Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater
Abstract
1. Introduction
2. Experimental
2.1. Reagent and Chemicals
2.2. Instruments
2.3. Synthesis of Fe3O4@MWCNT
2.4. Synthesis of Fe3O4@MWCNTs-CdS Nanocomposite
2.5. Preparation of Samples
2.6. Magnetic Adsorption Procedure
3. Result and Discussion
3.1. Characterization
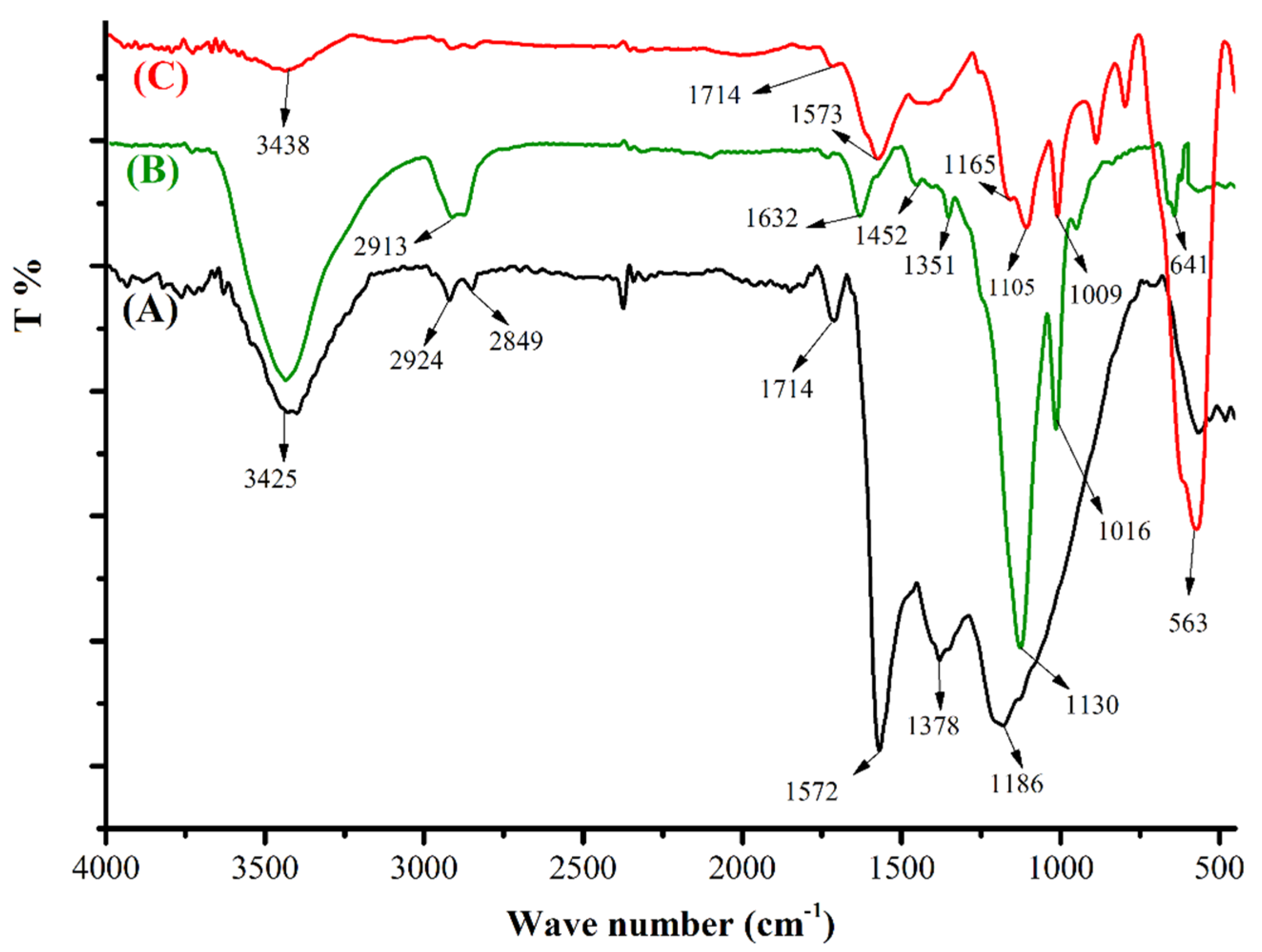
3.1.1. FTIR Spectroscopy
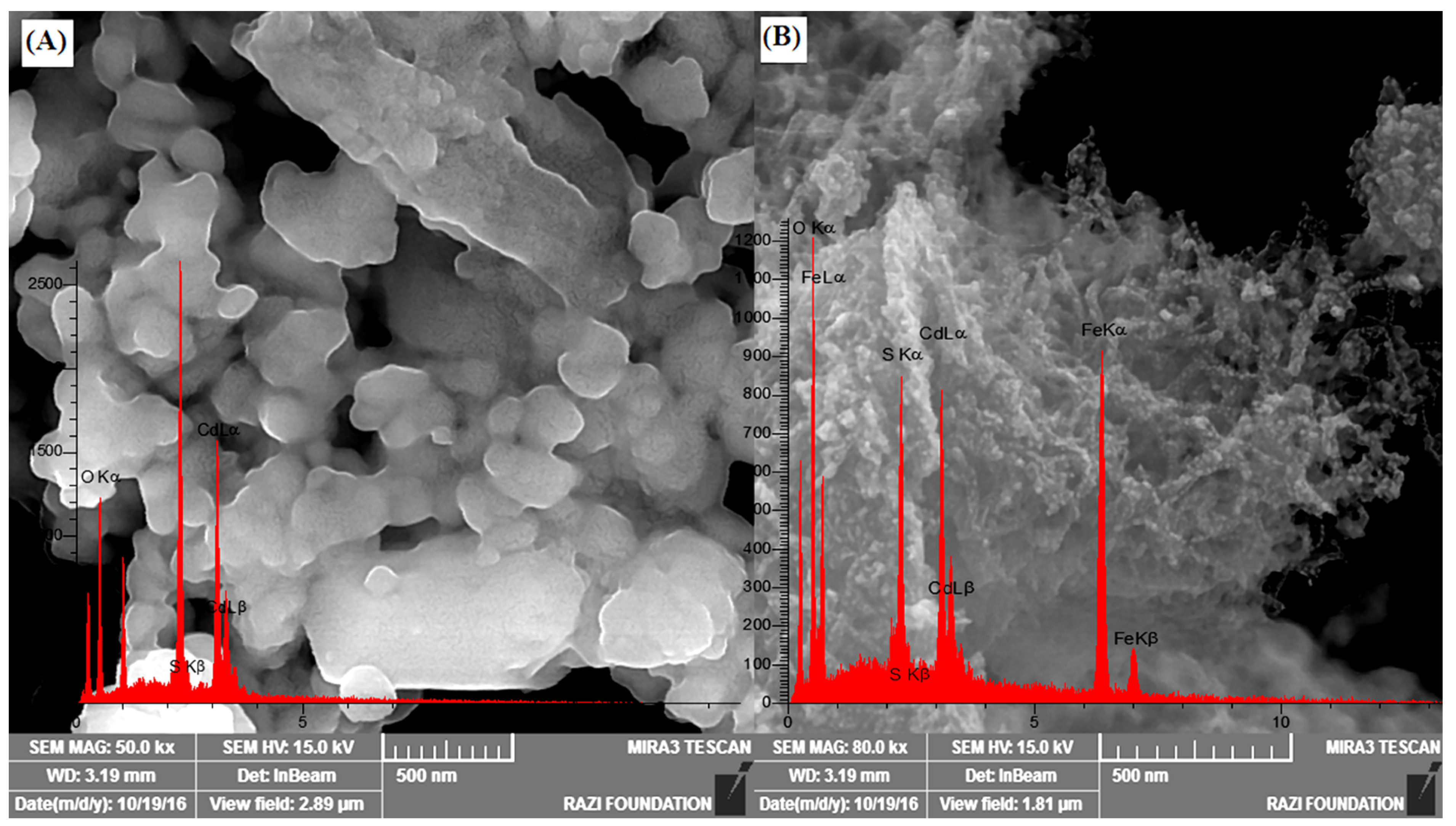
3.1.2. FESEM Microscopy
3.1.3. EDX Spectroscopy
3.2. Effective Parameters
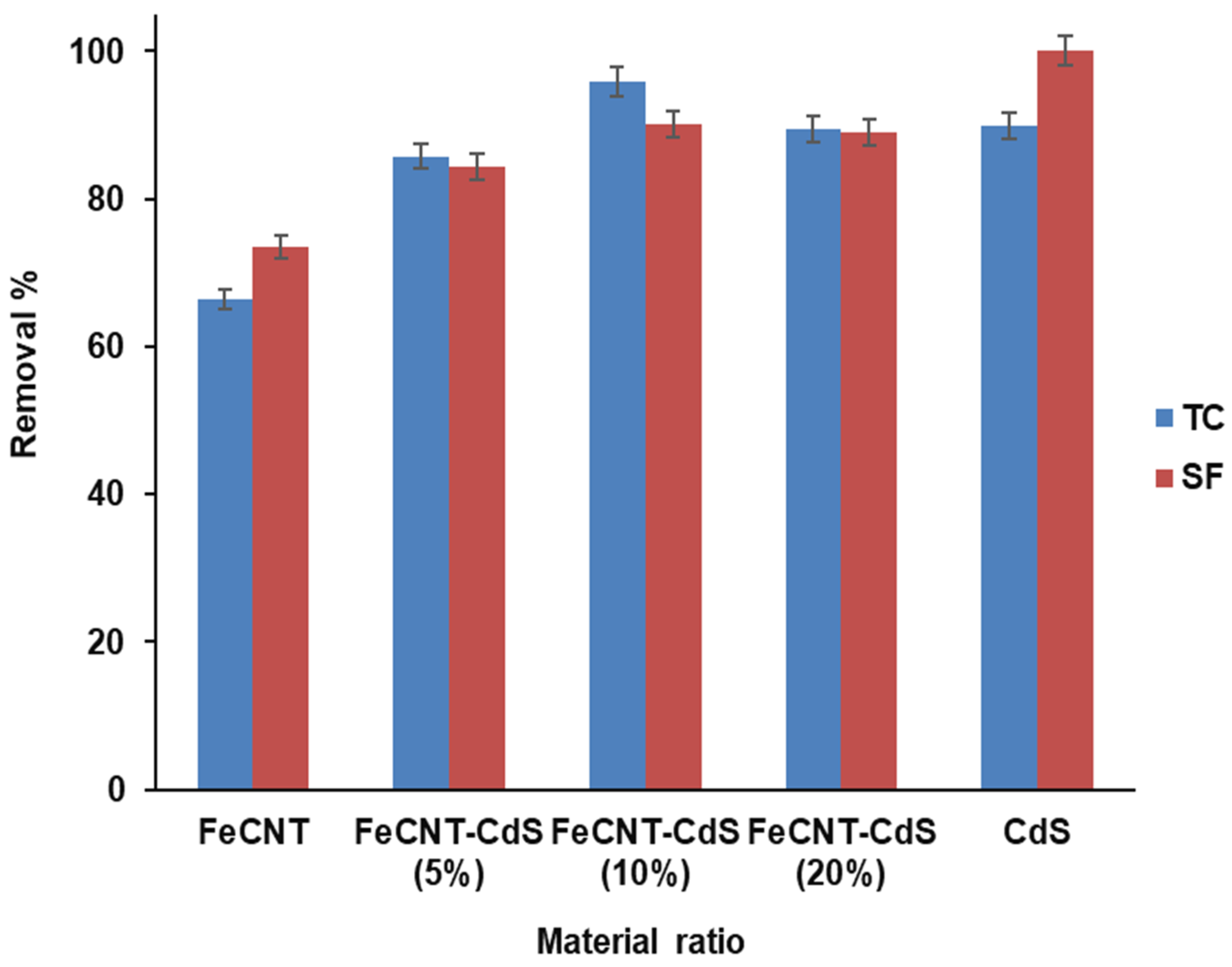
3.2.1. Material Ratio
3.2.2. Real Sample
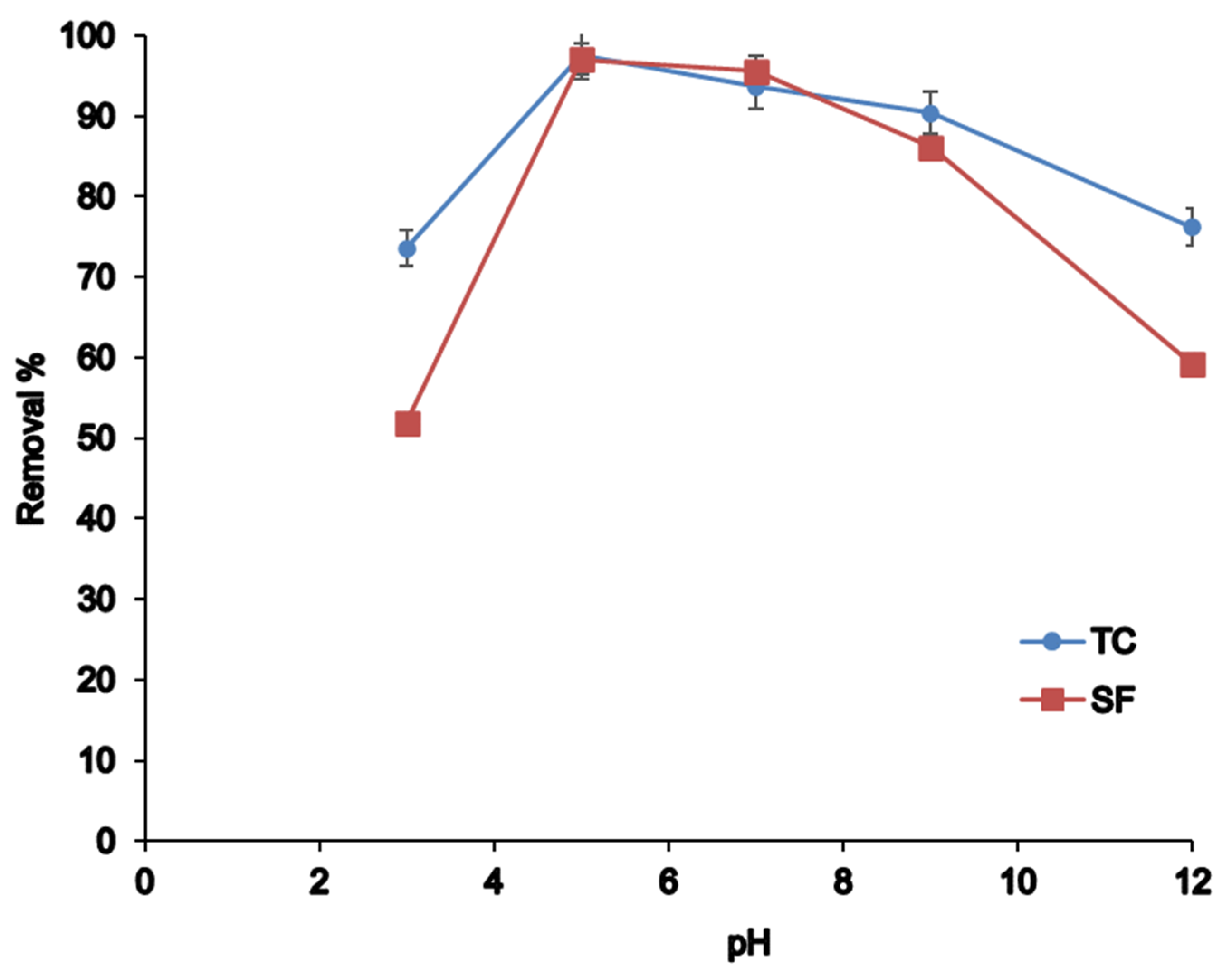
3.2.3. pH Effect
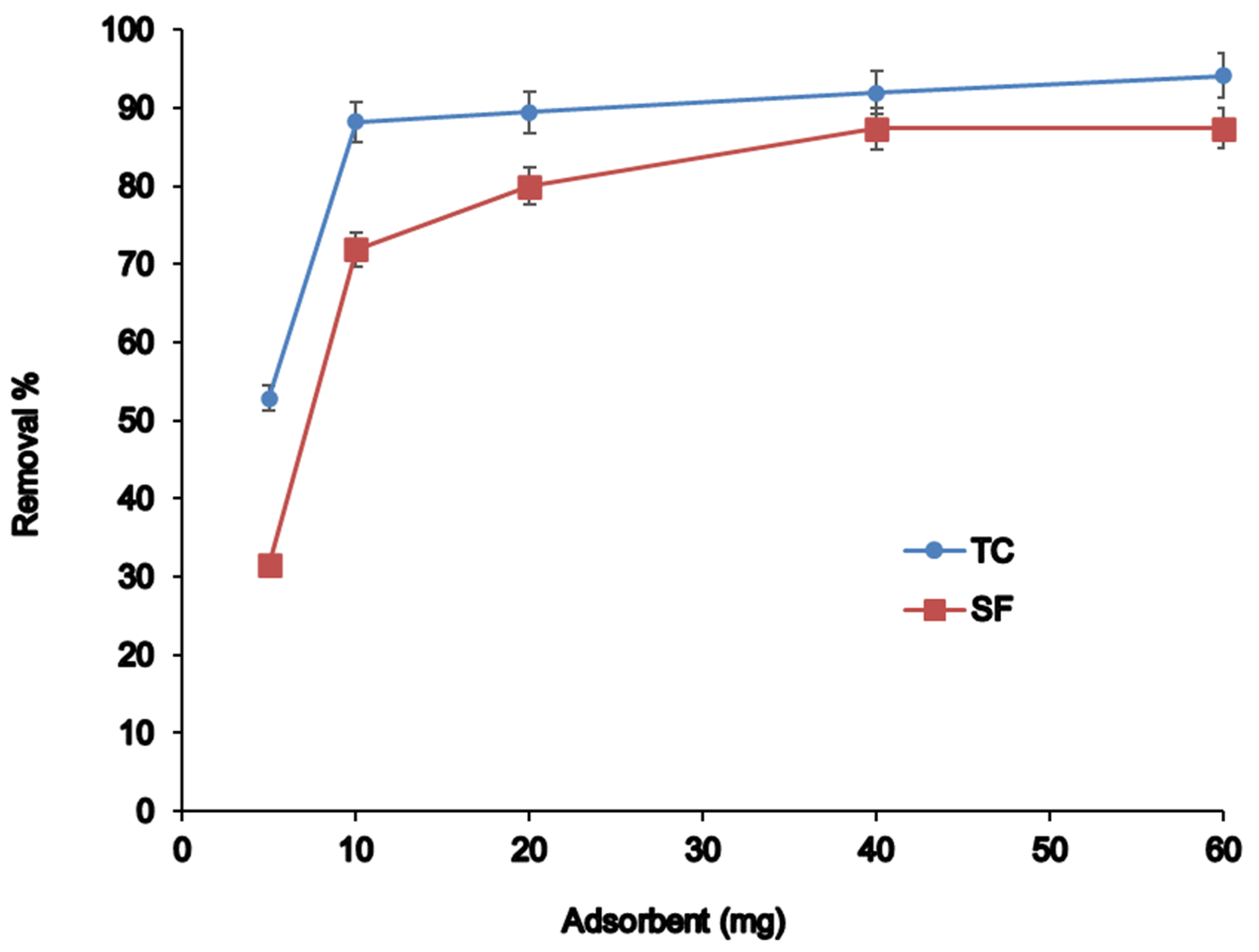
3.2.4. Effect of Adsorbent Amount
3.2.5. Salt Effect
3.2.6. Effect of Contact Time
3.3. Kinetic Study
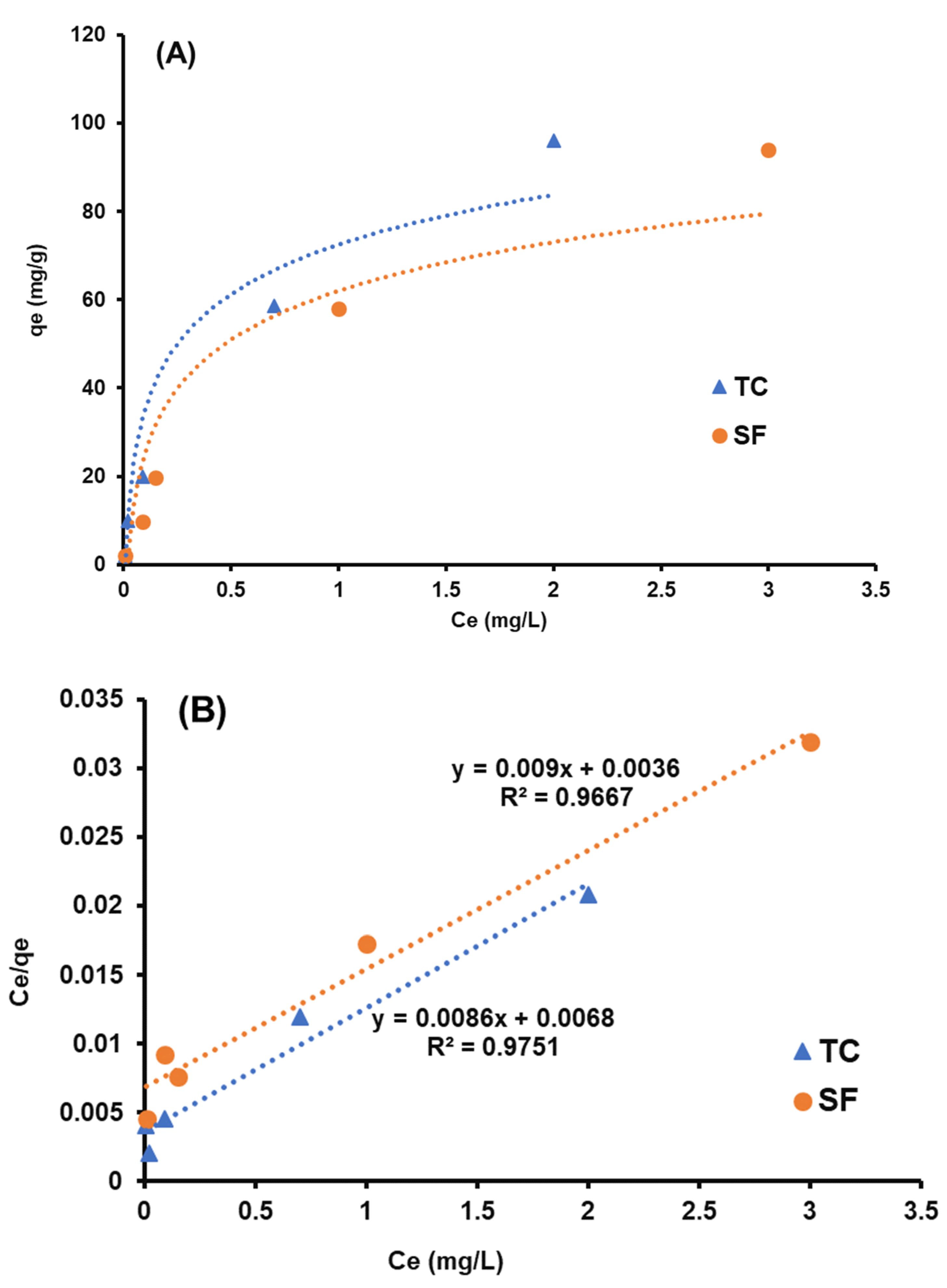
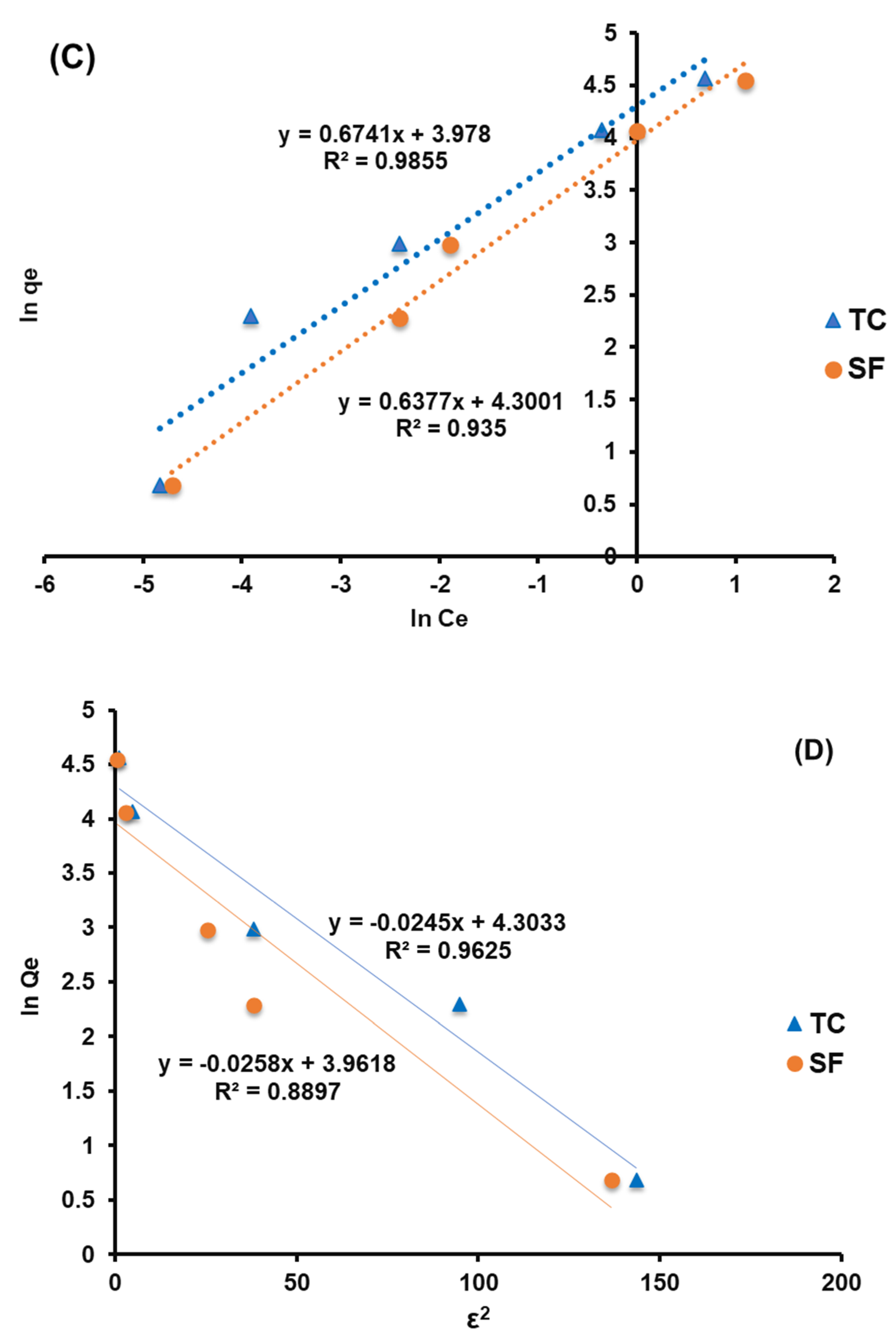
3.4. Isotherm Study
3.5. Effect of Temperature and Thermodynamic
3.6. Reusability and Stability
3.7. Adsorption Mechanism
3.8. Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Verawaty, M.; APRIANI, N.; TARIGAN, L.R.; APRIAN, E.T.R.I.; LAURENTA, W.C. Antibiotics resistant Escherichia coli isolated from aquatic ecosystems in Palembang, South Sumatra, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 86–97. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ-ment and human health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ma, Y.; Zhang, J.; Fan, N.; Huang, B.; Jin, R. Journal of Water Process Engineering A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-silva, S.; Segundo, M.A.; et al. AC SC. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Luo, Y.; Zhu, Y.; Lai, X.; Shang, J.; Chen, J.; Liao, Q. Chemosphere Effects of suspended particulate matter from natural lakes in conjunction with coagulation to tetracycline removal from water. Chemosphere 2021, 277, 130327. [Google Scholar] [CrossRef] [PubMed]
