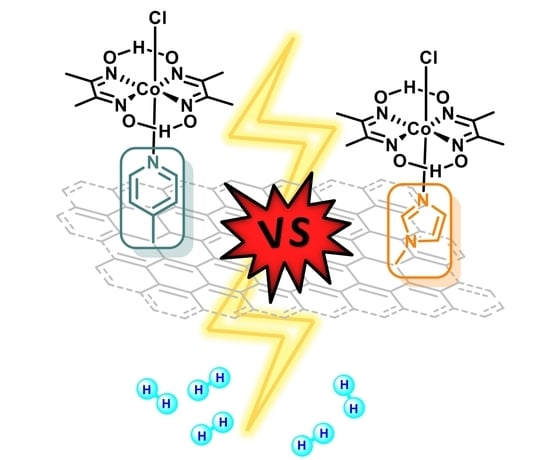
Pyridine vs. Imidazole Axial Ligation on Cobaloxime Grafted Graphene: Hydrogen Evolution Reaction Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Experimental
2.2.1. Synthetic Procedures
(4-(Pyridin-4-yl-carbamoyl)phenyl)carbamate (2)
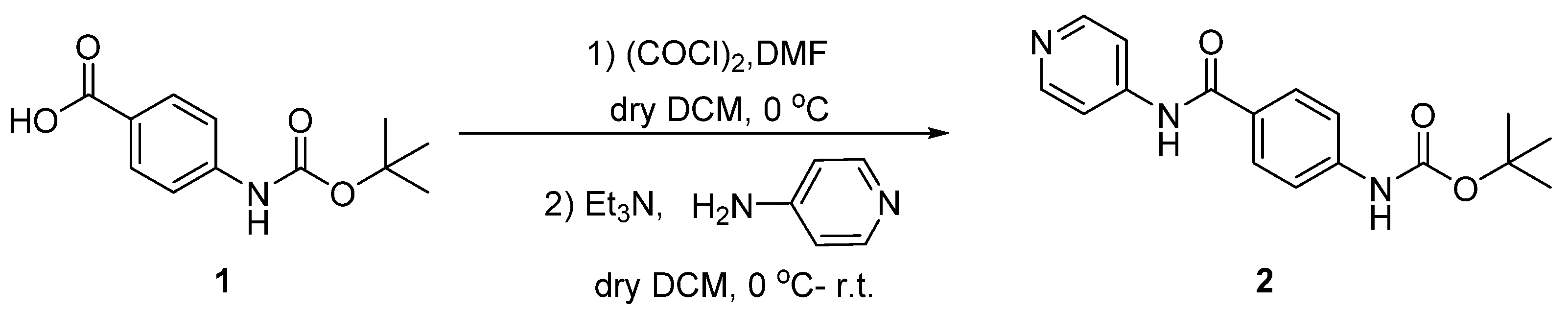
4-Amino-N-(pyridin-4-yl)benzamide (3)

N-(3-(1H-Imidazol-1-yl)propyl)-4-aminobenzamide (5)

Synthesis of Cobaloxime Complexes (6) and (7) [13]
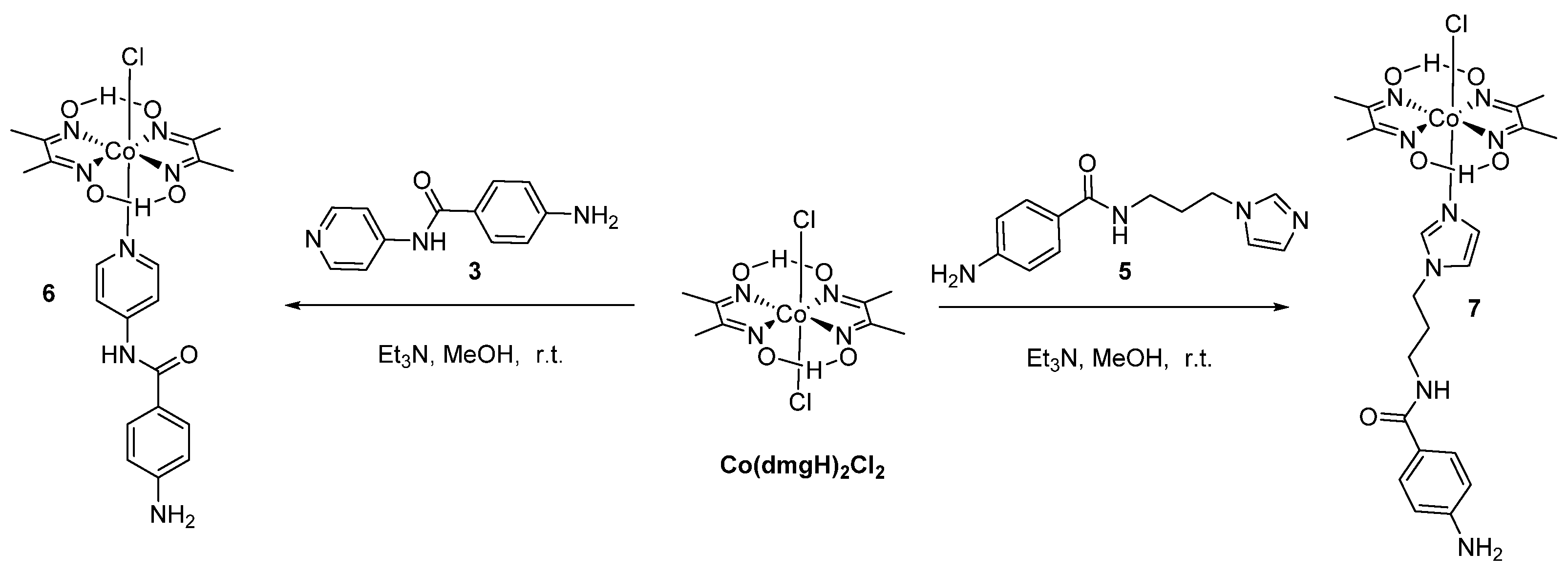
2.2.2. Synthetic Procedures
Graphene Exfoliation [14]
Preparation of Pyr-Graphene and Imi-Graphene [15]
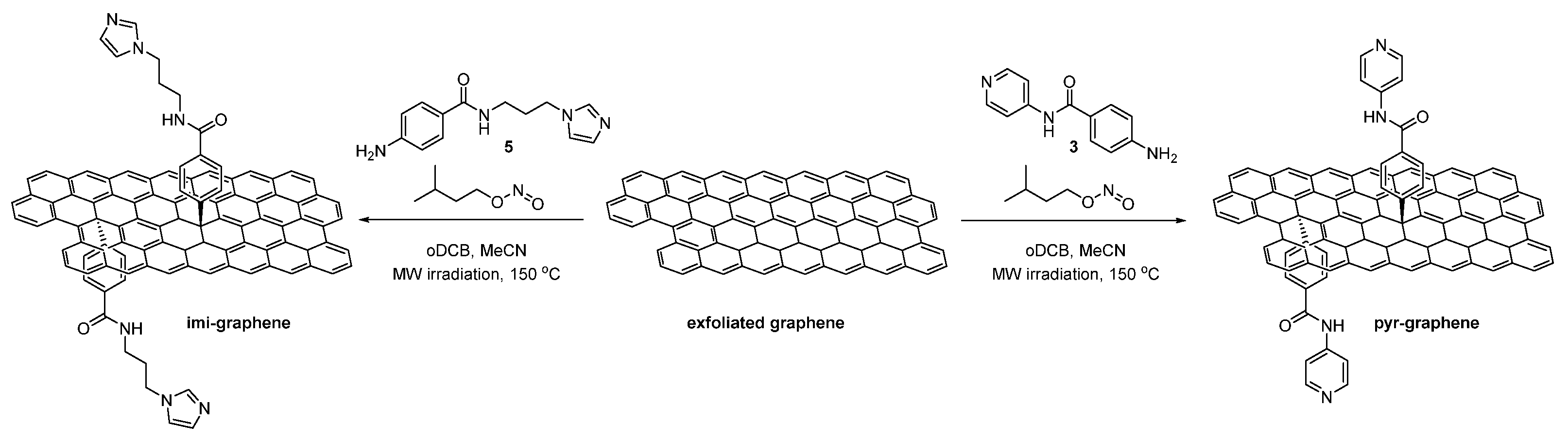
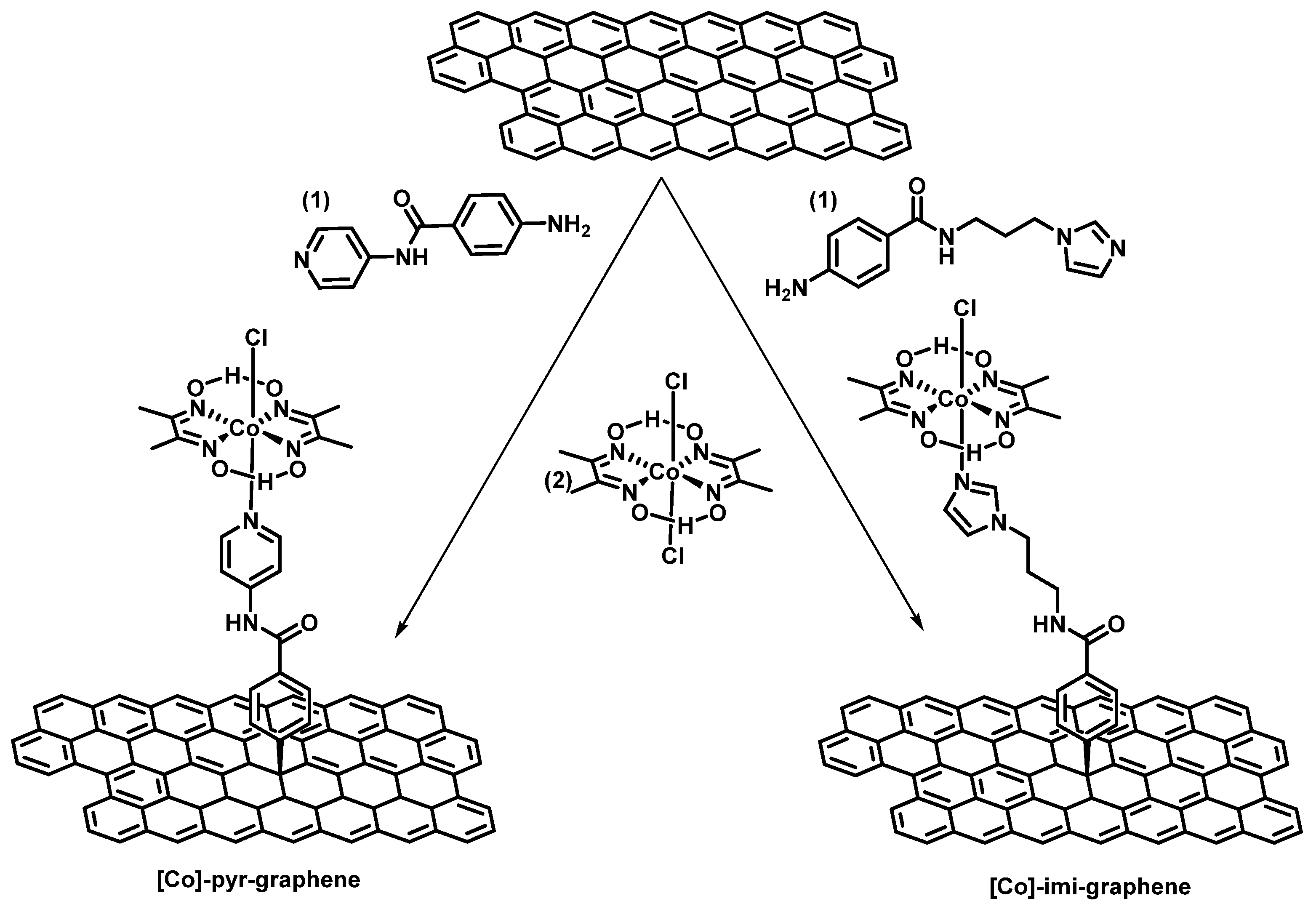
Preparation of Cobaloxime-Based Materials [Co]-Pyr-Graphene and [Co]-Imi-Graphene
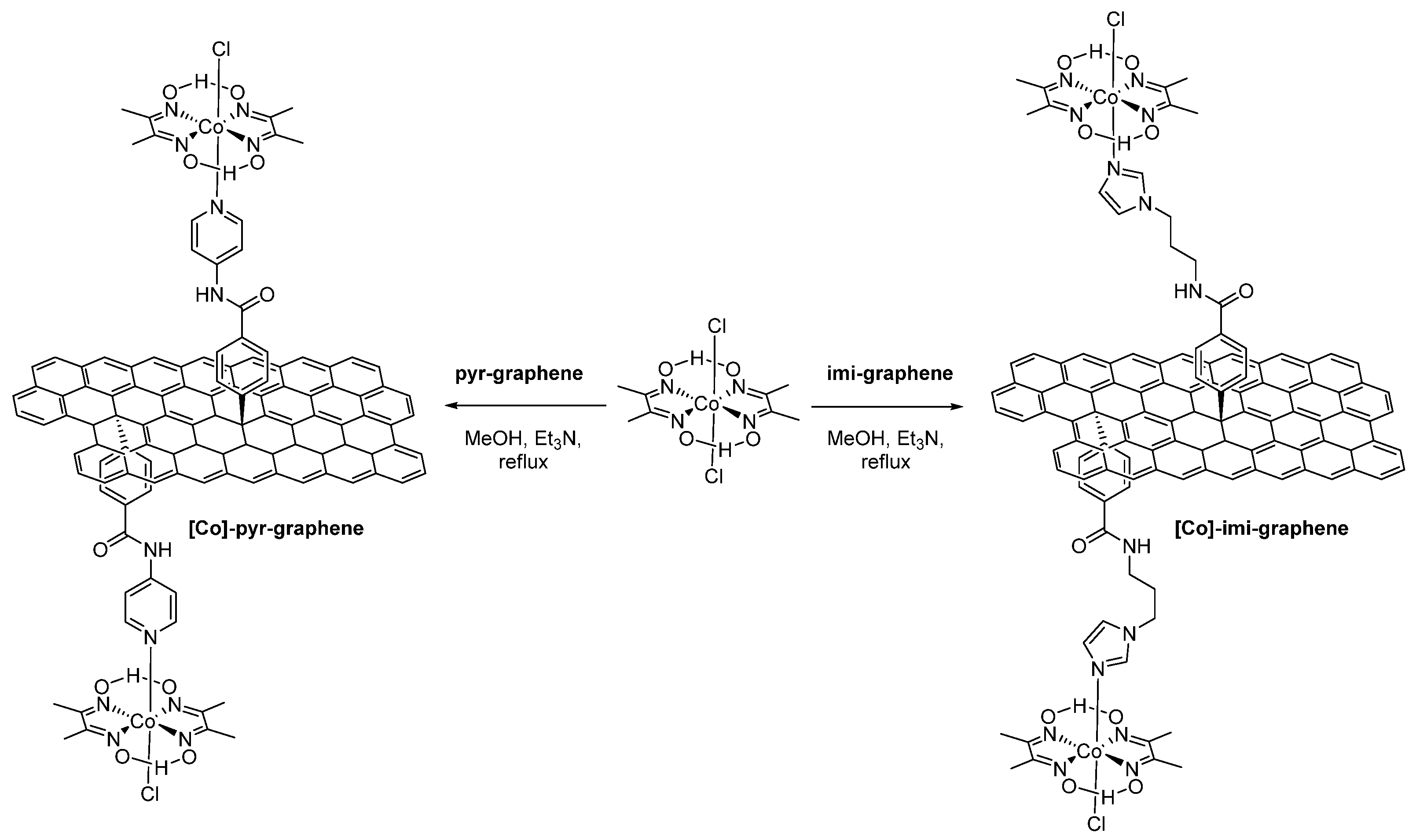
3. Results and Discussion
3.1. Raman Spectroscopy
3.2. Thermogravimetric Analysis
3.3. Electrochemical Characterization
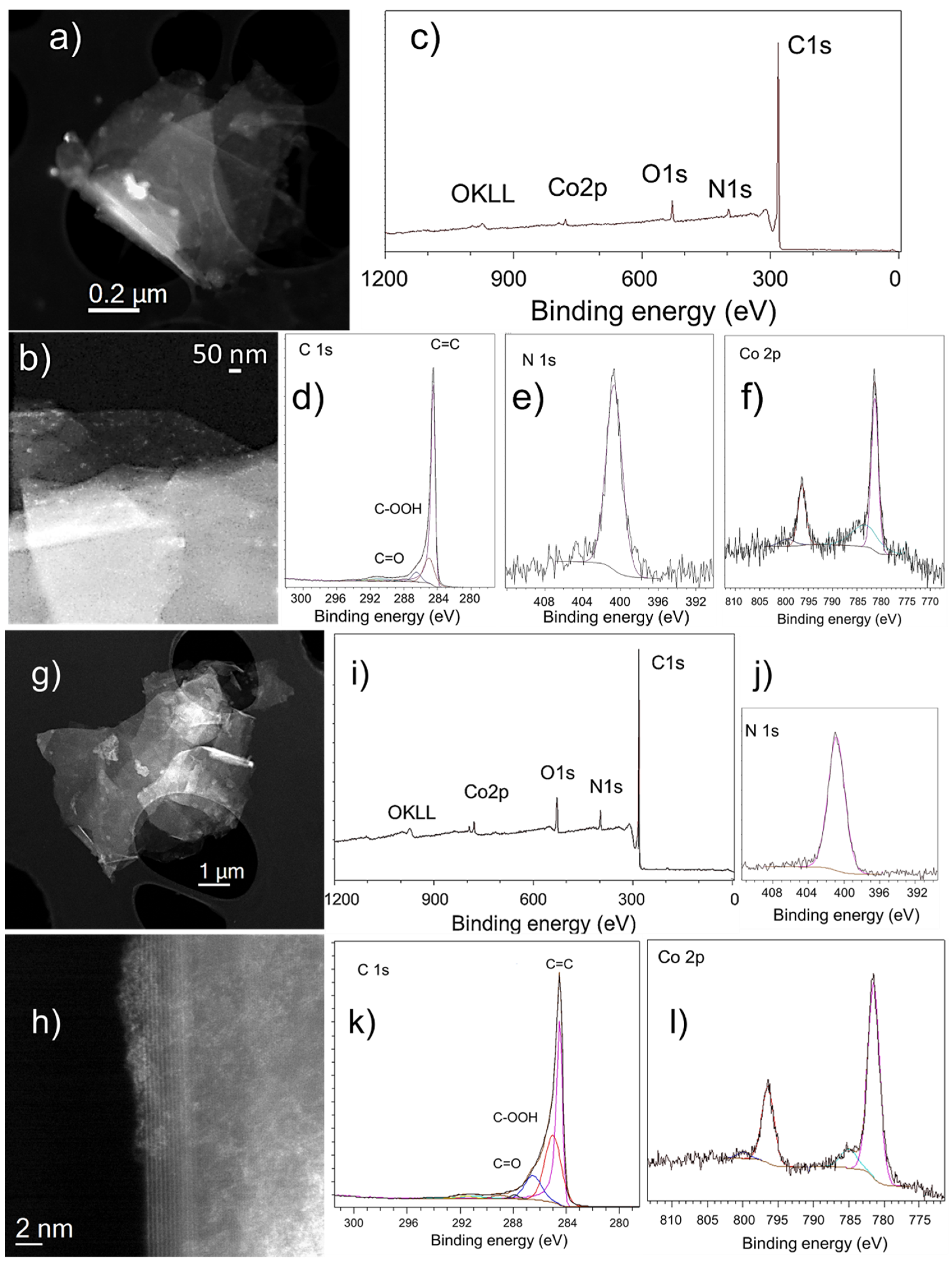
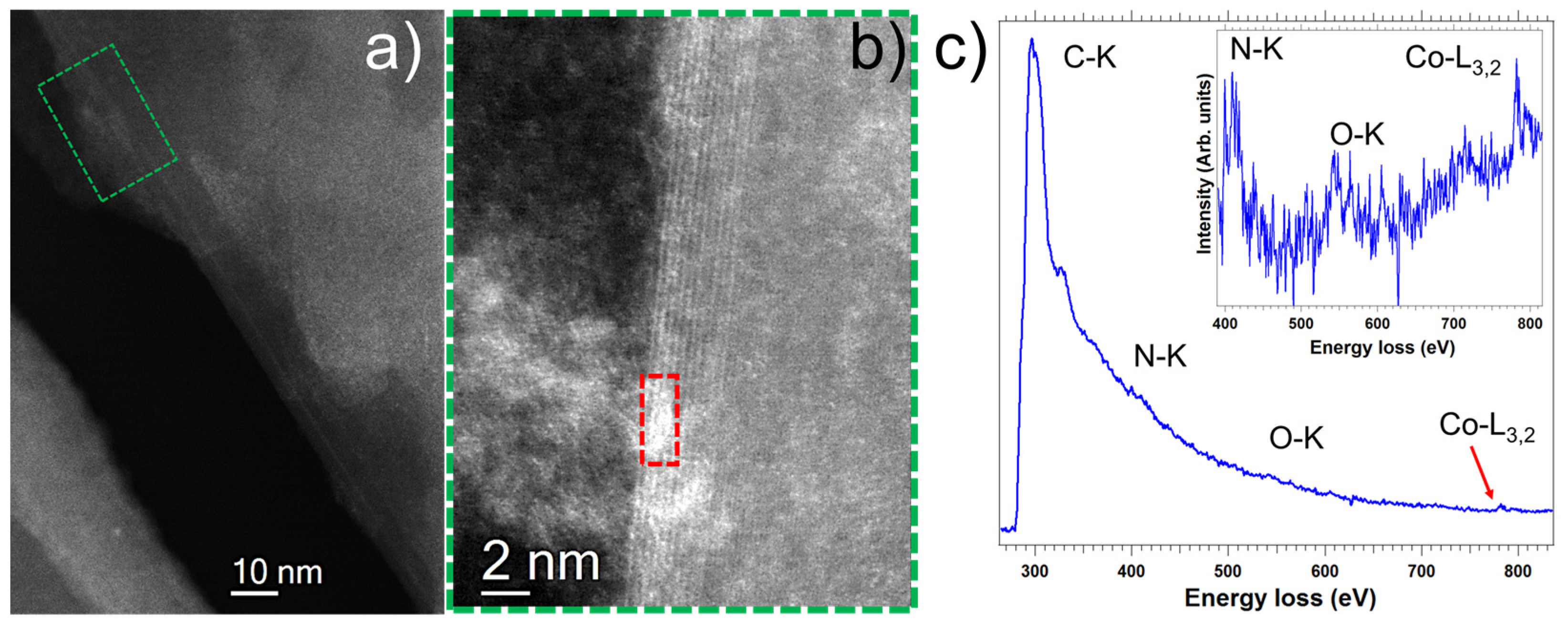
3.4. Imaging
3.5. Catalytic Studies on the Hydrogen Evolution Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

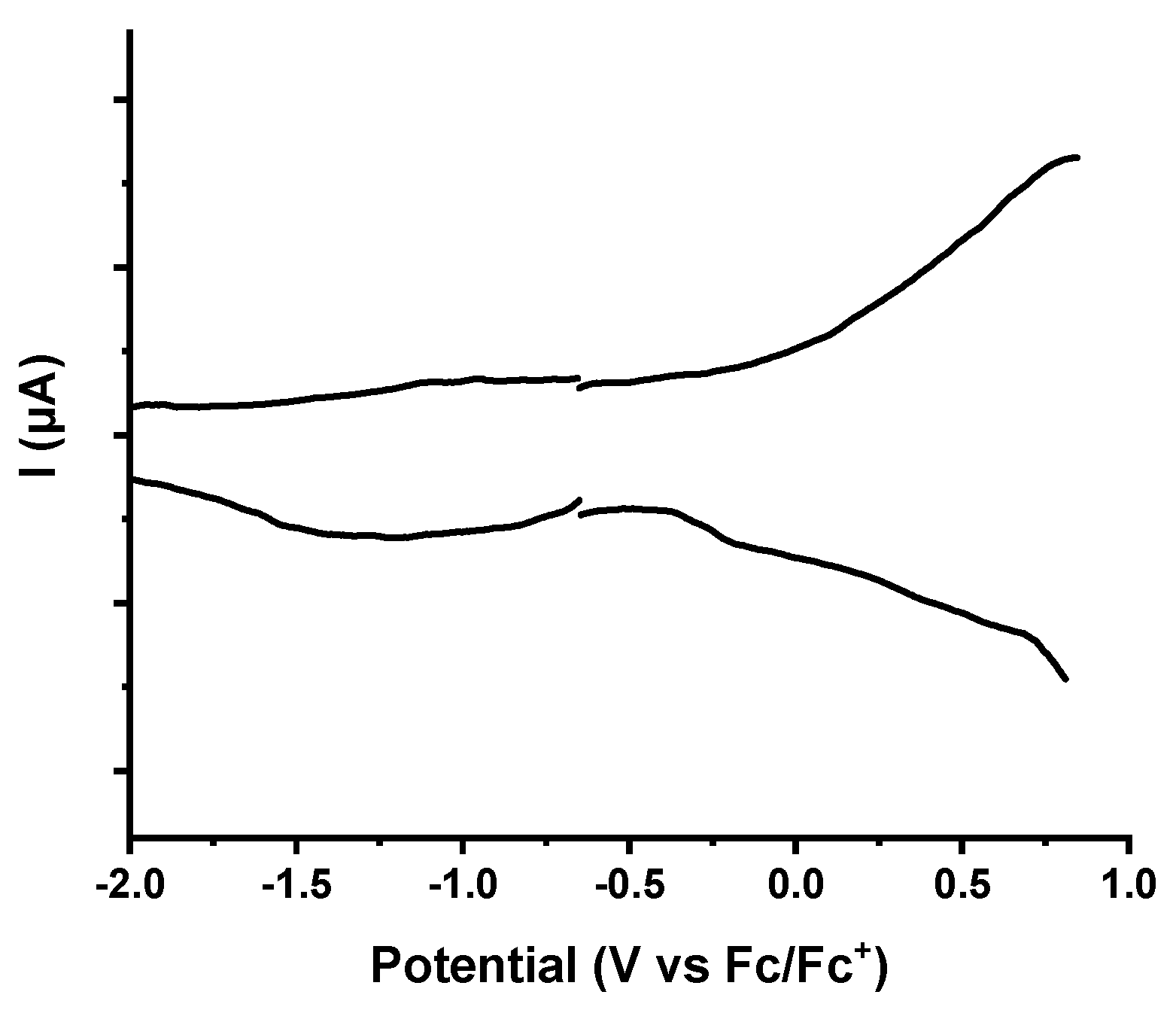
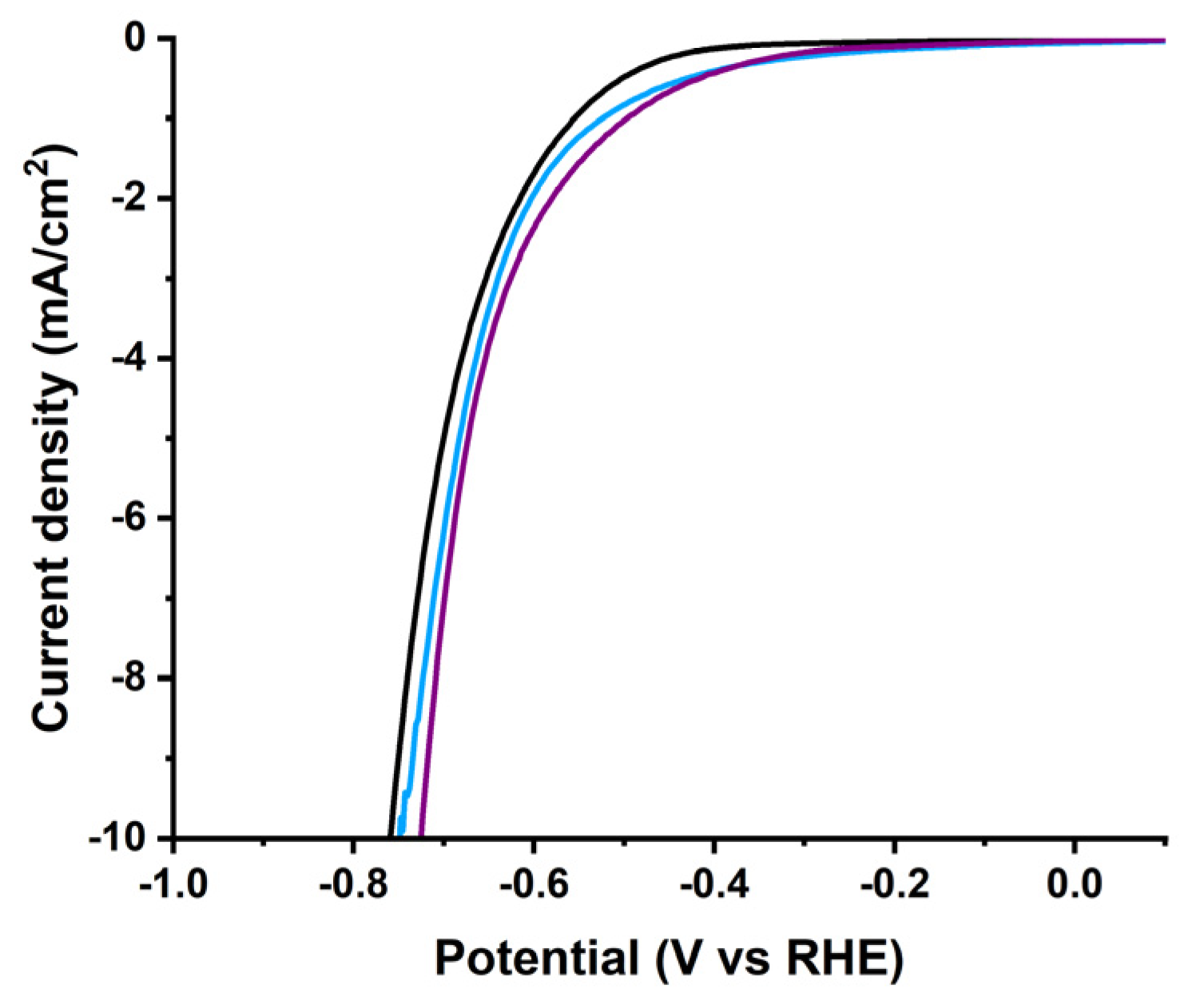
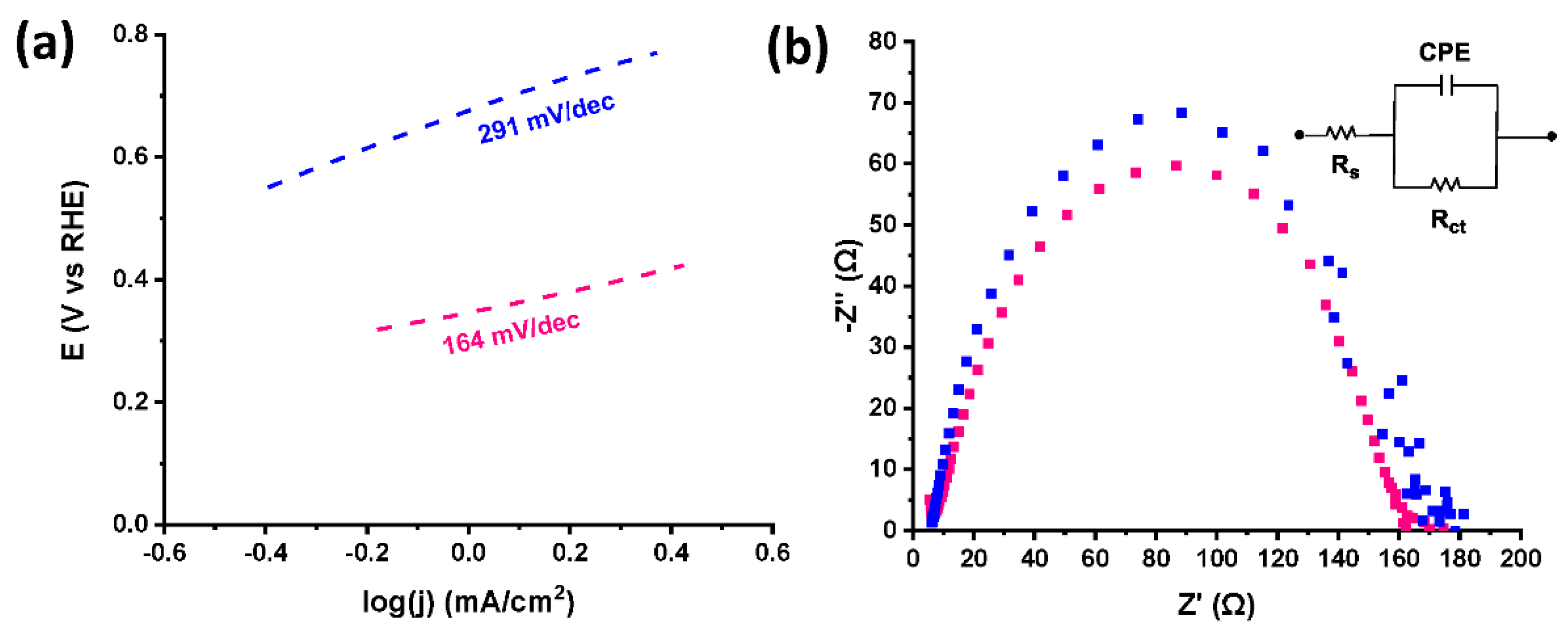
References
