Efficient Activation of Peroxymonosulfate by Biochar-Loaded Zero-Valent Copper for Enrofloxacin Degradation: Singlet Oxygen-Dominated Oxidation Process
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CuC
2.3. Jar Experiments
2.4. Determination of PMS Concentration
2.5. Characterization Methods
2.6. Analytical Methods
3. Result and Discussions
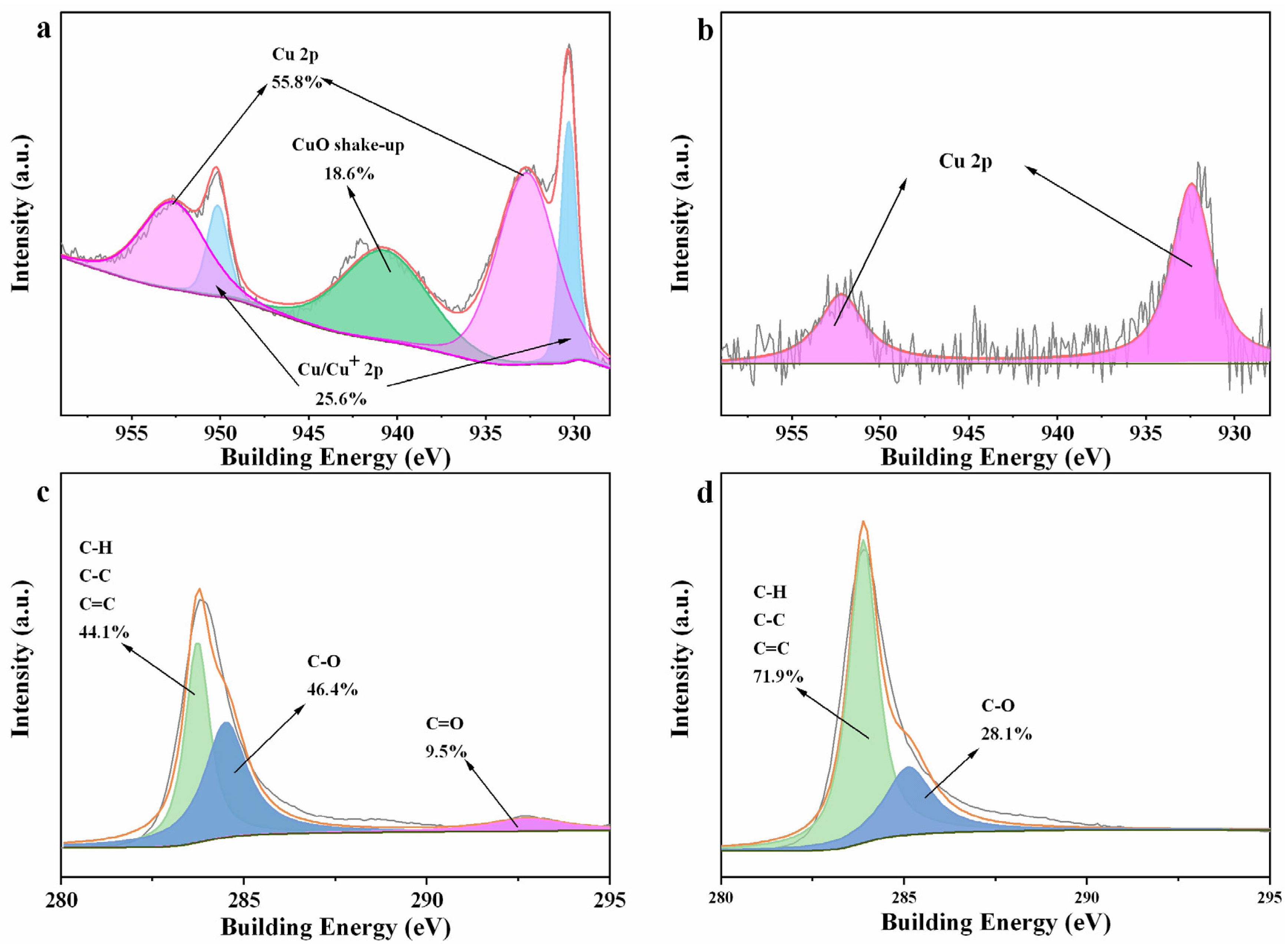
3.1. Characterization of the Samples
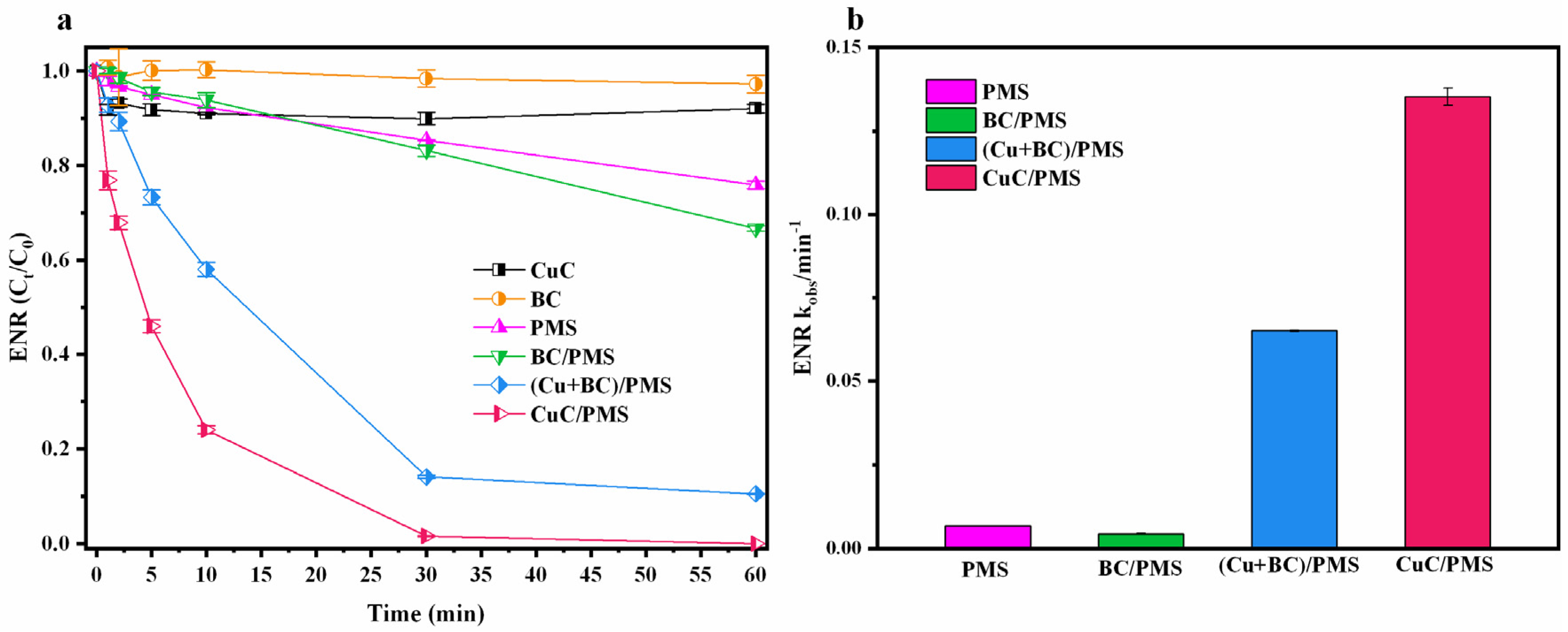
3.2. Degradation of ENR in Different Systems
3.3. Degradation of ENR by the CuC/PMS System under Various Experimental Conditions
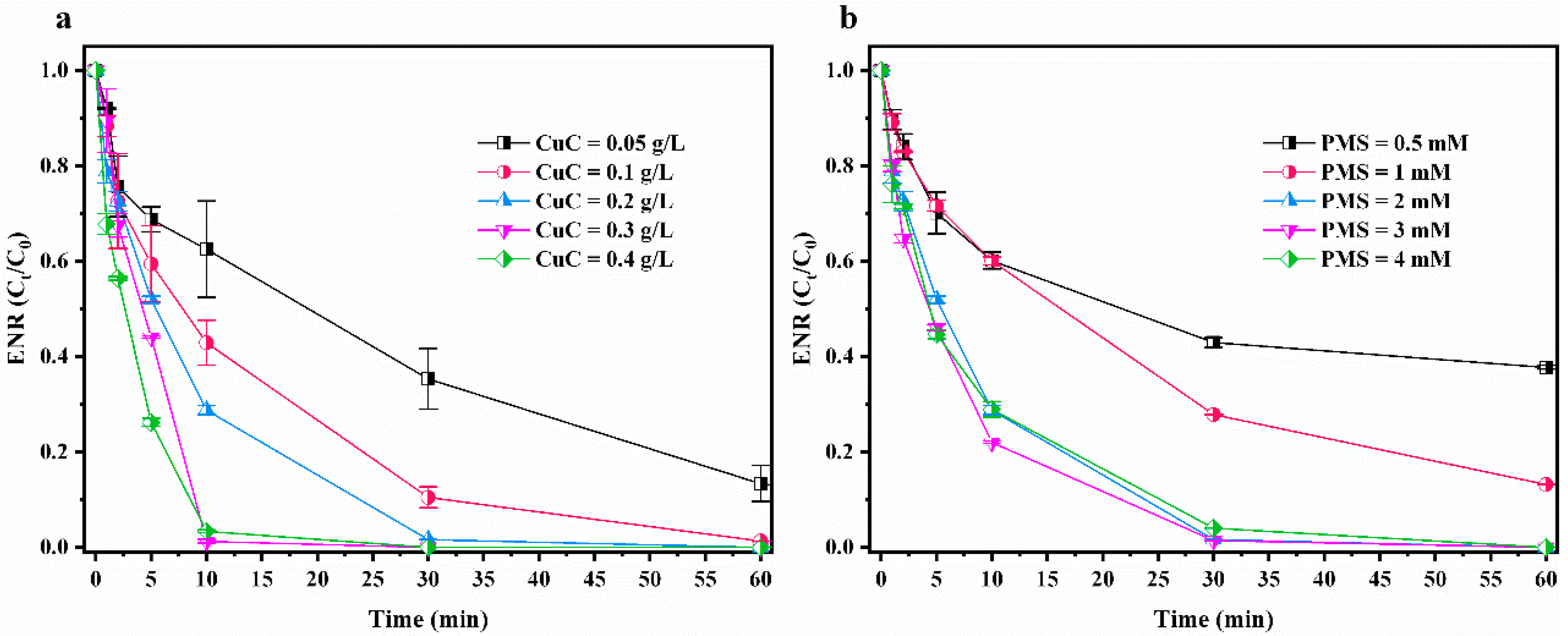
3.3.1. CuC and PMS Dosage
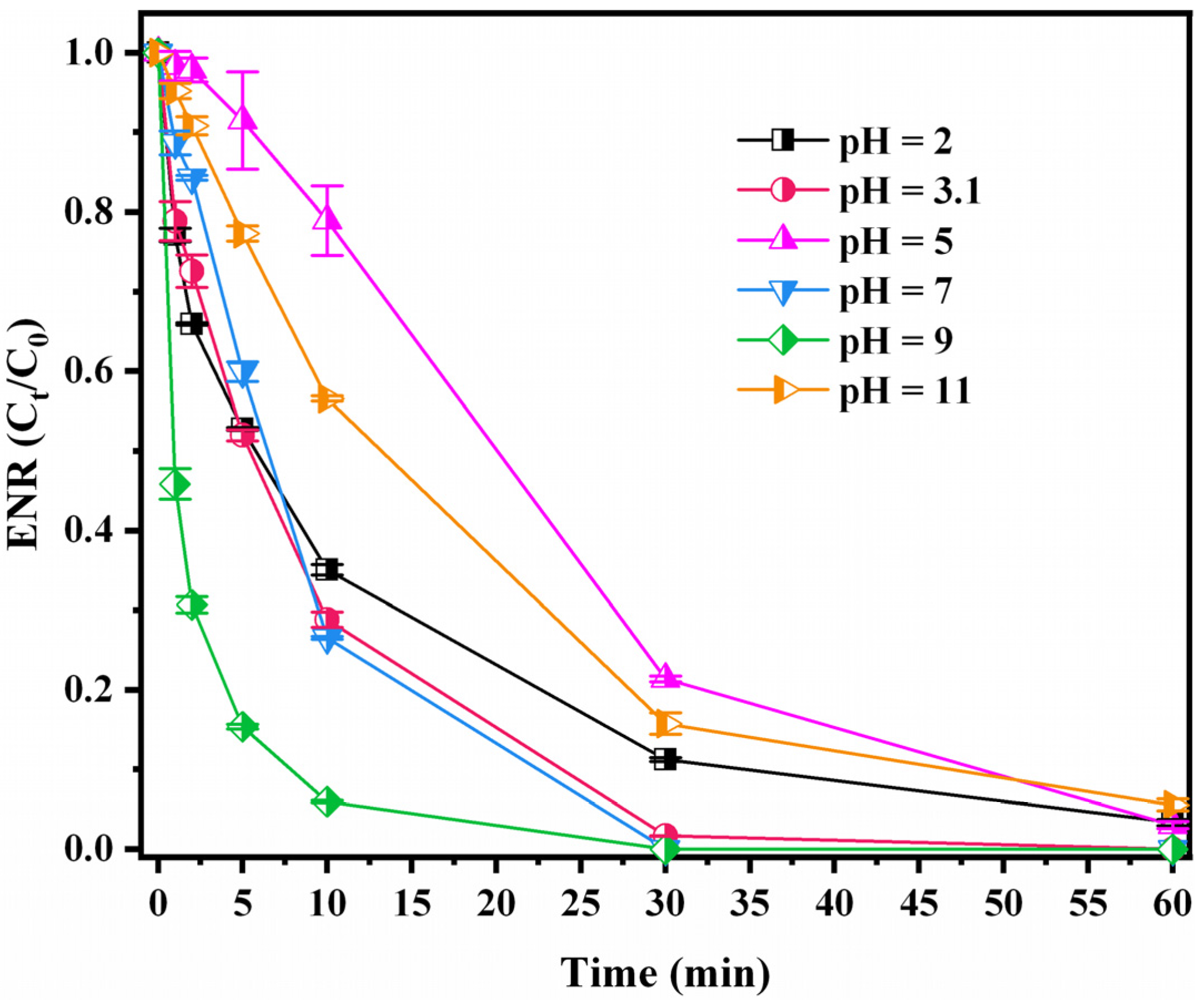
3.3.2. pH
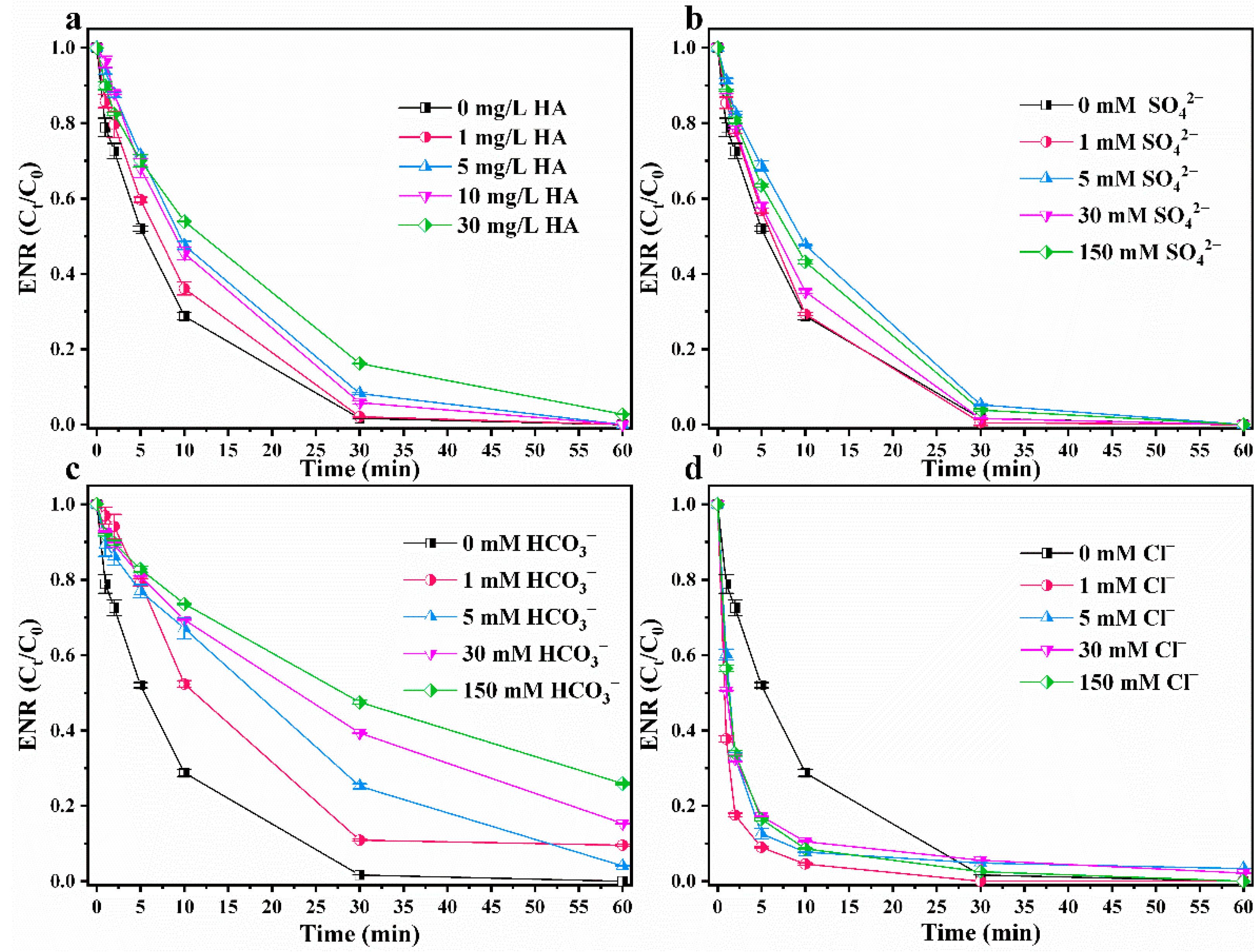
3.3.3. Coexisting Ions and Humic Acid
3.4. Mechanism Study
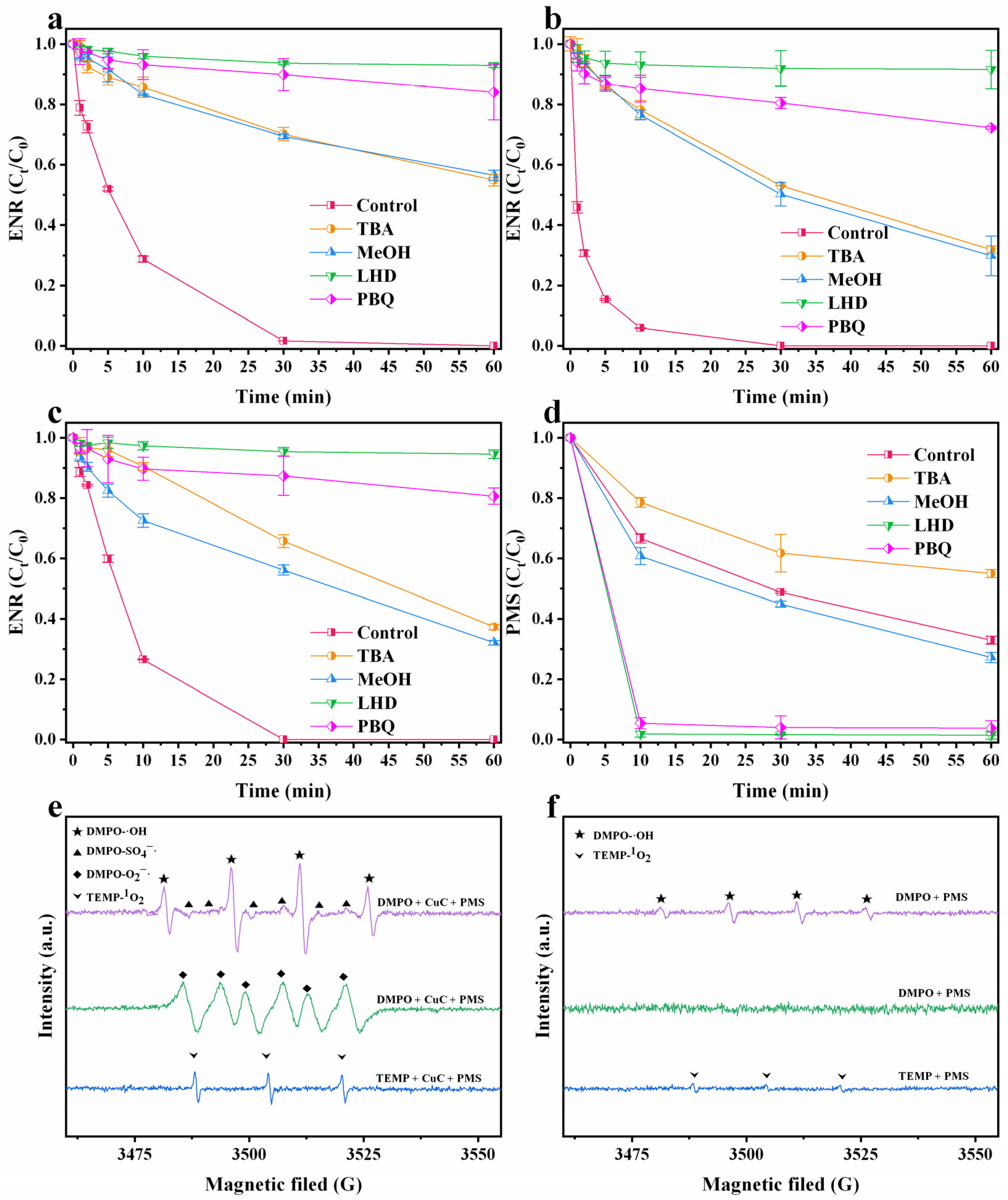
3.4.1. Identification of the Reactive Oxygen Species
3.4.2. Activation Process
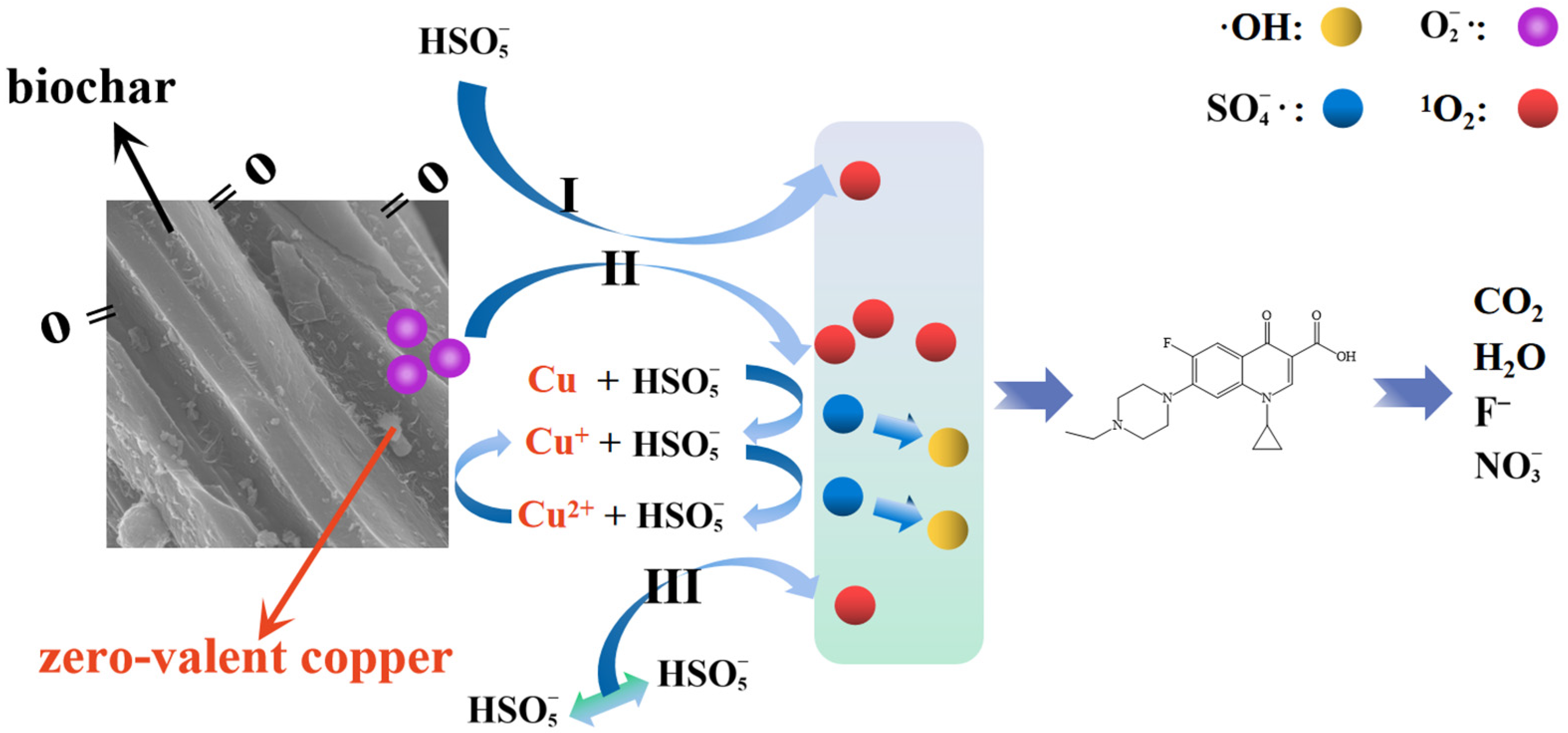
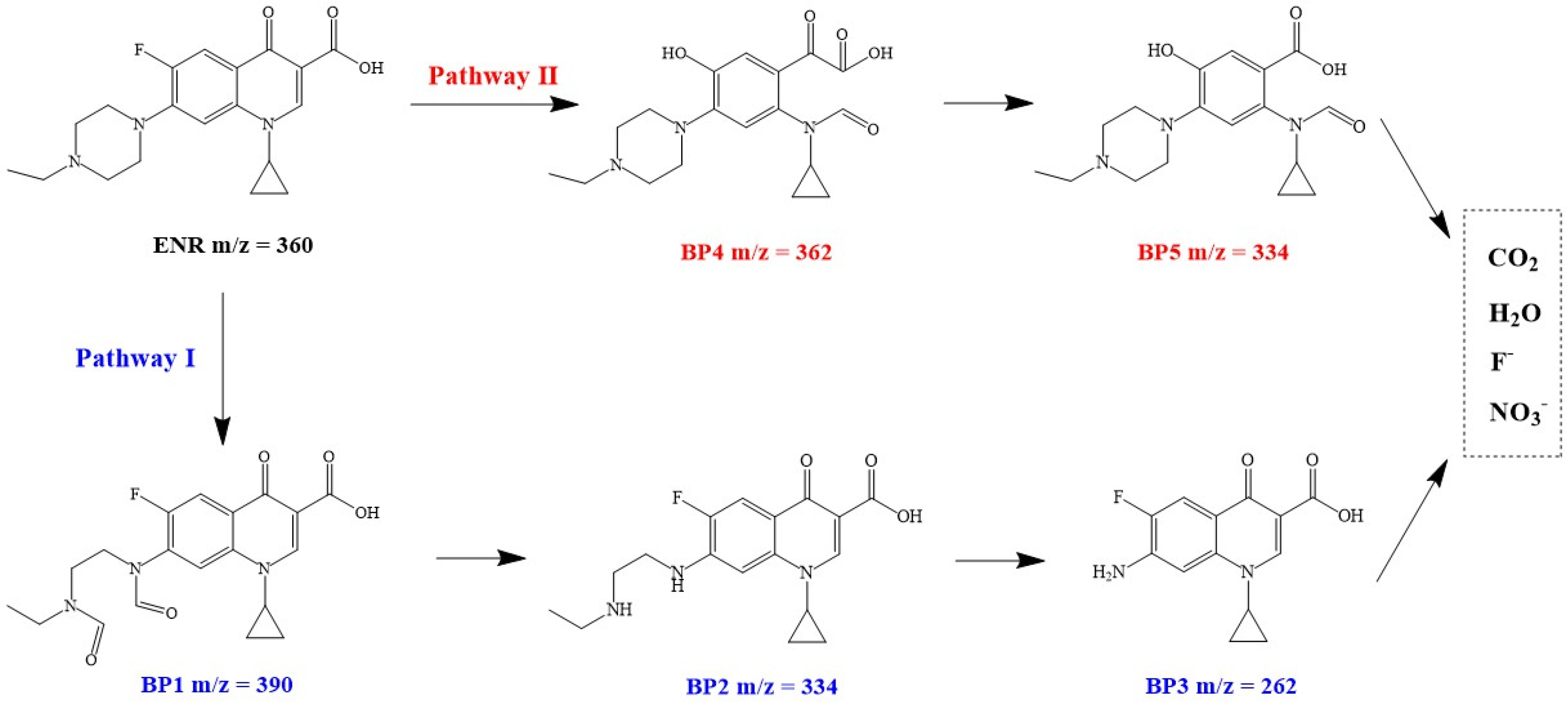
3.5. Proposed ENR Degradation Mechanism in CuC/PMS System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Santos, D.; Flores, E.M.M.; Ince, N.H. Pharmaceuticals and personal care products (PPCPs): Environmental and public health risks. Environ. Prog. Sustain. Energy 2022, 41, e13821. [Google Scholar] [CrossRef]
- Badawy, S.; Yang, Y.Q.; Liu, Y.N.; Marawan, M.A.; Ares, I.; Martinez, M.A.; Martinez-Larranaga, M.R.; Wang, X.; Anadon, A.; Martinez, M. Toxicity induced by ciprofloxacin and enrofloxacin: Oxidative stress and metabolism. Crit. Rev. Toxicol. 2021, 51, 754–787. [Google Scholar] [CrossRef] [PubMed]
