A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery
Abstract
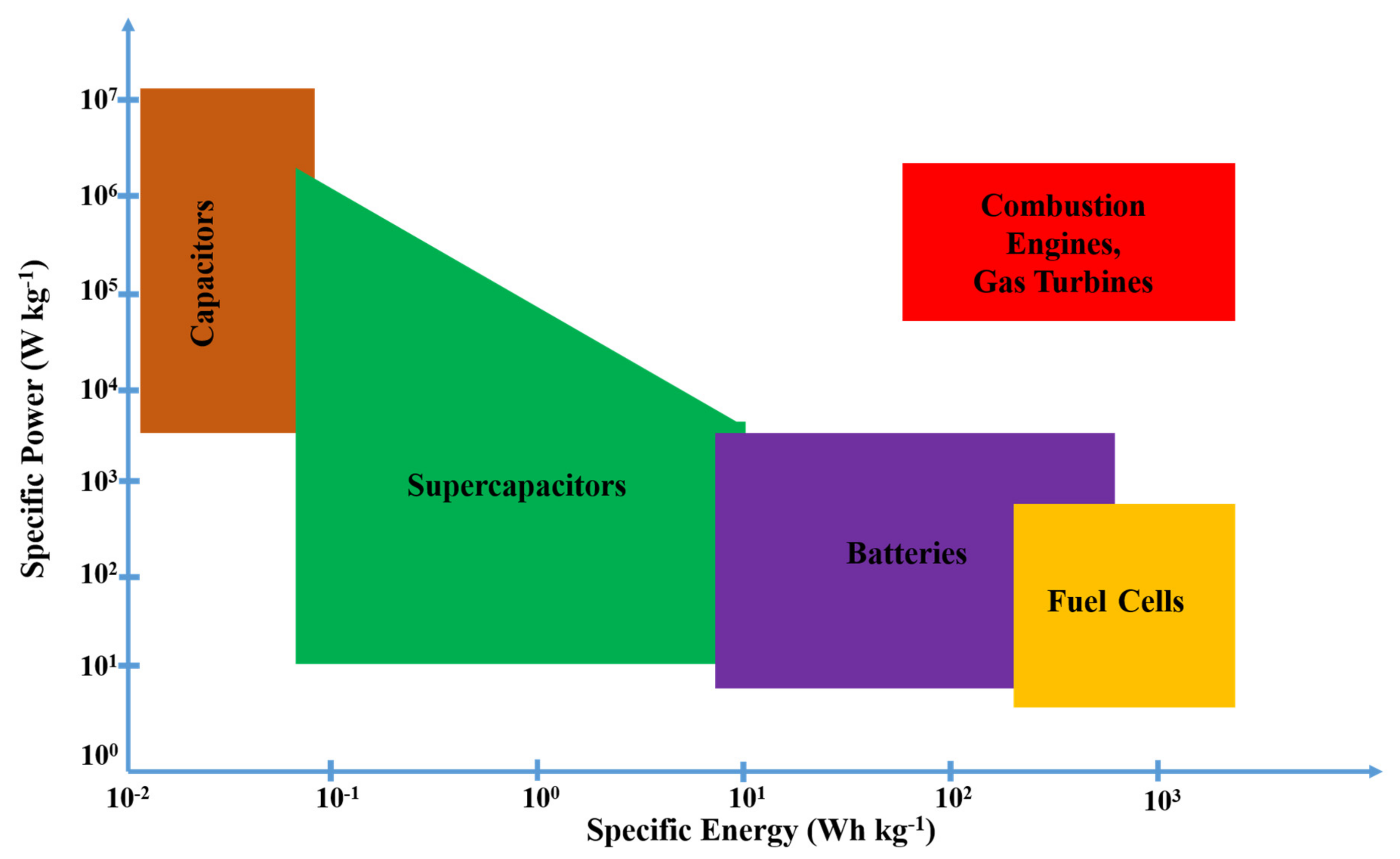
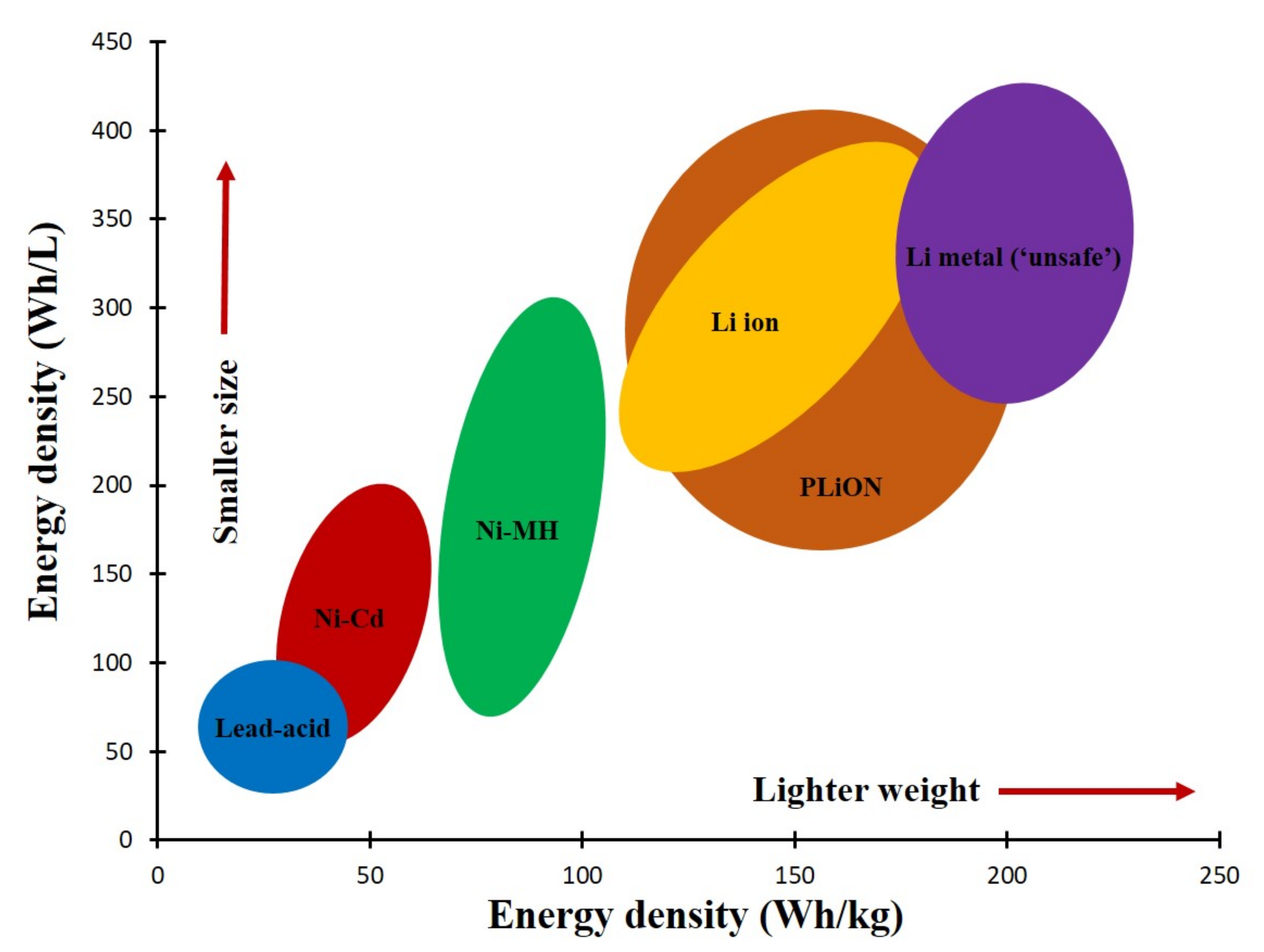
1. Introduction
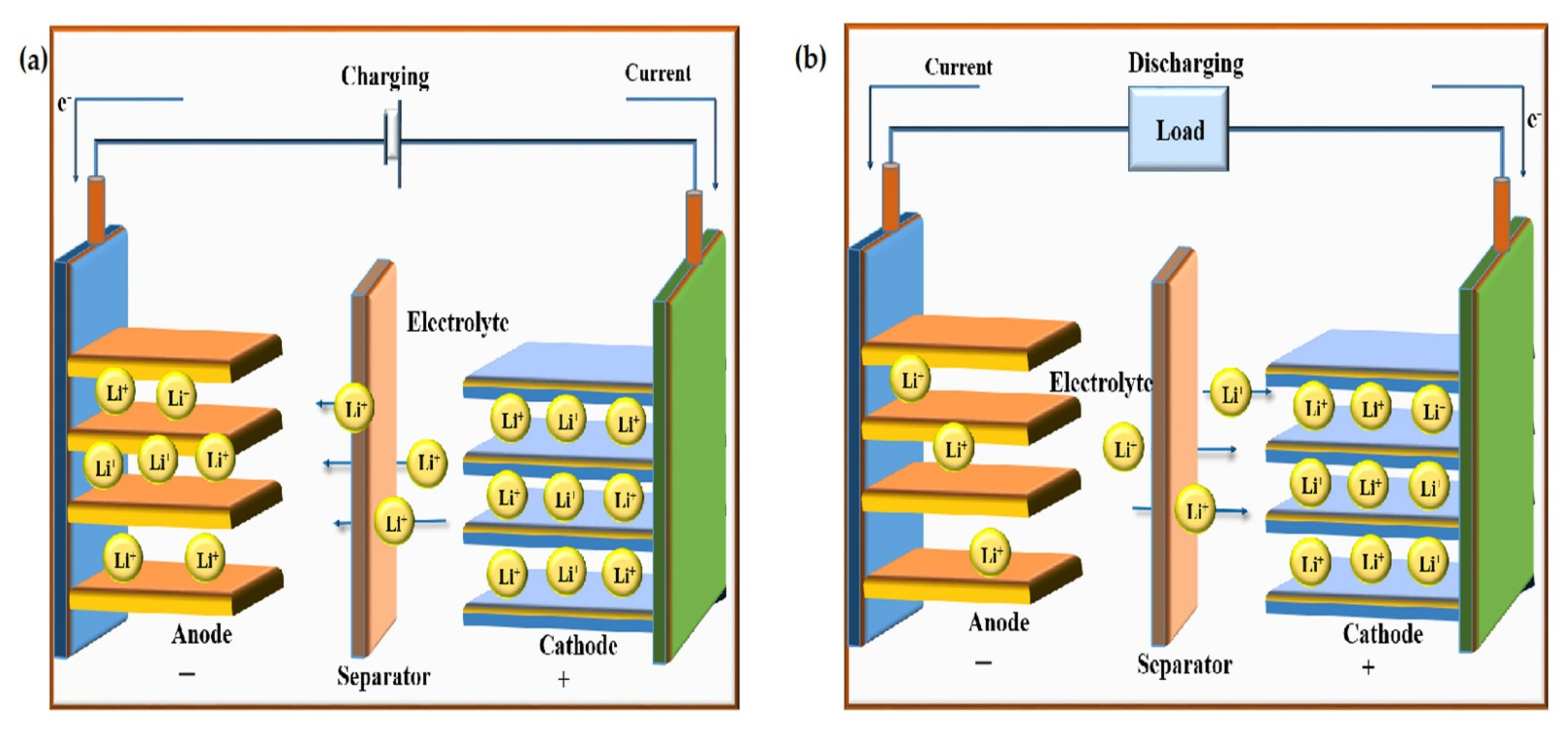
2. Working of the Lithium-Ion Battery
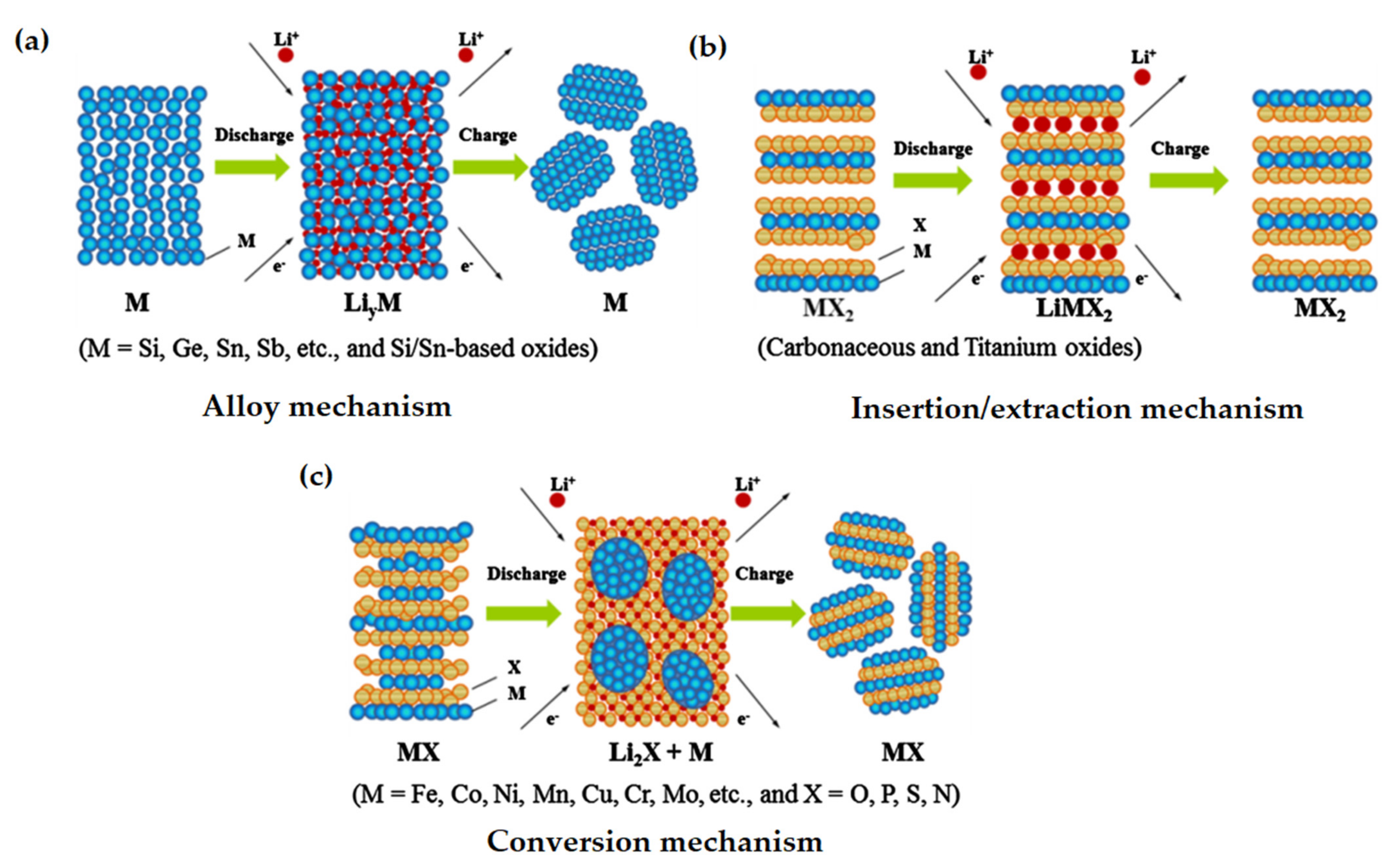
3. Anode Materials
4. Nanocomposite NiO as Anode of LIB
4.1. Nanocomposite of NiO with Carbon
4.2. Nanocomposite of NiO with Graphene Nanosheet
4.3. Nanocomposite of NiO with Carbon Nanotube (CNT)
4.4. Nanocomposite of NiO with Reduced Graphene Oxide
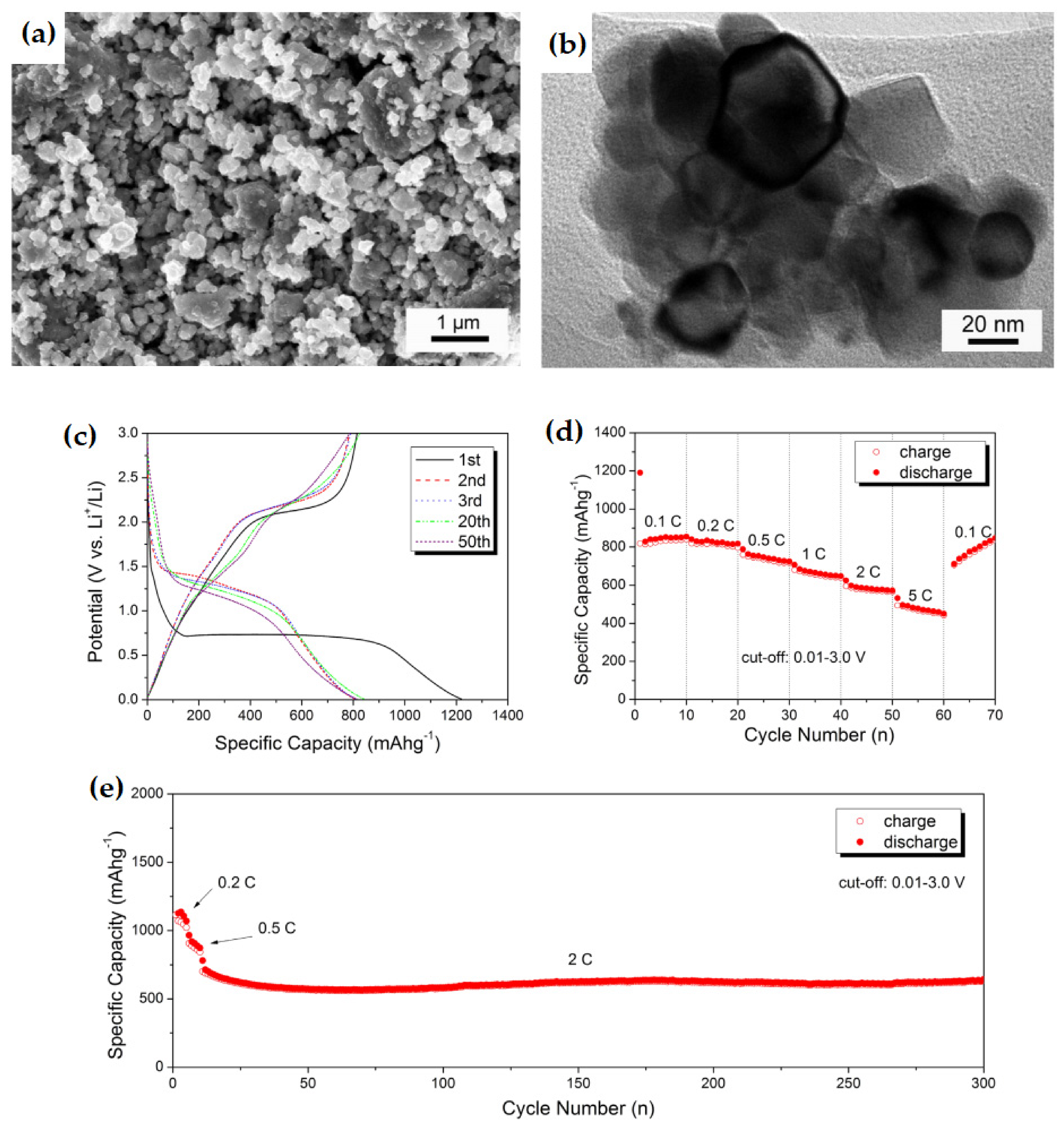
4.5. Nanocomposite of NiO with Ni
5. Conclusions
- As compared to commercial graphite, NiO has two times higher specific capacity, is environmentally friendly, and has high stability. When NiO is incorporated with carbon to synthesize nanocomposite it shows enhanced cycling performance. In addition, NiO with CNT, MWCNT, RGO, GNS, HCS, etc. exhibited remarkable improvements in performance because these carbon structures provide cushioning and act as a support matrix, hence improving the conductivity as well as impeding the detachment of active material from the electrode.
- The doping of Ni into NiO causes the formation of Ni-NiO nanocomposite which facilitates the ionic conductivity of the material due to the presence of metallic Ni within the structure. The presence of metallic nickel assists the reverse reaction during charging decomposition of SEI and Li2O. The incorporation of Ni into NiO shows better electrochemical performance than the carbonaceous compound.
- When Ni-NiO nanocomposite powder is used as anode the powder structure can smoothly buffer the volume change as a result of continuous expansion/contraction. Therefore, performance enhancement and increased conductivity can be observed. The electrode surface area, electrode/electrolyte contact, and short Li+ diffusion distance can be increased due to the nanocomposite structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Global Energy Review 2020. Available online: https://www.iea.org/reports/global-energy-review-2020 (accessed on 15 June 2022).
- Agency, I.E. World Energy Outlook; OECD/IEA: Paris, France, 2009; ISBN 926428205X. [Google Scholar]
- Nardo, M.; Saisana, M.; Saltelli, A.; Tarantola, S.; Hoffman, H.; Giovannini, E. Handbook on Constructing Composite Indicators: Methodology and User Guide. Organisation for Economic Cooperation and Development (OECD); Statistics Working Paper JT00188147; OECD: Paris, France, 2005. [Google Scholar]
- Höhne, N.; Gidden, M.J.; den Elzen, M.; Hans, F.; Fyson, C.; Geiges, A.; Jeffery, M.L.; Gonzales-Zuñiga, S.; Mooldijk, S.; Hare, W. Wave of Net Zero Emission Targets Opens Window to Meeting the Paris Agreement. Nat. Clim. Chang. 2021, 11, 820–822. [Google Scholar] [CrossRef]
- Baker, J.N.; Collinson, A. Electrical Energy Storage at the Turn of the Millennium. Power Eng. J. 1999, 13, 107–112. [Google Scholar] [CrossRef]
- Zhang, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T.; et al. Carbon Science in 2016: Status, Challenges and Perspectives. Carbon 2016, 98, 708–732. [Google Scholar] [CrossRef]
- Chemistry of Materials Editorial. Available online: https://doi.org/10.1021/acs.chemmater (accessed on 10 August 2022).
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A Materials Perspective on Li-Ion Batteries at Extreme Temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific Publishing Company: Singapore, 2011; pp. 171–179. [Google Scholar]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0<x~l): A New Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium Insertion into Manganese Spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Eichinger, G. High Energy Density Lithium Cells: Part I. Electrolytes and Anodes. J. Electroanal. Chem. Interfacial Electrochem. 1976, 68, 1–18. [Google Scholar] [CrossRef]
- Yazami, R.; Touzain, P.H. A Reversible Graphite-Lithium Negative Electrode for Electrochemical Generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Basu, S.; Zeller, C.; Flanders, P.J.; Fuerst, C.D.; Johnson, W.D.; Fischer, J.E. Synthesis and Properties of Lithium-Graphite Intercalation Compounds. Mater. Sci. Eng. 1979, 38, 275–283. [Google Scholar] [CrossRef]
- Yoshino, A. Secondary Battery. U.S. Patent (19) 4,668,595, 26 May 1987. [Google Scholar]
- Yoshino, A. The Birth of the Lithium-ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical Energy Storage for Transportation—Approaching the Limits of, and Going beyond, Lithium-Ion Batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novµk, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Search Results 1998, 10, 725–763. [Google Scholar] [CrossRef]
- van Schalkwijk, W.A.; Scrosati, B. Advances in Lithium-Ion Batteries Kluwer Academic; Springer: New York, NY, USA, 2002. [Google Scholar]
- Ma, D.; Cao, Z.; Hu, A. Si-Based Anode Materials for Li-Ion Batteries: A Mini Review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem.-Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Balogun, M.S.; Qiu, W.; Zhao, R.; Liu, P.; Tong, Y. Sulfurization of FeOOH Nanorods on a Carbon Cloth and Their Conversion into Fe2O3/Fe3O4-S Core-Shell Nanorods for Lithium Storage. Chem. Commun. 2015, 51, 13016–13019. [Google Scholar] [CrossRef]
