Adsorption of Mercury on Oxidized Graphenes
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
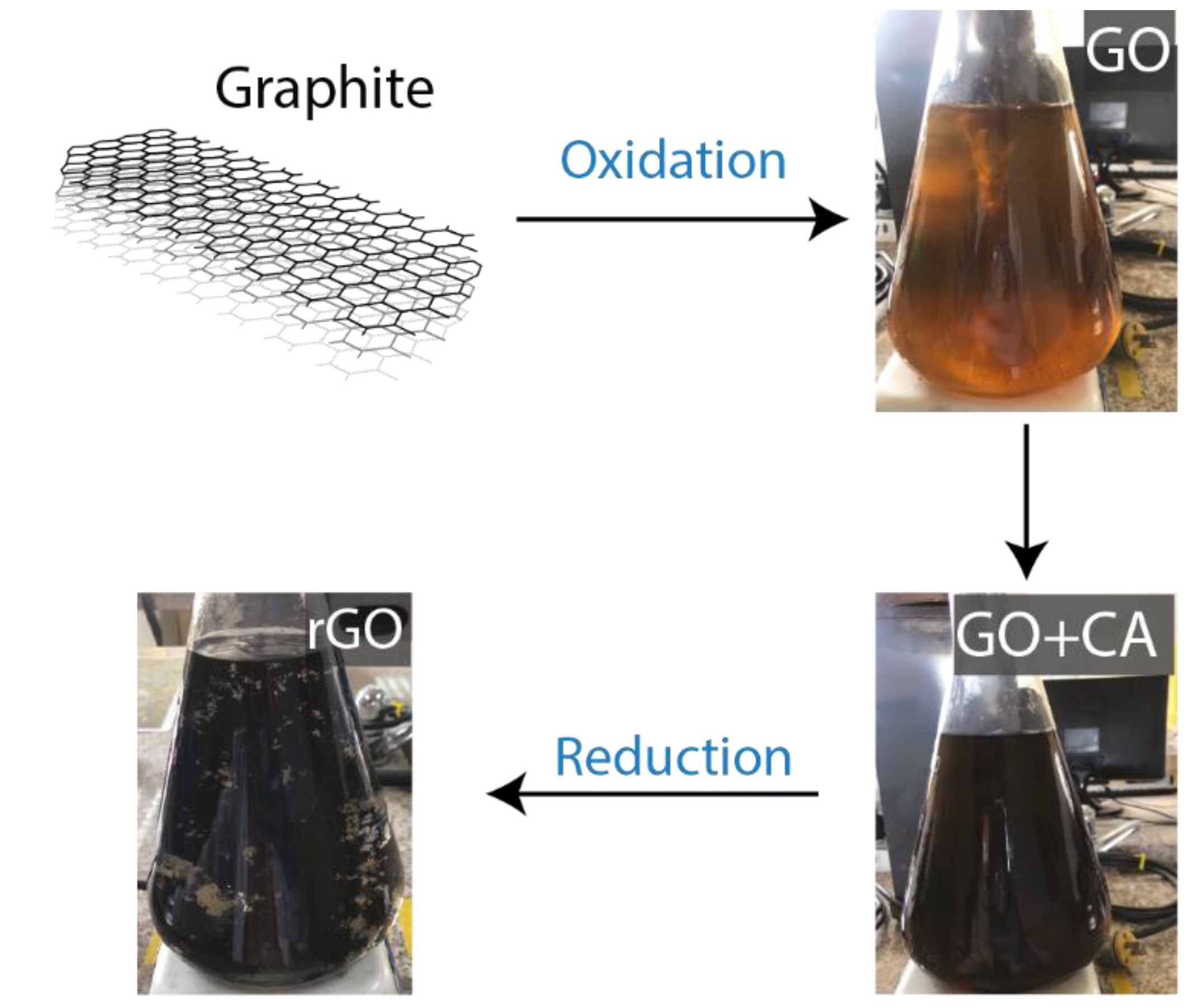
2.2. Synthesis of Oxidized Graphenes
2.3. Characterization Techniques
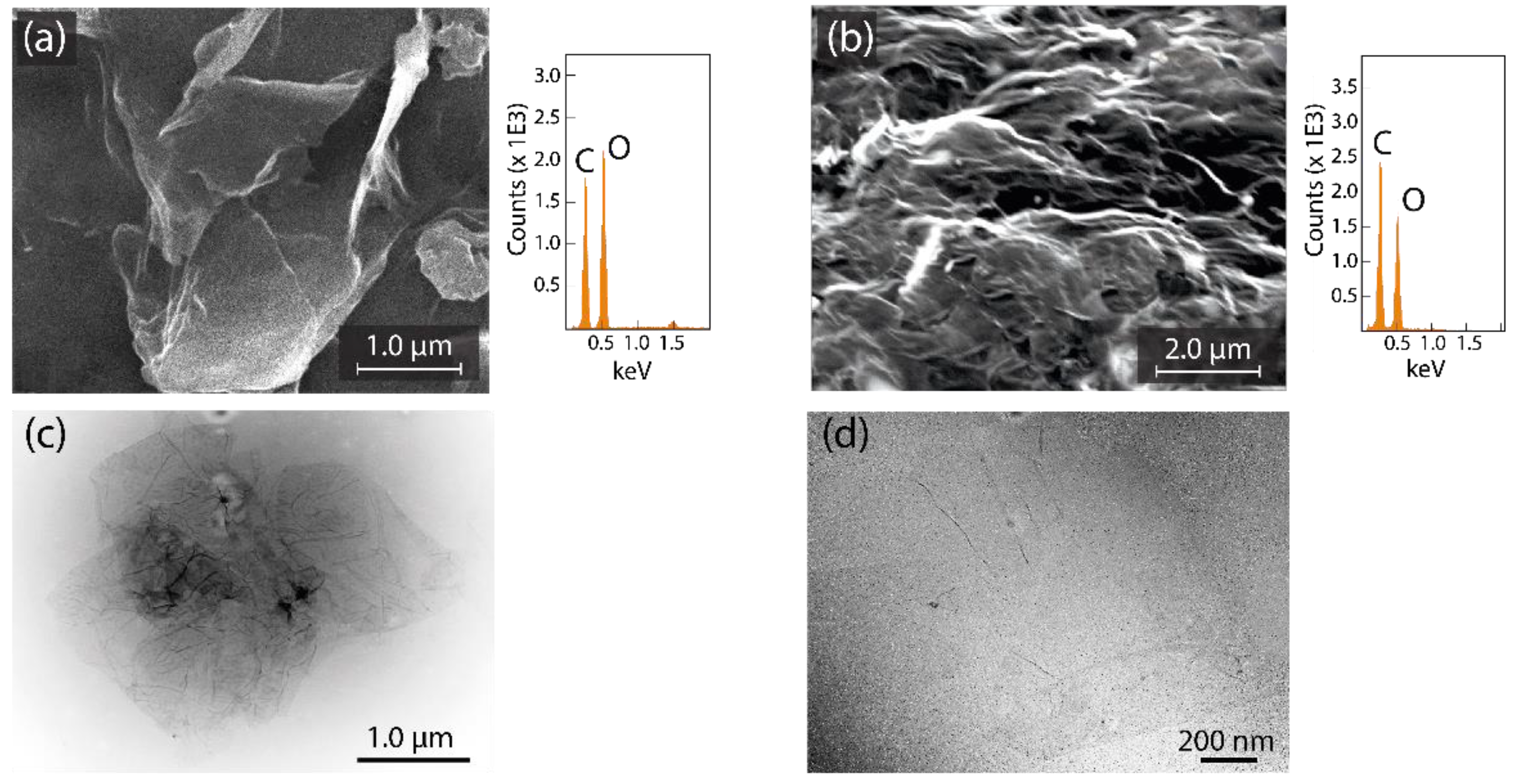
- A scanning electron microscope (SEM, JSM-IT100 InTouchScope) equipped with a JEOL dispersive X-ray spectrometer (EDS) (accelerating voltage of 15 kV) as well as a transmission electron microscope (TEM, JEM 1400 Plus) (operating at 80 kV) were used to observe the surface morphology of GO and rGO.
