Abstract
Graphitic C3N4-based materials are promising for photocatalytic H2 evolution applications, but they still suffer from low photocatalytic activity due to the insufficient light absorption, unfavorable structure and fast recombination of photogenerated charge. Herein, a novel anion–cation co-doped g-C3N4 porous nanotube is successfully synthesized using a self-assembly impregnation-assisted polymerization method. Ni ions on the surface of the self-assembly nanorod precursor can not only cooperate with H3P gas from the thermal cracking of NaH2PO2 as an anion–cation co-doping source, but, more importantly, suppress the shape-collapsing effect of the etching of H3P gas due to the strong coordinate bonding of Ni-P, which leads to a Ni and P co-doped g-C3N4 porous nanotube (PNCNT). Ni and P co-doping can build a new intermediate state near the conduction band in the bandgap of the PNCNT, and the porous nanotube structure gives it a higher BET surface area and light reflection path, showing a synergistic ability to broaden the visible-light absorption, facilitate photogenerated charge separation and the light-electron excitation rate of g-C3N4 and provide more reaction sites for photocatalytic H2 evolution reaction. Therefore, as expected, the PNCNT exhibits an excellent photocatalytic H2 evolution rate of 240.91 μmol·g−1·h−1, which is 30.5, 3.8 and 27.8 times as that of the pure g-C3N4 nanotube (CNT), single Ni-doped g-C3N4 nanotube (NCNT) and single P-doped g-C3N4 nanotube (PCNT), respectively. Moreover, the PNCNT shows good stability and long-term photocatalytic H2 production activity, which makes it a promising candidate for practical applications.
1. Introduction
The crises of resource scarcity and environmental disruption caused by the excessive use of fossil fuels have profoundly affected the sustainable development of human beings. As a well-known clean energy, hydrogen (H2) energy is widely regarded as an ideal substitute for fossil fuels due to its high energy density and lack of carbon dioxide emission. In recent decades, obtaining H2 from photocatalytic water splitting reaction has received an increasing amount of attention due to its characteristics of clean and low energy consumption. Among the reported visible-light-driven photocatalysts, graphite carbon nitride (g-C3N4) has attracted increasing attention in the field of photocatalytic H2 production [1,2,3] due to its suitable conduction band potential for water reduction reaction, preeminent chemical and thermal stability. However, the photocatalytic activity of pristine bulk g-C3N4 is greatly restricted by its intrinsically low specific surface area and inadequate visible-light response range, and the rapid recombination of photogeneration electron–hole pairs originates from its organic π conjugated structure [4,5,6,7]. Therefore, a variety of g-C3N4-based photocatalysts with superior photocatalytic activity have been synthesized through multiple strategies, such as element doping [8,9], heterojunction construction [10,11,12,13] and morphology regulation [14,15,16].
Morphological control through constructing 3D structures, especially hollow tubular structures, has obtained extensive attention in developing high-efficiency g-C3N4-based photocatalysts through element doping. Compared with the traditional bulk g-C3N4, the hollow g-C3N4 nanotube structure can enhance the light absorption ability through diffuse reflection and promote the migration of photogenerated carriers along the one-dimensional path, which offers a faster electron transfer channel and is beneficial to the recovery needed for practical applications [17]. For instance, Chen et al. [18] converted 2D g-C3N4 nanosheets into 1D nanotubes through the induction of phosphine gas. The formation of the nanotubes increased the SBET of the photocatalyst and created a porous surface. However, this method is complicated and has a long preparation period. Zhang et al. [19] provided a simple thermal polymerization synthesis method to build g-C3N4 nanotubes by adding a mixture of melamine and cyanic acid in a fixed proportion to form a supramolecular precursor, but this strategy requires the use of both raw materials, which is often limited by the economy in the practical application process. Li et al. [20] further simplified the preparation process and directly prepared g-C3N4 nanotubes using the in situ transformation of melamine and molecular self-assembly to form supramolecular intermediates. It is worth noting that the structure of ordered intermediates is formed through the strong saturation from hydrogen bonds in the process of self-assembly, resulting in the g-C3N4 nanotubes with specific morphologies, which is very important to enhance the ability of photocatalytic reactions. The prepared porous nanotube structure broadens light absorption and facilitates the transfer of the photocarrier along the one-dimensional structure of nanotubes, resulting in higher photogenerated carrier separation efficiency.
In addition, various existing researches have proven that implanting the anion–cation into the g-C3N4 frameworks could regulate their bandgap structure for broader visible-light absorption, facilitate the bulk phase and interface charge separation and lead to enhanced photocatalytic activity for H2 evolution [21,22,23]. For instance, Qiao et al. [24] found that a new intermediate energy level was formed in the bandgap of g-C3N4 via the insertion of P into its skeleton, which received the electrons generated by light excitation from VB and thus improved the separation of photocarriers. Fang et al. [25] demonstrated that the insertion of Fe(III) into the framework of g-C3N4 through impregnation, which enhanced the sensitivity of the catalyst to visible light, induced an interfacial charge transfer (IFCT) for faster charge separation and obtained a superior photocatalytic activity. Moreover, relative to the doping of a single element into g-C3N4, the co-doping of anion–cation has been proven to be a more promising strategy for improving photocatalytic activity, which is ascribed to its synergistic effect in modifying the electronic structure, reducing defect positions, accelerating charge transfer and broadening the visible light absorption of g-C3N4 [26,27,28]. To date, various g-C3N4-based photocatalysts with different combinations of anion–cation co-doping have been developed and show obvious enhanced photocatalytic activity in comparison to the doping of a single element. For example, Xu et al. [29] reported a P/K-co-doped porous g-C3N4 prepared by one-step thermal polymerization with K2HPO4 as a double-functional dopant, which showed 2.8 times more photocatalytic activation than the pristine g-C3N4. The improvement of photocatalytic activity is attributed to the narrowed bandgap, which allows for more efficient use of visible light and faster mass/charge transfer. In addition, Na-O [30], Fe-P [31], K-I [32], Na-S [33], Co-O [34] and other co-doping systems have also been successfully incorporated into g-C3N4 to improve its photocatalytic performance.
