Amphiphilic Cationic Peptide-Coated PHA Nanosphere as an Efficient Vector for Multiple-Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
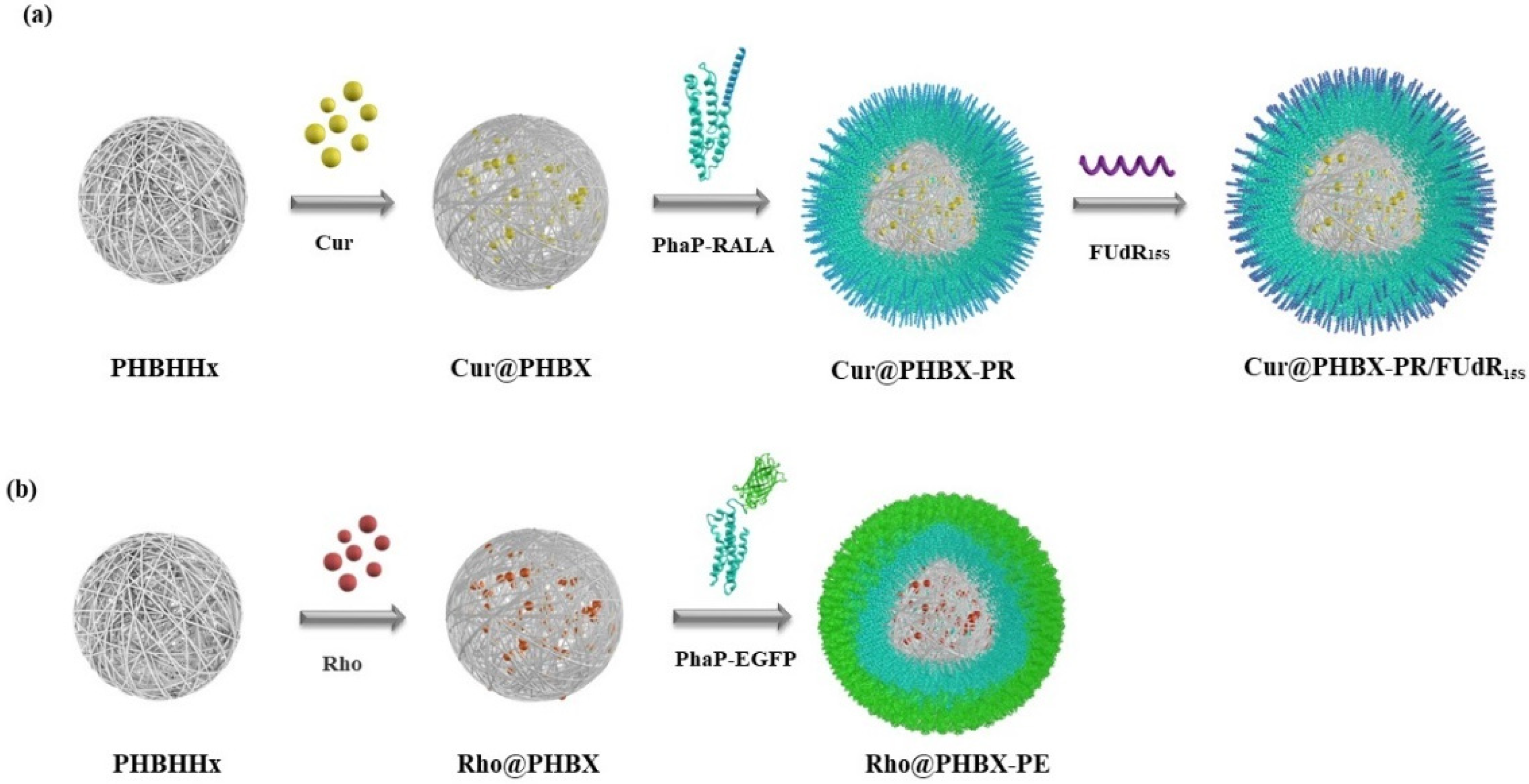
2.2. Preparation of Drug-Loaded PHBX NPs
2.3. Synthesis, Purification, and Characterization of Protein
2.4. Self-Assembly of Cur@PHBX-PR/FUdR15S
2.5. Characterization of Cur@PHBX-PR/FUdR15S
2.6. Drug Loading (DL) of Cur@PHBX-PR/FUdR15S
2.7. Stability Analysis of Cur@PHBX-PR/FUdR15S
2.8. In Vitro Drug Release of Cur@PHBX-PR/FUdR15S
2.9. Cell Cultures
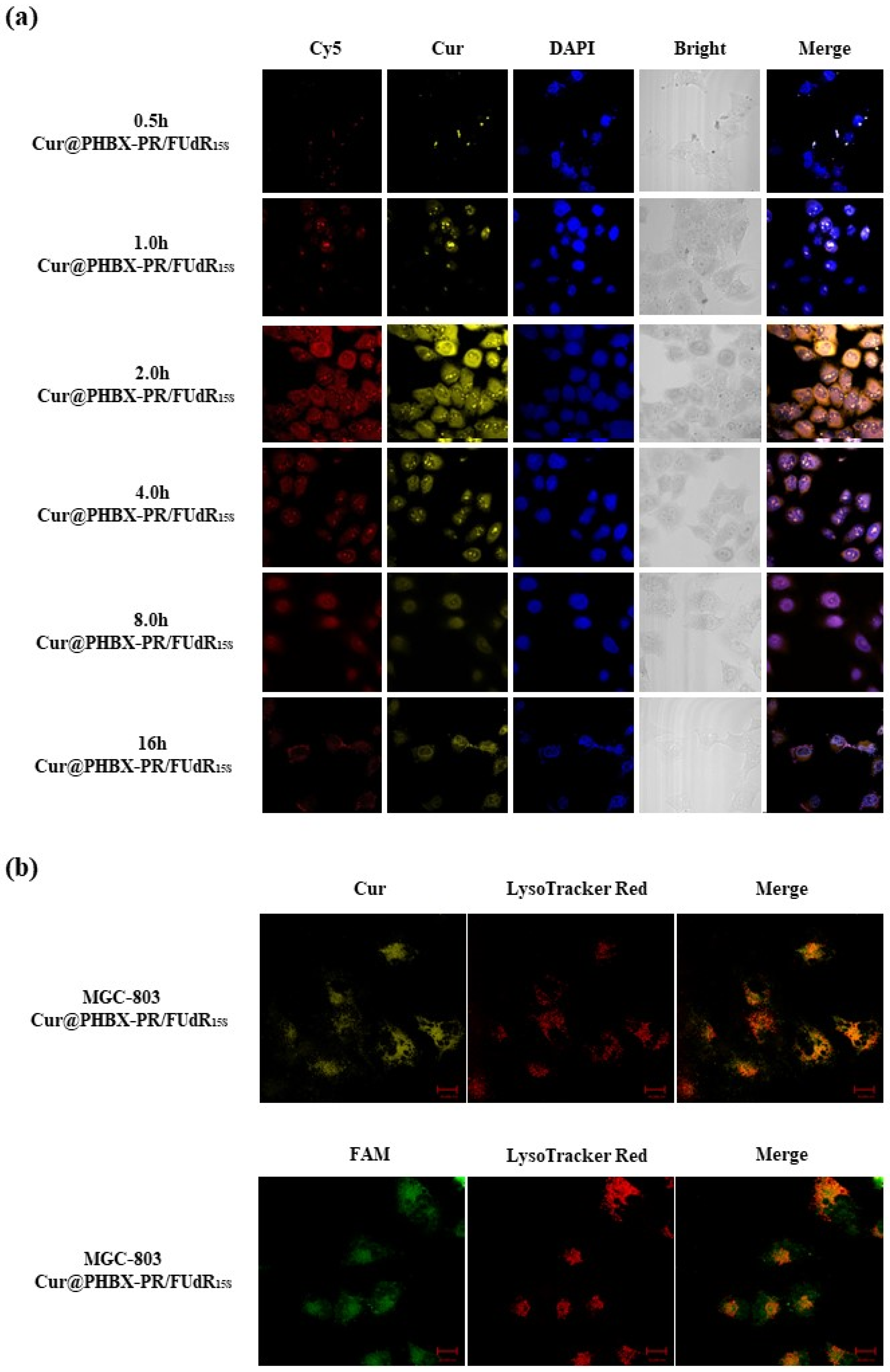
2.10. Evaluation of Cellular Uptake
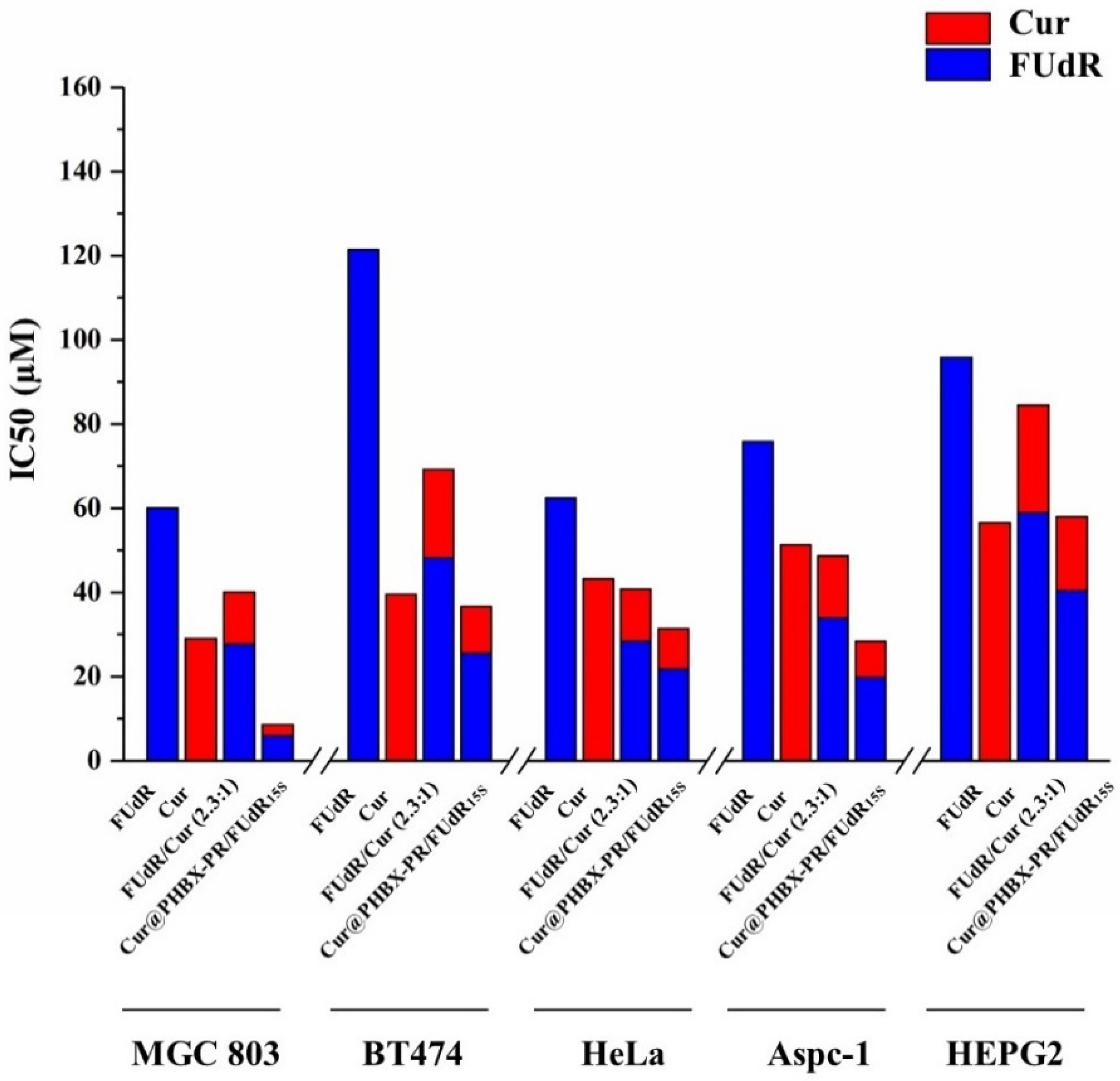
2.11. In Vitro Cytotoxicity
2.12. Western Blot Analysis
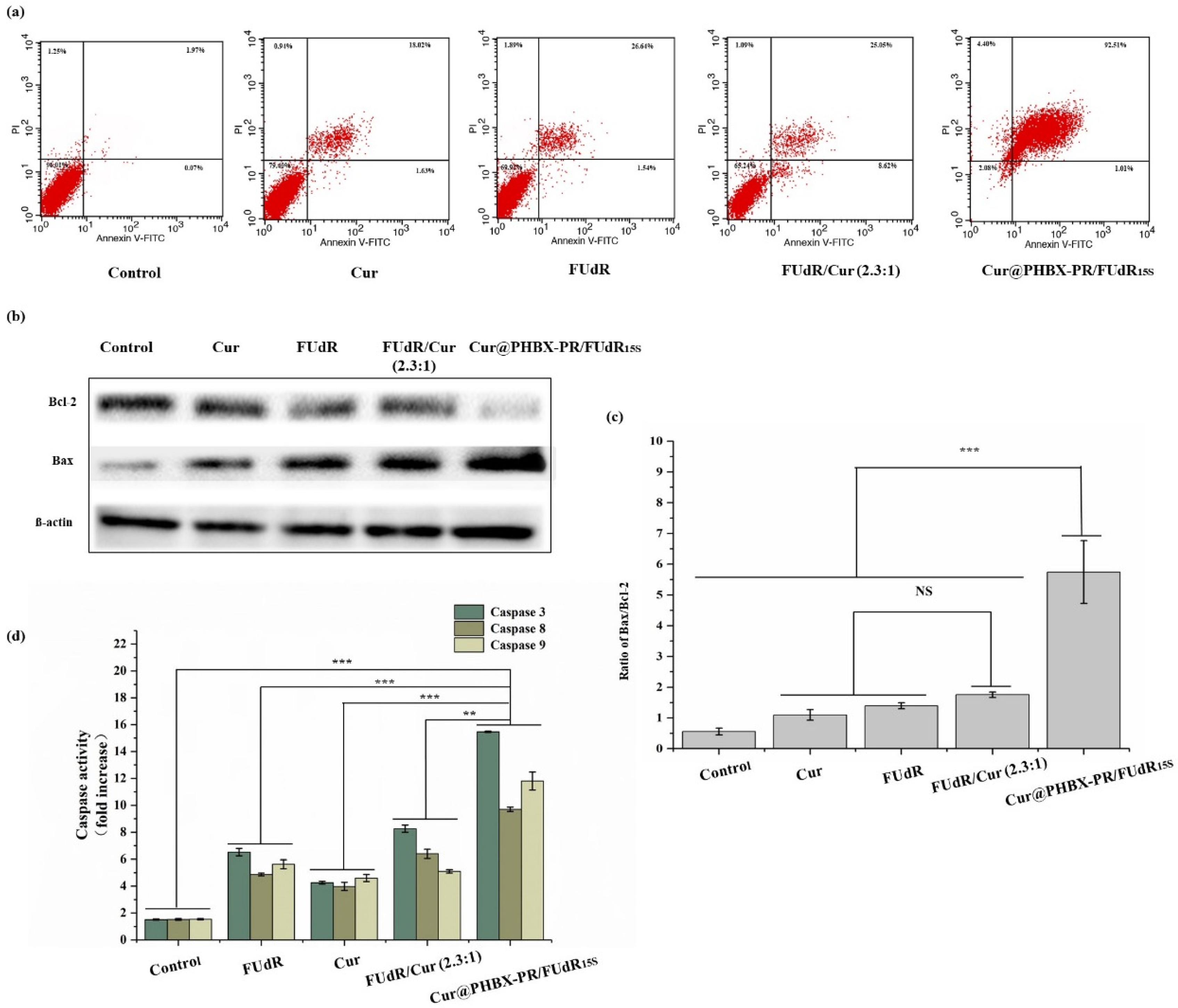
2.13. Caspase Activity Assays
2.14. Statistical Analysis
3. Results and Discussion
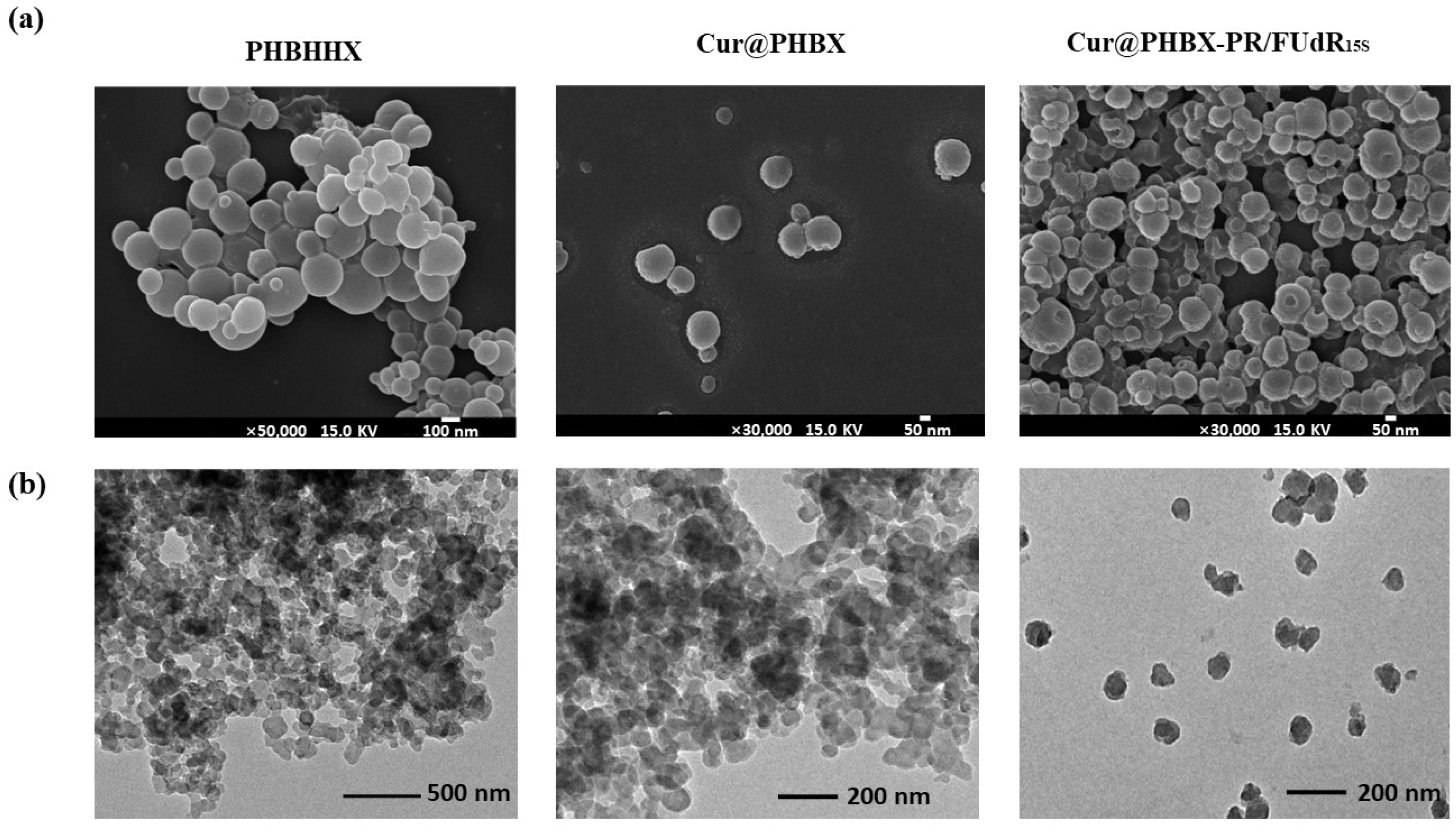
3.1. Preparation and Characterization of Cationic Peptide-Coated PHA Nanosphere
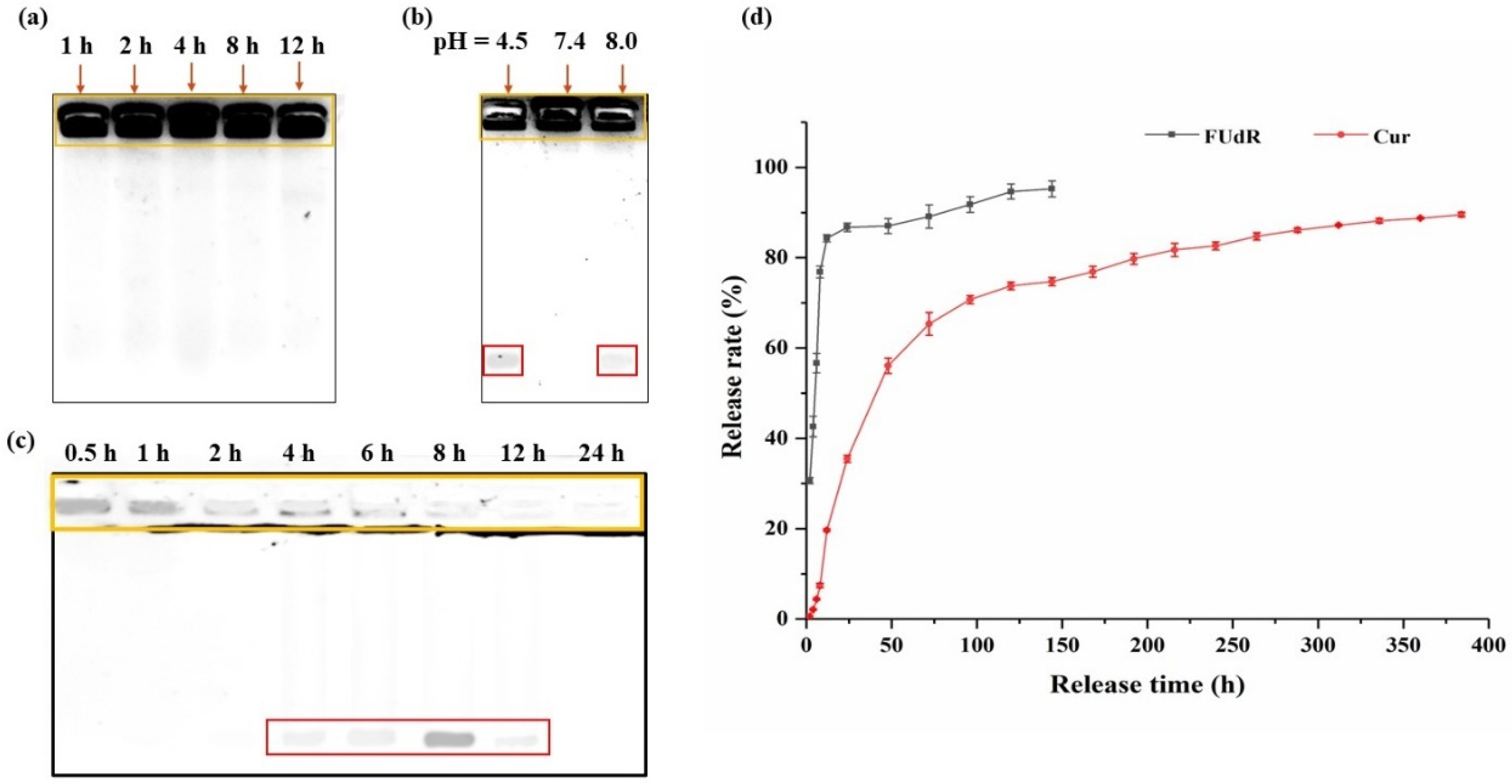
3.2. Analysis of Stability of Cur@PHBX-PR/FUdR15S
3.3. In Vitro Drug Release
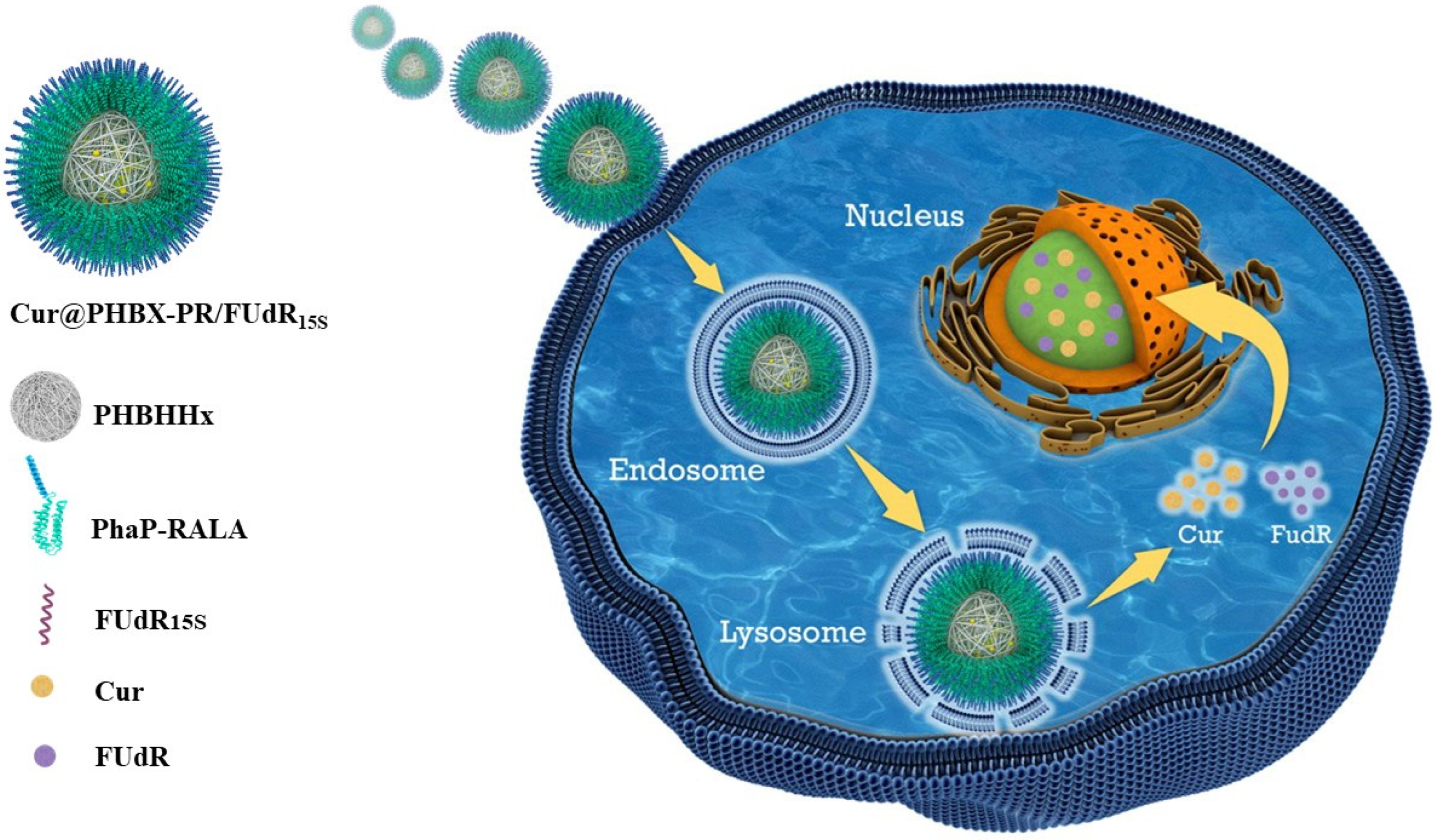
3.4. Cellular Uptake
3.5. Studies on Cytotoxicity and Synergistic Effect
3.6. Exploration of Anticancer Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2015, 98, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; de la Torre, L.G. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2020, 9, 1208–1237. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-mediated drug delivery systems for anticancer agents: An overview and perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Kashanian, S. Novel amphiphilic chitosan nanocarriers for sustained oral delivery of hydrophobic drugs. Eur. J. Pharm. Sci. 2017, 99, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Pérez, M.; Cieza, R.J.; Ngo, B.K.D.; Grunlan, M.A.; Domenech, M. Amphiphilic silicones to reduce the absorption of small hydrophobic molecules. Acta Biomater. 2021, 121, 339–348. [Google Scholar] [CrossRef]
- Son, I.; Lee, Y.; Baek, J.; Park, M.; Han, D.; Min, S.K.; Lee, D.; Kim, B.S. pH-Responsive amphiphilic polyether micelles with superior stability for smart drug delivery. Biomacromolecules 2021, 22, 2043–2056. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Duong, H.T.T.; Tran, P.-T.; Thambi, T.; Ho, D.-K.; Murgia, X. Self-assembled amphiphilic starch based drug delivery platform: Synthesis, preparation, and interactions with biological barriers. Biomacromolecules 2021, 22, 572–585. [Google Scholar] [CrossRef]
- Li, W.-M.; Su, C.-W.; Chen, Y.-W.; Chen, S.-Y. In situ DOX-calcium phosphate mineralized CPT-amphiphilic gelatin nanoparticle for intracellular controlled sequential release of multiple drugs. Acta Biomater. 2015, 15, 191–199. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Niu, D.; Zhang, J.; Zhang, W.; Yao, Y.; Li, P.; Gong, J. Amphiphilic core shell nanoparticles containing dense polyethyleneimine shells for efficient delivery of microRNA to Kupffer cells. Int. J. Nanomed. 2016, 11, 2785–2797. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef] [PubMed]
- Panday, R.; Poudel, A.J.; Li, X.; Adhikari, M.; Ullah, M.W.; Yang, G. Amphiphilic core-shell nanoparticles: Synthesis, biophysical properties, and applications. Colloids Surf. B Biointerfaces 2018, 172, 68–81. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Kourilova, X.; Pernicova, I.; Sedlar, K.; Musilova, J.; Sedlacek, P.; Kalina, M.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates (PHA) by a thermophilic strain of Schlegelella thermodepolymerans from xylose rich substrates. Bioresour. Technol. 2020, 315, 123885. [Google Scholar] [CrossRef]
- Peng, K.; Wu, C.; Wei, G.; Jiang, J.; Zhang, Z.; Sun, X. Implantable sandwich PHBHHx film for burst-free controlled delivery of thymopentin peptide. Acta Pharm. Sin. B 2018, 8, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Sun, X.; Gong, T.; Wu, C.-Y.; Zhang, T.; Tan, J.; Zhang, Z.-R. Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater. 2013, 9, 5063–5069. [Google Scholar] [CrossRef]
