Multilayer Graphene Oxide Supported ZIF-8 for Efficient Removal of Copper Ions
Abstract
:Highlights
- A multilayer graphene based adsorbent was prepared by a simple method;
- Adsorbents have high adsorption capacity and a high specific surface area;
- The multilayer structure of graphene layer provides a framework for ZIF, adding more adsorption sites.
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of MGO@ZIF-8
2.3. Assessment of MGO@ZIF-8 for Cu2+ Adsorbing Capacity
2.4. Standard Curve of Cu2+
2.5. Adsorption Kinetics
2.6. Adsorption Isotherms
3. Results and Discussion
3.1. Characterization of Adsorbents
3.1.1. SEM and AFM Analysis
3.1.2. XRD Diffraction and FT-IR Spectroscopy
3.1.3. Specific Surface Area and BET Analysis
3.1.4. XPS Analysis
3.2. Adsorption Mechanism
3.2.1. pH Effect on Cu2+ Adsorption by MGO@ZIF-8
3.2.2. Temperature Effect on Cu2+ Adsorption by MGO@ZIF-8
3.2.3. Effect of Adsorbent Amount on Cu2+ Adsorption of MGO@ZIF-8
3.2.4. Time-Dependent Adsorption Behavior
3.2.5. Analysis of Orthogonal Experiment Results
3.2.6. Adsorption Kinetics
3.2.7. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ansone-Bertina, L.; Ozols, V.; Arbidans, L.; Dobkevica, L.; Sarsuns, K.; Vanags, E.; Klavins, M. Metal–Organic Frameworks (MOFs) Containing Adsorbents for Carbon Capture. Energies 2022, 15, 3473. [Google Scholar] [CrossRef]
- Atasoy, A.D.; Bilgic, B. Adsorption of Copper and Zinc Ions from Aqueous Solutions Using Montmorillonite and Bauxite as Low-Cost Adsorbents. Mine. Water Environ. 2017, 37, 205–210. [Google Scholar] [CrossRef]
- Casolla, B.; Kuchcinski, G.; Bodenant, M.; Leys, D.; Labreuche, J.; Cordonnier, C.; Hénonet, H. Prédicteurs de pronostic fonctionnel à 1 an chez les survivants d’un infarctus étendu de l’artère cérébrale moyenne traité par hemicraniectomie décompressive. Rev. Neurol. 2021, 176, S80. [Google Scholar] [CrossRef]