More recently, the combination of element doping and nanotube control has been demonstrated to be a more efficient way to improve photocatalytic activity and benefit the light absorbance of g-C3N4. For example, our previous research reported g-C3N4 nanotubes with carbon-ring doping prepared through the self-assembly assisted thermal polymerization method, which showed superior photocatalytic H2 production performance with outstanding recycling stability [35]. He et al. [36] prepared a hierarchical g-C3N4 tube with oxygen doping and carbon defects through the self-templating method, which achieved satisfactory recycling photocatalytic H2 evolution due to the larger surface area, light scattering, the shorter charge transfer distance and the creation of midgap states. Moreover, Sun et al. [37] found that the photocatalytic H2 evolution ability of g-C3N4 was significantly enhanced through the modification of anion doping and tube formation. The P/S co-doped g-C3N4 effectively expanded light absorption; thus, more charge carriers were excited in order to be distributed in the hydrogen production process. Cabot et al. [38] successfully prepared Ni/O co-doped nanotubes, which promoted the rapid separation of charge carriers in the ORR process and improved the ability of catalysts to produce hydrogen peroxide under light irradiation. Inspired by these results, it can be speculated that g-C3N4 nanotubes with anion–cation doping will combine the advantages of the above-mentioned co-doping and tubular structure and result in a high-efficiency g-C3N4 photocatalyst for H2 production, but this is still rarely reported.
Herein, an anion–cation co-doped g-C3N4 nanotube (PNCNT) was successfully prepared through self-assembly impregnation-assisted thermal polymerization method. The crystalline phase, morphology features and surface states of the PNCNT were characterized, and the results revealed that the P and Ni atoms were co-doped into the g-C3N4 framework, and the Ni atom could prevent the cracking of hollow tubular structures. The optical, photochemical, luminescent and electronic distribution properties were measured, and it was found that P/Ni-co-doped and porous hollow tubular structures can synergistically enhance photogenerated carrier transport, light absorption and surface catalytic reactions. Therefore, the PNCNT showed a photocatalytic H2 evolution efficiency of up to 954.5 μmol/g after irradiation for 4 h under visible light, which is approximately 29.1 times as that of a CNT. This work enriches and expands the idea of developing high-performance g-C3N4-based photocatalysts through anion–cation co-doping along with morphological control.
2. Experiments
2.1. Synthesis of Self-Assembled Nanorod Precursor
An amount of 2 g melamine was added into 120 mL deionized water with strong stirring for dissolution in a water bath at 85 °C. The dissolved solution was transferred into a Teflon-lined autoclave and reacted at 180 °C for 8 h. The obtained reaction products were preserved as precursors after washing with deionized water and anhydrous ethanol several times before drying in an oven.
2.2. Synthesis of g-C3N4 Nanotubes (CNTs)
The precursors were loaded into a porcelain crucible and calcined to 500 °C in a tube furnace under a nitrogen atmosphere, and the heating rate was maintained at 2.5 °C/min. The obtained products were ground into fine powders in an agate mortar, denoted as CNTs.
2.3. Synthesis of Single P-Doped g-C3N4 Nanotubes (PCNT), Single Ni-Doped g-C3N4 Nanotubes (NCNT) and P/Ni Co-Doped g-C3N4 Nanotubes (PNCNT-x)
First, the precursors were added into the NiCl2·6H2O solution under strong stirring for 2 h in order to make them homogeneous. The solution mixture was separated through high-speed centrifugal force, and the precipitated material was dried at 60 °C. Second, the composite mixed with NaH2PO2 was calcinated under the same condition with a CNT. The final resultant sample was named PNCNT-x (x = 5, 10, 15), where x represents 0.5, 1 and 1.5 wt% Ni added to the solution. In addition, the same procedure was used to synthesize the single P-doped g-C3N4 nanotubes (PCNT) and the single Ni-doped g-C3N4 nanotubes (NCNT) without adding the NiCl2·6H2O solution or NaH2PO2, respectively, before calcination to clarify the effect of single P-doped and single Ni-doped load on the performance of the photocatalyst.
2.4. Characterization
The morphology and composition of the catalyst were analyzed using a scanning electron microscope (SEM) equipped with an EDX detector (for the neatness of the manuscript, PNCNT-10 samples were selected to represent PNCNT in the following characterization). Transmission electron microscopy (TEM) was carried out to further observe the surface microstructure. The crystalline structure was measured with an X-ray diffraction (XRD) with a wide-angle scanning range with Cu Kα radiation. The N2 adsorption/desorption analysis was conducted by Micromeritics ASAP 2460 specific surface area analyzer. FTIR spectra were measured to reflect the structural information of the catalyst by a Thermo spectrometer, through setting the scanning range from 4000 cm−1 to 500 cm−1 for a total of 36 scans. X-ray photoelectron spectroscopy (XPS) was obtained on a Thermo Nexsa photoelectron spectrometer equipped with an Al Kα radiation source. The photoluminescence (PL) spectra and the time-resolved PL spectra of the catalyst were recorded to evaluate the transfer and recombination of the photocarriers with a FLS100 fluorescence lifetime spectrophotometer. UV-vis diffuse reflectance spectra (DRS) were used to analyze the light absorption capacity of the catalyst.
2.5. Photocatalytic H2 Evolution Activity
Photocatalytic H2 evolution measurements were accomplished in the Labsolar-6A system (Perfectlight, Beijing). A 300 W Xenon lamp (PLS-SXE300, Perfectlight, Beijing) equipped with a cutoff filter (λ ≥ 400 nm) was used as the light source. Typically, a quartz vessel contained 50mg catalyst and 100 mL aqueous solution with 10 vol.% triethanolamine (TEOA) as a scavenger to quench photo-induced holes was prepared for a photocatalytic reaction. Additionally, 1 wt% Pt was loaded onto the surface of the catalyst through the conventional photo-reduction method. Before every reaction, the optical power meter was used to calibrate the same light intensity, control the reaction temperature at 5 °C through the cooling water circulation system and bubble nitrogen in the system for 30 min to ensure that the reaction system was maintained under anaerobic conditions. During the reaction, the gaseous product was sampled per hour through a rubber plug and detected using gas chromatography (Techcomp GC7900), which was equipped with a TCD detector with N2 as the carrier gas.