- Le Goff, A.; Artero, V.; Jousselme, B.; Tran, P.D.; Guillet, N.; Métayé, R.; Fihri, A.; Palacin, S.; Fontecave, M. From hydrogenases to noble metal-free catalytic nanomaterials for H2 production and uptake. Science 2009, 326, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Dolui, D.; Khandelwal, S.; Majumder, P.; Dutta, A. The odyssey of cobaloximes for catalytic H2 production and their recent revival with enzyme-inspired design. Chem. Commun. 2020, 56, 8166–8181. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Vijaikanth, V. Electrochemistry and electrocatalytic activity of cobaloxime complexes. ChemistrySelect 2022, 7, e202104044. [Google Scholar] [CrossRef]
- Andreidis, E.S.; Jacques, P.-A.; Tran, P.D.; Leyris, A.; Chavarot-Kerlidou, M.; Jousselme, B.; Matheron, M.; Pecaut, J.; Palacin, S.; Fontecave, M.; et al. Molecular engineering of a cobalt-based electrocatalytic nanomaterial for H₂ evolution under fully aqueous conditions. Nat. Chem. 2013, 5, 48–53. [Google Scholar] [CrossRef]
- Kaeffer, N.; Morozan, A.; Artero, V. Oxygen tolerance of a molecular engineered cathode for hydrogen evolution based on a cobalt diimine–dioxime catalyst. J. Phys. Chem. B 2015, 119, 13707–13713. [Google Scholar] [CrossRef]