- Burch, K.D.; Han, B. Removal efficiency of commonly prescribed antibiotics via tertiary wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6301–6310. [Google Scholar] [CrossRef] [PubMed]
- Wan Ibrahim, W.A.; Rashidi Nodeh, H.; Aboul-Enein, H.Y.; Sanagi, M.M. Magnetic solid-phase extraction based on modified ferum oxides for enrichment, preconcentration, and isolation of pesticides and selected pollutants. Crit. Rev. Anal. Chem. 2015, 45, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Tan, Z.; Kuykendall, T.R.; Aloni, S.; Xun, S.; Lin, E.; Battaglia, V.; Zhang, Y. Fe3O4 nanoparticle-integrated graphene sheets for high-performance half and full lithium ion cells. Phys. Chem. Chem. Phys. 2011, 13, 7170–7177. [Google Scholar] [CrossRef]
- Ji, F.; Li, S.; Yang, H.; Wang, Z.; Li, A. Versatile Photocrosslinked Protein Hydrogel Matrix for Magnetic-Nanoparticle-Doping and Biomineralization. J. Nanosci. Nanotechnol. 2016, 16, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Naghiha, R.; Kheirizadeh, M.; Sadatfaraji, H.; Mirzaei, A.; Alvand, Z.M. Microwave assisted extraction as an efficient approach for biosynthesis of zinc oxide nanoparticles: Synthesis, characterization, and biological properties. Mater. Sci. Eng. C 2017, 78, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Cristea, C.; Tertis, M.; Galatus, R. Magnetic Nanoparticles for Antibiotics Detection. Nanomaterials 2017, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Xiao, C.; Chen, L.; Yang, P.; Liu, Q.; Wang, J.; Liu, X. Rapid Determination of Trace Sulfonamides in Milk by Graphene Oxide-Based Magnetic Solid Phase Extraction Coupled with HPLC–MS/MS. Food Anal. Methods 2016, 9, 2521–2530. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, L.; Wu, G.; Xia, Y.; Lu, W.; Li, J. Determination of six sulfonylurea herbicides in environmental water samples by magnetic solid-phase extraction using multi-walled carbon nanotubes as adsorbents coupled with high-performance liquid chromatography. J. Chromatogr. A 2016, 1466, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, F.; Zhang, X.; Wang, L.; Wang, C.; Zhao, Q.; Xu, Y.; Ding, L.; Ren, N. Determination of roxithromycin from human plasma samples based on magnetic surface molecularly imprinted polymers followed by liquid chromatography-tandem mass spectromer. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1021, 221–228. [Google Scholar] [CrossRef]
- Ding, J.; Gao, Q.; Li, X.S.; Huang, W.; Shi, Z.G.; Feng, Y.Q. Magnetic solid-phase extraction based on magnetic carbon nanotube for the determination of estrogens in milk. J. Sep. Sci. 2011, 34, 2498–2504. [Google Scholar] [CrossRef]
- Fakhri, A.; Shahidi, S.; Agarwal, S.; Gupta, V.K. Electrocatalytic Oxidation Behavior of Cefixime Antibiotic at Bimetallic Pt-W Nanoparticle-Decorated Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode and its Determination. Int. J. Electrochem. Sci. 2016, 11, 1530–1540. [Google Scholar]
- Foroutan, R.; Peighambardoust, S.J.; Esvandi, Z.; Khatooni, H.; Ramavandi, B. Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: A comparative study. J. Environ. Chem. Eng. 2021, 9, 104752. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.Z.; He, J.X.; Wang, S. Multiwalled carbon nanotubes as sorbent for on-line coupling of solid-phase extraction to high-performance liquid chromatography for simultaneous determination of 10 sulfonamides in eggs and pork. J. Chromatogr. A 2006, 1127, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Sun, H.