Toxicological Assessment of Cellulose Nanomaterials: Oral Exposure
Abstract
1. Introduction
2. Production and Application of CNMs
2.1. Overview of Sources and Production of CNMs
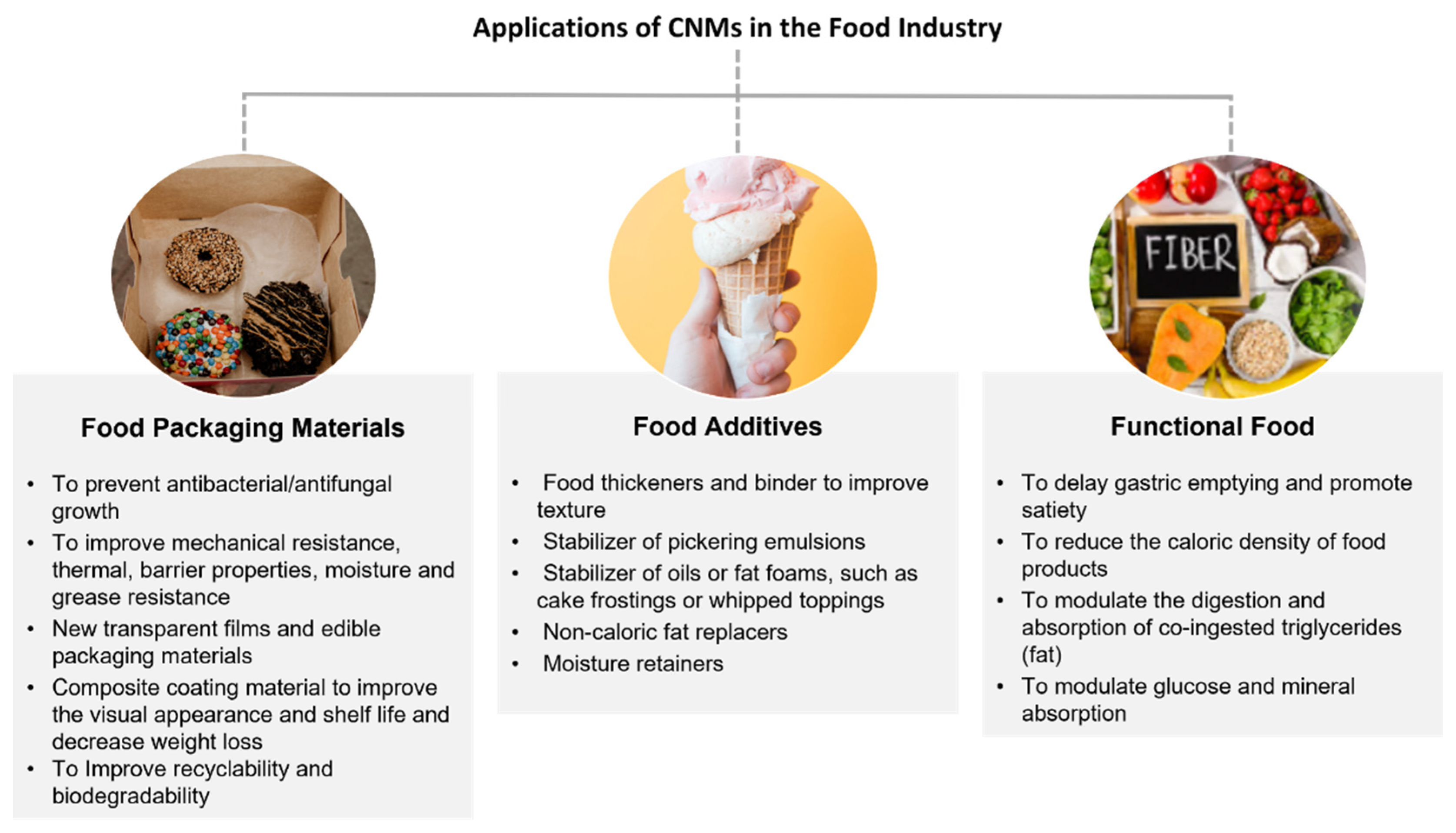
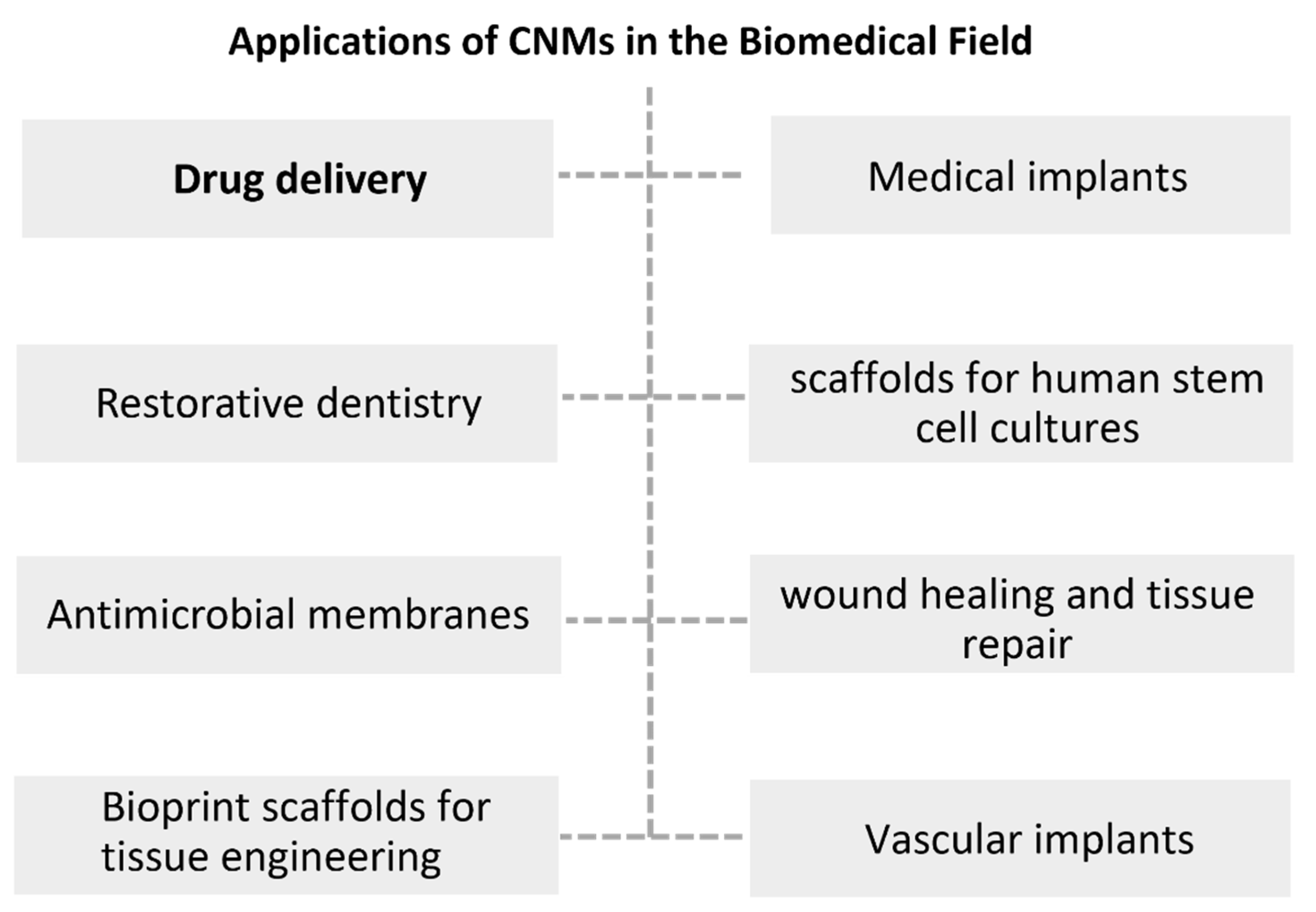
2.2. CNMs in Food Technology and Biomedicine
3. CNMs’ Digestion and Fate in the GIT
4. Hazard Assessment of CNMs
4.1. In Vitro Toxicity of CNMs in GIT Cells
4.1.1. Cellulose Nanocrystals (CNCs)
4.1.2. Cellulose Nanofibres (CNFs)
4.2. In Vivo Toxicity of CNM
4.2.1. Cellulose Nanocrystals (CNCs)
4.2.2. Cellulose Nanofibres (CNFs)
5. Relevant Features to Be Considered in the Toxicity Assessment of CNMs
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO/TS 20477:2017; Nanotechnologies—Standard Terms and Their Definition for Cellulose Nanomaterial. ISO: Geneva, Switzerland, 2017.
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2020, 62, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in drug delivery and antimicrobially active materials. Polymers 2020, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in Biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Mohammad, M. Recent advances in cellulose-based hydrophobic food packaging. Emerg. Mater. 2021, 5, 703–718. [Google Scholar] [CrossRef]
- Hasan, N.; Rahman, L.; Kim, S.H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- Andrade, D.R.M.; Mendonça, M.H.; Helm, C.V.; Magalhães, W.L.E.; de Muniz, G.I.B.; Kestur, S.G. Assessment of nano cellulose from peach palm residue as potential food additive: Part II: Preliminary studies. J. Food Sci. Technol. 2015, 52, 5641–5650. [Google Scholar] [CrossRef]
- Deloid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: Interference of triglyceride hydrolysis by nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Chang, C.; Wang, Y. Recent developments and prospective food-related applications of cellulose nanocrystals: A review. Cellulose 2020, 27, 2991–3011. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017, 10, 977. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Kim, B. Cellulose nanomaterials: Life cycle risk assessment, and environmental health and safety roadmap. Environ. Sci. Nano 2015, 2, 477–499. [Google Scholar] [CrossRef]
- Souza, E.; Gottschalk, L.; Freitas-Silva, O. Overview of nanocellulose in food packaging. Recent Pat. Food Nutr. Agric. 2019, 11, 154–167. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Ong, K.J.; Ede, J.D.; Wegner, T.H.; Goergen, M. Toward cellulose nanomaterial commercialization: Knowledge gap analysis for safety data sheets according to the globally harmonized system. Tappi J. 2016, 15, 425–437. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.M.; Cowie, J. Market projections of cellulose nanomaterial-enabled products? Part 1: Applications. Tappi J. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Markets and Markets. Nanocellulose Market Research Report. Available online: https://www.marketsandmarkets.com/Market-Reports/nano-cellulose-market-56392090.html?gclid=CjwKCAiA9tyQBhAIEiwA6tdCrB0hVUbEjxuVLOlp5V3y_uAzeVaU5LVfd_0tZYTML8HFdge4oI9N_RoCa_IQAvD_BwE# (accessed on 20 January 2022).
