ZIF-78 Heterogeneous Catalyst with dual Lewis Acidic and Basic Sites for Carbon Dioxide Fixation via Cyclic Carbonate Synthesis. J. CO2 Util. 2017, 22, 178–183. [Google Scholar] [CrossRef]

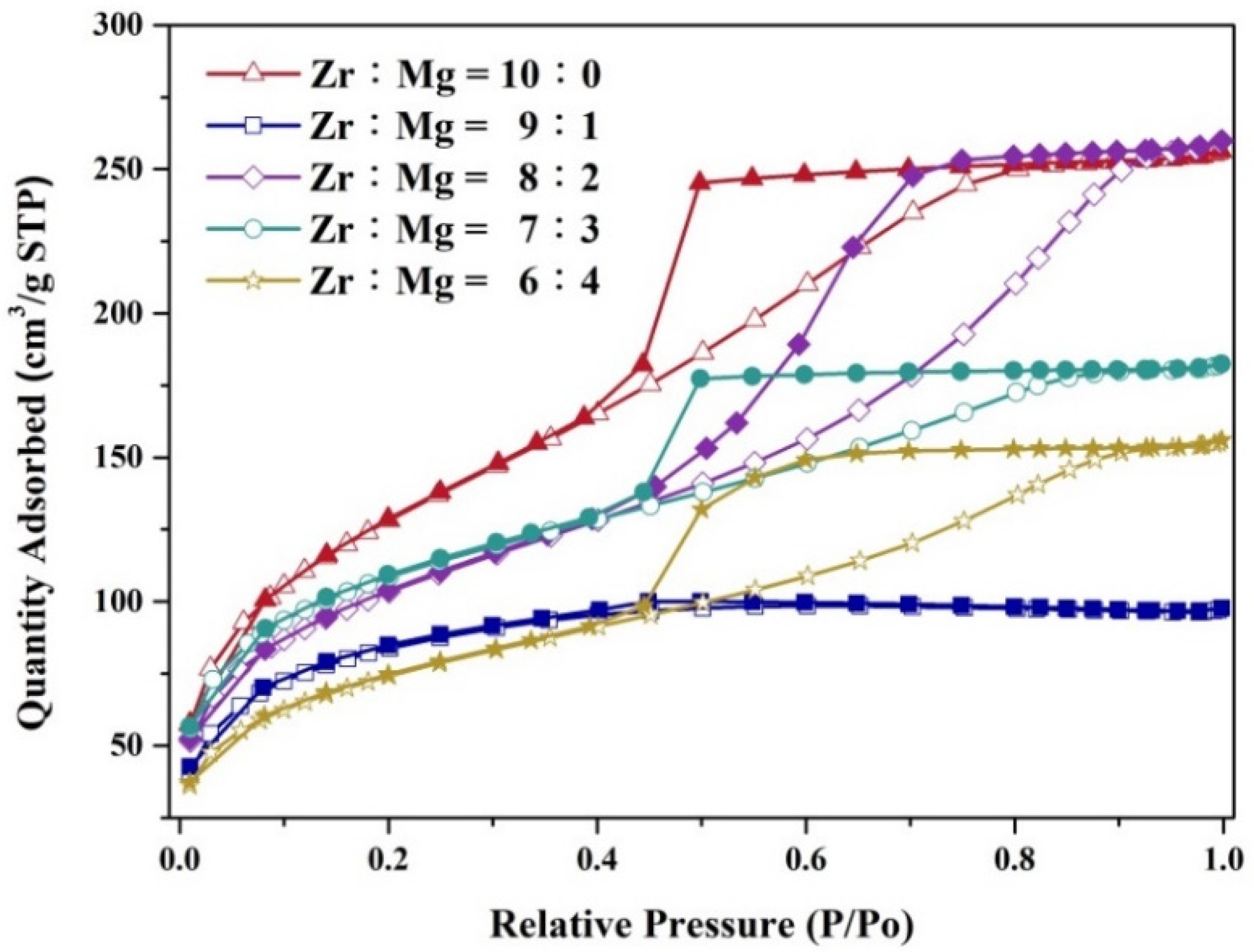
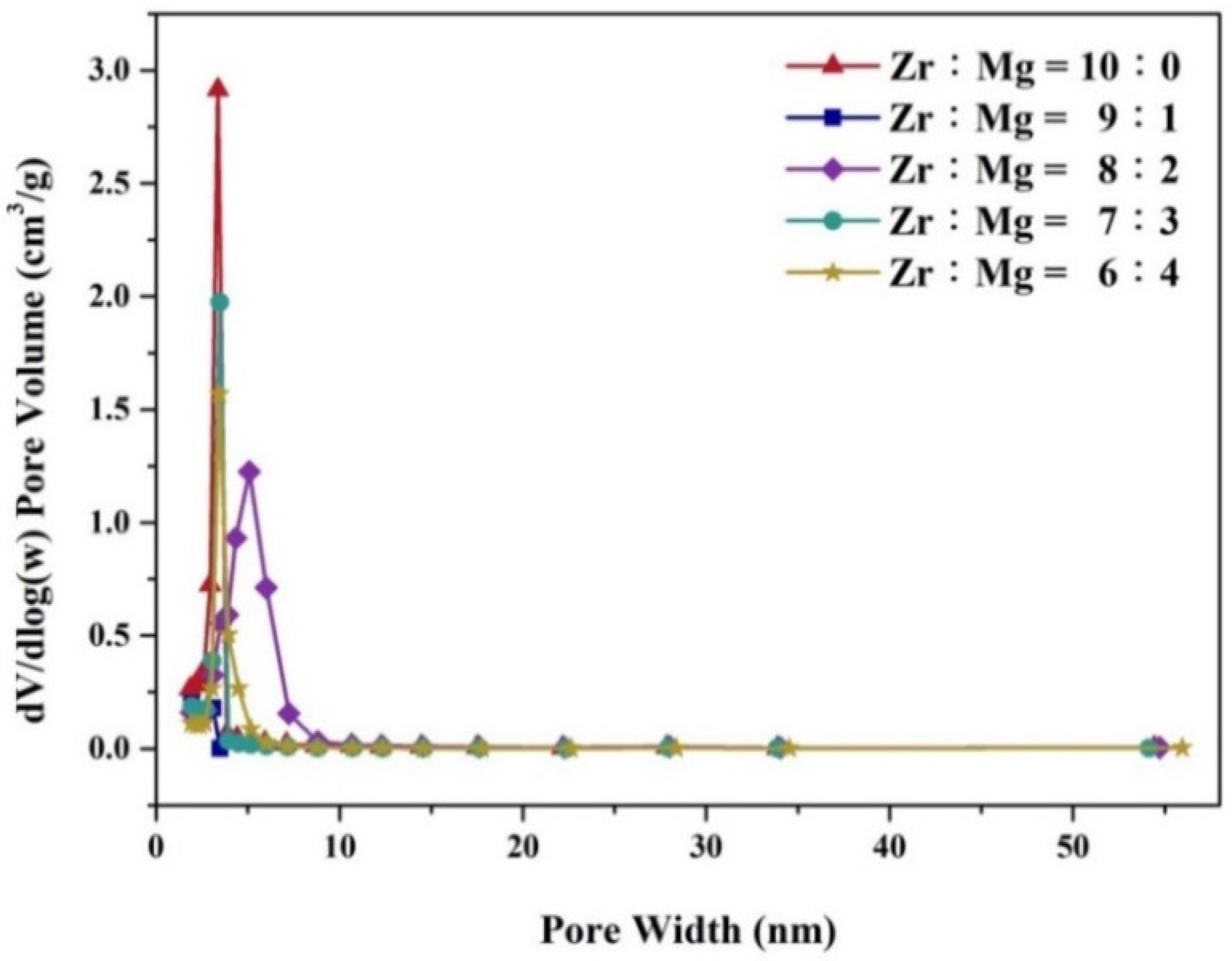
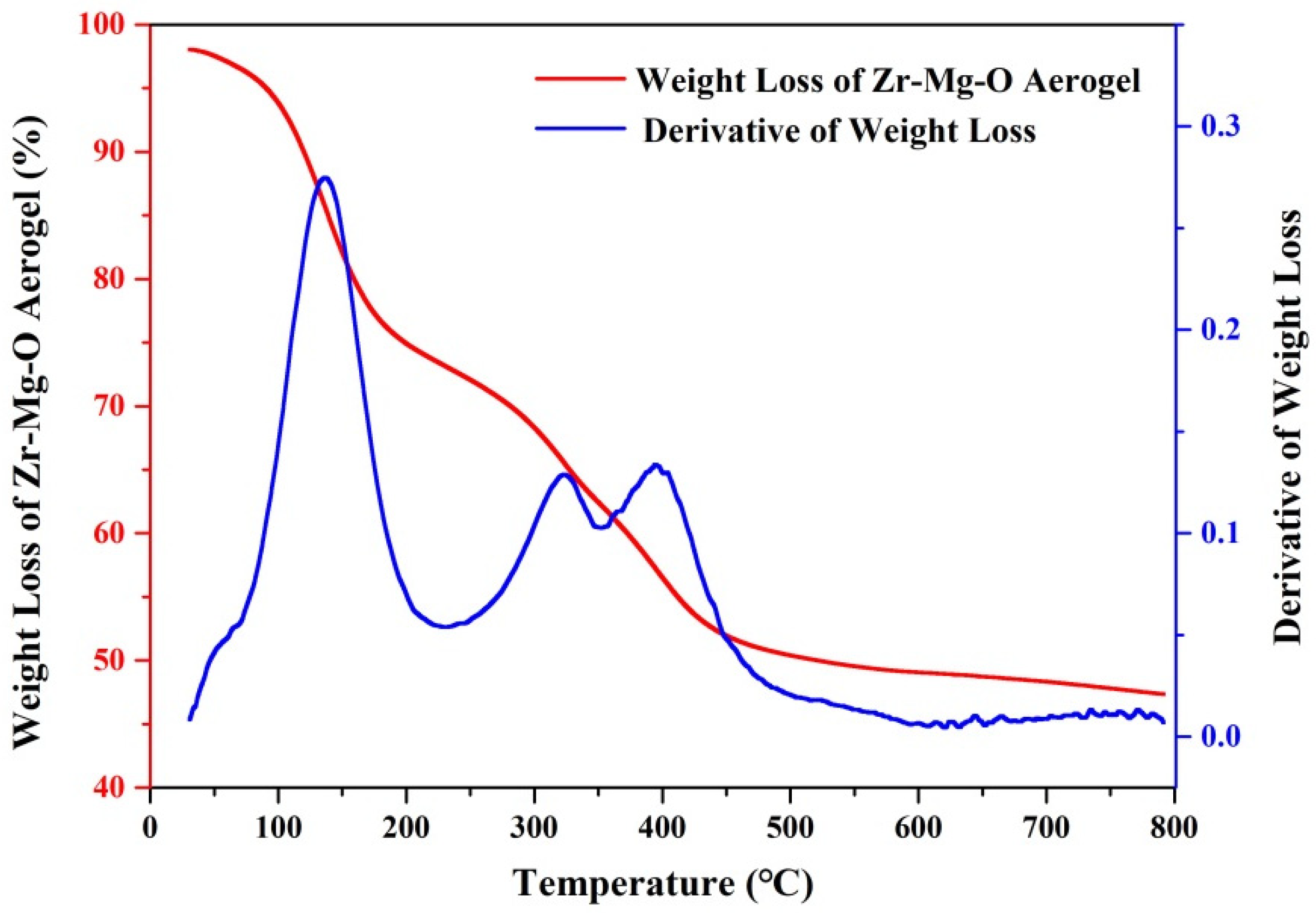
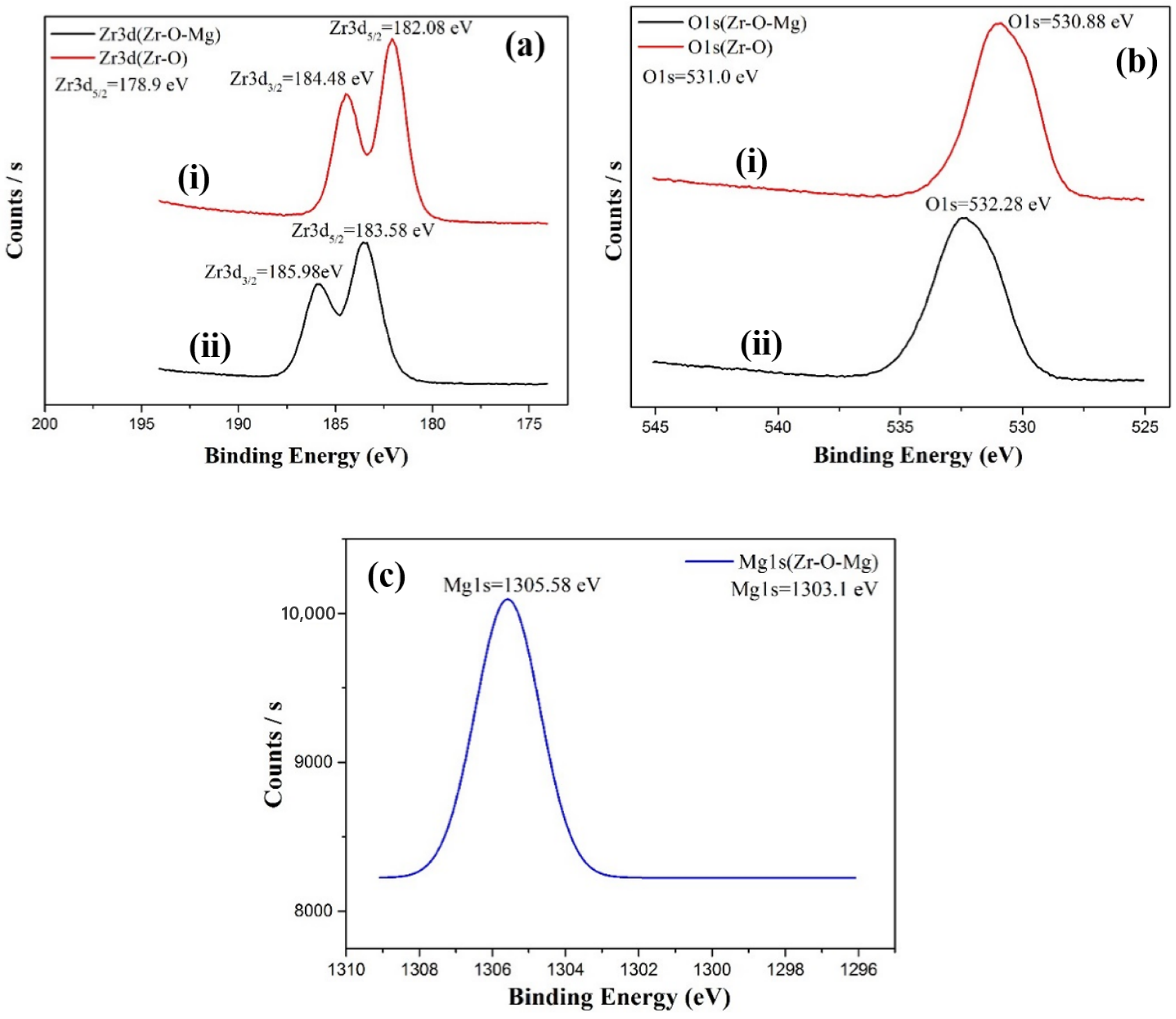
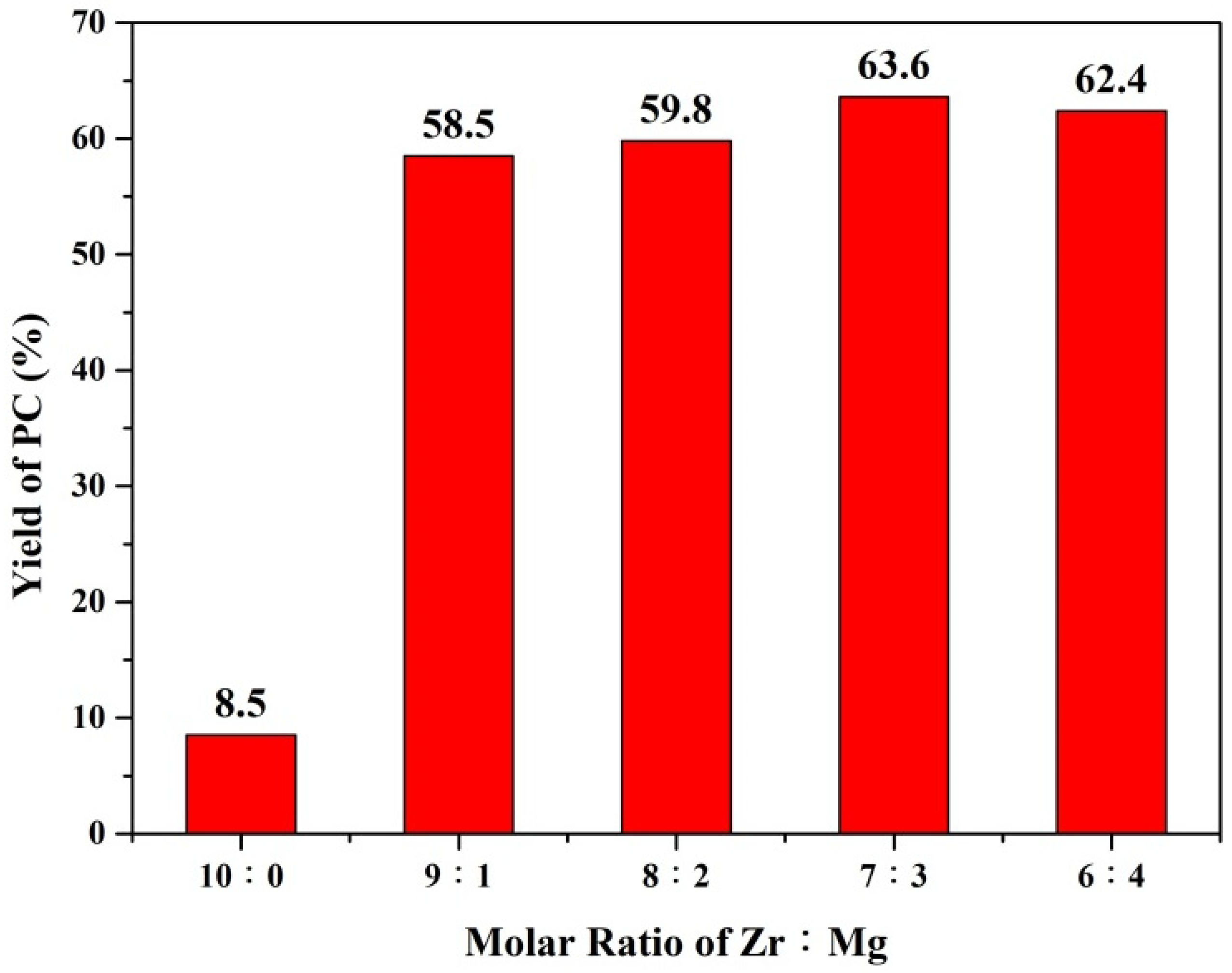
