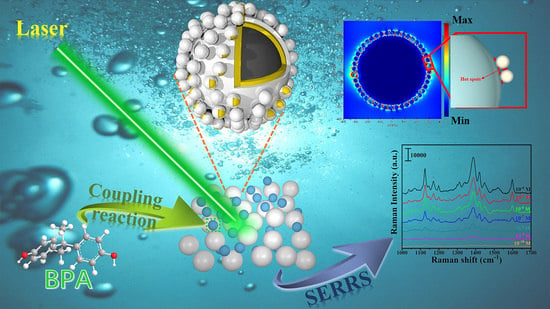
Magnetic-Core–Shell–Satellite Fe3O4-Au@Ag@(Au@Ag) Nanocomposites for Determination of Trace Bisphenol A Based on Surface-Enhanced Resonance Raman Scattering (SERRS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Synthesis of PEI-DTC Aqueous Solution, Fe3O4 Hollow Spheres, Au Seeds, and Au@Ag Nanocrystals
2.3. Synthesis of Fe3O4-Au (FA) NCs
2.4. Synthesis of FA@Ag@(Au@Ag) (CSSN) NCs
2.5. Pauly’s Reagents and Coupling Reaction
2.6. FDTD Algorithm Method
2.7. SERRS Measurements
3. Results and Discussion
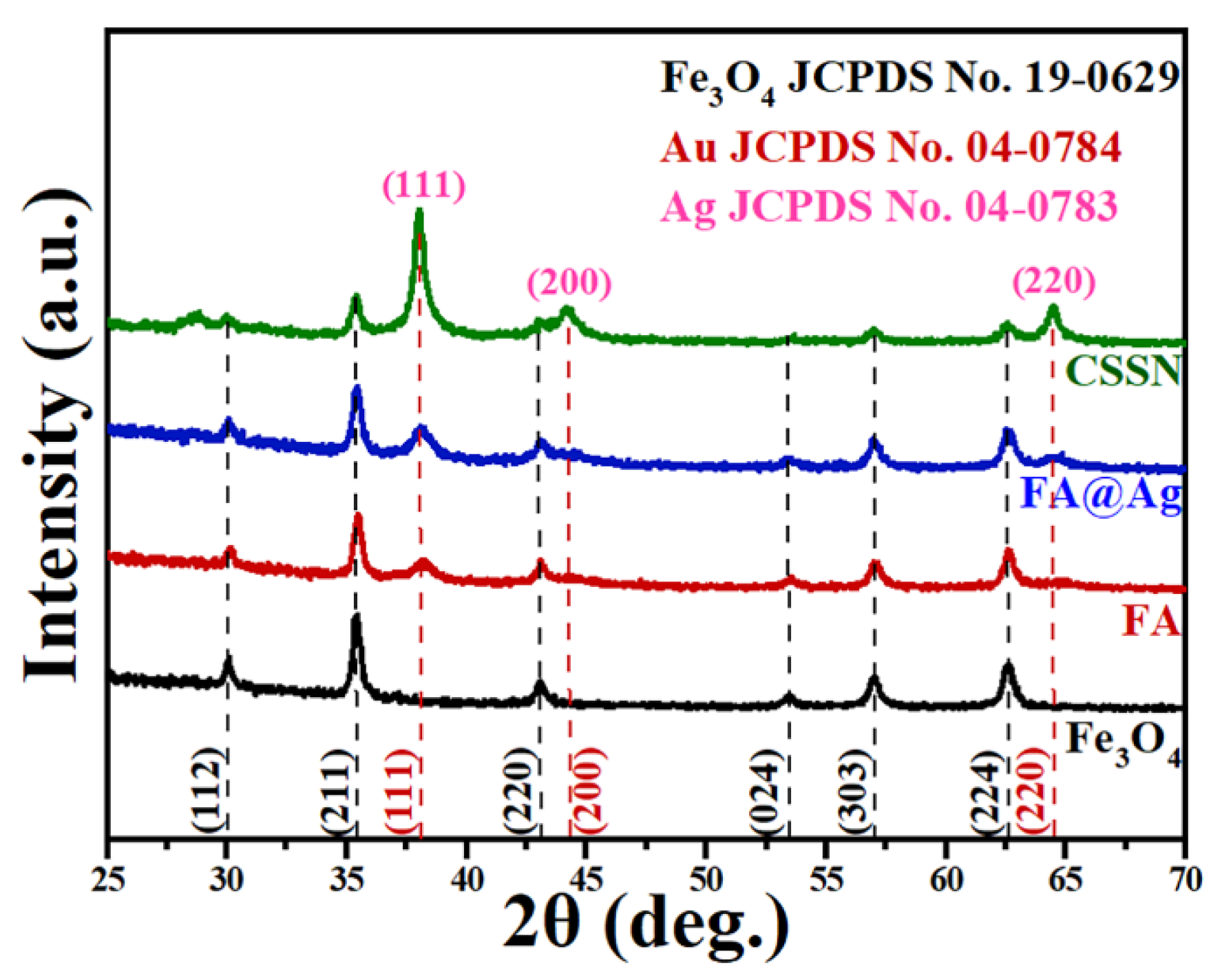
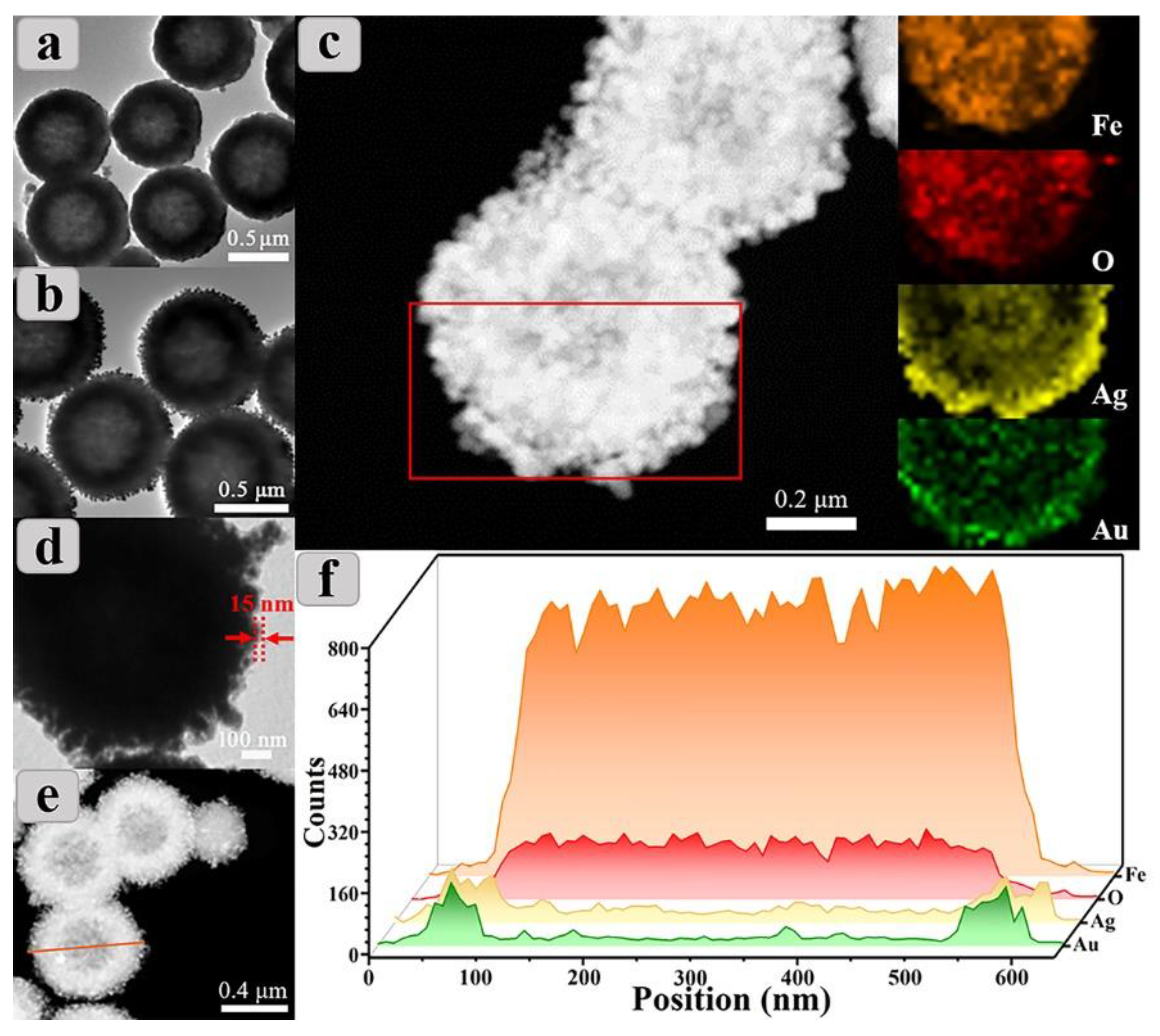
3.1. Structure and Magnetic Properties of CSSN NCs
3.2. Choice of Excitation Source
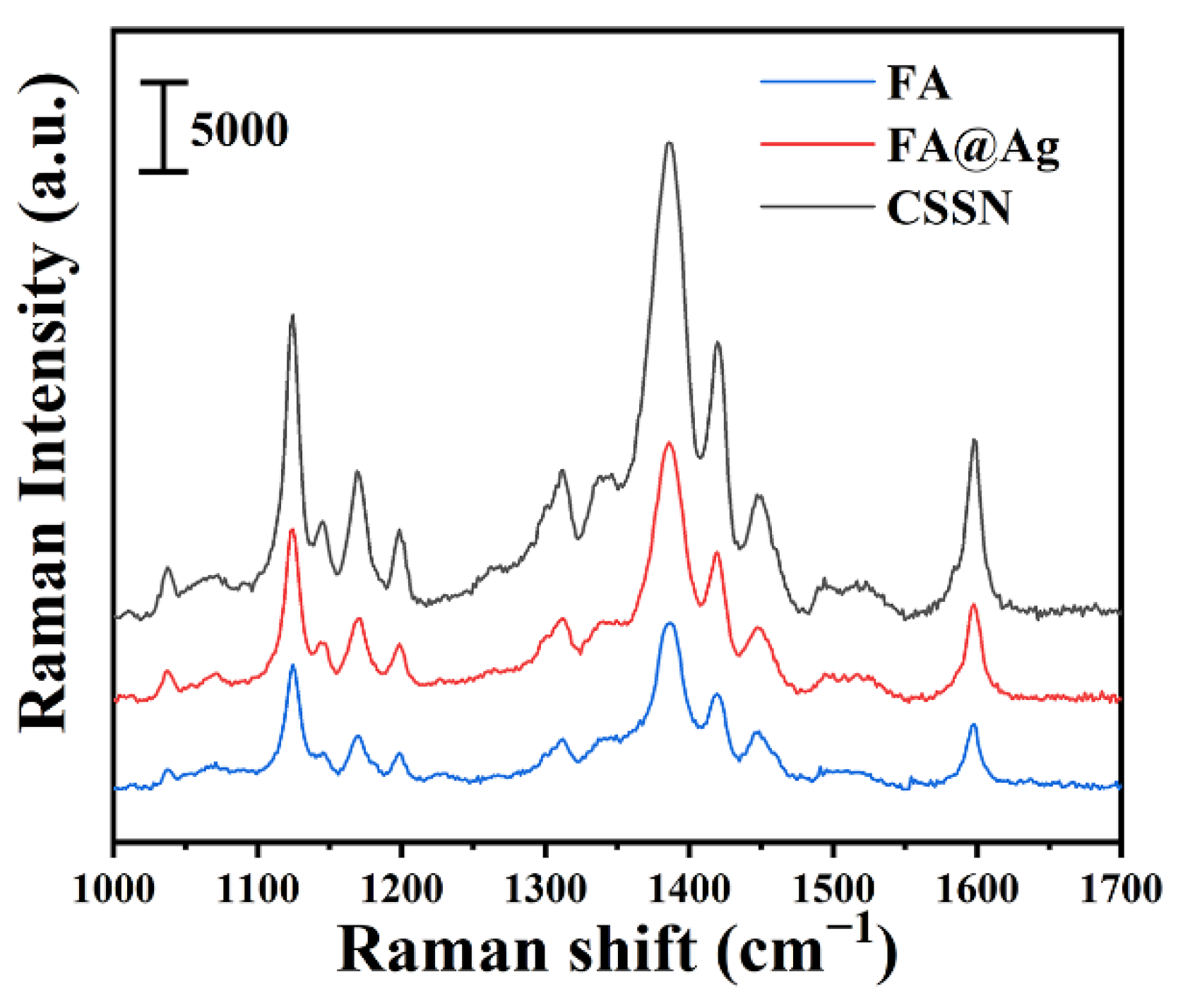
3.3. SERRS Spectra of BPA Azo Product on FA, FA@Ag, and CSSN NCs
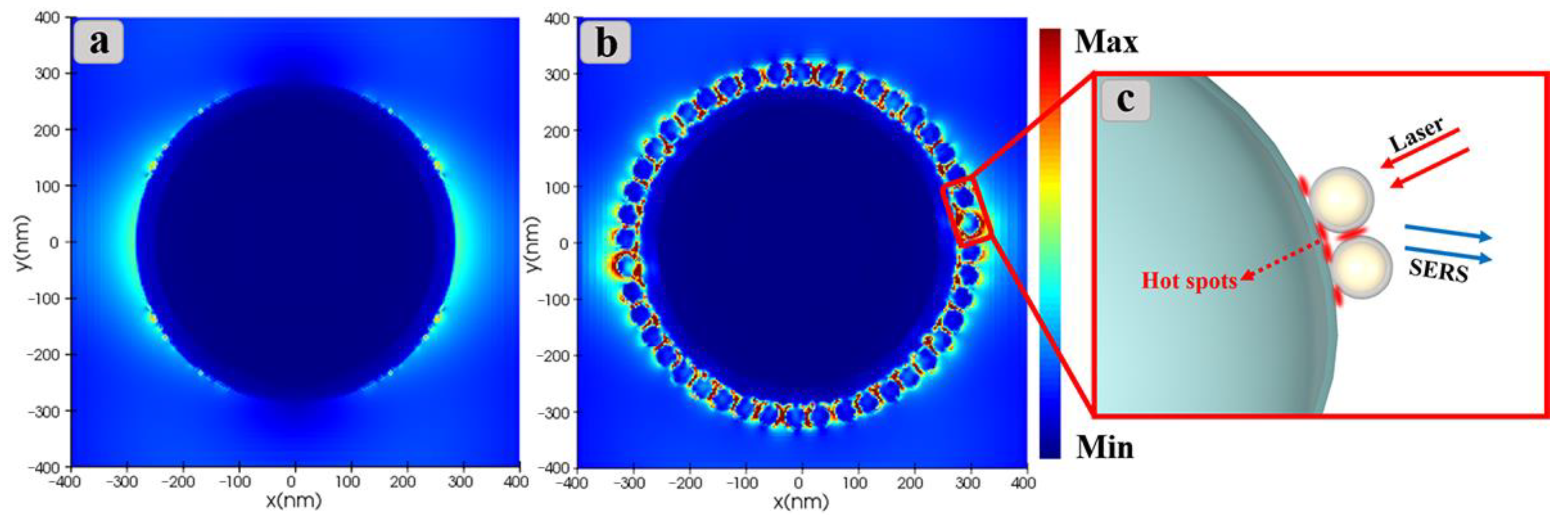
3.4. Mechanism of SERRS Enhancement
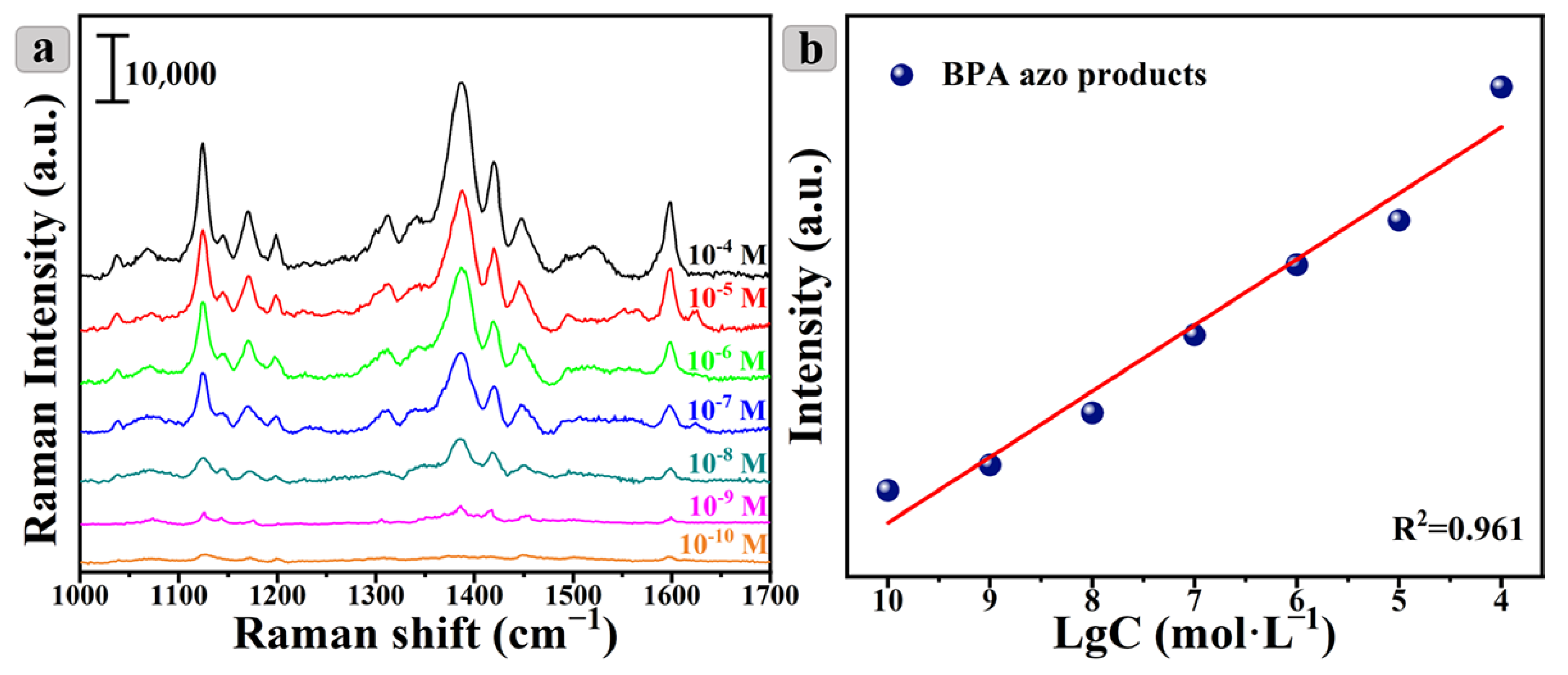
3.5. Quantitative Detection of BPA Azo Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismanto, A.; Hadibarata, T.; Kristanti, R.A.; Maslukah, L.; Safinatunnajah, N.; Kusumastuti, W. Endocrine disrupting chemicals (EDCs) in environmental matrices: Occurrence, fate, health impact, physio-chemical and bioremediation technology. Environ. Pollut. 2022, 302, 119061. [Google Scholar] [CrossRef] [PubMed]
- Al Sharabati, M.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable polymers and their nano-composites for the removal of endocrine-disrupting chemicals (EDCs) from wastewater: A review. Environ. Res. 2021, 202, 111694. [Google Scholar] [CrossRef] [PubMed]
- Astrahan, P.; Korzen, L.; Khanin, M.; Sharoni, Y.; Israel, A. Seaweeds fast EDC bioremediation: Supporting evidence of EE2 and BPA degradation by the red seaweed Gracilaria sp., and a proposed model for the remedy of marine-borne phenol pollutants. Environ. Pollut. 2021, 278, 116853. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules 2021, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Ouladsmane, M.; Alammari, A.M.; Azam, M. Bisphenol A leaches from packaging to fruit juice commercially available in markets. Food Packag. Shelf 2021, 28, 100678. [Google Scholar] [CrossRef]
- Sousa, S.; Maia, M.L.; Delerue-Matos, C.; Calhau, C.; Domingues, V.F. The role of adipose tissue analysis on Environmental Pollutants Biomonitoring in women: The European scenario. Sci. Total Environ. 2022, 806, 150922. [Google Scholar] [CrossRef] [PubMed]
