Black Phosphorus/Carbon Nanoframes for Efficient Flexible All-Solid-State Supercapacitor
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Preparation of Flexible All-Solid-State Supercapacitors
3. Results and Discussions
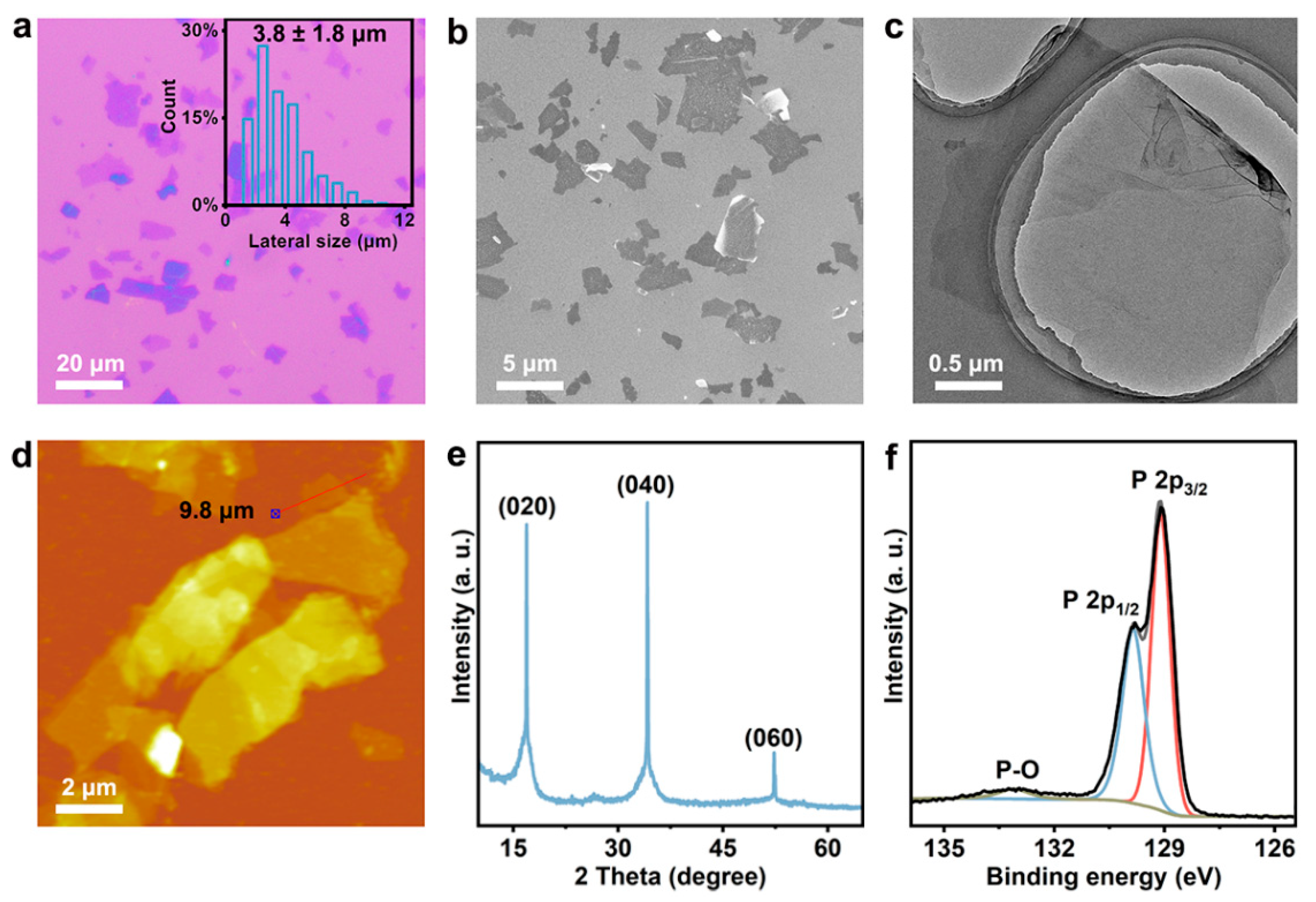
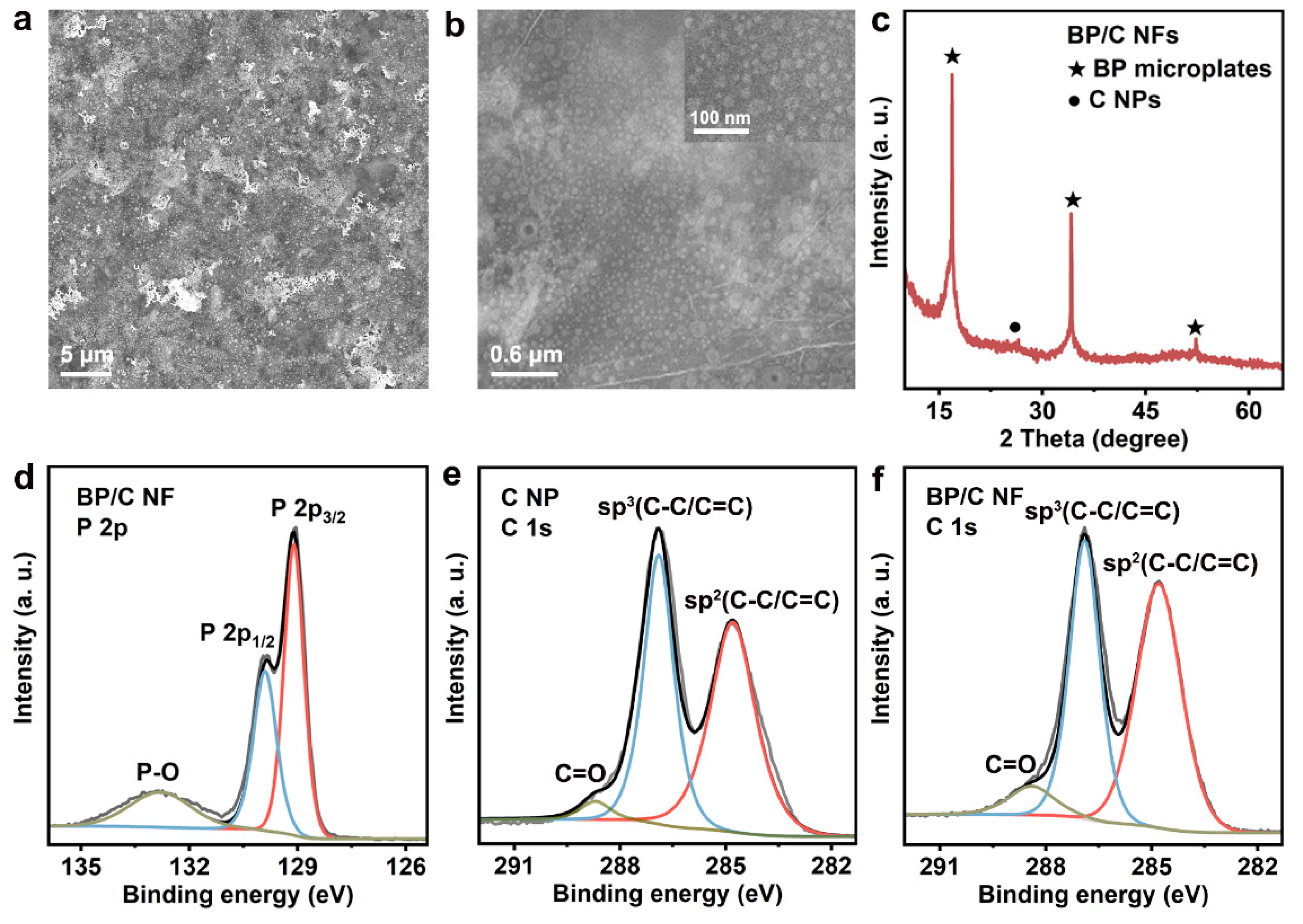
3.1. Material Synthesis and Characterization
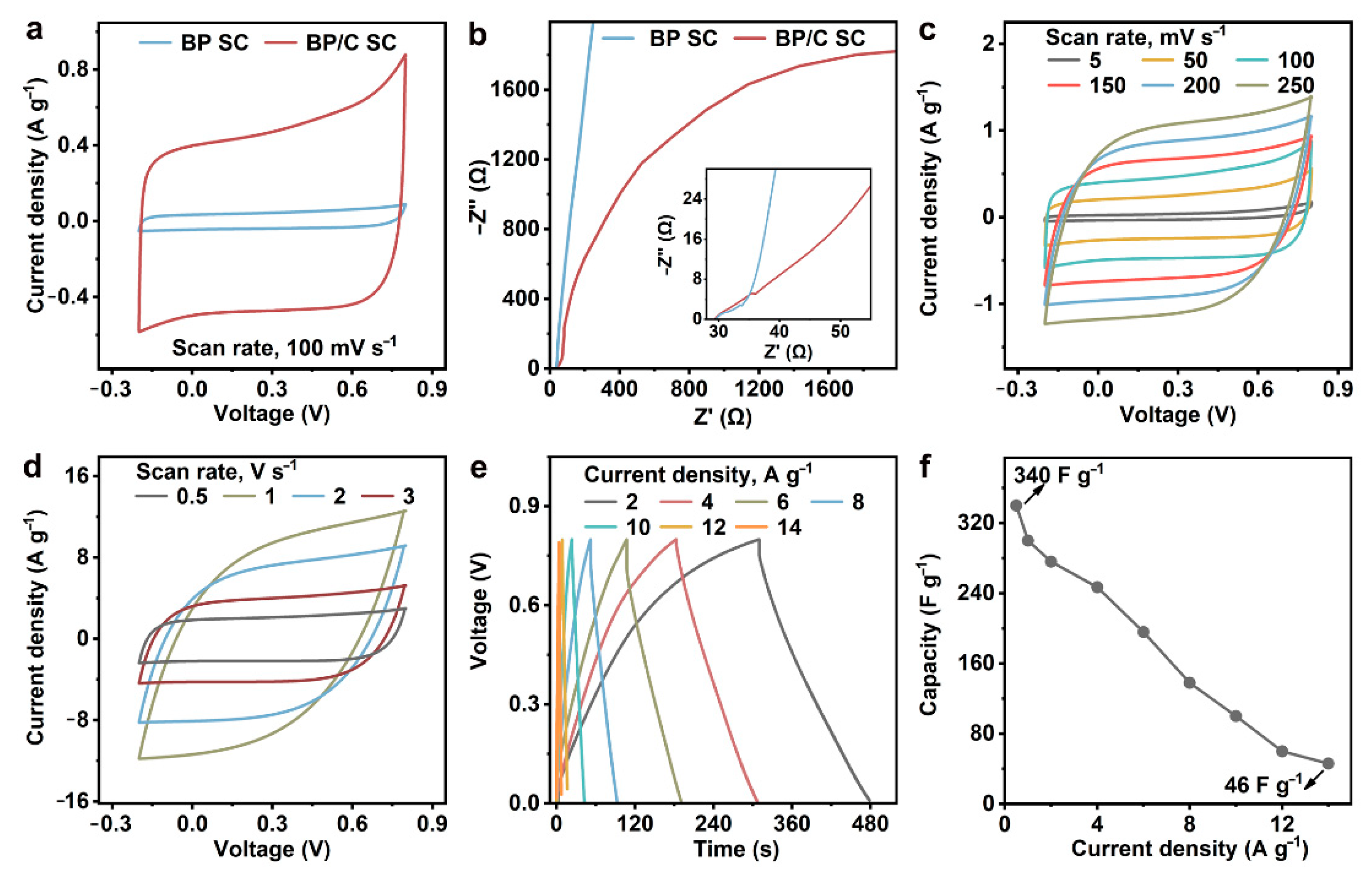
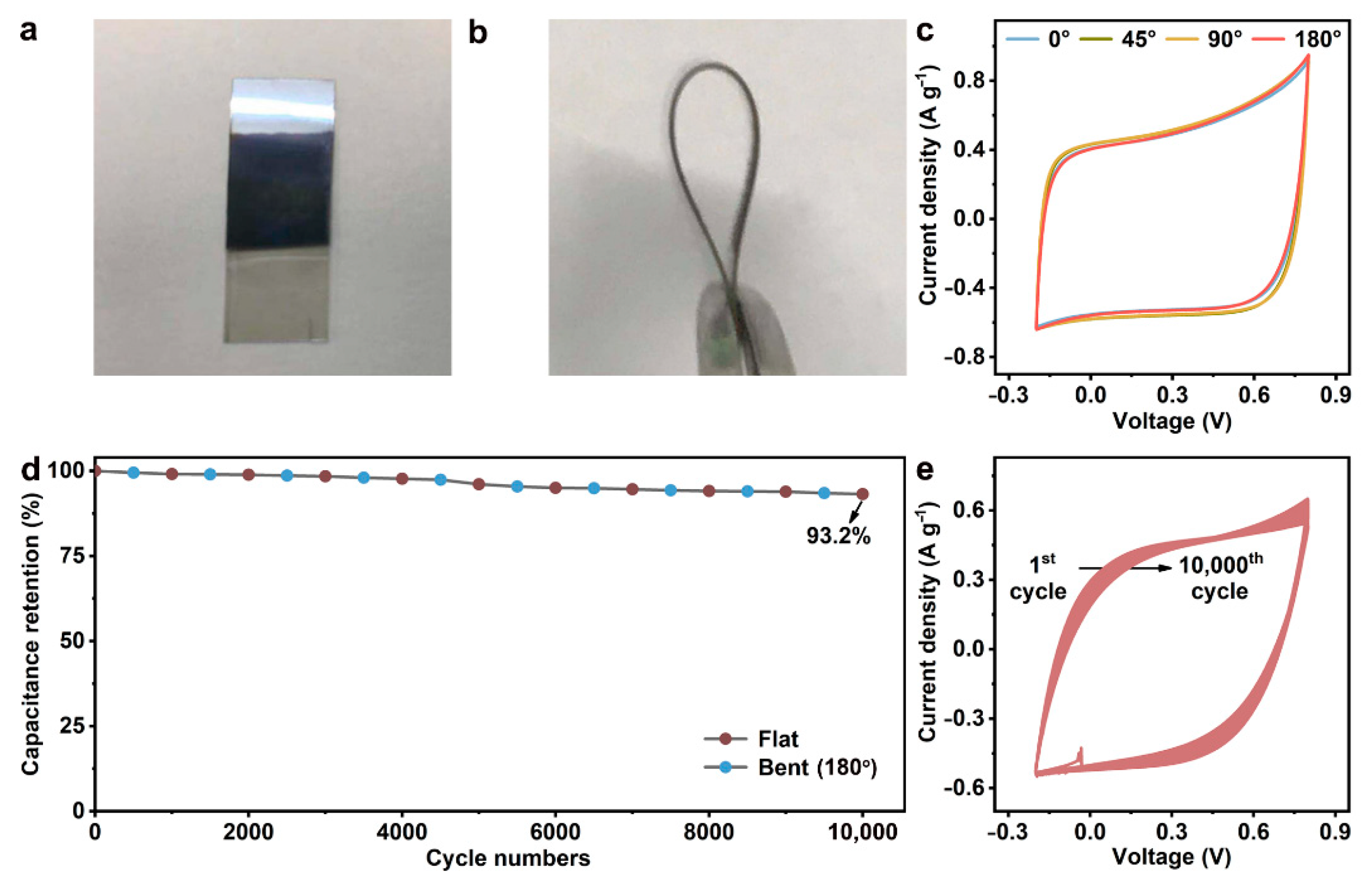
3.2. Performance Evaluation of BP/C SC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubal, D.P.; Chodankar, N.R.; Kim, D.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Cherusseri, J.; Pandey, D.; Kumar, K.S.; Thomas, J.; Zhai, L. Flexible supercapacitor electrodes using metal-organic frameworks. Nanoscale 2020, 12, 17649–17662. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, M.; Zhou, L.; Zhang, R.; Zhang, C.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@layered double hydroxide core-shell nanoarrays. Nano Energy 2016, 20, 294–304. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on carbon fibers modified with carbon nanotubes for ultrafast flexible supercapacitors in ionic liquid electrolytes with wide voltage windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, X.; Wang, F.; Lv, G.; Yu, T.; Lv, M.; Wang, J.; Zhai, Y.; Hu, J. ZIF-67-assisted construction of hollow core/shell cactus-like MnNiCo trimetal electrodes and Co, N dual-doped carbon electrodes for high-performance hybrid supercapacitors. J. Mater. Chem. A 2020, 8, 14287–14298. [Google Scholar] [CrossRef]