- Jung, H.; Park, M.; Yoon, Y.-G.; Kim, G.-B.; Joo, S.-K. Amorphous Silicon Anode for Lithium-Ion Rechargeable Batteries. J. Power Sources 2003, 115, 346–351. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yan, Z.; Cheng, F.; Chen, J. Structure Design and Mechanism Analysis of Silicon Anode for Lithium-Ion Batteries. Sci. China Mater. 2019, 62, 1515–1536. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Lyu, J.; Zhao, T.; Li, T.; Choi, B.G. Tin-Based Anode Materials with Well-Designed Architectures for next-Generation Lithium-Ion Batteries. J. Power Sources 2016, 321, 11–35. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, S.; Xing, Y.; Wu, X. Nanocone-Arrays Supported Tin-Based Anode Materials for Lithium-Ion Battery. J. Power Sources 2011, 196, 9780–9785. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Chen, Y.; Zhang, F. Carbon-Coated Copper–Tin Alloy Anode Material for Lithium Ion Batteries. J. Alloys Compd. 2009, 478, 694–698. [Google Scholar] [CrossRef]
- Nithyadharseni, P.; Reddy, M.V.; Nalini, B.; Kalpana, M.; Chowdari, B.V.R. Sn-Based Intermetallic Alloy Anode Materials for the Application of Lithium Ion Batteries. Electrochim. Acta 2015, 161, 261–268. [Google Scholar] [CrossRef]
- Shi, L.; Li, H.; Wang, Z.; Huang, X.; Chen, L. Nano-SnSb Alloy Deposited on MCMB as an Anode Material for Lithium Ion Batteries. J. Mater. Chem. 2001, 11, 1502–1505. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Zeng, Y.; Qiu, W.; Luo, Y.; Onasanya, A.; Olaniyi, T.K.; Tong, Y. Three-Dimensional Nickel Nitride (Ni3N) Nanosheets: Free Standing and Flexible Electrodes for Lithium Ion Batteries and Supercapacitors. J. Mater. Chem. A 2016, 4, 9844–9849. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Yu, M.; Huang, Y.; Li, C.; Fang, P.; Liu, Y.; Lu, X.; Tong, Y. Binder-Free Fe2N Nanoparticles on Carbon Textile with High Power Density as Novel Anode for High-Performance Flexible Lithium Ion Batteries. Nano Energy 2015, 11, 348–355. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium Ion Battery Applications of Molybdenum Disulfide (MoS2) Nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Qiu, W.; Jiao, J.; Xia, J.; Zhong, H.; Chen, L. A Self-Standing and Flexible Electrode of Yolk–Shell CoS2 Spheres Encapsulated with Nitrogen-Doped Graphene for High-Performance Lithium-Ion Batteries. Chem. Eur. J. 2015, 21, 4359–4367. [Google Scholar] [CrossRef]
- Plichta, E.J.; Behl, W.K. The Rechargeable Ambient Temperature Rocking-Chair Lithium Cell Employing a Solution of Lithium Hexafluoroarsenate in Acetonitrile as the Electrolyte. J. Electrochem. Soc. 1993, 140, 46. [Google Scholar] [CrossRef]
- Hayner, C.M.; Zhao, X.; Kung, H.H. Materials for Rechargeable Lithium-Ion Batteries. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 445–471. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Sen, M.S.E.-M. Nanocomposite Materials. In Nanotechnology and the Environment; IntechOpen: Rijeka, Croatia, 2020; Chapter 6; ISBN 978-1-78985-671-2. [Google Scholar]
- Henrique Cury Camargo, P.; Gundappa Satyanarayana, K.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Hassan, F.M.; Elsayed, A.R.; Chabot, V.; Batmaz, R.; Xiao, X.; Chen, Z. Subeutectic Growth of Single-Crystal Silicon Nanowires Grown on and Wrapped with Graphene Nanosheets: High-Performance Anode Material for Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 13757–13764. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A Pomegranate-Inspired Nanoscale Design for Large-Volume-Change Lithium Battery Anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Hwang, T.H.; Lee, Y.M.; Kong, B.-S.; Seo, J.-S.; Choi, J.W. Electrospun Core–Shell Fibers for Robust Silicon Nanoparticle-Based Lithium Ion Battery Anodes. Nano Lett. 2012, 12, 802–807. [Google Scholar] [CrossRef]
- Cui, L.-F.; Hu, L.; Choi, J.W.; Cui, Y. Light-Weight Free-Standing Carbon Nanotube-Silicon Films for Anodes of Lithium Ion Batteries. ACS Nano 2010, 4, 3671–3678. [Google Scholar] [CrossRef]
- Zhang, H.; Braun, P.V. Three-Dimensional Metal Scaffold Supported Bicontinuous Silicon Battery Anodes. Nano Lett. 2012, 12, 2778–2783. [Google Scholar] [CrossRef]
- Kim, H.; Seo, M.; Park, M.; Cho, J. A Critical Size of Silicon Nano-anodes for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2010, 49, 2146–2149. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Xu, X.; Cao, C.; Xia, M.; Luo, Y. Scalable 2D Mesoporous Silicon Nanosheets for High-Performance Lithium-Ion Battery Anode. Small 2018, 14, 1703361. [Google Scholar] [CrossRef]
- Hwang, S.S.; Cho, C.G.; Kim, H. Polymer Microsphere Embedded Si/Graphite Composite Anode Material for Lithium Rechargeable Battery. Electrochim. Acta 2010, 55, 3236–3239. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, W.; Yang, J.; Zhang, L. Designing Si/Porous-C Composite with Buffering Voids as High Capacity Anode for Lithium-Ion Batteries. Electrochim. Acta 2014, 125, 206–217. [Google Scholar] [CrossRef]
- Wolf, H.; Pajkic, Z.; Gerdes, T.; Willert-Porada, M. Carbon-Fiber-Silicon-Nanocomposites for Lithium-Ion Battery Anodes by Microwave Plasma Chemical Vapor Deposition. J. Power Sources 2009, 190, 157–161. [Google Scholar] [CrossRef]