- Din, S.T.U.; Lee, H.; Yang, W. Z-Scheme Heterojunction of 3-Dimensional Hierarchical Bi3O4Cl/Bi5O7I for a Significant Enhancement in the Photocatalytic Degradation of Organic Pollutants (RhB and BPA). Nanomaterials 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef]
- Makowska, K.; Staniszewska, M.; Bodziach, K.; Calka, J.; Gonkowski, S. Concentrations of bisphenol a (BPA) in fresh pork loin meat under standard stock-farming conditions and after oral exposure—A preliminary study. Chemosphere 2022, 295, 133816. [Google Scholar] [CrossRef]
- Escarda-Castro, E.; Herraez, M.P.; Lombo, M. Effects of bisphenol A exposure during cardiac cell differentiation. Environ. Pollut. 2021, 286, 117567. [Google Scholar] [CrossRef]
- Santhi, V.A.; Hairin, T.; Mustafa, A.M. Simultaneous determination of organochlorine pesticides and bisphenol A in edible marine biota by GC-MS. Chemosphere 2012, 86, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Murugananthan, M.; Yoshihara, S.; Rakuma, T.; Shirakashi, T. Mineralization of bisphenol A (BPA) by anodic oxidation with boron-doped diamond (BDD) electrode. J. Hazard. Mater. 2008, 154, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Maqbool, A.; Sun, K.; Si, Y. Immobilization of Trametes Versicolor laccase on Cu-alginate beads for biocatalytic degradation of bisphenol A in water: Optimized immobilization, degradation and toxicity assessment. J. Environ.Chem. Eng. 2022, 10, 107089. [Google Scholar] [CrossRef]
- Xue, C.S.; Erika, G.; Jiri, H. Surface plasmon resonance biosensor for the ultrasensitive detection of bisphenol A. Anal. Bioanal. Chem. 2019, 411, 5655–5658. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, S.; Shi, T.; Li, C.; Wang, Y.; Zhang, H. Competitive plasmonic biomimetic enzyme-linked immunosorbent assay for sensitive detection of bisphenol A. Food Chem. 2021, 344, 128602. [Google Scholar] [CrossRef]
- Dai, J.; Baker, G.L.; Bruening, M.L. Use of Porous Membranes Modified with Polyelectrolyte Multilayers as Substrates for Protein Arrays with Low Nonspecific Adsorption. Anal. Chem. 2006, 78, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, L.; Ling, S.; Ouyang, H.; Liang, A.; Jiang, Z. Aptamer-Adjusted Carbon Dot Catalysis-Silver Nanosol SERS Spectrometry for Bisphenol A Detection. Nanomaterials 2022, 12, 1374. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Musaev, A.G.; Simonenko, T.L.; Gorobtsov, P.Y.; Volkov, I.A.; Gulin, A.A.