Bio-Based Carbon Materials for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
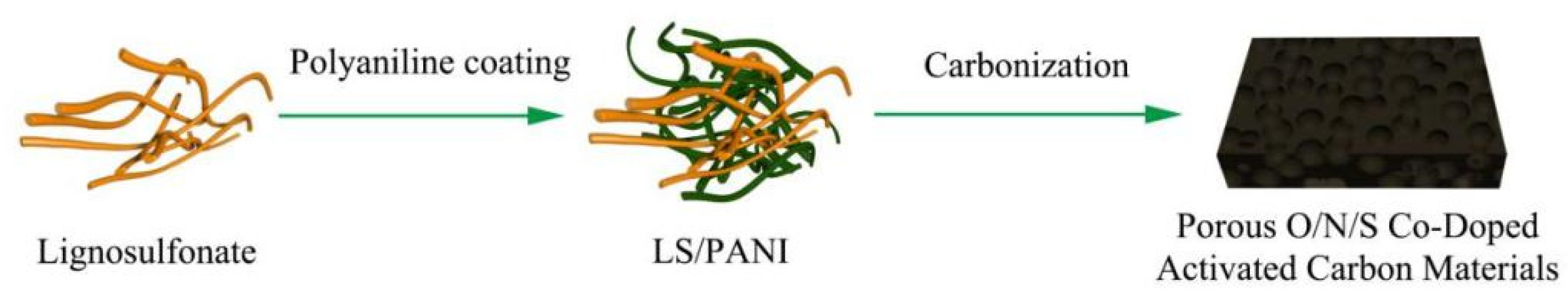
2.2. Preparation of LS/PANI
2.3. Preparation of Porous O/N/S Co-Doped Activated Carbon Materials