- Fang, S.; Tong, Z.; Nie, P.; Liu, G.; Zhang, X. Raspberry-Like Nanostructured Silicon Composite Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 18766–18773. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yi, R.; Chen, S.; Xu, T.; Gordin, M.L.; Wang, D. Facile Synthesis of Graphene-Silicon Nanocomposites with an Advanced Binder for High-Performance Lithium-Ion Battery Anodes. Solid State Ion. 2014, 254, 65–71. [Google Scholar] [CrossRef]
- Yang, L.Y.; Li, H.Z.; Liu, J.; Sun, Z.Q.; Tang, S.S.; Lei, M. Dual Yolk-Shell Structure of Carbon and Silica-Coated Silicon for High-Performance Lithium-Ion Batteries. Sci. Rep. 2015, 5, 10908. [Google Scholar] [CrossRef] [PubMed]
- Dylla, A.G.; Henkelman, G.; Stevenson, K.J. Lithium Insertion in Nanostructured TiO2(B) Architectures. Acc. Chem. Res. 2013, 46, 1104–1112. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Gu, L.; Guo, Y.-G.; Li, H.; He, X.-Q.; Tsukimoto, S.; Ikuhara, Y.; Wan, L.-J. Rutile-TiO2 Nanocoating for a High-Rate Li4Ti5O12 Anode of a Lithium-Ion Battery. J. Am. Chem. Soc. 2012, 134, 7874–7879. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. Enhanced Electrochemical Performance of Li-Ion Batteries with Nanoporous Titania as Negative Electrodes. J. Energy Chem. 2015, 24, 157–170. [Google Scholar] [CrossRef]
- Paul, S.; Rahman, M.A.; Islam, M.S.; Islam, M.R.; Siddiqui, S. Nanostructured Anatase TiO2 as Anode of High-performance Lithium-ion Batteries. Battery Energy 2022, 0220018. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Deng, J.; Qi, D.; Leow, W.R.; Wei, J.; Yin, S.; Dong, Z.; Yazami, R.; Chen, Z. Unravelling the Correlation between the Aspect Ratio of Nanotubular Structures and Their Electrochemical Performance to Achieve High-rate and Long-life Lithium-ion Batteries. Angew. Chem. 2014, 126, 13706–13710. [Google Scholar] [CrossRef]
- Myung, S.-T.; Kikuchi, M.; Yoon, C.S.; Yashiro, H.; Kim, S.-J.; Sun, Y.-K.; Scrosati, B. Black Anatase Titania Enabling Ultra High Cycling Rates for Rechargeable Lithium Batteries. Energy Environ. Sci. 2013, 6, 2609–2614. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Bae, J.-Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 Hollow Nanofibers as an Anode Material for High Power Lithium Ion Batteries. Energy Environ. Sci. 2011, 4, 4532–4536. [Google Scholar] [CrossRef]
- Plylahan, N.; Letiche, M.; Barr, M.K.S.; Djenizian, T. All-Solid-State Lithium-Ion Batteries Based on Self-Supported Titania Nanotubes. Electrochem. Commun. 2014, 43, 121–124. [Google Scholar] [CrossRef]
- Kumar, P.S.; Aravindan, V.; Sundaramurthy, J.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S.; Madhavi, S. High Performance Lithium-Ion Cells Using One Dimensional Electrospun TiO2 Nanofibers with Spinel Cathode. RSC Adv. 2012, 2, 7983–7987. [Google Scholar] [CrossRef]
- Borghols, W.J.H.; Lützenkirchen-Hecht, D.; Haake, U.; van Eck, E.R.H.; Mulder, F.M.; Wagemaker, M. The Electronic Structure and Ionic Diffusion of Nanoscale LiTiO2 Anatase. Phys. Chem. Chem. Phys. 2009, 11, 5742–5748. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of Hierarchically Structured Porous Materials from Energy Storage and Conversion, Catalysis, Photocatalysis, Adsorption, Separation, and Sensing to Biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tang, H.; Li, L.; Hu, Q.; Zhang, L.; Xue, H.; Pang, H. Hierarchically Nanostructured Transition Metal Oxides for Lithium-Ion Batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-Based Oxides for Electrochemical Energy Storage and Conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef]
- Kumar Rai, A.; Tuan Anh, L.; Park, C.J.; Kim, J. Electrochemical Study of NiO Nanoparticles Electrode for Application in Rechargeable Lithium-Ion Batteries. Ceram. Int. 2013, 39, 6611–6618. [Google Scholar] [CrossRef]
- Li, X.; Dhanabalan, A.; Wang, C. Enhanced Electrochemical Performance of Porous NiO-Ni Nanocomposite Anode for Lithium Ion Batteries. J. Power Sources 2011, 196, 9625–9630. [Google Scholar] [CrossRef]
- Varghese, B.; Reddy, M.V.; Yanwu, Z.; Lit, C.S.; Hoong, T.C.; Rao, G.V.S.; Chowdari, B.V.R.; Shen Wee, A.T.; Lim, C.T.; Sow, C.H. Fabrication of NiO Nanowall Electrodes for High Performance Lithium Ion Battery. Chem. Mater. 2008, 20, 3360–3367. [Google Scholar] [CrossRef]
- Cabanas-Polo, S.; Bermejo, R.; Ferrari, B.; Sanchez-Herencia, A.J. Ni-NiO Composites Obtained by Controlled Oxidation of Green Compacts. Corros. Sci. 2012, 55, 172–179. [Google Scholar] [CrossRef]
- Bandara, J.; Weerasinghe, H. Solid-State Dye-Sensitized Solar Cell with p-Type NiO as a Hole Collector. Sol. Energy Mater. Sol. Cells 2005, 85, 385–390. [Google Scholar] [CrossRef]
- Radovic, M.; Lara-Curzio, E. Mechanical Properties of Tape Cast Nickel-Based Anode Materials for Solid Oxide Fuel Cells before and after Reduction in Hydrogen. Acta Mater. 2004, 52, 5747–5756. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Wang, Q.; Chen, H. Fabrication and Lithium Storage Performance of Three-Dimensional Porous NiO as Anode for Lithium-Ion Battery. J. Power Sources 2010, 195, 7432–7437. [Google Scholar] [CrossRef]