- Raman spectra of GO and rGO were recorded using a Jasco NRS-500 spectrometer with a 532 nm laser wavelength (0.3 mW, 100X objective).
- Infrared spectra were recorded using a Fourier transform infrared spectrometer (Jasco FT/IR 4000).
- UV-visible measurements were recorded using UV–vis spectroscopy (Thermo Scientific, Waltham, MA, USA, Evolution 220).
- X-ray diffraction measurements were carried out using an X-ray diffractometer (PANalytical Pro X-ray) with a diffraction angle (2θ) window of 5–70° and using Cu Kα irradiation with the acceleration voltage of 60 kV and a current of 55 mA.
2.4. Adsorption Experiments
2.5. Gas-Phase Calculations
3. Results and Discussion
3.1. Characterization of Oxidized Graphenes
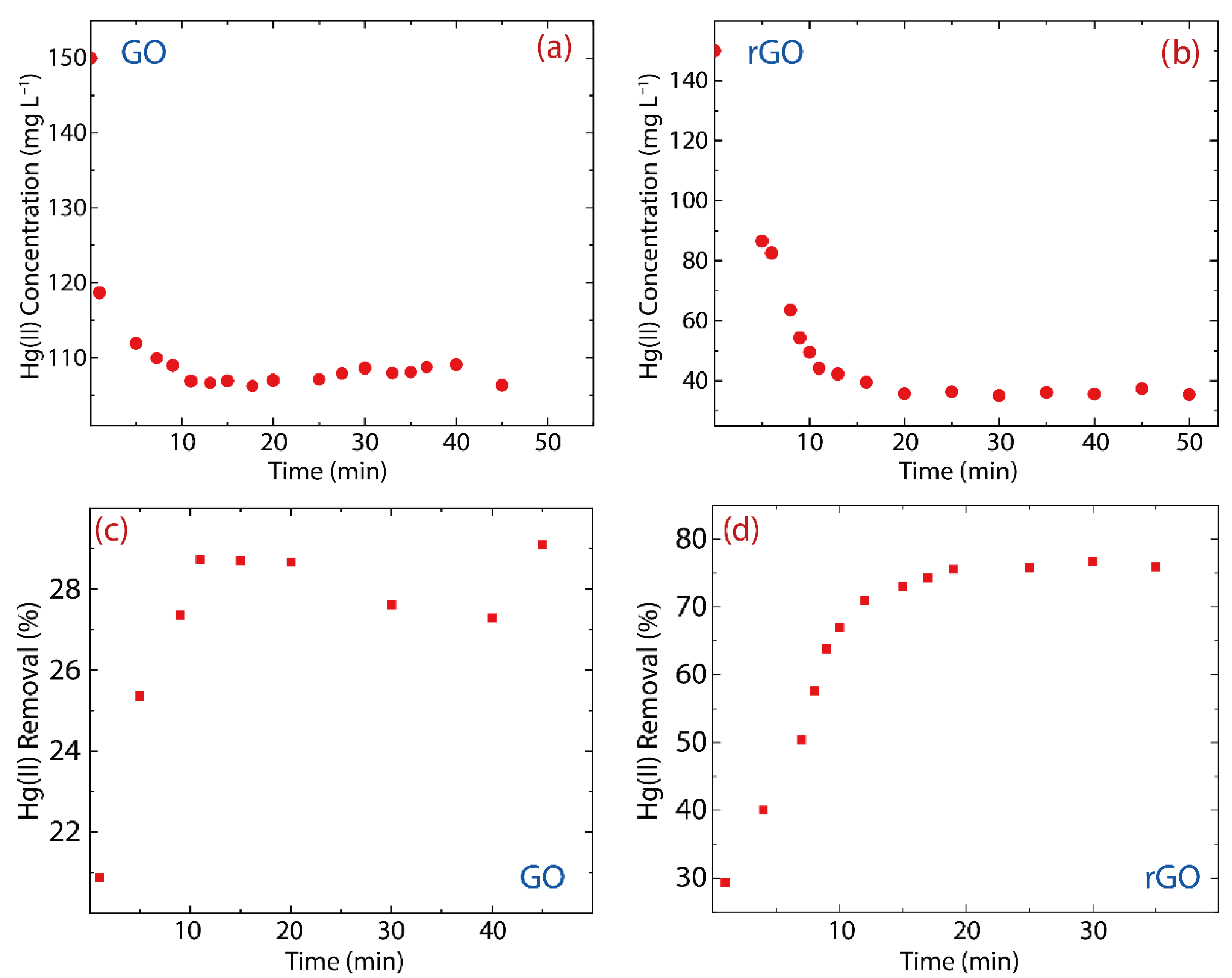
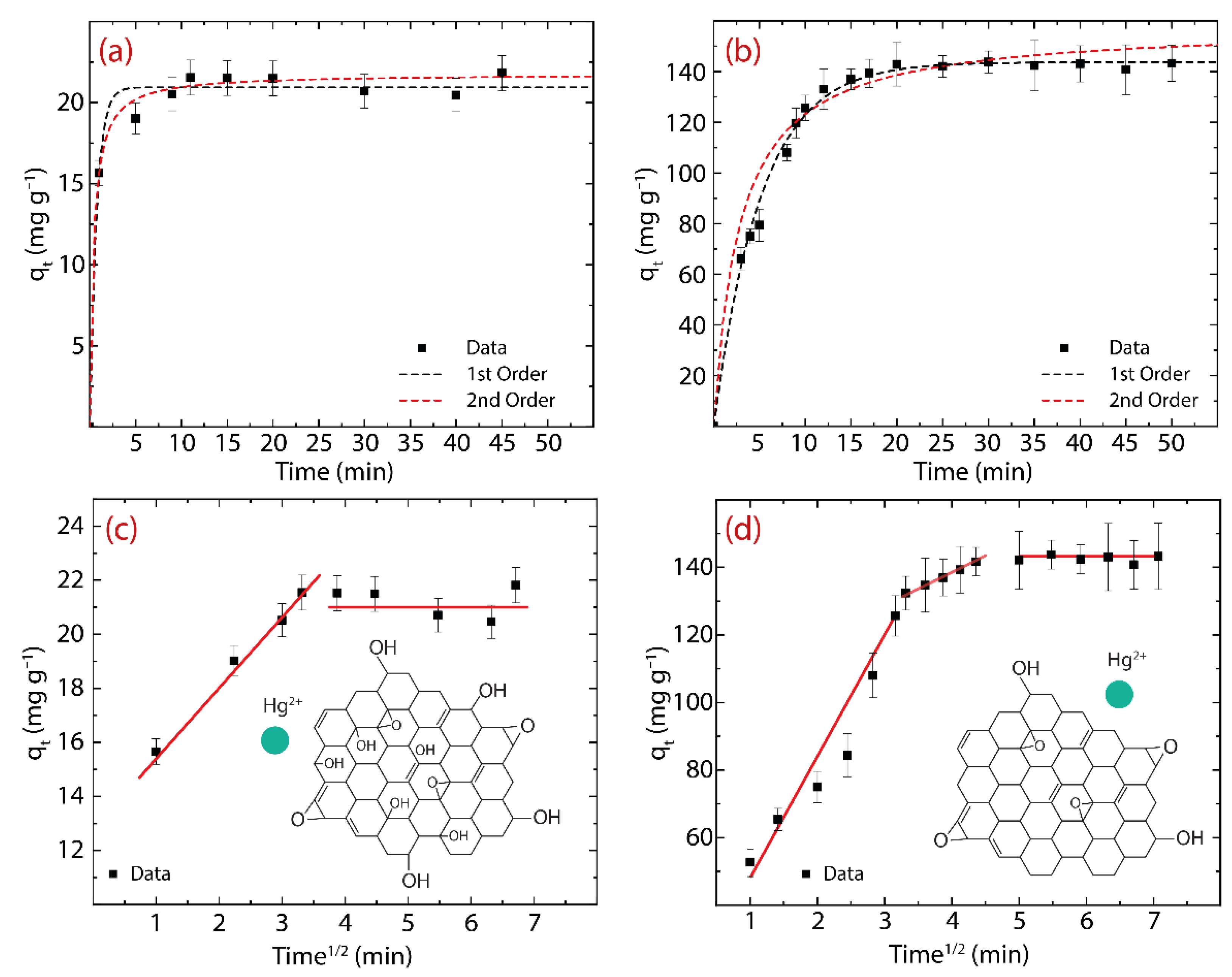
3.2. Adsorption Kinetics: GO vs. rGO
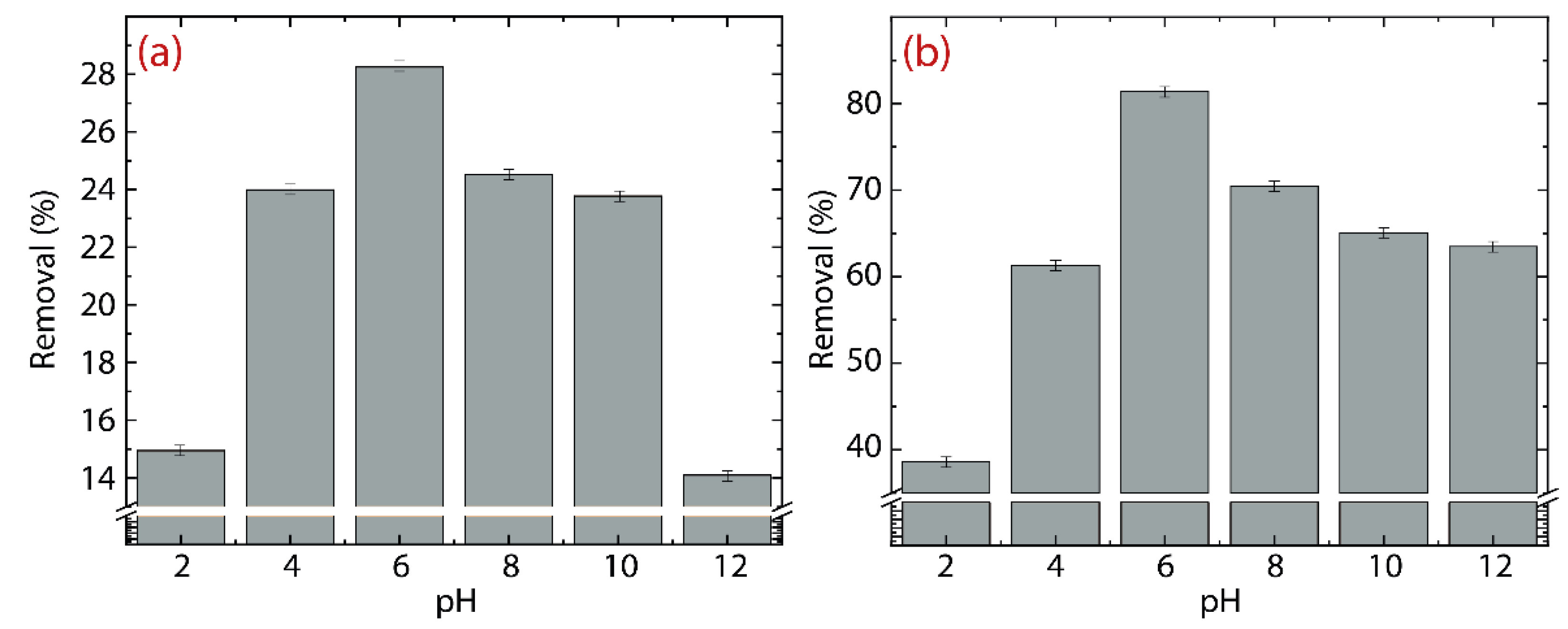
3.3. Effect of pH: GO vs. rGO
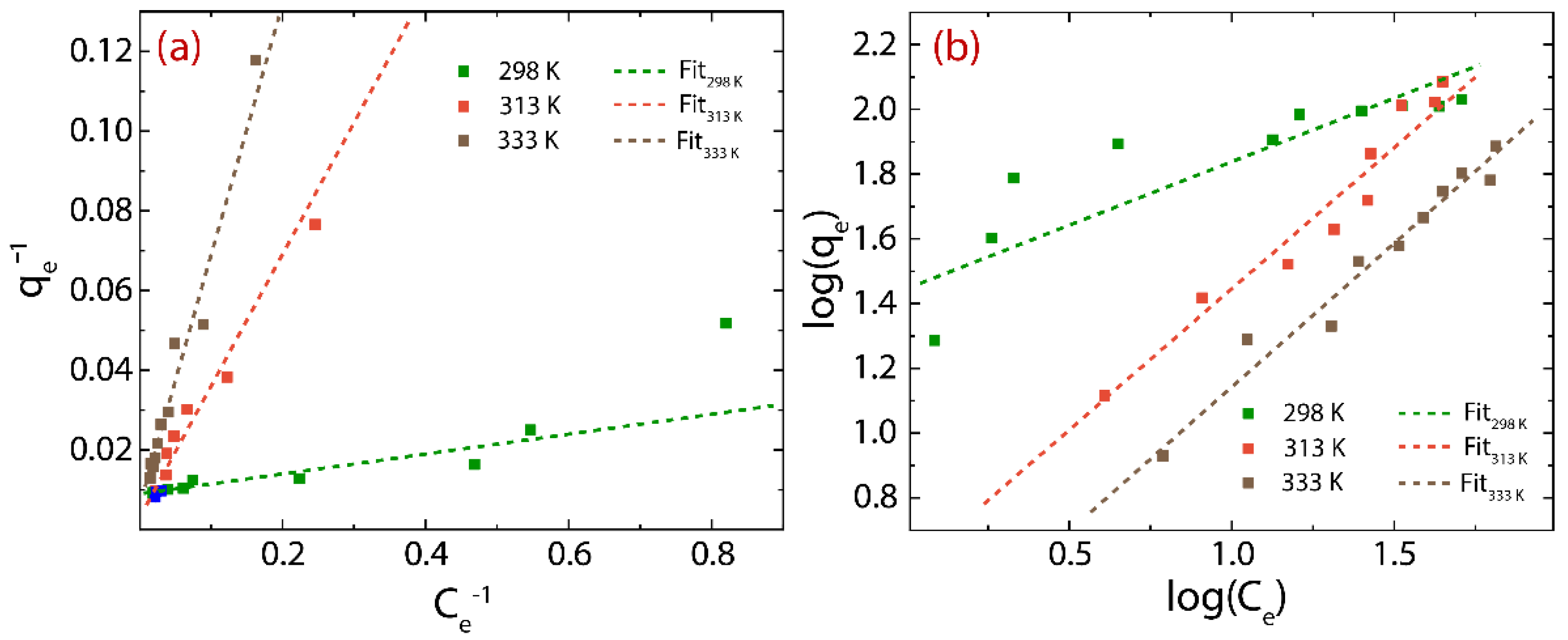
3.4. Adsorption Isotherms of rGO
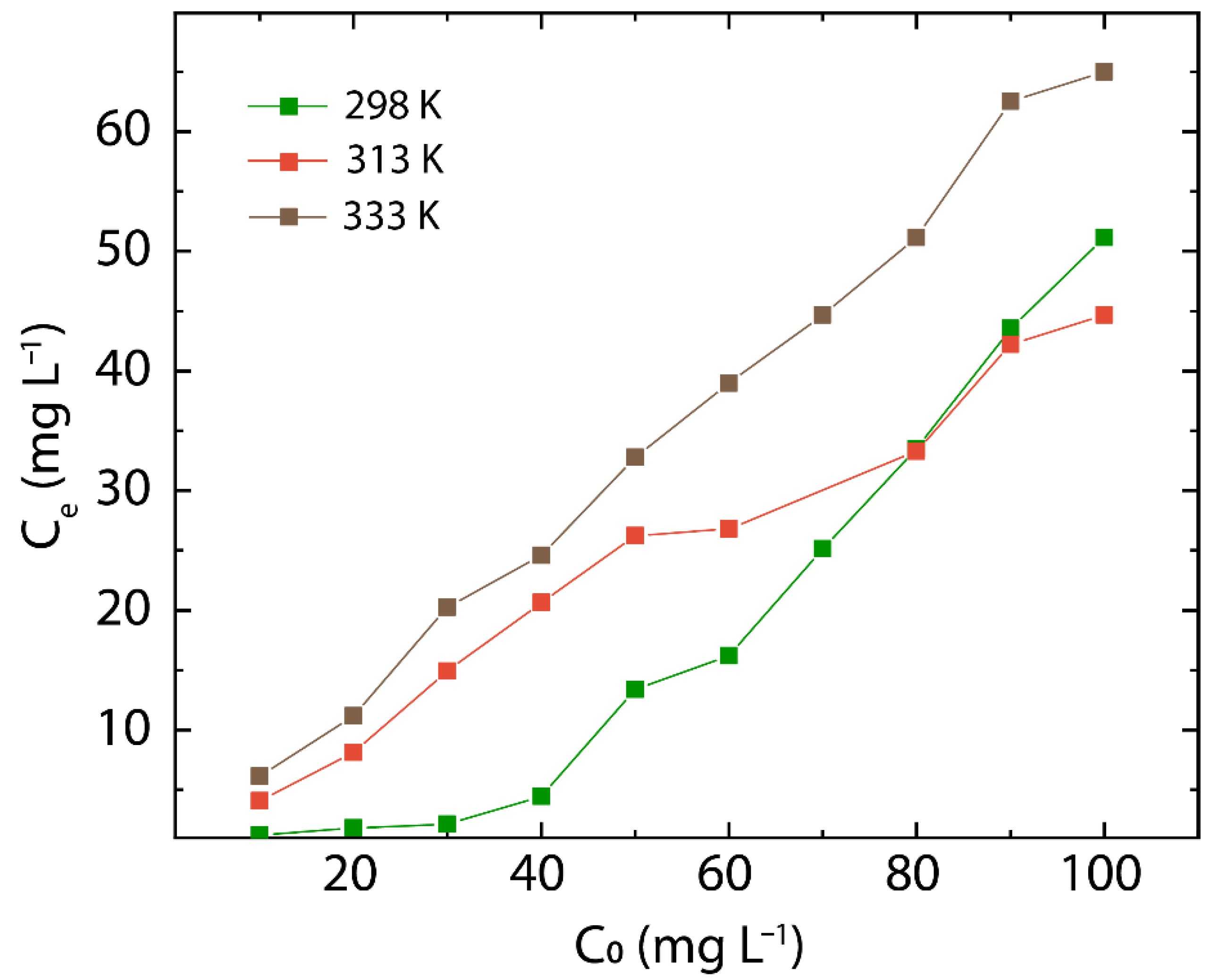
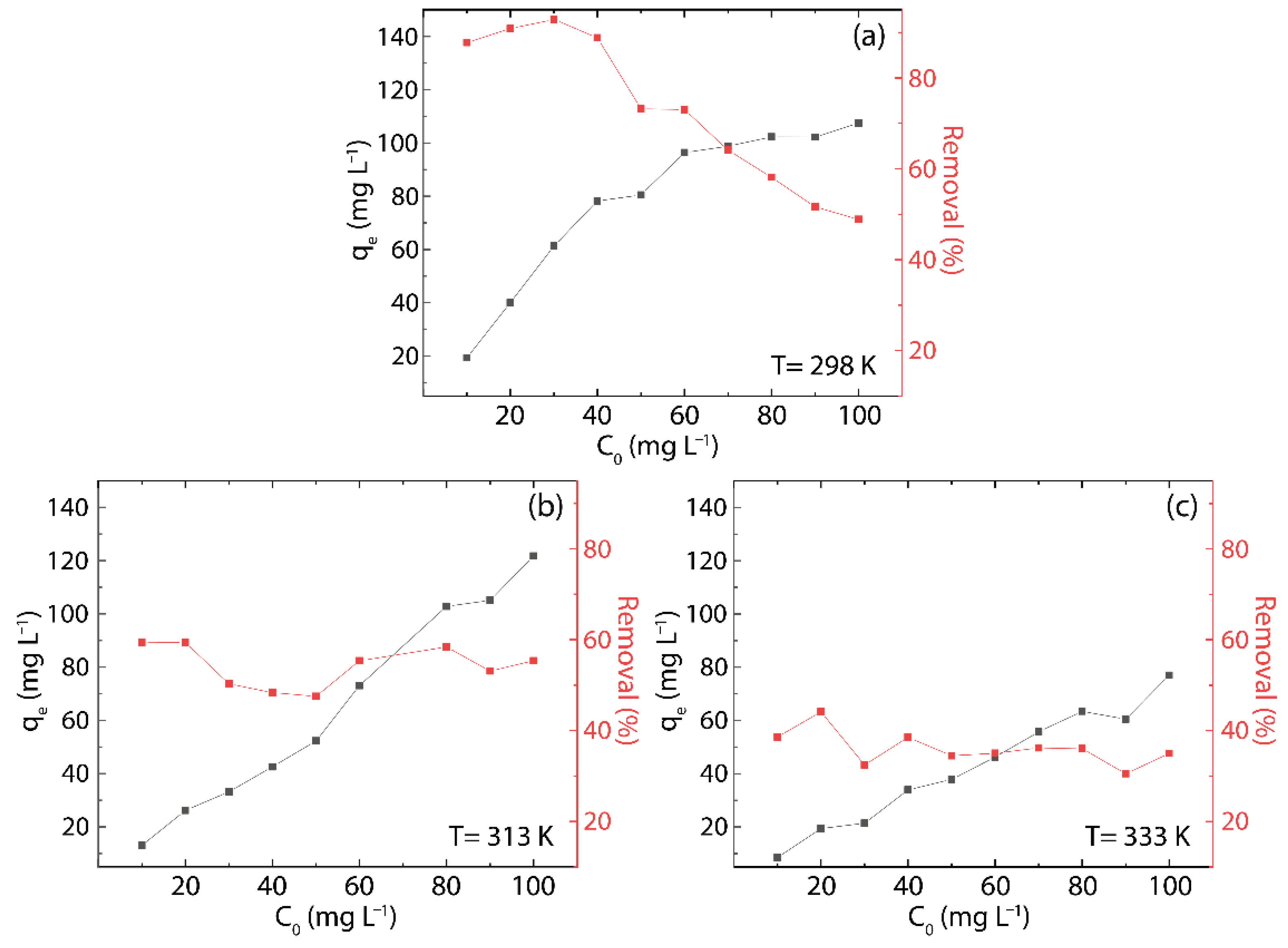
3.5. Effect of Initial Concentration in rGO
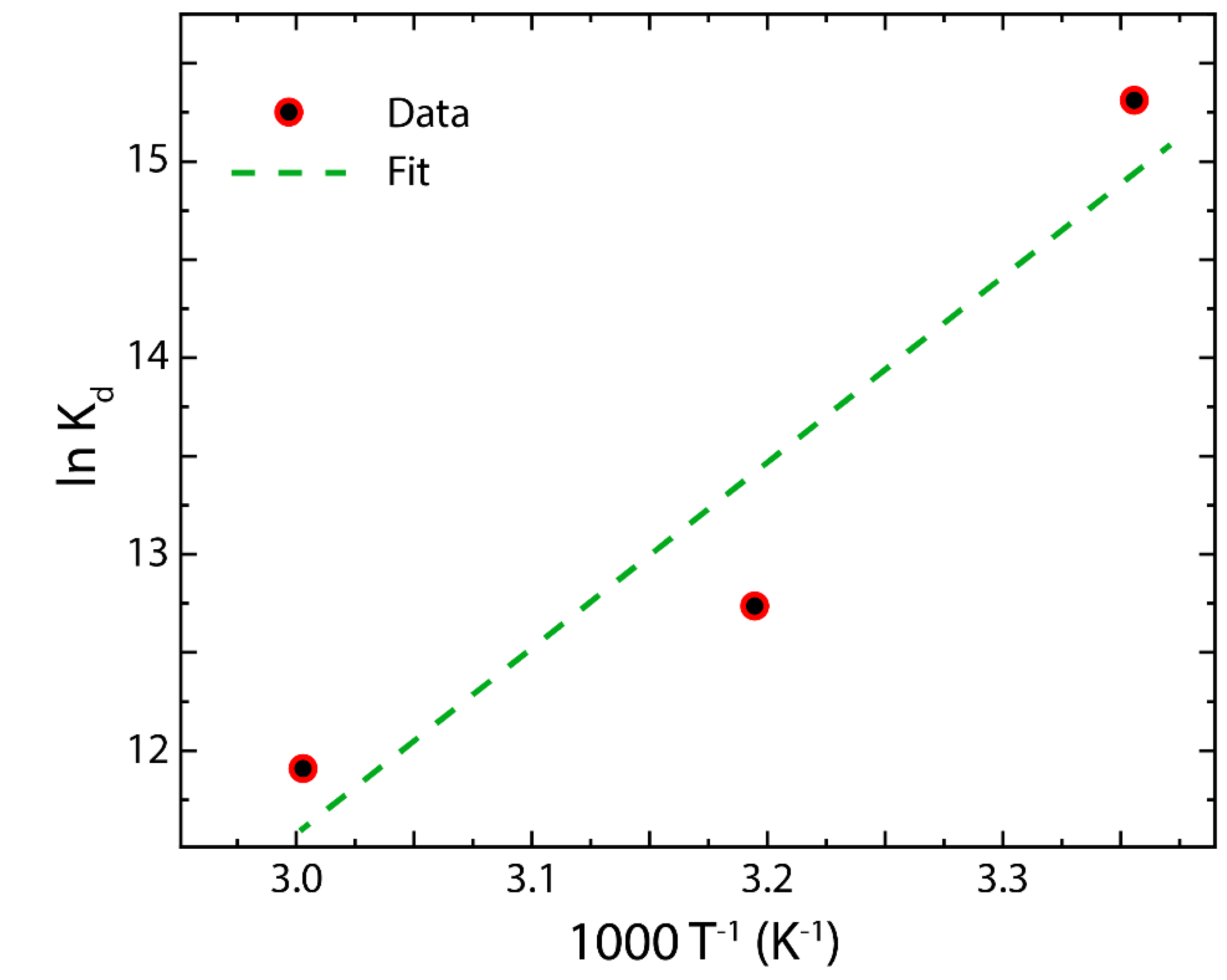
3.6. Adsorption Thermodynamics in rGO
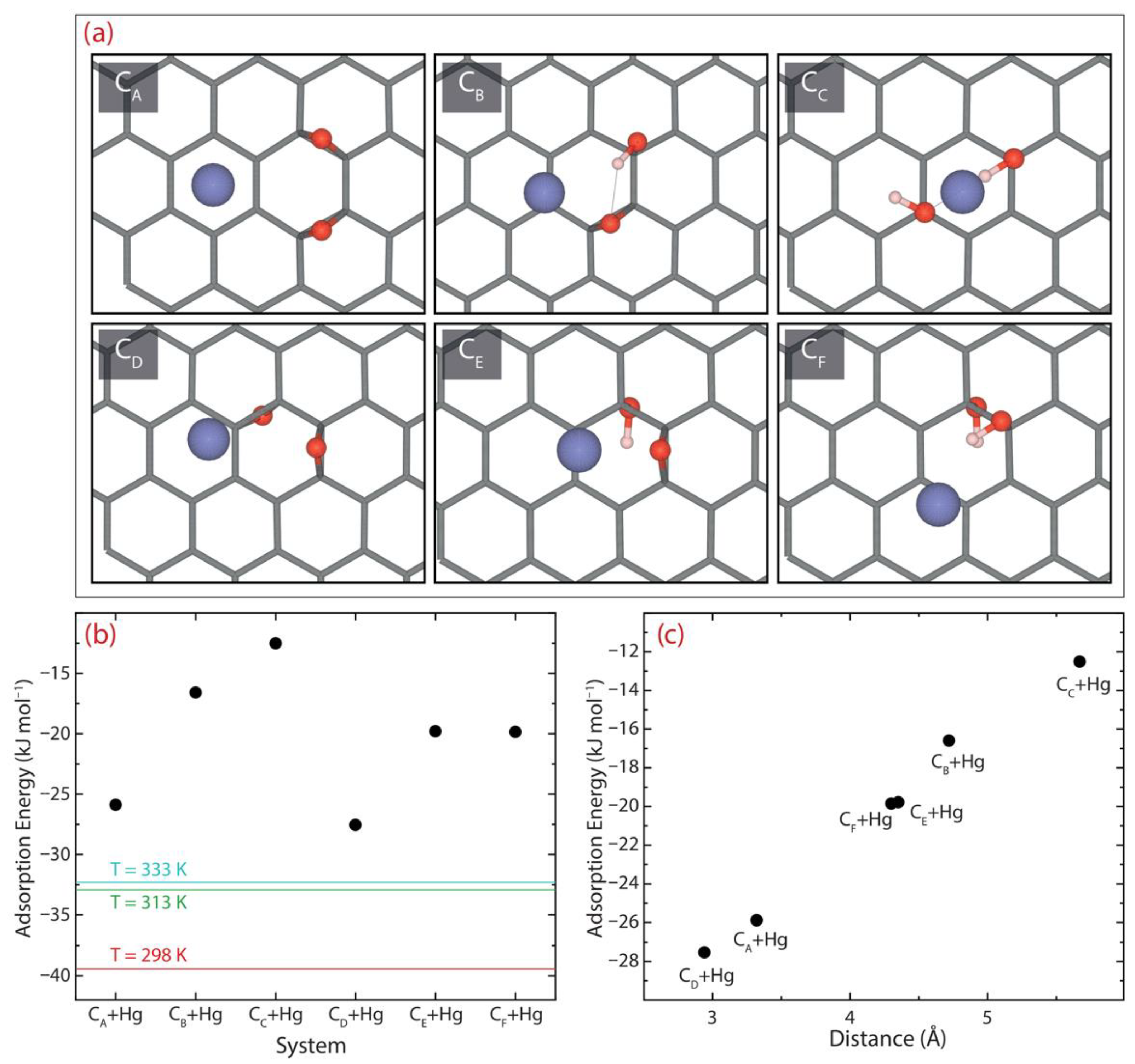
3.7. Theoretical Insights on the Adsorption Mechanism by Density Functional Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mojammal, A.H.M.; Back, S.K.; Seo, Y.C.; Kim, J.H. Mass balance and behavior of mercury in oil refinery facilities. Atmos. Pollut. Res. 2019, 10, 145–151. [Google Scholar] [CrossRef]
- Ortego, A.; Calvo, G.; Valero, A.; Iglesias-Émbil, M.; Valero, A.; Villacampa, M. Assessment of strategic raw materials in the automobile sector. Resour. Conserv. Recycl. 2020, 161, 104968. [Google Scholar] [CrossRef]
- Liu, G.; Cai, Y.; O’Driscoll, N.; Feng, X.; Jiang, G. Overview of mercury in the environment. Environ. Chem. Toxicol. Mercury 2012, 1–12. [Google Scholar]
- Hawley, D.M.; Hallinger, K.K.; Cristol, D.A. Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 2009, 18, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Tse, H.F. Health risk and significance of mercury in the environment. Environ. Sci. Pollut. Res. 2015, 22, 192–201. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatric Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Ahmadi, A.; Akbari, A.; Farjadfard, S.; Ramavandi, B. Adsorption mercury, cobalt, and nickel with a reclaimable and magnetic composite of hydroxyapatite/Fe3O4/polydopamine. J. Environ. Chem. Eng. 2021, 9, 105709. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; El-Naas, M.H. Comparative study between adsorption and membrane technologies for the removal of mercury. Sep. Purif. Technol. 2021, 257, 117833. [Google Scholar] [CrossRef]
- Wu, S.; Yan, P.; Yu, W.; Cheng, K.; Wang, H.; Yang, W.; Zhou, J.; Xi, J.; Qiu, J.; Zhu, S.; et al. Efficient removal of mercury from flue gases by regenerable cerium-doped functional activated carbon derived from resin made by in situ ion exchange method. Fuel Processing Technol. 2019, 196, 106167. [Google Scholar] [CrossRef]
- De, S.; Hazra, T.; Dutta, A. Assessment of removal of mercury from landfill leachate by electrocoagulation. In Environmental Biotechnology for Soil and Wastewater Implications on Ecosystems; Springer: Singapore, 2019; pp. 21–27. [Google Scholar]
- Yoshida, Z.; Kihara, S. Electrodeposition of mercury on glassy carbon electrode from very dilute mercury (II) solution. J. Electroanal. Chem. Interfacial Electrochem. 1979, 95, 159–168. [Google Scholar] [CrossRef]
- Arias, F.A.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Gomez, C.V.; Arias, M.; Caputi, L.S. The adsorption of methylene blue on eco-friendly reduced graphene oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tene, T.; Bellucci, S.; Guevara, M.; Viteri, E.; Polanco, M.A.; Salguero, O.; Vera-Guzmán, E.; Valladares, S.; Scarcello, A.; Alessandro, F.; et al. Cationic pollutant removal from aqueous solution using reduced graphene oxide. Nanomaterials 2022, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Arias, F.A.; Guevara, M.; Nuñez, A.; Villamagua, L.; Tapia, C.; Pisarra, M.; Torres, F.J.; Caputi, L.S.; Gomez, C.V. Removal of mercury (II) from aqueous solution by partially reduced graphene oxide. Sci. Rep. 2022, 12, 6326. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.A.; Zaman, M.K. Mercury removal from water. Recent Dev. Mercury Sci. 2006, 120, 163–182. [Google Scholar]
- Veltri, F.; Alessandro, F.; Scarcello, A.; Beneduci, A.; Polanco, M.A.; Perez, D.C.; Gomez, C.V.; Tavolaro, A.; Giordano, G.; Caputi, L.S. Porous carbon materials obtained by the hydrothermal carbonization of orange juice. Nanomaterials 2020, 10, 655. [Google Scholar] [CrossRef]