- Rusch, M.; Spielmeyer, A.; Zorn, H.; Hamscher, G. Degradation and transformation of fluoroquinolones by microorganisms with special emphasis on ciprofloxacin. Appl. Microbiol. Biotechnol. 2019, 103, 6933–6948. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.L.; Shen, S.Z.; Zhou, J.; Ding, G.Y.; Zhang, K.Q. Systematic analysis of occurrence, density and ecological risks of 45 veterinary antibiotics: Focused on family livestock farms in Erhai Lake basin, Yunnan, China. Environ. Pollut. 2020, 267, 115539. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Giang, C.N.D.; Sebesvari, Z.; Renaud, F.; Rosendahl, I.; Minh, Q.H.; Amelung, W. Occurrence and dissipation of the antibiotics sulfamethoxazole, sulfadiazine, trimethoprim, and enrofloxacin in the Mekong Delta, Vietnam. PLoS ONE 2015, 10, e0131855. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.Y.; Li, P.; Zang, Y.G.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef]
- Wang, Q.J.; Mo, C.H.; Li, Y.W.; Gao, P.; Tai, Y.P.; Zhang, Y.; Ruan, Z.L.; Xu, J.W. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao. Environ. Pollut. 2010, 158, 2350–2358. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.G.; Wang, Q.D.; Li, X.G.; Guo, Y.X.; Song, W.; Li, Y.F. Ball milling boosted the activation of peroxymonosulfate by biochar for tetracycline removal. J. Environ. Chem. Eng. 2021, 9, 106870. [Google Scholar] [CrossRef]
- Sonawane, S.; Rayaroth, M.P.; Landge, V.K.; Fedorov, K.; Boczkaj, G. Thermally activated persulfate-based Advanced Oxidation Processes—Recent progress and challenges in mineralization of persistent organic chemicals: A review. Curr. Opin. Chem. Eng. 2022, 37, 100839. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Matta, R.; Tlili, S.; Chiron, S.; Barbati, S. Removal of carbamazepine from urban wastewater by sulfate radical oxidation. Environ. Chem. Lett. 2011, 9, 347–353. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, R.; Guo, Y.; Xiao, D.; Cao, Y.; Wang, Z.; Liu, J. Sulfate radical-induced degradation of 2,4,6-trichlorophenol: A de novo formation of chlorinated compounds. Chem. Eng. J. 2013, 217, 169–173. [Google Scholar] [CrossRef]