- Liang, C.; Gao, M.; Pan, H.; Liu, Y.; Yan, M. Lithium Alloys and Metal Oxides as High-Capacity Anode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2013, 575, 246–256. [Google Scholar] [CrossRef]
- Zhang, W.J. Lithium Insertion/Extraction Mechanism in Alloy Anodes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 877–885. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, Z.; Mcdowell, M.T.; Cui, Y.; Bao, Z. Self-Healing Chemistry Enables the Stable Operation of Silicon Microparticle Anodes for High-Energy Lithium-Ion Batteries. Nat. Chem. 2013, 5, 1042–1048. [Google Scholar] [CrossRef]
- An, K.; Barai, P.; Smith, K.; Mukherjee, P.P. Probing the Thermal Implications in Mechanical Degradation of Lithium-Ion Battery Electrodes. J. Electrochem. Soc. 2014, 161, A1058–A1070. [Google Scholar] [CrossRef]
- Arorat, P.; White, R.E.; Doyle, M. Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 3647. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wen, C. A Study of the Capacity Fade of Porous NiO/Ni Foam as Negative Electrode for Lithium-Ion Batteries. Ionics 2016, 22, 173–184. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Internal Short Circuit in Li-Ion Cells. J. Power Sources 2009, 191, 568–574. [Google Scholar] [CrossRef]
- Cai, W.; Wang, H.; Maleki, H.; Howard, J.; Lara-Curzio, E. Experimental Simulation of Internal Short Circuit in Li-Ion and Li-Ion-Polymer Cells. J. Power Sources 2011, 196, 7779–7783. [Google Scholar] [CrossRef]
- Kim, H.J.; Krishna, T.N.V.; Zeb, K.; Rajangam, V.; Muralee Gopi, C.V.V.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A Comprehensive Review of Li-Ion Battery Materials and Their Recycling Techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Hein, S.; Danner, T.; Westhoff, D.; Prifling, B.; Scurtu, R.; Kremer, L.; Hoffmann, A.; Hilger, A.; Osenberg, M.; Manke, I.; et al. Influence of Conductive Additives and Binder on the Impedance of Lithium-Ion Battery Electrodes: Effect of Morphology. J. Electrochem. Soc. 2020, 167, 013546. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Yuan, S.; Ge, M.; Ren, M.; Sun, C.; Zhou, Z. Nanosheet-Based NiO Microspheres: Controlled Solvothermal Synthesis and Lithium Storage Performances. J. Phys. Chem. C 2010, 114, 251–255. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wen, C. Nanogravel Structured NiO/Ni Foam as Electrode for High-Performance Lithium-Ion Batteries. Ionics 2015, 21, 2709–2723. [Google Scholar] [CrossRef]
- Bell, J.; Ye, R.; Ahmed, K.; Liu, C.; Ozkan, M.; Ozkan, C.S. Free-Standing Ni-NiO Nanofiber Cloth Anode for High Capacity and High Rate Li-Ion Batteries. Nano Energy 2015, 18, 47–56. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Y.; Wang, Y.; Yang, X.; Wang, S.; Guo, H. Hollow NiO Nanotubes Synthesized by Bio-Templates as the High Performance Anode Materials of Lithium-Ion Batteries. Electrochim. Acta 2013, 114, 42–47. [Google Scholar] [CrossRef]
- Wu, M.S.; Lin, Y.P. Monodispersed Macroporous Architecture of Nickel-Oxide Film as an Anode Material for Thin-Film Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 2068–2073. [Google Scholar] [CrossRef]
- Su, D.; Kim, H.S.; Kim, W.S.; Wang, G. Mesoporous Nickel Oxide Nanowires: Hydrothermal Synthesis, Characterisation and Applications for Lithium-Ion Batteries and Supercapacitors with Superior Performance. Chem. Eur. J. 2012, 18, 8224–8229. [Google Scholar] [CrossRef]
- Singh, J.; Lee, S.; Kim, S.; Singh, S.P.; Kim, J.; Rai, A.K. Fabrication of 1D Mesoporous NiO Nano-Rods as High Capacity and Long-Life Anode Material for Lithium Ion Batteries. J. Alloys Compd. 2021, 850, 156755. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Liu, J.; Qiao, S.; Ahn, H. Highly Ordered Mesoporous NiO Anode Material for Lithium Ion Batteries with an Excellent Electrochemical Performance. J. Mater. Chem. 2011, 21, 3046–3052. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J.; Chen, Y.; Sun, R.; Liu, Y.; Yang, Y.; Yang, Y.; Yang, G. Preparation of Hierarchical Hexagonal Nanoplates NiO Composite with Microcrystalline Graphite for Highly Reversible Lithium Storage. J. Alloys Compd. 2020, 815, 152333. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, J.; Chen, D.; Jiao, X. 3D Hierarchical Porous NiO Nanoflowers as an Advanced Anode Material with Remarkable Lithium Storage Performance. RSC Adv. 2016, 6, 30395–30400. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Thorat, G.M.; Mun, J.; Seo, J.G. Self-Assembled Hierarchical 3D—NiO Microspheres with Ultra-Thin Porous Nanoflakes for Lithium-Ion Batteries. J. Power Sources 2016, 302, 13–21. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Hierarchically Ordered Porous Nickel Oxide Array Film with Enhanced Electrochemical Properties for Lithium Ion Batteries. Electrochem. Commun. 2010, 12, 890–893. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, B.; Butburee, T.; Zhu, J.; Han, S.; Wang, L. Hierarchical Macro/Mesoporous NiO as Stable and Fast-Charging Anode Materials for Lithium-Ion Batteries. Microporous Mesoporous Mater. 2017, 238, 78–83. [Google Scholar] [CrossRef][Green Version]
- Li, T.; Ni, S.; Lv, X.; Yang, X.; Duan, S. Preparation of NiO-Ni/Natural Graphite Composite Anode for Lithium Ion Batteries. J. Alloys Compd. 2013, 553, 167–171. [Google Scholar] [CrossRef]