- Bo, S.; Ren, W.; Lei, C.; Xie, Y.; Cai, Y.; Wang, S.; Gao, J.; Ni, Q.; Yao, J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr(IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, D.C.; Hua, S.H.; Chun, H.; Kim, Y.S. Sonochemical Preparation of a Magnet-Responsive Fe3O4@ZIF-8 Adsorbent for Efficient Cu2+ Removal. Nanomaterials 2022, 12, 753. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Shen, J.; Sun, Q. Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+. Materials 2020, 13, 5047. [Google Scholar] [CrossRef]
- Bhagat, S.K.; Pyrgaki, K.; Salih, S.Q.; Tiyasha, T.; Beyaztas, U.; Shahid, S.; Yaseen, Z.M. Prediction of copper ions adsorption by attapulgite adsorbent using tuned-artificial intelligence model. Chemosphere 2021, 276, 130162. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkuş, B.; Bozkurt, H. Development of a new adsorbent from pumpkin husk by KOH-modification to remove copper ions. Environ. Sci. Pollut. Res. 2018, 26, 11514–11523. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Nie, H. Crumpled graphene balls as rapid and efficient adsorbents for removal of copper ions. J. Colloid Interface Sci. 2018, 530, 46–51. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Wang, J.; Lu, J.; Zhou, Y. Polydopamine modified cyclodextrin polymer as efficient adsorbent for removing cationic dyes and Cu2+. J. Hazard. Mater. 2019, 389, 121897. [Google Scholar] [CrossRef]
- Chu, Y.; Zhu, S.; Wang, F.; Lei, W.; Xia, M.; Liao, C. Tyrosine-Immobilized Montmorillonite: An Efficient Adsorbent for Removal of Pb2+ and Cu2+ from Aqueous Solution. J. Chem. Eng. Data 2019, 64, 3535–3546. [Google Scholar] [CrossRef]
- Jorge, E.Y.C.; Sánchez, R.M.; Chacón, J.J.; Diaz-Castañon, S.; Piñar, F.C. Magnetite nanoparticles as adsorbent material for Cu2+ ions from aqueous solution. Part. Sci. Technol. 2017, 36, 778–784. [Google Scholar] [CrossRef]
- Dou, W.; Liu, J.; Li, M. Competitive adsorption of Cu2+ in Cu2+, Co2+ and Ni2+ mixed multi–metal solution onto graphene oxide (GO)–based hybrid membranes. J. Mol. Liq. 2020, 322, 114516. [Google Scholar] [CrossRef]
- Du, T.; Wang, J.; Zhang, T.; Zhang, L.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Zhou, M.; Wang, J. An Integrating Platform of Ratiometric Fluorescent Adsorbent for Unconventional Real-Time Removing and Monitoring of Copper Ions. ACS Appl. Mater. Interfaces 2020, 12, 13189–13199. [Google Scholar] [CrossRef] [PubMed]