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.J.T.; Louro, H.; Silva, M.J. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]
- Catalán, J.; Norppa, H. Safety aspects of bio-based nanomaterials. Bioengineering 2017, 4, 94. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Kim, B. Environmental health and safety of cellulose nanomaterials and composites. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH: Weinheim, Germany, 2017; Volume 1, pp. 683–729. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Endes, C.; Mueller, S.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D.; Foster, E.J. Elucidating the potential biological impact of cellulose nanocrystals. Fibers 2016, 4, 21. [Google Scholar] [CrossRef]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 78. [Google Scholar] [CrossRef]
- Ede, J.D.; Ong, K.J.; Goergen, M.; Rudie, A.; Pomeroy-Carter, C.A.; Shatkin, J.A. Risk analysis of cellulose nanomaterials by inhalation: Current state of science. Nanomaterials 2019, 9, 337. [Google Scholar] [CrossRef]
- Sai, T.; Fujita, K. A review of pulmonary toxicity studies of nanocellulose. Inhal. Toxicol. 2020, 32, 231–239. [Google Scholar] [CrossRef]
- Stoudmann, N.; Schmutz, M.; Hirsch, C.; Nowack, B.; Som, C. Human hazard potential of nanocellulose: Quantitative insights from the literature. Nanotoxicology 2020, 14, 1241–1257. [Google Scholar] [CrossRef]
- Park, E.J.; Khaliullin, T.O.; Shurin, M.R.; Kisin, E.R.; Yanamala, N.; Fadeel, B.; Chang, J.; Shvedova, A.A. Fibrous nanocellulose, crystalline nanocellulose, carbon nanotubes, and crocidolite asbestos elicit disparate immune responses upon pharyngeal aspiration in mice. J. Immunotoxicol. 2018, 15, 12–23. [Google Scholar] [CrossRef]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of cellulose nanomaterials, their capabilities and applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Tibolla, H.; Pelissari, F.M.; Martins, J.T.; Lanzoni, E.M.; Vicente, A.A.; Menegalli, F.C.; Cunha, R.L. Banana starch nanocomposite with cellulose nanofibers isolated from banana peel by enzymatic treatment: In vitro cytotoxicity assessment. Carbohydr. Polym. 2019, 207, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Islam Sarker, M.Z.; Fazita, M.R.N.; Syakir, M.I.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose production: Exploring the enzymatic route and residues of pulp and paper industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Gamelas, J.A.F.; Pedrosa, J.; Lourenço, A.F.; Mutjé, P.; González, I.; Chinga-Carrasco, G.; Singh, G.; Ferreira, P.J.T. On the morphology of cellulose nanofibrils obtained by TEMPO-mediated oxidation and mechanical treatment. Micron 2015, 72, 28–33. [Google Scholar] [CrossRef]
- Levanič, J.; Šenk, V.P.; Nadrah, P.; Poljanšek, I.; Oven, P.; Haapala, A. Analyzing TEMPO-Oxidized cellulose fiber morphology: New insights into optimization of the oxidation process and nanocellulose dispersion quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Wang, W. Toxicological studies and some functional properties of carboxymethylated cellulose nanofibrils as potential food ingredient. Int. J. Biol. Macromol. 2021, 190, 887–893. [Google Scholar] [CrossRef]
- Lopes, V.R.; Strømme, M.; Ferraz, N. In vitro biological impact of nanocellulose fibers on human gut bacteria and gastrointestinal cells. Nanomaterials 2020, 10, 1159. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Gómez, H.C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Pinto, F.; Lourenço, A.F.; Pedrosa, J.F.S.; Gonçalves, L.; Ventura, C.; Vital, N.; Bettencourt, A.; Fernandes, S.N.; da Rosa, R.R.; Godinho, M.H.; et al. Analysis of the in vitro toxicity of nanocelluloses in human lung cells as compared to multi-walled carbon nanotubes. Nanomaterials 2022, 12, 1432. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose nanocrystals for health care applications. In Applications of Nanomaterials, 1st ed.; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 415–459. [Google Scholar] [CrossRef]
- Abitbol, T.; Palermo, A.; Moran-Mirabal, J.M.; Cranston, E.D. Fluorescent labeling and characterization of cellulose nanocrystals with varying charge contents. Biomacromolecules 2013, 14, 3278–3284. [Google Scholar] [CrossRef]
- Reid, M.S.; Karlsson, M.; Abitbol, T. Fluorescently labeled cellulose nanofibrils for detection and loss analysis. Carbohydr. Polym. 2020, 250, 116943. [Google Scholar] [CrossRef]
- Patel, I.; Woodcock, J.; Beams, R.; Stranick, S.J.; Nieuwendaal, R.; Gilman, J.W.; Mulenos, M.R.; Sayes, C.M.; Salari, M.; DeLoid, G.; et al. Fluorescently Labeled Cellulose Nanofibers for Environmental Health and Safety Studies. Nanomaterials 2021, 11, 1015. [Google Scholar] [CrossRef]
- Salari, M.; Bitounis, D.; Bhattacharya, K.; Pyrgiotakis, G.; Zhang, Z.; Purington, E.; Gramlich, W.; Grondin, Y.; Rogers, R.; Bousfield, D.; et al. Development & characterization of fluorescently tagged nanocellulose for nanotoxicological studies. Environ. Sci. Nano 2019, 6, 1516–1526. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Drozd, N.N.; Paderin, N.M.; Tarabukin, D.V.; Udoratina, E.V. Hemocompatibility, biodegradability and acute toxicity of acetylated cellulose nanocrystals of different types in comparison. Carbohydr. Polym. 2021, 269, 118307. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion properties of nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef] [PubMed]
- Aimonen, K.; Suhonen, S.; Hartikainen, M.; Lopes, V.R.; Norppa, H.; Ferraz, N.; Catalán, J. Role of surface chemistry in the in vitro lung response to nanofibrillated cellulose. Nanomaterials 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef]
- Otuechere, C.A.; Adewuyi, A.; Adebayo, O.L.; Ebigwei, I.A. In vivo hepatotoxicity of chemically modified nanocellulose in rats. Hum. Exp. Toxicol. 2019, 39, 212–223. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Filipič, M.; Jose Frutos, M.; Galtier, P.; et al. Re-evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives. EFSA J. 2018, 16, e05047. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- Deloid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in: In vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano 2019, 6, 2105–2115. [Google Scholar] [CrossRef]
- FDA. SCOGS (Select Committee on GRAS Substances). Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 7 September 2022).
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- European Medicine Agency. Reflection Paper on Surface Coatings: General Issues for Consideration Regarding Parenteral Administration of Coated Nanomedicine Products. 2013. Available online: https://www.ema.europa.eu/en/surface-coatings-general-issues-consideration-regarding-parenteral-administration-coated (accessed on 10 May 2022).
- European Medicine Agency. Reflection Paper on the Data Requirements for Intravenous Iron-Based Nano-Colloidal Products Developed with Reference to an Innovator Medicinal Product. 2015. Available online: https://www.ema.europa.eu/en/data-requirements-intravenous-iron-based-nano-colloidal-products-developed-reference-innovator (accessed on 10 May 2022).
- Lu, Q.; Yu, X.; Yagoub, A.E.G.A.; Wahia, H.; Zhou, C. Application and challenge of nanocellulose in the food industry. Food Biosci. 2021, 43, 101285. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of food packaging based on nanocellulose: Current advances and challenges. Nanomaterials 2020, 10, 1726. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose bio-based composites for food packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Seoane, I.T.; Cerrutti, P.; Vazquez, A.; Manfredi, L.B.; Cyras, V.P. Polyhydroxybutyrate-Based Nanocomposites with Cellulose Nanocrystals and Bacterial Cellulose. J. Polym. Environ. 2017, 25, 586–598. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and conductive nanocellulose-based films for active and intelligent food packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef]
- Fotie, G.; Amoroso, L.; Muratore, G.; Piergiovanni, L. Carbon dioxide diffusion at different relative humidity through coating of cellulose nanocrystals for food packaging applications. Food Packag. Shelf Life 2018, 18, 62–70. [Google Scholar] [CrossRef]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and antimicrobial films based on brewers spent grain arabinoxylans, nanocellulose and feruloylated compounds for active packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.M.; Dadashi, S. Thermal and antimicrobial properties of chitosan-nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef]
- Ye, D.; Bramini, M.; Hristov, D.R.; Wan, S.; Salvati, A.; Åberg, C.; Dawson, K.A. Low uptake of silica nanoparticles in Caco-2 intestinal epithelial barriers. Beilstein J. Nanotechnol. 2017, 8, 1396–1406. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.M.; Zerafat, M.M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef]