To evaluate the apparent quantum efficiency (AQE) of the photocatalyst, a PCX-50C (Perfectlight, Beijing, China) system equipped with a 300 W Xe-lamp and a 400 nm band pass filter was used to perform photocatalytic H2 production under monochromatic light. The AQE for H2 evolution was calculated using the following equation:
2.6. Photoelectrochemical Measure Experiments
The transient photocurrent time (I-t) and electrochemical impedance spectra (EIS) of the catalyst were investigated using the CHI-730E electrochemical workstation. In an electrochemical workstation with a typical three-electrode system, an Ag/AgCl electrode was used as a reference electrode, the catalyst loaded onto the fluorine-doped tin oxide (FTO) glass as the working electrode and a Pt plate as the counter electrode, respectively. Moreover, the Xe-lamp with a cutoff filter (λ > 400 nm) was utilized as the light source and 0.1 M Na2SO4 aqueous solution as the electrolyte.
The working electrode preparation process was conducted according to the following steps: 5 mg of photocatalyst was dispersed in a 410 μL mixed solution, consisting of 10 μL of Nafion solution, 300 μL of water and 100 μL of isopropanol under ultrasonic treatment for 60 min. Then, 20 μL of the resultant catalyst solution was spread onto the FTO glass with a fixed area and dried on a heating plate.
3. Results
3.1. Photocatalyst Structure
The morphology and microstructure of the as-prepared samples were investigated using SEM and TEM. As shown in Figure 1, the nanorod precursor prepared through the hydrothermal self-assembly of melamine presented an average length of 3–6 μm. The nanorod precursor was turned into a collapsed nanotube structure after thermal polymerization in a N2 atmosphere (CNT), which was due to the weak binding force of hydrogen bonds. With the addition of NaH2PO2 into the thermal polymerization process, the nanorod precursor was completely broken and converted into fragmentized sheets (PCNT) due to the etching effect of H3P gas from the cracking of NaH2PO2. After the impregnation of the Ni ion into the nanorod precursor, both the NCNT and PNCNT showed a clear and intact nanotube morphology, suggesting that the Ni ion could suppress the shape collapsing and destruction of the nanorod precursor under the thermal polymerization process, which might be attributed to the formation of Ni-P and strong coordinate bonding between Ni and melamine. Additionally, compared with NCNT, an amount of mesopores were formed on the tube wall of PNCNT, which could offer more reaction sites for photocatalytic H2 reaction (Figure 1f). As shown in the EDX spectroscopy of PNCNT (Figure S1), Ni and P were highly dispersed on the surface of the nanotube, demonstrating the co-doping of Ni and P on the surface of PNCNT. The TEM images (Figure 2) of PNCNT clearly reveal the hollow tubular structure of PNCNT with abundant nanopore for surface photocatalytic H2 evolution reaction.
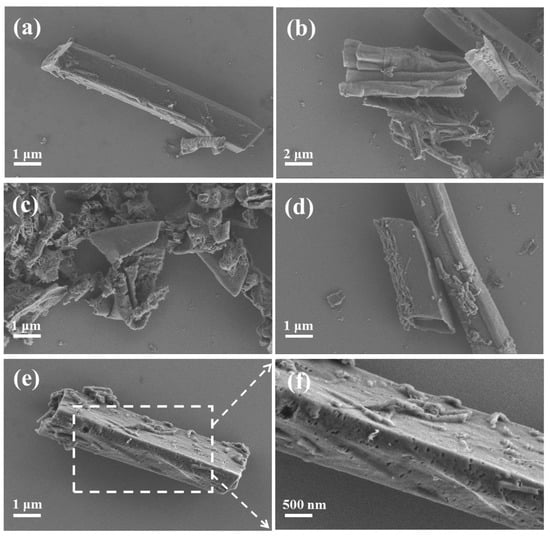
Figure 1.
The SEM images of precursor (a), CNT (b), PCNT (c), NCNT (d), PNCNT (e) and the surface situation of PNCNT samples (f).
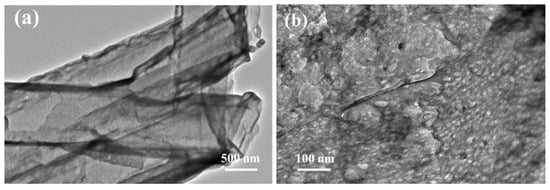
Figure 2.
TEM images of hollow tubular morphology (a) and surface porous state (b) of PNCNT samples.
To further study the porous hollow tubular structure of the PNCNT, the N2 adsorption–desorption isotherms of the CNT, NCNT, PCNT and PNCNT samples were measured (Figure 3). Table S1 lists the corresponding Brunauer–Emmett–Teller (BET) surface areas, pore volumes and pore diameters. It is clear that all the as-prepared samples displayed type IV isotherms with H3 hysteresis loops, indicating the mesoporous characteristic pore structure of the as-prepared g-C3N4-based materials. The BET surface area of the CNT was 4.115 m2/g, which showed a slight enhancement to 8.763 m2/g and 8.748 m2/g with single Ni-doping and P-doping, respectively, implying that single Ni-doping or P-doping is highly beneficial to enhance the surface area of the CNT, but the effect is not significant. In contrast, with the co-doping of Ni and P, the BET surface of the PNCNT rapidly increased to 22.257 m2/g, which is much larger than that of the PCNT and NCNT, suggesting a synergistic effect of building pores on the surface of the CNT tubular structure. The pore size distribution of the PCNT and NCNT was concentrated in the range of 50–300 nm, while the PNCNT showed a clearly additional pore size distribution at 1–50 nm, which is consistent with the SEM and TEM results and demonstrates that the synergistic effect of Ni and P co-doping has an excellent nanopore-producing ability. The additional pore size distribution might be ascribed to the ammonia gas emission along with H3P etching in the calcination process. The nanoporous structure of the g-C3N4 nanotube can increase the activity site number and light reflection path for the photocatalytic H2 evolution reaction.
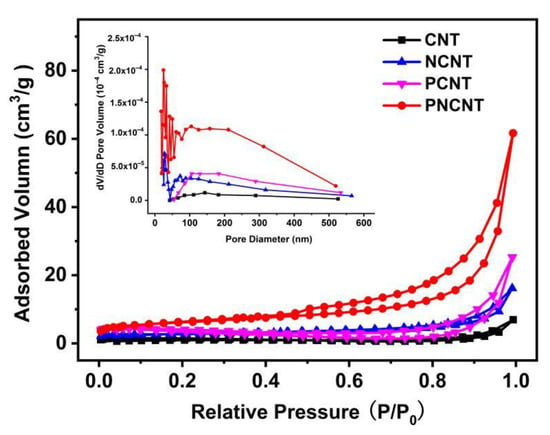
Figure 3.