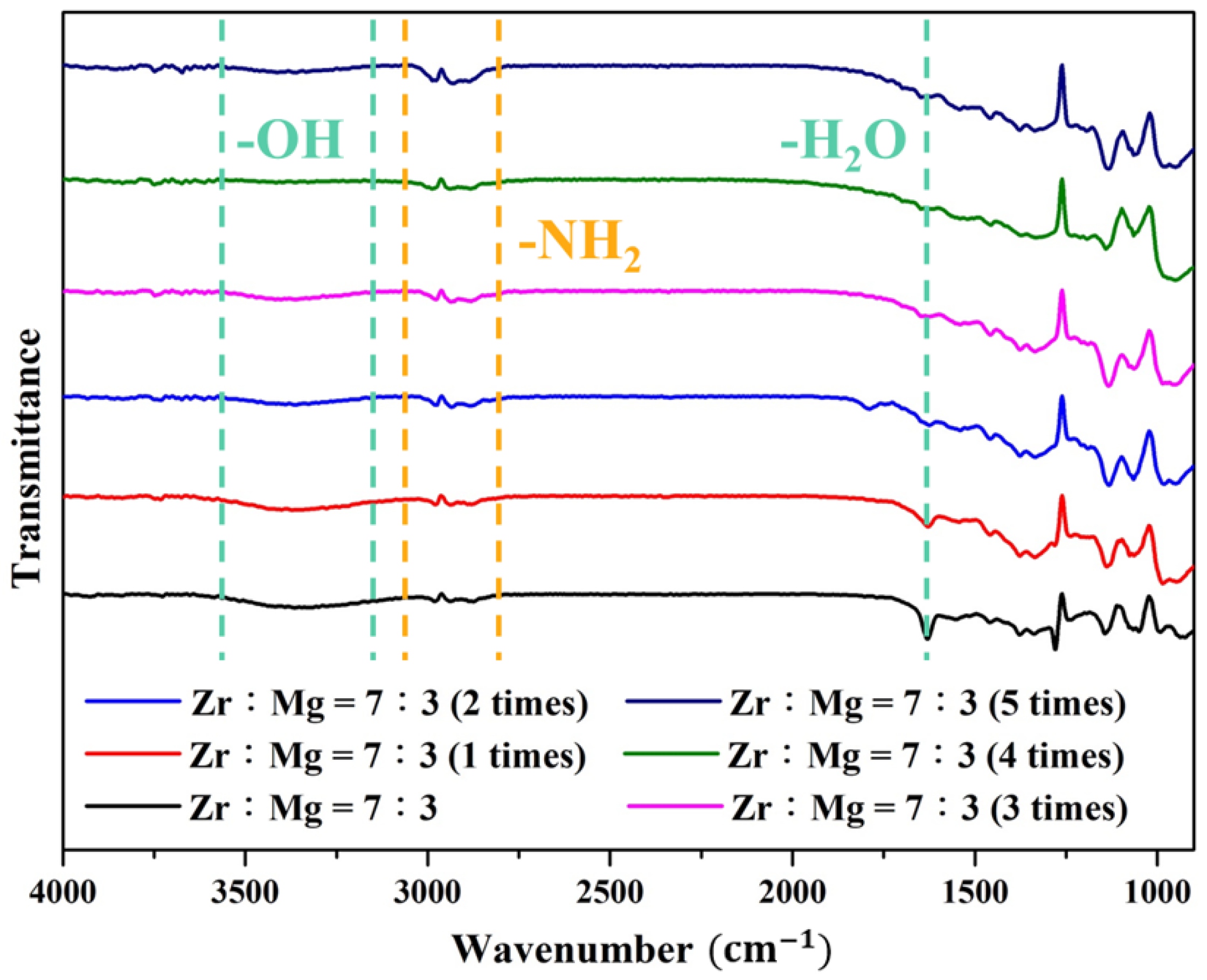
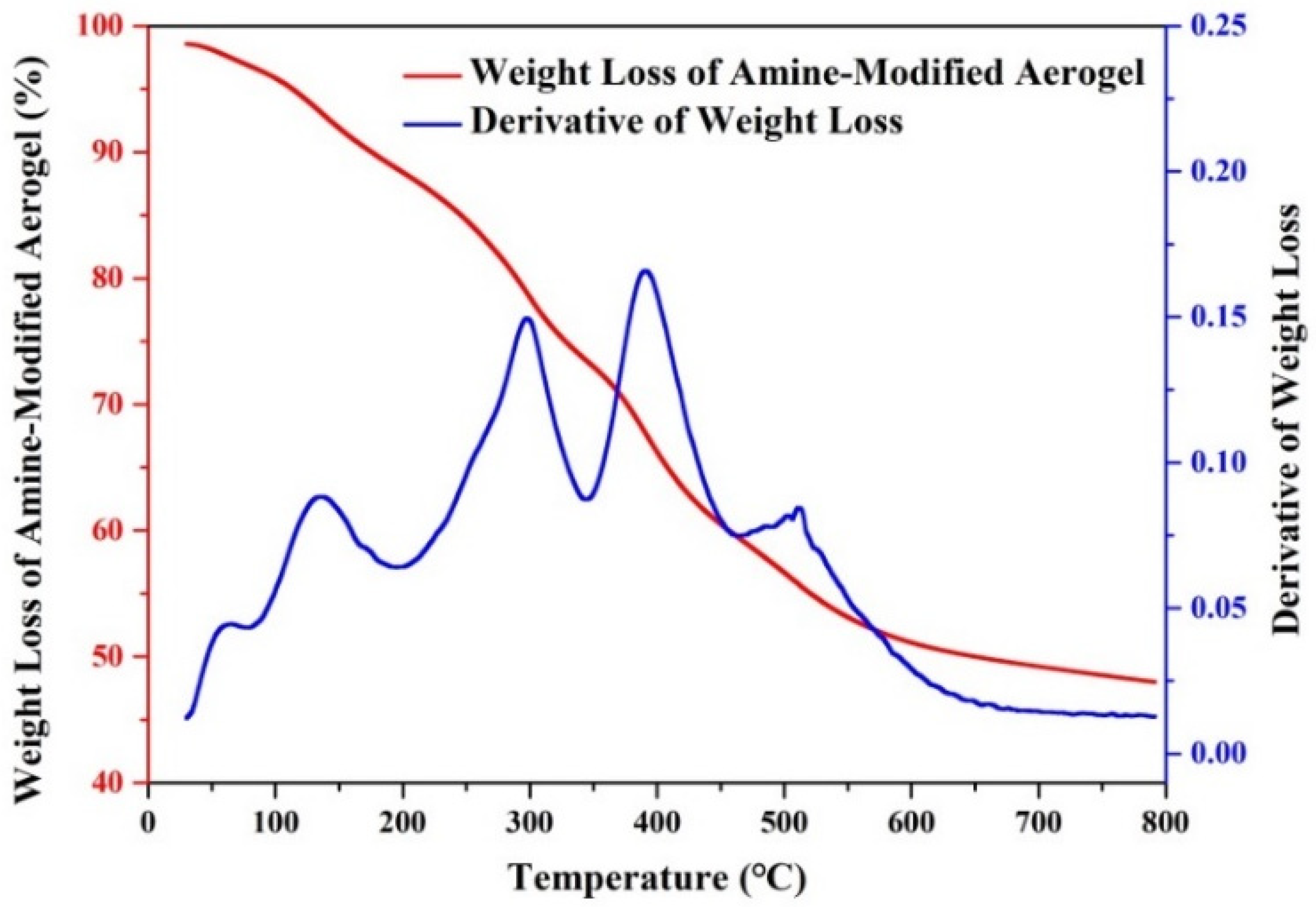

| Molar Ratio of Zr:Mg | Average Pore Diameter (nm) | BET S.S.A (m2/g) |
|---|---|---|
| 10:0 | 3.41 | 464.48 |
| 9:1 | 2.12 | 283.04 |
| 8:2 | 4.4 | 364.45 |
| 7:3 | 3.04 | 370.67 |
| 6:4 | 3.68 | 260.77 |
| Molar Ratio of Zr:Mg | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| 10:0 | 91.0 | 9.4 | 8.5 |
| 9:1 | 92.9 | 62.9 | 58.5 |