- Dong, F.; Li, S.; Jin, C.; Liu, Z.; Zhu, K.; Zou, H.; Wang, X. Effect of nanocellulose/chitosan composite coatings on cucumber quality and shelf life. Toxicol. Environ. Chem. 2016, 98, 450–461. [Google Scholar] [CrossRef]
- Deng, Z.; Jung, J.; Simonsen, J.; Zhao, Y. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocoll. 2018, 84, 229–237. [Google Scholar] [CrossRef]
- Ma, Z.S.; Wang, Y.J.; Chen, S.S.; Zhang, N.; Sui, S.Y.; Zhang, L.P. Effects of mung bean hull nanocrystalline cellulose on the performance of whey protein concentrate edible film. Mod. Food Sci. Technol. 2016, 32, 40–45. [Google Scholar] [CrossRef]
- Chen, S.; Tao, H.; Wang, Y.; Ma, Z. Process optimization of soy protein isolate-based edible films containing nanocrystalline cellulose from sunflower seed hull and chitosan. Trans. Chin. Soc. Agric. Eng. 2016, 32, 306–314. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Casari, A.C.A.; Mariano, M.; Yamashita, F.; Mei, L.H.I.; Soldi, V.; Martelli, S.M. Effect of a gelatin-based edible coating containing cellulose nanocrystals (CNC) on the quality and nutrient retention of fresh strawberries during storage. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012024. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 2014, 30, 9327–9335. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Hedjazi, S.; Razavi, S.H. A comparison of Canthaxanthine Pickering emulsions, stabilized with cellulose nanocrystals of different origins. Int. J. Biol. Macromol. 2018, 106, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Cock, J.; Serpa, A.; Vélez, L.; Gañán, P.; Gómez Hoyos, C.; Castro, C.; Duizer, L.; Goff, H.D.; Zuluaga, R. Influence of cellulose nanofibrils on the structural elements of ice cream. Food Hydrocoll. 2019, 87, 204–213. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Liu, Y.; Li, J.; Yu, H. Soy protein isolate/cellulose nanofiber complex gels as fat substitutes: Rheological and textural properties and extent of cream imitation. Cellulose 2015, 22, 2619–2627. [Google Scholar] [CrossRef]
- Qi, W.; Wu, J.; Shu, Y.; Wang, H.; Rao, W.; Xu, H.N.; Zhang, Z. Microstructure and physiochemical properties of meat sausages based on nanocellulose-stabilized emulsions. Int. J. Biol. Macromol. 2020, 152, 567–575. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Douglas Goff, H.; Liu, W.; Chen, M.; Zhong, F. The resilience of nanocrystalline cellulose viscosity to simulated digestive processes and its influence on glucose diffusion. Carbohydr. Polym. 2018, 200, 436–445. [Google Scholar] [CrossRef]
- Liu, L.; Kerr, W.L.; Kong, F.; Dee, D.R.; Lin, M. Influence of nano-fibrillated cellulose (NFC) on starch digestion and glucose absorption. Carbohydr. Polym. 2018, 196, 146–153. [Google Scholar] [CrossRef]
- Laurén, P. Biomedical Applications of Nanofibrillar Cellulose. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2018. [Google Scholar]
- Tan, T.H.; Lee, H.V.; Yehya Dabdawb, W.A.; Hamid, S.B.B.O.A.A. A review of nanocellulose in the drug-delivery system. In Materials for Biomedical Engineering: Nanomaterials-Based Drug Delivery; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–164. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A review on cellulose nanocrystals as promising biocompounds for the synthesis of nanocomposite hydrogels. Carbohydr. Polym. 2019, 216, 247–259. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.S.; Abdat, M.; N.A.M., N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R.; et al. A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced Functional Materials Based on Nanocellulose for Pharmaceutical/Medical Applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Svagan, A.J.; Benjamins, J.W.; Al-Ansari, Z.; Shalom, D.B.; Müllertz, A.; Wågberg, L.; Löbmann, K. Solid cellulose nanofiber based foams—Towards facile design of sustained drug delivery systems. J. Control. Release 2016, 244, 74–82. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef]
- Paukkonen, H.; Ukkonen, A.; Szilvay, G.; Yliperttula, M.; Laaksonen, T. Hydrophobin-nanofibrillated cellulose stabilized emulsions for encapsulation and release of BCS class II drugs. Eur. J. Pharm. Sci. 2017, 100, 238–248. [Google Scholar] [CrossRef]
- Svagan, A.J.; Müllertz, A.; Löbmann, K. Floating solid cellulose nanofibre nanofoams for sustained release of the poorly soluble model drug furosemide. J. Pharm. Pharmacol. 2017, 69, 1477–1484. [Google Scholar] [CrossRef]
- Ching, Y.C.; Gunathilake, T.M.S.U.; Chuah, C.H.; Ching, K.Y.; Singh, R.; Liou, N.S. Curcumin/Tween 20-incorporated cellulose nanoparticles with enhanced curcumin solubility for nano-drug delivery: Characterization and in vitro evaluation. Cellulose 2019, 26, 5467–5481. [Google Scholar] [CrossRef]
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible Double-Membrane Hydrogels from Cationic Cellulose Nanocrystals and Anionic Alginate as Complexing Drugs Codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889. [Google Scholar] [CrossRef]
- Abo-Elseoud, W.S.; Hassan, M.L.; Sabaa, M.W.; Basha, M.; Hassan, E.A.; Fadel, S.M. Chitosan nanoparticles/cellulose nanocrystals nanocomposites as a carrier system for the controlled release of repaglinide. Int. J. Biol. Macromol. 2018, 111, 604–613. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Ferreira Cury, B.S.; dos Santos, A.M.; Franco, D.F.; Barud, H.S.; da Silva Filho, E.C. Resistant starch/pectin free-standing films reinforced with nanocellulose intended for colonic methotrexate release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Pereira, F.V.; Mota, F.A.P.; Watanabe, E.; Soares, S.M.C.S.; Santos, M.H. Dental glass ionomer cement reinforced by cellulose microfibers and cellulose nanocrystals. Mater. Sci. Eng. C 2016, 58, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Koivuniemi, R.; Hakkarainen, T.; Kiiskinen, J.; Kosonen, M.; Vuola, J.; Valtonen, J.; Luukko, K.; Kavola, H.; Yliperttula, M. Clinical Study of Nanofibrillar Cellulose Hydrogel Dressing for Skin Graft Donor Site Treatment. Adv. Wound Care 2020, 9, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Laurén, P.; Lou, Y.R.; Raki, M.; Urtti, A.; Bergström, K.; Yliperttula, M. Technetium-99m-labeled nanofibrillar cellulose hydrogel for in vivo drug release. Eur. J. Pharm. Sci. 2014, 65, 79–88. [Google Scholar] [CrossRef]
- Durand, H.; Smyth, M.; Bras, J. Nanocellulose: A New Biopolymer for Biomedical Application. In Biopolymers for Biomedical and Biotechnological Applications; Rehm, B., Moradali, M.F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2021; pp. 129–179. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Piergiovanni, L.; Fotie, G.; Amoroso, L.; Akgun, B.; Limbo, S. Are cellulose nanocrystals “alien particles” to human experience? Packag. Technol. Sci. 2019, 32, 637–640. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho Moniz, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Quiros Pesudo, L.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef]
- Cockburn, A.; Bradford, R.; Buck, N.; Constable, A.; Edwards, G.; Haber, B.; Hepburn, P.; Howlett, J.; Kampers, F.; Klein, C.; et al. Approaches to the safety assessment of engineered nanomaterials (ENM) in food. Food Chem. Toxicol. 2012, 50, 2224–2242. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Khare, S.; DeLoid, G.M.; Molina, R.M.; Gokulan, K.; Couvillion, S.P.; Bloodsworth, K.J.; Eder, E.K.; Wong, A.R.; Hoyt, D.W.; Bramer, L.M.; et al. Effects of ingested nanocellulose on intestinal microbiota and homeostasis in Wistar Han rats. NanoImpact 2020, 18, 100216. [Google Scholar] [CrossRef]
- Koshani, R.; Madadlou, A. A viewpoint on the gastrointestinal fate of cellulose nanocrystals. Trends Food Sci. Technol. 2018, 71, 268–273. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. The behavior of nanocellulose in gastrointestinal tract and its influence on food digestion. J. Food Eng. 2021, 292, 110346. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Wang, Y.; Kapronezai, K.; Lorente, L.R.; Zhang, R.; Pyrgiotakis, G.; Konduru, N.V.; Ericsson, M.; White, J.C.; De La Torre-Roche, R.; et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 40. [Google Scholar] [CrossRef]
- Sohal, I.S.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol. 2018, 15, 29. [Google Scholar] [CrossRef]
- NANoREG Partners. NANoREG Deliverable D 5.03; European Union: Brussels, Belgium, 2016. [Google Scholar]
- Peters, R.; Kramer, E.; Oomen, A.G.; Herrera Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.P.; Weigel, S.; et al. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef]
- Sieg, H.; Kästner, C.; Krause, B.; Meyer, T.; Burel, A.; Böhmert, L.; Lichtenstein, D.; Jungnickel, H.; Tentschert, J.; Laux, P.; et al. Impact of an artificial digestion procedure on aluminum-containing nanomaterials. Langmuir 2017, 33, 10726–10735. [Google Scholar] [CrossRef]
- Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the characteristics and cytotoxicity of titanium dioxide nanomaterials following simulated in vitro digestion. Nanomaterials 2020, 10, 1516. [Google Scholar] [CrossRef]