- Kim, M.H.; Jeong, I.T.; Park, S.B.; Kim, J.W. Analysis of environmental impact of activated carbon production from wood waste. Environ. Eng. Res. 2019, 24, 117–126. [Google Scholar] [CrossRef]
- Ali, I.; Zakharchenko, E.; Myasoedova, G.; Molochnikova, N.; Rodionova, A.; Baulin, V.; Burakov, A.; Burakova, I.; Babkin, A.; Neskoromnaya, E.; et al. Preparation and characterization of oxidized graphene for actinides and rare earth elements removal in nitric acid solutions from nuclear wastes. J. Mol. Liq. 2021, 335, 116260. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Viteri, E.; Maldonado, A.; Pisarra, M.; Sindona, A.; Gomez, C.V.; Bellucci, S. Calibration of Fermi Velocity to Explore the Plasmonic Character of Graphene Nanoribbon Arrays by a Semi-Analytical Model. Nanomaterials 2022, 12, 2028. [Google Scholar] [CrossRef]
- Villamagua, L.; Carini, M.; Stashans, A.; Gomez, C.V. Band gap engineering of graphene through quantum confinement and edge distortions. Ric. Di Mat. 2016, 65, 579–584. [Google Scholar] [CrossRef]
- Gomez, C.V.; Pisarra, M.; Gravina, M.; Sindona, A. Tunable plasmons in regular planar arrays of graphene nanoribbons with armchair and zigzag-shaped edges. Beilstein J. Nanotechnol. 2017, 8, 172–182. [Google Scholar] [CrossRef]
- Sindona, A.; Pisarra, M.; Bellucci, S.; Tene, T.; Guevara, M.; Gomez, C.V. Plasmon oscillations in two-dimensional arrays of ultranarrow graphene nanoribbons. Phys. Rev. B 2019, 100, 235422. [Google Scholar] [CrossRef]
- Gomez, C.V.; Robalino, E.; Haro, D.; Tene, T.; Escudero, P.; Haro, A.; Orbe, J. Structural and electronic properties of graphene oxide for different degree of oxidation. Mater. Today Proc. 2016, 3, 796–802. [Google Scholar] [CrossRef]
- Fiallos, D.C.; Gómez, C.V.; Tubon Usca, G.; Pérez, D.C.; Tavolaro, P.; Martino, G.; Caputi, L.S.; Tavolaro, A. Removal of Acridine Orange from Water by Graphene Oxide. In Proceedings of the AIP Conference Proceedings, Porto Palace Conference Centre & Hotel, Thessaloniki, Greece, 5–12 July 2014; American Institute of Physics: College Park, MD, USA, 2015; Volume 1646. [Google Scholar]
- Gomez, C.V.; Guevara, M.; Tene, T.; Villamagua, L.; Usca, G.T.; Maldonado, F.; Tapia, C.; Cataldo, A.; Bellucci, S.; Caputi, L.S. The liquid exfoliation of graphene in polar solvents. Appl. Surf. Sci. 2021, 546, 149046. [Google Scholar] [CrossRef]
