Facile Synthesis of Black Phosphorus Nanosheet@NaReF4 Nanocomposites for Potential Bioimaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Black Phosphorus Nanosheets
2.4. Synthesis of NaGdF4 Nanoparticles
2.5. Synthesis of NaYF4:Yb,Er (18.2 mol %) Nanoparticles
2.6. Synthesis of Ligand-Free Nanoparticles
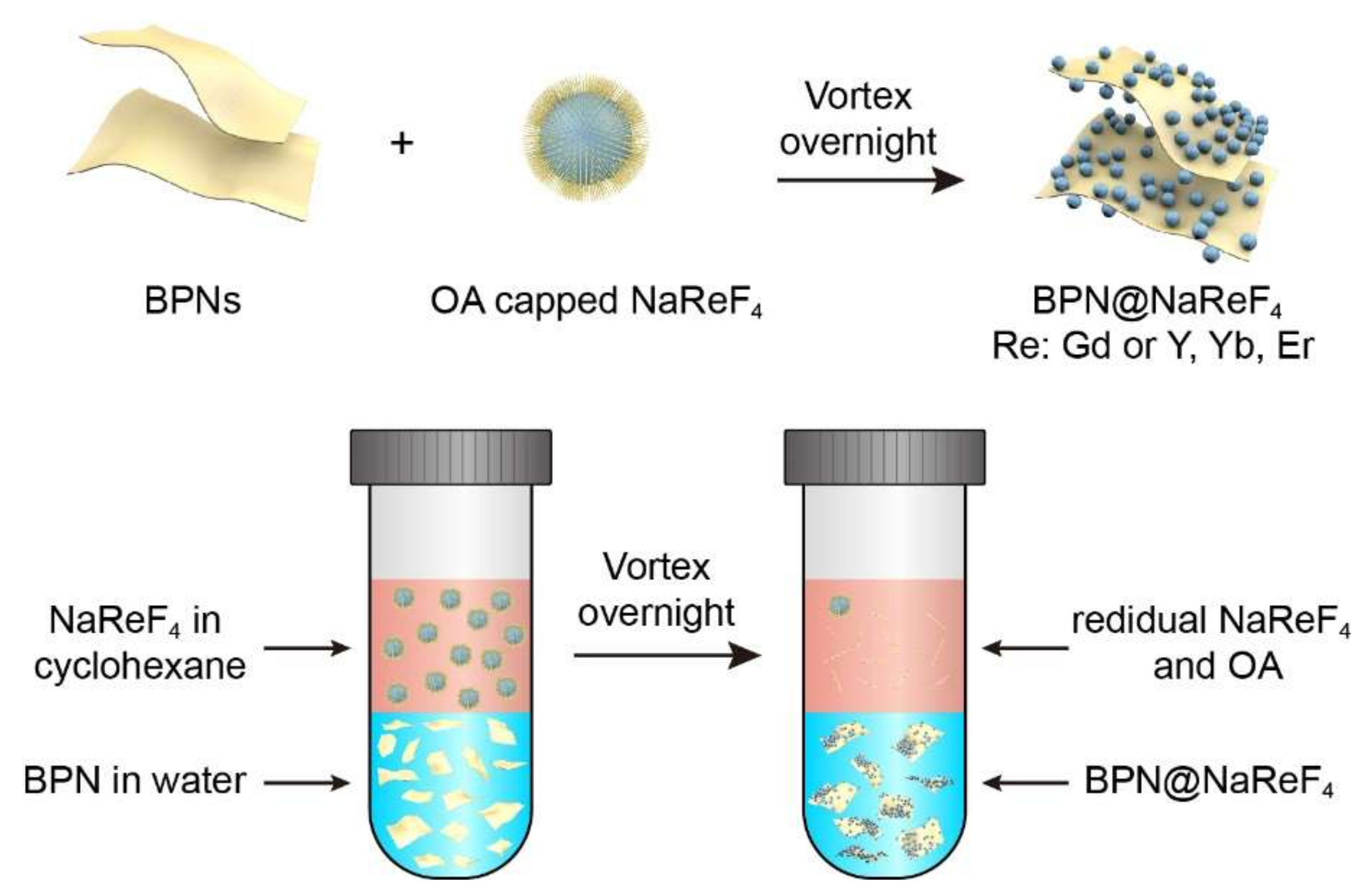
2.7. Synthesis of BPN@NaReF4 Nanocomposites
3. Results
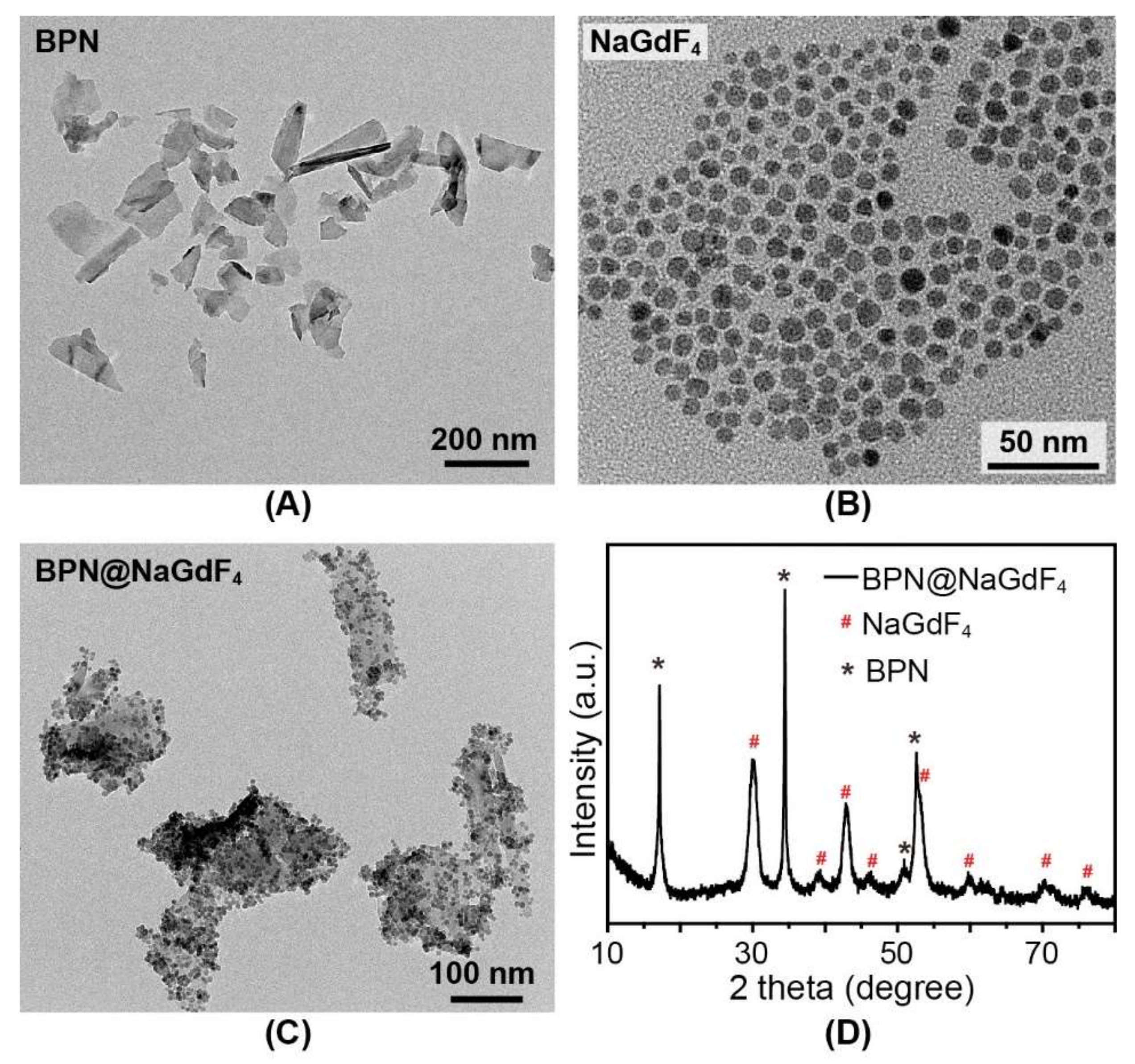
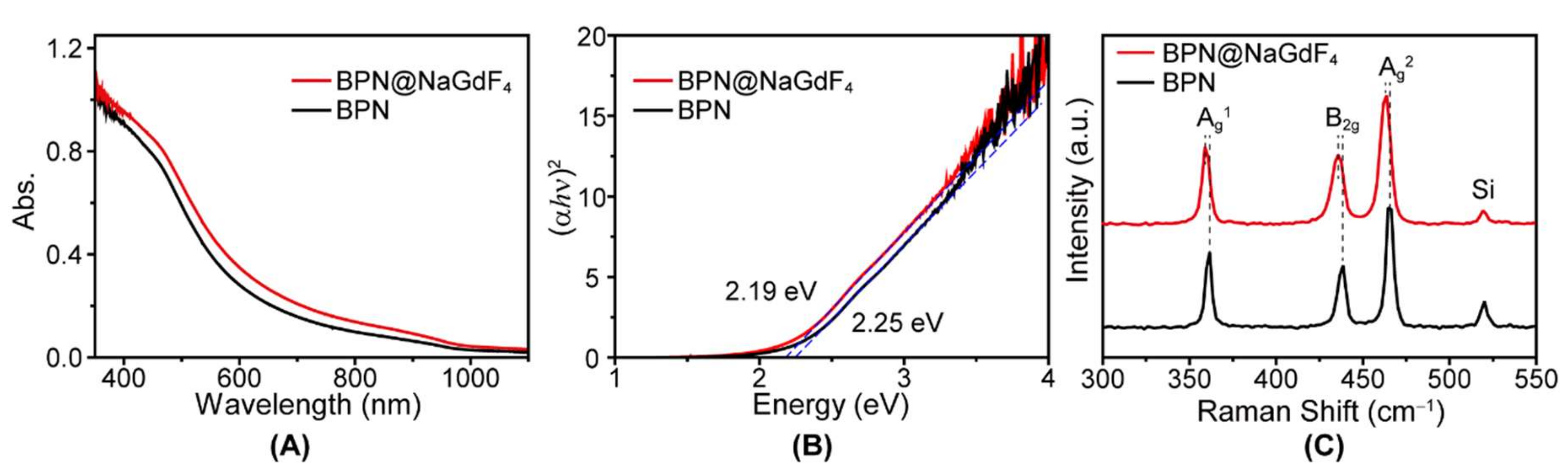
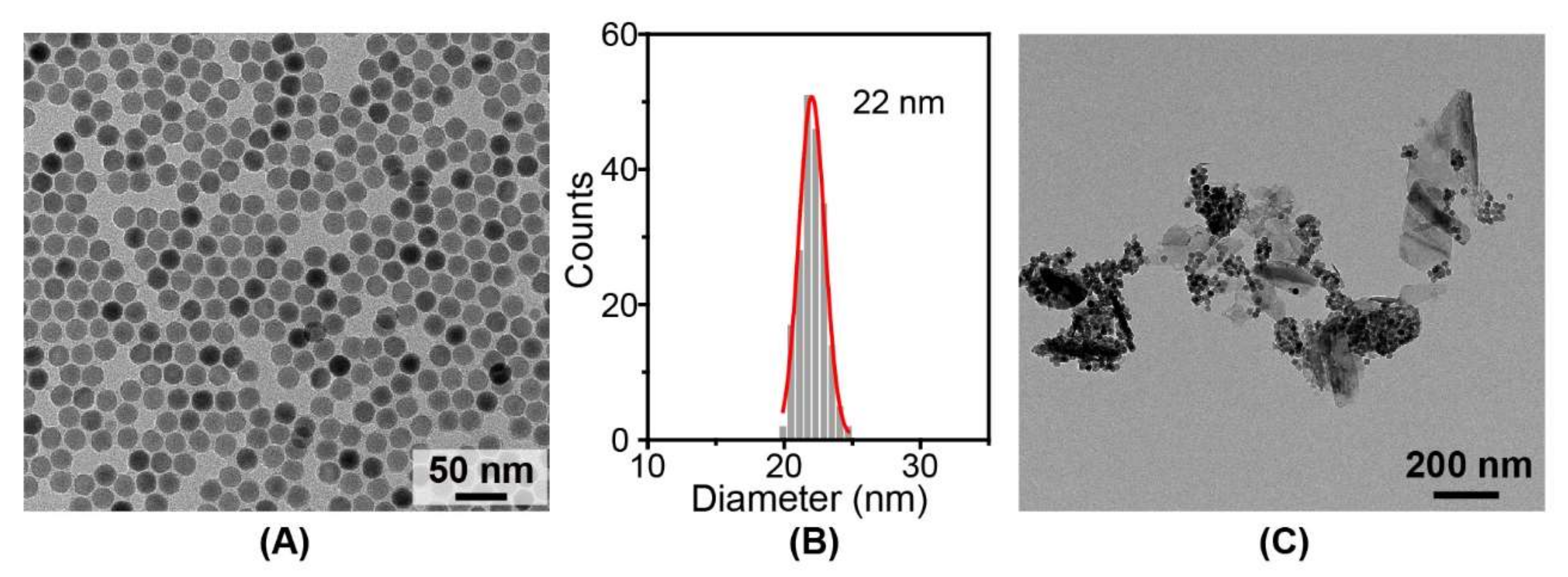
3.1. Characterization of BPN@NaGdF4 Nanocomposites
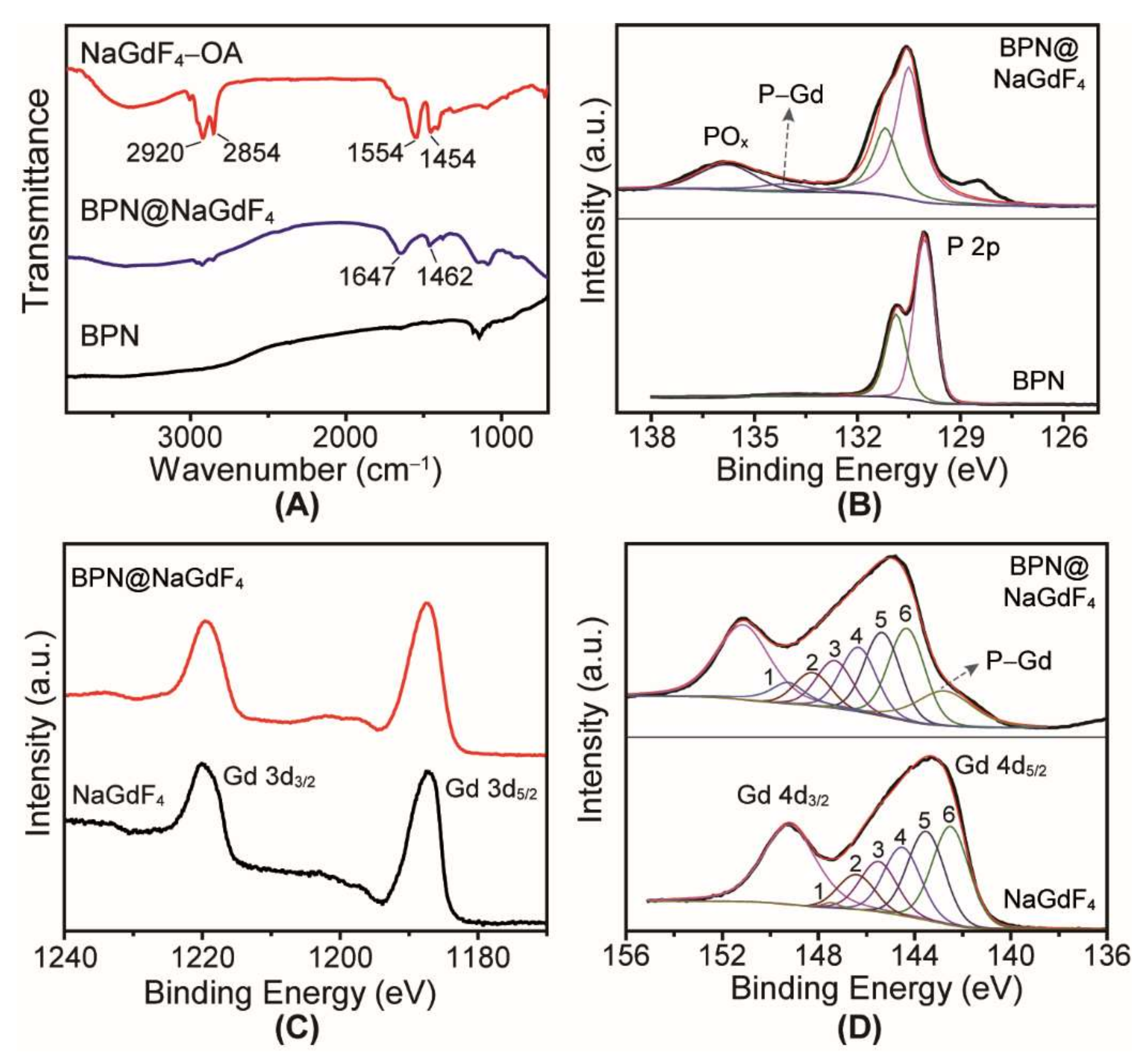
3.2. Mechanism of BPN@NaGdF4 Preparation
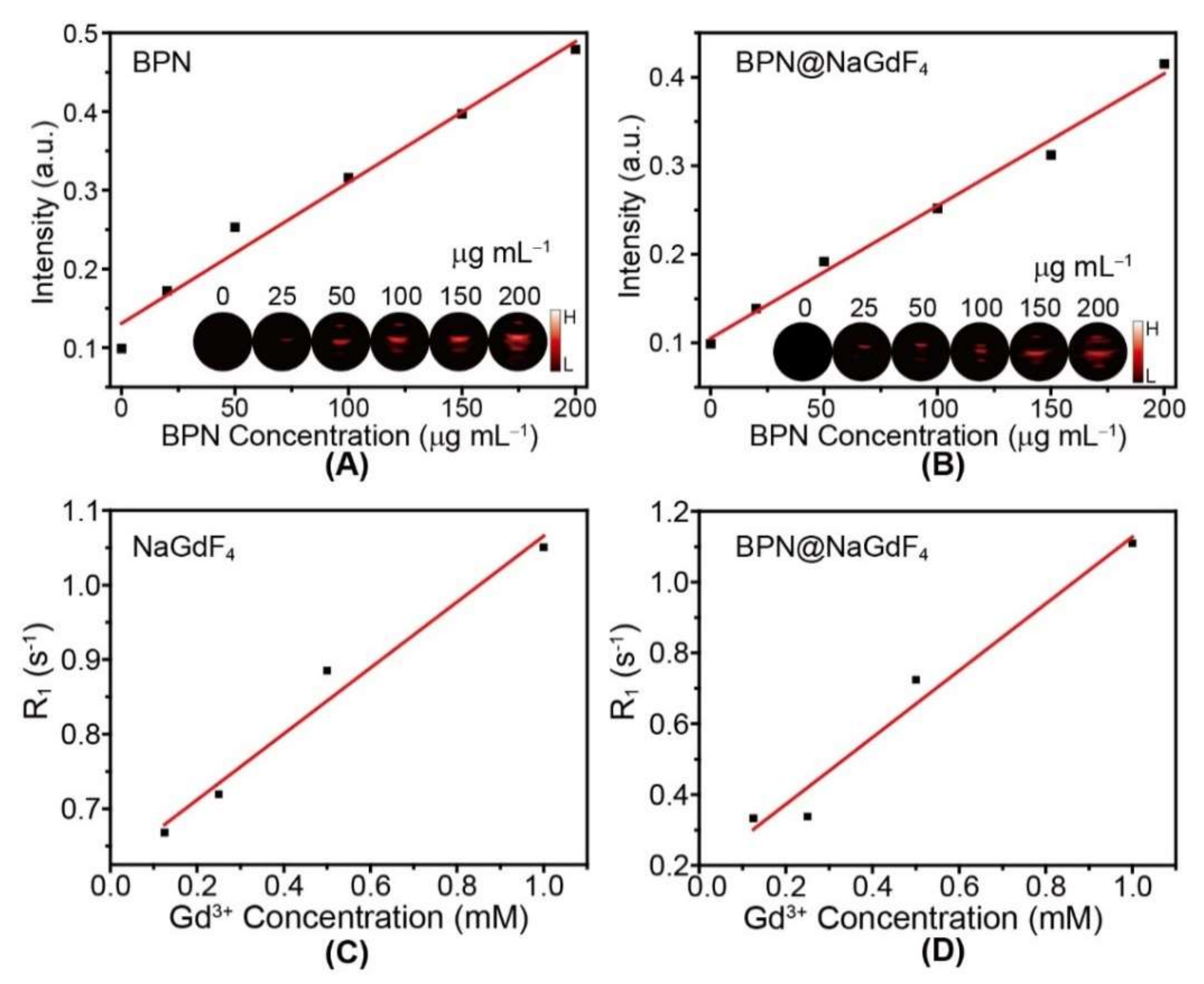
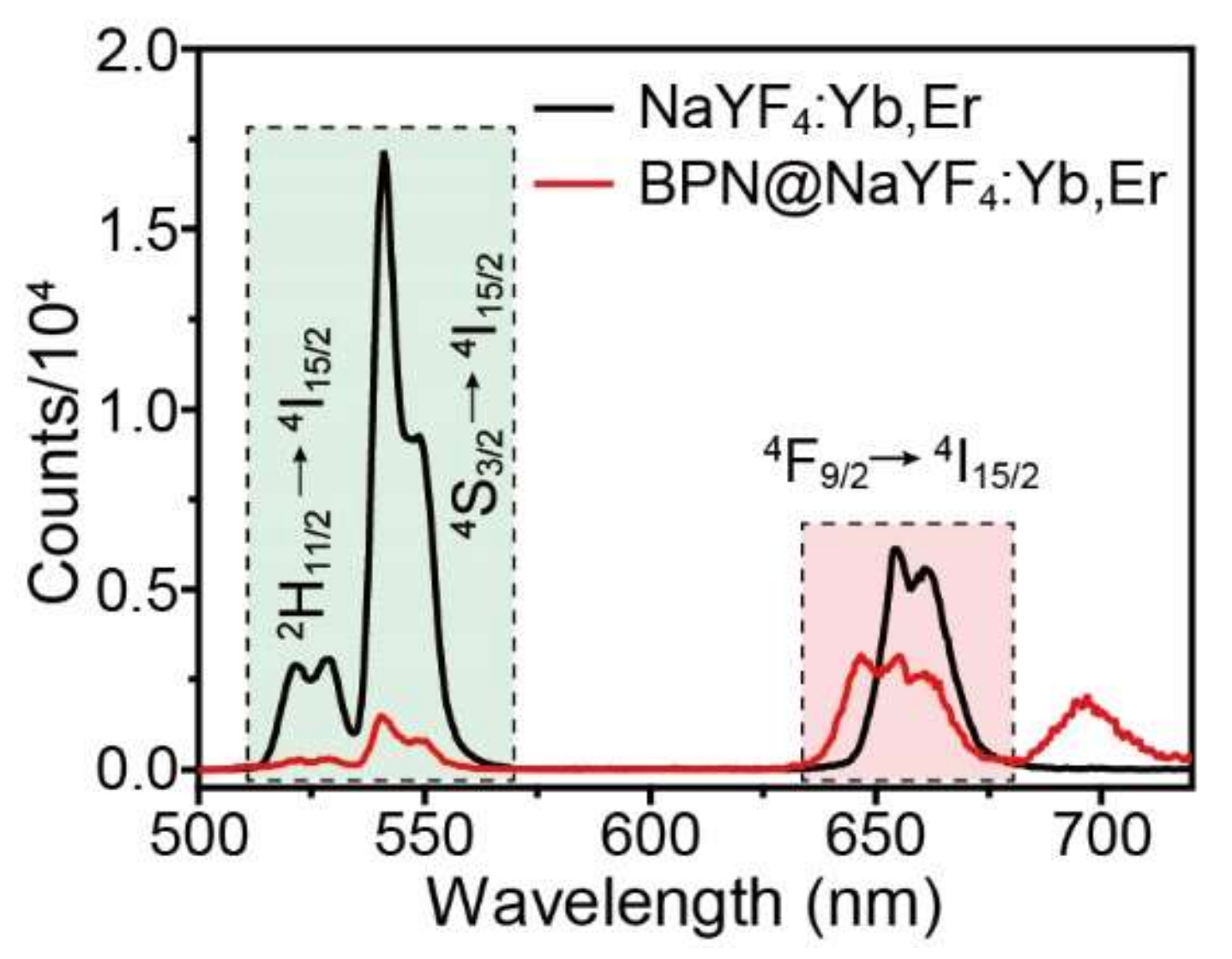
3.3. Bioimaging Properties of BPN@NaReF4 Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Debnath, P.C.; Park, K.; Song, Y.W. Recent Advances in Black-Phosphorus-Based Photonics and Optoelectronics Devices. Small Methods 2018, 2, 1700315. [Google Scholar] [CrossRef]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Fan, T.; Zhou, Y.; Zhang, H.; Mei, L. 2D Black Phosphorus–Based Biomedical Applications. Adv. Funct. Mater. 2019, 29, 1808306. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, C.; Zhang, X.; Kong, N.; Tao, W. Black Phosphorus in Biological Applications: Evolutionary Journey from Monoelemental Materials to Composite Materials. Acc. Mater. Res. 2021, 2, 489–500. [Google Scholar] [CrossRef]
- Liu, W.; Dong, A.; Wang, B.; Zhang, H. Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy. Adv. Sci. 2021, 8, 2003033. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2016, 29, 1603276. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, G.; Yang, P.; Lv, R.; Gai, S.; Li, C.; He, F.; Lin, J. Assembly of Au Plasmonic Photothermal Agent and Iron Oxide Nanoparticles on Ultrathin Black Phosphorus for Targeted Photothermal and Photodynamic Cancer Therapy. Adv. Funct. Mater. 2017, 27, 1700371. [Google Scholar] [CrossRef]
- Ge, X.; Xia, Z.; Guo, S. Recent Advances on Black Phosphorus for Biomedicine and Biosensing. Adv. Funct. Mater. 2019, 29, 1900318. [Google Scholar] [CrossRef]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene Is the New Graphene in Biomedical Applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black Phosphorus and its Biomedical Applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Ohulchanskyy, T.Y.; Chen, G. Lanthanide-Doped Near-Infrared Nanoparticles for Biophotonics. Adv. Mater. 