- Dahal, B.; Chhetri, K.; Muthurasu, A.; Mukhiya, T.; Tiwari, A.P.; Gautam, J.; Lee, J.Y.; Chung, D.C.; Kim, H.Y. Biaxial stretchability in high-performance, all-solid-state supercapacitor with a double-layer anode and a faradic cathode based on graphitic-2200 knitted carbon fiber. Adv. Energy Mater. 2021, 11, 2002961. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Ali, M.; Lee, H.; Ko, M.J. In situ grown MWCNTs/MXenes nanocomposites on carbon cloth for high-performance flexible supercapacitors. Adv. Funct. Mater. 2020, 30, 2002739. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Liu, R.; He, H.; Hao, J.; Lu, Y.; Wang, Y.; Liang, G.; Yuan, G.; Guo, Z. Engineering textile electrode and bacterial cellulose nanofiber reinforced hydrogel electrolyte to enable high-performance flexible all-solid-state supercapacitors. Adv. Energy Mater. 2021, 11, 2003010. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Han, L.; Meng, J.; Lu, J.; Song, Q. All Si3N4 nanowires membrane based high-performance flexible solid-state asymmetric supercapacitor. Small 2021, 17, 2008056. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-based materials for flexible all-solid-state supercapacitors. Adv. Mater. 2018, 30, 1705489. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Cesar, R.; Moreira, C.M.; Santos, J.H.; De Souza, L.G.; Pires, B.M.; Vicentini, R.; Nunes, W.; Zanin, H. Reviewing the fundamentals of supercapacitors and the difficulties involving the analysis of the electrochemical findings obtained for porous electrode materials. Energy Storage Mater. 2020, 27, 555–590. [Google Scholar]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Zhou, Q.; Fan, T.; Li, Y.; Chen, D.; Liu, S.; Li, X. Hollow-structure NiCo hydroxide/carbon nanotube composite for high-performance supercapacitors. J. Power Sources 2019, 426, 111–115. [Google Scholar]