Nitrogen adsorption–desorption isotherms (inset shows the pore size distribution curves) of CNT, NCNT, PCNT and PNCNT samples.
The crystalline and chemical composition of the CNT, PCNT, NCNT and PNCNT was investigated using XRD and FTIR measurements. As shown in Figure 4a, CNT and NCNT maintained the typical phase structure of g-C3N4 with characteristic diffraction peaks at 13.4 and 27.5°, which is related to the (100) inter-planar graphitic stacking and the (002) inter-planar stacking peak, indicating that neither the single Ni doping nor the P/Ni co-doping process destroyed the main crystalline structure of the g-C3N4 nanotubes. However, the XRD spectra of the PCNT exhibited a significant decrease in the intensity of both the diffraction peaks of the (002) and (100) planes, which suggests that the inter-planar structure of the g-C3N4 was changed or destroyed due to the H3P etching originating from the cracking of NaH2PO2 in the thermal polymerization process.
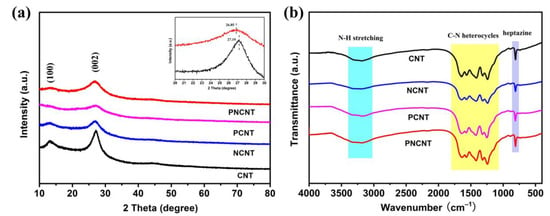
Figure 4.
XRD pattern (a) and FTIR spectra (b) of CNT, NCNT, PCNT and PNCNT samples.
On the contrary, with the impregnation of the Ni ion in the nanotube precursor, the PNCNT also maintained the typical characteristic diffraction phase of g-C3N4, which might test the theory that the addition of Ni is beneficial to reduce the structure etching from H3P in order to maintain the nanotube structure. As depicted in Figure 4b, the CNT, PCNT, NCNT and PNCNT revealed almost identical FTIR spectra; a group of strong vibration peaks at 810, 1200–1700 and 3200–3400 cm−1 were classified as the typical heterocycle skeletal vibration of g-C3N4, indicating that the single or co-doping process does not break the main chemical skeleton of g-C3N4. In addition, the peaks located at 810 cm−1 and 1200–1700 cm−1 responded to the breathing vibration of the heptazine units of the g-C3N4 and -CN heterocycles, respectively. Additionally, the wide peak located at 3200–3400 cm−1 represented the hydroxyl of the surface-absorbed water and uncondensed amino groups (-NHx).
XPS measurement was carried out to investigate the surface chemical states of the as-prepared sample and confirm the co-doping of Ni/P into the g-C3N4 framework. The results are displayed in Figure 5. It is apparent that Figure S2 depicts the photoelectron peaks of C, N and O existing in both the XPS survey spectra of the CNT and PNCNT. Except for C, N and O, the signal related to P and Ni elements can be found in the PNCNT XPS survey spectra, indicating the co-doping of P and Ni into the framework of the g-C3N4 nanotube. Regarding the C 1s high-resolution spectra (Figure 5a), the characteristic peaks at approximately 284.8, 286.4 and 288.3 eV corresponded to the sp2 C-C/C=C, C-NHx bond and sp2-bonded carbon (N-C=N), respectively. The N 1s high-resolution spectra (Figure 5b) were separated into four peaks centered at binding energies of 398.7, 400.1, 401.3 and 404.3 eV, corresponding to N-C=N, N-(C)3, N-Hx and π-excitation, respectively. It is worth noting that all the peaks of N 1s in the PNCNT displayed a weak positive shift in comparison to the CNT, which was caused by the strong interfacial interactions from the surface electron transfer from the g-C3N4 to the Ni atom. In the P 2p high-resolution spectra of the PNCNT, two peaks located at 28.97 and 133.47 eV were clearly observed, which is considered to be the Pδ- and typical P-N coordination. This implies that the P atom replaces the C atom rather than the N atom in the g-C3N4 framework because the binding energy of the P-N band is 1–2 eV higher than that of P-C [39]. Two characteristic peaks with satellite peaks of Ni 2p3/2 (855.6 eV) and Ni 2p1/2 (873.5 eV) were clearly observed in the high-resolution Ni 2p XPS spectra. The peak located at 855.6 eV is assigned to Niδ+, which can be bonded with Pδ- to easily form a P–Ni bond to induce electronic interactions. Meanwhile, the peak located at 873.5 eV is assigned to the coordination bond of Ni–N in the triangular hole in the plane of the g-C3N4 aromatic ring. Based on the above results and discussion, a potential structure of the PNCNT is proposed and shown in Scheme 1.
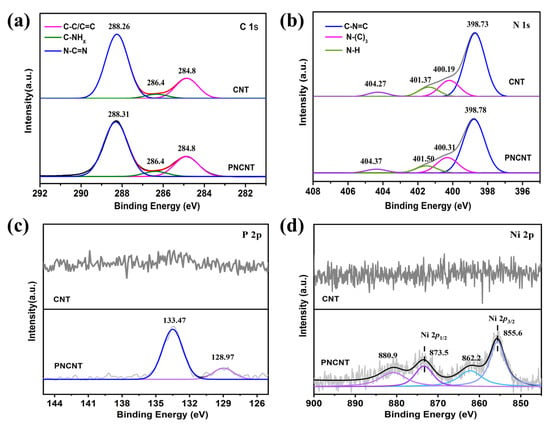
Figure 5.
The high-resolution C 1s (a), N 1s (b), P 2p (c) and Ni 2p (d) XPS spectra of CNT and PNCNT samples.
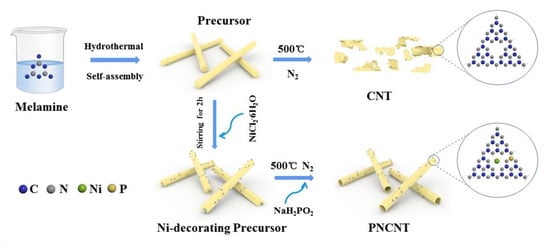
Scheme 1.
The synthesis process of the PNCNT samples.
