Biosynthesis of ZnONP Using Chamaecostus cuspidatus and Their Evolution of Anticancer Property in MCF-7 and A549 Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material and Preparation of Leaf Extracts
2.2. Green Synthesis of ZnONP Using C. cuspidatus
2.3. Characterization of ZnONP
2.4. Antioxidant Activity of ZnONP
2.5. Cell Culture
2.6. MTT Assay Determination
2.7. Analytical Statistics
3. Results
3.1. Visual Observation of ZnONP Synthesized by C. cuspidatus
3.2. Characterization of ZnONP
3.2.1. UV Spectrophotometry
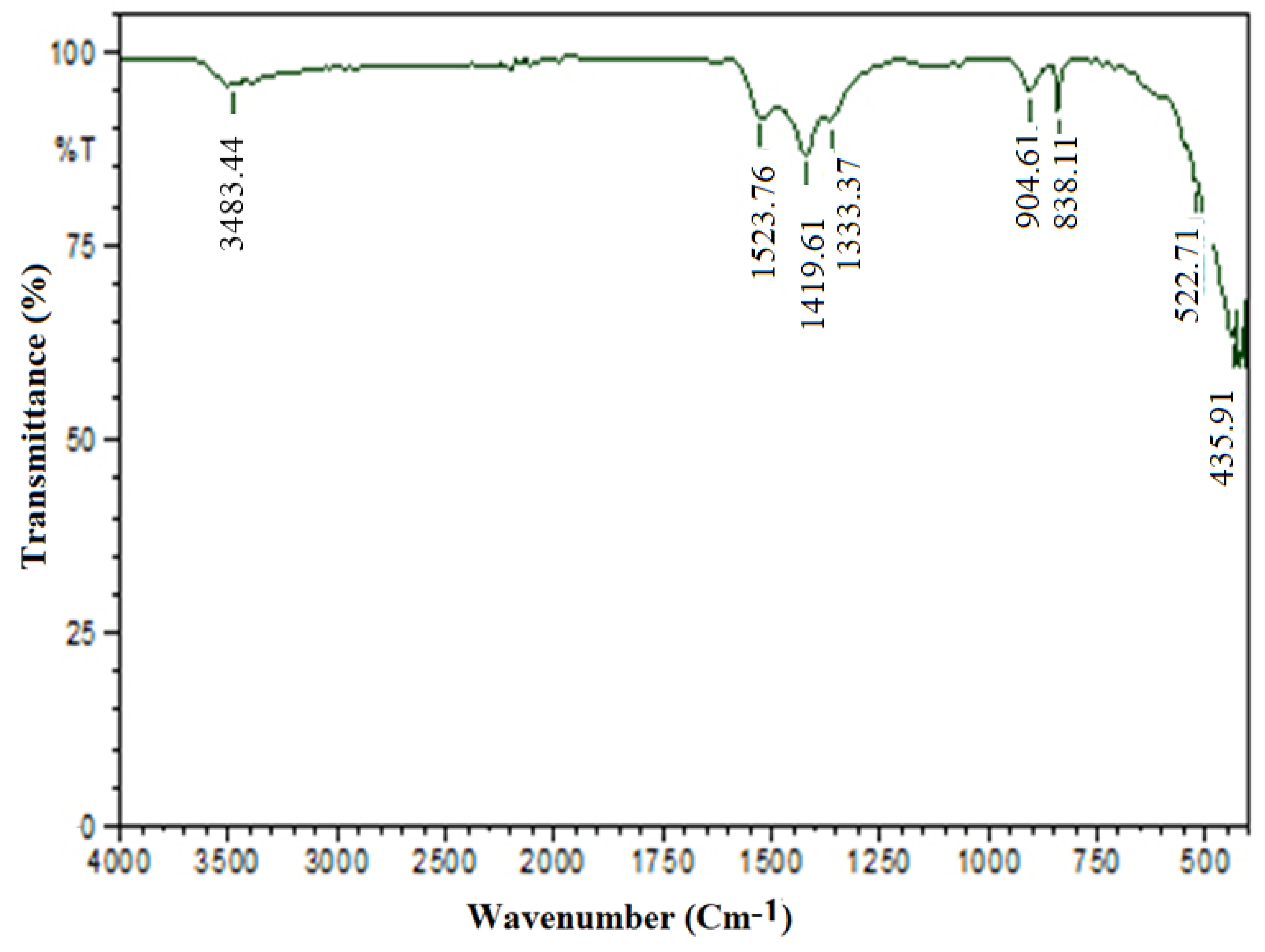
3.2.2. FT-IR Spectrum
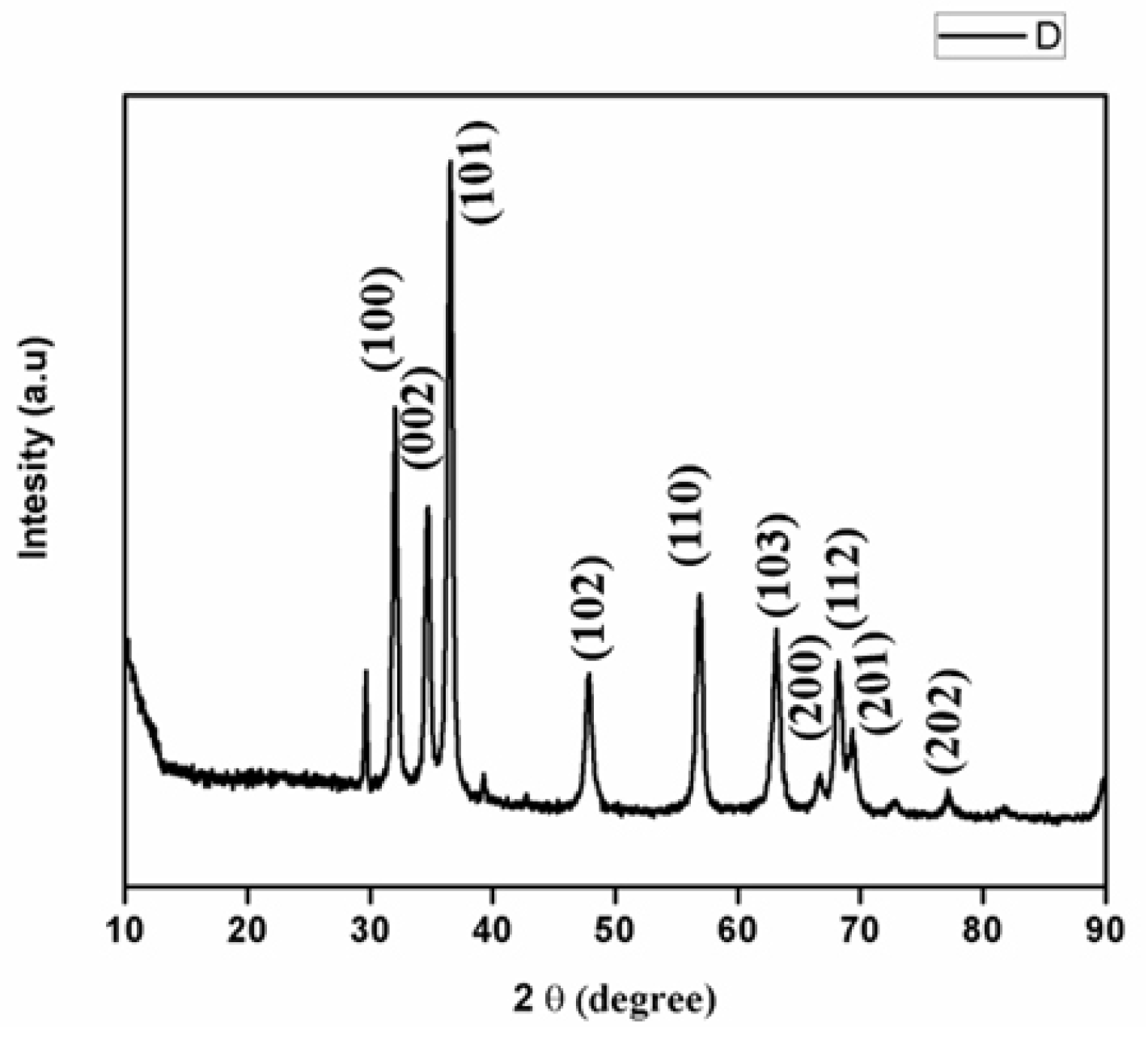
3.2.3. X-ray Diffraction Analysis of ZnONP
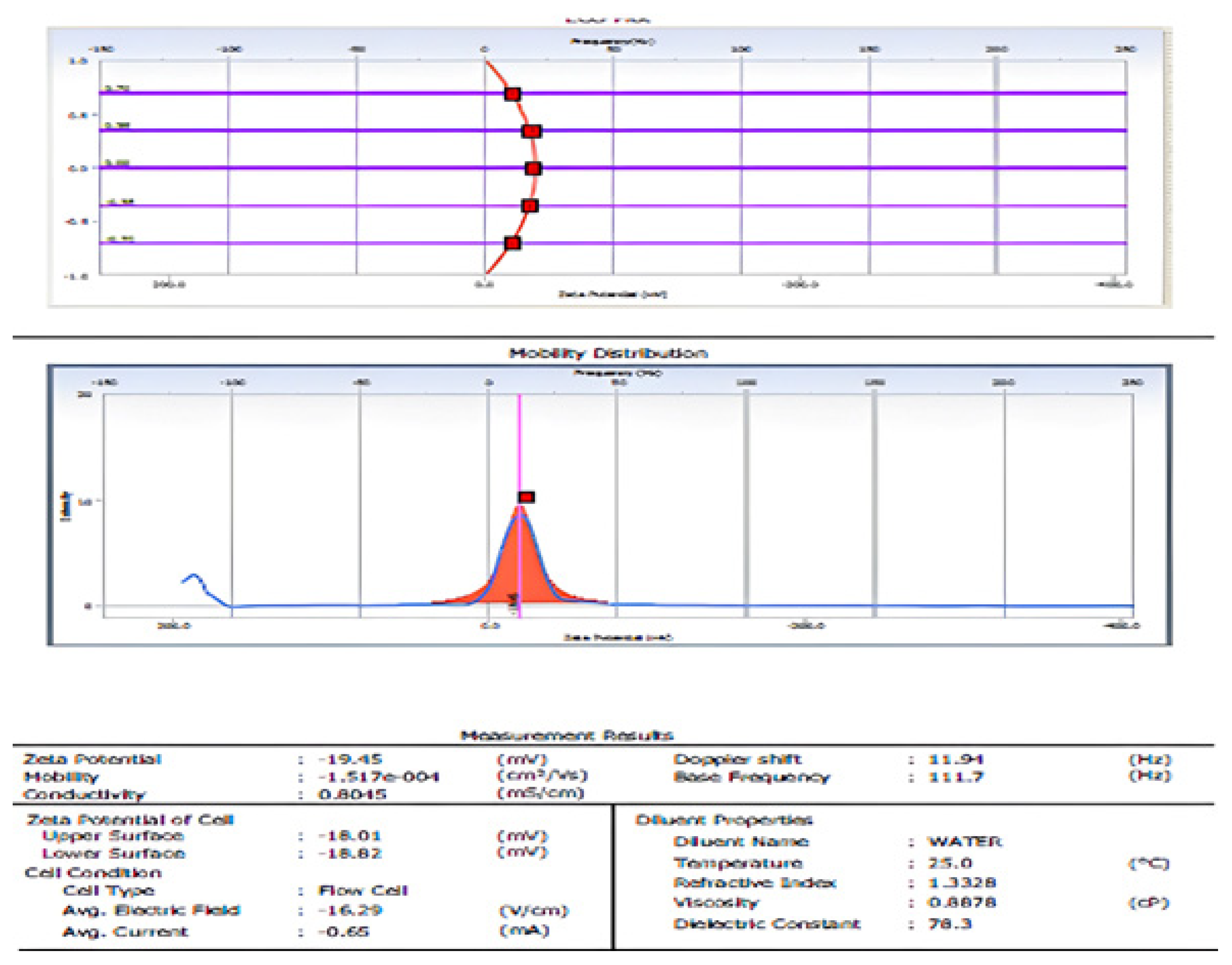
3.2.4. Dynamic Light Scattering (DLS) and ζ-Potential of ZnONP
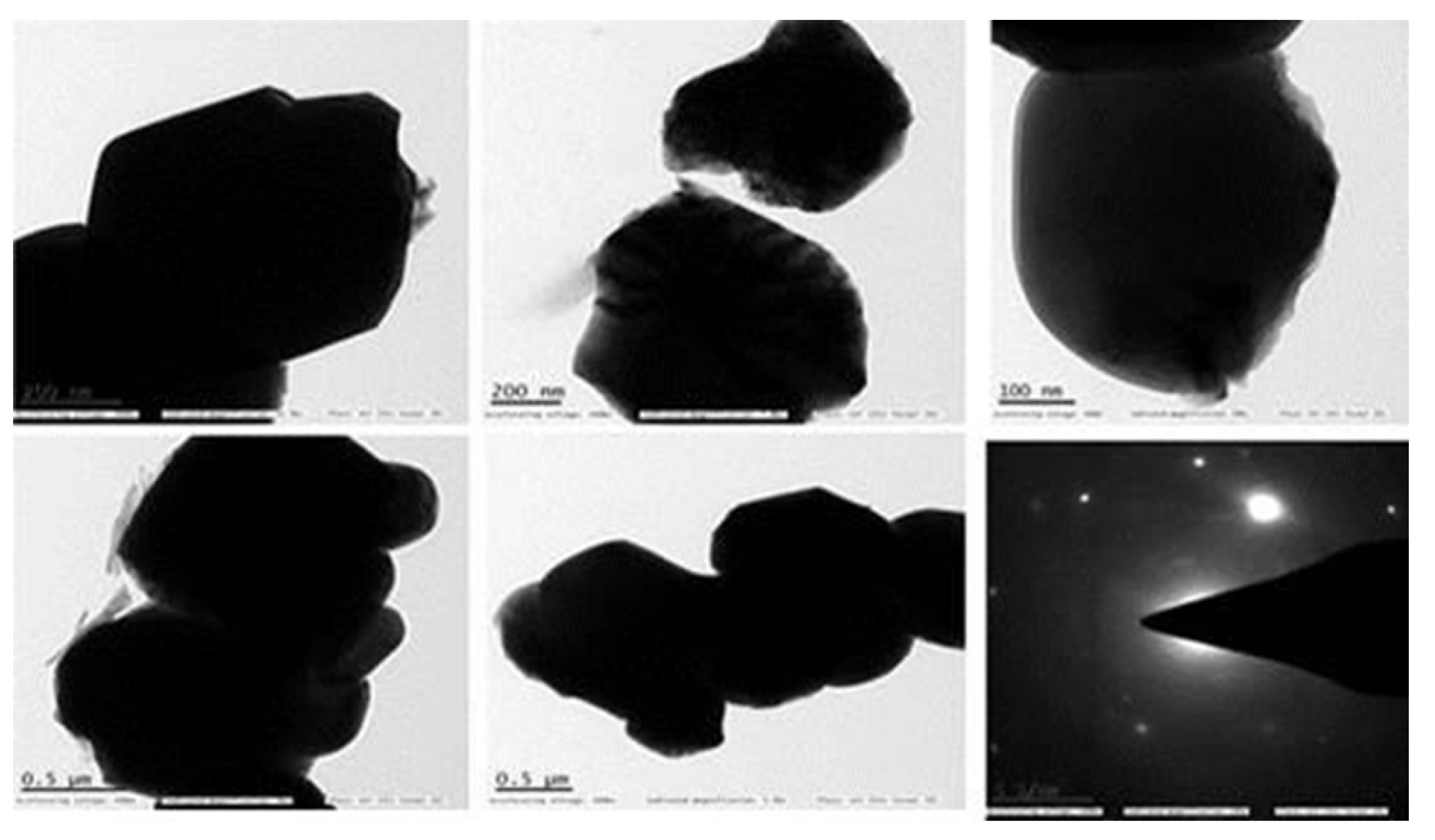
3.2.5. TEM
3.2.6. FESEM and EDX Analysis
3.2.7. Atomic Force Microscopy (AFM) Analysis
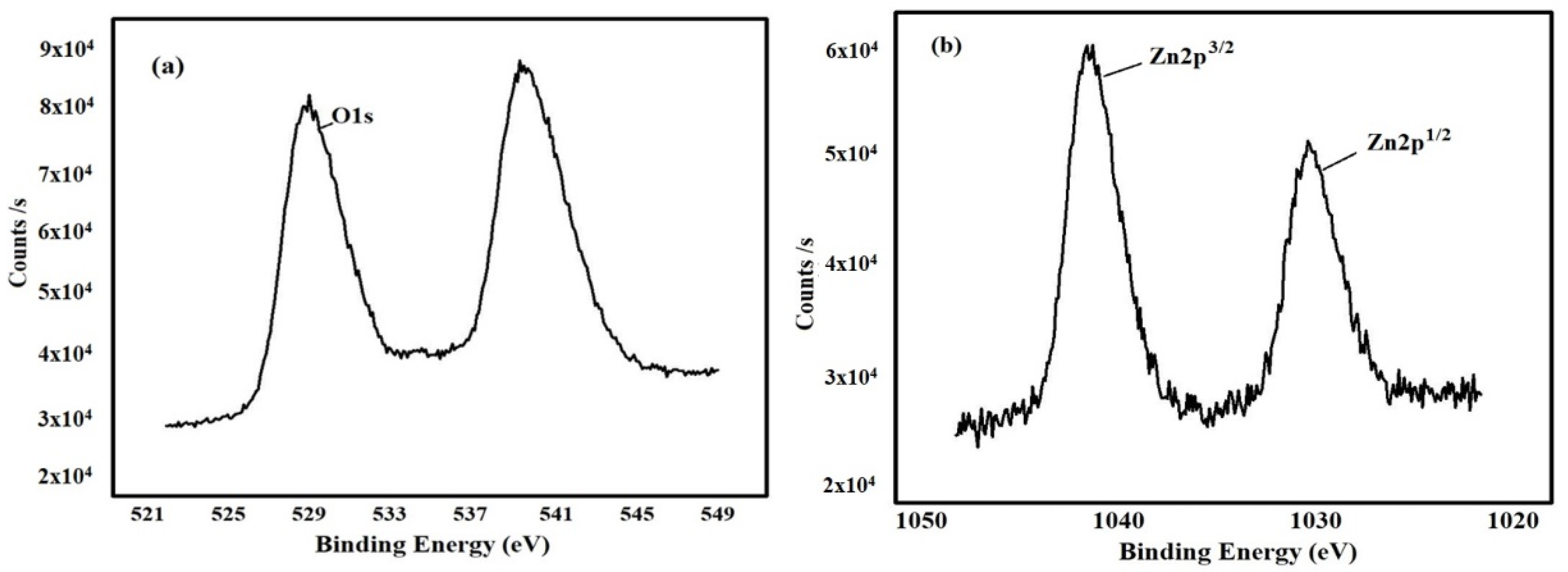
3.2.8. X-ray Photoelectron Spectroscopy (XPS) Analysis
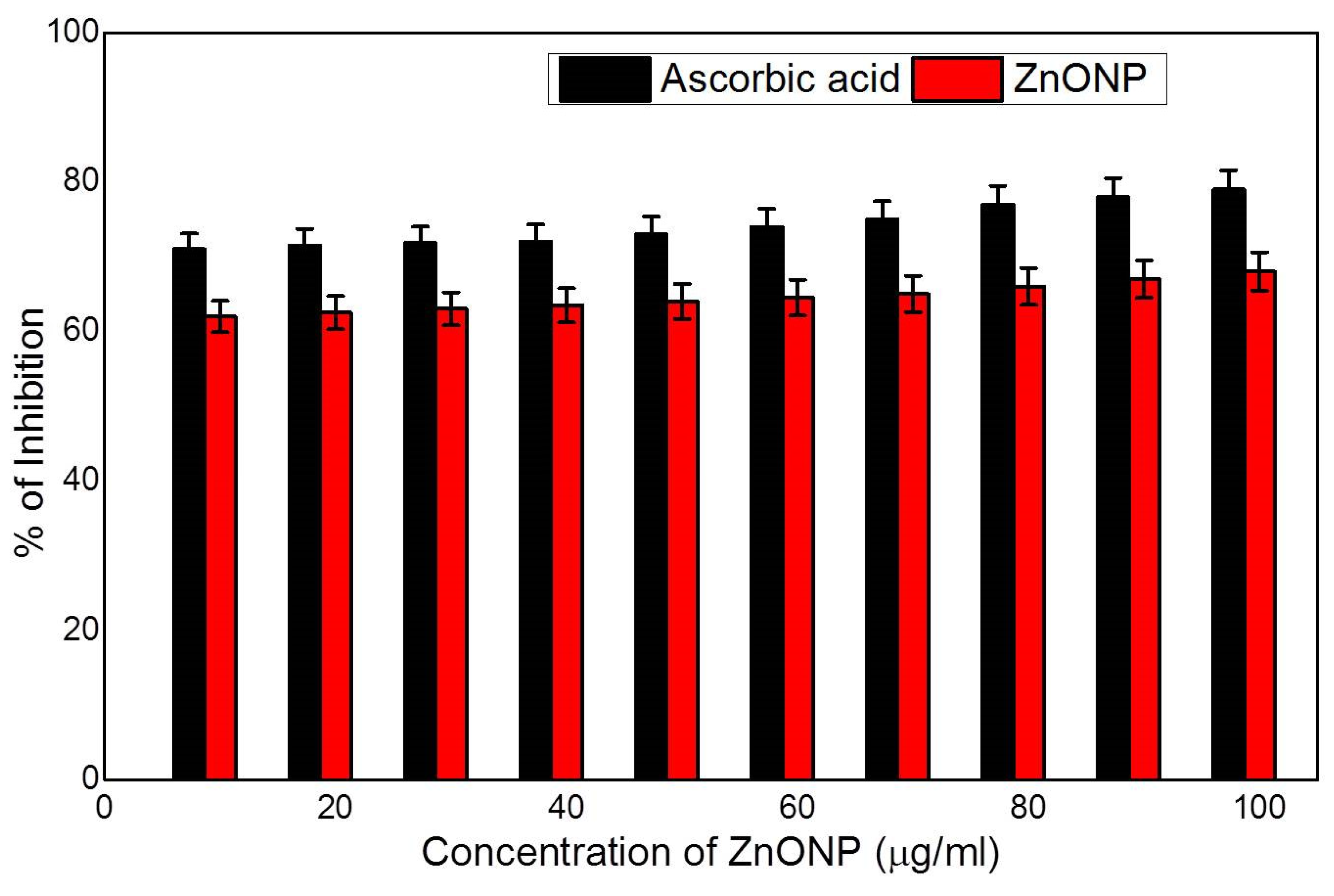
3.3. Antioxidant Activity
3.4. Anticancer Activity in MCF-10A, MCF-7, and A549 Cells Studied by MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, A.; Ray Banerjee, E. Introduction to Nanoscience, Nanotechnology and Nanoparticles BT—Nanomaterials and Biomedicine: Therapeutic and Diagnostic Approach; Ray Banerjee, E., Ed.; Springer: Singapore, 2020; pp. 1–39. ISBN 