- Fronczak, M.; Demby, K.; Strachowski, P.; Strawski, M.; Bystrzejewski, M. Graphitic Carbon Nitride Doped with the s-Block Metals: Adsorbent for the Removal of Methyl Blue and Copper (II) Ions. Langmuir 2018, 34, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, E.C.; Santos Júnior, L.S.; Santos, M.R.M.C.; Fonseca, M.G.; Sousa, K.S.; Santana, S.A.A.; Airoldi, C. Thermochemistry of interaction between cellulose modified with 2-aminomethyl pyridine and divalent cations. J. Therm. Anal. Calorim. 2013, 114, 423–429. [Google Scholar] [CrossRef]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscop-ic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef]
- Georgiou, E.; Mihajlović, M.; Petrović, J.; Anastopoulos, I.; Dosche, C.; Pashalidis, I. Single-stage production of miscanthus hydrochar at low severity conditions and application as adsorbent of copper and ammonium ions. Bioresour. Technol. 2021, 337, 125458. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Pashaei, S.; Hosseinzadeh, S.; Khodaparast, Z.; Ramin, S.; Saadat, Y. Preparation of novel multi-walled carbon nanotubes nanocomposite adsorbent via RAFT technique for the adsorption of toxic copper ions. Sci. Total Environ. 2018, 640–641, 303–314. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, J.; Huang, X. Allylthiourea functionalized magnetic adsorbent for the extraction of cadmium, copper and lead ions prior to their determination by atomic absorption spectrometry. Mikrochim. Acta 2019, 186, 51. [Google Scholar] [CrossRef]
- Hussain, M.S.; Musharraf, S.G.; Bhanger, M.I.; Malik, M.I. Salicylaldehyde derivative of nano-chitosan as an efficient adsorbent for lead(II), copper(II), and cadmium(II) ions. Int. J. Biol. Macromol. 2020, 147, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Kanchan, D.; Joshi, M.; Jethva, H.; Parikh, K. Dielectric relaxation, complex impedance and modulus spectroscopic studies of mix phase rod like cobalt sulfide nanoparticles. Mater. Res. Bull. 2017, 93, 63–73. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Szcześ, A.; Sternik, D. The preparation of calcium phosphate adsorbent from natural calcium resource and its application for copper ion removal. Environ. Sci. Pollut. Res. 2020, 28, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Klimmek, S.; Stan, H.-J.; Wilke, A.; Bunke, G.; Buchholz, R. Comparative Analysis of the Biosorption of Cadmium, Lead, Nickel, and Zinc by Algae. Environ. Sci. Technol. 2001, 35, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Majdańska, M.; Budnyak, T.M. Lanthanum and copper ions recovery from nickel-metal hydride cells leaching solutions by the oxide adsorbent Pyrolox®. J. Environ. Chem. Eng. 2019, 7, 103003. [Google Scholar] [CrossRef]
- Lin, Y.; Hong, Y.; Song, Q.; Zhang, Z.; Gao, J.; Tao, T. Highly efficient removal of copper ions from water using poly(acrylic acid)-grafted chitosan adsorbent. Colloid Polym. Sci. 2017, 295, 627–635. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Hong, L. Diethylenetriamine-grafted poly(glycidyl methacrylate) adsorbent for effective copper ion adsorption. J. Colloid Interf. Sci. 2006, 303, 99–108. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Ly, Q.S. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Liu, J.; Yuan, W. Desorption of copper ions from the polyamine-functionalized adsorbents: Behaviors and mechanisms. Adsorpt. Sci. Technol. 2016, 34, 455–468. [Google Scholar] [CrossRef]
- Niuniavaite, D.; Baltakys, K.; Dambrauskas, T.; Eisinas, A. Cu2+, Co2+ and Cr3+ adsorption by synthetic dibasic calcium silicate hydrates and their thermal stability in a 25–1000 °C temperature range. J. Therm. Anal. 2019, 138, 2241–2249. [Google Scholar] [CrossRef]
- Mariyam, S.; Zuhara, S.; Al-Ansari, T.; Mackey, H.; McKay, G. Novel high capacity model for copper binary ion exchange on e-waste derived adsorbent resin. Adsorption 2022, 28, 185–196. [Google Scholar] [CrossRef]
- Marjub, M.M.; Rahman, N.; Dafader, N.C.; Tuhen, F.S.; Sultana, S.; Ahmed, F.T. Acrylic acid-chitosan blend hydrogel: A novel polymer adsorbent for adsorption of lead (II) and copper (II) ions from wastewater. J. Polym. Eng. 2019, 39, 883–891. [Google Scholar] [CrossRef]