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Hydrothermal Synthesis of Ag Thin Films and Their SERS Application. Nanomaterials 2021, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bai, B.; You, O.; Li, Q.; Fan, S. Fano resonance boosted cascaded optical field enhancement in a plasmonic nanoparticle-in-cavity nanoantenna array and its SERS application. Light Sci. Appl. 2015, 4, e296. [Google Scholar] [CrossRef]
- Wu, R.; Jin, Q.; Storey, C.; Collins, J.; Gomard, G.; Lemmer, U.; Canham, L.; Kling, R.; Kaplan, A. Gold nanoplasmonic particles in tunable porous silicon 3D scaffolds for ultra-low concentration detection by SERS. Nanoscale Horiz. 2021, 6, 781–790. [Google Scholar] [CrossRef]
- Morton, S.M.; Silverstein, D.W.; Jensen, L. Theoretical studies of plasmonics using electronic structure methods. Chem. Rev. 2011, 111, 3962–3994. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yao, J.; Quan, Y.; Hu, M.; Su, R.; Gao, M.; Han, D.; Yang, J. Monitoring the charge-transfer process in a Nd-doped semiconductor based on photoluminescence and SERS technology. Light Sci. Appl. 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xu, M.; Xu, L.; Wei, T.; Ma, X.; Zheng, X.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Seo, M.K.; Shin, K.H.; Lee, Y.W.; Sohn, J.I. Hierarchically Assembled Plasmonic Metal-Dielectric-Metal Hybrid Nano-Architectures for High-Sensitivity SERS Detection. Nanomaterials 2022, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, C.; Yuan, Y.; Xu, M.; Yao, J. The moveable “hot spots” effect in an Au nanoparticles-Au plate coupled system. Nanoscale 2020, 12, 23789–23798. [Google Scholar] [CrossRef]
- Shao, Y.; Li, S.; Niu, Y.; Wang, Z.; Zhang, K.; Mei, L.; Hao, Y. Three-Dimensional Dendritic Au-Ag Substrate for On-Site SERS Detection of Trace Molecules in Liquid Phase. Nanomaterials 2022, 12, 2002. [Google Scholar] [CrossRef]
- Baffou, G.; Bordacchini, I.; Baldi, A.; Quidant, R. Simple experimental procedures to distinguish photothermal from hot-carrier processes in plasmonics. Light Sci. Appl. 2020, 9, 108. [Google Scholar] [CrossRef]
- Weber, M.L.; Willets, K.A. Correlated Super-Resolution Optical and Structural Studies of Surface-Enhanced Raman Scattering Hot Spots in Silver Colloid Aggregates. J. Phys. Chem. Lett. 2011, 2, 1766–1770. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, Q.; Song, Y.; Zhang, B.; Ren, T.; Yang, H.; Wang, F. Polyethyleneimine mediated interaction for highly sensitive, magnetically assisted detection of tetracycline hydrochloride. Appl. Surf. Sci. 2020, 505, 144543. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X.; Ge, B.; Zhou, J.; Wang, M.; Zhang, L.; Jiao, T. Facile Preparation of Self-Assembled Black Phosphorus-Dye Composite Films for Chemical Gas Sensors and Surface-Enhanced Raman Scattering Performances. ACS Sustain. Chem. Eng. 2020, 8, 4521–4536. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Kou, Q.; Wang, D.; Han, D.; Lu, Z.; Chen, Y.; Chen, L.; Wang, Y.; Zhang, Y.; et al. Fe3O4/Au binary nanocrystals: Facile synthesis with diverse structure evolution and highly efficient catalytic reduction with cyclability characteristics in 4-nitrophenol. Powder Technol. 2018, 338, 26–35. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, S.; Guo, S.; Song, X.; Ding, B.; Yang, Z. Bimetallic Ag/Au nanoparticles: A low temperature ripening strategy in aqueous solution. Colloid. Surface. A 2010, 372, 204–209. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Fu, Z.; Qin, D. Galvanic replacement-free deposition of Au on Ag for core-shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, B.; Hu, M.; Zhou, N.; Huang, Z.; Meng, G. In-Situ Monitoring the SERS Spectra of para-Aminothiophenol Adsorbed on Plasmon-Tunable Au@Ag Core-Shell Nanostars. Nanomaterials 2022, 12, 1156. [Google Scholar] [CrossRef]
- Svedendahl, M.; Verre, R.; Käll, M. Refractometric biosensing based on optical phase flips in sparse and short-range-ordered nanoplasmonic layers. Light Sci. Appl. 2014, 3, e220. [Google Scholar] [CrossRef]
- Yin, W.; Wu, L.; Ding, F.; Li, Q.; Wang, P.; Li, J.; Lu, Z.; Han, H. Surface-imprinted SiO2@Ag nanoparticles for the selective detection of BPA using surface enhanced Raman scattering. Sensor. Actuat. B Chem. 2018, 258, 566–573. [Google Scholar] [CrossRef]
- Han, X.; Pienpinijtham, P.; Zhao, B.; Ozaki, Y. Coupling reaction-based ultrasensitive detection of phenolic estrogens using surface-enhanced resonance Raman scattering. Anal. Chem. 2011, 83, 8582–8588. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Covian, L.; Montes-Garcia, V.; Girard, A.; Fernandez-Abedul, M.T.; Perez-Juste, J.; Pastoriza-Santos, I.; Faulds, K.; Graham, D.; Blanco-Lopez, M.C. Au@Ag SERRS tags coupled to a lateral flow immunoassay for the sensitive detection of pneumolysin. Nanoscale 2017, 9, 2051–2058. [Google Scholar] [CrossRef]
- Ishmukhametov, I.; Batasheva, S.; Rozhina, E.; Akhatova, F.; Mingaleeva, R.; Rozhin, A.; Fakhrullin, R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers 2022, 14, 344. [Google Scholar] [CrossRef]