- Cayambe, M.; Zambrano, C.; Tene, T.; Guevara, M.; Usca, G.T.; Brito, H.; Molina, R.; Coello-Fiallos, D.; Caputi, L.; Gomez, C.V. Dispersion of graphene in ethanol by sonication. Mater. Today Proc. 2021, 37, 4027–4030. [Google Scholar] [CrossRef]
- Tubon Usca, G.; Vacacela Gomez, C.; Guevara, M.; Tene, T.; Hernandez, J.; Molina, R.; Tavolaro, A.; Miriello, D.; Caputi, L.S. Zeolite-assisted shear exfoliation of graphite into few-layer graphene. Crystals 2019, 9, 377. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Valarezo, A.; Salguero, O.; Arias, F.A.; Arias, M.; Scarcello, A.; Caputi, L.; Gomez, C.V. Drying-time study in graphene oxide. Nanomaterials 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Tene, T.; Usca, G.T.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Gomez, C.V. Toward large-scale production of oxidized graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Yu, Y.; Yang, W.; Sun, Y. Cross-linked sulfydryl-functionalized graphene oxide as ultra-high capacity adsorbent for high selectivity and ppb level removal of mercury from water under wide pH range. Environ. Pollut. 2021, 271, 116378. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-dimensional graphene for efficient aqueous heavy metal adsorption: Rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Yap, P.L.; Tung, T.T.; Kabiri, S.; Matulick, N.; Tran, D.N.; Losic, D. Polyamine-modified reduced graphene oxide: A new and cost-effective adsorbent for efficient removal of mercury in waters. Sep. Purif. Technol. 2020, 238, 116441. [Google Scholar] [CrossRef]
- Sitko, R.; Musielak, M.; Serda, M.; Talik, E.; Zawisza, B.; Gagor, A.; Malecka, M. Thiosemicarbazide-grafted graphene oxide as superior adsorbent for highly efficient and selective removal of mercury ions from water. Sep. Purif. Technol. 2021, 254, 117606. [Google Scholar] [CrossRef]
- Li, M.; Wang, B.; Yang, M.; Li, Q.; Calatayud, D.G.; Zhang, S.; Wang, H.; Wang, L.; Mao, B. Promoting mercury removal from desulfurization slurry via S-doped carbon nitride/graphene oxide 3D hierarchical framework. Sep. Purif. Technol. 2020, 239, 116515. [Google Scholar] [CrossRef]