- Chen, H.; Wang, W.; Yang, L.; Dong, L.; Wang, D.; Xu, X.; Wang, D.; Huang, J.; Lv, M.; Wang, H. A review of cobalt-containing nanomaterials, carbon nanomaterials and their composites in preparation methods and application. Nanomaterials 2022, 12, 2042. [Google Scholar]
- Yan, L.; Li, D.; Yan, T.; Chen, G.; Shi, L.; An, Z.; Zhang, D. N,P,S-Codoped hierarchically porous carbon spheres with well-balanced gravimetric/volumetric capacitance for supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 5265–5272. [Google Scholar]
- Li, Y.; Han, X.; Yi, T.; He, Y.; Li, X. Review and prospect of NiCo2O4-based composite materials for supercapacitor electrodes. J. Energy Chem. 2019, 31, 54–78. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Yang, X.; Yang, J.; Chen, X.; Ying, J.Y. Graphene-wrapped nickel sulfide nanoprisms with improved performance for Li-ion battery anodes and supercapacitors. Nano Energy 2016, 26, 425–437. [Google Scholar]
- Zhao, J.; Hou, S.; Bai, Y.; Lian, Y.; Zhou, Q.; Ban, C.; Wang, Z.; Zhang, H. Multilayer dodecahedrons Zn-Co sulfide for supercapacitors. Electrochim. Acta 2020, 354, 136714. [Google Scholar] [CrossRef]
- Wan, L.; Yuan, Y.; Liu, J.; Chen, J.; Zhang, Y.; Du, C.; Xie, M. A free-standing Ni-Mn-S@NiCo2S4 core-shell heterostructure on carbon cloth for high-energy flexible supercapacitors. Electrochim. Acta 2021, 368, 137579. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for supercapacitors: Status, challenges and prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ge, Y.; Chao, Y.; Wang, C.; Wallace, G.G. Recent progress in 2D materials for flexible supercapacitors. J. Energy Chem. 2018, 27, 57–72. [Google Scholar] [CrossRef]
- Nakhanivej, P.; Yu, X.; Park, S.K.; Kim, S.; Hong, J.; Kim, H.J.; Lee, W.; Hwang, J.Y.; Yang, J.E.; Wolverton, C. Revealing molecular-level surface redox sites of controllably oxidized black phosphorus nanosheets. Nat. Mater. 2019, 18, 156–162. [Google Scholar] [CrossRef]