-Z.; Zhang, Y.-G.; Lin, Y.-L.; Li, J.-L.; Wang, E.-K.; Yu, Y.; Tan, M.; Wang, J.-B. Preparation, characterization and photocatalytic properties of CdS nanoparticles dotted on the surface of carbon nanotubes. Nanotechnology 2008, 19, 115709. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, S.; Wang, S.; Liang, X.; Guo, Y. Cadmium sulfide nanoparticles as a novel coating for solid-phase microextraction. J. Sep. Sci. 2015, 38, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Dong, P.; Pu, L.; Tan, H.; Li, J. Cadmium sulfide nanoparticles with controllable morphology, photoluminescence and photocatalytic activity templated by worm-like dendronized poly(amido amine)s. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 25–35. [Google Scholar] [CrossRef]
- Maji, S.K.; Dutta, A.K.; Srivastava, D.N.; Paul, P.; Mondal, A.; Adhikary, B. Peroxidase-like behavior, amperometric biosensing of hydrogen peroxide and photocatalytic activity by cadmium sulfide nanoparticles. J. Mol. Catal. A Chem. 2012, 358, 1–9. [Google Scholar] [CrossRef]
- Lv, J.; Li, D.; Dai, K.; Liang, C.; Jiang, D.; Lu, L.; Zhu, G. Multi-walled carbon nanotube supported CdS-DETA nanocomposite for efficient visible light photocatalysis. Mater. Chem. Phys. 2017, 186, 372–381. [Google Scholar] [CrossRef]
- Ye, A.; Fan, W.; Zhang, Q.; Deng, W.; Wang, Y. CdS–graphene and CdS–CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal. Sci. Technol. 2012, 2, 969–978. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.C.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, R. Voltammetric determination of the endocrine disruptor diethylstilbestrol by using a glassy carbon electrode modified with a composite consisting of platinum nanoparticles and multiwalled carbon nanotubes. Microchim. Acta 2016, 183, 3069–3076. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, A.; Peng, F.; Yu, H.; Yang, J. Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II). J. Colloid Interface Sci. 2007, 316, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Hamadi, A.; Foroutan, R.; Peighambardoust, S.J.; Ramavandi, B. Decontamination of Cd2+ and Pb2+ from aqueous solution using a magnetic nanocomposite of eggshell/starch/Fe3O4. J. Water Process Eng. 2022, 48, 102911. [Google Scholar] [CrossRef]
- Alvand, Z.M.; Rajabi, H.R.; Mirzaei, A.; Masoumiasl, A.; Sadatfaraji, H. Rapid and green synthesis of cadmium telluride quantum dots with low toxicity based on a plant-mediated approach after microwave and ultrasonic assisted extraction: Synthesis, characterization, biological potentials and comparison study. Mater. Sci. Eng. C 2019, 98, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ren, H.; Huang, X.; Li, M.; Tang, Y.; Guo, F. Separation and Puri fi cation Technology Low cost red mud modi fi ed graphitic carbon nitride for the removal of organic pollutants in wastewater by the synergistic e ff ect of adsorption and photocatalysis. Sep. Purif. Technol. 2020, 237, 116477. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, Y.; Xu, K.; Li, N.; Zhang, H.; Yang, Q. A novel polymeric ionic liquid-coated magnetic multiwalled carbon nanotubes for the solid-phase extraction of Cu, Zn-superoxide dismutase. Anal. Chim. Acta 2016, 939, 54–63. [Google Scholar] [CrossRef]
- Sankhla, A.; Sharma, R.; Yadav, R.S.; Kashyap, D.; Kothari, S.L.; Kachhwaha, S. Biosynthesis and characterization of cadmium sulfide nanoparticles—An emphasis of zeta potential behavior due to capping. Mater. Chem. Phys. 2016, 170, 44–51. [Google Scholar] [CrossRef]