- Mone, M.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G. Chitosan Grafted with Biobased 5-Hydroxymethyl-Furfural as Adsorbent for Copper and Cadmium Ions Removal. Polymers 2020, 12, 1173. [Google Scholar] [CrossRef]
- Mongioví, C.; Lacalamita, D.; Morin-Crini, N.; Gabrion, X.; Placet, V.; Ribeiro, A.R.L.; Ivanovska, A.; Kostić, M.; Bradu, C.; Staelens, J.-N.; et al. Use of chènevotte, a valuable co-product of industrial hemp fiber, as adsorbent for copper ions: Kinetic studies and modeling. Arab. J. Chem. 2022, 15, 103742. [Google Scholar] [CrossRef]
- Niu, Y.; Ying, D.; Li, K.; Wang, Y.; Jia, J. Fast removal of copper ions from aqueous solution using an eco–friendly fibrous adsor-bent. Chemosphere 2016, 161, 501–509. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Abdullah, T.A.T.; Hoshina, H.; Sattayaporn, S.; Seko, N. Selectivity of Copper by Amine-Based Ion Recognition Polymer Adsorbent with Different Aliphatic Amines. Polymers 2019, 11, 1994. [Google Scholar] [CrossRef]
- Prasad, J.; Singh, A.K.; Haldar, K.K.; Tomar, M.; Gupta, V.; Singh, K. CoFe2O4 nanoparticles decorated MoS2-reduced graphene oxide nanocomposite for improved microwave absorption and shielding performance. RSC Adv. 2019, 9, 21881–21892. [Google Scholar] [CrossRef]
- Qiu, L.; Phule, A.D.; Wen, S.; Zhang, X.; Chen, Q.; Zhang, Z.X. Multifunctional Adsorbent: Oleophobic Latex Sponge for Removing Dyes and Cu2+ from Sewage Waste. Macromol. Mater. Eng. 2021, 306, 2100096. [Google Scholar] [CrossRef]
- Quintero-Álvarez, F.G.; Rojas-Mayorga, C.K.; Mendoza-Castillo, D.I.; Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Hamad, H. Physicochemical modeling of the adsorption of pharmaceuticals on MIL-100-Fe and MIL-101-Fe MOFs. Adsorpt. Sci. Technol. 2022, 2022, 4482263. [Google Scholar] [CrossRef]
- Razak, M.R.; Yusof, N.A.; Aris, A.Z.; Nasir, H.M.; Haron, M.J.; Ibrahim, N.A.; Johari, I.S.; Kamaruzaman, S. Phosphoric acid modified kenaf fiber (K-PA) as green adsorbent for the removal of copper (II) ions towards industrial waste water effluents. React. Funct. Polym. 2020, 147, 104466. [Google Scholar] [CrossRef]