- Nfodzo, P.; Choi, H. Triclosan decomposition by sulfate radicals: Effects of oxidant and metal doses. Chem. Eng. J. 2011, 174, 629–634. [Google Scholar] [CrossRef]
- Zheng, H.; Bao, J.G.; Huang, Y.; Xiang, L.J.; Faheem; Ren, B.X.; Du, J.K.; Nadagouda, M.N.; Dionysiou, D.D. Efficient degradation of atrazine with porous sulfurized Fe2O3 as catalyst for peroxymonosulfate activation. Appl. Catal. B 2019, 259, 118056. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.H.; Wu, D.L.; Zhou, Z.Y.; Deng, Y.; Zhang, T.; Shih, K.M. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Chi, H.; He, X.; Zhang, J.; Ma, J. Efficient degradation of refractory organic contaminants by zero-valent copper/hydroxylamine/peroxymonosulfate process. Chemosphere 2019, 237, 124431. [Google Scholar] [CrossRef]
- Al-Shamsi, M.A.; Thomson, N.R. Treatment of organic compounds by activated persulfate using nanoscale zerovalent iron. Ind. Eng. Chem. Res. 2013, 52, 13564–13571. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Lee, C.-S.; Aravind, U.K.; Aravindakumar, C.T.; Chang, Y.-S. Oxidative degradation of benzoic acid using Fe0- and sulfidized Fe0-activated persulfate: A comparative study. Chem. Eng. J. 2017, 315, 426–436. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Ma, J.; Pang, S.; Zhang, T. A review on advanced oxidation processes homogeneously initiated by copper(II). Chem. Eng. J. 2022, 427, 131721. [Google Scholar] [CrossRef]
- Chi, H.; Wang, Z.; He, X.; Zhang, J.; Wang, D.; Ma, J. Activation of peroxymonosulfate system by copper-based catalyst for degradation of naproxen: Mechanisms and pathways. Chemosphere 2019, 228, 54–64. [Google Scholar] [CrossRef]