- Jia, S.; Yang, Z.; Yang, W.; Zhang, T.; Zhang, S.; Yang, X.; Dong, Y.; Wu, J.; Wang, Y. Removal of Cu(II) and tetracycline using an aromatic rings-functionalized chitosan-based flocculant: Enhanced interaction between the flocculant and the antibiotic. Chem. Eng. J. 2016, 283, 495–503. [Google Scholar] [CrossRef]
- Bidhendi, M.E.; Poursorkh, Z.; Sereshti, H.; Nodeh, H.R. Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study. Int. J. Environ. Res. Public Health 2020, 17, 4223. [Google Scholar] [CrossRef]
- Naghipour, D.; Amouei, A.; Ghasemi, K.T.; Taghavi, K. Removal of cefixime from aqueous solutions by the biosorbent prepared from pine cones: Kinetic and isotherm studies. Desalin. Water Treat. 2020, 201, 219–227. [Google Scholar] [CrossRef]
- Shivashankar, M.; Mandal, B.K. Design and Evaluation of Chitosan-Based Novel pH- Sensitive Drug Carrier for Sustained Release of Cefixime. Trop. J. Pharm. Res. 2013, 12, 155–161. [Google Scholar]
- Singh, K.; Mohan, M.; Nautiyal, S. Comparing Cefixime, Cefpodoxime and Ofloxacin as Anti-Microbial Agents Comparing Cefixime, Cefpodoxime and Ofloxacin as Anti-Microbial Agents and their Effects on Gut Microbiota. Int. J. Sci. Res. Sci. Technol. 2020, 131–152. [Google Scholar] [CrossRef]
- Duc, T.; Thuy, T.; Thuy, T.; Truong, T.; Ha, T.; Son, T.; Dung, V.; Yamaguchi, A.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of beta-lactam ce fi xime onto nanosilica fabricated from rice HUSK with surface modi fi cation by polyelectrolyte. J. Mol. Liq. 2019, 298, 111981. [Google Scholar] [CrossRef]
