Conservation of Archaeological Bones: Assessment of Innovative Phosphate Consolidants in Comparison with Paraloid B72
Abstract
:1. Introduction
- Formulation of the DAP treatment. To increase the strengthening efficacy, studies reported in the literature have focused on pre-treatment with suspensions of nano-Ca(OH)2 [26,32] and/or nano-HAP [20]. However, several alternative strategies to improve the consolidating efficacy of the DAP treatment, when applied onto heritage stones and mortars, have been proposed in the literature (e.g., increasing the DAP concentration [37], adding Ca2+ ions [38] or adding alcohol to the DAP solution [39,40]), but they have not yet been tested in the case of bone conservation.
- Systematic comparison with Paraloid B72. Even though a comparison between DAP and PB72 has been reported in one of the first studies on the topic [24] and, recently, PB72 has been compared to the DAP-based treatment in terms of impact on radiocarbon dating [20], still, to our best knowledge, no systematic comparison between increases in bone mechanical properties brought about by DAP and PB72 has been reported in the literature.
- Choice of the archeological samples. The majority of the studies reported in the literature has been carried out on fresh bone and/or bone powders, while studies on archeological bones have been mostly limited to specimens up to 3000 years old. Here, we applied the treatments on archaeological bones dating to 1–0.8 million years ago, which corresponds to a very challenging state of conservation.
2. Materials and Methods
2.1. Bone Samples
2.2. Consolidating Treatments
2.2.1. DAP
- DAP concentration: previous studies have shown that the higher the DAP concentration, the more abundant the formation of the new consolidating phases and the strengthening efficacy [37], but also the higher the tendency of the new phases to crack during drying [40] and the higher the risk that unreacted DAP remains in the substrate, if not properly removed [44].
- Addition of CaCl2. The addition of a calcium source promotes and accelerates formation of the new consolidating phases [38], also having a positive effect on the consolidating ability [45]. Besides formation of HAP, the addition of CaCl2 has been found to promote formation of another calcium phosphate mineral, octacalcium phosphate (OCP, Ca8H2(PO4)6·5H2O) [38].
2.2.2. Paraloid B72
2.3. Characterization Tests
2.3.1. FT-IR Microscopy
2.3.2. FEG-SEM Observation
2.3.3. Knoop Microhardness
- to compare the consolidating ability of DAP and PB72, microhardness was determined before and after treatment with the two consolidants (3 replicates per condition), applying a load of 1 N for 30 s.
- to evaluate the effect of rinsing the DAP-treated sample at the end of the treatment and to check that the hardened consolidant was not soluble in water, microhardness was determined before treatment, after the DAP-treatment (before rinsing with water) and then again after rinsing with water, applying a load of 2 N for 30 s.
2.3.4. Vickers Microhardness
2.3.5. Scratch Resistance
2.3.6. Scotch Tape Test
3. Results and Discussion
3.1. Identification of the Most Promising DAP Formulation
3.2. Comparison between DAP and PB72
3.2.1. Knoop Microhardness
3.2.2. Vickers Microhardness
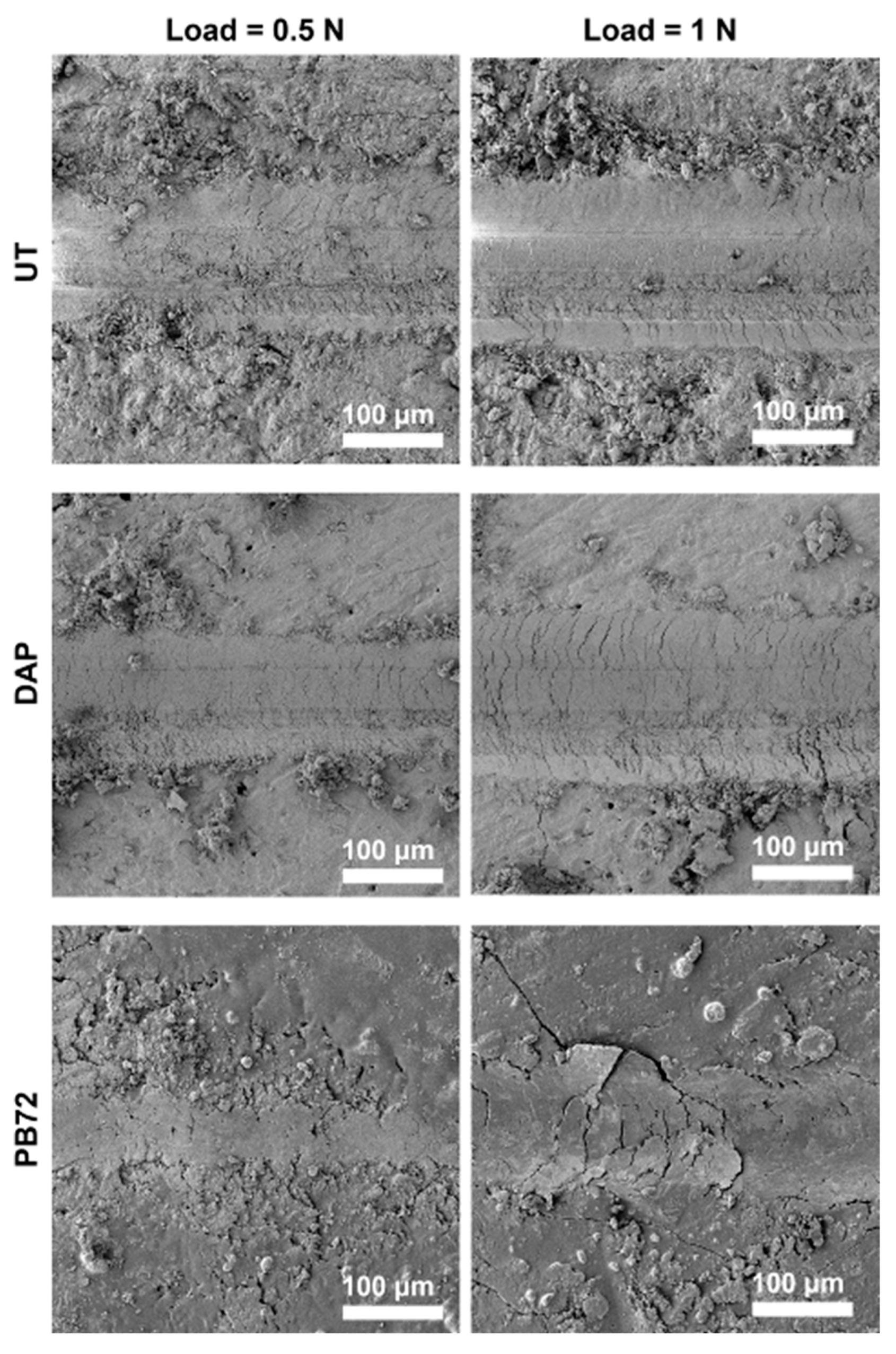
3.2.3. Scratching Test
3.2.4. Scotch Tape Test
4. Conclusions
- (1)