- Shi, J.T.; Dai, B.R.; Fang, X.Y.; Xu, L.J.; Wu, Y.; Lu, H.Q.; Cui, J.Q.; Han, S.G.; Gan, L. Waste preserved wood derived biochar catalyst for promoted peroxymonosulfate activation towards bisphenol A degradation with low metal ion release: The insight into the mechanisms. Sci. Total Environ. 2022, 813, 152673. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Zhang, Y.; Zhang, G.; Li, W.; Wei, C.; Liang, J.; Liu, Y.; Shu, S. Degradation of 2,4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper. J. Hazard. Mater. 2018, 344, 1209–1219. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, B.; Zhang, J.; Zhang, Y.L.; Zhang, G.C.; Wei, C.M.; Liang, J.; Liu, Y.; Zhang, W. Radicals induced from peroxomonosulfate by nanoscale zero-valent copper in the acidic solution. Water Sci. Technol. 2016, 74, 1946–1952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niu, X.Y.; Yang, Q.N.; Wang, Z.; Zhu, Y.J.; Zhang, C.; Liang, P.; Zhang, Z.H. Dealloyed nanoporous copper as a highly active catalyst in Fenton-like reaction for degradation of organic pollutants. Chem. Eng. J. 2022, 431, 133834. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Guo, J.; Zhou, L.; Lan, Y.; Chen, C. MOFs-derived magnetic C@Cu–Ni bimetal particles: An efficient peroxymonosulfate activator for 2,4,6-trichlorophenol degradation. Chemosphere 2021, 269, 129394. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yin, K.; Li, N.; Liu, H.; Chen, J.; Zhou, X.; Zhang, Y. Efficient activation of peroxymonosulfate by copper supported on polyurethane foam for contaminant degradation: Synergistic effect and mechanism. Chem. Eng. J. 2022, 427, 131741. [Google Scholar] [CrossRef]
- Reinsch, H. “Green” Synthesis of Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4290–4299. [Google Scholar] [CrossRef]