978-981-15-5274-8. [Google Scholar]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of zinc oxide nanoparticles using Ixora coccinea leaf extract—A green approach. Open J. Synth. Theory Appl. 2016, 5, 51001. [Google Scholar] [CrossRef]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Rajendar, V.; Park, S.-H. Novel synthesis and structural analysis of zinc oxide nanoparticles for the non-enzymatic glucose biosensor. Mater. Sci. Eng. C 2017, 75, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 2022, 24, 221. [Google Scholar] [CrossRef]
- Bayrami, A.; Haghgooie, S.; Pouran, S.R.; Arvanag, F.M.; Habibi-Yangjeh, A. Synergistic antidiabetic activity of ZnO nanoparticles encompassed by Urtica dioica extract. Adv. Powder Technol. 2020, 31, 2110–2118. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Hafeez, M.; Shaheen, R.; Akram, B.; Abdin, Z.U.; Haq, S.; Mahsud, S.; Ali, S.; Khan, R.T. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater. Res. Express 2020, 7, 025019. [Google Scholar] [CrossRef]
- Modan, E.M.; Plăiașu, A.G. Advantages and disadvantages of chemical methods in the elaboration of nanomaterials. Ann. Dunarea De Jos Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Degefa, A.; Bekele, B.; Jule, L.T.; Fikadu, B.; Ramaswamy, S.; Dwarampudi, L.P.; Nagaprasad, N.; Ramaswamy, K. Green Synthesis, characterization of zinc oxide nanoparticles, and ex-amination of properties for dye-sensitive solar cells using various vegetable extracts. J. Nanomater. 