- Kaeffer, N.; Morozan, A.; Fize, J.; Martinez, E.; Guetaz, L.; Artero, V. The dark side of molecular catalysis: Diimine–dioxime cobalt complexes are not the actual hydrogen evolution electrocatalyst in acidic aqueous solutions. ACS Catal. 2016, 6, 3727–3737. [Google Scholar] [CrossRef]
- Reuillard, B.; Warnan, J.; Leung, J.J.; Wakerley, D.W.; Reisner, E. A poly(cobaloxime)/carbon nanotube electrode: Freestanding buckypaper with polymer-enhanced H2-evolution performance. Angew. Chem. Int. Ed. 2016, 55, 3952–3957. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Reisner, E. Development and understanding of cobaloxime activity through electrochemical molecular catalyst screening. Phys. Chem. Chem. Phys. 2014, 16, 5739–5746. [Google Scholar] [CrossRef]
- Panagiotopoulos, A.; Ladomenou, K.; Sun, D.; Artero, V.; Coutsolelos, A.G. Photochemical hydrogen production and cobaloximes: The influence of the cobalt axial N-ligand on the system stability. Dalton Trans. 2016, 45, 6732–6738. [Google Scholar] [CrossRef]
- Mu, F.; Coffing, S.L.; Riese, D.J.; Geahlen, R.L.; Verdier-Pinard, P.; Hamel, E.; Johnson, J.; Cushman, M. Design, synthesis, and biological evaluation of a series of lavendustin A analogues that inhibit EGFR and syk tyrosine kinases, as well as tubulin polymerization. J. Med. Chem. 2001, 44, 441–452. [Google Scholar] [CrossRef]