- Khorshidi, P.; Shirazi, R.H.S.M.; Miralinaghi, M.; Moniri, E.; Saadi, S. Adsorptive removal of mercury (II), copper (II), and lead (II) ions from aqueous solutions using glutathione-functionalized NiFe2O4/graphene oxide composite. Res. Chem. Intermed. 2020, 46, 3607–3627. [Google Scholar] [CrossRef]
- Available online: https://gaussian.com/gaussian16/ (accessed on 30 August 2022).
- Sindona, A.; Pisarra, M.; Gravina, M.; Gomez, C.V.; Riccardi, P.; Falcone, G.; Plastina, F. Statistics of work and orthogonality catastrophe in discrete level systems: An application to fullerene molecules and ultra-cold trapped Fermi gases. Beilstein J. Nanotechnol. 2015, 6, 755–766. [Google Scholar] [CrossRef]
- Chai, J.-D.; Martin, H.-G. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Yang, Y.; Michael, N.W.; Kenneth, M.M., Jr. Assessment of the “6-31+ G**+ LANL2DZ” mixed basis set coupled with density functional theory methods and the effective core potential: Prediction of heats of formation and ionization potentials for first-row-transition-metal complexes. J. Phys. Chem. A 2009, 113, 9843–9851. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the Raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Calizo, I.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 2007, 7, 2645–2649. [Google Scholar] [CrossRef]
- López-Díaz, D.; Holgado, M.L.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Ke, F.; Lin, C.; Zhao, H.; Gan, L.; Luo, Z.; Zhao, R.; Heinz, T.F.; Hu, Z. Evolution of the Raman spectrum of graphene grown on copper upon oxidation of the substrate. Nano Res. 2014, 7, 1613–1622. [Google Scholar] [CrossRef]
- Solin, S.A.; Ramdas, A.K. Raman spectrum of diamond. Phys. Rev. B 1970, 1, 1687. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Tang, K.; Zhang, K.; Zou, Z.; Gao, X. High-strength chitin based hydrogels reinforced by tannic acid functionalized graphene for congo red adsorption. J. Polym. Environ. 2020, 28, 984–994. [Google Scholar] [CrossRef]
- Habte, A.T.; Delele, W.A. Synthesis and characterization of reduced graphene oxide (rGO) started from graphene oxide (GO) using the tour method with different parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Maddinedi, S.B.; Mandal, B.K.; Vankayala, R.; Kalluru, P.; Pamanji, S.R. Bioinspired reduced graphene oxide nanosheets using Terminalia chebula seeds extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 117–124. [Google Scholar] [CrossRef]