- Zhu, X.J.; Hu, J.; Dai, H.L.; Ding, L.; Jiang, L. Reduced Graphene Oxide and Nanosheet-Based Nickel Oxide Microsphere Composite as an Anode Material for Lithium Ion Battery. Electrochim. Acta 2012, 64, 23–28. [Google Scholar] [CrossRef]
- Mai, Y.J.; Tu, J.P.; Gu, C.D.; Wang, X.L. Graphene Anchored with Nickel Nanoparticles as a High-Performance Anode Material for Lithium Ion Batteries. J. Power Sources 2012, 209, 1–6. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y. NiO Nanosheets Grown on Graphene Nanosheets as Superior Anode Materials for Li-Ion Batteries. Nanoscale 2011, 3, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Tu, J.P.; Xia, X.H.; Wang, X.L.; Xiang, J.Y.; Zhang, L. Porous NiO/Poly(3,4-Ethylenedioxythiophene) Films as Anode Materials for Lithium Ion Batteries. J. Power Sources 2010, 195, 1207–1210. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Xia, X.H.; Wang, X.L.; Xiang, J.Y. Nickel Foam-Supported Porous NiO/Polyaniline Film as Anode for Lithium Ion Batteries. Electrochem. Commun. 2008, 10, 1288–1290. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chou, S.L.; Zhong, C.; Wang, J.Z.; Wexler, D.; Liu, H.K. Spray Pyrolyzed NiO-C Nanocomposite as an Anode Material for the Lithium-Ion Battery with Enhanced Capacity Retention. Solid State Ion. 2010, 180, 1646–1651. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, C.Q.; Chen, X.T.; Yuan, Y.F.; Wu, H.M. Spherical NiO-C Composite for Anode Material of Lithium Ion Batteries. Electrochim. Acta 2007, 52, 4177–4181. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, J.; Wang, Z.; Li, G.; Zhang, Y.; He, Y. One-Pot Synthesis of NiO/C Composite Nanoparticles as Anode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2016, 671, 60–65. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, J.; Zhao, P.; Li, D.; Dong, Y.; Zhang, L. Synthesis and Research of Egg Shell-Yolk NiO/C Porous Composites as Lithium-Ion Battery Anode Material. Electrochim. Acta 2017, 245, 941–948. [Google Scholar] [CrossRef]
- Liu, X.; Or, S.W.; Jin, C.; Lv, Y.; Feng, C.; Sun, Y. NiO/C Nanocapsules with Onion-like Carbon Shell as Anode Material for Lithium Ion Batteries. Carbon 2013, 60, 215–220. [Google Scholar] [CrossRef]
- Tao, L.; Zai, J.; Wang, K.; Wan, Y.; Zhang, H.; Yu, C.; Xiao, Y.; Qian, X. 3D-Hierarchical NiO-Graphene Nanosheet Composites as Anodes for Lithium Ion Batteries with Improved Reversible Capacity and Cycle Stability. RSC Adv. 2012, 2, 3410–3415. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.Y.; Shan, W.F.; Xing, L.L.; Xue, X.Y. Uniformly Loading NiO Nanowalls on Graphene and Their Extremely High Capacity and Cyclability as Anodes of Lithium-Ion Batteries. Mater. Lett. 2014, 118, 66–68. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, S.; Chen, X.; Tang, T.; Klingeler, R.; Mijowska, E. Ultrathin NiO Confined within Hollow Carbon Sphere for Efficient Electrochemical Energy Storage. J. Alloys Compd. 2019, 797, 702–709. [Google Scholar] [CrossRef]
- Jae, W.; Song, J.; Hong, J.J.; Kim, J. Raspberry-like Hollow Ni/NiO Nanospheres Anchored on Graphitic Carbon Sheets as Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2019, 805, 957–966. [Google Scholar] [CrossRef]
- Fan, Z.; Liang, J.; Yu, W.; Ding, S.; Cheng, S.; Yang, G.; Wang, Y.; Xi, Y.; Xi, K.; Kumar, R.V. Ultrathin NiO Nanosheets Anchored on a Highly Ordered Nanostructured Carbon as an Enhanced Anode Material for Lithium Ion Batteries. Nano Energy 2015, 16, 152–162. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Tan, Q.; Chen, Y. Designed Synthesis of Ultrafine NiO Nanocrystals Bonded on a Three Dimensional Graphene Framework for High-Capacity Lithium-Ion Batteries. New J. Chem. 2018, 42, 9901–9910. [Google Scholar] [CrossRef]
- Tian, S.; Zheng, G.; Liu, Q.; Ren, M.; Yin, J. Preparation of RGO/NiO Anode for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2019, 14, 9459–9467. [Google Scholar] [CrossRef]
- Idris, N.H.; Wang, J.; Chou, S.; Zhong, C.; Rahman, M.M.; Liu, H. Effects of Polypyrrole on the Performance of Nickel Oxide Anode Materials for Rechargeable Lithium-Ion Batteries. J. Mater. Res. 2011, 26, 860–866. [Google Scholar] [CrossRef]
- Wu, G.T.; Wang, C.S.; Zhang, X.B.; Yang, H.S.; Qi, Z.F.; Li, W.Z. Lithium Insertion into CuO/Carbon Nanotubes. J. Power Sources 1998, 75, 175–179. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Yao, J.; Wexler, D.; Ahn, J. Hydrothermal Synthesis of Carbon Nanotube/Cobalt Oxide Core-Shell One-Dimensional Nanocomposite and Application as an Anode Material for Lithium-Ion Batteries. Electrochem. Commun. 2009, 11, 546–549. [Google Scholar] [CrossRef]
- Xu, X.; Tan, H.; Xi, K.; Ding, S.; Yu, D.; Cheng, S.; Yang, G.; Peng, X.; Fakeeh, A.; Kumar, R.V. Bamboo-like Amorphous Carbon Nanotubes Clad in Ultrathin Nickel Oxide Nanosheets for Lithium-Ion Battery Electrodes with Long Cycle Life. Carbon 2015, 84, 491–499. [Google Scholar] [CrossRef]
- Zhong, J.; Chae, O.B.; Shi, W.; Fan, J.; Mi, H.; Oh, S.M. Ultrathin NiO Nanoflakes Perpendicularly Oriented on Carbon Nanotubes as Lithium Ion Battery Anode. J. Mater. Res. 2013, 28, 2577–2583. [Google Scholar] [CrossRef]