- Vieira, A.; Vital, N.; Rolo, D.; Roque, R.; Gonçalves, L.M.; Bettencourt, A.; Silva, M.J.; Louro, H. Investigation of the genotoxicity of digested titanium dioxide nanomaterials in human intestinal cells. Food Chem. Toxicol. 2022, 161, 112841. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, T.; DeLoid, G.M.; Gaffrey, M.J.; Weitz, K.K.; Thrall, B.D.; Qian, W.J.; Demokritou, P. Cytotoxicity and cellular proteome impact of cellulose nanocrystals using simulated digestion and an in vitro small intestinal epithelium cellular model. NanoImpact 2020, 20, 100269. [Google Scholar] [CrossRef]
- Sarkar, A.; Li, H.; Cray, D.; Boxall, S. Composite whey protein-cellulose nanocrystals at oil-water interface: Towards delaying lipid digestion. Food Hydrocoll. 2018, 77, 436–444. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-fibrillated cellulose on lipid digestion and absorption. Int. J. Biol. Macromol. 2019, 139, 361–366. [Google Scholar] [CrossRef]
- Lin, Y.J.; Shatkin, J.A.; Kong, F. Evaluating mucoadhesion properties of three types of nanocellulose in the gastrointestinal tract in vitro and ex vivo. Carbohydr. Polym. 2019, 210, 157–166. [Google Scholar] [CrossRef]
- Ede, J.D.; Ong, K.J.; Mulenos, M.R.; Pradhan, S.; Gibb, M.; Sayes, C.M.; Shatkin, J.A. Physical, chemical, and toxicological characterization of sulfated cellulose nanocrystals for food-related applications using in vivo and in vitro strategies. Toxicol. Res. 2020, 9, 808–822. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. Influence of nanocellulose on in vitro digestion of whey protein isolate. Carbohydr. Polym. 2019, 210, 399–411. [Google Scholar] [CrossRef]
- McClements, D.J.; DeLoid, G.; Pyrgiotakis, G.; Shatkin, J.A.; Xiao, H.; Demokritou, P. The role of the food matrix and gastrointestinal tract in the assessment of biological properties of ingested engineered nanomaterials (iENMs): State of the science and knowledge gaps. NanoImpact 2016, 3–4, 47–57. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Xiao, H.; Bhattacharya, K.; Bitounis, D.; Demokritou, P.; McClements, D.J. Development of a standardized food model for studying the impact of food matrix effects on the gastrointestinal fate and toxicity of ingested nanomaterials. NanoImpact 2019, 13, 13–25. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, T.; DeLoid, G.M.; Gaffrey, M.J.; Weitz, K.K.; Thrall, B.D.; Qian, W.J.; Demokritou, P. Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model. NanoImpact 2020, 17, 100202. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Deloid, G.M.; Bitounis, D.; De La Torre-Roche, R.; White, J.C.; Zhang, Z.; Ho, C.G.; Ng, K.W.; Eitzer, B.D.; Demokritou, P. Co-exposure to the food additives SiO2 (E551) or TiO2 (E171) and the pesticide boscalid increases cytotoxicity and bioavailability of the pesticide in a tri-culture small intestinal epithelium model: Potential health implications. Environ. Sci. Nano 2019, 6, 2786–2800. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Liu, C.; Li, M.; Sun, Q.; Xiong, L. Interaction of cellulose nanocrystals and amylase: Its influence on enzyme activity and resistant starch content. Food Chem. 2018, 245, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Y.J.; Nagy, T.; Kong, F.; Guo, T.L. Subchronic exposure to cellulose nanofibrils induces nutritional risk by non-specifically reducing the intestinal absorption. Carbohydr. Polym. 2020, 229, 115536. [Google Scholar] [CrossRef]
- Elespuru, R.; Pfuhler, S.; Aardema, M.J.; Chen, T.; Doak, S.H.; Doherty, A.; Farabaugh, C.S.; Kenny, J.; Manjanatha, M.; Mahadevan, B.; et al. Genotoxicity assessment of nanomaterials: Recommendations on best practices, assays, and methods. Toxicol. Sci. 2018, 164, 391–416. [Google Scholar] [CrossRef]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of nanomaterials: Advanced in vitro models and high throughput methods for human hazard assessment—A review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef]
- Vital, N.; Pinhão, M.; Yamani, N.E.; Rundén-Pran, E.; Louro, H.; Dušinská, M.; Silva, M.J. Hazard Assessment of Benchmark Metal-Based Nanomaterials Through a Set of In Vitro Genotoxicity Assays. In Nanotoxicology in Safety Assessment of Nanomaterials, 1st ed.; Louro, H., Silva, M.J., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2022; Volume 1357, pp. 351–375. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Ansón-Casaos, A.; Grasa, L.; Abenia, L.; Salvador, A.; Colom, E.; Mesonero, J.E.; García-Bordejé, J.E.; Benito, A.M.; Maser, W.K. Unique properties and behavior of nonmercerized type-II cellulose nanocrystals as carbon nanotube biocompatible dispersants. Biomacromolecules 2019, 20, 3147–3160. [Google Scholar] [CrossRef]
- Lin, Y.J.; Qin, Z.; Paton, C.M.; Fox, D.M.; Kong, F. Influence of cellulose nanocrystals (CNC) on permeation through intestinal monolayer and mucus model in vitro. Carbohydr. Polym. 2021, 263, 117984. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Alam, M.N.; Sim, G.; Tufenkji, N.; Van De Ven, T.G.M. Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 2015, 7, 16647–16657. [Google Scholar] [CrossRef]
- Hanif, Z.; Ahmed, F.R.; Shin, S.W.; Kim, Y.K.; Um, S.H. Size- and dose-dependent toxicity of cellulose nanocrystals (CNC) on human fibroblasts and colon adenocarcinoma. Colloids Surf. B Biointerfaces 2014, 119, 162–165. [Google Scholar] [CrossRef]
- Yusefi, M.; Soon, M.L.K.; Teow, S.Y.; Monchouguy, E.I.; Neerooa, B.N.H.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J.; Shameli, K. Fabrication of cellulose nanocrystals as potential anticancer drug delivery systems for colorectal cancer treatment. Int. J. Biol. Macromol. 2022, 199, 372–385. [Google Scholar] [CrossRef]
- Vakili, M.R.; Mohammed-Saeid, W.; Aljasser, A.; Hopwood-Raja, J.; Ahvazi, B.; Hrynets, Y.; Betti, M.; Lavasanifar, A. Development of mucoadhesive hydrogels based on polyacrylic acid grafted cellulose nanocrystals for local cisplatin delivery. Carbohydr. Polym. 2021, 255, 117332. [Google Scholar] [CrossRef]
- Hanif, Z.; Khan, Z.A.; Tariq, M.Z.; Choi, D.; Park, S.J. Coatable tannic acid-deposited cellulose nanocrystals for Fe(III) sensing and its application to a facile, scalable and portable sensing platform. Dyes Pigment. 2021, 196, 109732. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liu, X.; Liao, Y.P.; Ma, T.; Meng, H.; Xia, T. Nanocellulose Length Determines the Differential Cytotoxic Effects and Inflammatory Responses in Macrophages and Hepatocytes. Small 2021, 17, 2102545. [Google Scholar] [CrossRef]
- O’Connor, B.; Berry, R.; Goguen, R. Commercialization of Cellulose Nanocrystal (NCCTM) Production: A Business Case Focusing on the Importance of Proactive EHS Management. In Nanotechnology Environmental Health and Safety: Risks, Regulation, and Management, 2nd ed.; Hull, M.S., Bowman, D.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 225–246. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Martins, J.T.; Vicente, A.A.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: Characterization and cytotoxicity assessment. Food Hydrocoll. 2018, 75, 192–201. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Sun, L.; Kong, F.; Lin, M.; Mustapha, A. Preparation of cellulose nanofibril/titanium dioxide nanoparticle nanocomposites as fillers for PVA-based packaging and investigation into their intestinal toxicity. Int. J. Biol. Macromol. 2020, 156, 1174–1182. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Dhital, R.; Kong, F.; Lin, M.; Mustapha, A. Antimicrobial effect and toxicity of cellulose nanofibril/silver nanoparticle nanocomposites prepared by an ultraviolet irradiation method. Colloids Surf. B Biointerfaces 2019, 180, 212–220. [Google Scholar] [CrossRef]
- Pradhan, S.H.; Mulenos, M.R.; Steele, L.R.; Gibb, M.; Ede, J.D.; Ong, K.J.; Shatkin, J.A.; Sayes, C.M. Physical, chemical, and toxicological characterization of fibrillated forms of cellulose using an in vitro gastrointestinal digestion and co-culture model. Toxicol. Res. 2020, 9, 290–301. [Google Scholar] [CrossRef]
- De Lima Pizi Cândido, A.; Fregonezi, N.F.; Carvalho, A.J.F.; Trovatti, E.; Resende, F.A. TEMPO-Oxidized Cellulose Nanofibers In Vitro Cyto-genotoxicity Studies. BioNanoScience 2020, 10, 766–772. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; Guguen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef]
- Fujita, K.; Obara, S.; Maru, J.; Endoh, S. Genotoxicity assessment of cellulose nanofibrils using a standard battery of in vitro and in vivo assays. Toxicol. Rep. 2022, 9, 68–77. [Google Scholar] [CrossRef]
- Pitkänen, M.; Kangas, H.; Laitinen, O.; Sneck, A.; Lahtinen, P.; Peresin, M.S.; Niinimäki, J. Characteristics and safety of nano-sized cellulose fibrils. Cellulose 2014, 21, 3871–3886. [Google Scholar] [CrossRef]
- Doak, S.H.; Manshian, B.; Jenkins, G.J.S.; Singh, N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat. Res. 2012, 745, 104–111. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Genotoxicity of Manufactured Nanomaterials: Report of the OECD Expert Meeting; OECD: Paris, France, 2014; Volume 43, pp. 1–37. [Google Scholar]