2021, 33, 2000678. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Biyikli, L.; Sellars, M.J.; Khodaparast, G.A.; Richardson, F.S.; Quagliano, J.R. Energy Transfer and Upconversions in Cubic Cs2NaYCl6:Er3+ and Cs2NaErCl6. Phys. Rev. B 1997, 56, 4518–4528. [Google Scholar] [CrossRef]
- Li, T.; Dorn, H.C. Biomedical Applications of Metal-Encapsulated Fullerene Nanoparticles. Small 2017, 13, 1603152. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Qiao, R.; Fang, F.; Wang, X.; Dong, C.; Liu, K.; Liu, C.; Liu, Z.; Lei, H.; Wang, F.; et al. NaGdF4 Nanoparticle-Based Molecular Probes for Magnetic Resonance Imaging of Intraperitoneal Tumor Xenografts in Vivo. ACS Nano 2013, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zhang, S.; Bu, W.; Zheng, X.; Wang, L.; Xiao, Q.; Ni, D.; Zhang, J.; Zhou, L.; Peng, W.; et al. Ultrasmall NaGdF4 Nanodots for Efficient MR Angiography and Atherosclerotic Plaque Imaging. Adv. Mater. 2014, 26, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gu, W.; Wang, H.; Qi, Y.; Deng, Y.; Xiao, N.; Liu, Y.; Xu, Q.; Ye, L. Effect of surface functionalities on relaxometric properties of MR contrast agents based on NaGdF4 nanoparticles. RSC Adv. 2013, 3, 5386–5392. [Google Scholar] [CrossRef]