2.4. Material Characterizations
2.5. Preparation of Working Electrodes
2.6. Electrochemical Measurements
3. Results and Discussion
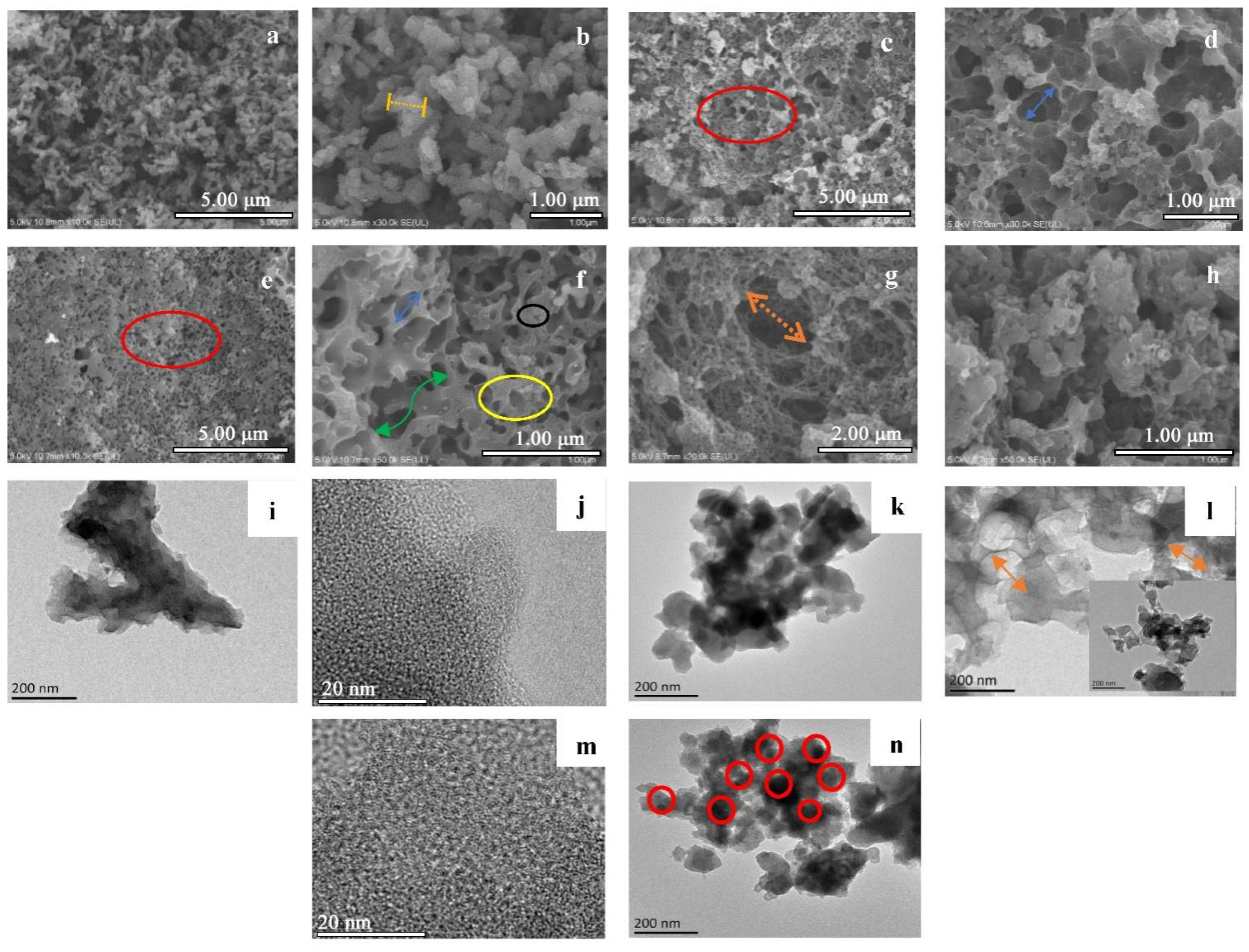
3.1. Morphological Analysis