3.2. Photocatalytic Activity of H2 Evolution
To assess the activity of the as-prepared samples, 10 vol.% TEOA was used as the sacrificial agent in aqueous solution in a photocatalytic H2 evolution system with visible-light irradiation, and the results are shown in Figure 6a. Clearly, the CNT showed an extremely low H2 production of 32.78 μmol/g under a four-hour irradiation (Figure 6a), which is due to its inadequate visible-light response and severe charge recombination. After single P-doping to the framework of g-C3N4, the PCNT showed only a negligible improvement in photocatalytic H2 production to 33.65 μmol/g, indicating that high-charge recombination from the damage of the nanotube and crystalline structure is responsible for limiting its activity. Comparatively, the photocatalytic H2 production of the NCNT increased to 252.2 μmol/g due to the catalytic effect of the Ni atom and nanotube, which induced fast charge separation. The PNCNT exhibited a superior photocatalytic H2 production up to 954.5 μmol/g, which was approximately 29.1, 28.4 and 3.8 times as that of the CNT, PCNT and NCNT, respectively. Moreover, the photocatalytic H2 production of the PNCNT was higher than the sum of the PCNT and NCNT under the same illumination time. These results confirm that P/Ni co-doping is an effective strategy to enhance the photocatalytic H2 evolution activity of the CNT. The synergistic effect of anion–cation co-doping plays a more important role in improving the photocatalytic activity than two single-doping processes. However, an excess amount of Ni doping leads to a decrease in photocatalytic H2 production concentration (594.51 μmol/g), which may result from excessive Ni acting as a recombination site on the g-C3N4 nanotube surface (Figure S3). Additionally, the increasing trend of the hydrogen concentration produced by the catalyst under illumination with time conformed to the pseudo-first-order kinetic equation and the fitted k value showed a consistent result with photocatalytic H2 production (Figure 6b). In addition, the apparent quantum efficiency (AQE) of the PNCNT was calculated to be 1.66% at 400 nm. Notably, the PNCNT exhibited remarkable stability in the photocatalytic H2 production reaction (Figure 6c). The photocatalytic H2 evolution rate of the PNCNT gradually decreased during an 8 h reaction due to the consumption of TEOA. With the re-addition of TEOA, the PNCNT recovered to the same photocatalytic H2 evolution rates, which proved the good photo-stability and reusability of the PNCNT. Additionally, the PNCNT was collected after the cycle experiment, and the crystal structure stability was assessed through XRD measurement (Figure 6d). It is clear that there was no obvious change in the XRD pattern of the PNCNT before and after the cycle test, further demonstrating the superior stability of the PNCNT under a photocatalytic H2 production reaction. Hence, PNCNT, as a well-performing photocatalyst, showed unique advantages in H2 evolution compared with other g-C3N4-based compositions (Table S3).
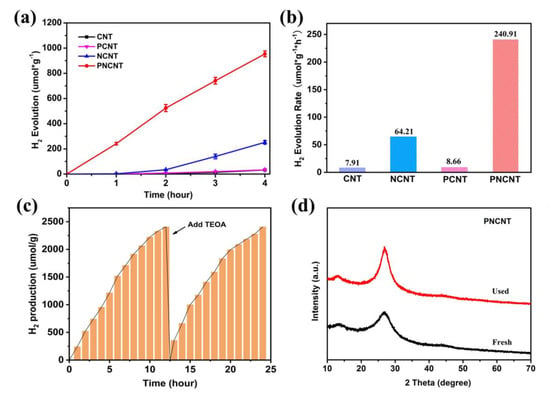
Figure 6.
Time courses of photocatalytic H2 evolution (a) and H2 evolution rate (b) of CNT, NCNT, PCNT, PNCNT samples under visible light; photocatalytic H2 evolution stability test of PNCNT (c), the XRD pattern of fresh and used PNCNT after photocatalytic H2 evolution stability tests (d).
3.3. The Optical Properties and Electrochemical Analysis
The optical properties of photocatalysts were studied using UV-Vis diffuse reflectance spectra. As shown in Figure 7a, the CNT reached an absorption of ca. 450 nm, which corresponded to a band-to-band excitation and an intrinsic bandgap of g-C3N4. Compared with the CNT, both the PCNT and NCNT showed a trailing absorption along with the intrinsic bandgap, which is related to the formation of P-mid-states and the Ni-induced interfacial charge transfer effect, respectively. Compared with the PCNT and NCNT, the PNCNT showed a mild red shift of the intrinsic bandgap and an obvious trailing absorption in the whole visible-light range, demonstrating that there is a synergistic effect between P and Ni co-doping that vastly extends the visible-light absorption ability of the CNT, which might result from the connection of mid-states and the interfacial charge transfer effect forming a new Urbach tail for sub-bandgap (Es). To study the band structure of the PNCNT, the intrinsic bandgap value was calculated using the Tauc equation. Meanwhile, the sub-bandgap value of the PNCNT was estimated using the plots of (αhν)1/2 vs. the energy of the absorbed light due to the indirect bandgap semiconductor property of g-C3N4, and the results are shown in Figure S4a. The CNT showed only an intrinsic bandgap of 2.81 eV, while the PNCNT displayed not only a narrowed bandgap of 2.67 eV but also a sub-bandgap of 2.04 eV.
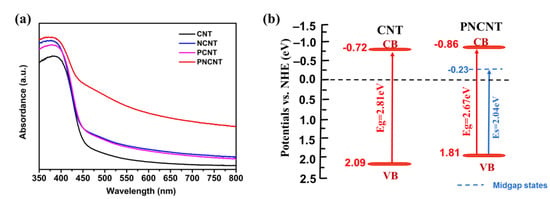
Figure 7.
UV-vis diffuse reflection spectra of CNT, NCNT, PCNT and PNCNT samples (a) and the band structure of CNT and PNCNT samples (b).