- Wen, M.; Liu, D.; Kang, Y.; Wang, J.; Huang, H.; Li, J.; Chu, P.K.; Yu, X. Synthesis of high-quality black phosphorus sponges for all-solid-state supercapacitors. Mater. Horiz. 2019, 6, 176–181. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, Z.; Chen, L.; Zhou, F.; Zheng, S.; Ren, W.; Cheng, H.; Bao, X. One-step device fabrication of phosphorene and graphene interdigital micro-supercapacitors with high energy density. ACS Nano 2017, 11, 7284–7292. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Bian, S.; Liu, Q.; Gao, Y.; Wang, X.; Chu, P.K.; Yu, X.F. Photoelectrochemical synthesis of ammonia with black phosphorus. Adv. Funct. Mater. 2020, 30, 2002731. [Google Scholar] [CrossRef]
- Gong, X.; Guan, L.; Li, Q.; Li, Y.; Zhang, T.; Pan, H.; Sun, Q.; Shen, Y.; Grätzel, C.; Zakeeruddin, S.M.; et al. Black phosphorus quantum dots in inorganic perovskite thin films for efficient photovoltaic application. Sci. Adv. 2020, 6, y5661. [Google Scholar] [CrossRef]
- Duan, Z.; Wang, Y.; Bian, S.; Liu, D.; Zhang, Y.; Zhang, X.; He, R.; Wang, J.; Qu, G.; Chu, P.K. Size-dependent flame retardancy of black phosphorus nanosheets. Nanoscale 2022, 14, 2599–2604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Zhao, Q.; Yu, X.; Zhang, S.; Xing, B. Unique interaction between layered black phosphorus and nitrogen dioxide. Nanomaterials 2022, 12, 2011. [Google Scholar] [CrossRef] [PubMed]
- Sajedi-Moghaddam, A.; Mayorga-Martinez, C.C.; Sofer, Z.; Bousa, D.; Saievar-Iranizad, E.; Pumera, M. Black phosphorus nanoflakes/polyaniline hybrid material for high-performance pseudocapacitors. J. Phys. Chem. C 2017, 121, 20532–20538. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Qian, J.; Lau, S.P. Emerging opportunities for black phosphorus in energy applications. Mater. Today Energy 2019, 12, 1–25. [Google Scholar] [CrossRef]