- Batten, M.P.; Rubio-Martinez, M.; Hadley, T.; Carey, K.-C.; Lim, K.-S.; Polyzos, A.; Hill, M.R. Continuous flow production of metal-organic frameworks. Curr. Opin. Chem. Eng. 2015, 8, 55–59. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.R.; Zhou, Q.X. Waterborne isocyanate-free polyurethane epoxy hybrid coatings synthesized from sustainable fatty acid diamine. Green Chem. 2020, 22, 1329–1337. [Google Scholar] [CrossRef]
- Wang, S.X.; Gao, S.S.; Tian, J.Y.; Wang, Q.; Wang, T.Y.; Hao, X.J.; Cui, F.Y. A stable and easily prepared copper oxide catalyst for degradation of organic pollutants by peroxymonosulfate activation. J. Hazard. Mater. 2020, 387, 121995. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q.; et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Liang, C.J.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Zeng, G.M.; Wang, X.; Hu, X.J.; Gu, Y.L.; Yang, Z.Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Graouer-Bacart, M.; Sayen, S.; Guillon, E. Macroscopic and molecular approaches of enrofloxacin retention in soils in presence of Cu(II). J. Colloid Interface Sci. 2013, 408, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, T.; Zhou, Y.; Fang, L.; Shao, Y. Degradation of atenolol by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism. Chemosphere 2013, 93, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl. Catal. B 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Faheem; Du, J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role. Environ. Sci. Pollut. Res. 2020, 27, 37312. [Google Scholar]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Brandt, C.; van Eldik, R. Transition metal-catalyzed oxidation of Sulfur(IV) oxides. atmospheric-relevant processes and mechanisms. Chem. Rev. 1995, 95, 119–190. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef]