| 8:2 | 94.7 | 63.2 | 59.8 |
| 7:3 | 97.6 | 65.2 | 63.6 |
| 6:4 | 98.4 | 63.4 | 62.4 |
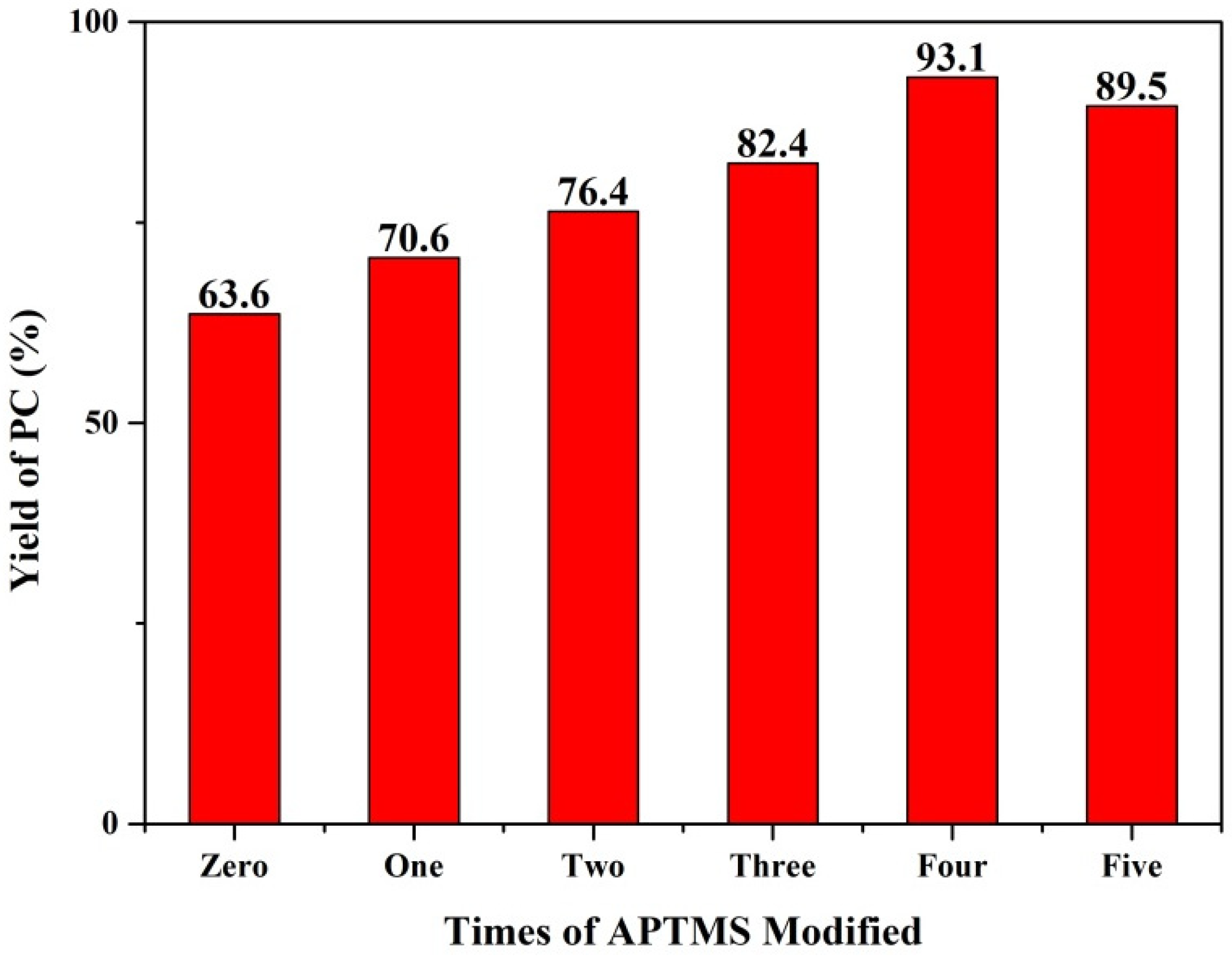
| Times of APTMS Modified | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| Zero | 97.6 | 65.2 | 63.6 |
| One | 98.3 | 71.8 | 70.6 |
| Two | 97.9 | 78.1 | 76.4 |
| Three | >99.9 | 82.4 | 82.4 |
| Four | 97.7 | 95.2 | 93.1 |
| Five | 98.3 | 91 | 89.5 |
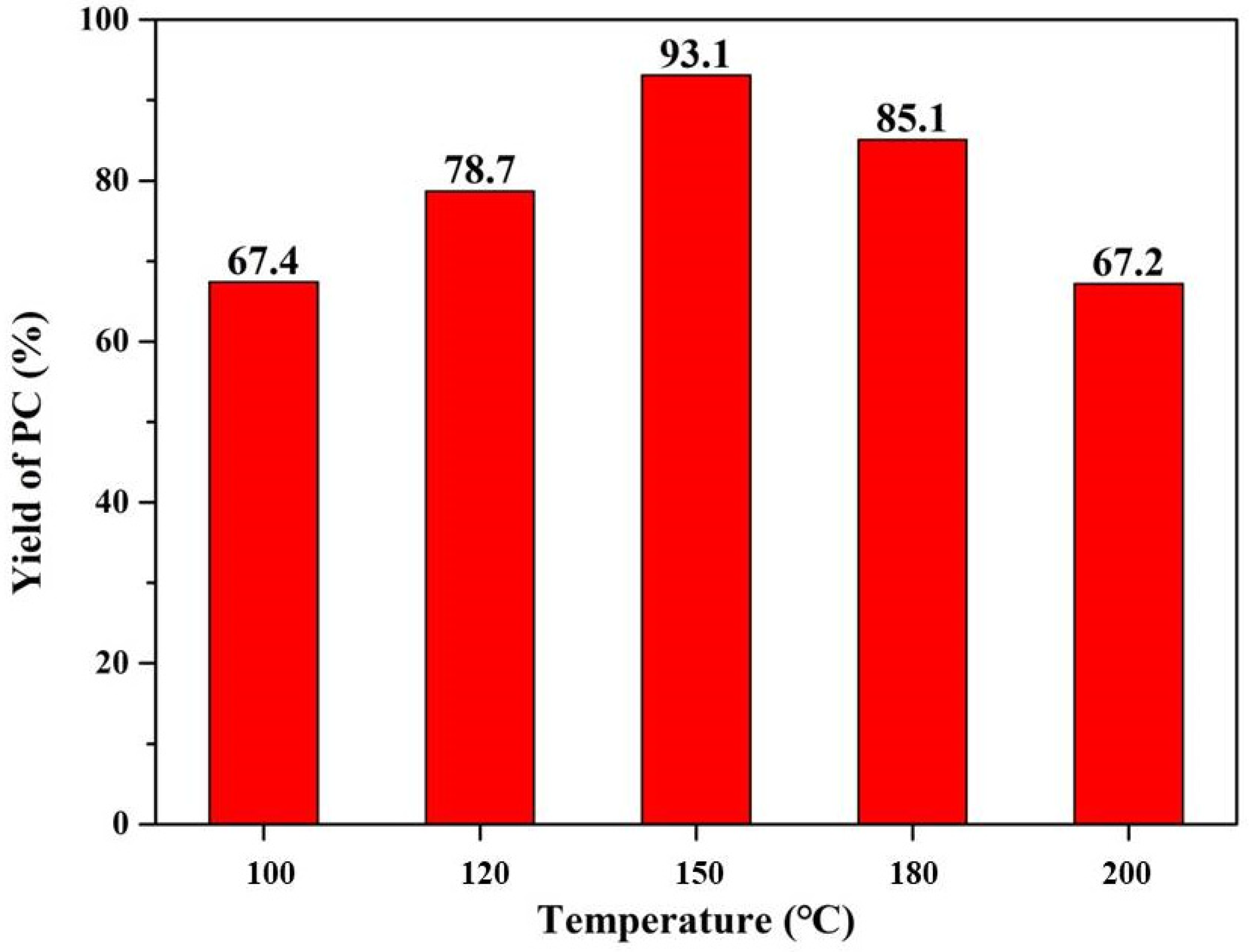
