Influence of Stress on Electronic and Optical Properties of Rocksalt and Wurtzite MgO–ZnO Nanocomposites with Varying Concentrations of Magnesium and Zinc
Abstract
1. Introduction
2. Computational Methods and Models
2.1. Methodology
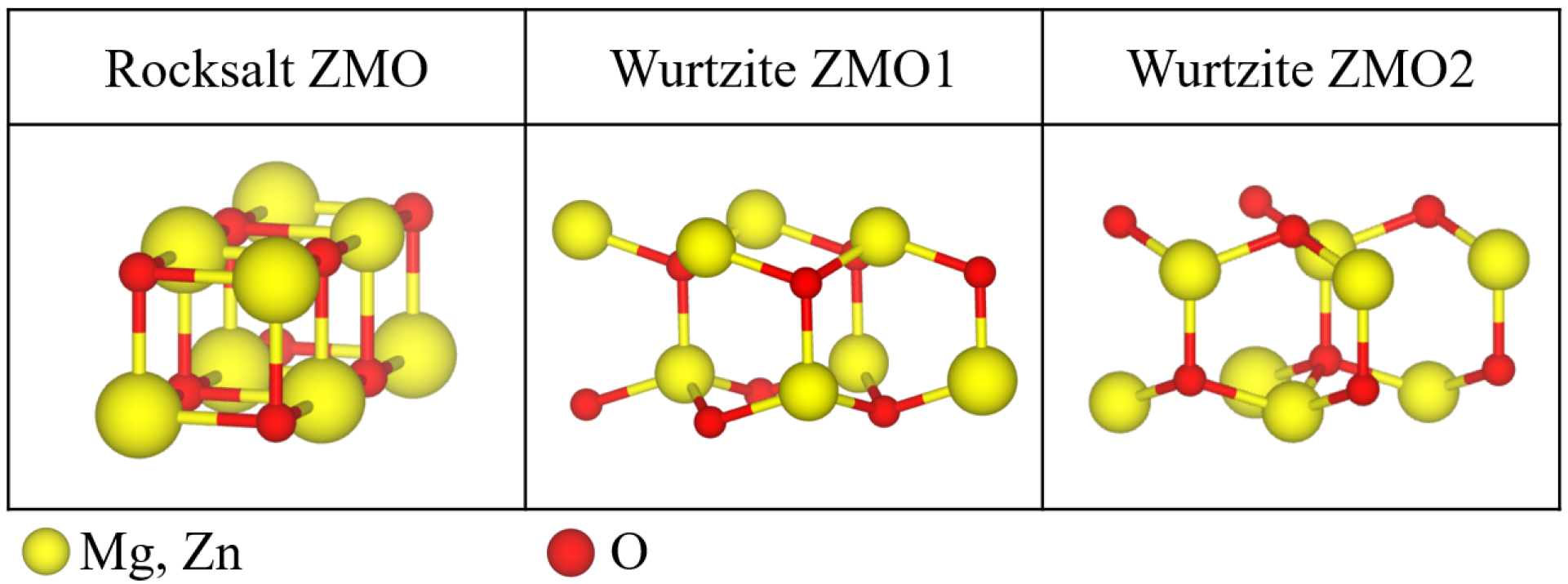
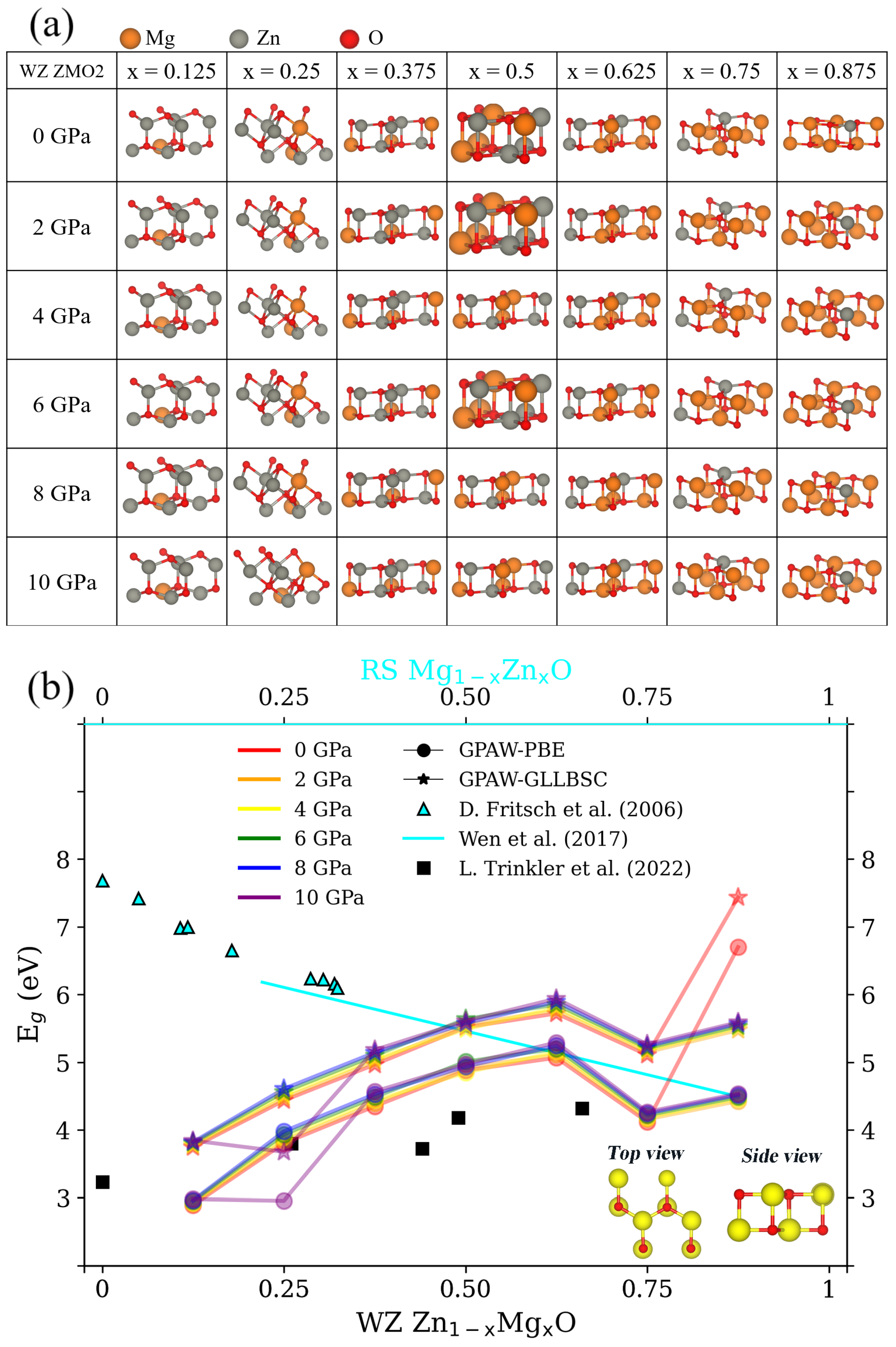
2.2. ZMO Models
3. Results and Discussion
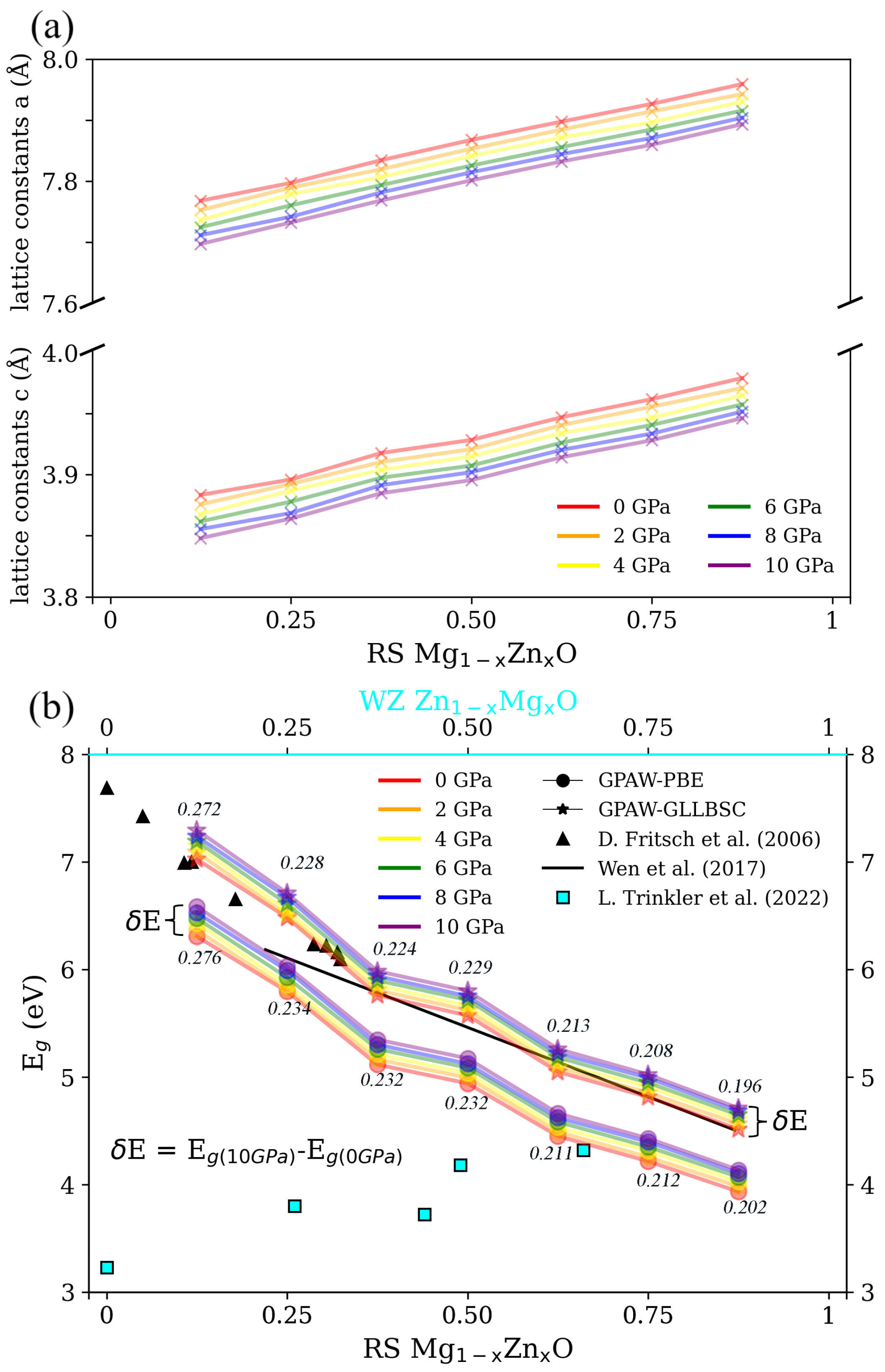
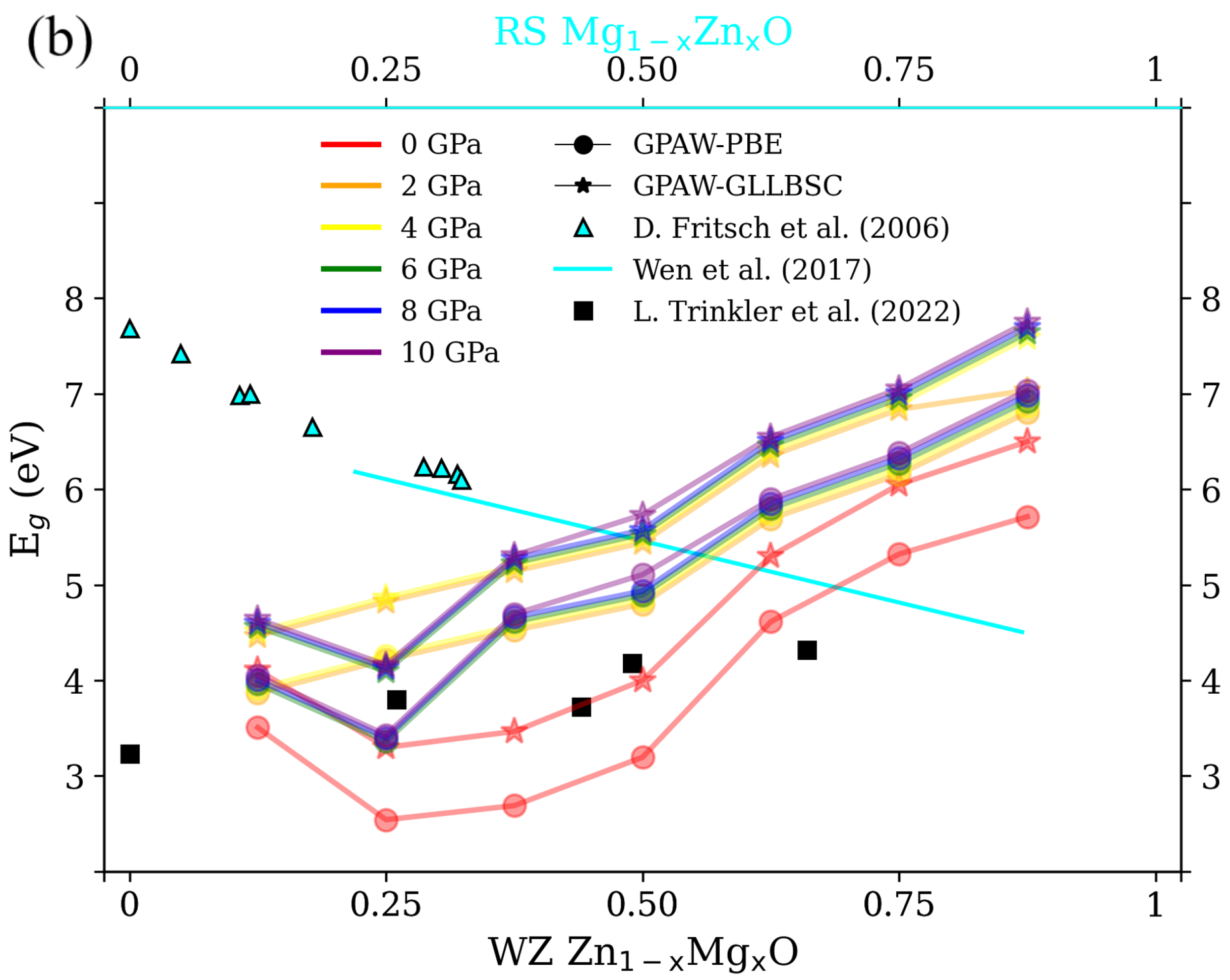
3.1. Structural Properties and for RS MgZnO
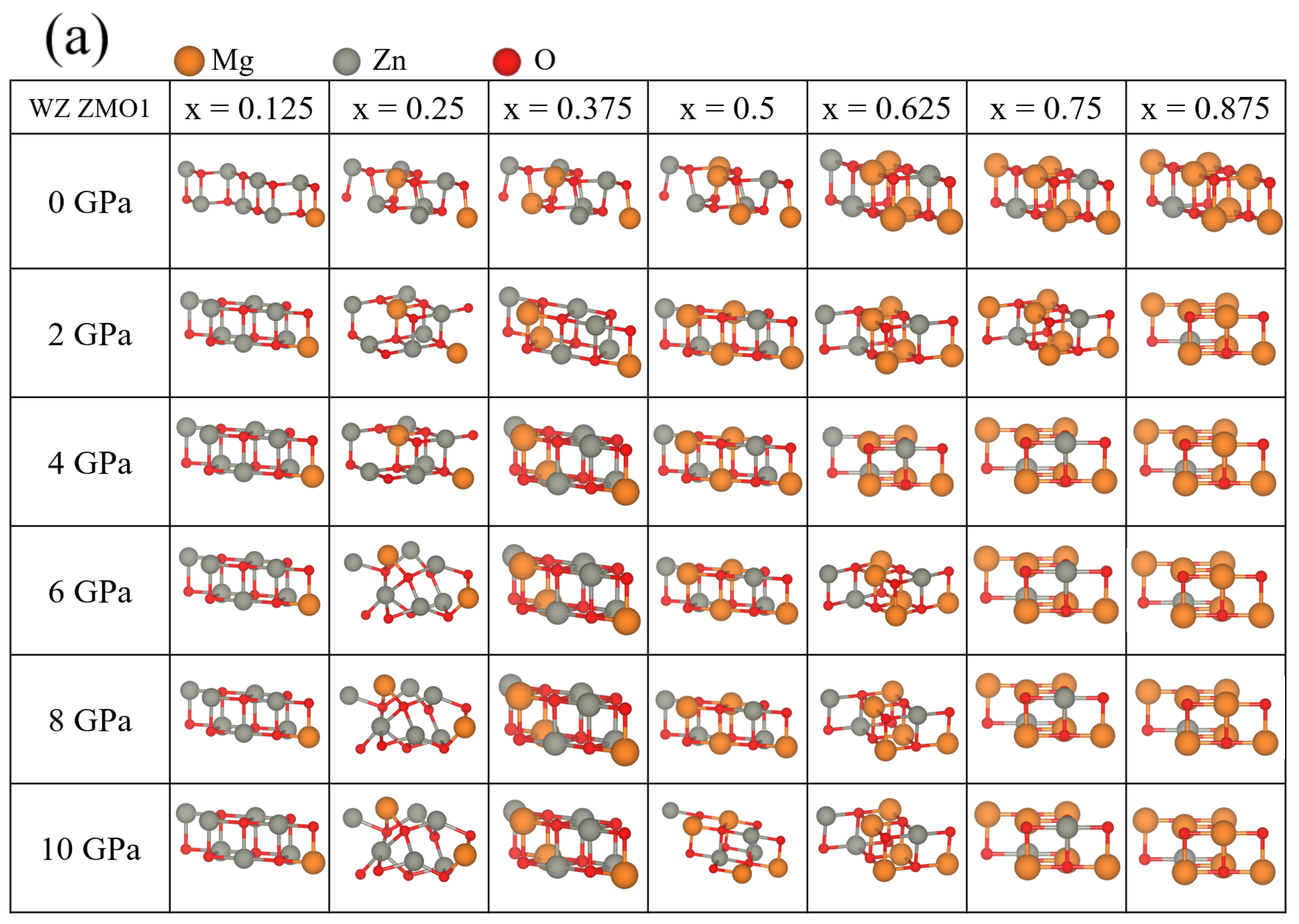
3.2. Structural Properties and for WZ ZnMgO
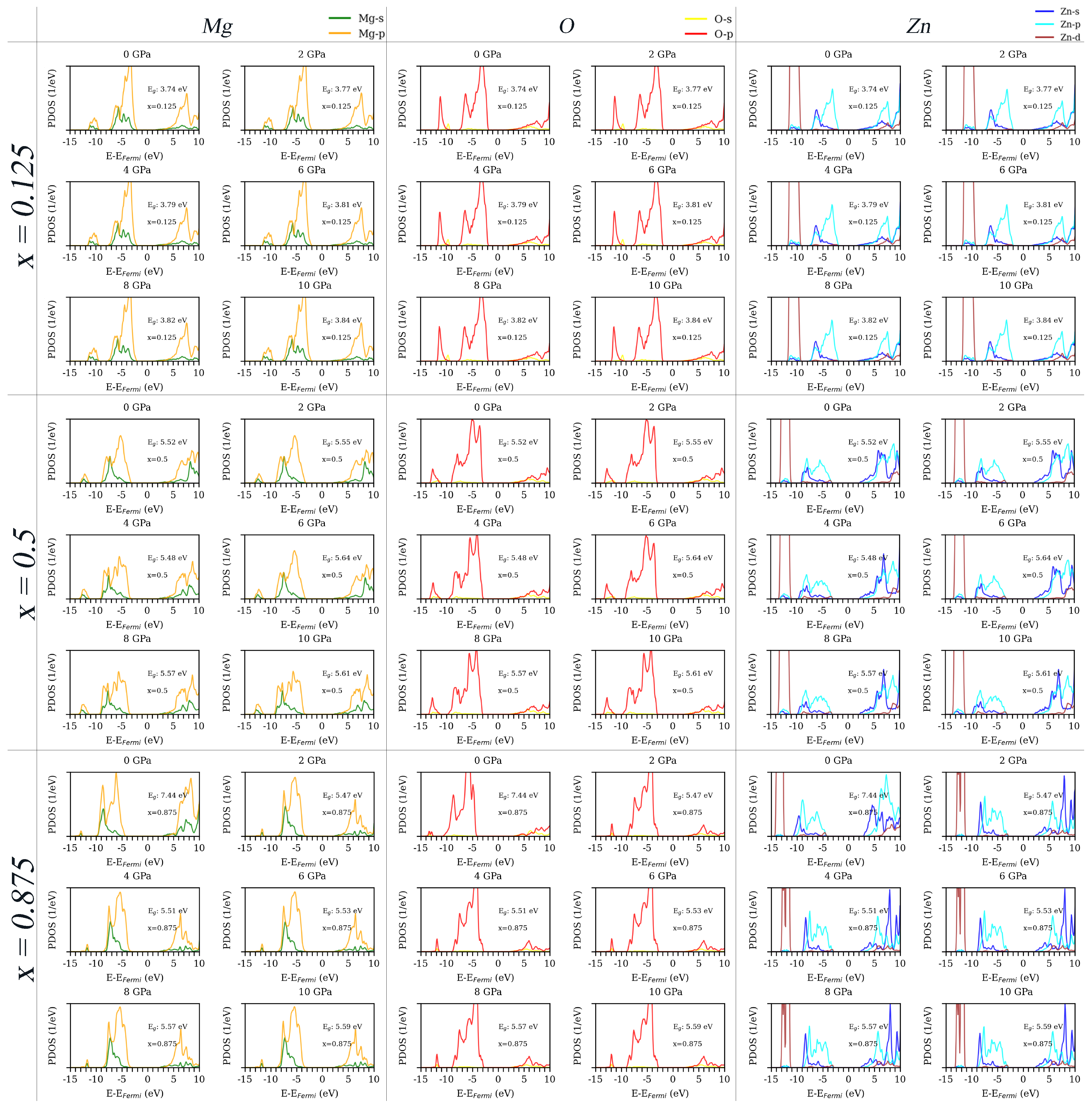
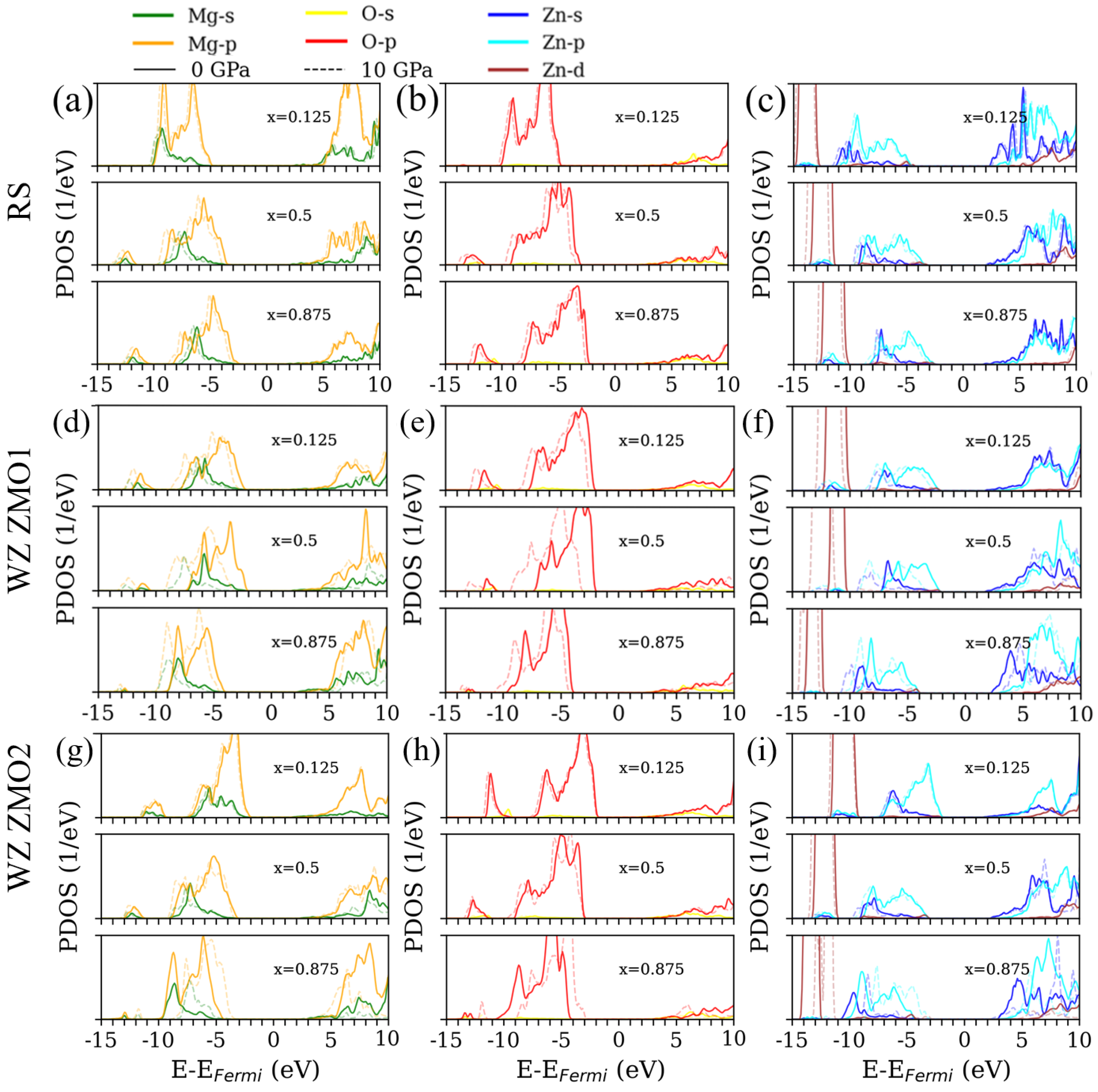
3.3. Density of States
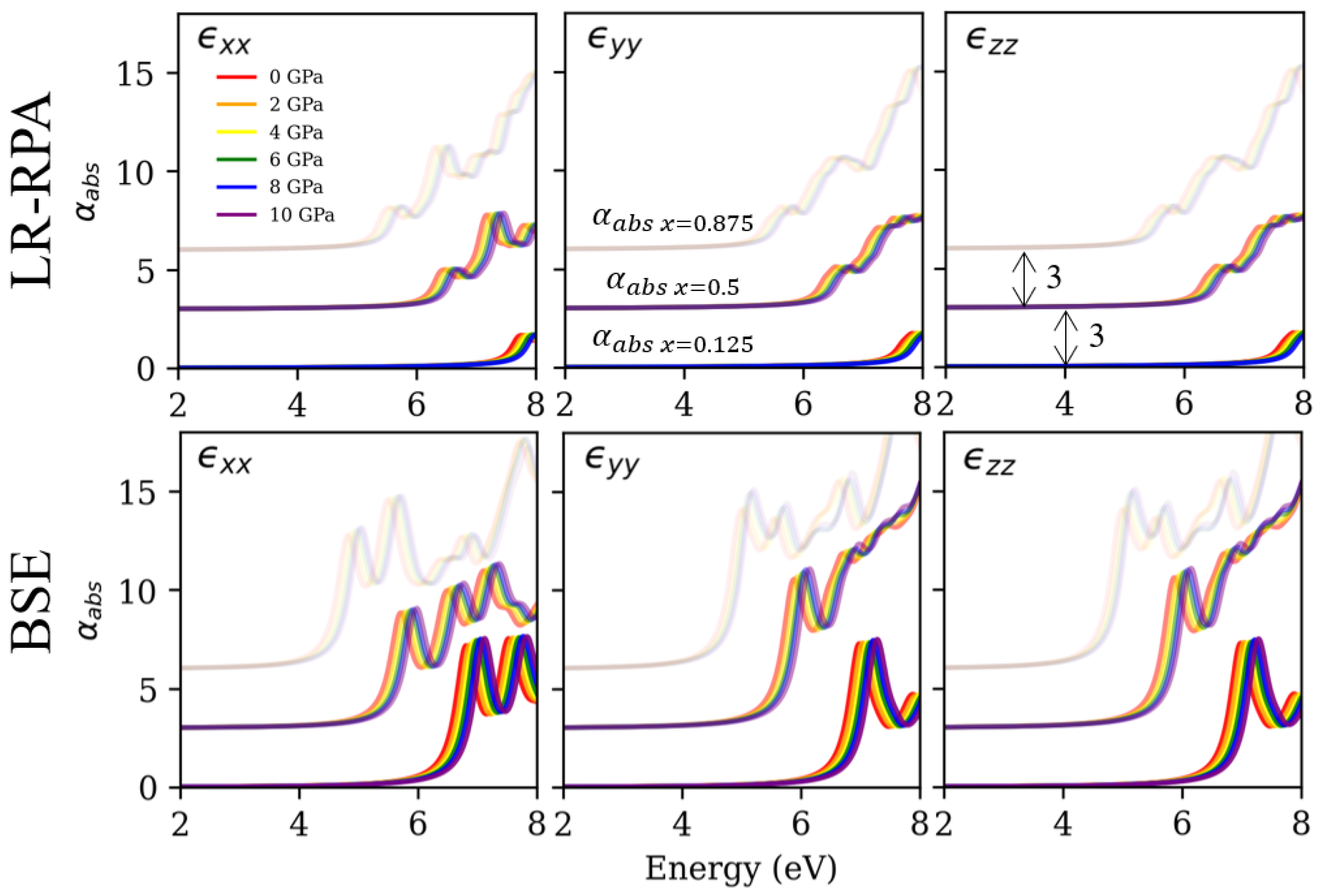
3.4. Absorption Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Band Gap Energy (Eg) for Rocksalt Mg1−xZnxO and Wurtzite Zn1−xMgxO
| x | 0 GPa | 2 GPa | 4 GPa | 6 GPa | 8 GPa | 10 GPa |
|---|---|---|---|---|---|---|
| PBE, GLLBSC (eV) | ||||||
| 0.125 | 6.31, 7.02 | 6.36, 7.09 | 6.43, 7.15 | 6.47, 7.19 | 6.53, 7.24 | 6.58, 7.30 |
| 0.25 | 5.79, 6.48 | 5.82, 6.50 | 5.86, 6.54 | 5.92, 6.61 | 5.99, 6.67 | 6.03, 6.71 |
| 0.375 | 5.11, 5.76 | 5.16, 5.81 | 5.21, 5.85 | 5.26, 5.90 | 5.30, 5.94 | 5.35, 5.98 |
| 0.5 | 4.94, 5.57 | 4.99, 5.62 | 5.03, 5.66 | 5.09, 5.71 | 5.12, 5.75 | 5.17, 5.80 |
| 0.625 | 4.45, 5.05 | 4.49, 5.09 | 4.53, 5.13 | 4.58, 5.18 | 4.62, 5.22 | 4.66, 5.26 |
| 0.75 | 4.22, 4.81 | 4.25, 4.85 | 4.31, 4.91 | 4.35, 4.95 | 4.40, 4.99 | 4.43, 5.02 |
| 0.875 | 3.93, 4.51 | 3.98, 4.56 | 4.02, 4.60 | 4.07, 4.65 | 4.10, 4.68 | 4.13, 4.71 |
| x | 0 GPa | 2 GPa | 4 GPa | 6 GPa | 8 GPa | 10 GPa |
|---|---|---|---|---|---|---|
| PBE, GLLBSC (eV) | ||||||
| 0.125 | 3.51, 4.11 | 3.87, 4.46 | 3.92, 4.51 | 3.96, 4.56 | 4.00, 4.60 | 4.05, 4.64 |