- Zhang, Q.; Wang, M.; Mu, G.; Ren, H.; He, C.; Xie, Q.; Liu, Q.; Wang, J.; Cha, R. Adsorptivity of cationic cellulose nanocrystals for phosphate and its application in hyperphosphatemia therapy. Carbohydr. Polym. 2021, 255, 117335. [Google Scholar] [CrossRef]
- Otuechere, C.A.; Adewuyi, A.; Salau, T.O.B.; Neupane, N.P.; Adebayo, O.L.; Egunjobi, M.; Verma, A. Polyathia longifolia: Redox potential of a cellulose nanocrystal derivative and ADMET predictions of selected compounds. Biocatal. Agric. Biotechnol. 2022, 40, 102295. [Google Scholar] [CrossRef]
- Ong, K.J.; Shatkin, J.A.; Nelson, K.; Ede, J.D.; Retsina, T. Establishing the safety of novel bio-based cellulose nanomaterials for commercialization. NanoImpact 2017, 6, 19–29. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhou, Y.X.; Saqib, M.N.; Chen, M.; Douglas Goff, H.; Ma, J.; Zhong, F. Enhancing the prebiotic effect of cellulose biopolymer in the gut by physical structuring via particle size manipulation. Food Res. Int. 2020, 131, 108935. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Nagano, T.; Yano, H. Dietary cellulose nanofiber modulates obesity and gut microbiota in high-fat-fed mice. Bioact. Carbohydr. Diet. Fibre 2020, 22, 100214. [Google Scholar] [CrossRef]
- Ong, K.J.; Ede, J.D.; Pomeroy-Carter, C.A.; Sayes, C.M.; Mulenos, M.R.; Shatkin, J.A. A 90-day dietary study with fibrillated cellulose in Sprague-Dawley rats. Toxicol. Rep. 2020, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Ifuku, S.; Maeda, H.; Morimoto, M.; Takashima, O.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Saimoto, H.; et al. Suppressive effects of cellulose nanofibers—Made from adlay and seaweed—On colon inflammation in an inflammatory bowel-disease model. Bioact. Carbohydr. Diet. Fibre 2013, 2, 65–72. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Morimoto, M.; Takashima, O.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Anti-inflammatory effects of cellulose nanofiber made from pear in inflammatory bowel disease model. Bioact. Carbohydr. Diet. Fibre 2014, 3, 1–10. [Google Scholar] [CrossRef]
- Bitounis, D.; Pyrgiotakis, G.; Bousfield, D.; Demokritou, P. Dispersion preparation, characterization, and dosimetric analysis of cellulose nano-fibrils and nano-crystals: Implications for cellular toxicological studies. NanoImpact 2019, 15, 100171. [Google Scholar] [CrossRef]
- Cohen, J.M.; Beltran-Huarac, J.; Pyrgiotakis, G.; Demokritou, P. Effective delivery of sonication energy to fast settling and agglomerating nanomaterial suspensions for cellular studies: Implications for stability, particle kinetics, dosimetry and toxicity. NanoImpact 2018, 10, 81–86. [Google Scholar] [CrossRef]
- Ventura, C.; Lourenço, A.F.; Sousa-Uva, A.; Ferreira, P.J.T.; Silva, M.J. Evaluating the genotoxicity of cellulose nanofibrils in a co-culture of human lung epithelial cells and monocyte-derived macrophages. Toxicol. Lett. 2018, 291, 173–183. [Google Scholar] [CrossRef]
- Hadrup, N.; Knudsen, K.B.; Berthing, T.; Wolff, H.; Bengtson, S.; Kofoed, C.; Espersen, R.; Højgaard, C.; Winther, J.R.; Willemoës, M.; et al. Pulmonary effects of nanofibrillated celluloses in mice suggest that carboxylation lowers the inflammatory and acute phase responses. Environ. Toxicol. Pharmacol. 2019, 66, 116–125. [Google Scholar] [CrossRef]
- Jensen, A.K.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.R.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Towards a Method for Detecting the Potential Genotoxicity of Nanomaterials: Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media Report; The Generic NANOGENOTOX Dispersion Protocol Standard Operation Procedure (SOP) and Background Documentation; NANOGENOTOX: Copenhagen, Denmark, 2011. [Google Scholar]
- Louro, H.; Saruga, A.; Santos, J.; Pinhão, M.; Silva, M.J. Biological impact of metal nanomaterials in relation to their physicochemical characteristics. Toxicol. In Vitro 2019, 56, 172–183. [Google Scholar] [CrossRef]
- Louro, H. Relevance of physicochemical characterization of nanomaterials for understanding nano-cellular interactions. In Cellular and Molecular Toxicology of Nanoparticles, 1st ed.; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1048, pp. 123–142. [Google Scholar] [CrossRef]
- Tavares, A.M.; Louro, H.; Antunes, S.; Quarré, S.; Simar, S.; De Temmerman, P.J.; Verleysen, E.; Mast, J.; Jensen, K.A.; Norppa, H.; et al. Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi-walled carbon nanotubes in human lymphocytes. Toxicol. In Vitro 2014, 28, 60–69. [Google Scholar] [CrossRef]
- Louro, H.; Pinhão, M.; Santos, J.; Tavares, A.; Vital, N.; Silva, M.J. Evaluation of the cytotoxic and genotoxic effects of benchmark multi-walled carbon nanotubes in relation to their physicochemical properties. Toxicol. Lett. 2016, 262, 123–134. [Google Scholar] [CrossRef]
- Ventura, C.; Pereira, J.F.S.; Matos, P.; Marques, B.; Jordan, P.; Sousa-Uva, A.; Silva, M.J. Cytotoxicity and genotoxicity of MWCNT-7 and crocidolite: Assessment in alveolar epithelial cells versus their coculture with monocyte-derived macrophages. Nanotoxicology 2019, 14, 479–503. [Google Scholar] [CrossRef]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef]
- Čolić, M.; Tomić, S.; Bekić, M. Immunological aspects of nanocellulose. Immunol. Lett. 2020, 222, 80–89. [Google Scholar] [CrossRef]
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J.H.T. Probing inhibitory effects of nanocrystalline cellulose: Inhibition versus surface charge. Nanoscale 2012, 4, 1373–1379. [Google Scholar] [CrossRef]
- Harper, B.J.; Clendaniel, A.; Sinche, F.; Way, D.; Hughes, M.; Schardt, J.; Simonsen, J.; Stefaniak, A.B.; Harper, S.L. Impacts of chemical modification on the toxicity of diverse nanocellulose materials to developing zebrafish. Cellulose 2016, 23, 1763–1775. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef]
- Balea, A.; Blanco, A.; Delgado-Aguilar, M.; Monte, M.C.; Tarrés, Q.; Fuente, E.; Mutjé, P.; Negro, C. Nanocellulose characterization challenges. BioResources 2021, 16, 4382–4410. [Google Scholar] [CrossRef]
- Campano, C.; Lopez-Exposito, P.; Gonzalez-Aguilera, L.; Blanco, Á.; Negro, C. In-depth characterization of the aggregation state of cellulose nanocrystals through analysis of transmission electron microscopy images. Carbohydr. Polym. 2021, 254, 117271. [Google Scholar] [CrossRef]
- Duan, Y.; Coreas, R.; Liu, Y.; Bitounis, D.; Zhang, Z.; Parviz, D.; Strano, M.; Demokritou, P.; Zhong, W. Prediction of protein corona on nanomaterials by machine learning using novel descriptors. NanoImpact 2020, 17, 100207. [Google Scholar] [CrossRef]
- Kim, S.M.; Gwak, E.J.; Jeong, S.H.; Lee, S.M.; Sim, W.J.; Kim, J.S. Toxicity evaluation of cellulose nanofibers (Cnfs) for cosmetic industry application. J. Toxicol. Risk Assess. 2019, 5, 29. [Google Scholar] [CrossRef]
- Kämpfer, A.A.M.; Busch, M.; Schins, R.P.F. Advanced in vitro testing strategies and models of the intestine for nanosafety research. Chem. Res. Toxicol. 2020, 33, 1163–1178. [Google Scholar] [CrossRef]
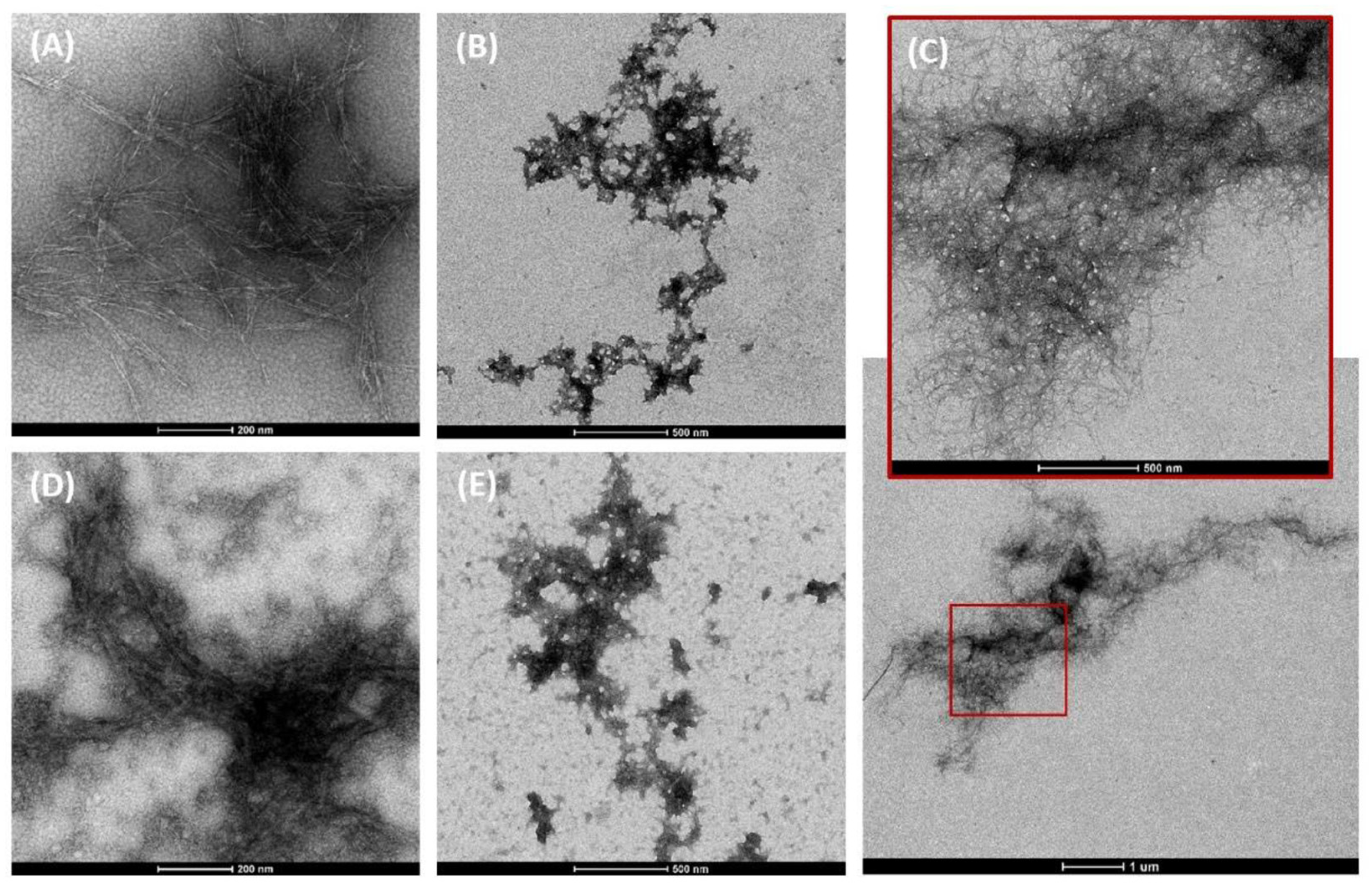
| Effects | Description | Reference |
|---|---|---|
| Influence of the food matrix on the physicochemical properties of CNMs | ||
| Interaction of the food matrix with CNMs | Larger particle agglomerates were observed in the food matrix when in the presence of CNCs, suggesting possible binding of CNCs to the nutrient particles (e.g., fat droplets) | [134] |
| Influence of CNMs on the digestion of food components | ||
| CNMs’ interactions with fat | Reduction in triglyceride hydrolysis by CNFs and CNCs. CNMs can interact with fatty foods, thereby substantially reducing the digestion and absorption of fat | [10] |
| Impact of CNCs on lipid digestion | CNCs sequester bile salt and bind with protein-coated lipid droplets via bridging effects, restricting the available surface area for lipase | [135] |
| Influence of CNCs on the digesta’s viscosity and the subsequent release and diffusion of glucose | CNCs modulate the viscosity of the digesta. The release and diffusion rates of glucose were significantly reduced in the CNC–food system, and the digestion and diffusion of starch and glucose were delayed. | [92] |
| CNFs’ effects on lipid digestion and absorption and related mechanisms | CNFs slightly reduced lipase activity and increased intestinal digesta viscosity, and had a bile-acid-retardation effect; CNFs led to higher cholesterol adsorption compared to cellulose, but did not affect cholesterol micellar solubility; CNFs did not affect the total amounts of free fatty acids produced during lipid digestion | [136] |
| Influence of digestion on the physicochemical and biological properties of CNMs | ||