- You, M.; Peng, G.; Li, J.; Ma, P.; Wang, Z.; Shu, W.; Peng, S.; Chen, G.-Q. Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials 2011, 32, 2305–2313. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Chen, J.; Zhang, J.; Yao, Y.; Chen, G.-Q. A novel self-cleaving phasin tag for purification of recombinant proteins based on hydrophobic polyhydroxyalkanoate nanoparticles. Lab Chip 2008, 8, 1957–1962. [Google Scholar] [CrossRef]
- Yao, Y.C.; Zhan, X.Y.; Zhang, J.; Zou, X.H.; Wang, Z.H.; Xiong, Y.C.; Chen, J.; Chen, G.Q. A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused with targeted cell ligands. Biomaterials 2008, 29, 4823–4830. [Google Scholar] [CrossRef]
- Li, M.C.; Liu, Q.Q.; Lu, X.Y.; Zhang, Y.L.; Wang, L.L. Heterologous expression of human costimulatory molecule B7-2 and construction of B7-2 immobilized polyhydroxyalkanoate nanoparticles for use as an immune activation agent. BMC Biotechnol. 2012, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Fominaya, J.; Gasset, M.; García, R.; Roncal, F.; Albar, J.P.; Bernad, A. An optimized amphiphilic cationic peptide as an efficient non-viral gene delivery vector. J. Gene Med. 2000, 2, 455–464. [Google Scholar] [CrossRef]
- McCarthy, H.O.; McCaffrey, J.; McCrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.H.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release 2014, 189, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Yakkundi, A.; McKeen, H.D.; McClements, L.; McKeogh, T.J.; McCrudden, C.M.; Arthur, K.; Robson, T.; McCarthy, H.O. RALA-mediated