- Bae, S.H.; Karthikeyan, K.; Lee, Y.S.; Oh, I.K. Microwave Self-Assembly of 3D Graphene-Carbon Nanotube-Nickel Nanostructure for High Capacity Anode Material in Lithium Ion Battery. Carbon 2013, 64, 527–536. [Google Scholar] [CrossRef]
- Xu, C.; Sun, J.; Gao, L. Large Scale Synthesis of Nickel Oxide/Multiwalled Carbon Nanotube Composites by Direct Thermal Decomposition and Their Lithium Storage Properties. J. Power Sources 2011, 196, 5138–5142. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; You, X.; Zhang, M.; Walle, M.D.; Wang, J.; Li, Y.; Liu, Y.N. 3D Well-Interconnected NiO–Graphene–Carbon Nanotube Nanohybrids as High-Performance Anode Materials for Li-Ion Batteries. J. Nanopart. Res. 2016, 18, 247. [Google Scholar] [CrossRef]
- Zhang, J.; Tahmasebi, A.; Omoriyekomwan, J.E.; Yu, J. Microwave-Assisted Synthesis of Biochar-carbon-Nanotube-NiO Composite as High-Performance Anode Materials for Lithium-Ion Batteries. Fuel Process. Technol. 2021, 213, 106714. [Google Scholar] [CrossRef]
- Ren, H.; Wen, Z.; Chen, S.; Liu, J.; Joo, S.W.; Huang, J. Preparation of Reduced Graphene Oxide@nickel Oxide Nanosheets Composites with Enhanced Lithium-Ion Storage Performance. Mater. Chem. Phys. 2019, 232, 229–239. [Google Scholar] [CrossRef]
- Li, M.; Dong, B.; Gao, G.; Ding, S. Synthesis of Nickel Oxide/Reduced Graphene Oxide Composite with Nanosheet-on-Sheet Nanostructure for Lithium-Ion Batteries. Mater. Lett. 2015, 155, 30–33. [Google Scholar] [CrossRef]
- Zhu, H.; Zeng, X.; Han, T.; Li, X.; Zhu, S.; Sun, B.; Zhou, P.; Liu, J. A Nickel Oxide Nanoflakes/Reduced Graphene Oxide Composite and Its High-Performance Lithium-Storage Properties. J. Solid State Electrochem. 2019, 23, 2173–2180. [Google Scholar] [CrossRef]
- Du, M.; Li, Q.; Pang, H. Oxalate-Derived Porous Prismatic Nickel/Nickel Oxide Nanocomposites toward Lithium-Ion Battery. J. Colloid Interface Sci. 2020, 580, 614–622. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, D.; Kenzhina, I.; Zdorovets, M. Study of the Use of Ionizing Radiation to Improve the Efficiency of Performance of Nickel Nanostructures as Anodes of Lithium-Ion Batteries. Mater. Res. Express 2019, 6, 055026. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Akhmetzhan, S.; Ivanitskaya, N.; Zdorovets, M. Study of the Effect of Irradiation with Ca5+ Ions on the Increase in Ni Nanotubes Lifetime, Applicable as the Basis for Lithium-Ion Batteries. Mater. Res. Express 2019, 6, 085074. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, D.; Zdorovets, M. Investigation of the Effect of Ionizing Radiation on the Structural and Conductive Characteristics of Ni Nanostructures. Vacuum 2019, 163, 103–109. [Google Scholar] [CrossRef]
- Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Kadyrzhanov, K.K.; Uglov, V.V.; Kenzhina, I.E.; Shumskaya, E.E.; Kaniukov, E.Y. Obtaining of Ni Nanotubes with Specified Properties. Mater. Res. Express 2018, 5, 035024. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M. Study of the Applicability of Directional Modification of Nanostructures to Improve the Efficiency of Their Performance as the Anode Material of Lithium-Ion Batteries. Mater. Res. Express 2019, 6, 075066. [Google Scholar] [CrossRef]
- Meng, X.; Deng, D. Bio-Inspired Synthesis of 3-D Network of NiO-Ni Nanowires on Carbonized Eggshell Membrane for Lithium-Ion Batteries. Chem. Eng. Sci. 2019, 194, 134–141. [Google Scholar] [CrossRef]
- Liang, J.; Liu, X.; Zhang, T.; Yu, K.; Liang, C. Hemp Straw Carbon and Ni/NiO Embedded Structure Composites as Anode Materials for Lithium Ion Batteries. J. Alloys Compd. 2022, 889, 161776. [Google Scholar] [CrossRef]
- Zhang, Q.-t.; Bai, Y.-b.; Ma, Y.-h.; Tang, X.-x. Preparation of Ni and NiO Nanoparticle Composite Structure and Study on Lithium Storage Performance. J. Lanzhou Univ. Technol. 2020, 46, 72–76. [Google Scholar]
- Duraisamy, E.; Sujithkrishnan, E.; Kannadasan, K.; Prabunathan, P.; Elumalai, P. Facile Metal Complex-Derived Ni/NiO/Carbon Composite as Anode Material for Lithium-Ion Battery. J. Electroanal. Chem. 2021, 887, 115168. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Z.; Wu, W.; Li, Y.; Mi, H.; Ren, X.; Zhang, P.; Sun, L. Facile Synthesis of a Scale-like NiO/Ni Composite Anode with Boosted Electrochemical Performance for Lithium-Ion Batteries. J. Alloys Compd. 2021, 862, 158012. [Google Scholar] [CrossRef]
- Yue, G.H.; Zhao, Y.C.; Wang, C.G.; Zhang, X.X.; Zhang, X.Q.; Xie, Q.S. Flower-like Nickel Oxide Nanocomposites Anode Materials for Excellent Performance Lithium-Ion Batteries. Electrochim. Acta 2015, 152, 315–322. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, B.; Zhang, C.Q.; Li, Y.; Yuan, Y.F.; Wu, H.M. Electrochemical Properties of NiO-Ni Nanocomposite as Anode Material for Lithium Ion Batteries. J. Power Sources 2006, 161, 541–544. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, H.; Teng, Y.; Du, Z.; Wang, J.; Zhang, T. Synthesis of NiO/Ni Nanocomposite Anode Material for High Rate Lithium-Ion Batteries. Mater. Lett. 2015, 142, 67–70. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, C.Q.; Xiang, J.Y. Net-Structured NiO–C Nanocomposite as Li-Intercalation Electrode Material. Electrochem. Commun. 2007, 9, 1180–1184. [Google Scholar] [CrossRef]