3.1.1. Scanning Electron Microscopy (SEM)
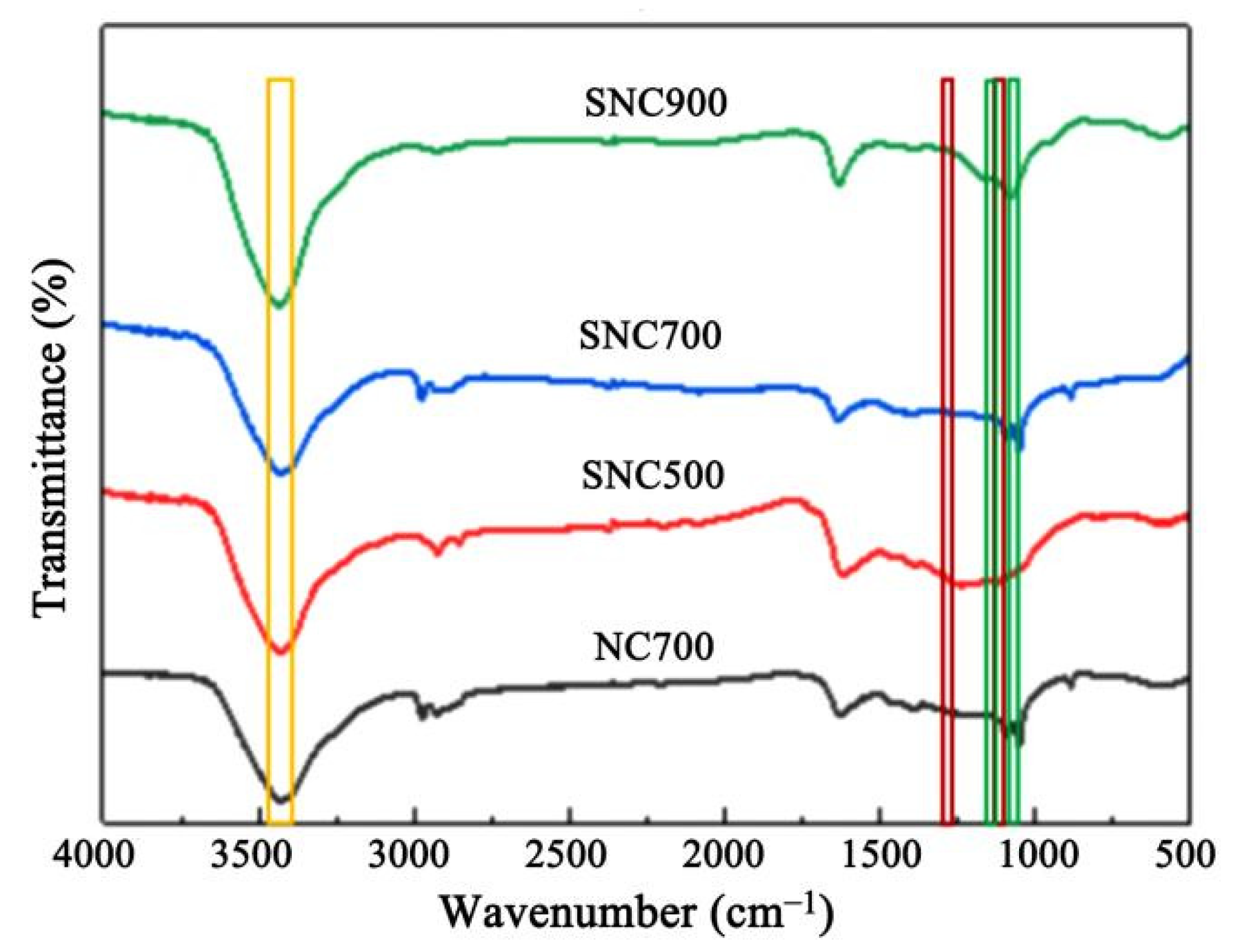
3.1.2. FT-IR Analysis
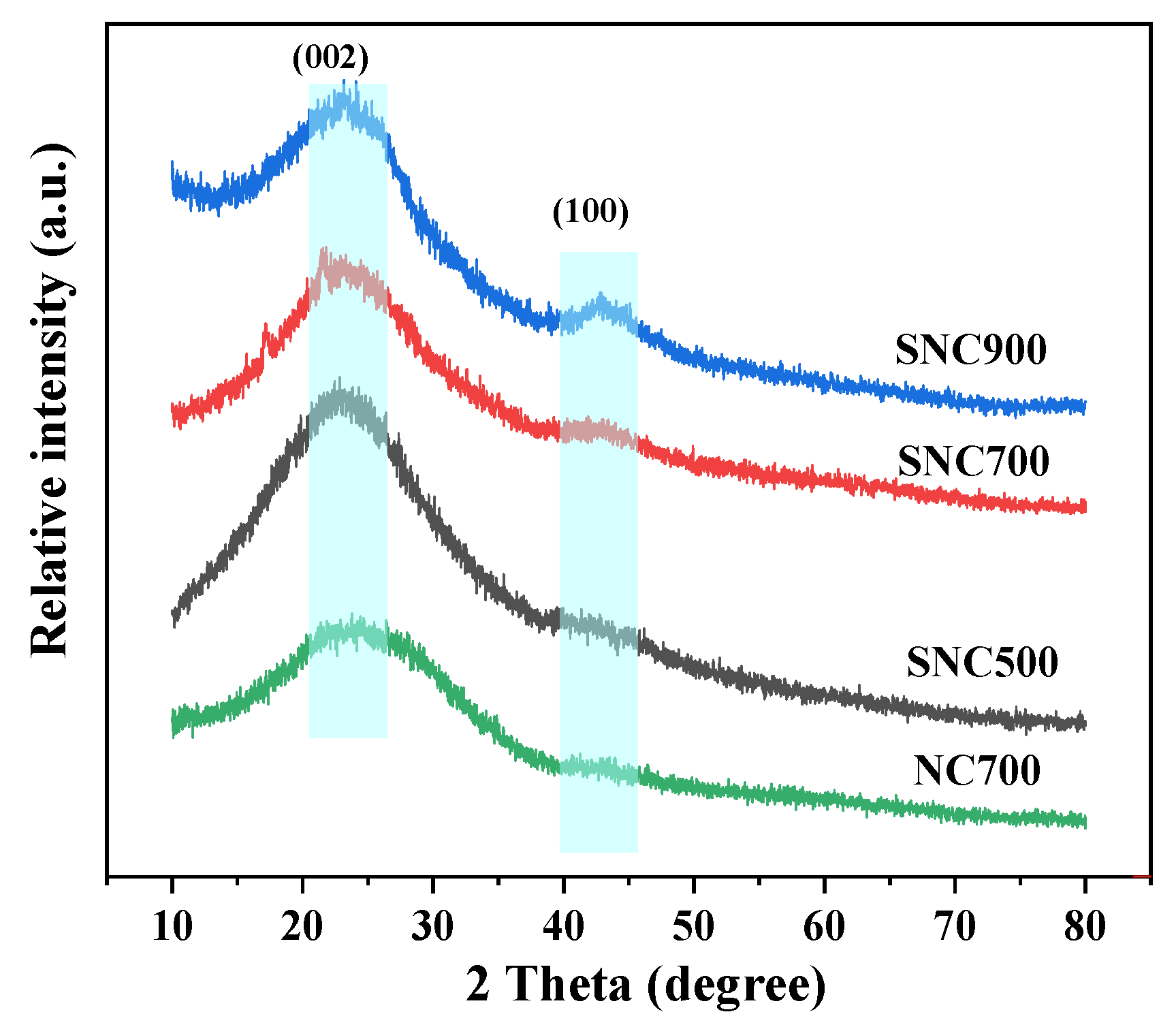
3.1.3. XRD Analysis
3.1.4. Raman Spectroscopy Analysis