2021, 202, 3941923. [Google Scholar]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A. Chapter 11—Multimodal applications of phytonanoparticles. In Phy-Tonanotechnology; Thajuddin, N., Mathew, S., Eds.; Micro and nanotechnologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–219. ISBN 978-0-12-822348-2. [Google Scholar]
- Falih, A.; Ahmed, N.M.; Rashid, M. Green synthesis of zinc oxide nanoparticles by fresh and dry Alhagi plant. Mater. Today Proc. 2021, 49, 3624–3629. [Google Scholar] [CrossRef]
- Al Awadh, A.A.; Shet, A.R.; Patil, L.R.; Shaikh, I.A.; Alshahrani, M.M.; Nadaf, R.; Mahnashi, M.H.; Desai, S.V.; Muddapur, U.M.; Achappa, S.; et al. Sustainable synthesis and characterization of zinc oxide nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals 2022, 12, 1142. [Google Scholar] [CrossRef]
- Naga Jyothi, A.; Priyanka, E.; Eswar Tony, D.; Nadendla, R.R. Chamaecostus cuspidatus—A short review on anti-diabetic plant. Indo Am. J. Pharm. Sci. 2015, 2, 1110–1113. [Google Scholar]
- Rani, D. Phytochemical and pharmacological overview of Chemoecostus cuspidatus. Plant Arch. 2019, 19, 4565–4573. [Google Scholar]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A. Synthesis of zinc oxide nanoparticles using leaf extract of Lippia adoensis (Koseret) and evaluation of its antibacterial activity. J. Chem. 2020, 2020, 7459042. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 4, 1–23. [Google Scholar] [CrossRef]