| Temperature (°C) | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| 100 | 90.9 | 74.1 | 67.4 |
| 120 | 98.9 | 79.6 | 78.7 |
| 150 | 97.9 | 95.2 | 93.1 |
| 180 | >99.9 | 85.1 | 85.1 |
| 200 | >99.9 | 67.2 | 67.2 |
| Reaction Time (h) | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| 6 | 99.4 | 84.4 | 83.9 |
| 9 | 98.5 | 85.4 | 84.1 |
| 15 | 97.7 | 95.2 | 93.1 |
| 24 | >99.9 | 40.6 | 40.6 |
| Catalyst | PO (mmol) | Cat. Amount (g) | Temp. (°C) | PCO2 (kg/cm2) | Time (h) | Co-Catalyst/Solvent | PC Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MgO | 25 | 0.5 | 150 | 80 | 4 | DMF | 32.1 | [44] |
| ZrO2 | 25 | 0.5 | 150 | 80 | 4 | DMF | 10.9 | [44] |
| Al2O3 | 25 | 0.5 | 150 | 80 | 4 | DMF | 6.6 | [44] |
| Al-Mg-O | 4 | 0.5 | 100 | 5 | 24 | DMF | 88 | [45] |
| MOF-5 | 20 | 0.1 | 50 | 60 | 4 | - | 0.1 | [59] |