- Hirsch, A.; Hauke, F. Post-graphene 2D chemistry: The emerging field of molybdenum disulfide and black phosphorus functionalization. Angew. Chem. Int. Ed. 2018, 57, 4338–4354. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, W.; Xu, M.; Bai, S.; Chen, Y.; Tang, Z.; Wang, C.; Yang, Y.; Zhang, X.; Yuan, Y.; et al. Two-dimensional black phosphorus: Properties, fabrication and application for flexible supercapacitors. Chem. Eng. J. 2021, 412, 128744. [Google Scholar] [CrossRef]
- Hao, C.; Yang, B.; Wen, F.; Xiang, J.; Li, L.; Wang, W.; Zeng, Z.; Xu, B.; Zhao, Z.; Liu, Z. Flexible all-solid-state supercapacitors based on liquid-exfoliated black-phosphorus nanoflakes. Adv. Mater. 2016, 28, 3194–3201. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, X.; Liu, Y.; Yang, X.; Wang, J.; Liu, Q.; Luo, Q.; Jing, C.; Yu, X.; Qu, G.; et al. Surface and interface control of black phosphorus. Chem 2022, 8, 632–662. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Guo, Z.; Huang, H.; Xiao, Q.; Zhang, H.; Yu, X.F. Solvothermal synthesis and ultrafast photonics of black phosphorus quantum dots. Adv. Opt. Mater. 2016, 4, 1223–1229. [Google Scholar] [CrossRef]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z.; et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef]
- Hu, Y.; Ji, Q.; Huang, M.; Chang, L.; Zhang, C.; Wu, G.; Zi, B.; Bao, N.; Chen, W.; Wu, Y. Light-driven self-oscillating actuators with phototactic locomotion based on black phosphorus heterostructure. Angew. Chem. Int. Ed. 2021, 60, 20511–20517. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.T.; Tadich, A.; Carvalho, A.; Ziletti, A.; O Donnell, K.M.; Koenig, S.P.; Coker, D.F.; Özyilmaz, B.; Neto, A.H.C.; Fuhrer, M.S. Creating a stable oxide at the surface of black phosphorus. ACS Appl. Mater. Interfaces 2015, 7, 14557–14562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, T.; Jiang, D.; Duan, H.; Sun, Z.; Zhang, M.; Jin, H.; Guan, R.; Liu, Y.; Chen, M.; et al. Stabilizing black phosphorus nanosheets via edge-selective bonding of sacrificial C60 molecules. Nat. Commun. 2018, 9, 4177. [Google Scholar] [CrossRef] [PubMed]
- Tofan, D.; Sakazaki, Y.; Walz Mitra, K.L.; Peng, R.; Lee, S.; Li, M.; Velian, A. Surface modification of black phosphorus with group 13 lewis acids for ambient protection and electronic tuning. Angew. Chem. Int. Ed. 2021, 60, 8329–8336. [Google Scholar] [CrossRef]
- Abate, Y.; Akinwande, D.; Gamage, S.; Wang, H.; Snure, M.; Poudel, N.; Cronin, S.B. Recent progress on stability and passivation of black phosphorus. Adv. Mater. 2018, 30, 1704749. [Google Scholar] [CrossRef]
- Cao, J.; He, P.; Brent, J.R.; Yilmaz, H.; Lewis, D.J.; Kinloch, I.A.; Derby, B. Supercapacitor electrodes from the in situ reaction between two-dimensional sheets of black phosphorus and graphene oxide. ACS Appl. Mater. Interfaces 2018, 10, 10330–10338. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, X.; Liu, M.; Wang, C.; Chu, F. Synthesis of 3D phosphorus doped graphene foam in carbon cloth to support V2O5/CoMoS4 hybrid for flexible all-solid-state asymmetry supercapacitors. J. Power Sources 2020, 453, 227902. [Google Scholar] [CrossRef]

| Electrode Material | Capacitance (F g−1) at a Scan Rate (mV s−1) | Stability Test | Capacitance Retention | Ref. |
|---|---|---|---|---|
| BP/C | 372 (5) | 4 A g−1 galvanostatic | 89.1% (10,000th) | This work |