- Asghari, K.; Shargh, Z.; Fatehfar, S.; Chodari, L.; Sameei, P. The impact of zinc on the molecular signaling pathways in the diabetes disease. J. Trace Elements Med. Biol. 2022, 72, 126985. [Google Scholar] [CrossRef] [PubMed]
- Elshayb, O.; Farroh, K.; Amin, H.; Atta, A. Green synthesis of zinc oxide nanoparticles: Fortification for rice grain yield and nutrients uptake enhancement. Molecules 2021, 26, 584. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S. A Review on green synthesis of zinc oxide nanoparticles using plant extracts and its biomedical applications. BioNanoScience 2020, 10, 848–863. [Google Scholar] [CrossRef]
- He, T.; Long, J.; Li, J.; Liu, L.; Cao, Y. Toxicity of ZnO nanoparticles (NPs) to A549 cells and A549 epithelium in vitro: Interactions with dipalmitoyl phosphatidylcholine (DPPC). Environ. Toxicol. Pharmacol. 2017, 56, 233–240. [Google Scholar] [CrossRef]
- Fahimmunisha, B.A.; Ishwarya, R.; AlSalhi, M.S.; Devanesan, S.; Govindarajan, M.; Vaseeharan, B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: A novel drug delivery approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101465. [Google Scholar] [CrossRef]
- Singh, T.A.; Das, J.; Sil, P.C. Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv. Colloid Interface Sci. 2020, 286, 102317. [Google Scholar] [CrossRef]