- Among the investigated parameters (DAP concentration, addition of a calcium source or addition of ethanol to the DAP solution), the DAP concentration apparently had the highest impact. In fact, application of a 3 M DAP solution allowed the achievement of a good increase in cohesion, thanks to newly formed HAP, as assessed by micro-FTIR and FEG-SEM. Consequently, the 3 M DAP formulation was systematically compared to Paraloid B72.
- (2)
- In terms of effectiveness, both consolidants exhibited significant consolidating ability. In particular, the DAP treatment increased the bone surface properties (microhardness and resistance to scratch) and also the resistance to material loss by peeling off, which is more dependent on in-depth consolidation. The performance of Paraloid B72 was highly influenced by the formation of a layer of acrylic resin on the bone surface: the measurements of microhardness (especially with the Knoop indenter) and resistance to scratch essentially regarded only the surface coating, with limited influence of the bone substrate. Nonetheless, Paraloid B72 was able to substantially decrease the material loss by peeling off (even more effectively than DAP), thus indicating that the consolidant was able to penetrate in depth in the bone samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, M.; Nielsen-Marsh, C.M.; Hiller, J.; Smith, C.; Roberts, J.P.; Prigodich, R.V.; Wess, T.J.; Csapo, J.; Millard, A.; Turner-Walker, G. The survival of organic matter in bone: A review. Archaeometry 2002, 44, 383–394. [Google Scholar] [CrossRef]
- Currey, J. The structure of bone tissue. In Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002; pp. 224–225. [Google Scholar] [CrossRef]
- Reiche, I.; Vignaud, C.; Menu, M. The crystallinity of ancient bone and dentine: New insights by transmission electron microscopy. Archaeometry 2002, 44, 447–459. [Google Scholar] [CrossRef]
- Nielsen-Marsh, C.; Smith, C.; Jans, M.; Nord, A.; Kars, H.; Collins, M. Bone diagenesis in the European Holocene II: Taphonomic and environmental considerations. J. Archaeol. Sci. 2007, 34, 1523–1531. [Google Scholar] [CrossRef]
- Smith, C.; Nielsen-Marsh, C.; Jans, M.; Collins, M. Bone diagenesis in the European Holocene I: Patterns and mechanisms. J. Archaeol. Sci. 2007, 34, 1485–1493. [Google Scholar] [CrossRef]
- Turner-Walker, G.; Nielsen-Marsh, C.M.; Syversen, U.; Kars, H.; Collins, M.J. Sub-micron spongiform porosity is the major ultra-structural alteration occurring in archaeological bone. Int. J. Osteoarchaeol. 2002, 12, 407–414. [Google Scholar] [CrossRef]
- López-Polín, L. Interventive conservation treatments (or preparation) of Pleistocene bones: Criteria for covering information from the archaeopalaeontological record. Quat. Int. 2015, 388, 199–205. [Google Scholar] [CrossRef]
- López-Polín, L.; Bertral, A.; Font, B.; Ollé, A. Preparation and Conservation treatments of the Pleistocene fossil vertebrate remains from the cave site of Tossal de la Font (Vilafamés, Castelló, Spain). Paleontol. Evol. 2009, 4, 225–231. [Google Scholar]
- Beiner, G.G.; Rabinovich, R. An elephant task—Conservation of elephant remains from Revadim Quarry, Israel. J. Inst. Conserv. 2013, 36, 56–64. [Google Scholar] [CrossRef]
- Larkin, N.R. Literally a ‘mammoth task’: The conservation, preparation and curation of the West Runton Mammoth skeleton. Quat. Int. 2010, 228, 233–240. [Google Scholar] [CrossRef]