delivery of FKBPL nucleic acid therapeutics. Nanomedicine 2015, 10, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; McErlean, E.M.; Dunne, N.J.; Kett, V.L.; Coulter, J.A.; Robson, T.; McCarthy, H.O. Gene therapy with RALA/iNOS composite nanoparticles significantly enhances survival in a model of metastatic prostate cancer. Cancer Nanotechnol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Xu, C.F.; Chen, G.J.; Luo, Y.L.; Zhang, Y.; Zhao, G.; Lu, Z.D.; Czarna, A.; Gu, Z.; Wang, J. Rational designs of in vivo CRISPR-Cas delivery systems. Adv. Drug Deliv. Rev. 2021, 168, 3–29. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Han, M.; Wang, X.; Du, J.; Zhang, H.; Li, W. Co-delivery of 5-fluorodeoxyuridine and doxorubicin via gold nanoparticle equipped with affibody-DNA hybrid strands for targeted synergistic chemotherapy of HER2 overexpressing breast cancer. Sci. Rep. 2020, 10, 22015. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, J.; Zhang, C.; Han, M.; Wang, X.; Fu, S.; Du, J.; Zhang, H.; Li, W. Affibody-conjugated RALA polymers delivering oligomeric 5-Fluorodeoxyuridine for targeted therapy of HER2 overexpressing gastric cancer. Macromol. Biosci. 2020, 20, 2000083. [Google Scholar] [CrossRef]