The VB positions of the CNT and PNCNT were determined to be 2.09 and 1.81 V vs. NHE, respectively, using XPS valence band spectra. Then, the CB potentials of the samples were obtained according to the following equation:
ECB = EVB − Eg
The CB potentials of the CNT and PNCNT were calculated to be −0.72 eV and −0.86 eV. Based on previous research, P-doping and Ni-induced IFCT were near and below the CB position, so the mid-state potentials of the PNCNT were determined to be −0.23 V vs. NHE, which is more negative than the potential of the H+/H2 reaction, indicating that the electrons on the mid-states are able to evolve H2 in thermodynamics. Overall, the mid-states broadened the visible-light absorption and accelerated the transmission of the photo-induced holes from VB to the mid-gap states simultaneously. In order to further investigate the light response of the synergistic effect between P/Ni co-doping, the PL and TRPL spectra and photochemical spectroscopy were applied to study the photogenerated charge transfer behavior. Figure 8a displays the steady-state PL spectra of the CNT, PCNT, NCNT and PNCNT, and the excitation wavelength was set to 325 nm. All the prepared samples showed a broad emission PL peak centered at 470 nm, which is related to the direct electron–hole recombination of intrinsic band-to-band transition. The PL emission peak position was a little higher than the intrinsic bandgap value of g-C3N4, which is due to the Stokes shift of photoelectric conversion. The peak intensity of the PCNT and NCNT showed an obvious decrease after single doping with P or Ni and showed a sharp quenching with P/Ni co-doping (PNCNT), proving that the charge recombination is inhibited to a greater extent by the co-doping method. Furthermore, the charge separation dynamics of the CNT and PNCNT was investigated using time-resolved PL spectra, and the results are shown in Figure 8b. The lifetime of the photocarriers was fitted using triple-exponential fitting equations, and the average emission lifetimes (τA) of the CNT and PNCNT were calculated to be 18.766 ns and 19.382 ns, respectively, indicating that co-doping benefits the improvement of charge separation efficiency. In addition, compared with single P-doping or Ni-doping, the PNCNT with the P/Ni co-doping showed a much higher photocurrent and smaller arc radius than that of the PCNT and NCNT (Figure 8c,d), demonstrating that co-doping is a more effective route to facilitate photoelectric conversion and interfacial charge transfer, which further confirms the synergistic effect for accelerating charge separation.
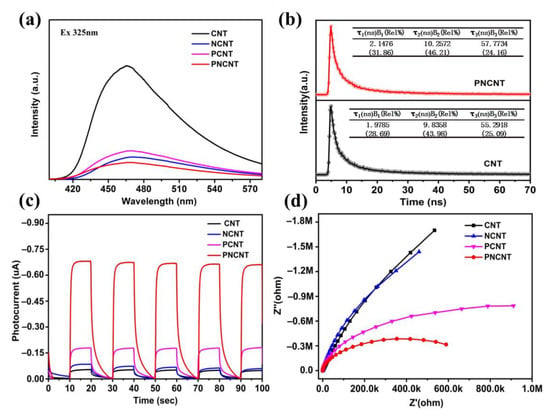
Figure 8.
Photoluminescence spectra (a), PE-TA spectra of CNT and PNCNT (b), the periodic on/off photocurrent response under visible-light irradiation (c) and electrochemical impedance spectroscopy plots (d) of CNT, NCNT, PCNT and PNCNT samples.
The single-electron state of the CNT, PCNT, NCNT and PNCNT was characterized by electron paramagnetic resonance (EPR) spectra to determine the effect of single and co-doping on the unpaired electrons of heptazine π-conjugated rings. As shown in Figure 9, a single Lorentzian line centered at g = 2.0034 could be clearly observed for all the samples, which corresponded to the unpaired electron on the sp2-carbon atoms of the heptazine rings. The PNCNT displayed a significant enhancement in the EPR signal in comparison to the CNT, PCNT and NCNT, suggesting the synergistic effect for the further delocalization of the heptazine π-conjugated rings. More importantly, it was observed that the PNCNT, as expected, still exhibited an increased and highest signal intensity during the irradiation process, suggesting that co-doping is more favorable to the sensitive light response of photo-induced free radical pairs, which promotes the conversion of solar energy to hydrogen energy.
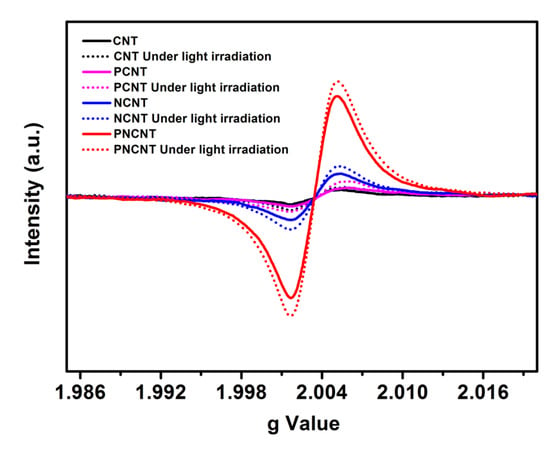
Figure 9.
EPR pattern in dark and light irradiation conditions of CNT, NCNT, PCNT and PNCNT samples.
Therefore, a possible photocatalytic reaction mechanism of the PNCNT for photocatalytic H2 evolution was proposed and is illustrated in Scheme 2. The co-doping of P and Ni will create suitable mid-states near and below the CB of the g-C3N4 nanotube and lead to a sub-bandgap, which can greatly broaden its visible-light absorption. Under visible-light irradiation, the PNCNT is excited by intrinsic and sub-bandgaps to generate electrons on the CB and mid-states and leave holes on the VB. As the potential of mid-states is more positive than the CB, the photogenerated e− is easily transferred from the CB of the PNCNT to the Es and reacts with water to produce H2.
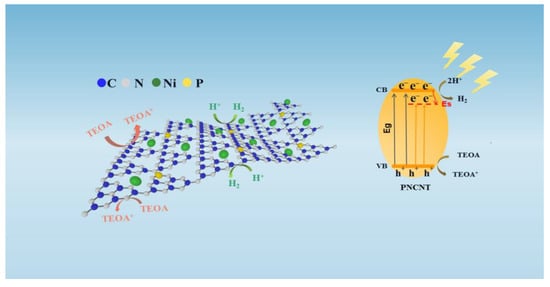
Scheme 2.
The mechanism of photocatalytic H2 evolution on the PNCNT samples.