| Effect of digestion conditions on the mucoadhesion of CNMs | CNMs have mucoadhesive properties in the digestive tract, with the level of adhesion depending on the type and concentration of CNMs, as well as the gastrointestinal compartment; CNCs showed the highest viscosity synergism in the stomach, while TEMPO–CNFs displayed synergism only under gelling concentrations | [137] |
| Effects of in vitro digestion on CNMs’ size and surface charge | No effect | [138] |
| Effects of digestion on CNMs’ size and viscosity | No observable changes in the particle size of CNCs, CNFs, and CNF–TEMPO in all digestive compartments; In the stomach, CNF–TEMPO aggregated and formed phase separation, resulting in decreased viscosity and increased particle size; CNCs formed hydrogel networks, causing increased viscosity | [139] |
| CNMs | Endpoint (Assay) | Cellular Model | Source; Isolation Method; Isolation Conditions | Characteristics | Dispersion Method | Concentrations Tested | Endotoxin and Sterility Check | In Vitro Digestion | Exposure Duration (h) | Main Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CNCs | |||||||||||
| CNC1 CNC2 CNC3 CNC4 | Cytotoxicity (WST-1) | HCT116 | Fluka Avicel PH-101; AH (CNC1: H2SO4; CNC2 H2SO4/HCl; CNC3:NAOH/H2SO4/HCl;CNC4: HC) | CNC1 L: 256 ± 64.8 nm; CNC2 L: 140.5 ± 37.5 nm; CNC3 L: 108.4 ± 94.8 nm; CNC4 L: 1174 ± 338.7 nm; | NA | 10–1000 μg/mL | NA | No | 24 h | Cytotoxic effects at concentrations equal to or above 500 μg/mL | [154] |
| CNCs with variable COOH contents | Cytotoxicity (LDH release; MTS; live/dead staining), cell uptake | Caco-2 | Softwood pulp; AH (HCl) | d: 125 nm to 234 nm; L: 97 nm to 110 nm; w: 1 nm to 8 nm; –COOH content: 1.7 to 6.6 mmol/g | NA | 100–300 μg/mL | NA | No | 24 h | Charge-dependent decrease in mitochondrial activity for -COOH contents higher than 3.8 mmol/g; no cellular membrane disruption and low cell uptake of CNCs | [153] |
| CNC-AH30S CNC-AH60S | Cytotoxicity (resazurin) | Caco-2 | Dry grape pomace residue; AH (H2SO4) | CNC-AH30S: L: 307 nm; w: 8 nm; L/d: 38; CI: 70.62% CNC-AH60S: L: 323 nm; w: 7 nm; L/d: 46; CI: 74.89% | Magnetic stirring followed by ultrasonication (15 min; 37 KHz; 104 W) | 50–200 μg/mL | NA | No | 48 h | No effects | [149] |
| CNC30 CNC60 CNC90 | Cytotoxicity (MTT) | Caco-2 | Wheat bran; AH (H2SO4) | CNC30: L: 644.77 ± 225.2 nm; w: 33.80 ± 9.83 nm; L/d: 20.39 ± 8.4; ZP: −36.5 ± 0.8 mV; Y: 37.11 ± 1.43% CNC60: L: 568.81 ± 229.66 nm; w: 21.57 ± 9.71 nm; L/d: 30.01 ± 13.88; ZP: −39.8 ± 1 mV; Y: 35.11 ± 0.95% CNC90: L: 486.18 ± 177.36 nm; w: 16.94 ± 7.30 nm; L/d: 32.11 ± 13.19; ZP: −39.6 ± 1.2 mV; Y: 28.7 ± 1.54% | NA | 50–5000 μg/mL | NA | No | 24 h | Cytotoxicity at 5000 μg/mL | [150] |
| CNC-25 | Cytotoxicity (LDH release), oxidative stress (flow cytometry with CellROX® green reagent) TEER | Caco-2/HT29MTX/Raji B | Softwood bleached kraft fibre; AH (H2SO4) | L: 267 ± 91 nm; d: 25.2 ± 9 nm; L/d: 11.5 ± 3.2; SSA: 93 m2/g | NA | 0,75% and 1.5% (w/w) | Endotoxin levels using the EndoZyme® recombinant factor C (rFC) assay; microbiological assessment | Yes | 24 h | Moderate cytotoxicity increase and significant ROS increase at the highest dose in the fasting food model (<1.1-fold) | [62] |
| FITC-CNC | Cytotoxicity (LDH release) TEER | Caco-2/HT29MTX/Raji B | Softwood bleached kraft fibre; AH (H2SO4) | L: 267 ± 91 nm; d: 25.2 ± 9 nm; L/d: 11.5 ± 3.2; SSA: 93 m2/g | Homogenisation (vortex, 20 s) | 0.75% w/w (7500 μg/mL) and 1.5% w/w (15,000 μg/mL) | Endotoxin level using the EndoZyme® recombinant factor C (rFC) assay; microbiological assessment | Yes | 24 h | No cytotoxic effects; epithelial integrity maintained | [52] |
| CNCs | Cytotoxicity (LDH release), TEER, oxidative stress (flow cytometry with CellROX® green reagent), proteomics (liquid chromatography with mass spectrometry) | Caco-2/HT29MTX/Raji B | Softwood bleached kraft fibre; AH (H2SO4) | L: 267 ± 91 nm; d: 25.2 ± 9 nm; L/d: 11.5 ± 3.2; SSA: 93 m2/g | NA | 156.25; 312.5 μg/mL | Endotoxin levels using the EndoZyme® recombinant factor C (rFC) assay; microbiological assessment | Yes | 24 h | Cytotoxicity and ROS induction at the highest dose in the fasting food model (<1.1-fold); 125 significantly differentially expressed proteins; epithelial integrity maintained | [134] |
| CNCs | Cytotoxicity (MTS), oxidative stress (flow cytometry with CellROX® green reagent), TEER, inflammation (IL-6 expression) | Caco-2/HT29MTX/Raji B | Wood pulp; AH (H2SO4) | L: 25 to 250 nm; w: <10 nm; w: 893 ± 251 nm; PDI: 0.51 ± 0.02; ZP: −50.8 ± 6 mV, rod-shaped | Homogenisation (Vortex-Genie 2, 10 min) | 0.02% (w/w) | Assessment of impurities and microbiological contaminants | Yes + lysosomal digestion | 1 h, 6 h, 24 h, or 48 h | No effects in any of the endpoints analysed | [138] |
| CNCs-1 CNCs-2 CNCs-3 | Cytotoxicity (ATP, MTT; activations of caspases), oxidative stress (H2DCFA; glutathione; mtROS), lysosomal damage, release of IL-1β and TNF-α | Hepa 1–6 cells and Kupffer cells (KUP5) | NA | CNCs-1: L: 149.0 ± 50 nm; w: 16 ± 5.0 nm; L/d: 9.3 ± 1.9; d: 121.9 ± 19.9; ZP: −14.3 ± 1.6 mV; CNCs-2: L: 279.1 ± 116.3 nm; w: 22.4 ± 7.2 nm; L/d: 12.7 ± 2.2; d: 249.5 ± 44.8; ZP: −12.8 ± 1.5 mV; CNCs-3: L: 715.0 ± 315.0 nm; w: 27.0 ± 8.0 nm; L/d: 26.5 ± 5.9; d: 780.2 ± 57.7; ZP: −11.7 ± 1.2 mV; | Vortexing + bath ultrasonication (15 min, 42 Khz, 100 W) | 25–200 µg/mL | Endotoxin levels using the limulus amebocyte lysate (LAL) | NO | 24 h | No cytotoxic effects in Hepa 1–6 cells; decreased cell viability and significant morphological alteration in KUP5 cells; mtROS induction, caspase-3/7 activation, apoptotic cell death, lysosomal damage, cathepsin B release, NLRP3 inflammasome and caspase-1 activation, leading to IL-1β production in KUP5 cells, cellular uptake, mtROS generation, and caspase-3/7-mediated apoptotic cell death in Hepa 1–6 cells | [158] |
| CNCs | Cytotoxicity (MTT) | Caco-2/TC7 (undifferentiated or differentiated) | Microcrystalline cellulose (MCC) from cotton linters; AH (H2SO4) | CNC type I: L: 200−300 nm; w: 5−10 nm; ZP: >20 mV CNC type II: L: 20−100 nm; w: 15−20 nm; ZP: >20 mV | NA | 0–5 μg/mL | NA | No | 72 h | No cytotoxic effects | [151] |
| CNCs | Cytotoxicity (MTT), cellular permeation | Caco-2 differentiated | Wood pulp; AH (H2SO4) | L: 150−200 nm; w: 5−20 nm; d: 98.9 ± 2.5 nm. | NA | 1–10,000 μg/mL | NA | Yes, but not for biological studies | 24 h | No cytotoxicity and no cellular permeation | [152] |
| CNCs | Cytotoxicity (clonogenic assay) | HCT116 HT-29 CCD112 colon fibroblasts | Rice straw waste; AH (H2SO4) | d: 109.64 ± 2.8 nm; ZP: −42.76 ± 1.4 mV | NA | 7.8–500 µg/mL | NA | No | 72 h | No cytotoxicity, except in HT-29 cells at the highest dose | [155] |
| CNCs CNCs grafted with poly(acrylic acid) | Cytotoxicity (MTT) | HCT116 | Bleached wood pulp; AH (H2SO4) | L: 100–200 nm; w: 5–15 nm; density: 1.6 g/cm3, CI: >80% SSA: 200–300 m2/g; ZP: −28.37 ± 0.65 mV | NA | 7.03–450 µg/mL | NA | No | 72 h | No cytotoxic effects | [156] |
| CNCs/tannic acid | Cytotoxicity (MTT) | HepG2 | Microcrystalline cellulose Avicel® PH-101; AH (H2SO4) | L: 220 ± 67 nm; d: 156.72 ± 57.47 nm; ZP: 41.62 ± 0.42 mV | NA | 10–30,000 µg/mL | NA | No | 24 h | No cytotoxic effects | [157] |
| CNFs | |||||||||||
| CNF | Cytotoxicity (resazurin; alamarBlue) | HepaRG HepG2 | Bleached birch pulp; controlled homogenisation process using an industrial fluidiser | w: 20–30 nm | NA | 0.1–1% (w/w) | Sterilised by autoclaving (121 °C, 20 min). | No | 30 and 5 days | No cytotoxic effects | [165] |
| CNFs-N 0.1%; N 1%; N 10% CNFs-NM 0.1%; NM 1%; NM 10% | Cytotoxicity (MTT) | Caco-2 | Unripe banana peel bran; AH (H2SO4 and/or MT: HPH) | d: 2.89 nm to 4.65 nm; L: 310.77 nm to 619.57 nm; L/d: 93.95 to 143.51; z-potential: 37.60 mV to 67.37 mV; Y: 27.07 to 71.51 | NA | 50–5000 μg/mL | NA | No | 24 h | Cytotoxicity above 2000 mg/mL | [160] |
| CNF1 15% CNF2 35% | Cytotoxicity (MTT) | Caco-2 | Banana peel bran with different concentrations (15% and 35%); enzymatic hydrolysis | CNF1: L: 1490.0 ± 107.3 nm; d: 3.7 ± 0.4 nm; L/d: 404 ± 63.9; ZP: −29.1.5 ± 0.7 mV; CI 61.5 ± 1.1 CNF2: L: 1544.5 ± 40.6 nm; d: 8.8 ± 0.7 nm; L/d: 170.2 ± 14.7; ZP: −31.5 ± 2.9 mV; CI: 66.2.5 ± 4.1 | NA | 50–5000 μg/mL | NA | No | 24 h | Cytotoxicity at 5000 μg/mL (74.59% and 73.13%) | [35] |
| CNFs | Cytotoxicity (LDH release), oxidative stress (flow cytometry with CellROX® green reagent), TEER | Caco-2/HT29MTX/Raji B | CNFs: softwood bleached kraft fibre; mechanical ultrafine friction grinding; autoclaved | L: 6710 ± 5611 nm; d: 64 ± 29 nm; NtN: 335.60 ± 232.66 nm; L/d: 107.6 ± 54.5; SSA: 34 m2/g | NA | 0,75% and 1.5% (w/w) | Endotoxin level using the EndoZyme® recombinant factor C (rFC) assay; microbiological assessment | Yes | 24 h | No effects | [62] |
| CNF/Ag | Cytotoxicity (MTT; WST-8) | Caco-2 FHC | CNF slurry | L: 95.22 ± 0.29 nm; ZP: −21.13 mV | NA | 50–1000 μg/mL | NA | No | 24 h | No cytotoxic effects | [17] |