- Das, T.N.; Huie, R.E.; Neta, P. Reduction potentials of SO3•-, SO5•-, and S4O6•3− radicals in aqueous solution. J. Phys. Chem. A 1999, 103, 3581–3588. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Selective degradation of sulfonamide antibiotics by peroxymonosulfate alone: Direct oxidation and nonradical mechanisms. Chem. Eng. J. 2018, 334, 2539–2546. [Google Scholar] [CrossRef]
- Qin, J.; Dai, L.; Shi, P.; Fan, J.; Min, Y.; Xu, Q. Rational design of efficient metal-free catalysts for peroxymonosulfate activation: Selective degradation of organic contaminants via a dual nonradical reaction pathway. J. Hazard. Mater. 2020, 398, 122808. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, J. Nitrogen-doped graphene as peroxymonosulfate activator and electron transfer mediator for the enhanced degradation of sulfamethoxazole. Chem. Eng. J. 2019, 375, 122041. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Rivas, F.J.; Solís, R.R. Chloride promoted oxidation of tritosulfuron by peroxymonosulfate. Chem. Eng. J. 2018, 349, 728–736. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-I.; Weon, S.; Choi, W.; Hwang, Y.S.; Seo, J.; Lee, C.; Kim, J.-H. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds. Environ. Sci. Technol. 2016, 50, 10134–10142. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Liu, M.; Zhao, X. Insights into heterogeneous catalytic activation of peroxymonosulfate by Pd/g-C3N4: The role of superoxide radical and singlet oxygen. Catal. Commun. 2017, 102, 85–88. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Liu, J.; Chen, M.; Ahmed, Z.; Zhu, N. Efficient removal of bisphenol A by superoxide radical and singlet oxygen generated from peroxymonosulfate activated with Fe0-montmorillonite. Chem. Eng. J. 2018, 350, 484–495. [Google Scholar] [CrossRef]