- Eklund, J.A. The Effects of Preparation and Conservation Treatments on DNA; University College of London: London, UK, 2007. [Google Scholar]
- France, C.A.; Giaccai, J.A.; Doney, C.R. The effects of Paraloid B-72 and Butvar B-98 treatment and organic solvent removal on δ13C, δ15N, and δ18O values of collagen and hydroxyapatite in a modern bone. Am. J. Phys. Anthropol. 2015, 157, 330–338. [Google Scholar] [CrossRef]
- López-Polín, L. Possible interferences of some conservation treatments with subsequent studies on fossil bones: A conservator’s overview. Quat. Int. 2012, 275, 120–127. [Google Scholar] [CrossRef]
- Moore, K.M.; Murray, M.L.; Schoeninger, M.J. Dietary reconstruction from bones treated with preservatives. J. Archaeol. Sci. 1989, 16, 437–446. [Google Scholar] [CrossRef]
- Turner-Walker, G.; Hung, Y.; Yang, Y. How reversible are consolidants used on fragile archaeological bones? A practical evaluation of B-72 impregnation. J. Innov. Technol. 2020, 2, 19–26. [Google Scholar] [CrossRef]
- Beaubien, H.F. Field Conservation of Skeletal Remains: Stabilization Treatment Techniques and Implications for Future Analysis. Adv. Archaeol. Pract. 2019, 7, 23–29. [Google Scholar] [CrossRef]
- Koob, S.P. Paraloid-25 years of use as a consolidant. In Holding It All Together; Ambers, J., Higgitt, C., Harrison, L., Saunders, D., Eds.; Archetype Publications: London, UK, 2009; pp. 113–119. [Google Scholar]
- Peters, R.F. Bridge over Olduvai Gorge: Capacity building and conservation of lithics and fossils. In Proceedings of the ICOM-CC 18th Triennial Conference Preprints, Copenhagen, Denmark, 4–8 September 2017; Bridgland, J., Ed.; International Council of Museums ICOM: Copenhagen, Denmark, 2017. [Google Scholar]
- Brock, F.; Dee, M.; Hughes, A.; Snoeck, C.; Staff, R.; Ramsey, C.B. Testing the Effectiveness of Protocols for Removal of Common Conservation Treatments for Radiocarbon Dating. Radiocarbon 2018, 60, 35–50. [Google Scholar] [CrossRef]
- Porpora, F.; Zaro, V.; Liccioli, L.; Modi, A.; Meoli, A.; Marradi, G.; Barone, S.; Vai, S.; Dei, L.; Caramelli, D.; et al. Performance of innovative nanomaterials for bone remains consolidation and effect on 14C dating and on palaeogenetic analysis. Sci. Rep. 2022, 12, 6975. [Google Scholar] [CrossRef] [PubMed]
- Higham, T. Carbon-14 dating. In Encyclopedia of Archaeology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 955–957. [Google Scholar]
- Peng, X.; Wang, Y.; Ma, X.-F.; Bao, H.; Huang, X.; Zhou, H.; Luo, H.; Wang, X. Sol–Gel derived hybrid materials for conservation of fossils. J. Sol-Gel Sci. Technol. 2020, 94, 347–355. [Google Scholar] [CrossRef]
- Natali, I.; Tempesti, P.; Carretti, E.; Potenza, M.; Sansoni, S.; Baglioni, P.; Dei, L. Aragonite Crystals Grown on Bones by Reaction of CO2 with Nanostructured Ca(OH)2 in the Presence of Collagen. Implications in Archaeology and Paleontology. Langmuir 2014, 30, 660–668. [Google Scholar] [CrossRef]
- North, A.; Balonis, M.; Kakoulli, I. Biomimetic hydroxyapatite as a new consolidating agent for archaeological bone. Stud. Conserv. 2016, 61, 146–161. [Google Scholar] [CrossRef]
- Nesseri, E.; Boyatzis, S.C.; Boukos, N.; Panagiaris, G. Optimizing the biomimetic synthesis of hydroxyapatite for the consolidation of bone using diammonium phosphate, simulated body fluid, and gelatin. SN Appl. Sci. 2020, 2, 1892. [Google Scholar] [CrossRef]