- Sharma, P.; Hasan, M.R.; Mehto, N.K.; Deepak; Bishoyi, A.; Narang, J. 92 years of zinc oxide: Has been studied by the scientific community since the 1930s- An overview. Sens. Int. 2022, 3, 100182. [Google Scholar] [CrossRef]
- Yadwade, R.; Gharpure, S.; Ankamwar, B. Non-antimicrobial and non-anticancer properties of ZnO nanoparticles biosynthesized using different plant parts of Bixa orellana. ACS Omega. 2022, 7, 1914–1933. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. effective antimicrobial activity of green ZnO nanoparticles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and antioxidant properties. J. Photochem. Photobiol. B Biol. 2019, 197, 111541. [Google Scholar] [CrossRef] [PubMed]
- Selvakumari, D.; Deepa, R.; Mahalakshmi, V.; Subhashini, P.; Lakshminarayan, N. Anticancer activity of Zno nanoparticles on MCF7 (breast cancer cell) and A549 (lung cancer cell). ARPN J. Eng. Appl. Sci. 2015, 10, 5418–5421. [Google Scholar]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.-S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef]
- Koygun, G.; Arslan, E.; Zengin, G.; Orlando, G.; Ferrante, C. Comparison of anticancer activity of Dorycnium pentaphyllum extract on MCF-7 and MCF-12A cell line: Correlation with invasion and adhesion. Biomolecules 2021, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green fabrication of zinc oxide nanoparticles using Phlomis leaf extract: Characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Ashwini, J.; Aswathy, T.R.; Rahul, A.B.; Thara, G.M.; Nair, A.S. Synthesis and characterization of zinc oxide nanoparticles using Acacia caesia bark extract and its photocatalytic and antimicrobial activities. Catalysts 2021, 11, 1507. [Google Scholar] [CrossRef]
- Li, S.; Silvers, S.J.; El-Shall, M.S. Preparation, characterization and optical properties of zinc oxide nanoparticles. MRS Proc. 1996, 452, 389–394. [Google Scholar] [CrossRef]
- Kalpana, V.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Saraswathi, S.; Tatsugi, J.; Shin, P.-K.; Santhakumar, K. Facile biosynthesis, characterization, and solar assisted photocatalytic effect of ZnO nanoparticles mediated by leaves of L. speciosa. J. Photochem. Photobiol. B Biol. 2017, 167, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bakur, A.; Elshaarani, T.; Niu, Y.; Chen, Q. Comparative study of antidiabetic, bactericidal, and antitumor activities of MEL@AgNPs, MEL@ZnONPs, and Ag–ZnO/MEL/GA nanocomposites prepared by using MEL and gum arabic. RSC Adv. 2019, 9, 9745–9754. [Google Scholar] [CrossRef]
- Abdelkader, D.H.; Negm, W.A.; Elekhnawy, E.; Eliwa, D.; Aldosari, B.N.; Almurshedi, A.S. Zinc oxide nanoparticles as potential delivery carrier: Green synthesis by Aspergillus niger endophytic fungus, characterization, and in vitro/in vivo antibacterial activity. Pharmaceuticals 