- Shahzad, A.; Jang, J.; Lim, S.-R.; Lee, D.S. Unique selectivity and rapid uptake of molybdenum-disulfide-functionalized MXene nanocomposite for mercury adsorption. Environ. Res. 2019, 182, 109005. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Sun, X.-F.; Xie, Y.; Li, W.; Ji, T. High-Performance Hydrogel Adsorbent Based on Cellulose, Hemicellulose, and Lignin for Copper (II) Ion Removal. Polymers 2021, 13, 3063. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kong, A.; Ji, Y.; He, B.; Wang, H.; Li, J. Adsorption for copper(II) ion with chitosan-SP/PET composite adsorbent enhanced by electric field. Adsorpt. Sci. Technol. 2019, 37, 274–287. [Google Scholar] [CrossRef]
- Sun, H.; Xia, N.; Liu, Z.; Kong, F.; Wang, S. Removal of copper and cadmium ions from alkaline solutions using chitosan-tannin functional paper materials as adsorbent. Chemosphere 2019, 236, 124370. [Google Scholar] [CrossRef] [PubMed]
- Yermiyahu, Z.; Lapides, I.; Yariv, S. Thermo-XRD analysis of the adsorption of Congo-red by montmorillonite saturated with different cations. J. Therm. Anal. 2002, 69, 317–332. [Google Scholar] [CrossRef]
- Tan, P.; Wen, J.; Hu, Y.; Tan, X. Adsorption of Cu2+ and Cd2+ from aqueous solution by novel electrospun poly (vinyl alcohol)/graphene oxide nanofibers. RSC Adv. 2016, 6, 79641–79650. [Google Scholar] [CrossRef]
- Tang, Y.-B.; Yin, L.-C.; Yang, Y.; Bo, X.-H.; Cao, Y.-L.; Wang, H.; Zhang, W.; Bello, I.; Lee, S.-T.; Cheng, H.-M.; et al. Tunable Band Gaps and p-Type Transport Properties of Boron-Doped Graphenes by Controllable Ion Doping Using Reactive Microwave Plasma. ACS Nano 2012, 6, 1970–1978. [Google Scholar] [CrossRef]
- Wang, H.; Fang, S.; Zuo, M.; Li, Z.; Yu, X.; Tang, X.; Sun, Y.; Yang, S.; Zeng, X.; Lin, L. Removal of copper ions by cellulose nanocrystal-based hydrogel and reduced adsorbents for its catalytic properties. Cellulose 2022, 29, 4525–4537. [Google Scholar] [CrossRef]