- Arias, F.A.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef]
- Wijaya, R.; Andersan, G.; Santoso, S.P.; Irawaty, W. Green reduction of graphene oxide using kaffir lime peel extract (Citrus hystrix) and its application as adsorbent for methylene blue. Sci. Rep. 2020, 10, 667. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Sani, A.; Singh, P.; Raizada, P.; Lima, E.C.; Anastopoulos, I.; Giannakoudakis, D.A.; Sivamani, S.; Dontsova, T.A.; Hosseini-Bandegharaei, A. Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions. Bioresour. Technol. 2020, 297, 122452. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Ashok, K.G. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Sharma, P.; Hussain, N.; Borah, D.J.; Das, M.R. Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: A comparative study. J. Chem. Eng. Data 2013, 58, 3477–3488. [Google Scholar] [CrossRef]
- Ghasemi, S.S.; Hadavifar, M.; Maleki, B.; Mohammadnia, E. Adsorption of mercury ions from synthetic aqueous solution using polydopamine decorated SWCNTs. J. Water Process Eng. 2019, 32, 100965. [Google Scholar] [CrossRef]
- Coello-Fiallos, D.; Tene, T.; Guayllas, J.; Haro, D.; Haro, A.; Gomez, C.V. DFT comparison of structural and electronic properties of graphene and germanene: Monolayer and bilayer systems. Mater. Today Proc. 2017, 4, 6835–6841. [Google Scholar] [CrossRef]
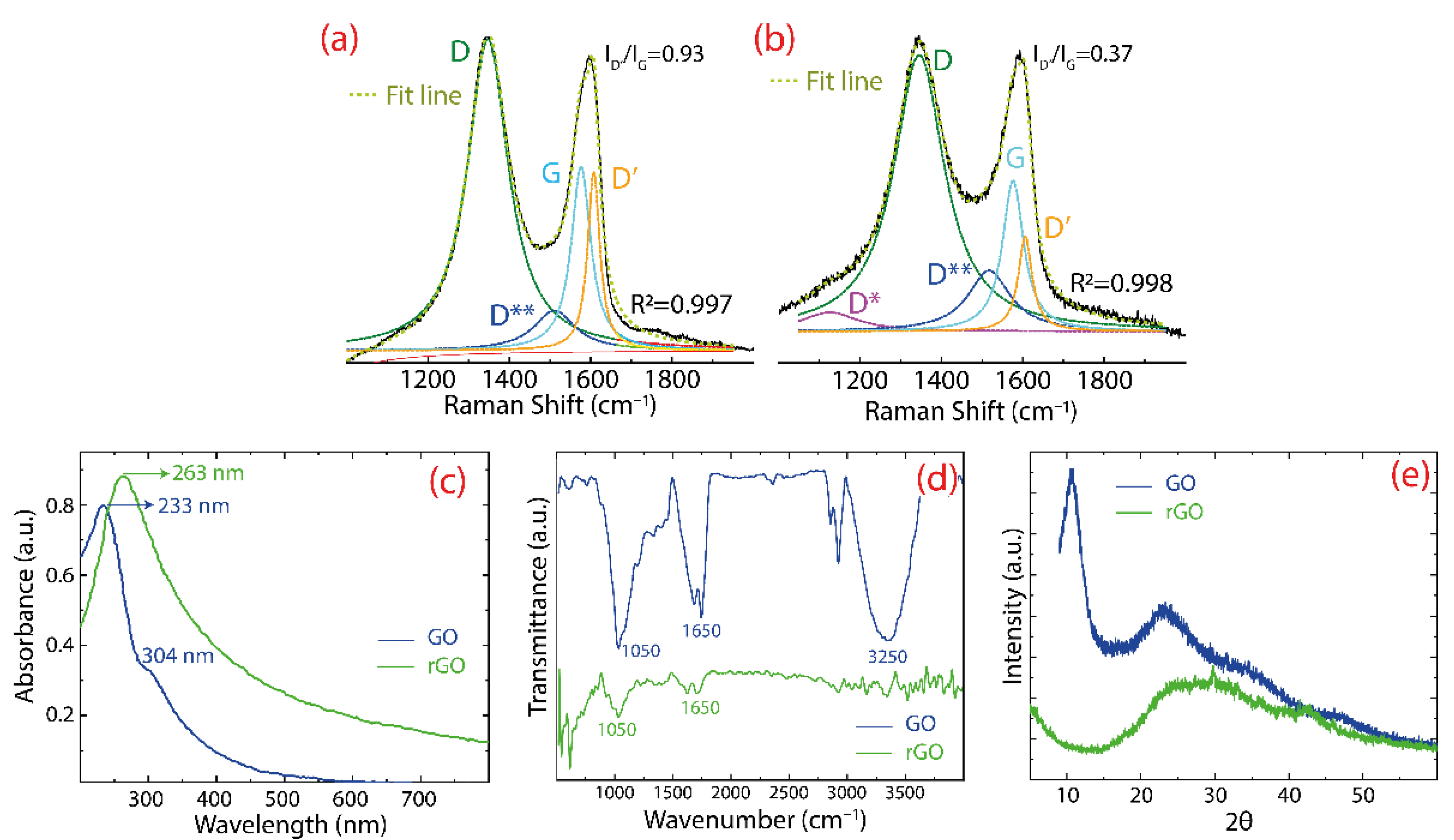
| D*—FWHM (cm−1) | D—FWHM (cm−1) | D**—FWHM (cm−1) | G—FWHM (cm−1) | D’—FWHM (cm−1) | |
|---|---|---|---|---|---|
| GO | --- | 1348-122 | 1511-122 | 1576-58 | 1608-36 |
| rGO | 1122-79 | 1340-131 | 1522-127 | 1578-51 | 1605-38 |
| Parameters | Hg(II) on GO | Hg(II) on rGO |
|---|---|---|
| (mg g−1) | 21.12 | 142.26 |
| Pseudo-first-order model | ||
| (mg g−1) | 20.9 ± 0.7 | 143.7 ± 5.7 |
| (min−1) | 13.4 ± 0.4 | 0.194 ± 0.030 |
| SSE | 3.743 | 1826 |
| R2 | 0.985 | 0.931 |
| RMSE | 0.856 | 8.546 |
| Pseudo-second-order model | ||
| (mg g−1) | 21.8 ± 0.7 | 151.3 ± 9.45 |
| (g mg−1 min−1) | 0.118 ± 0.001 | 0.002 ± 0.001 |
| SSE | 5.861 | 2480 |
| R2 | 0.990 | 0.949 |
| RMSE | 0.680 | 9.96 |
| Hg(II) on GO | Hg(II) on rGO | |
|---|---|---|
| Parameters | Value | Value |
| (mg g−1 min1/2) | 6.97 ± 0.67 | 7.82 ± 1.25 |
| (mg g−1) | 8.65 ± 0.82 | 44.28 ± 7.75 |
| 0.586 | 0.411 | |
| R2 | 0.996 | 0.963 |