- Xu, M.; Yang, G.; Bi, H.; Xu, J.; Dong, S.; Jia, T.; Wang, Z.; Zhao, R.; Sun, Q.; Gai, S.; et al. An Intelligent Nanoplatform for Imaging-Guided Photodynamic/Photothermal/Chemo-Therapy Based on Upconversion Nanoparticles and CuS Integrated Black Phosphorus. Chem. Eng. J. 2020, 382, 122822. [Google Scholar] [CrossRef]
- Lv, R.; Yang, D.; Yang, P.; Xu, J.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. Integration of Upconversion Nanoparticles and Ultrathin Black Phosphorus for Efficient Photodynamic Theranostics under 808 nm Near-Infrared Light Irradiation. Chem. Mater. 2016, 28, 4724–4734. [Google Scholar] [CrossRef]
- Wang, D.; Yi, P.; Wang, L.; Zhang, L.; Li, H.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Revisiting the Growth of Black Phosphorus in Sn-I Assisted Reactions. Front. Chem. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Deng, R.R.; Liu, X.G. Preparation of Core-Shell NaGdF4 Nanoparticles Doped with Luminescent Lanthanide Ions to be Used as Upconversion-Based Probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef]
- Guo, S.; Xie, X.; Huang, L.; Huang, W. Sensitive Water Probing through Nonlinear Photon Upconversion of Lanthanide-Doped Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of Ligand-Free Colloidally Stable Water Dispersible Brightly Luminescent Lanthanide-Doped Upconverting Nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wang, D.; Zhang, J.; Li, X.; Liu, D.; Li, H.