- Zhang, C.; Han, M.; Zhang, F.; Yang, X.; Du, J.; Zhang, H.; Li, W.; Chen, S. Enhancing antitumor efficacy of nucleoside analog 5-fluorodeoxyuridine on HER2-overexpressing breast cancer by affibody-engineered DNA nanoparticle. Int. J. Nanomed. 2020, 15, 885–900. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Kulkarni, A.R.; Aminabhavi, T.M. Blend microspheres of poly(3-hydroxybutyrate) and cellulose acetate phthalate for colon delivery of 5-fluorouracil. Ind. Eng. Chem. Res. 2011, 50, 10414–10423. [Google Scholar] [CrossRef]
- Ravichandiran, V.; Masilamani, K.; Senthilnathan, B.; Maheshwaran, A.; Wong, T.W.; Roy, P. Quercetin-decorated curcumin liposome design for cancer therapy: In-vitro and in-vivo studies. Curr. Drug Deliv. 2017, 14, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, S.; Zhang, F.; Han, M.; Wang, X.; Du, J.; Zhang, H.; Li, W. Affibody modified G-quadruplex DNA micelles incorporating polymeric 5-Fluorodeoxyuridine for targeted delivery of curcumin to enhance synergetic therapy of HER2 positive gastric cancer. Nanomaterials 2022, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, H.; Han, M.; Yang, X.; Pei, C.; Xu, Z.; Du, J.; Li, W.; Chen, S. DNA–affibody nanoparticle delivery system for cisplatin-based breast cancer chemotherapy. RSC Adv. 2019, 9, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug delivery system based on pH-sensitive biocompatible poly(2-vinyl pyridine)-b-poly(ethylene oxide) nanomicelles loaded with curcumin and 5-fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Ni, W.; Li, Z.; Liu, Z.; Ji, Y.; Wu, L.; Sun, S.; Jian, X.; Gao, X. Dual-targeting