- Samadi, S.; Jadidi, K.; Khanmohammadi, B.; Tavakoli, N. Heterogenization of chiral mono oxazoline ligands by grafting onto mesoporous silica MCM-41 and their application in copper-catalyzed asymmetric allylic oxidation of cyclic olefins. J. Catal. 2016, 340, 344–353. [Google Scholar] [CrossRef]
- De Vita, D.; Angeli, A.; Pandolfi, F.; Bortolami, M.; Costi, R.; Di Santo, R.; Suffredini, E.; Ceruso, M.; Del Prete, S.; Capasso, C.; et al. Inhibition of the α-carbonic anhydrase from Vibrio cholerae with amides and sulfonamides incorporating imidazole moieties. J. Enzyme Inhib. Med. Chem. 2017, 32, 798–804. [Google Scholar] [CrossRef]
- Lazarides, T.; Peuntinger, K.; Dafnomili, D.; Charalambidis, G.; Landrou, G.; Kahnt, A.; Sabatini, R.; McCamant, D.W.; Gryko, D.T.; Coutsolelos, A.G.; et al. Photoinduced charge transfer in porphyrin–cobaloxime and corrole–cobaloxime hybrids. J. Phys. Chem. C 2013, 117, 1647–1655. [Google Scholar] [CrossRef]
- Stergiou, A.; Sideri, I.K.; Kafetzi, M.; Ioannou, A.; Arenal, R.; Mousdis, G.; Pispas, S.; Tagmatarchis, N. Methylammonium lead bromide perovskite nano-crystals grown in a poly[styrene-co-(2-(dimethylamino)ethyl methacrylate)] matrix immobilized on exfoliated graphene nano-sheets. Nanomaterials 2022, 12, 1275. [Google Scholar] [CrossRef]