| Flat-bend | 93.2% (10,000th) | |||
| BP nanoflakes | 45.8 (10) | Flat-bend | 84.5% (1000th) | [37] |
| BP sponges | 80 (10) | 0.1 V s−1 cycle | 80% (15,000th) | [26] |
| BP/GO | 104.4 (250) | 5 A g−1 galvanostatic | 92.7% (5000th) | [46] |
| BP/G | 37.5 (5) | Flat-bend | 89.5% (2000th) | [27] |
| BP/PANI | 354 (300) | 0.3 A g−1 galvanostatic | 87% (175th) | [33] |
| N,P,S-HCS | 31.3 (500) | 10 A g−1 galvanostatic | 95% (1000th) | [16] |
| PGO/CC | 211.7 (1000) | 10 A g−1 galvanostatic | 89.3% (10,000th) | [47] |
| Ni-Mn-S@NiCo2S4 | 939 (1000) | 5 A g−1 galvanostatic | 90.3% (5000th) | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; Liu, D.; Ye, Z.; Sun, C.; Wang, Z.; Chen, K.; Li, Y.; Huang, H.; Zeng, X.; Wang, J.; et al. Black Phosphorus/Carbon Nanoframes for Efficient Flexible All-Solid-State Supercapacitor. Nanomaterials 2022, 12, 3311. https://doi.org/10.3390/nano12193311
Duan Z, Liu D, Ye Z, Sun C, Wang Z, Chen K, Li Y, Huang H, Zeng X, Wang J, et al. Black Phosphorus/Carbon Nanoframes for Efficient Flexible All-Solid-State Supercapacitor. Nanomaterials. 2022; 12(19):3311. https://doi.org/10.3390/nano12193311
Chicago/Turabian StyleDuan, Zunbin, Danni Liu, Zhaoer Ye, Caixia Sun, Zikun Wang, Kezhen Chen, Yang Li, Hao Huang, Xiaoliang Zeng, Jiahong Wang, and et al. 2022. "Black Phosphorus/Carbon Nanoframes for Efficient Flexible All-Solid-State Supercapacitor" Nanomaterials 12, no. 19: 3311. https://doi.org/10.3390/nano12193311
APA StyleDuan, Z., Liu, D., Ye, Z., Sun, C., Wang, Z., Chen, K., Li, Y., Huang, H., Zeng, X., Wang, J., Sun, R., & Yu, X.-F. (2022). Black Phosphorus/Carbon Nanoframes for Efficient Flexible All-Solid-State Supercapacitor. Nanomaterials, 12(19), 3311. https://doi.org/10.3390/nano12193311