| T (K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| (L g−1) | (mg g−1) | R2 | (mg(1-n) g−1 L1/n) | n | R2 | |
| 298 | 4.71 | 110.21 | 0.933 | 0.592 | 1.44 | 0.936 |
| 313 | 1.56 | 217.34 | 0.947 | 0.872 | 0.57 | 0.979 |
| 333 | 0.58 | 255.04 | 0.964 | 0.885 | 0.26 | 0.978 |
| Adsorbents | Adsorption Capacity (mg g−1) | Time (min) | Ref. |
|---|---|---|---|
| S-GO | 3490 | 240 | [32] |
| GONR (Hg and As) | 33.02 | 12 | [33] |
| GO-TSC | 231 | 30 | [34] |
| S-doped g-C3N4/LGO | 46 | 120 | [35] |
| GSH-NiFe2O4/GO | 272.94 | 90 | [36] |
| HT-rGO-N | 75.80 | 10 | [37] |
| | 110.21 217.34 255.04 | 20 | This work |
| T (K) | (kJ mol−1) | (kJ mol−1) | (kJ mol−1 K−1) |
| 298 | −39.43 | ||
| 313 | −32.93 | −98.31 | 0.085 |
| 333 | −32.30 |
| Adsorption Energy | |||
|---|---|---|---|
| System | eV | kJ mol−1 | kcal mol−1 |
| CA + Hg | −0.27 | −25.88 | −6.19 |
| CB + Hg | −0.17 | −16.60 | −3.97 |
| CC + Hg | −0.13 | −12.51 | −2.99 |
| CD + Hg | −0.29 | −27.54 | −6.85 |
| CE + Hg | −0.21 | −19.79 | −4.73 |
| CF + Hg | −0.21 | −19.85 | −4.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Bellucci, S.; Guevara, M.; Arias Arias, F.; Sáez Paguay, M.Á.; Quispillo Moyota, J.M.; Arias Polanco, M.; Scarcello, A.; Vacacela Gomez, C.; Straface, S.; et al. Adsorption of Mercury on Oxidized Graphenes. Nanomaterials 2022, 12, 3025. https://doi.org/10.3390/nano12173025
Tene T, Bellucci S, Guevara M, Arias Arias F, Sáez Paguay MÁ, Quispillo Moyota JM, Arias Polanco M, Scarcello A, Vacacela Gomez C, Straface S, et al. Adsorption of Mercury on Oxidized Graphenes. Nanomaterials. 2022; 12(17):3025. https://doi.org/10.3390/nano12173025
Chicago/Turabian StyleTene, Talia, Stefano Bellucci, Marco Guevara, Fabian Arias Arias, Miguel Ángel Sáez Paguay, John Marcos Quispillo Moyota, Melvin Arias Polanco, Andrea Scarcello, Cristian Vacacela Gomez, Salvatore Straface, and et al. 2022. "Adsorption of Mercury on Oxidized Graphenes" Nanomaterials 12, no. 17: 3025. https://doi.org/10.3390/nano12173025
APA StyleTene, T., Bellucci, S., Guevara, M., Arias Arias, F., Sáez Paguay, M. Á., Quispillo Moyota, J. M., Arias Polanco, M., Scarcello, A., Vacacela Gomez, C., Straface, S., Caputi, L. S., & Torres, F. J. (2022). Adsorption of Mercury on Oxidized Graphenes. Nanomaterials, 12(17), 3025. https://doi.org/10.3390/nano12173025