- Pagona, G.; Zervaki, G.E.; Sandanayaka, A.S.D.; Ito, O.; Charalambidis, G.; Hasobe, T.; Coutsolelos, A.G.; Tagmatarchis, N. Carbon Nanohorn–Porphyrin Dimer Hybrid Material for Enhancing Light-Energy Conversion. J. Phys. Chem. C 2012, 116, 9439–9449. [Google Scholar] [CrossRef]
- Paulus, G.L.C.; Wang, Q.H.; Strano, M.S. Covalent electron transfer chemistry of graphene with diazonium salts. Acc. Chem. Res. 2013, 46, 160–170. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Koehler, F.M.; Jacobsen, A.; Ensslin, K.; Stampfer, C.; Stark, W.J. Selective chemical modification of graphene surfaces: Distinction between single- and bilayer graphene. Small 2010, 6, 1125–1130. [Google Scholar] [CrossRef]
- Schirowski, M.; Hauke, F.; Hirsch, A. Controlling the degree of functionalization: In-depth quantification and side-product analysis of diazonium chemistry on SWCNTs. Chem. Eur. J. 2019, 25, 12761–12768. [Google Scholar] [CrossRef] [Green Version]
- Michael Elliott, C.; Hershenhart, E.; Finke, R.G.; Smith, B.L. Coenzyme B12 model studies: An electrochemical comparison of both alkylcobaloxime and nonalkyl cobaloxime and Co[C2(DO)(DOH)pn] complexes to coenzyme B12. J. Am. Chem. Soc. 1981, 103, 5558–5566. [Google Scholar] [CrossRef]