- Sunding, M.; Hadidi, K.; Diplas, S.; Løvvik, O.; Norby, T.; Gunnæs, A. XPS characterisation of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J. Electron. Spectrosc. Relat. Phenom. 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Yaras, A.; Arslanoğlu, H. Valorization of Paper Mill Sludge as Adsorbent in Adsorption Process of Copper (II) Ion from Synthetic Solution: Kinetic, Isotherm and Thermodynamic Studies. Arab. J. Sci. Eng. 2017, 43, 2393–2402. [Google Scholar] [CrossRef]
- Zeatoun, L.; Yousef, S. The use of activated and non-activated tar sands as adsorbents for copper ion removal. Adsorpt. Sci. Technol. 2004, 22, 223–235. [Google Scholar] [CrossRef]
- Zhan, M.; Jia, H.; Fan, J.; Yu, H.; Amador, E.; Chen, W. Two D-π-A Schiff-Base-Functionalized Silica Gel Adsorbents for Preconcentration of Copper Ions in Foods and Water for Detection. Anal. Chem. 2019, 91, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Wang, Z.; Song, L.; Yao, J. In situ growth of ZIF-8 within wood channels for water pollutants removal. Sep. Purif. Technol. 2021, 266, 118527. [Google Scholar] [CrossRef]
- Liu, H.; Guo, J.; Zhou, Y.; Qian, J. Preparation and Adsorption Performance of Efficient Adsorbent for Heavy Metal Copper(II) Using Graphene-Oxide-Based Composites. ChemistrySelect 2020, 5, 11354–11360. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal-Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef]


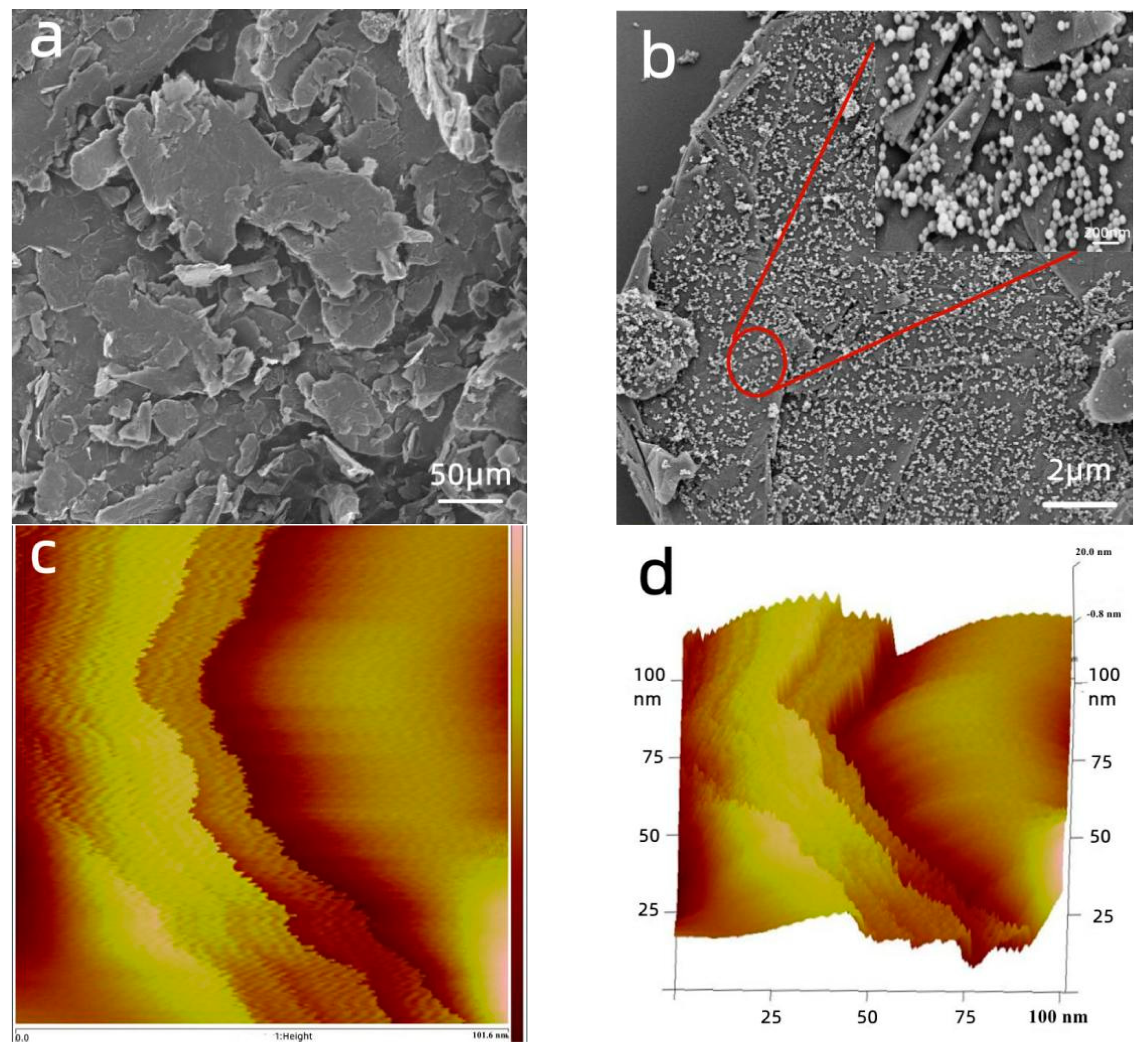

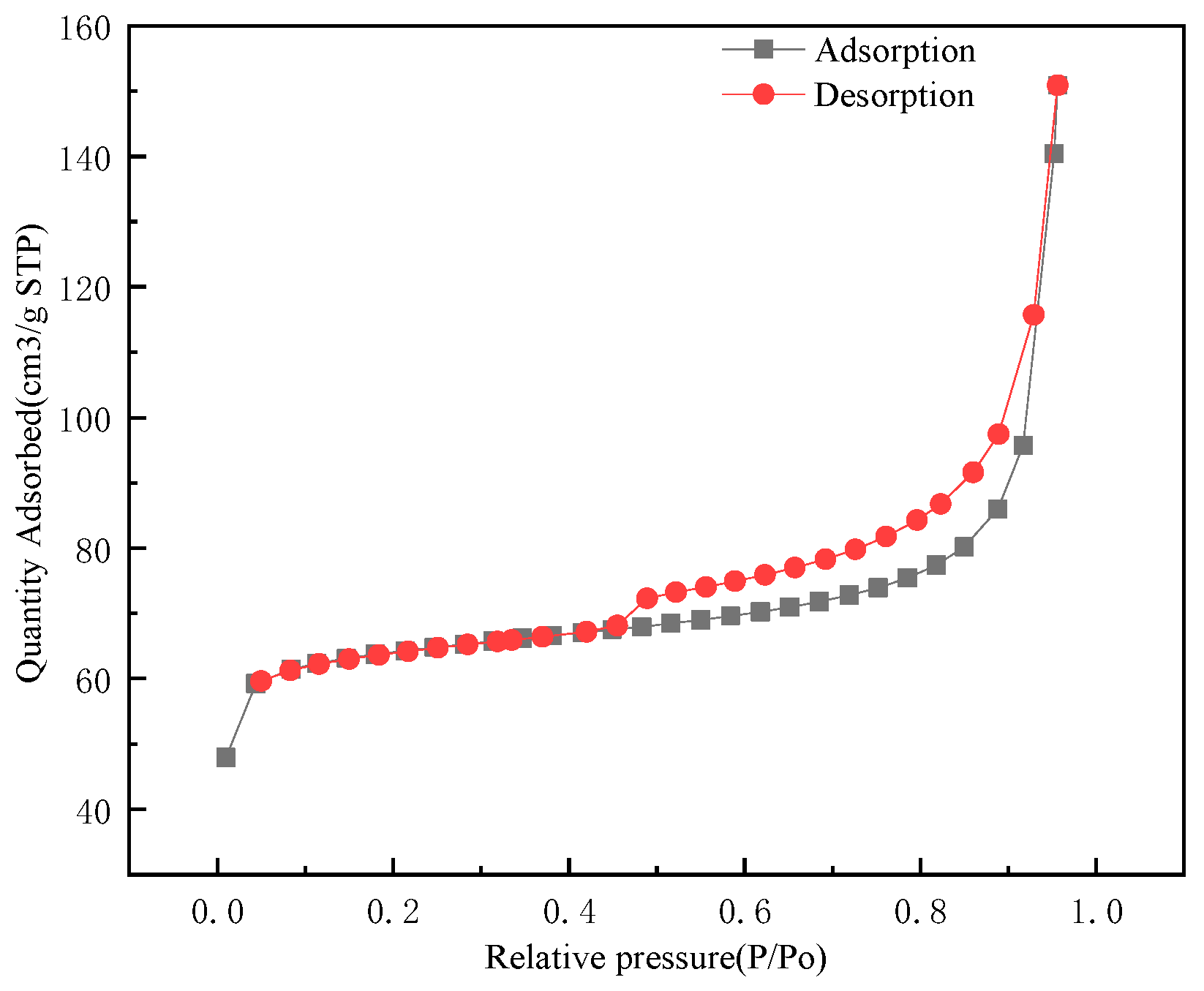
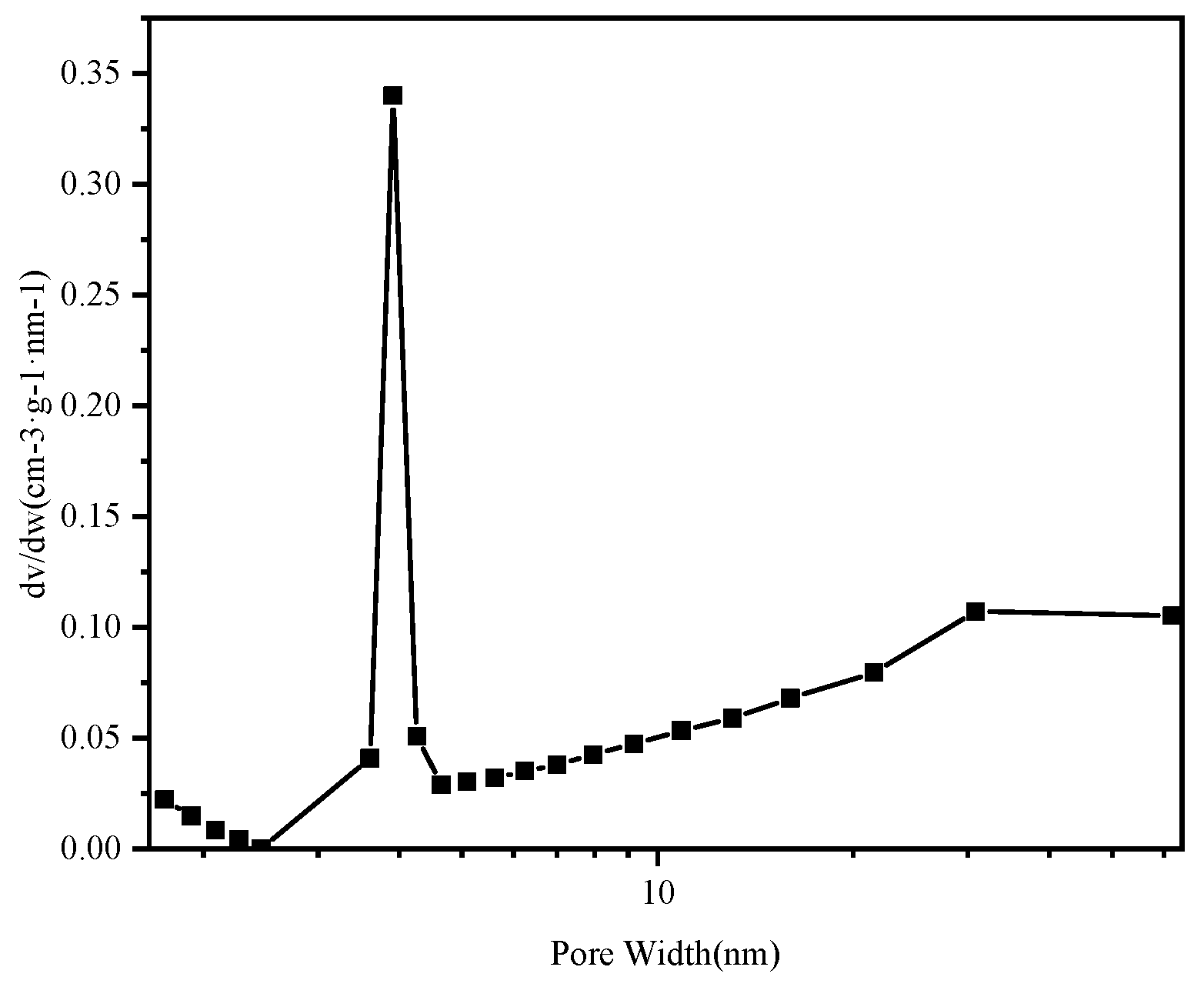

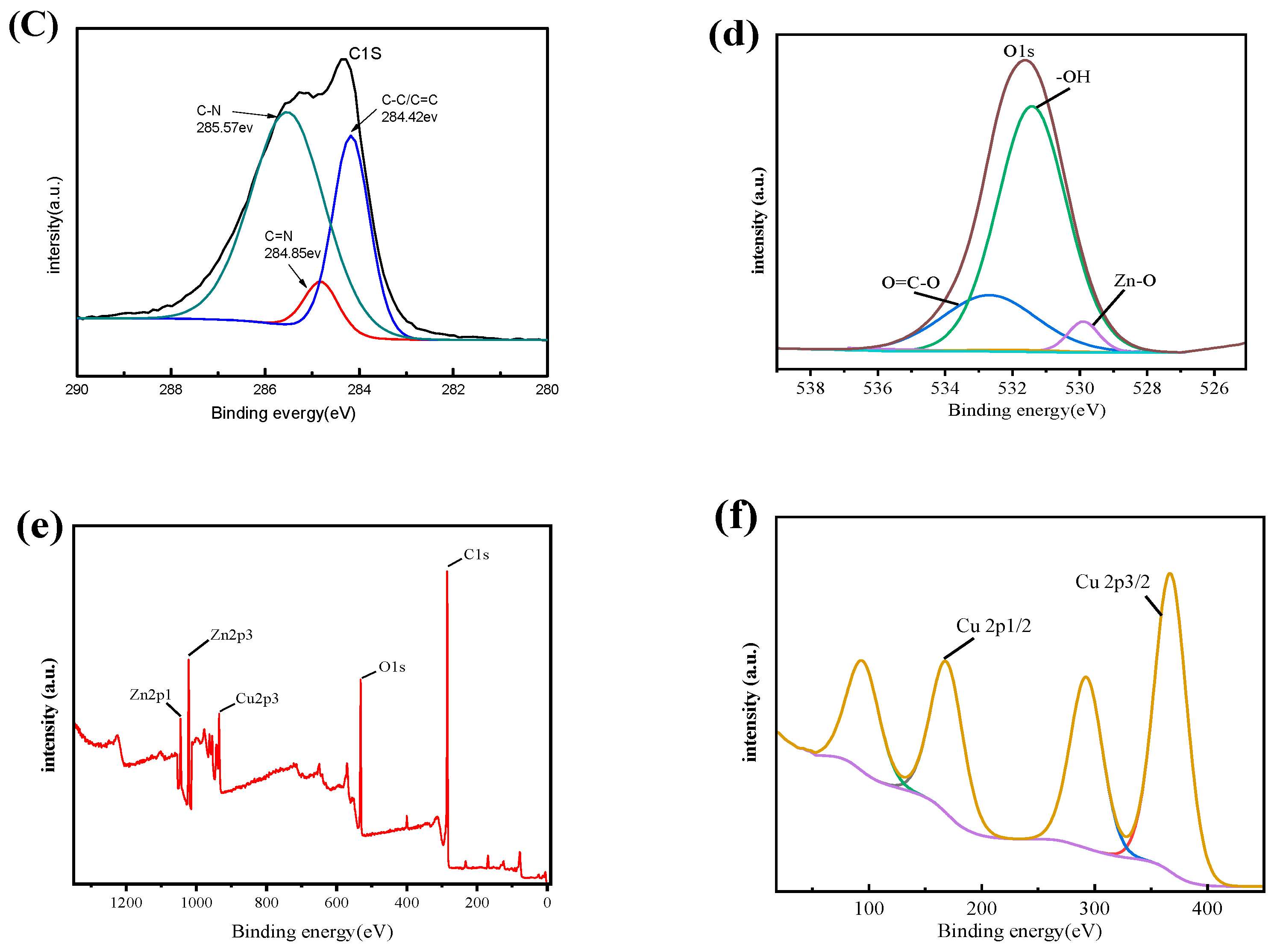
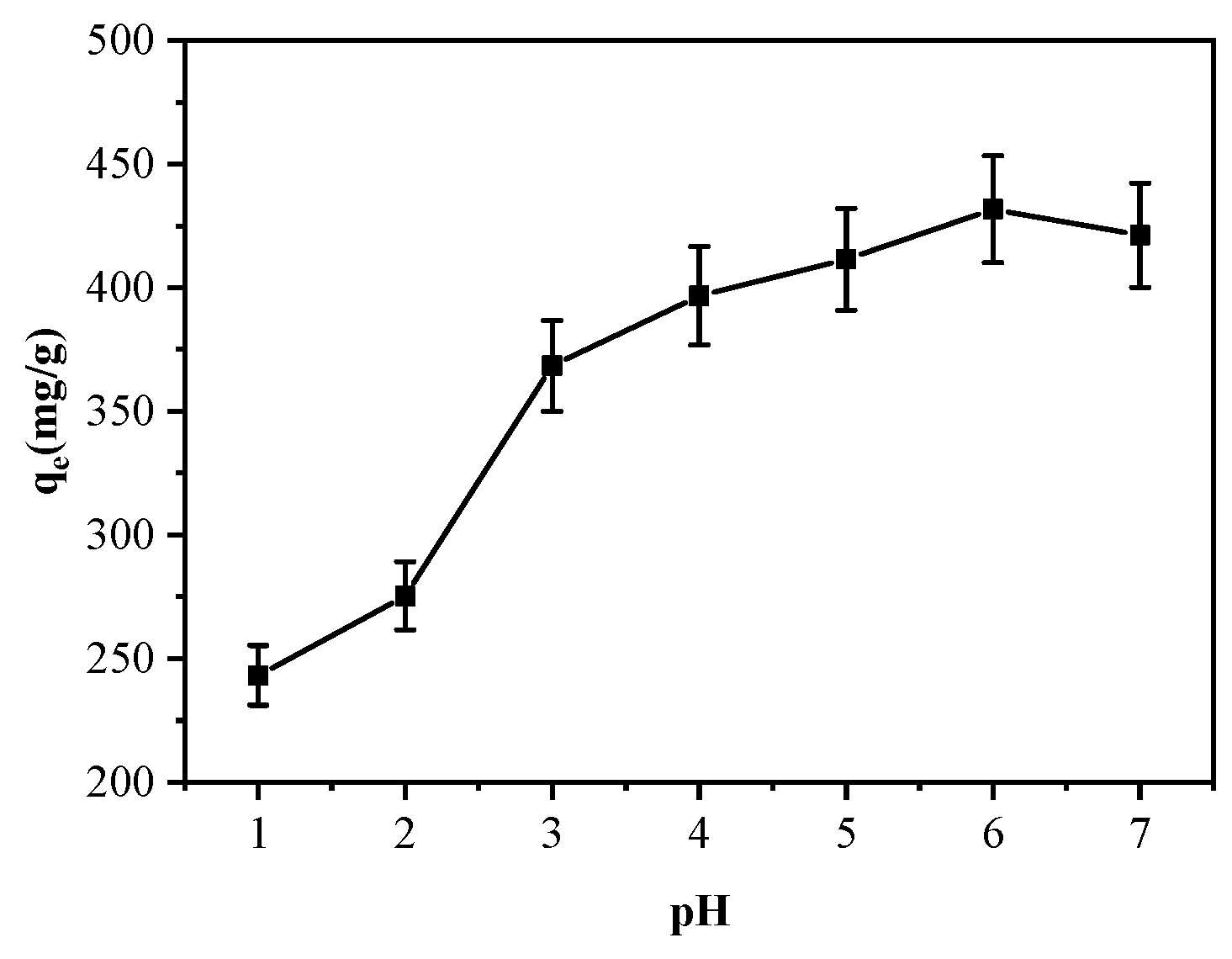
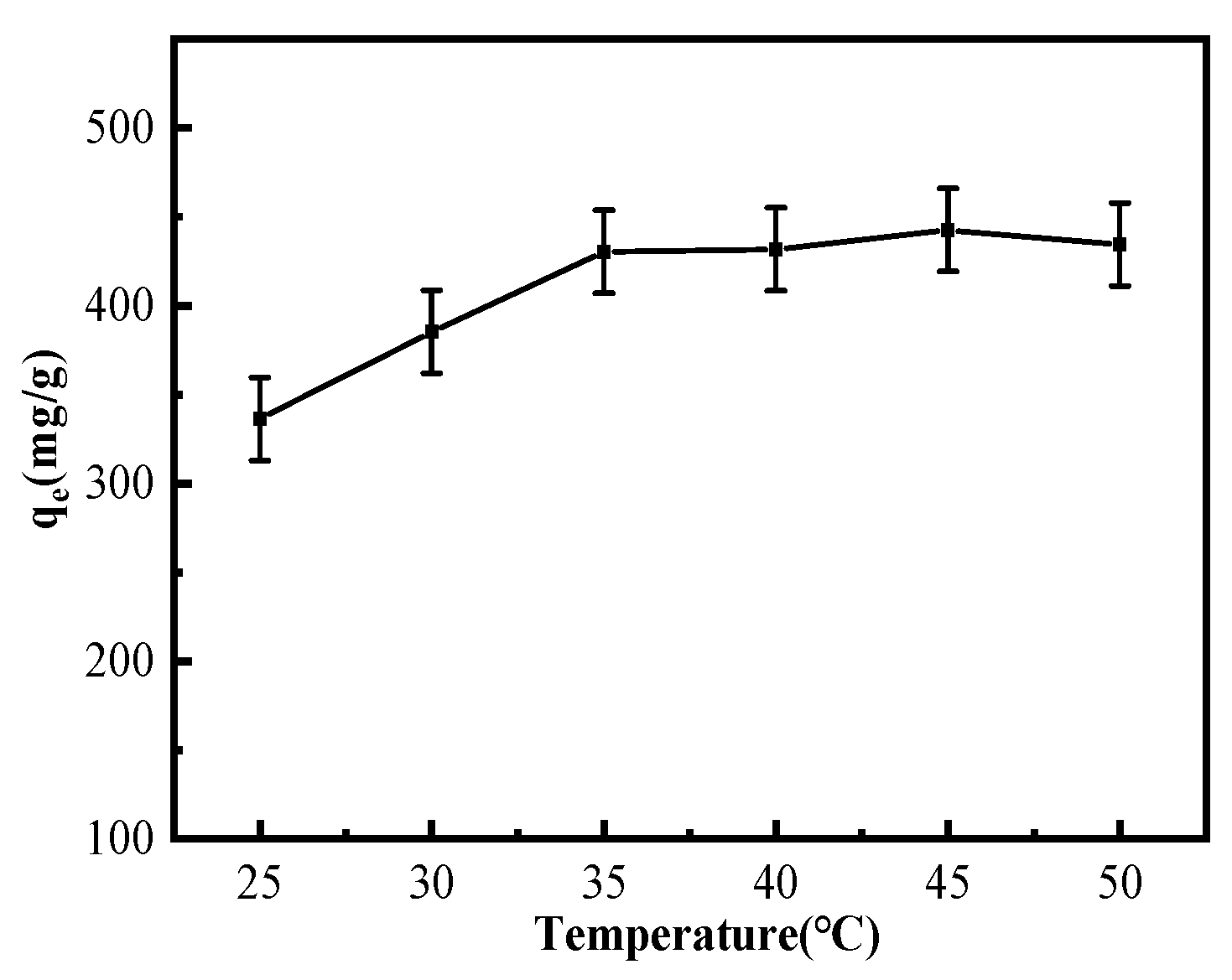
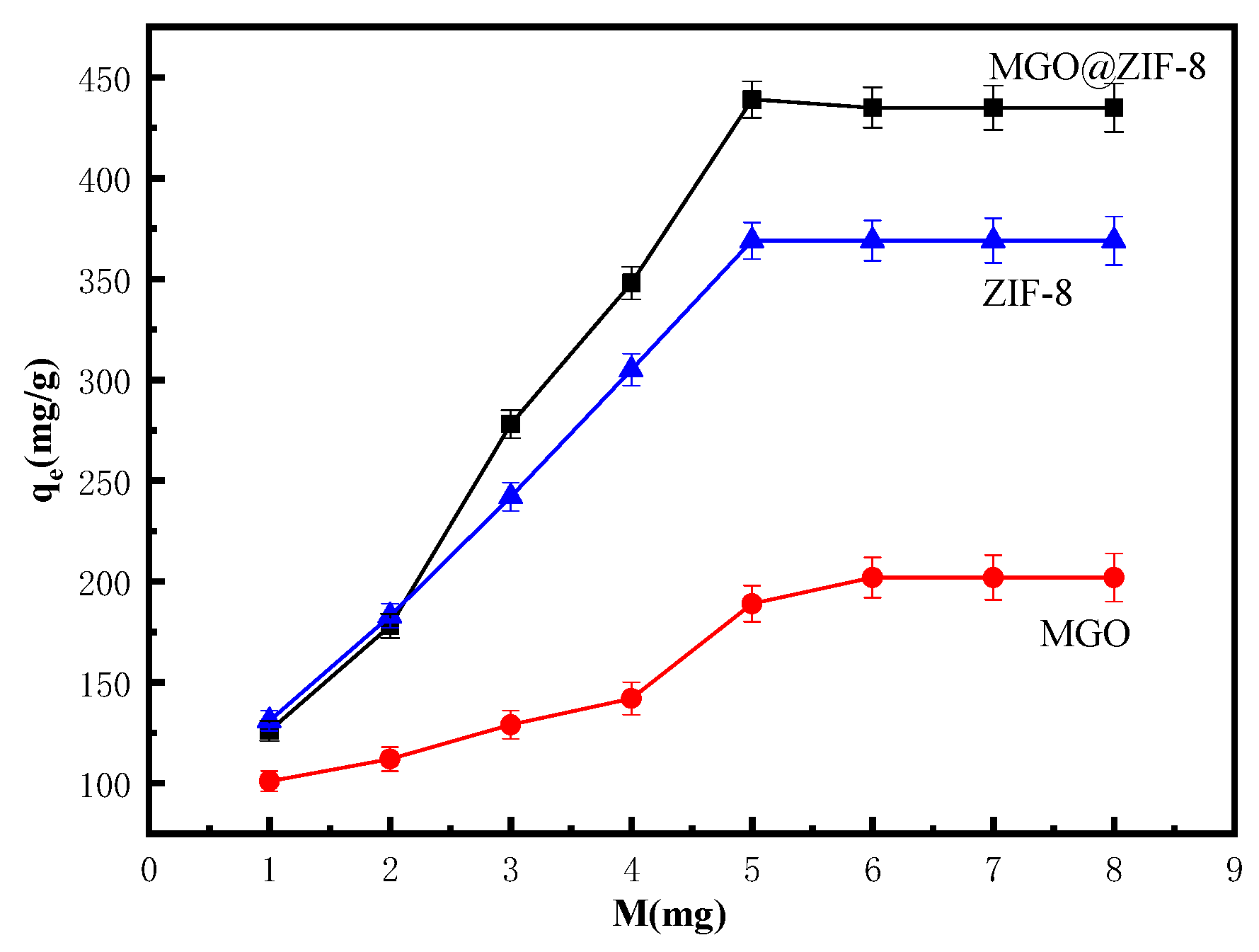
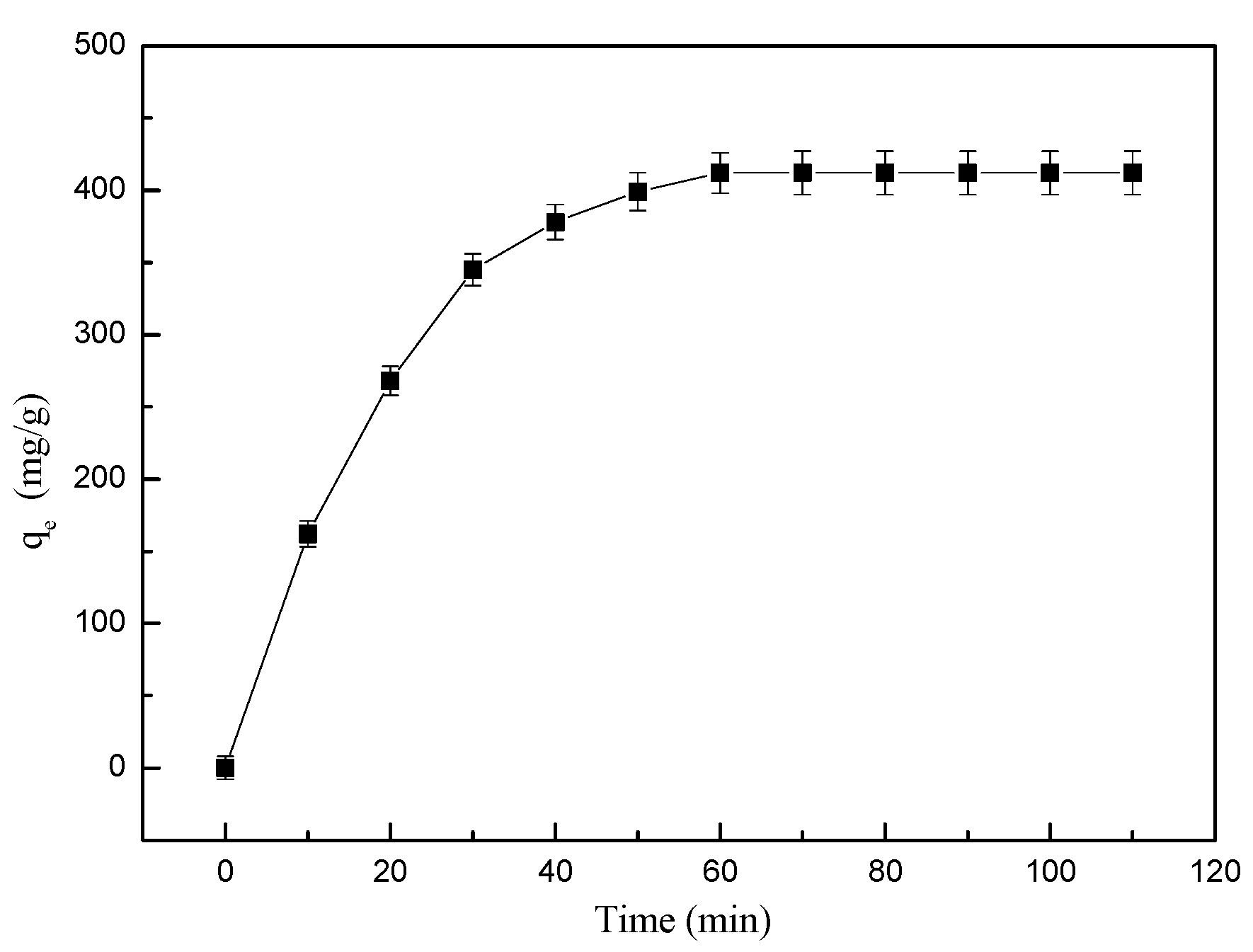

| Level | pH | Time (min) | Temperature (°C) | Amount of Adsorbent (mg) | Results (mg/g) |
|---|---|---|---|---|---|
| 1 | 5 | 50 | 30 | 4 | Q |
| 2 | 6 | 60 | 35 | 5 | |
| 3 | 7 | 70 | 40 | 6 |
| Number | Influence Factor | Adsorbing Capacity (mg/g) | |||
|---|---|---|---|---|---|
| pH | Time (min) | Temperature (°C) | Amount of Adsorbent (mg) | ||
| 1 | 5 | 50 | 30 | 4 | 390.05 |
| 2 | 5 | 70 | 35 | 5 | 437.21 |
| 3 | 5 | 70 | 40 | 6 | 410.37 |
| 4 | 6 | 50 | 35 | 4 | 420.45 |