2022, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Burducea, I.; Ionescu, C.; Straticiuc, M.; Craciun, L.S.; Racolta, P.M.; Jipa, A.L. Characterization of indium nitride and zinc oxide thin films by AFM and RBS. Rom. J. Phys. 2013, 58, 345–353. [Google Scholar]
- Al-Gaashani, R.; Radiman, S.; Daud, A.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Visinescu, D.B.; Jurca, B.; Ianculescu, A.; Carp, O. Starch—A suitable fuel in new low-temperature combustion-based synthesis of zinc aluminate oxides. Polyhedron 2011, 30, 2824–2831. [Google Scholar] [CrossRef]
- Armelao, L.; Bottaro, G.; Bovo, L.; Maccato, C.; Tondello, E.; Anselmi, F.; Bersani, S.; Caliceti, P. Proteins conjugation with ZnO sol–gel nanopowders. J. Sol-Gel Sci. Technol. 2011, 60, 352–358. [Google Scholar] [CrossRef]
- Rajendran, S.P.; Sengodan, K. Synthesis and characterization of zinc oxide and iron oxide nanoparticles using Sesbania grandiflora leaf extract as reducing agent. J. Nanosci. 2017, 2017, 8348507. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar] [CrossRef]
- Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; et al. Plant-mediated zinc oxide nanoparticles: Advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics 2021, 13, 1662. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A promising anticancer agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Anusuya, S.; Anbazhagan, V. Anticancer, anti-diabetic, antimicrobial activity of zinc oxide nanoparticles: A comparative analysis. J. Mol. Struct. 