- Ngameni, E.; Ngoune, J.; Nassi, A.; Belombe, M.M.; Roux, R. Electrochemical studies and electronic spectroscopic examination of some cobaloximatic complexes based on 2,3-butanedione dioxime or dimethylglyoxime with identical or mixed axial ligands. Electrochim. Acta 1996, 41, 2571–2577. [Google Scholar] [CrossRef]
- Mandal, D.; Gupta, B.D. Cobaloximes with dimesitylglyoxime: Synthesis, characterization, and spectral correlations with the related cobaloximes. Organometallics 2005, 24, 1501–1510. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Noda, S. The significance of properly reporting turnover frequency in electrocatalysis research. Angew. Chem. Int. Ed. 2021, 60, 23051–23067. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Vrubel, H.; Moehl, T.; Gratzel, M.; Hu, X. Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction. Chem. Commun. 2013, 49, 8985–8987. [Google Scholar] [CrossRef] [PubMed]
- Donck, S.; Fize, J.; Gravel, E.; Doris, E.; Artero, V. Supramolecular assembly of cobaloxime on nanoring-coated carbon nanotubes: Addressing the stability of the pyridine–cobalt linkage under hydrogen evolution turnover conditions. Chem. Commun. 2016, 52, 11783–11786. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.B.; Haikal, R.R.; Abugableac, A.A.; Alkordi, M.H. Microporous cobaloxime–graphene composite: A reloadable non-noble metal catalyst platform for the proton reduction reaction. J. Mater. Chem. A 2017, 5, 1957–1961. [Google Scholar] [CrossRef]
- Razavet, M.; Artero, V.; Fontecave, M. Proton Electroreduction Catalyzed by Cobaloximes: Functional Models for Hydrogenases. Inorg. Chem. 2005, 44, 4786–4795. [Google Scholar] [CrossRef]


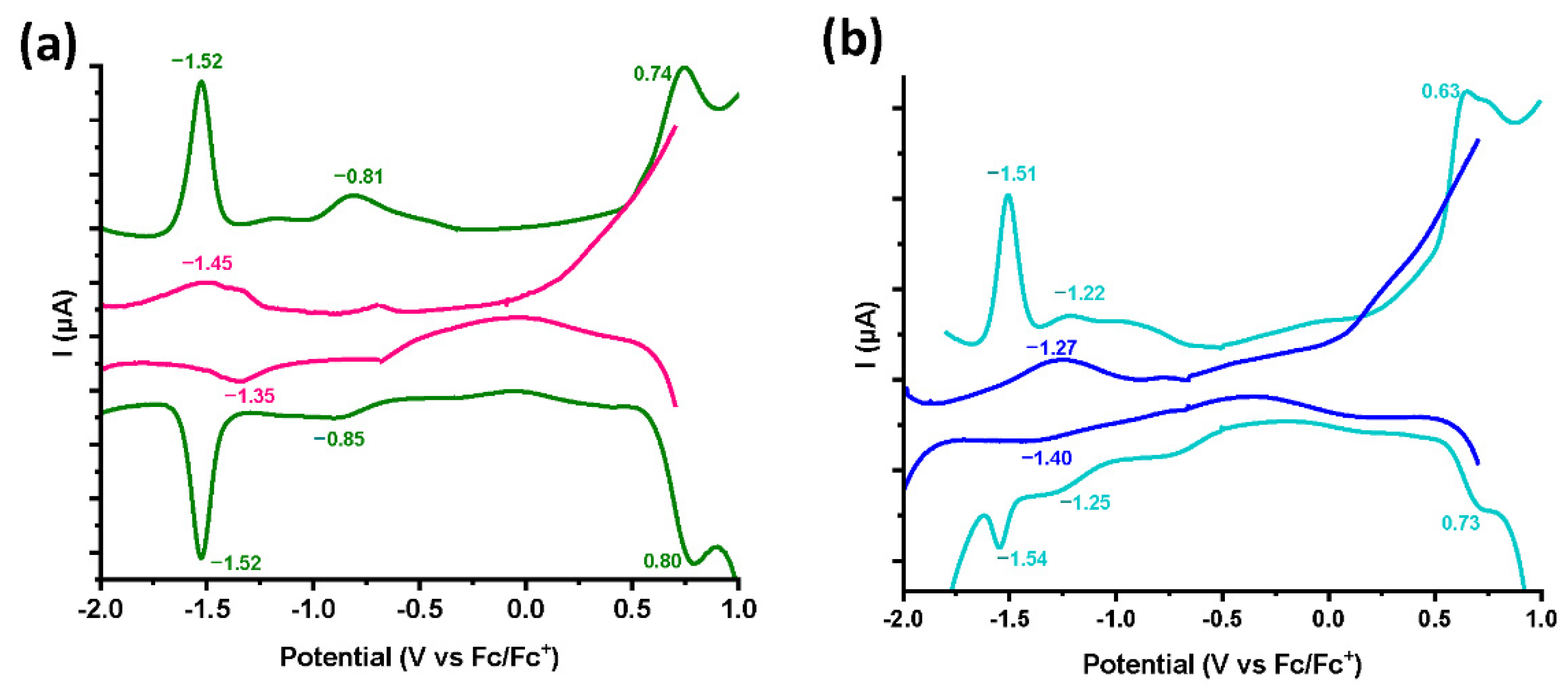


| Material/Compound | Co(III)/Co(II) E1/2Red (ΔEpa,pc)/ V vs. Fc/Fc+ | Co(II)/Co(I) E1/2Red (ΔEpa,pc)/ V vs. Fc/Fc+ | Co(IV)/Co(III) E1/2Ox (ΔEpa,pc)/ V vs. Fc/Fc+ |
|---|---|---|---|
| Pyridine/Co(III) | −0.83 (0.04) | −1.52 (0.0) | 0.77 (0.06) |
| Imidazole/Co(III) | −1.24 (0.06) | −1.53 (0.03) | 0.68 (0.1) |
| [Co]-pyr-graphene | - | −1.39 (0.1) | - |
| [Co]-imi-graphene | - | −1.34 (0.13) | - |
| Electrocatalyst | Onset PoAPPential (V vs. RHE) | Potential @ −10 mA/cm2 (V vs. RHE) | Tafel Slope (mV/dec) | Rct (Ω) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 c * | 10,000 c * | 0 c * | 10,000 c * | 0 c * | 10,000 c * | 0 c * | 10,000 c * | |
| exfoliated graphene | −0.588 | −0.497 | −0.887 | −0.887 | 233 | - | 87 | - |
| [Co]-pyr-graphene | −0.296 | −0.299 | −0.576 | −0.598 | 154 | 164 | 116 | 122 |