| 0.25 | 2.54, 3.30 | 4.21, 4.82 | 4.26, 4.87 | 3.36, 4.09 | 3.40, 4.13 | 3.43, 4.16 |
| 0.375 | 2.69, 3.46 | 4.52, 5.14 | 4.57, 5.19 | 4.61, 5.23 | 4.66, 5.28 | 4.69, 5.31 |
| 0.5 | 3.19, 4.00 | 4.80, 5.44 | 4.85, 5.49 | 4.89, 5.53 | 4.94, 5.57 | 5.11, 5.73 |
| 0.625 | 4.61, 5.30 | 5.69, 6.35 | 5.75, 6.40 | 5.80, 6.46 | 5.85, 6.50 | 5.90, 6.55 |
| 0.75 | 5.32, 6.05 | 6.16, 6.84 | 6.21, 6.89 | 6.27, 6.95 | 6.33, 7.00 | 6.38, 7.05 |
| 0.875 | 5.71, 6.50 | 6.80, 7.04 | 6.86, 7.59 | 6.93, 7.64 | 6.98, 7.70 | 7.04, 7.75 |
| x | 0 GPa | 2 GPa | 4 GPa | 6 GPa | 8 GPa | 10 GPa |
|---|---|---|---|---|---|---|
| PBE, GLLBSC (eV) | ||||||
| 0.125 | 2.89, 3.74 | 2.91, 3.77 | 2.93, 3.79 | 2.95, 3.81 | 2.96, 3.82 | 2.98, 3.84 |
| 0.25 | 3.80, 4.43 | 3.84, 4.47 | 3.88, 4.51 | 3.93, 4.56 | 3.98, 4.61 | 2.96, 3.67 |
| 0.375 | 4.35, 4.97 | 4.39, 5.01 | 4.44, 5.05 | 4.48, 5.10 | 4.53, 5.14 | 4.57, 5.18 |
| 0.5 | 4.88, 5.52 | 4.92, 5.55 | 4.84, 5.48 | 5.01, 5.64 | 4.93, 5.57 | 4.97, 5.61 |
| 0.625 | 5.07, 5.72 | 5.11, 5.77 | 5.16, 5.81 | 5.20, 5.85 | 5.24, 5.90 | 5.29, 5.94 |
| 0.75 | 4.12, 5.11 | 4.16, 5.15 | 4.18, 5.18 | 4.21, 5.21 | 4.24, 5.23 | 4.27, 5.26 |
| 0.875 | 6.70, 7.44 | 4.42, 5.47 | 4.46, 5.51 | 4.49, 5.53 | 4.52, 5.57 | 4.54, 5.59 |
Appendix B. Detailed Density of States for x = 0.125, 0.5 and 0.875 with the Increasing External Pressures from 0 to 10 GPa


References
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- Trinkler, L.; Aulika, I.; Krieke, G.; Nilova, D.; Ruska, R.; Butikova, J.; Berzina, B.; Chou, M.M.C.; Chang, L.; Wen, M.C.; et al. Characterization of wurtzite Zn1−xMgxO epilayers grown on ScAlMgO4 substrate by methods of optical spectroscopy. J. Alloys Compd. 2022, 912, 165178. [Google Scholar] [CrossRef]