2022, 1263, 133139. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaji, M.P.; Govindasamy, R.; Alharbi, N.S.; Kadaikunnan, S.; Thiruvengadam, M.; Baskar, V.; Devi Rajeswari, V. Biosynthesis of ZnONP Using Chamaecostus cuspidatus and Their Evolution of Anticancer Property in MCF-7 and A549 Cell Lines. Nanomaterials 2022, 12, 3384. https://doi.org/10.3390/nano12193384
Balaji MP, Govindasamy R, Alharbi NS, Kadaikunnan S, Thiruvengadam M, Baskar V, Devi Rajeswari V. Biosynthesis of ZnONP Using Chamaecostus cuspidatus and Their Evolution of Anticancer Property in MCF-7 and A549 Cell Lines. Nanomaterials. 2022; 12(19):3384. https://doi.org/10.3390/nano12193384
Chicago/Turabian StyleBalaji, Menaka Priya, Rajakumar Govindasamy, Naiyf S. Alharbi, Shine Kadaikunnan, Muthu Thiruvengadam, Venkidasamy Baskar, and Vijayarangan Devi Rajeswari. 2022. "Biosynthesis of ZnONP Using Chamaecostus cuspidatus and Their Evolution of Anticancer Property in MCF-7 and A549 Cell Lines" Nanomaterials 12, no. 19: 3384. https://doi.org/10.3390/nano12193384
APA StyleBalaji, M. P., Govindasamy, R., Alharbi, N. S., Kadaikunnan, S., Thiruvengadam, M., Baskar, V., & Devi Rajeswari, V. (2022). Biosynthesis of ZnONP Using Chamaecostus cuspidatus and Their Evolution of Anticancer Property in MCF-7 and A549 Cell Lines. Nanomaterials, 12(19), 3384. https://doi.org/10.3390/nano12193384