nanoparticles: Codelivery of curcumin and 5-fluorouracil for synergistic treatment of hepatocarcinoma. J. Pharm. Sci. 2019, 108, 1284–1295. [Google Scholar] [CrossRef]
- Masloub, S.M.; Elmalahy, M.H.; Sabry, D.; Mohamed, W.S.; Ahmed, S.H. Comparative evaluation of PLGA nanoparticle delivery system for 5-fluorouracil and curcumin on squamous cell carcinoma. Arch. Oral Biol. 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Gami, P.; Kundu, D.; Seera, S.D.K.; Banerjee, T. Chemically crosslinked xylan–β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 158, 18–31. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, P.; Cao, S.; Zhao, L. The combination of curcumin and 5-fluorouracil in cancer therapy. Arch. Pharmacal Res. 2018, 41, 1–13. [Google Scholar] [CrossRef]
- Guo, P.; Pi, C.; Zhao, S.; Fu, S.; Yang, H.; Zheng, X.; Zhang, X.; Zhao, L.; Wei, Y. Oral co-delivery nanoemulsion of 5-fluorouracil and curcumin for synergistic effects against liver cancer. Expert Opin. Drug Deliv. 2020, 17, 1473–1484. [Google Scholar] [CrossRef]
- Wang, D.; Yu, D.; Liu, X.; Wang, Q.; Chen, X.; Hu, X.; Wang, Q.; Jin, C.; Wen, L.; Zhang, L. Targeting laryngeal cancer cells with 5-fluorouracil and curcumin using mesoporous silica nanoparticles. Technol. Cancer Res. Treat. 