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Ultrafast Cathodic Exfoliation of Few-Layer Black Phosphorus in Aqueous Solution. ACS Appl. Nano Mater. 2019, 2, 3793–3801. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Pimenta, M.A.; de Matos, C.J.S. Raman Spectroscopy in Black Phosphorus. J. Raman Spectrosc. 2018, 49, 76–90. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.F. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Woomer, A.H.; Farnsworth, T.W.; Hu, J.; Wells, R.A.; Donley, C.L.; Warren, S.C. Phosphorene: Synthesis, Scale-Up, and Quantitative Optical Spectroscopy. ACS Nano 2015, 9, 8869–8884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, Q.; Tong, Y.; Wang, J. Light-Induced Ambient Degradation of Few-Layer Black Phosphorus: Mechanism and Protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D.; Wu, X.; Ji, H.; Wan, L. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef]
- Sofer, Z.; Luxa, J.; Bouša, D.; Sedmidubský, D.; Lazar, P.; Hartman, T.; Hardtdegen, H.; Pumera, M. The Covalent Functionalization of Layered Black Phosphorus by Nucleophilic Reagents. Angew. Chem. Int. Ed. 2017, 56, 9891–9896. [Google Scholar] [CrossRef]
- Yubao, L.; Klein, C.P.A.T.; Xingdong, Z.; de Groot, K. Formation of a Bone Apatite-Like Layer on the Surface of Porous Hydroxyapatite Ceramics. Biomaterials 1994, 15, 835–841. [Google Scholar] [CrossRef]
- Ren, W.; Wen, S.; Tawfik, S.A.; Su, Q.P.; Lin, G.; Ju, L.A.; Ford, M.J.; Ghodke, H.; van Oijen, A.M.; Jin, D. Anisotropic Functionalization of Upconversion Nanoparticles. Chem. Sci. 2018, 9, 4352–4358. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, R.; Liu, W.; Donovan, M.J.; Wang, L.; Ismail, I.; Li, J.; Li, J.; Qu, F.; Tan, W. Engineering DNA on the Surface of Upconversion Nanoparticles for Bioanalysis and Therapeutics. ACS Nano 2021, 15, 17257–17274. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; Lu, J.; Liu, D.; Yang, N.; Huang, H.; Chu, P.K.; Yu, X.F. Lanthanide-Coordinated Black Phosphorus. Small 2018, 14, 1801405. [Google Scholar] [CrossRef] [PubMed]
- Ryder, C.R.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.J.; Schatz, G.C.; Hersam, M.C. Covalent Functionalization and Passivation of Exfoliated Black Phosphorus via Aryl Diazonium Chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Thiede, T.B.; Krasnopolski, M.; Milanov, A.P.; de los Arcos, T.; Ney, A.; Becker, H.