- Harun, K.; Salleh, N.; Bahri, D.; Yaakob, M.; Mohamad, A.A. DFT+U calculations for electronic, structural, and optical properties of ZnO wurtzite structure: A review. Results Phys. 2020, 16, 102829. [Google Scholar] [CrossRef]
- Chen, L.; Xu, C.; Zhang, X.F. DFT calculations of vibrational spectra and nonlinear optical properties for MgO nanotube clusters. J. Mol. Struct. THEOCHEM 2008, 863, 55–59. [Google Scholar] [CrossRef]
- Nourozi, B.; Aminian, A.; Fili, N.; Zangeneh, Y.; Boochani, A.; Darabi, P. The electronic and optical properties of MgO mono-layer: Based on GGA-mBJ. Results Phys. 2019, 12, 2038–2043. [Google Scholar] [CrossRef]
- Shayeganfar, F.; Beheshtiyan, J.; Neek-Amal, M.; Shahsavari, R. Electro- and opto-mutable properties of MgO nanoclusters adsorbed on mono- and double-layer graphene. Nanoscale 2017, 9, 4205–4218. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Mujasam Batoo, K.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Rad, A.S.; Ayub, K. Nonlinear optical, IR and orbital properties of Ni doped MgO nanoclusters: A DFT investigation. Comput. Theor. Chem. 2018, 1138, 39–47. [Google Scholar] [CrossRef]
- González, R.; Monge, M.; Santiuste, J.M.; Pareja, R.; Chen, Y.; Kotomin, E.; Kukla, M.; Popov, A. Photoconversion of F-type centers in thermochemically reduced MgO single crystals. Phys. Rev. B 1999, 59, 4786. [Google Scholar] [CrossRef]
- Kuzovkov, V.; Popov, A.; Kotomin, E.; Monge, M.; Gonzalez, R.; Chen, Y. Kinetics of nanocavity formation based on F-center aggregation in thermochemically reduced MgO single crystals. Phys. Rev. B 2001, 64, 064102. [Google Scholar] [CrossRef]
- Popov, A.; Monge, M.; González, R.; Chen, Y.; Kotomin, E. Dynamics of F-center annihilation in thermochemically reduced MgO single crystals. Solid State Commun. 2001, 118, 163–167. [Google Scholar] [CrossRef]