| 5 | 6 | 60 | 40 | 5 | 400.52 |
| 6 | 6 | 70 | 30 | 6 | 440.83 |
| 7 | 7 | 50 | 40 | 5 | 403.16 |
| 8 | 7 | 60 | 30 | 6 | 384.91 |
| 9 | 7 | 70 | 35 | 2 | 397.42 |
| K1 | 412.333 | 404.333 | 404.667 | 395.677 | —— |
| K2 | 420.000 | 407.000 | 418.000 | 426.667 | —— |
| K3 | 394.667 | 415.667 | 404.333 | 404.667 | —— |
| R | 25.333 | 11.334 | 13.667 | 31.000 | —— |
| Sorbent | Adsorption Capacity | pH | Temperature (°C) | Reference |
|---|---|---|---|---|
| CD-CA/PDA | 73.46 | 6 | 25 | [10] |
| Magnetite Nanoparticles | 6.28 | 7 | 30 | [12] |
| Fe3O4@zeolite NaA | 86.54 | 4 | 25.15 | [6] |
| Tyr-Mt | 28.31 | 5 | 15 | [11] |
| Modified latex sponge | 125.8 | 5.85 | 40 | [38] |
| PVA/GO | 44.7 | 5.8 | 50 | [47] |
| GO/PEI/CMC | 302.04 | 5.0–5.5 | 25 | [55] |
| MGO@ZIF-8 | 431.63 | 6 | 35 | This work |
| Copper Ion | Qe (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg/g) | R2 | K2 (g/(mg·min)) | Qe (mg/g) | R2 | ||
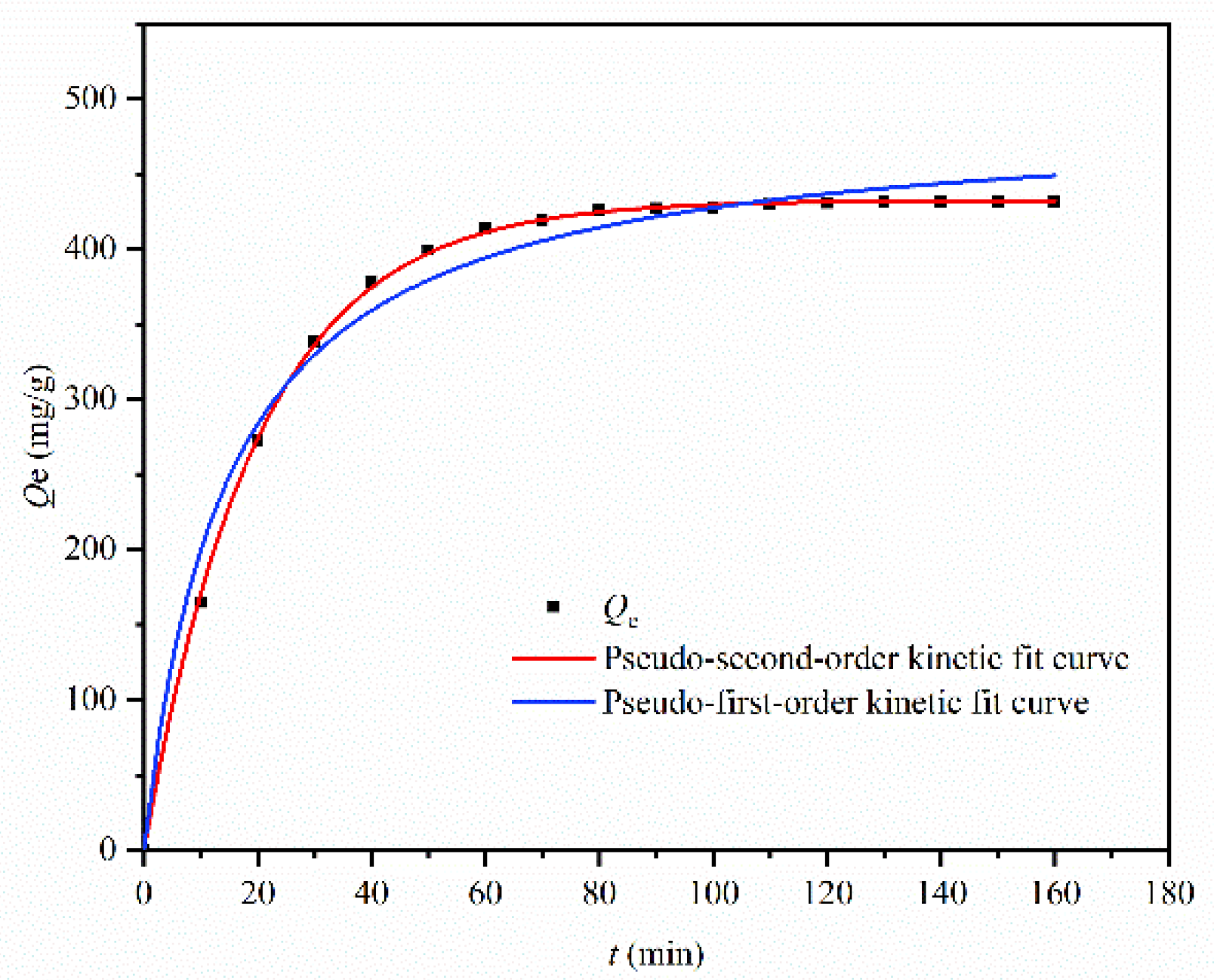
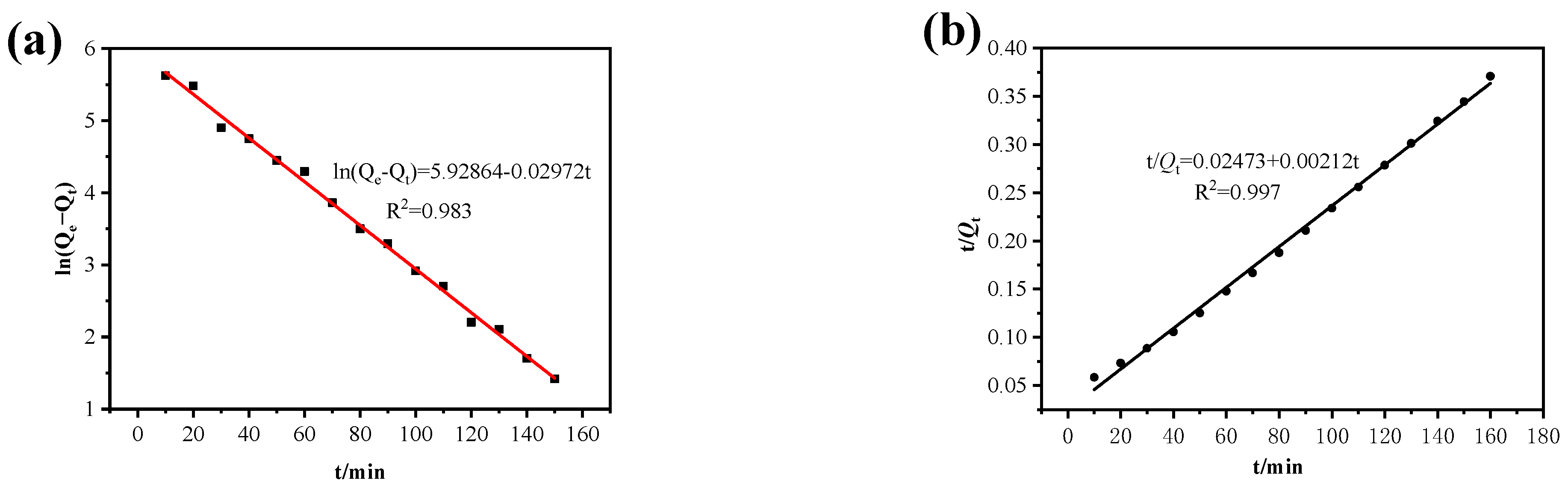
| Cu | 431.63 | 0.05 | 489.82 | 0.983 | 0.0014 | 432.50 | 0.997 |
| —— | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| T/K | Qe | KL | R2 | KF | 1/n | R2 |
| 308 | 458.20 | 0.016 | 0.999 | 0.27318 | 0.653 | 0.963 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Zhang, Y.; Wang, X.; Hu, L.; Shi, C. Multilayer Graphene Oxide Supported ZIF-8 for Efficient Removal of Copper Ions. Nanomaterials 2022, 12, 3162. https://doi.org/10.3390/nano12183162
Lv X, Zhang Y, Wang X, Hu L, Shi C. Multilayer Graphene Oxide Supported ZIF-8 for Efficient Removal of Copper Ions. Nanomaterials. 2022; 12(18):3162. https://doi.org/10.3390/nano12183162
Chicago/Turabian StyleLv, Xifeng, Yishi Zhang, Xiaodong Wang, Libing Hu, and Chunhui Shi. 2022. "Multilayer Graphene Oxide Supported ZIF-8 for Efficient Removal of Copper Ions" Nanomaterials 12, no. 18: 3162. https://doi.org/10.3390/nano12183162
APA StyleLv, X., Zhang, Y., Wang, X., Hu, L., & Shi, C. (2022). Multilayer Graphene Oxide Supported ZIF-8 for Efficient Removal of Copper Ions. Nanomaterials, 12(18), 3162. https://doi.org/10.3390/nano12183162