4. Conclusions
In summary, a P/Ni co-doped porous g-C3N4 nanotube was successfully prepared using the molecular self-assembly impregnation method because Ni-doping can suppress the shape collapse and destruction of the nanotube under the thermal polymerization process through strong coordinate bonding and the formation of Ni-P. P/Ni co-doping can not only enhance visible-light absorption using a sub-bandgap, suppress charge recombination and facilitate photoelectric conversion, but it also creates more pores for photocatalytic reaction sites. As expected, the PNCNT showed an outstanding photocatalytic performance in the H2 evolution of 240.91 μmol·g−1·h−1 under visible light irradiation, which was 30.5 times higher than that of the CNT. Moreover, the PNCNT had good stability in photocatalytic H2 evolution when exposed to testing under visible-light irradiation for a long period of time. This work provides a deep insight and a convenient route for constructing high-performance g-C3N4-based photocatalysts with the combination of surface electronic regulation and nanostructure engineering.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12172929/s1, Figure S1: The EDX spectrum of PNCNT samples; Figure S2: The XPS survey spectra of CNT and PNCNT samples; Figure S3: Time courses of photocatalytic H2 evolution (a) and H2 evolution rate (b) of CNT, NCNT, PCNT and PNCNT-x samples under visible light; Figure S4: Plots of (ahv)1/2 vs. photon energy (a) and XPS-VB of CNT and PNCNT (b); Table S1: The BET surface area, pore volume and average pore size of CNT, NCNT, PCNT and PNCNT samples; Table S2: Portion of each peak in the C 1s XPS spectra of CNT and PNCNT; Table S3: Comparison with other g-C3N4-based photocatalysts for HER rate. References [40,41,42,43,44,45] were cited in the Supplementary Materials.
Author Contributions
Data collection and curation and Writing—original draft, X.Z.; Methodology and Investigation, T.L.; Conceptualization and Funding acquisition, C.H.; Data collection, X.Y.; Data collection, K.Q.; Conceptualization, Supervision, Project administration, Funding acquisition and Writing—review and editing, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation, grant numbers 52150056 and 52000044; the Natural Science Foundation of Guangdong Province, grant numbers 2021A1515012610; the Science and Technology Program of Guangzhou, grant number 202102010418; and the Graduate Innovative Research Grant Program of Guangzhou University, grant number 2021GDJC-M48.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2021, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Heng, S.; Wang, W.; Guo, H.; Li, H.; Dang, D. Binary Type-II Heterojunction K7HNb6O19/g-C3N4: An Effective Photocatalyst for Hydrogen Evolution without a Co-Catalyst. Nanomaterials 2022, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, G.; Chen, X.; Lin, S.; Mohlmann, L.; Dolega, G.; Lipner, G.; Antonietti, M.; Blechert, S.; Wang, X. Co-monomer control of carbon nitride semiconductors to optimize hydrogen evolution with visible light. Angew. Chem. Int. Ed. Engl. 2012, 51, 3183–3187. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Enhanced catalytic activity of potassium-doped graphitic carbon nitride induced by lower valence position. Appl. Catal. B 2015, 164, 77–81. [Google Scholar] [CrossRef]
- Xing, W.; Cheng, K.; Zhang, Y.; Ran, J.; Wu, G. Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production. Nanomaterials 2021, 11, 1480. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; Wang, Y.; Fang, J.; Qi, Y.; Zhou, Y.; Liu, L.; Zhuo, S. A self-assembly strategy to synthesize carbon doped carbon nitride microtubes with a large π-electron conjugated system for efficient H2 evolution. Chem. Eng. J. 2022, 447, 137436. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, J.; Zhai, X.; Guan, F.; Wang, X.; Zhang, J.; Hou, B. Photocatalytic Degradation and Antibacterial Properties of Fe(3+)-Doped Alkalized Carbon Nitride. Nanomaterials 2020, 10, 1751. [Google Scholar] [CrossRef]
- Lin, B.; Li, S.; Peng, Y.; Chen, Z.; Wang, X. MOF-derived core/shell C-TiO2/CoTiO3 type II heterojunction for efficient photocatalytic removal of antibiotics. J. Hazard. Mater. 2021, 406, 124675. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2019, 10, 1. [Google Scholar] [CrossRef]
- Lv, C.; Qin, S.; Lei, Y.; Li, X.; Huang, J.; Liu, J. Direct Z-Scheme Heterojunction Catalysts Constructed by Graphitic-C3N4 and Photosensitive Metal-Organic Cages for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 890. [Google Scholar] [CrossRef]
- Xia, Y.; Liang, R.; Yang, M.Q.; Zhu, S.; Yan, G. Construction of Chemically Bonded Interface of Organic/Inorganic g-C3N4/LDH Heterojunction for Z-Schematic Photocatalytic H2 Generation. Nanomaterials 2021, 11, 2762. [Google Scholar] [CrossRef]
- Niu, H.; Wan, X.; Wang, X.; Shao, C.; Robertson, J.; Zhang, Z.; Guo, Y. Single-Atom Rhodium on Defective g-C3N4: A Promising Bifunctional Oxygen Electrocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 3590–3599. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon Quantum Dot Implanted Graphite Carbon Nitride Nanotubes: Excellent Charge Separation and Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2018, 57, 5765–5771. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Z.; Jin, C.; Li, W.; Wei, Q.; Chen, Y.; Wang, T. Flower-Like Dual-Defective Z-Scheme Heterojunction g-C3N4/ZnIn2S4 High-Efficiency Photocatalytic Hydrogen Evolution and Degradation of Mixed Pollutants. Nanomaterials 2021, 11, 2483. [Google Scholar] [CrossRef]
- Yu, D.; Jia, T.; Deng, Z.; Wei, Q.; Wang, K.; Chen, L.; Wang, P.; Cui, J. One-Dimensional P-Doped Graphitic Carbon Nitride Tube: Facile Synthesis, Effect of Doping Concentration, and Enhanced Mechanism for Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1759. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-Doped Graphitic Carbon Nitride Nanotubes with Amino-rich Surface for Efficient CO2 Capture, Enhanced Photocatalytic Activity, and Product Selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, C.; Shi, R.; Liu, Q.; Waterhouse, G.I.N.; Wu, L.; Tung, C.-H.; Zhang, T. Supramolecular precursor strategy for the synthesis of holey graphitic carbon nitride nanotubes with enhanced photocatalytic hydrogen evolution performance. Nano Res. 2019, 12, 2385–2389. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Wu, J.; Yan, P.; Xu, L.; Lei, Y.; Yuan, S.; et al. Self-assembled synthesis of defect-engineered graphitic carbon nitride nanotubes for efficient conversion of solar energy. Appl. Catal. B 2018, 225, 154–161. [Google Scholar] [CrossRef]