-W.; Rogalla, D.; Winter, J.; Devi, A.; Fischer, R.A. Evaluation of Homoleptic Guanidinate and Amidinate Complexes of Gadolinium and Dysprosium for MOCVD of Rare-Earth Nitride Thin Films. Chem. Mater. 2011, 23, 1430–1440. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Q.; Ye, J.; Ge, X.; Wang, J.; Song, J.; Yang, H. Ag+-Coupled Black Phosphorus Vesicles with Emerging NIR-II Photoacoustic Imaging Performance for Cancer Immune-Dynamic Therapy and Fast Wound Healing. Angew. Chem. Int. Ed. 2020, 59, 22202–22209. [Google Scholar] [CrossRef]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L.; Zhao, C.; Li, Z. One-Pot Solventless Preparation of PEGylated Black Phosphorus Nanoparticles for Photoacoustic Imaging and Photothermal Therapy of Cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Li, Z.; Cui, H.; Zhou, Y.; Li, W.; Tao, W.; Zhang, H.; Wang, H.; Chu, P.K.; et al. TiL4-Coordinated Black Phosphorus Quantum Dots as an Efficient Contrast Agent for in Vivo Photoacoustic Imaging of Cancer. Small 2017, 13, 1602896. [Google Scholar] [CrossRef]
- Chan, K.W.-Y.; Wong, W.-T. Small Molecular Gadolinium(III) Complexes as MRI Contrast Agents for Diagnostic Imaging. Coord. Chem. Rev. 2007, 251, 2428–2451. [Google Scholar] [CrossRef]