- Salah Uddin, G.M.; Saha, S.; Karmaker, S.; Saha, T.K. Adsorption of cefixime trihydrate onto chitosan 10b from aqueous solution: Kinetic, equilibrium and thermodynamic studies. Cellul. Chem. Technol. 2021, 55, 771–784. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Küçükyıldız, G.; Erim, B.; Alp, E. Easy preparation of magnetic nanoparticles-rGO-chitosan composite beads: Optimization study on cefixime removal based on RSM and ANN by using Genetic Algorithm Approach. J. Mol. Struct. 2021, 1224, 129182. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Latifi, P.; Ahmadi, A.; Alizadeh, M.; Ramavandi, B. Carbon nanotubes/β-cyclodextrin/MnFe2O4 as a magnetic nanocomposite powder for tetracycline antibiotic decontamination from different aqueous environments. J. Environ. Chem. Eng. 2021, 9, 106344. [Google Scholar] [CrossRef]
- Naghipour, D.; Hoseinzadeh, L.; Taghavi, K.; Jaafari, J.; Amouei, A. Effective removal of tetracycline from aqueous solution using biochar prepared from pine bark: Isotherms, kinetics and thermodynamic analyses. Int. J. Environ. Anal. Chem. 2021, 101, 1–14. [Google Scholar] [CrossRef]


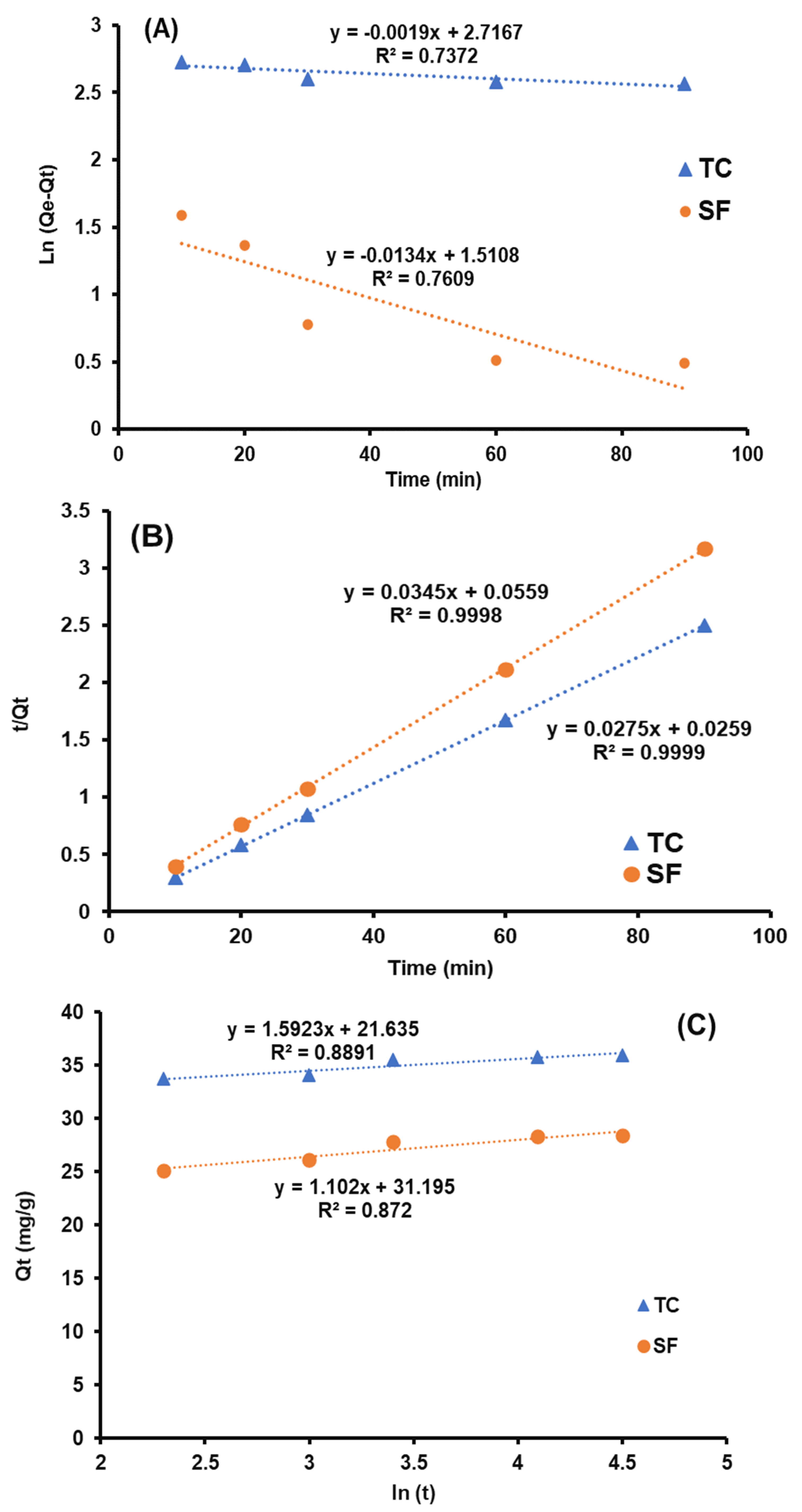
| Kinetic Models | Model Constants | T.C. | S.F. |
|---|---|---|---|
| k1 (min−1) | 0.001 | 0.013 | |
| Pseudo-first order | qe (mg·g−1) | 14.61 | 4.43 |
| R2 | 0.732 | 0.699 | |
| k2 (g mg−1 min−1) | 0.007 | 0.001 | |
| Pseudo-second order | qe (mg·g−1) | 37.03 | 29.41 |
| R2 | 0.999 | 0.999 | |
| α | 13.61 | 28.65 | |
| Elovich | β | 0.61 | 0.90 |
| R2 | 0.889 | 0.872 |
| Models | Parameters | T.C. | S.F. |
|---|---|---|---|
| Langmuir | Qm (mg·g–1) | 116.27 | 105.26 |
| R2 | 0.971 | 0.966 | |
| KL (min–1) | 1.26 | 3.06 | |
| Freundlich | KF [(mg·g−1) (L/mg)]1/n | 48.59 | 71.59 |
| n | 1.49 | 0.93 | |
| R2 | 0.935 | 0.985 | |
| Kad | 0.025 | 0.024 | |
| D-R | R2 | 0.962 | 0.900 |
| Qs (mg·g–1) | 72.31 | 51.07 |
| Adsorbate | Temp. °C | qe (mg/g) | ∆G (KJ/mol) | ∆H (KJ/mol.K) | ∆S (KJ/mol.K) |