- Kou, Y.; Wu, T.; Zheng, H.; Kadasala, N.R.; Yang, S.; Guo, C.; Chen, L.; Liu, Y.; Yang, J. Recyclable magnetic MIP-based SERS sensors for selective, sensitive, and reliable detection of paclobutrazol residues in complex environments. ACS Sustain. Chem. 2020, 8, 14549–14556. [Google Scholar] [CrossRef]
- Dong, S.; Rene, E.R.; Zhao, L.; Xiaoxiu, L.; Ma, W. Design and preparation of functional azo linked polymers for the adsorptive removal of bisphenol A from water: Performance and analysis of the mechanism. Environ. Res. 2022, 206, 112601. [Google Scholar] [CrossRef] [PubMed]
- Jaguey-Hernandez, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Anorve-Morga, J.; Gonzalez-Olivares, L.G.; Castaneda-Ovando, A. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Res. Int. 2021, 144, 110341. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Liu, G.; Dong, B.; Zhou, J.; Wang, A.; Wang, J.; Jin, R.; Lv, H.; Dou, Z.; Huang, W. Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci. Rep. 2015, 5, 13515. [Google Scholar] [CrossRef]
- Qu, J.; Liu, G.; Wang, Y.; Hong, R. Preparation of Fe3O4–chitosan nanoparticles used for hyperthermia. Adv. Powder Technol. 2010, 21, 461–467. [Google Scholar] [CrossRef]
- Pinto, P.S.; Lanza, G.D.; Souza, M.N.; Ardisson, J.D.; Lago, R.M. Surface restructuring of red mud to produce FeOx(OH)y sites and mesopores for the efficient complexation/adsorption of beta-lactam antibiotics. Environ. Sci. Pollut. R. 2018, 25, 6762–6771. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Kou, Q.; Chen, Y.; Han, D.; Wang, D.; Lu, Z.; Chen, L.; Yang, J.; Xing, G. Eco-friendly seeded Fe3O4-Ag nanocrystals: A new type of highly efficient and low cost catalyst for methylene blue reduction. RSC Adv. 2018, 8, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Kou, Q.; Liu, Y.; Han, D.; Wang, D.; Sun, Y.; Zhang, Y.; Wang, Y.; Lu, Z.; et al. Enhanced Catalytic Reduction of 4-Nitrophenol Driven by Fe3O4-Au Magnetic Nanocomposite Interface Engineering: From Facile Preparation to Recyclable Application. Nanomaterials 2018, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, B.; Chen, Y.; Wu, T.; Kou, Y.; Xue, X.; Chen, L.; Liu, Y.; Duan, Q. Facile synthesis of Fe3O4@Au core-shell nanocomposite as a recyclable magnetic surface enhanced Raman scattering substrate for thiram detection. Nanotechnology 2019, 30, 465703. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Li, C. Multifunctional Fe3O4@Ag/SiO2/Au core-shell microspheres as a novel SERS-activity label via long-range plasmon coupling. Langmuir 2013, 29, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Kleber, M.; Jahn, R. Review of XRD-based quantitative analyses of clay minerals in soils: The suitability of mineral intensity factors. Geoderma 2002, 109, 191–205. [Google Scholar] [CrossRef]
- Hill, R.J.; Foxworthy, A.M.; White, R.J. PEAKS®: A PC-based method for quantitative X-ray diffraction phase analysis of lead-acid battery materials. J. Power Sources 1990, 32, 315–328. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Zhou, T.; Huang, J.; Wang, Y.; Li, B.; Chen, L.; Yang, J.; Liu, Y. Aptamer-conjugated magnetic Fe3O4@Au core-shell multifunctional nanoprobe: A three-in-one aptasensor for selective capture, sensitive SERS detection and efficient near-infrared light triggered photothermal therapy of Staphylococcus aureus. Sensor. Actuat. B Chem. 2022, 350, 130879. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Qi, M.; Tang, Z.; Xu, Y. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118380. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, H.; Yi, J. MOF-derived Au-loaded Co3O4 porous hollow nanocages for acetone detection. Sensor. Actuat. B Chem. 2021, 344, 130182. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, E.-M.; Lee, C.-M.; Kim, D.W.; Lim, S.T.; Sohn, M.-H.; Jeong, H.-J. Synthesis of PEG-Iodine-Capped Gold Nanoparticles and Their Contrast Enhancement in In Vitro and In Vivo for X-Ray/CT. J. Nanomater. 2012, 2012, 504026. [Google Scholar] [CrossRef]
- Liu, Y.; Kou, Q.; Wang, D.; Chen, L.; Sun, Y.; Lu, Z.; Zhang, Y.; Wang, Y.; Yang, J.; Xing, S.G. Rational synthesis and tailored optical and magnetic characteristics of Fe3O4–Au composite nanoparticles. J. Mater. Sci. 2017, 52, 10163–10174. [Google Scholar] [CrossRef]
- Guo, X.; Sun, X.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Zhan, W. Enhanced catalytic performance for selective oxidation of propene with O2 over bimetallic Au–Cu/SiO2 catalysts. Rare Met. 2021, 40, 1056–1066. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Xu, G.; Guo, N.; Zhang, Q.; Wang, T.; Song, P.; Xia, L. A sensitive surface-enhanced resonance Raman scattering sensor with bifunctional negatively charged gold nanoparticles for the determination of Cr(VI). Sci. Total Environ. 2022, 830, 154598. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Zhang, Y.; Kou, Q.; Zhang, Y.; Wang, Y.; Chen, L.; Sun, Y.; Zhang, H.; MeeJung, Y. Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Molecules 2018, 23, 1330. [Google Scholar] [CrossRef]