- Liu, X.R.; Liu, Y.; Qin, H.H.; Ye, Z.W.; Wei, X.J.; Miao, W.; Yang, D.H.; Mao, S. Selective removal of phenolic compounds by peroxydisulfate activation: Inherent role of hydrophobicity and interface ROS. Environ. Sci. Technol. 2022, 56, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Yan, J.; Qian, L.; Chen, Y.; Han, L.; Su, A.; Zhang, W.; Ni, H.; Chen, M. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 2017, 184, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Huang, W.; Wei, X.; Huang, W. Biochar supported CuO composites used as an efficient peroxymonosulfate activator for highly saline organic wastewater treatment. Sci. Total Environ. 2020, 721, 137764. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.; Ma, Y.; Xing, S. Enhanced superoxide radical production for ofloxacin removal via persulfate activation with Cu-Fe oxide. Chem. Eng. J. 2018, 354, 473–480. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Devaraj, M.; Saravanan, R.; Deivasigamani, R.; Gupta, V.K.; Gracia, F.; Jayadevan, S. Fabrication of novel shape Cu and Cu/Cu2O nanoparticles modified electrode for the determination of dopamine and paracetamol. J. Mol. Liq. 2016, 221, 930–941. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.Q.; Wang, C.H.; Zhang, M.; Khan, M.A.N.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J.; Li, J.S. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef]
- Mi, X.Y.; Wang, P.F.; Xu, S.Z.; Su, L.N.; Zhong, H.; Wang, H.T.; Li, Y.; Zhan, S.H. Almost 100% peroxymonosulfate conversion to singlet oxygen on single-atom CoN2+2 sites. Angew. Chem. Int. Ed. 2021, 60, 4588–4593. [Google Scholar] [CrossRef]