2020, 19, 1533033820962114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine 2021, 85, 153547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Ge, X.; Zhang, F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019, 109, 726–733. [Google Scholar] [CrossRef] [PubMed]
- García-Sáez, A.J. The secrets of the Bcl-2 family. Cell Death Differ. 2012, 19, 1733–1740. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, C.; Fu, S.; Liu, H.; Han, M.; Fan, X.; Zhang, H.; Li, W. Amphiphilic Cationic Peptide-Coated PHA Nanosphere as an Efficient Vector for Multiple-Drug Delivery. Nanomaterials 2022, 12, 3024. https://doi.org/10.3390/nano12173024
Zhang F, Zhang C, Fu S, Liu H, Han M, Fan X, Zhang H, Li W. Amphiphilic Cationic Peptide-Coated PHA Nanosphere as an Efficient Vector for Multiple-Drug Delivery. Nanomaterials. 2022; 12(17):3024. https://doi.org/10.3390/nano12173024
Chicago/Turabian StyleZhang, Fanghua, Chao Zhang, Shuangqing Fu, Huandi Liu, Mengnan Han, Xueyu Fan, Honglei Zhang, and Wei Li. 2022. "Amphiphilic Cationic Peptide-Coated PHA Nanosphere as an Efficient Vector for Multiple-Drug Delivery" Nanomaterials 12, no. 17: 3024. https://doi.org/10.3390/nano12173024
APA StyleZhang, F., Zhang, C., Fu, S., Liu, H., Han, M., Fan, X., Zhang, H., & Li, W. (2022). Amphiphilic Cationic Peptide-Coated PHA Nanosphere as an Efficient Vector for Multiple-Drug Delivery. Nanomaterials, 12(17), 3024. https://doi.org/10.3390/nano12173024