- Hoang, T.V.A.; Nguyen, T.K.A.; Dao, D.Q.; Nguyen, P.A.; Jeong, D.H.; Shin, E.W. Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors. Nanomaterials 2022, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Palani, G.; Apsari, R.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Kannan, K.; Shivanna, A.T.; Idris, A.M.; Yadav, C.H. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications-A Review. Nanomaterials 2022, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, Z.; Huang, S.; Zhang, Y.; Zhang, M.; Zeng, Y.J.; Ruan, S. Ce-Doped Graphitic Carbon Nitride Derived from Metal Organic Frameworks as a Visible Light-Responsive Photocatalyst for H2 Production. Nanomaterials 2019, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Ma, T.Y.; Gao, G.; Du, X.-W.; Qiao, S.Z. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lok, L.-W.; Veksha, A.; Giannis, A.; Lim, T.-T. Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C3N4 under solar irradiation: Performance and mechanistic studies. Chem. Eng. J. 2018, 333, 739–749. [Google Scholar] [CrossRef]
- Long, D.; Chen, W.; Zheng, S.; Rao, X.; Zhang, Y. Barium- and Phosphorus-Codoped g-C3N4 Microtubes with Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. Ind. Eng. Chem. Res. 2020, 59, 4549–4556. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Hou, Y.-N.; Tang, Y.; Dong, Y.; Wang, S.; Hu, X.; Zhang, Z.; Wang, X.; Qiu, J. Nanopore-confined g-C3N4 nanodots in N, S co-doped hollow porous carbon with boosted capacity for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 7133–7141. [Google Scholar] [CrossRef]
- Shen, H.; Li, M.; Guo, W.; Li, G.; Xu, C. P, K co-doped porous g-C3N4 with enhanced photocatalytic activity synthesized in vapor and self-producing NH3 atmosphere. Appl. Surf. Sci. 2020, 507, 145086. [Google Scholar] [CrossRef]
- Fang, W.; Liu, J.; Yu, L.; Jiang, Z.; Shangguan, W. Novel (Na, O) co-doped g-C3N4 with simultaneously enhanced absorption and narrowed bandgap for highly efficient hydrogen evolution. Appl. Catal. B 2017, 209, 631–636. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, T.; Liu, Q.; Zhang, Z.; Fang, X. Insight into the Enhanced Photocatalytic Activity of Potassium and Iodine Codoped Graphitic Carbon Nitride Photocatalysts. J. Phys. Chem. C 2016, 120, 25328–25337. [Google Scholar] [CrossRef]
- Chen, K.-L.; Zhang, S.-S.; Yan, J.-Q.; Peng, W.; Lei, D.-P.; Huang, J.-H. Excellent visible light photocatalytic efficiency of Na and S co-doped g-C3N4 nanotubes for H2 production and organic pollutant degradation. Int. J. Hydrogen Energy 2019, 44, 31916–31929. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, H.; Zhang, C.; Wang, Y.; Wei, Q.; Zhang, R. Interfacial Electronic Effects in Co@N-Doped Carbon Shells Heterojunction Catalyst for Semi-Hydrogenation of Phenylacetylene. Nanomaterials 2021, 11, 2776. [Google Scholar] [CrossRef]
- Li, S.; Peng, Y.; Hu, C.; Chen, Z. Self-assembled synthesis of benzene-ring-grafted g-C3N4 nanotubes for enhanced photocatalytic H2 evolution. Appl. Catal. B-Environ. 2020, 279, 119401. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, W.; Chen, Q.; Pu, Y.; He, H.; Zhuang, W.; He, J.; Huang, L. A hierarchical carbon nitride tube with oxygen doping and carbon defects promotes solar-to-hydrogen conversion. J. Mater. Chem. A 2020, 8, 3160–3167. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, X.; Li, L.; Sun, T. P, S Co-doped g-C3N4 isotype heterojunction composites for high-efficiency photocatalytic H2 evolution. J. Alloys Compd. 2020, 827, 154259. [Google Scholar] [CrossRef]
- Du, R.; Xiao, K.; Li, B.; Han, X.; Zhang, C.; Wang, X.; Zuo, Y.; Guardia, P.; Li, J.; Chen, J.; et al. Controlled oxygen doping in highly dispersed Ni-loaded g-C3N4 nanotubes for efficient photocatalytic H2O2 production. Chem. Eng. J. 2022, 441, 135999. [Google Scholar] [CrossRef]
- Fang, H.-B.; Zhang, X.-H.; Wu, J.; Li, N.; Zheng, Y.-Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Q.; Ma, L.; Wu, H.; Li, Y.; Han, J.; Chen, G.; Xing, W. Strengthening reactive metal–support interaction to stabilize Ni species on the nitrogen vacancies of g-C3N4 for boosting photocatalytic H2 production. Catal. Sci. Technol. 2021, 11, 7134–7140. [Google Scholar] [CrossRef]
- Pi, W.; Humayun, M.; Li, Y.; Yuan, Y.; Cao, J.; Ali, S.; Wang, M.; Li, H.; Khan, A.; Zheng, Z.; et al. Properly aligned band structures in B-TiO2/MIL53(Fe)/g-C3N4 ternary nanocomposite can drastically improve its photocatalytic activity for H2 evolution: Investigations based on the experimental results. Int. J. Hydrogen Energy 2021, 46, 21912–21923. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Mu, L.; Hu, M.; Dong, B.; Zhang, F. Synthesis of a novel nitrogen-doped K2Ti6O13 nanorod with visible-light-driven water splitting performance promoted by fabrication of 1D/2D heterostructure. Appl. Surf. Sci. 2022, 581, 152345. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Wang, J.; He, Z.; Li, Z. Double Z-scheme photocatalyst C3N4 nanotube/N-doped carbon dots/Ni2P with enhanced visible-light photocatalytic activity for hydrogen generation. Appl. Surf. Sci. 2020, 534, 147603. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Wang, J.; He, Z.; Li, Z. Novel B-N-Co surface bonding states constructed on hollow tubular boron doped g-C3N4/CoP for enhanced photocatalytic H2 evolution. J. Colloid Interface Sci. 2021, 595, 69–77. [Google Scholar] [CrossRef]
- Wu, Y.; Song, M.; Chai, Z.; Wang, X. Enhanced photocatalytic activity of Ag/Ag2Ta4O11/g-C3N4 under wide-spectrum-light irradiation: H2 evolution from water reduction without co-catalyst. J. Colloid Interface Sci. 2019, 550, 64–72. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).