- Wang, N.; NuLi, Y.; Su, S.; Yang, J.; Wang, J. Effects of Binders on the Electrochemical Performance of Rechargeable Magnesium Batteries. J. Power Sources 2017, 341, 219–229. [Google Scholar] [CrossRef]
- Song, J.; Zhou, M.; Yi, R.; Xu, T.; Gordin, M.L.; Tang, D.; Yu, Z.; Regula, M.; Wang, D. Interpenetrated Gel Polymer Binder for High-performance Silicon Anodes in Lithium-ion Batteries. Adv. Funct. Mater. 2014, 24, 5904–5910. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterisation and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.M.; Song, G. A Review on Binder-free NiO-Ni Foam as Anode of High Performance Lithium-ion Batteries. Energy Storage 2021, 4, e278. [Google Scholar] [CrossRef]




| Materials | Specific Capacity (mAh g−1) | Remarks | Ref. |
|---|---|---|---|
| Si/graphite composite with polymer microsphere | Charge and discharge capacity of 1493 mAh g−1 and 1091 mAh g−1, respectively with 73.0% 1st cycle coulombic efficiency. | The capacity drops rapidly with continuous cycling and became approx. 790 mAh g−1 at 50 cycles. | [46] |
| Si/porous-C composite with voids | A reversible capacity of 980 mAh g−1 after 80 cycles, little capacity decay per cycle (0.17%), excellent rate capability of 721 mAh g−1 at a high current density of 2000 mA g−1. | Rapid capacity depletion as a result of crack formation and mechanical degradation of active electrode material over cycling. | [47] |
| 3D-Carbon fiber/Si nanocomposite | The reversible capacity in the 1st cycle at a 0.05 C rate was between 2.5 Ah g−1 and 3 Ah g−1. | High reversible specific capacity, low cyclability. | [48] |
| Raspberry-like HSi/C nanocomposite | Reversible specific capacity of 886.2 mAh g−1 at 0.5 A g−1 current density after 200 cycles with high rate capability and cycle ability of 516.7 mAh g−1 at 2 A g−1 after 500 cycles. | The specific capacity decreases rapidly with cycling and increasing current density and electrodes become pulverized. | [49] |
| Si/graphene nanocomposite | The initial discharge capacity of 769 mAh g−1, at 4000 mA g−1 current rate. | Improved reversible specific capacity, low initial capacity, capacity decreased with high C-rate. | [50] |
| Dual yolk-shell Si/C structure | Stable specific capacity of 956 mAh g−1 at 0.46 A g−1 after 430 cycles with 83% capacity retention. | Reversible capacity, capacity drastically decays with cycling. | [51] |
| Materials | Specific Capacity (mAh g−1) | Remarks | Ref. |
|---|---|---|---|
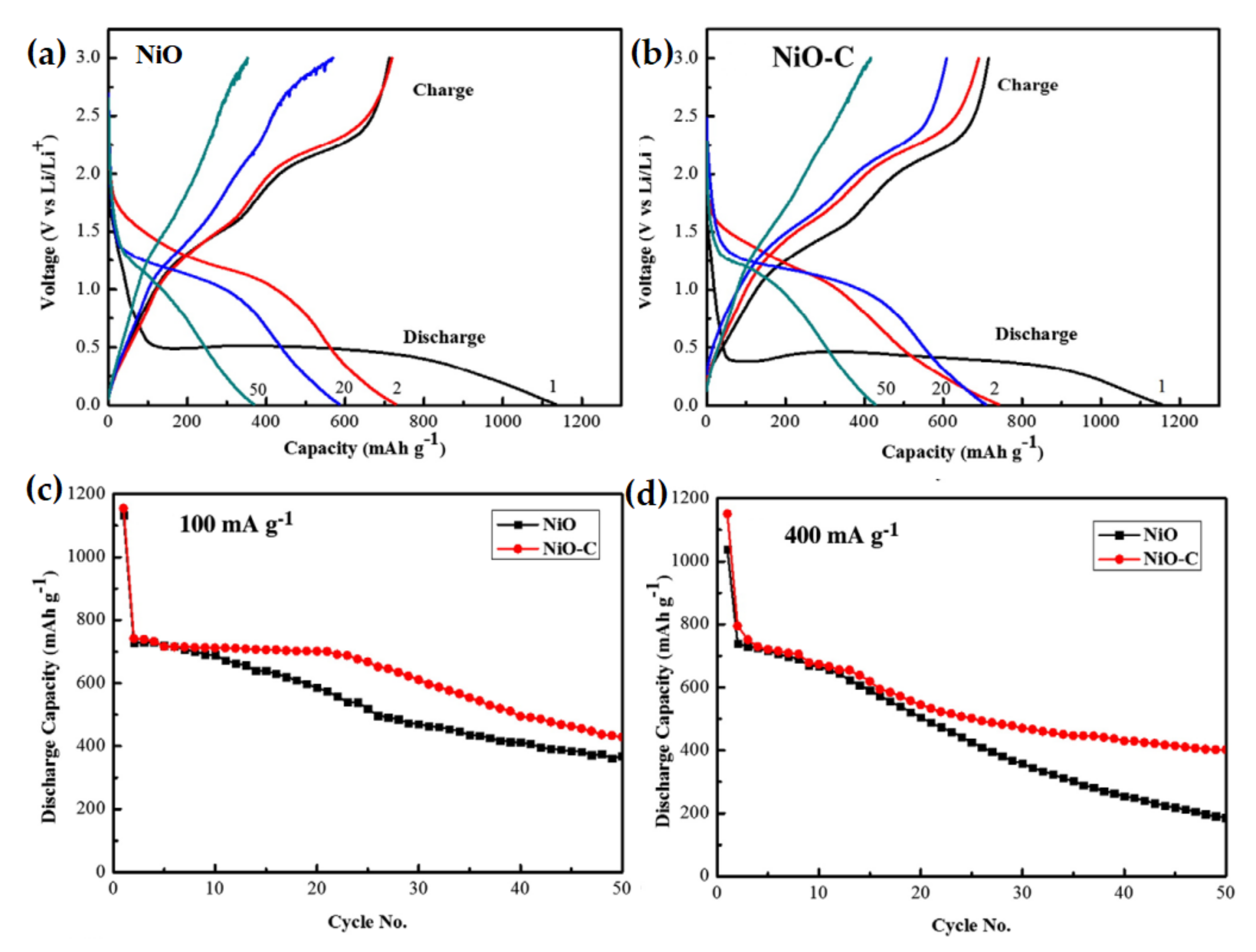
| NiO-C nanocomposite | A high initial capacity of 1102 mAh g−1. After 50 cycles, 37% of initial discharge capacity was retained. | High initial specific capacity, small capacity retention ability. | [102] |
| Net-structured NiO-C | A reversible capacity of 429 mAh g−1 even after 40 cycle at 71.8 mA g−1 current density. | Stable cycle performance due to carbon inclusion. | [140] |
| Spherical shaped NiO-C nanocomposite | High specific capacity of 430 mAh g−1 after 40 cycles with 66.6% initial coulombic efficiency at 0.5C rate. | Good cyclic performance and high initial coulombic efficiency. | [103] |
| NiO/C nanocomposite | A high reversible capacity of 585.9 mAh g−1 after 50 cycles. | High specific discharge, remarkable cyclic stability and good rate performance. | [104] |
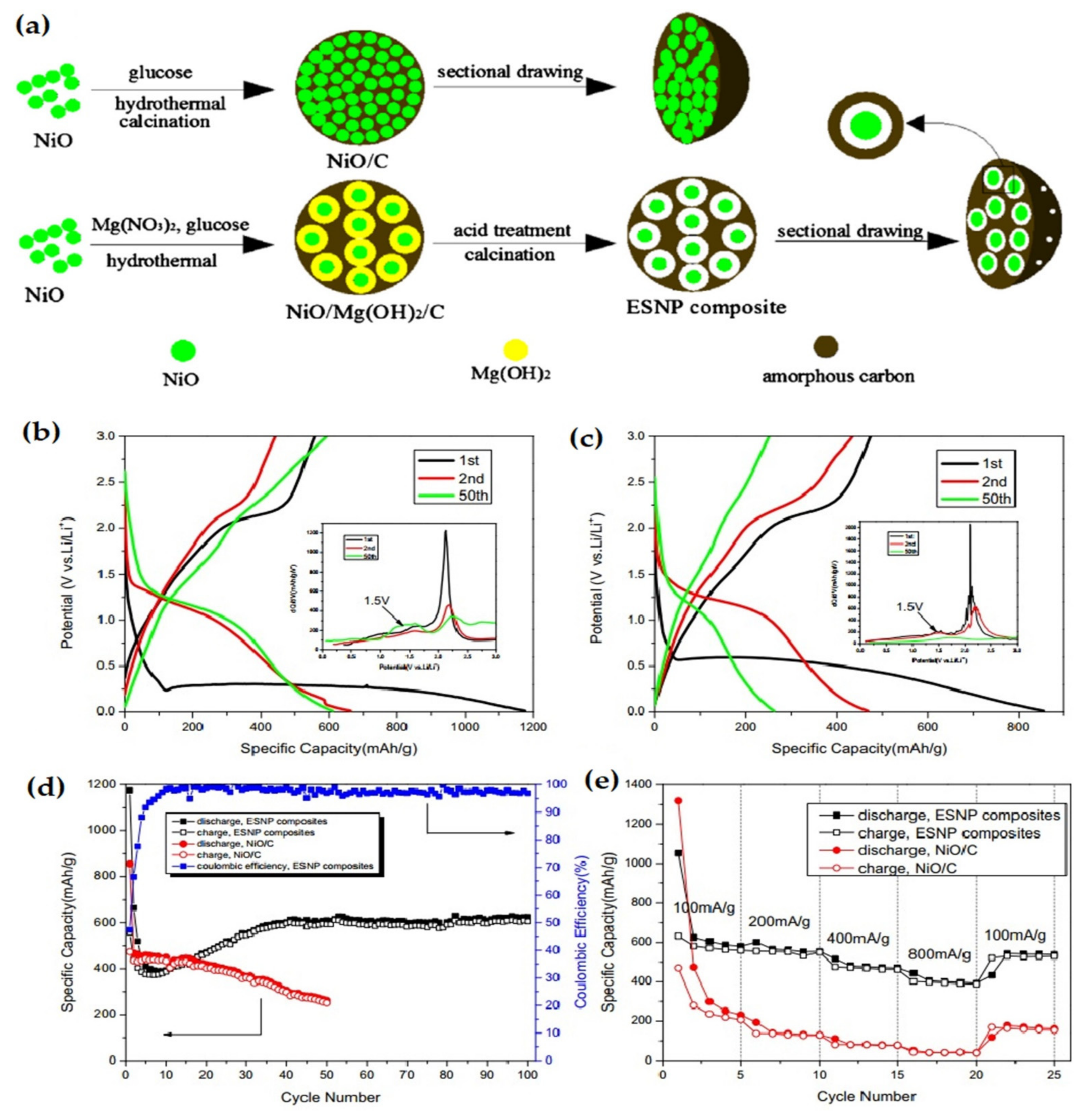
| Egg shell-yolk structured NiO/C porous composite | The first specific discharge capacity was 1175.2 mAh g−1 with 0.22 V discharge voltage. It maintained 625.3 mAh g−1 capacity after 100 cycles. | High capacity retention ability, good rate capability. | [105] |
| NiO/C nanocapsules | Initial discharge capacity of 1689.4 mAh g−1 at 0.5 C rate with a high reversible capacity of 1157.7 mAh g−1 after 50 cycles. | Outstanding discharge capacity, high rate capability, and exceptional cycling stability | [106] |
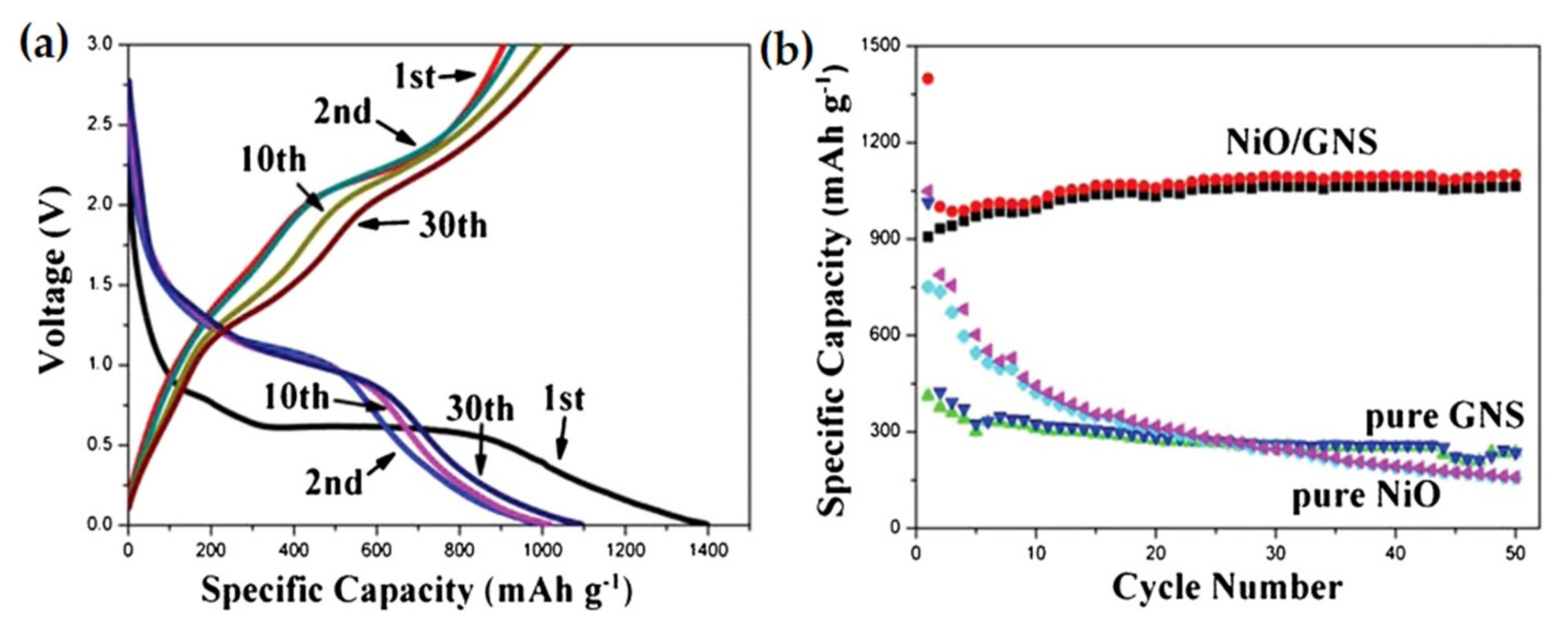
| 3D-hierarchical NiO-graphene nanosheet (GNS) composite | A high specific discharge capacity of 1400 mAh g−1. Even after 50 cycles, the composite can retain 1065 mAh g−1 specific capacity at 200 mA g−1 current density. | High discharge capacity, outstanding rate performance. | [107] |
| NiO nanowalls/GNS nanocomposites | A high reversible capacity of 844.9 mAh g−1 at 0.1C rate with little capacity fading of 7.1% after 50 cycles. | High capacity, cyclic stability and little capacity decay. | [108] |
| NiO@hollow carbon sphere | The structure provides an initial reversible capacity of 598 mAh g−1 at 0.1A g−1 current density. Even after 400 cycles delivers discharge capacity of 243 mAh g−1 at high current density of 2 A g−1. | Outstanding reversible capacity, stable cycle performance and rate capability. | [109] |
| Hollow nanospheric NiO/GCS | High reversible capacities of 1073.6 mAh g−1 and 966.6 mAh g−1 after 300 cycles at 0.5C and 1C rates | Highly reversible capacity, excellent cyclic performance and rate capability. | [110] |
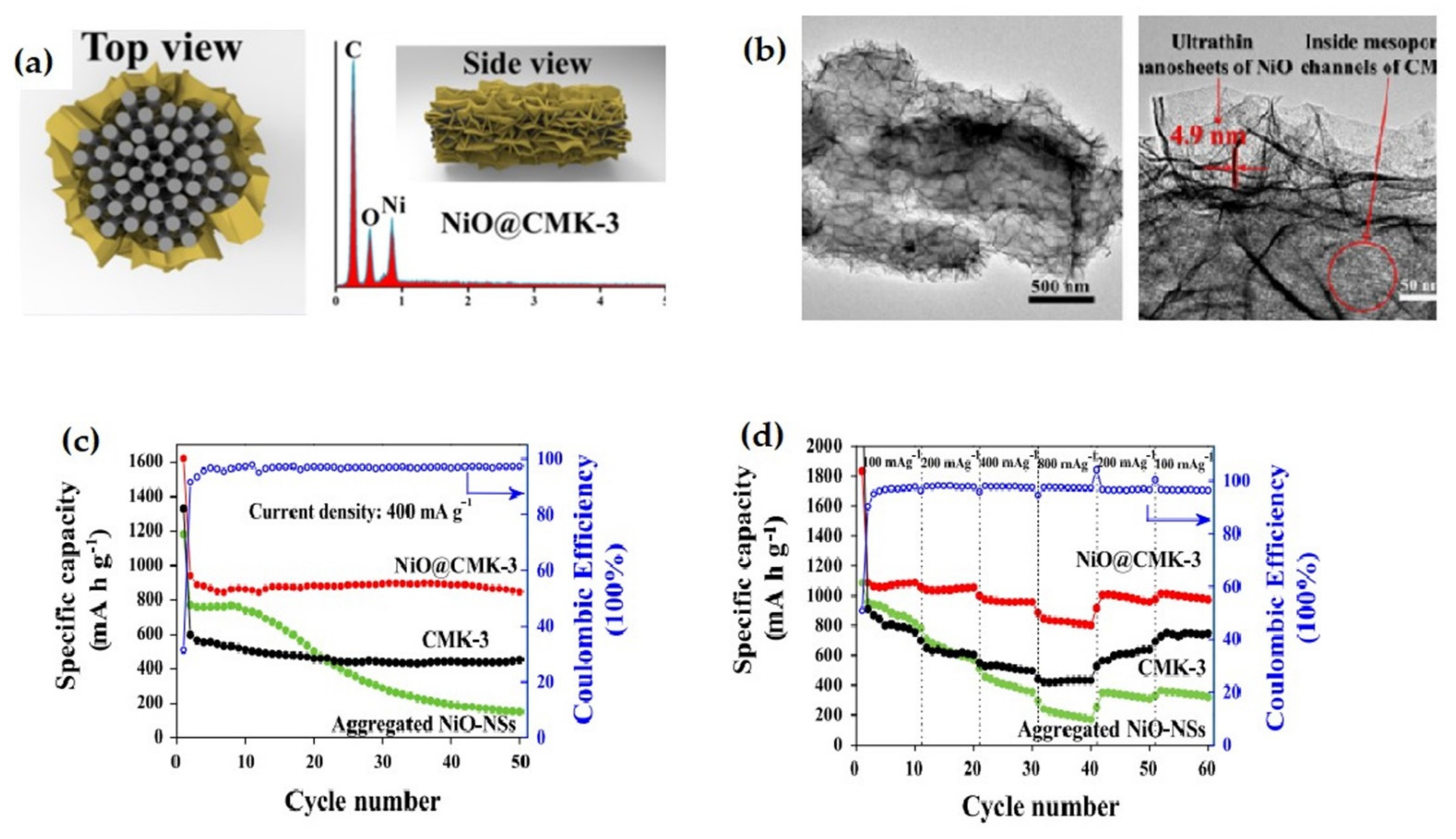
| NiO nanosheets@CMK-3 composite | The composite delivers discharge and charge capacity of 1641 and 1097 mAh g−1, merely 9.8% capacity fading after 50 cycles at a 400 mA g−1 rate. | High average specific capacity, remarkable cycling performance, excellent rate capacity. | [111] |