- Salvatore, A.; Vai, S.; Caporali, S.; Caramelli, D.; Lari, M.; Carretti, E. Evaluation of Diammonium hydrogen phosphate and Ca(OH)2 nanoparticles for consolidation of ancient bones. J. Cult. Herit. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Alderson, S.; Down, J.L.; Maines, C.A.; Williams, R.S.; Young, G.S. Potential substitutes for discontinued poly(vinyl acetate) resins used in conservation. J. Am. Inst. Conserv. 2019, 58, 158–179. [Google Scholar] [CrossRef]
- Bisulca, C.; Elkin, L.K.; Davidson, A.; Kronthal Elkin, L. Consolidation of fragile fossil bone from Ukhaa Tolgod, Mongolia (Late cretaceous) with Conservare OH100. Source J. Am. Inst. Conserv. 2009, 48, 37–50. [Google Scholar] [CrossRef]
- Davidson, A.; Brown, G.W. Paraloid B-72: Practical Tips for the vertbrate fossil preparator. Collet. Forum 2012, 26, 99–119. [Google Scholar]
- López-Polín, L.; de Castro, J.B.; Carbonell, E. The preparation and conservation treatments of the human fossils from Lower Pleistocene unit TD6 (Gran Dolina site, Atapuerca)—The 2003–2009 record. Quat. Int. 2017, 433, 251–262. [Google Scholar] [CrossRef]
- Russell, R.; Strilisky, B. Keep it together: An evaluation of the tensile strengths of three select adhesives used in fossil preparation. Collect. Forum 2016, 30, 85–95. [Google Scholar] [CrossRef]
- Yang, F.; He, D.; Liu, Y.; Li, N.; Wang, Z.; Ma, Q.; Dong, G. Conservation of bone relics using hydroxyapatite as protective material. Appl. Phys. A 2016, 122, 479. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Arcos Navarrete, D. Biological Apatites in Bone and Teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Royal Society for Chemistry: London, UK, 2015; pp. 1–29. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Gazzano, M. Ion substitution in biological and synthetic apatites. In Biomineralization and Biomaterials: Fundamentals and Applications; Aparicio, C., Ginebra, M.P., Eds.; Woodhead Publishing (Elsevier): Sawston, UK, 2016; pp. 235–266. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Gong, W.; Yang, S.; Zheng, L.; Xiao, H.; Zheng, J.; Wu, B.; Zhou, Z. Consolidating effect of hydroxyapatite on the ancient ivories from Jinsha ruins site: Surface morphology and mechanical properties study. J. Cult. Herit. 2018, 35, 116–122. [Google Scholar] [CrossRef]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef]
- Naidu, S.; Scherer, G.W. Nucleation, growth and evolution of calcium phosphate films on calcite. J. Colloid Interface Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Sassoni, E.; Franzoni, E.; Scherer, G.W. Hydroxyapatite coatings for marble protection: Optimization of calcite covering and acid resistance. Appl. Surf. Sci. 2016, 368, 241–257. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Calcium phosphate coatings for marble conservation: Influence of ethanol and isopropanol addition to the precipitation medium on the coating microstructure and performance. Corros. Sci. 2018, 136, 255–267. [Google Scholar] [CrossRef]
- Vallverdú, J.; Saladié, P.; Rosas, A.; Huguet, R.; Cáceres, I.; Mosquera, M.; Garcia-Tabernero, A.; Estalrrich, A.; Lozano-Fernández, I.; Pineda-Alcalá, A.; et al. Age and Date for Early Arrival of the Acheulian in Europe (Barranc de la Boella, la Canonja, Spain). PLoS ONE 2014, 9, e103634. [Google Scholar] [CrossRef]