- Li, X.; Xiao, C.; Ruan, X.; Hu, Y.; Zhang, C.; Cheng, J.; Chen, Y. Enrofloxacin degradation in a heterogeneous electro-Fenton system using a tri-metal-carbon nanofibers composite cathode. Chem. Eng. J. 2022, 427, 130927. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: Possible degradation pathways, mineralization activity and an in depth mechanism insight. Appl. Catal. B 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment—unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
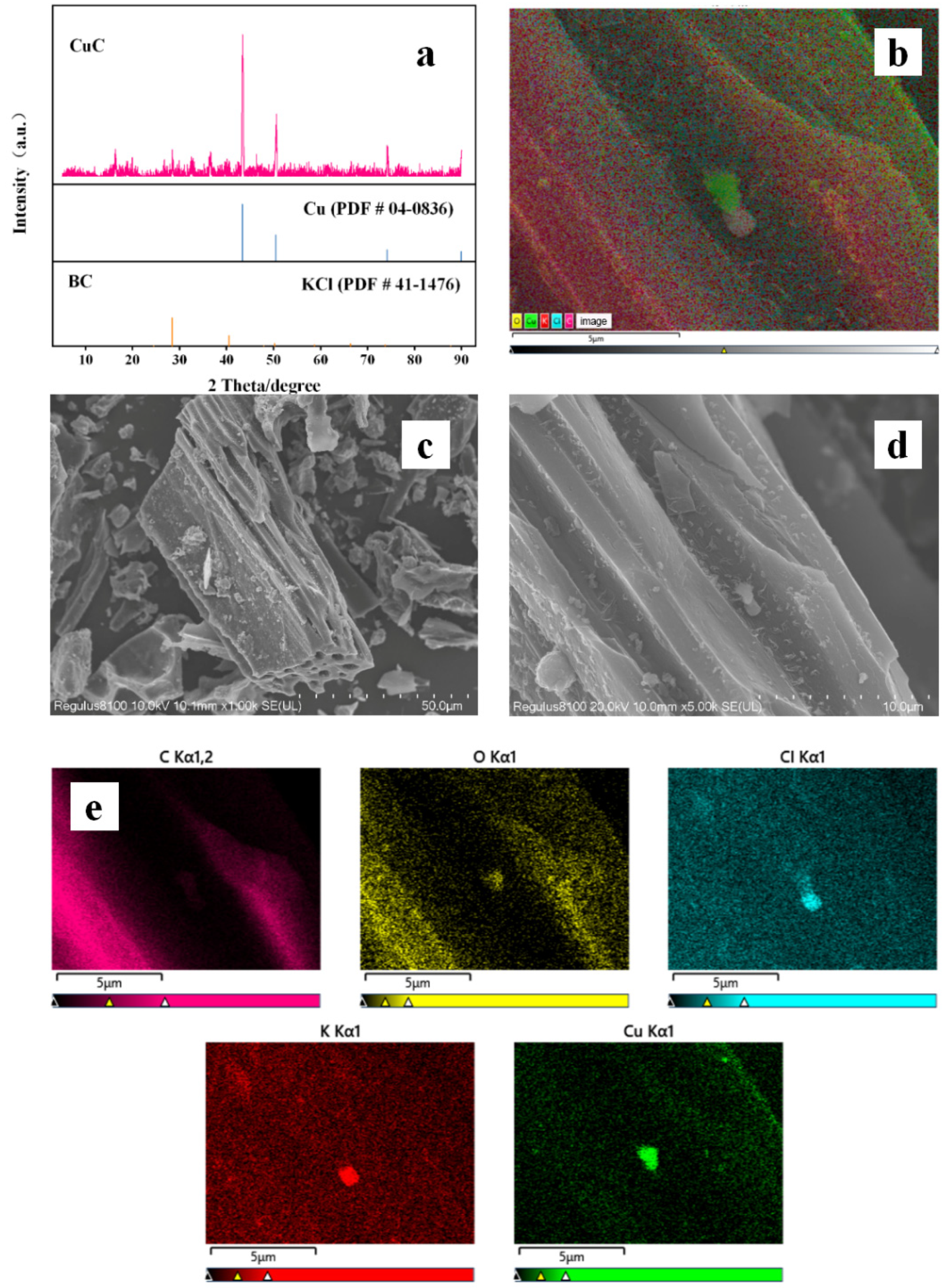
| EDS | |||||
|---|---|---|---|---|---|
| C | O | Cu | K | Cl | |
| Fresh CuC | 83.6 | 6.9 | 2.4 | 3.1 | 4.1 |
| Used CuC | 88.6 | 7.8 | 0.5 | 0.6 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Chen, T.; Hou, C.; Huang, B.; Du, J.; Liu, N.; Zhou, X.; Zhang, Y. Efficient Activation of Peroxymonosulfate by Biochar-Loaded Zero-Valent Copper for Enrofloxacin Degradation: Singlet Oxygen-Dominated Oxidation Process. Nanomaterials 2022, 12, 2842. https://doi.org/10.3390/nano12162842
Zhao J, Chen T, Hou C, Huang B, Du J, Liu N, Zhou X, Zhang Y. Efficient Activation of Peroxymonosulfate by Biochar-Loaded Zero-Valent Copper for Enrofloxacin Degradation: Singlet Oxygen-Dominated Oxidation Process. Nanomaterials. 2022; 12(16):2842. https://doi.org/10.3390/nano12162842
Chicago/Turabian StyleZhao, Jiang, Tianyin Chen, Cheng Hou, Baorong Huang, Jiawen Du, Nengqian Liu, Xuefei Zhou, and Yalei Zhang. 2022. "Efficient Activation of Peroxymonosulfate by Biochar-Loaded Zero-Valent Copper for Enrofloxacin Degradation: Singlet Oxygen-Dominated Oxidation Process" Nanomaterials 12, no. 16: 2842. https://doi.org/10.3390/nano12162842
APA StyleZhao, J., Chen, T., Hou, C., Huang, B., Du, J., Liu, N., Zhou, X., & Zhang, Y. (2022). Efficient Activation of Peroxymonosulfate by Biochar-Loaded Zero-Valent Copper for Enrofloxacin Degradation: Singlet Oxygen-Dominated Oxidation Process. Nanomaterials, 12(16), 2842. https://doi.org/10.3390/nano12162842