- Uklein, A.; Multian, V.; Kuz’micheva, G.; Linnik, R.; Lisnyak, V.; Popov, A.; Gayvoronsky, V.Y. Nonlinear optical response of bulk ZnO crystals with different content of intrinsic defects. Opt. Mater. 2018, 84, 738–747. [Google Scholar] [CrossRef]
- Kuang, D.; Cheng, J.; Li, X.; Li, Y.; Li, M.; Xu, F.; Xue, J.; Yu, Z. Dual-ultraviolet wavelength photodetector based on facile method fabrication of ZnO/ZnMgO core/shell nanorod arrays. J. Alloys Compd. 2021, 860, 157917. [Google Scholar] [CrossRef]
- Abed, C.; Ali, M.B.; Addad, A.; Elhouichet, H. Growth, structural and optical properties of ZnO-ZnMgO-MgO nanocomposites and their photocatalytic activity under sunlight irradiation. Mater. Res. Bull. 2019, 110, 230–238. [Google Scholar] [CrossRef]
- Alam, M.J.; Murkute, P.; Sushama, S.; Ghadi, H.; Paul, S.; Mondal, S.; Chakrabarti, S. Improving optical properties and controlling defect-bound states in ZnMgO thin films through ultraviolet–ozone annealing. Thin Solid Films 2020, 708, 138112. [Google Scholar] [CrossRef]
- Ren, S.; Wang, H.; Li, Y.; Li, H.; He, R.; Wu, L.; Li, W.; Zhang, J.; Wang, W.; Feng, L. Rapid thermal annealing on ZnMgO window layer for improved performance of CdTe solar cells. Sol. Energy Mater. Sol. Cells 2018, 187, 97–103. [Google Scholar] [CrossRef]
- Wen, M.; Lu, S.; Chang, L.; Chou, M.; Ploog, K. Epitaxial growth of rocksalt Zn1−xMgxO on MgO (100) substrate by molecular beam epitaxy. J. Cryst. Growth 2017, 477, 169–173. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, B.; Hu, Z.; Liu, Y.; Zhang, S.; Zeng, H. The impact of Mg content on the structural, electrical and optical properties of MgZnO alloys: A first principles study. Curr. Appl. Phys. 2015, 15, 423–428. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Chen, J.; Li, X.; Zeng, H. Rapid and High-Efficiency Laser-Alloying Formation of ZnMgO Nanocrystals. Sci. Rep. 2016, 6, 28131. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.B.; Le, N.M.; Jeong, S.H.; Lee, B.T. Tailoring of composition, band-gap, and structural phase in ZnMgO films by simply controlling growth temperature and oxygen partial pressure during sputter deposition. J. Alloys Compd. 2017, 709, 54–63. [Google Scholar] [CrossRef]
- Samanta, A.; Goswami, M.; Mahapatra, P. Multiferroicity in Mg–doped ZnO nanoparticles. Mater. Sci. Eng. B 2019, 245, 1–8. [Google Scholar] [CrossRef]
- Kutwade, V.V.; Gattu, K.P.; Dive, A.S.; Sonawane, M.E.; Tonpe, D.A.; Sharma, R. Enhanced photosensing by Mg-doped ZnO hexagonal rods via a feasible chemical route. J. Mater. Sci. Mater 2021, 32, 6475–6486. [Google Scholar] [CrossRef]
- Tian, F.; Duan, D.; Li, D.; Chen, C.; Sha, X.; Zhao, Z.; Liu, B.; Cui, T. Miscibility and ordered structures of MgO–ZnO alloys under high pressure. Sci. Rep. 2014, 4, 5759. [Google Scholar] [CrossRef]
- Yan, J.; Mortensen, J.J.; Jacobsen, K.W.; Thygesen, K.S. Linear density response function in the projector augmented wave method: Applications to solids, surfaces, and interfaces. Phys. Rev. B 2011, 83, 245122. [Google Scholar] [CrossRef]
- Hüser, F.; Olsen, T.; Thygesen, K.S. How dielectric screening in two-dimensional crystals affects the convergence of excited-state calculations: Monolayer MoS2. Phys. Rev. B 2013, 88, 245309. [Google Scholar] [CrossRef]
- Olsen, T.; Latini, S.; Rasmussen, F.; Thygesen, K.S. Simple Screened Hydrogen Model of Excitons in Two-Dimensional Materials. Phys. Rev. Lett. 2016, 116, 056401. [Google Scholar] [CrossRef]