| MOF-5 | 20 | 0.1 | 50 | 60 | 4 | n-Bu4NBr | 97.6 | [59] |
| ZIF-8 | 85 | 0.1 | 120 | 10 | 12 | - | 52.5 | [60] |
| ZIF-67 | 25 | 0.03 | 120 | 7 | 4 | - | 95 | [37] |
| ZIF-78 | 20 | 0.1 | 150 | 10 | 15 | - | 50.7 | [61] |
| Zr-Mg-O Aerogel | 20 | 0.2 | 150 | 10 | 15 | - | 63.6 | This work |
| APTMS-modified Al-Mg-O Aerogel | 20 | 0.2 | 150 | 10 | 15 | - | 93.1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-F.; Lai, Y.-R.; Sung, H.-L.; Chung, T.-W.; Lin, K.-Y.A. Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions. Nanomaterials 2022, 12, 3442. https://doi.org/10.3390/nano12193442
Lin Y-F, Lai Y-R, Sung H-L, Chung T-W, Lin K-YA. Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions. Nanomaterials. 2022; 12(19):3442. https://doi.org/10.3390/nano12193442
Chicago/Turabian StyleLin, Yi-Feng, Yu-Rou Lai, Hsiang-Ling Sung, Tsair-Wang Chung, and Kun-Yi Andrew Lin. 2022. "Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions" Nanomaterials 12, no. 19: 3442. https://doi.org/10.3390/nano12193442
APA StyleLin, Y.-F., Lai, Y.-R., Sung, H.-L., Chung, T.-W., & Lin, K.-Y. A. (2022). Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions. Nanomaterials, 12(19), 3442. https://doi.org/10.3390/nano12193442



_Lin.png)