|---|---|---|---|---|---|
| 25 | 59.92 | −35.23 | |||
| TC | 30 | 59.88 | −34.79 | −40.69 | −0.018 |
| 40 | 59.82 | −34.88 | |||
| 25 | 58.01 | −27.17 | |||
| SF | 30 | 57.68 | −27.24 | −21.77 | −0.017 |
| 40 | 57.03 | −27.44 |
| Adsorbent | Adsorbate | Qm (mg·g) | pH | Reference |
|---|---|---|---|---|
| Chitosan 10B | Cefixime | 37.04 | 5 | [43] |
| MGO-chitosan | Cefixime | 30.80 | 8 | [44] |
| CNT/cyclodexine/MnFeO | Tetracycline | 40.36 | 7 | [45] |
| biochar | Tetracycline | 58.47 | 5.1 | [46] |
| Polyelectrolyte-modified nanosilica | Cefixime | 10.40 | 4 | [42] |
| Fe-CNT-CdS | Tetracycline-cefixime | 116.27 105.26 | 5 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereshti, H.; Beyrak-Abadi, E.; Esmaeili Bidhendi, M.; Ahmad, I.; Shahabuddin, S.; Rashidi Nodeh, H.; Sridewi, N.; Wan Ibrahim, W.N. Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater. Nanomaterials 2022, 12, 3576. https://doi.org/10.3390/nano12203576
Sereshti H, Beyrak-Abadi E, Esmaeili Bidhendi M, Ahmad I, Shahabuddin S, Rashidi Nodeh H, Sridewi N, Wan Ibrahim WN. Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater. Nanomaterials. 2022; 12(20):3576. https://doi.org/10.3390/nano12203576
Chicago/Turabian StyleSereshti, Hassan, Elahe Beyrak-Abadi, Mehdi Esmaeili Bidhendi, Irfan Ahmad, Syed Shahabuddin, Hamid Rashidi Nodeh, Nanthini Sridewi, and Wan Nazihah Wan Ibrahim. 2022. "Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater" Nanomaterials 12, no. 20: 3576. https://doi.org/10.3390/nano12203576
APA StyleSereshti, H., Beyrak-Abadi, E., Esmaeili Bidhendi, M., Ahmad, I., Shahabuddin, S., Rashidi Nodeh, H., Sridewi, N., & Wan Ibrahim, W. N. (2022). Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater. Nanomaterials, 12(20), 3576. https://doi.org/10.3390/nano12203576