- Pineda, A.; Saladié, P.; Vergès, J.M.; Huguet, R.; Cáceres, I.; Vallverdú, J. Trampling versus cut marks on chemically altered surfaces: An experimental approach and archaeological application at the Barranc de la Boella site (la Canonja, Tarragona, Spain). J. Archaeol. Sci. 2014, 50, 84–93. [Google Scholar] [CrossRef]
- Pineda, A.; Saladié, P. Beyond the Problem of Bone Surface Preservation in Taphonomic Studies of Early and Middle Pleistocene Open-Air Sites; Springer: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.W.; Franzoni, E. Penetration depth and redistribution of an aqueous ammonium phosphate solution used for porous limestone consolidation by brushing and immersion. Constr. Build. Mater. 2017, 148, 571–578. [Google Scholar] [CrossRef]
- Naidu, S.; Liu, C.; Scherer, G.W. Hydroxyapatite-based consolidant and the acceleration of hydrolysis of silicate-based consolidants. J. Cult. Herit. 2015, 16, 94–101. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Graziani, G. Brushing, poultice or immersion? The role of the application technique on the performance of a novel hydroxyapatite-based consolidating treatment for limestone. J. Cult. Herit. 2015, 16, 173–184. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- ISO 20502; Fine ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Adhesion of Ceramic Coatings by Scratch Testing. International Organization for Standardization: Geneva, Switzerland, 2016.
- Drdácký, M.; Lesák, J.; Rescic, S.; Slížková, Z.; Tiano, P.; Valach, J. Standardization of peeling tests for assessing the cohesion and consolidation characteristics of historic stone surfaces. Mater. Struct. 2012, 45, 505–520. [Google Scholar] [CrossRef]
- Tao, J. FTIR and Raman Studies of Structure and Bonding in Mineral and Organic–Mineral Composites. Methods Enzymol. 2013, 532, 533–556. [Google Scholar] [PubMed]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile, T., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 6; pp. 123–148. [Google Scholar]
- Turner-Walker, G. The mechanical properties of artificially aged bone: Probing the nature of the collagen-mineral bond. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 310, 17–22. [Google Scholar] [CrossRef]
- Turner-Walker, G.; Parry, T. The Tensile Strength of Archaeological Bone. J. Archaeol. Sci. 1995, 22, 185–191. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Cortés, A.; Graziani, G.; Boi, M.; López-Polín, L.; Sassoni, E. Conservation of Archaeological Bones: Assessment of Innovative Phosphate Consolidants in Comparison with Paraloid B72. Nanomaterials 2022, 12, 3163. https://doi.org/10.3390/nano12183163
Díaz-Cortés A, Graziani G, Boi M, López-Polín L, Sassoni E. Conservation of Archaeological Bones: Assessment of Innovative Phosphate Consolidants in Comparison with Paraloid B72. Nanomaterials. 2022; 12(18):3163. https://doi.org/10.3390/nano12183163
Chicago/Turabian StyleDíaz-Cortés, Andrea, Gabriela Graziani, Marco Boi, Lucia López-Polín, and Enrico Sassoni. 2022. "Conservation of Archaeological Bones: Assessment of Innovative Phosphate Consolidants in Comparison with Paraloid B72" Nanomaterials 12, no. 18: 3163. https://doi.org/10.3390/nano12183163
APA StyleDíaz-Cortés, A., Graziani, G., Boi, M., López-Polín, L., & Sassoni, E. (2022). Conservation of Archaeological Bones: Assessment of Innovative Phosphate Consolidants in Comparison with Paraloid B72. Nanomaterials, 12(18), 3163. https://doi.org/10.3390/nano12183163