| FITC-CNF | Cytotoxicity (LDH release), TEER | Caco-2/HT29MTX/Raji B | CNFs: softwood bleached kraft fibre; mechanical ultrafine friction grinding; autoclaved. | L: 6710 ± 5611 nm; d: 64 ± 29 nm; NtN: 335.60 ± 232.66 nm; L/d: 107.6 ± 54.5; SSA: 34 m2/g | Homogenisation (vortex, 20 s) | 0.75% w/w (7500 μg/mL) and 1.5% w/w (15,000 μg/mL) | Endotoxin level using the EndoZyme® recombinant factor C (rFC) assay; microbiological assessment | Yes | 24 h | No cytotoxic effects; epithelial integrity maintained | [52] |
| CNF–U enzymatic pretreatment CNF–A carboxymethylated CNF–C cationic CNF–P phosphorylated CNF–S sulphoethylated | Cytotoxicity (resazurin live/dead staining), effects on the human gut bacteria Escherichia coli and Lactobacillus reuteri | Caco-2 | Never-dried bleached sulphite dissolved softwood pulp; enzymatic pretreatment; carboxymethylation, phosphorylation, sulphoethylation | CNF–U: w: 10–30 nm; ZP: −8.7 ± 1.3; CI: 61%; content functional groups: 30 mol/g CNF–A: w: 4–5 nm; ZP: −13.0 ± 0.8; CI: 52%; content functional groups: 570 mol/g CNF–C: w: 4–5 nm ZP: −11.7 ± 0.9; CI: 61%; content functional groups: 634 mol/g CNF–P: w: 4–5 nm ZP: −16.3 ± 1.6; CI: 45%; content functional groups: 1109 mol/g CNF–S: w: 4–5 nm ZP: −11.8 ± 0.6; CI: 56%; content functional groups: 444 mol/g | Ultrasonication (70% amplitude; 12 min, 20 KHz, 600 W) | 50–500 μg/mL | Sterilised by autoclaving (20 min, 121 °C, 15 KPa) | No | 24 h; 48 h | Cytotoxicity for carboxymethylated CNFs after 48 h at 500 μg/mL; bacteriostatic effect on Escherichia coli but not on Lactobacillus reuteri | [44] |
| Fibrillated Celluloses | Cytotoxicity (MTS), ROS, TEER, inflammation | Caco-2/HT29MTX/Raji B | Wood pulp | C20: d: 2.07 ± 0.03 μm; PDI: 0.871 ± 0.05; ZP: 46.4 ± 1.7 mV; C21: d: 1.24 ± 0.049 μm; PDI: 0.723 ± 0.132; ZP: −23.8 ± 1.83; C22: d: 2.46 ± 0.184 μm; PDI: 0.532 ± 0.25; ZP: −27.4 ± 6.98; C23: d: 1.449 ± 0.029 μm; PDI: 0.924 ± 0.083; ZP: −19.10 ± 0.93; C24: d: 0.809 ± 0.034 μm; PDI: 0.686 ± 0.036; ZP: −12.50 ± 0.83; C25: d: 0.646 ± 0.141 μm; PDI: 0.585 ± 0.208; ZP: −5.20 ± 0.19; | Homogenisation (Vortex-Genie 2, 10 min) | 0.4% (w/w) | Metal impurities | Yes + lysosomal digestion | 1, 6, 24, or 48 h | No effects | [163] |
| CNFs | Cytotoxicity (MTT) | HepG2 | Sugarcane bagasse; TEMPO oxidation, sterilised, mechanical | w: 20 nm | NA | 0.01–0.5 (w/w) | Sterilised before use following ISO10993-12 | No | 48 h | No cytotoxic effects | [164] |
| CNFs | Cytotoxicity (alamarBlue) | Caco-2 | softwood kraft pulp (3% w/w, slurry form); ultrafine grinder | L: several hundred μm w: 50 nm ZP: −48 to −5 Mv | Homogenisation (vortex mixer) | 0–500 μg/ml | NA | No | 48 h | No cytotoxic effects | [145] |
| Multiple CNFs, carboxymethylated | Cytotoxicity (MTT) | Caco-2 | Cotton linter pulp; mechanical stirring or carboxymethylated pretreatment; HPH | ZP: 12.4 ± 1.7, 21.8 ± 1.2, 26.7 ± 1.0, 34.2 ± 2.2 mV carboxyl content: 0, 0.36, 0.72, and 1.24 mmol/g | Vortex mixer | 100–1000 μg/mL | Sterilised by filtration | No | 24 h | No cytotoxic effects | [43] |
| mDTEB- CNF and CNFs | Cytotoxicity (LDH release; resazurin) | Caco-2/HT29MTX/Raji B | Wood pulp; mechanical treatment; autoclaved | mDTEB- CNF: d: 24.95 µm; ZP: −36.20 mV CNFs: d: 8.17 µm; ZP: −35.60 mV | NA | 0.75% (w/w) and 1.5% (w/w) | Endotoxin levels using the EndoZyme® recombinant factor C (rFC) assay; microbiological assessment | Yes | 24 h | No cytotoxic effects | [51] |
| CNFs | Cytotoxicity (ATP, MTT; activations of caspases), oxidative stress (H2DCFA; glutathione; mtROS), lysosomal damage, release of IL-1β and TNF-α | Hepa 1–6 cell Kupffer cells (KUP5) | NA | CNFs-1: L: 6091 ± 2732 nm; w: 72.6 ± 63.6 nm; L/d: 83.4 ± 51.5; d: 5354.2 ± 1897.5; ZP: −12.3 ± 2 mV; CNFs-2: L: 6710 ± 5610 nm; w: 38.7 ± 33.4 nm; L/d: 172.1 ± 105.8; d: 5590.5 ± 3676.4; ZP: −11.0 ± 2.6 mV; | Vortexing + bath ultrasonication (15 min, 42 Khz, 100 W) | 25–200 µg/mL | Endotoxin level using the limulus amebocyte lysate (LAL) | No | 24 h | No cytotoxic effect in Hepa 1–6 cells; decreased viability and alterations in KUP5 cells (ATP assay); no induction of abiotic ROS and abiotic GSH | [158] |
| CNFs/TiO2 | Cytotoxicity (MTT), toxicity to intestinal bacteria | Caco-2 FHC | Wood pulp; mechanical treatment followed by sonication | ZP: −36.50 ± 1.13 mV | NA | 50–1000 µg/mL | NA | No | No cytotoxic effects; no effects on the growth of Escherichia coli P-24, Lactobacillus acidophilus ADH, and Bifidobacterium animalis Bif-6 | [161] | |
| CNFs/Ag | Cytotoxicity (MTT; WST-8 assays) | FHC Caco-2 | CNF slurries | ZP: −23.03 ± 0.50 mV; size: w: 27.59 ± 10.53 nm | NA | 50–1000 µg/mL | NA | No | 24 h | Cytotoxic effects observed at higher concentrations | [162] |
| Tested CNM | In Vivo Model | Cellulose Source; Isolation Method: Isolation Conditions | Material Properties | Dispersion Method | Sterility or Endotoxin Assessment | Dose, Administration Route | Time of Exposure | Outcomes | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CNCs | Crl:CD(SD)BR rats | NA | L: 92 ± 6 nm; w: 6.3 ± 0.4 nm L/d: 14.6; SSA: 399 ± 23 m2/g; average sulphur content: 0.73% | Ultrasonication (1000 J/10 mL) | NA | 500, 1000, and 2000 mg/kg, oral gavage | 14 days, single dose (OECD test guidelines 425) | No toxic effects observed (LD50 > 2000 mg/kg) | [159] |
| CNCs | Crl:CD(SD)BR rats | NA | L: 92 ± 6 nm; w: 6.3 ± 0.4 nm L/d: 14.6; SSA: 399 ± 23 m2/g; average sulphur content: 0.73% | Ultrasonication (1000 J/10 mL) | NA | 500, 1000, and 2000 mg/kg, oral gavage | 28 days, daily (OECD test guidelines 407) | No toxic effects observed ((NOEL > 2000 mg/kg/day). Normal neurological, body weight, weight gain, and food consumption parameters | [159] |
| CNCs | Crl:CD(SD)BR rats | NA | L: 92 ± 6 nm; w: 6.3 ± 0.4 nm L/d: 14.6; SSA: 399 ± 23 m2/g; average sulphur content: 0.73% | Ultrasonication (1000 J/10 mL) | NA | 500, 1000, and 2000 mg/kg, oral gavage | ? (OECD test guidelines 474) | No genotoxic effects. No micronuclei at a maximum tested dose of 2000 mg/kg | [159] |
| CNFs | Rattus norvegicus albus, male | Peach palm, NA | NA | NA | NA | 7%, 14%, 21% (w/w), diet | 30 days, daily | Increased weight over time. No effects on glycaemic rate, triglyceride levels, total cholesterol (blood), or mineral/nutrients loss (faeces). No hepatic damage (histological analysis) | [9] |
| BioPlus® lignin- coated L-CNCs; BioPlus® lignin- coated L-CNFs | Albino Sprague Dawley rats, female | NA | L-CNCs: L: 317 ± 60 nm; w: 14 nm; ZP: −18 mV, rod-shaped; average sulphur content: 0.05%; CI: 98% L-CNFs: L: 500 nm to several microns; w: 14–200 nm; ZP: −22 mV; average sulphur content: 0.09%; CI: 97% | Ultrasonication (10 min, Sonics VCX-750) | NA | 5000 mg/kg, oral gavage | 14 days, single dose (up and down procedure in rats, OPPTS 870.1100) | No acute oral toxicity | [172] |
| CNFs | Wistar Han rats, male | Softwood bleached kraft fibre, mechanical ultrafine friction grinding; autoclaved | L: 6710 ± 5611 nm; d: 64 ± 29 nm; NtN: 335.60 ± 232.66 nm; L/d: 107.6 ± 54.5; SSA: 34 m2/g | NA | Endotoxin levels using the EndoZyme® recombinant factor C (rFC) assay; microbiological contaminations | 1% (w/w), oral gavage | Postprandial rise in serum triglycerides reduced by 36% | [10] 1 | |
| CNFs | Wistar Han rats, male | Softwood bleached kraft fibre, mechanical ultrafine friction grinding; autoclaved | L: 6710 ± 5611 nm; d: 64 ± 29 nm; NtN: 335.60 ± 232.66 nm; L/d: 107.6 ± 54.5; SSA: 34 m2/g | NA | Endotoxin levels using the EndoZyme® recombinant factor C (rFC) assay; microbiological contaminations | 1% (w/w), oral gavage | 35 days, twice a week | Reduction in weight gain (average 30–40% less weight), No damage to the liver, spleen, kidneys, and small intestine (histological analysis). No effect on hepatic, lipid, and renal markers; no alterations in electrolytes, blood cell counts, or haematological measurements | [62] 1 |
| CNFs | Wistar Han rats, male | Softwood bleached kraft fibre, mechanical ultrafine friction grinding; autoclaved | L: 6710 ± 5611 nm; d: 64 ± 29 nm; NtN: 335.60 ± 232.66 nm; L/d: 107.6 ± 54.5; SSA: 34 m2/g | NA | Endotoxin levels using the EndoZyme® recombinant factor C (rFC) assay; microbiological contaminations | 1% (w/w), oral gavage (CNFs alone or in food matrix) | 35 days, twice per week | Effects of CNF ingestion on bacterial genus and species diversity. Downregulation of claudins Cldn2 and Cldn3; gap-junction proteins Gja3 and Gjd2; integrins Itga2, Itga3, and Itgav; cadherins Cdh1; and adherens junction protein Nectin (Pvrl2)) in ileal mucosa. Upregulation of the claudin Cldn10 and cytokines IL-7, IL-18, IL-5, and IL-10 in the ileal mucosa | [122] 1 |