- Aziz, K.; Ekuma, C.E. Electronic and vibrational spectroscopy of miscible MgO–ZnO ternary alloys. J. Appl. Phys. 2020, 127, 075706. [Google Scholar] [CrossRef]
- Wang, J.; Tu, Y.; Yang, L.; Tolner, H. Theoretical investigation of the electronic structure and optical properties of zinc–doped magnesium oxide. J. Comput. Electron. 2016, 15, 1521–1530. [Google Scholar] [CrossRef]
- Marvinney, C.E.; Shen, X.; McBride, J.R.; Critchlow, D.; Li, Z.; Mayo, D.C.; Mu, R.R.; Pantelides, S.T.; Haglund, R.F. Effect of Material Structure on Photoluminescence of ZnO/MgO Core-Shell Nanowires. ChemNanoMat 2018, 4, 291–300. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, H.; Wang, H.Q.; Wang, X.D.; Geng, W.; Zhan, H.; Kisslinger, K.; Zhang, L.; Xu, M.; Chen, Q.Y.; et al. Interface structures of inclined ZnO thin film on (0 1 1)-MgO substrate with bulk-like optical properties. Appl. Surf. Sci. 2020, 509, 144781. [Google Scholar] [CrossRef]
- Schleife, A.; Bechstedt, F. Ab initio description of quasiparticle band structures and optical near–edge absorption of transparent conducting oxides. J. Mater. Res. 2012, 27, 2180–2189. [Google Scholar] [CrossRef]
- Mortensen, J.J.; Hansen, L.B.; Jacobsen, K.W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 2005, 71, 035109. [Google Scholar] [CrossRef]
- Enkovaara, J.; Rostgaard, C.; Mortensen, J.J.; Chen, J.; Dułak, M.; Ferrighi, L.; Gavnholt, J.; Glinsvad, C.; Haikola, V.; Hansen, H.A.; et al. Electronic structure calculations with GPAW: A real-space implementation of the projector augmented-wave method. J. Phys. Condens. Matter 2010, 22, 253202. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.H.; Mortensen, J.J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C.; et al. The atomic simulation environment—A Python library for working with atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef]