- Lin, S.; Guan, H.; Liu, Y.; Huang, S.; Li, J.; Hasi, W.; Xu, Y.; Zou, J.; Dong, B. Binary plasmonic assembly films with hotspot-type-dependent surface-enhanced Raman scattering properties. ACS Appl. Mater. Inter. 2021, 13, 53289–53299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Ma, H.; Su, H.; Li, A.; Ruan, W.; Zhao, B. Surface plasmon resonance from gallium-doped zinc oxide nanoparticles and their electromagnetic enhancement contribution to surface-enhanced Raman scattering. ACS Appl. Mater. Inter. 2021, 13, 35038–35045. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.J.C.; Yu, R.; Garcia de Abajo, F.J. Thermal manipulation of plasmons in atomically thin films. Light Sci. Appl. 2020, 9, 87. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ding, S.; Panneerselvam, R.; Tian, Z. Core-shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ho, W.K.H.; Zhang, Q.; Li, C.; Huang, Y.; Yan, J.; Yang, H.; Hao, J.; Wong, S.H.D.; Yang, M. Magnetic-Responsive Surface-Enhanced Raman Scattering Platform with Tunable Hot Spot for Ultrasensitive Virus Nucleic Acid Detection. ACS Appl. Mater. Inter. 2022, 14, 4714–4724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Chen, H.; Zhang, L.; Li, P.; Xiao, H.; Wu, W. One-dimensional nanohybrids based on cellulose nanocrystals and their SERS performance. Carbohyd. Polym. 2022, 284, 119140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Yuan, R.; Huang, J.A.; Yang, X. Gap controlled self-assembly Au@Ag@Au NPs for SERS assay of thiram. Food Chem. 2022, 390, 133164. [Google Scholar] [CrossRef]
- Li, X.; Lin, X.; Fang, G.; Dong, H.; Li, J.; Cong, S.; Wang, L.; Yang, S. Interfacial layer-by-layer self-assembly of PS nanospheres and Au@Ag nanorods for fabrication of broadband and sensitive SERS substrates. J. Colloid Interf. Sci. 2022, 620, 388–398. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X. Rapid and sensitive detection of Bisphenol A in water by LF-NMR based on magnetic relaxation switch sensor. Microchem. J. 2021, 163, 105911. [Google Scholar] [CrossRef]
- Quan, Y.; Yao, J.; Yang, S.; Chen, L.; Li, J.; Liu, Y.; Lang, J.; Shen, H.; Wang, Y.; Wang, Y.; et al. ZnO nanoparticles on MoS2 microflowers for ultrasensitive SERS detection of bisphenol A. Microchim. Acta 2019, 186, 593. [Google Scholar] [CrossRef]