| [Co]-imi-graphene | −0.377 | −0.342 | −0.766 | −0.717 | 302 | 291 | 133 | 141 |
| Pt/C | 0.02 | 0.01 | −0.03 | −0.03 | 30 | - | 6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sideri, I.K.; Charalambidis, G.; Coutsolelos, A.G.; Arenal, R.; Tagmatarchis, N. Pyridine vs. Imidazole Axial Ligation on Cobaloxime Grafted Graphene: Hydrogen Evolution Reaction Insights. Nanomaterials 2022, 12, 3077. https://doi.org/10.3390/nano12173077
Sideri IK, Charalambidis G, Coutsolelos AG, Arenal R, Tagmatarchis N. Pyridine vs. Imidazole Axial Ligation on Cobaloxime Grafted Graphene: Hydrogen Evolution Reaction Insights. Nanomaterials. 2022; 12(17):3077. https://doi.org/10.3390/nano12173077
Chicago/Turabian StyleSideri, Ioanna K., Georgios Charalambidis, Athanassios G. Coutsolelos, Raul Arenal, and Nikos Tagmatarchis. 2022. "Pyridine vs. Imidazole Axial Ligation on Cobaloxime Grafted Graphene: Hydrogen Evolution Reaction Insights" Nanomaterials 12, no. 17: 3077. https://doi.org/10.3390/nano12173077
APA StyleSideri, I. K., Charalambidis, G., Coutsolelos, A. G., Arenal, R., & Tagmatarchis, N. (2022). Pyridine vs. Imidazole Axial Ligation on Cobaloxime Grafted Graphene: Hydrogen Evolution Reaction Insights. Nanomaterials, 12(17), 3077. https://doi.org/10.3390/nano12173077