- Bahn, S.R.; Jacobsen, K.W. An object–oriented scripting interface to a legacy electronic structure code. Comput. Sci. Eng. 2002, 4, 56–66. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kuisma, M.; Ojanen, J.; Enkovaara, J.; Rantala, T.T. Kohn-Sham potential with discontinuity for band gap materials. Phys. Rev. B 2010, 82, 115106. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef]
- Taib, M.F.M.; Mustaffa, D.T.; Hussin, N.H.; Samat, M.H.; Ali, A.M.M.; Hassan, O.H.; Yahya, M.Z.A. First principles study on Zn doped MgO using Hubbard U correction. Mater. Res. Express 2019, 6, 094012. [Google Scholar] [CrossRef]
- Eya, H.I.; Ntsoenzok, E.; Dzade, N.Y. First–Principles Investigation of the Structural, Elastic, Electronic, and Optical Properties of α- and β-SrZrS3: Implications for Photovoltaic Applications. Materials 2020, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three–dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Van der Walt, S.; Colbert, S.C.; Varoquaux, G. The numpy array: A structure for efficient numerical computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Shimada, K.; Takahashi, N.; Nakagawa, Y.; Hiramatsu, T.; Kato, H. Nonlinear characteristics of structural properties and spontaneous polarization in wurtzite MgxZn1ࢤxO: A first-principles study. Phys. Rev. B 2013, 88, 075203. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Lv, Y.; Zhu, Y. Correlation Effects on Lattice Relaxation and Electronic Structure of ZnO within the GGA+U Formalism. J. Phys. Chem. C 2013, 117, 26029–26039. [Google Scholar] [CrossRef]