- Chen, K.-J.; Wolahan, S.M.; Wang, H.; Hsu, C.-H.; Chang, H.-W.; Durazo, A.; Hwang, L.-P.; Garcia, M.A.; Jiang, Z.K.; Wu, L.; et al. A Small MRI Contrast Agent Library of Gadolinium(III)-Encapsulated Supramolecular Nanoparticles for Improved Relaxivity and Sensitivity. Biomaterials 2011, 32, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Qin, J.; Zhang, C.; Li, Y. Facile Synthesis of Black Phosphorus Nanosheet@NaReF4 Nanocomposites for Potential Bioimaging. Nanomaterials 2022, 12, 3383. https://doi.org/10.3390/nano12193383
Wang D, Qin J, Zhang C, Li Y. Facile Synthesis of Black Phosphorus Nanosheet@NaReF4 Nanocomposites for Potential Bioimaging. Nanomaterials. 2022; 12(19):3383. https://doi.org/10.3390/nano12193383
Chicago/Turabian StyleWang, Dongya, Jingcan Qin, Chuan Zhang, and Yuehua Li. 2022. "Facile Synthesis of Black Phosphorus Nanosheet@NaReF4 Nanocomposites for Potential Bioimaging" Nanomaterials 12, no. 19: 3383. https://doi.org/10.3390/nano12193383
APA StyleWang, D., Qin, J., Zhang, C., & Li, Y. (2022). Facile Synthesis of Black Phosphorus Nanosheet@NaReF4 Nanocomposites for Potential Bioimaging. Nanomaterials, 12(19), 3383. https://doi.org/10.3390/nano12193383