| CNFs (fibrillated cellulose) | Albino Sprague Dawley rats | Wood pulp, mechanical homogenisation | L: 227.7 nm ± 103.3 μm w: 25.06 ± 6.29 nm d: 3330 ± 407 nm PI: 0.836 ± 0.190 ZP: −37.5 ± 1.67 mV | Homogenisation (Disruptor Genie 2, 10 min, 60 kHz; 240 W; 3000 rpm) | Assessment of impurities and microbiological contaminations | 2, 3, or 4% (1044, 1550, and 2194 mg/kg/day and 1302, 1886, and 2667 mg/kg/day, for male and female rats, respectively) | 90 consecutive days, repeated-dose exposure (OECD Test Guideline 408) | No toxicological effects. No-observed-adverse-effect level of 2194.2 mg/kg/day (males) and 2666.6 mg/kg/day (females). No observed clinical alterations in mortality, skin, fur, eyes, mucous membranes, secretions and excretions, body weight, food consumption, etc. No observed alterations in the cranial, thoracic, abdominal, and pelvic cavities, including associated organs and tissues. Histological vacuolisation of periportal hepatocytes (variably sized, clear cytoplasmic vacuoles) in the liver in the 4% CNCs and 4% cellulose groups, without hepatocyte degeneration or any other pathological observations. No alterations in haematological factors, serum chemistry, or urine parameters | [176] |
| CNCs | Albino Sprague Dawley rats | Wood pulp; AH (H2SO4) | L: 25 to 250 nm; w: <10 nm; d: 893 ± 251 nm; PDI: 0.51 ± 0.02; ZP: −50.8 ± 6 mV, rod-shaped | Homogenisation (Vortex Genie 2, 10 min) | Assessment of impurities and microbiological contaminations | 2, 3, and 4 w/w%, diet (1056, 1584, and 2085 mg/kg/day and 1278, 1930, and 2683 mg/kg/day for the male and female rats, respectively) | Pilot test: 7 and 14 days, daily (OECD Test Guideline 407) 90 consecutive days, repeated-dose exposure (OECD Test Guideline 408) | Pilot test: No adverse effects associated with feeding 5% CNCs over 7 days or up to 1.2% CNCs over 14 days. 90 consecutive days test: No toxicological effects. No-observed-adverse-effect level of 2085.3 (males) and 2682.8 (females) mg/kg/day. No observed clinical changes in mortality, skin, fur, eyes, mucous membranes, secretions, or excretions. Increased body weight and food consumption in female rats fed with 4% CNCs, with increased heart, liver and spleen weight Vacuolisation of periportal hepatocytes (variably sized, clear cytoplasmic vacuoles) in the liver in the 4% CNCs and 4% cellulose groups, without hepatocyte degeneration or any other pathological observations. No observed histological alterations in the other analysed organs (including the colon, kidneys, stomach, spleen, ileum with Peyer’s patches, jejunum, brain, reproductive organs, eyes, etc.). No alterations in haematological factors, serum chemistry, or urine parameters | [138] |
| CNFs | C57BL/6 mice | Softwood kraft pulp, ultrafine grinder | L: several hundred μm w: 50 nm ZP: −48 to −5 Mv | Homogenisation (vortex mixer) | NA | 30 mg/kg BW/day, oral gavage | 4–6 weeks | No toxicological effects. Dysregulated glucose homeostasis, decreased lean body mass (male), no differences in food consumption, no histological damage to the small intestine | [145] |
| CNCs modified with oxalate esters | Albino Sprague Dawley rats | Cotton seeds; AH (H2SO4) | Average particle size: 100 nm ZP: −50 mV to −10 mV | NA | NA | 50 and 100 mg/kg | 7 days, daily | Hepatic injury (100 mg/kg). Normal liver weight, alkaline phosphatase activity, and lipid peroxidation; increased aspartate aminotransferase, alanine aminotransferase, and myeloperoxidase activities; increased nitric oxide synthase, Bcl-2-associated X protein, and MPO (liver). No effect on SOD, GSH, H2O2, NO, and MDA; decreased CAT and GPx | [59] |
| Multiple CNFs, carboxymethylated | KM mice, female | Cotton linter pulp, mechanical stirring or carboxymethylated pretreatment; HPH | ZP: 12.4 ± 1.7, 21.8 ± 1.2, 26.7 ± 1.0, 34.2 ± 2.2 mV carboxyl content: 0, 0.36, 0.72, and 1.24 mmol/g | Homogenisation (vortex mixer) | Sterilised by filtration | 1% or 3.5% (w/w), oral gavage | 8 weeks, daily | No toxicological effects. Decreased body weight. No alterations in haematological measurements or serum lipid, hepatic, renal, and heart markers | [43] |
| R-CNCsulf R-CNCAC D-CNCAc | Mice, male and female | Cotton microcrystalline cellulose (R-CNCsulf: AH, 65 wt% H2SO4 1:20, 45 °C, 30–90 min; R-CNCAC and D-CNCAc: acetylated surface obtained via solvolysis in the acetic acid/phosphotungstic acid system) | R-CNCsulf: L: 195 ± 30 nm; d: 234 ± 3 nm; ZP: −53 ± 2 45 mV; H: 9.5 ± 2.0 nm R-CNCAC: L: 165 ± 35 nm; d: 200 ± 10 nm; ZP: −45 ± 6 mV; H = 8.5 ± 2.0 nm D-CNCAc: d: 110 ± 15 nm; ZP: −38 ± 4 mV; H: 3–12 nm | NA | Hydrosols filtered and treated with ultraviolet | 2000 mg/Kg, oral gavage | 14 days, single dose (OECD test guidelines 425) | No toxicological effects. No morphological changes of the liver, kidneys, heart, and spleen | [55] |
| CNCs; cationic CNCs | Murine models with chronic renal failure and hyperphosphatemia | Softwood bleached kraft pulp, AH (H2SO4); cationisation agent (EPTAC) in ultrasonic bath | Cationic CNCs: L: 150 nm d: 10 nm; ZP: 62.3 mV CNCs: ZP: −31.6 mV; | NA | NA | 0.6% (w/w), oral gavage | 14 days, daily | Elevated aspartate aminotransferase, cortical and cerebellar glutathione, and lipid peroxidation levels | [170] |
| CNCs | Albino Sprague Dawley rats, male | P. longifolia seeds; HCL followed by ultrasonication | d: 5–70 nm | NA | NA | 50 and 100 mg/kg BW, oral gavage | 14 days, daily | Elevated aspartate aminotransferase, cortical and cerebellar glutathione, and lipid peroxidation levels; no altered histology of neurons, hippocampus, and Purkinje layers; no alterations of body and organ weights, albumin, cortical and cerebellar catalase, and glutathione S-transferase levels | [171] |
| CNFs | C57BL/6 mice, female (inflammatory bowel disease model) | Wood, adlay (C. lacryma-jobi) chaff, and hijiki seaweed (Sargassum fusiforme); mechanical treatments | NA | NA | NA | 0.1% (w/w), via drinking water | 5 days, ad libitum | Colon lengths of CNFs obtained from adlay and hijiki seaweed were longer than controls and CNFs obtained from wood. In the colon of control CNFs and those obtained from wood, histological alterations were observed (i.e., erosion, shortening, or destruction of the crypts, and oedema), which were ameliorated by the other CNFs. Reduction in myeloperoxidase and NF-κB staining after exposure to CNFs obtained from adlay and hijiki seaweed compared to CNFs obtained from wood and control CNFs | [177] |
| CNFs | C57BL/6 mice, female (inflammatory bowel disease model) | Japanese pear and wood; mechanical treatments | NA | NA | NA | 5.7% (w/w) for CNFs from Japanese pears, 1% for CNFs from wood, via drinking water | 5 days, ad libitum | Colon lengths of CNFs obtained from pears were longer than those of controls and CNFs obtained from wood. In the colon of control CNFs and those obtained from wood, histological alterations were observed (e.g., erosion, shortening, or destruction of the crypts, and oedema), which were ameliorated by the other CNFs. Reduction in myeloperoxidase, collagen deposition, and NF-κB staining after exposure to CNFs obtained from pears and seaweed compared to those obtained from wood and controls | [178] |
| CNCs | C57BL/6 mice | NA | NA | NA | NA | 0.1 and 0.2% (w/w), via drinking water | 7 weeks, ad libitum | Administration of 0.2% CNCs decreased the relative abundance of Streptococcaceae and Rikenellaceae, and increased that of Lactobacillaceae; weight gain suppression in high-fat-diet-fed mice treated with 0.2% CN, with lower accumulation of epididymal and subcutaneous fat; no differences in levels of fasting blood glucose and glucose tolerance | [175] |
| CNCs | Healthy donors’ faecal matter; Wistar rats, male | Microcrystalline cellulose, AH (H2SO4) followed by ultrasonication | CNCs1: L: 346.28 ± 102.12 nm; L/d: 26.16; d: 367 ± 89 nm (DLS); d: 13.23 ± 0.84 nm (AFM); SSA: 8.28 m2/g CNCs2: L: 276.58 ± 135.22 nm; L/d: 32.77; d: 230 ± 68 nm (DLS); d: 8.44 ± 0.75 nm (AFM); SSA: 18.33 m2/g; CNCs3: L: 125.01 ± 25.43 nm; L/d: 38.58; d: 104 ± 25 nm (DLS); d: 3.24 ± 0.75 nm; SSA: 27.9 m2/g | NA | NA | 250 mg/kg BW, oral gavage | 14 days, twice per day | Size-dependent increase in short-chain fatty acids (including acetate, butyrate, and propionate); increased Bifidobacterium | [173] |
| Specific Issues in the Toxicology Assessment of CNMs |
|---|
| Chemical impurities |
| Presence of biological contaminants (e.g., endotoxins) |
| Physicochemical characteristics in cellular moieties |
| Dispersion and stability of CNMs in biological media |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital, N.; Ventura, C.; Kranendonk, M.; Silva, M.J.; Louro, H. Toxicological Assessment of Cellulose Nanomaterials: Oral Exposure. Nanomaterials 2022, 12, 3375. https://doi.org/10.3390/nano12193375
Vital N, Ventura C, Kranendonk M, Silva MJ, Louro H. Toxicological Assessment of Cellulose Nanomaterials: Oral Exposure. Nanomaterials. 2022; 12(19):3375. https://doi.org/10.3390/nano12193375
Chicago/Turabian StyleVital, Nádia, Célia Ventura, Michel Kranendonk, Maria João Silva, and Henriqueta Louro. 2022. "Toxicological Assessment of Cellulose Nanomaterials: Oral Exposure" Nanomaterials 12, no. 19: 3375. https://doi.org/10.3390/nano12193375
APA StyleVital, N., Ventura, C., Kranendonk, M., Silva, M. J., & Louro, H. (2022). Toxicological Assessment of Cellulose Nanomaterials: Oral Exposure. Nanomaterials, 12(19), 3375. https://doi.org/10.3390/nano12193375