- Fritsch, D.; Schmidt, H.; Grundmann, M. Pseudopotential band structures of rocksalt MgO, ZnO, and Mg1ࢤxZnxO. Appl. Phys. Lett. 2006, 88, 134104. [Google Scholar] [CrossRef]
- Drissi, N.; Gueddim, A.; Bouarissa, N. First-principles study of rocksalt MgxZn1ࢤxO: Band structure and optical spectra. Philos. Mag. 2020, 100, 1620–1635. [Google Scholar] [CrossRef]
- Calzolari, A.; Nardelli, M.B. Dielectric properties and Raman spectra of ZnO from a first principles finite-differences/finite-fields. Sci. Rep. 2013, 3, 2999. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-P.; Piskunov, S.; Trinkler, L.; Chou, M.M.-C.; Chang, L. Influence of Stress on Electronic and Optical Properties of Rocksalt and Wurtzite MgO–ZnO Nanocomposites with Varying Concentrations of Magnesium and Zinc. Nanomaterials 2022, 12, 3408. https://doi.org/10.3390/nano12193408
Lin Y-P, Piskunov S, Trinkler L, Chou MM-C, Chang L. Influence of Stress on Electronic and Optical Properties of Rocksalt and Wurtzite MgO–ZnO Nanocomposites with Varying Concentrations of Magnesium and Zinc. Nanomaterials. 2022; 12(19):3408. https://doi.org/10.3390/nano12193408
Chicago/Turabian StyleLin, Yin-Pai, Sergei Piskunov, Laima Trinkler, Mitch Ming-Chi Chou, and Liuwen Chang. 2022. "Influence of Stress on Electronic and Optical Properties of Rocksalt and Wurtzite MgO–ZnO Nanocomposites with Varying Concentrations of Magnesium and Zinc" Nanomaterials 12, no. 19: 3408. https://doi.org/10.3390/nano12193408
APA StyleLin, Y.-P., Piskunov, S., Trinkler, L., Chou, M. M.-C., & Chang, L. (2022). Influence of Stress on Electronic and Optical Properties of Rocksalt and Wurtzite MgO–ZnO Nanocomposites with Varying Concentrations of Magnesium and Zinc. Nanomaterials, 12(19), 3408. https://doi.org/10.3390/nano12193408