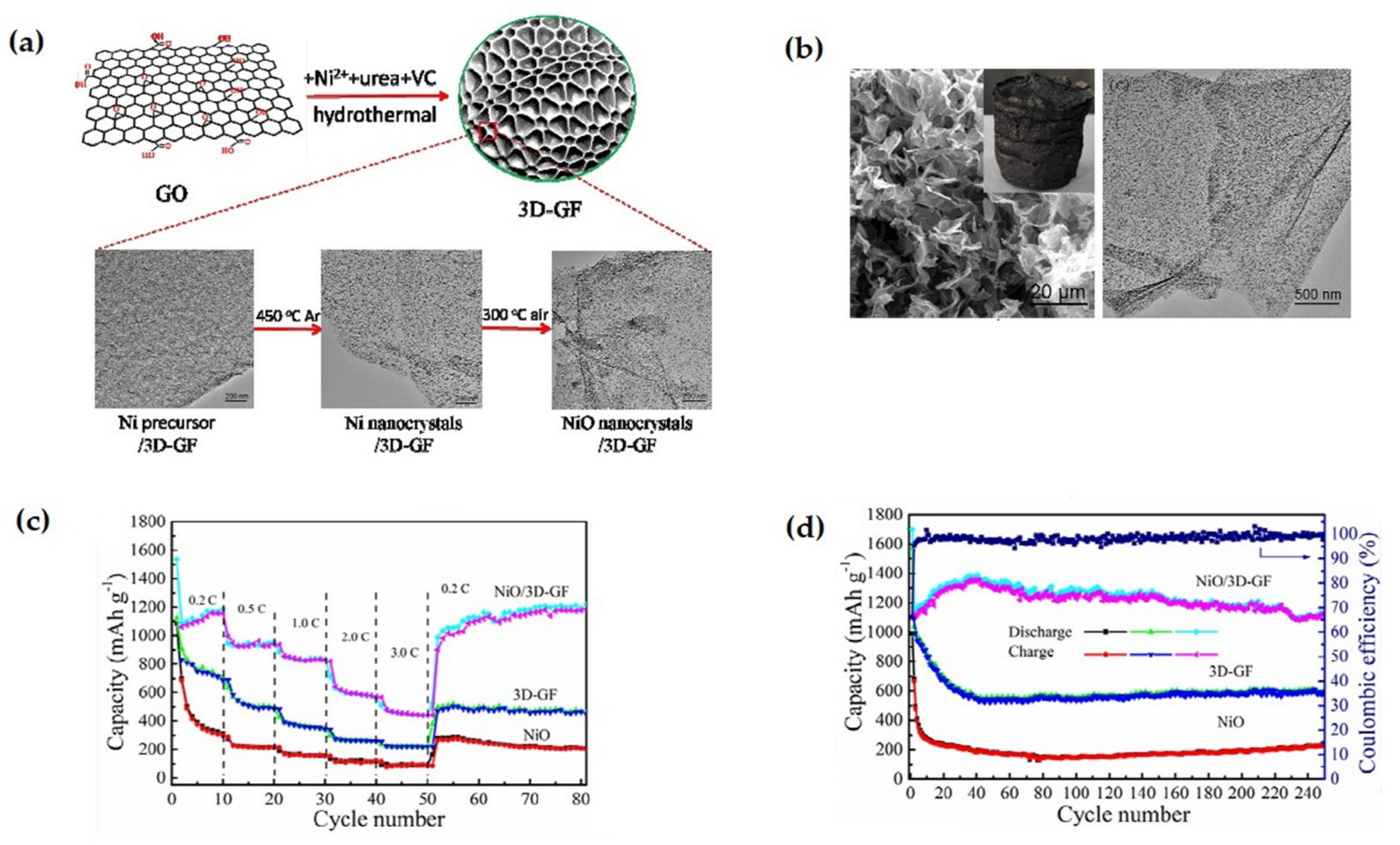
| NiO/3DGF nanocomposite | It shows an extremely high reversible capacity of 1104 mAh g−1 at 0.2C rate after 250 cycles, and an excellent rate capability with 440 mAh g−1 specific capacity at 3C rate. | Highly reversible capacity, excellent rate performance, superior capacity retention. | [112] |
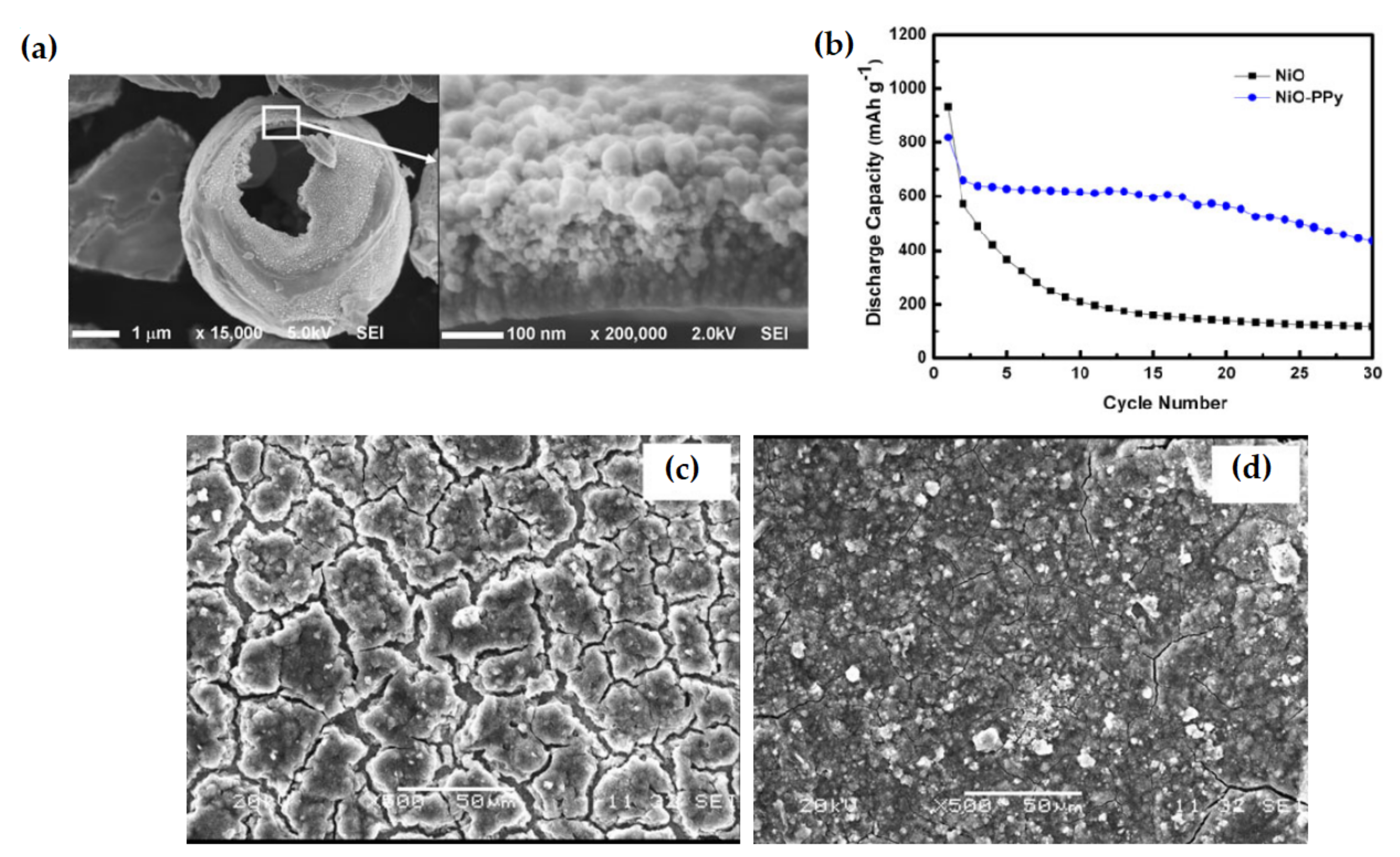
| NiO–PPy composite | The initial reversible capacity was 638 mAh g−1, which became 436 mAh g−1 after 30 cycles. The composite can retain 66% of capacity after 30 cycles. | Low decay in reversible capacity, good cycle ability and capacity retention. | [114] |
| Amorphous CNT-NiO nanosheet composite | A high discharge capacity of 1034 mAh g−1 was delivered after 300 cycles at a relatively 800 mA g−1 current density and 98.1% coulombic efficiency | High discharge capacity, coulombic efficiency, and high specific reversible capacity. | [117] |
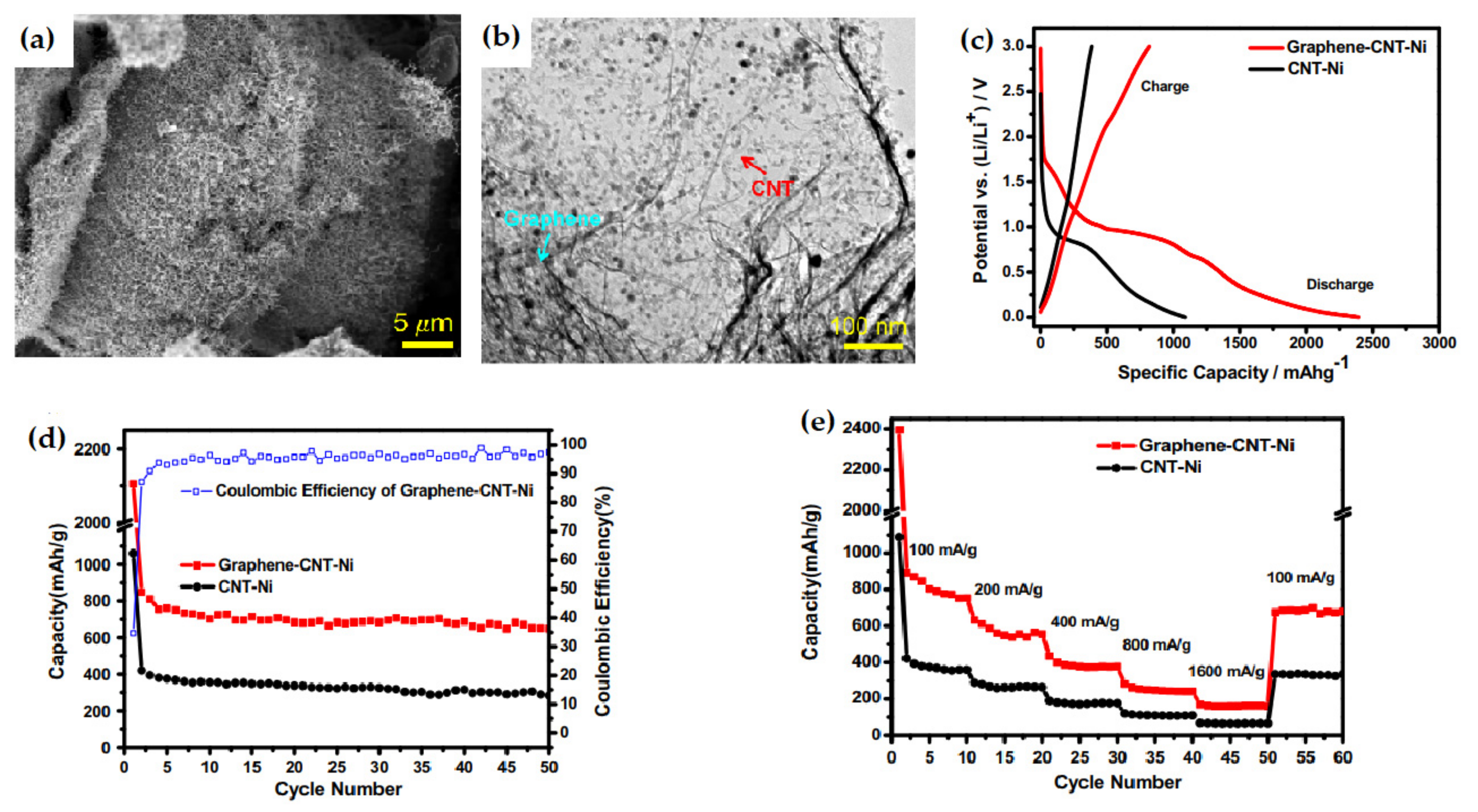
| 3D G-CNT-Ni nanostructures | It exhibited an initial capacity of 2395.2 mAh g−1 with a high reversible capacity of 648.2 mAh g−1 after 50 cycles. | High initial capacity, excellent stability, high reversible capacity. | [119] |
| MWCNT/NiO nanocomposite | Initial discharge and charge capacities of 1083.8 and 720.2 mAh g−1, respectively, with 66.45% coulombic efficiency. This sample maintained a stable ~800 mAh g−1 discharge capacity and 97% coulombic efficiency after 50 cycles. | High coulombic efficiency, stable discharge capacity, excellent cyclability. | [120] |
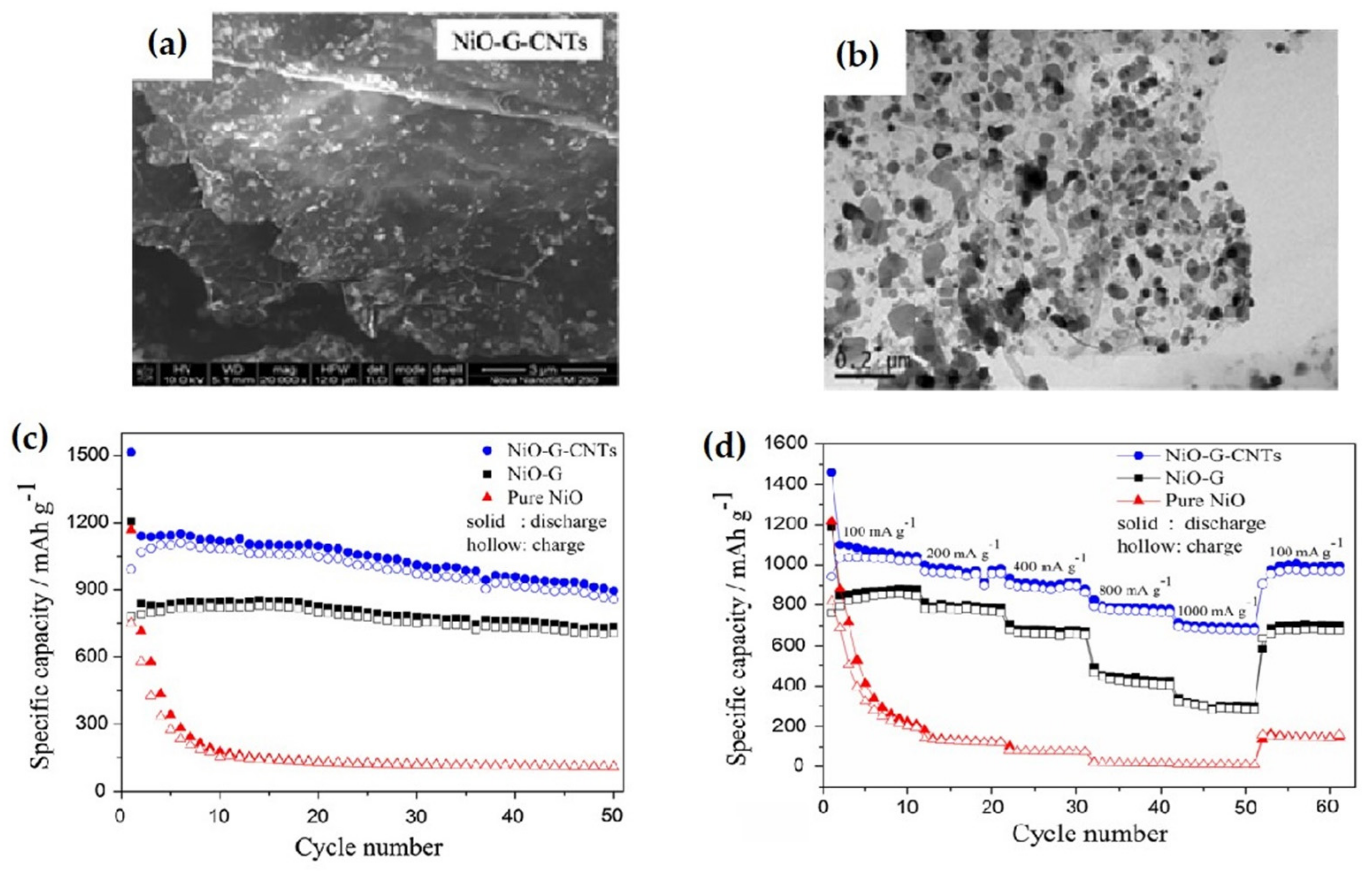
| NiO-G-CNTs nanohybrid composite | The nanohybrids delivered an initial discharge capacity of 1515.1 mAh g−1, a stable reversible specific capacity of 1022 mAh g−1, also after 50 cycles, a specific capacity of 858.1 mAh g−1 at 100 mA g−1 current density. | High initial discharge, stable reversible capacity, remarkable cycle stability and rate performance. | [121] |
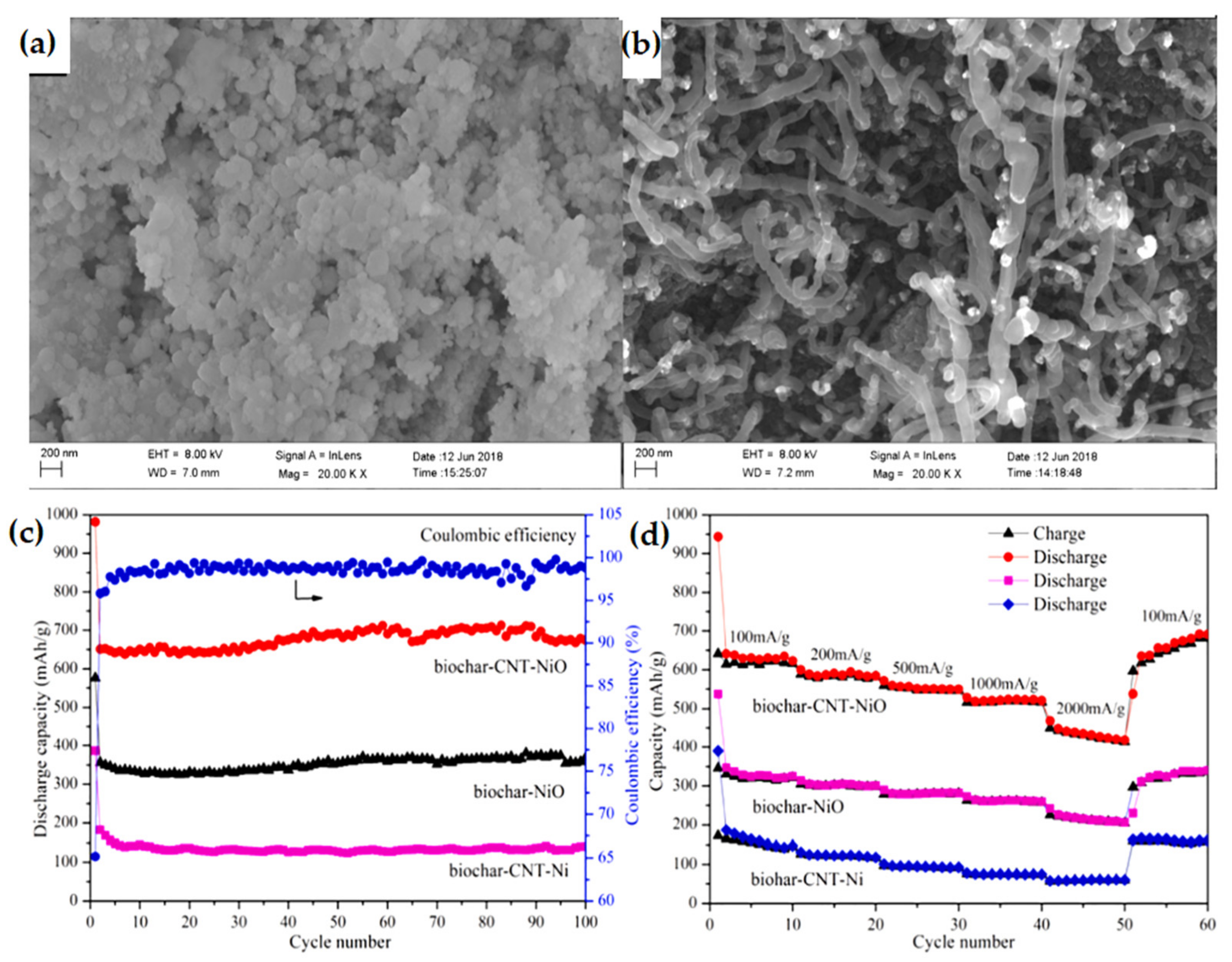
| Biochar-CNT-NiO composite | An initial discharge capacity of 981.0 mAh g−1 with 65.18% coulombic efficiency. | Highly stable cycle performance, outstanding rate capacity. | [122] |
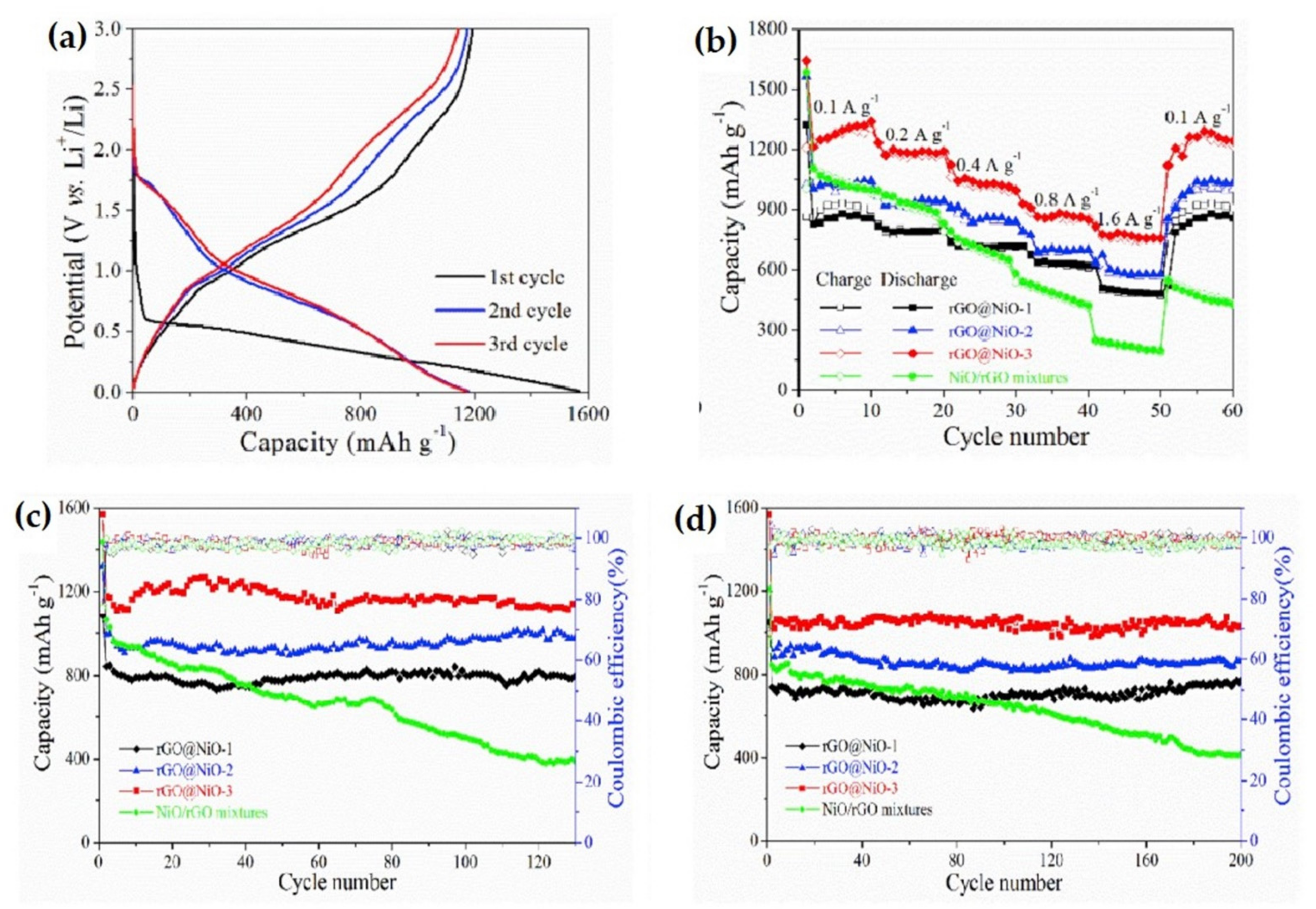
| RGO/NiO composite | The initial specific discharge/charge capacities were of 1641 mAh g−1 and 1097 mAh g−1, respectively, with 1041 mAh g−1 specific discharge capacity after 50 cycles at 100 mA g−1 current density and an excellent rate capacity of 727 mAh g−1 at 1600 mA g−1 current density. | High initial specific capacities, highly stable cycle performance and rate capability. | [97] |
| rGO/NiO nanosheet composite | Specific discharge/charge capacity of 1570 and 1193 mAh g−1 with 75.6% coulombic efficiency. | High coulombic efficiency, remarkable rate capability, high cycle stability. | [123] |
| NiO/rGO composite | It exhibited high reversible capacity of 1036.8 mAh g−1, even after 50 cycles. | High reversible capacity, can retain capacity closed to initial capacity. | [125] |
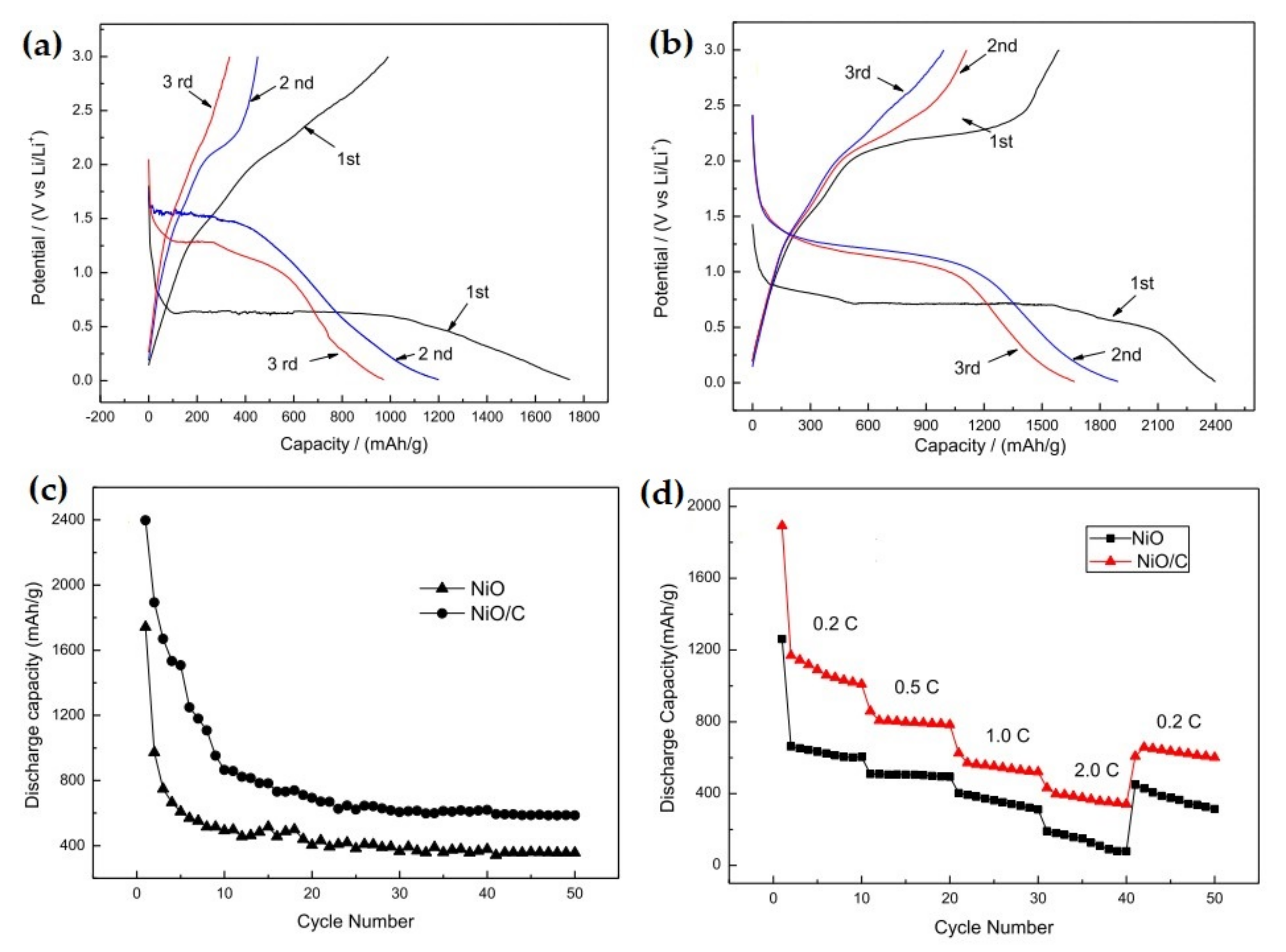
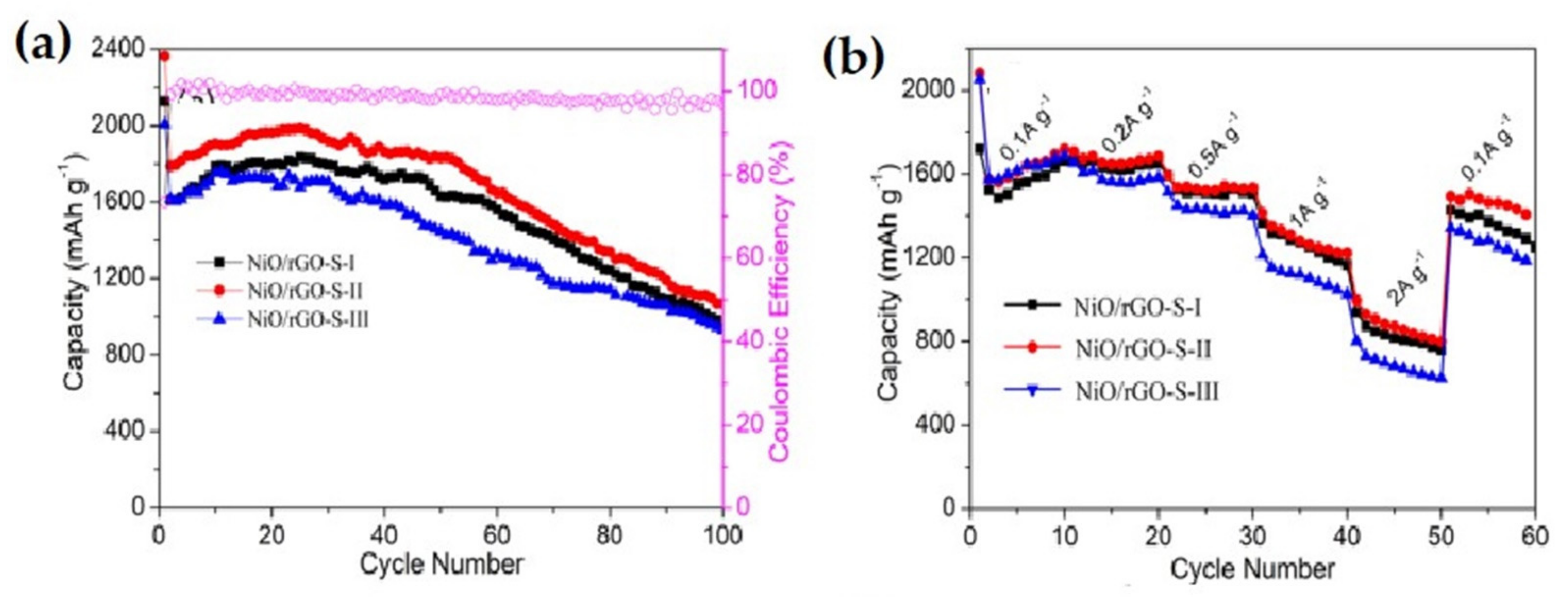
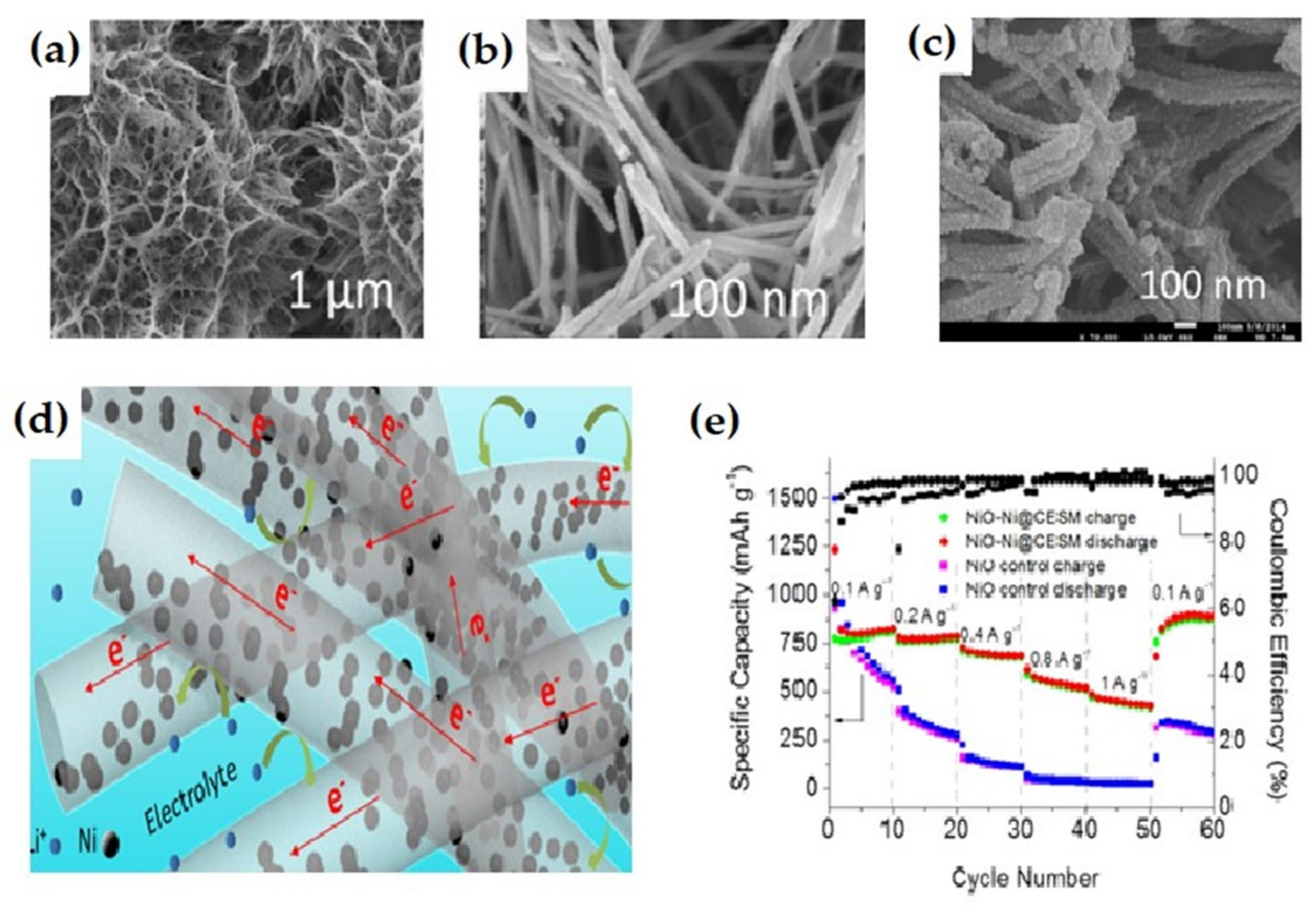
| 3D NiO-Ni nanowire composite | The composite offered a capacity of 827 mAh g−1 at 10th cycle, at 100 mA g−1 current density. At the 40th cycle the specific capacity reaches 424 mAh g−1, at 1000 mA g−1 current density, with a stable capacity retention up to 900 mAh g−1 | Highly stable cyclability, high capacity retention ability. | [132] |
| 3D-flower-like NiO/Ni nanocomposite | The Ni-doped NiO/Ni nanocomposite exhibited discharge/charge of 1316 mAh g−1 and 898 mAh g−1, respectively. | Stable reversible capacity, better capacity retention, superior cyclability, and rate performance | [137] |
| NiO-Ni nanocomposite | The first discharge capacity of 1152.4 mAh g−1 with 71.2% coulombic efficiency. | Higher reversible capacities and cycling ability | [138] |
| Spherical NiO/Ni nanocomposite | A high specific capacity of 800 mAh g−1 after 50 cycles, at a current density of 0.1C. with a reversible capacity of 450 mAh g−1, even at 5C rate, and 635 mAh g−1 capacity for 300 cycles at 2C, at 50 °C temperature. | High reversible capacity, excellent rate capability, and significantly long cycle stability. | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, S.-E.-T.; Rahman, M.A.; Kim, J.-H.; Sharif, S.B.; Paul, S. A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery. Nanomaterials 2022, 12, 2930. https://doi.org/10.3390/nano12172930
Siddiqui S-E-T, Rahman MA, Kim J-H, Sharif SB, Paul S. A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery. Nanomaterials. 2022; 12(17):2930. https://doi.org/10.3390/nano12172930
Chicago/Turabian StyleSiddiqui, Safina-E-Tahura, Md. Arafat Rahman, Jin-Hyuk Kim, Sazzad Bin Sharif, and Sourav Paul. 2022. "A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery" Nanomaterials 12, no. 17: 2930. https://doi.org/10.3390/nano12172930
APA StyleSiddiqui, S.-E.-T., Rahman, M. A., Kim, J.-H., Sharif, S. B., & Paul, S. (2022). A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery. Nanomaterials, 12(17), 2930. https://doi.org/10.3390/nano12172930