- Marks, H.L.; Pishko, M.V.; Jackson, G.W.; Coté, G.L. Rational Design of a Bisphenol A Aptamer Selective Surface-Enhanced Raman Scattering Nanoprobe. Anal. Chem. 2014, 86, 11614–11619. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Hsieh, C.; Hsieh, S. Rapid and Sensitive SERS Detection of Bisphenol A Using Self-assembled Graphitic Substrates. Sci. Rep. 2017, 7, 16698. [Google Scholar] [CrossRef] [PubMed]
- Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.C.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions. Nanomaterials 2021, 11, 881. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, R.; Liao, S.; Miao, Y.; Zhang, B.; Wang, F.; Yang, H. In situ reduced silver nanoparticles embedded molecularly imprinted reusable sensor for selective and sensitive SERS detection of Bisphenol A. Appl. Surf. Sci. 2018, 457, 323–331. [Google Scholar] [CrossRef]
- Ren, X.; Cheshari, E.C.; Qi, J.; Li, X. Silver microspheres coated with a molecularly imprinted polymer as a SERS substrate for sensitive detection of bisphenol A. Microchim. Acta 2018, 185, 242. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhou, T.; Zhao, W.; Zhang, M.; Zhang, Z.; Lai, W.; Kadasala, N.R.; Liu, H.; Liu, Y. Magnetic-Core–Shell–Satellite Fe3O4-Au@Ag@(Au@Ag) Nanocomposites for Determination of Trace Bisphenol A Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Nanomaterials 2022, 12, 3322. https://doi.org/10.3390/nano12193322
Huang J, Zhou T, Zhao W, Zhang M, Zhang Z, Lai W, Kadasala NR, Liu H, Liu Y. Magnetic-Core–Shell–Satellite Fe3O4-Au@Ag@(Au@Ag) Nanocomposites for Determination of Trace Bisphenol A Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Nanomaterials. 2022; 12(19):3322. https://doi.org/10.3390/nano12193322
Chicago/Turabian StyleHuang, Jie, Tianxiang Zhou, Wenshi Zhao, Min Zhang, Zhibo Zhang, Wangsheng Lai, Naveen Reddy Kadasala, Huilian Liu, and Yang Liu. 2022. "Magnetic-Core–Shell–Satellite Fe3O4-Au@Ag@(Au@Ag) Nanocomposites for Determination of Trace Bisphenol A Based on Surface-Enhanced Resonance Raman Scattering (SERRS)" Nanomaterials 12, no. 19: 3322. https://doi.org/10.3390/nano12193322
APA StyleHuang, J., Zhou, T., Zhao, W., Zhang, M., Zhang, Z., Lai, W., Kadasala, N. R., Liu, H., & Liu, Y. (2022). Magnetic-Core–Shell–Satellite Fe3O4-Au@Ag@(Au@Ag) Nanocomposites for Determination of Trace Bisphenol A Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Nanomaterials, 12(19), 3322. https://doi.org/10.3390/nano12193322