3.1.5. XPS Analysis
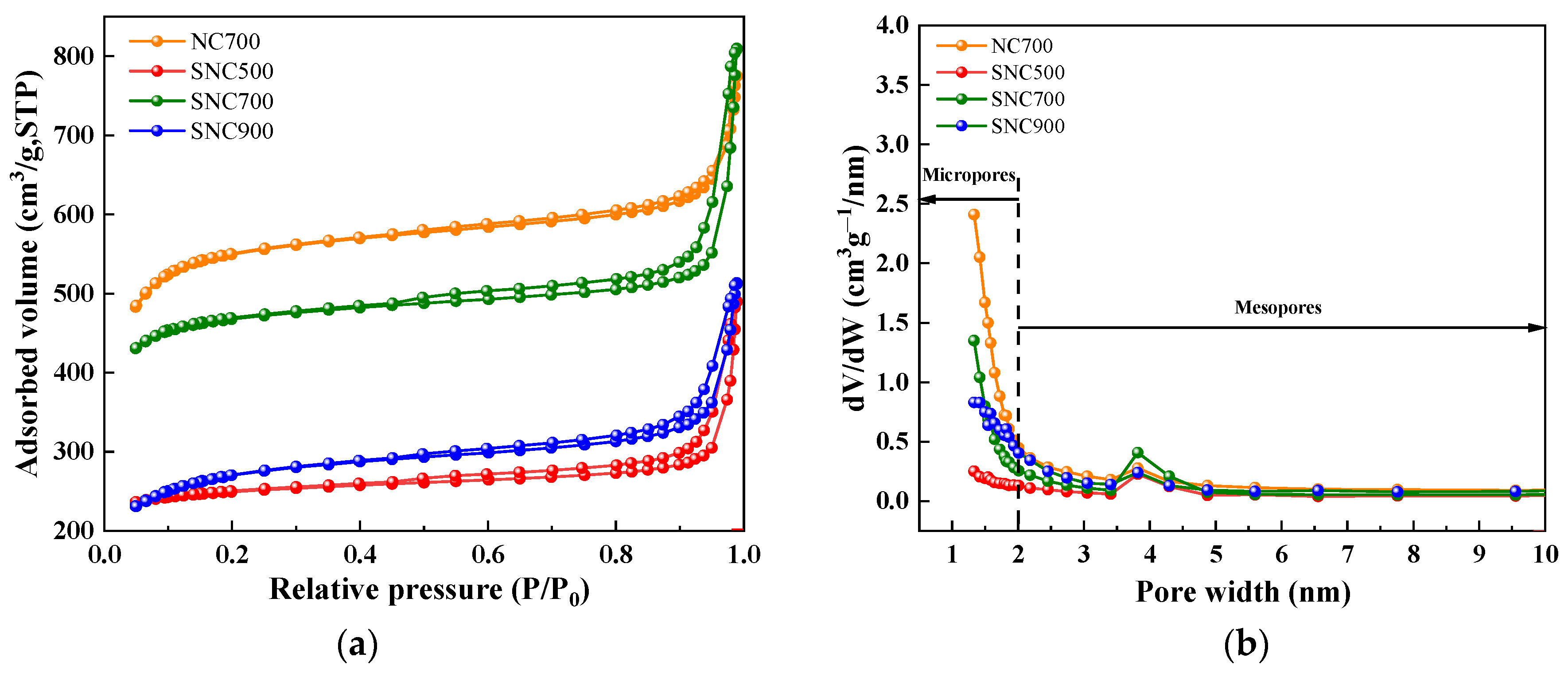
3.1.6. BET Specific Surface Area and Pore Size Analysis
3.2. Electrochemical Performance Analysis (Three-Electrode System)
3.2.1. Cyclic Voltammetry
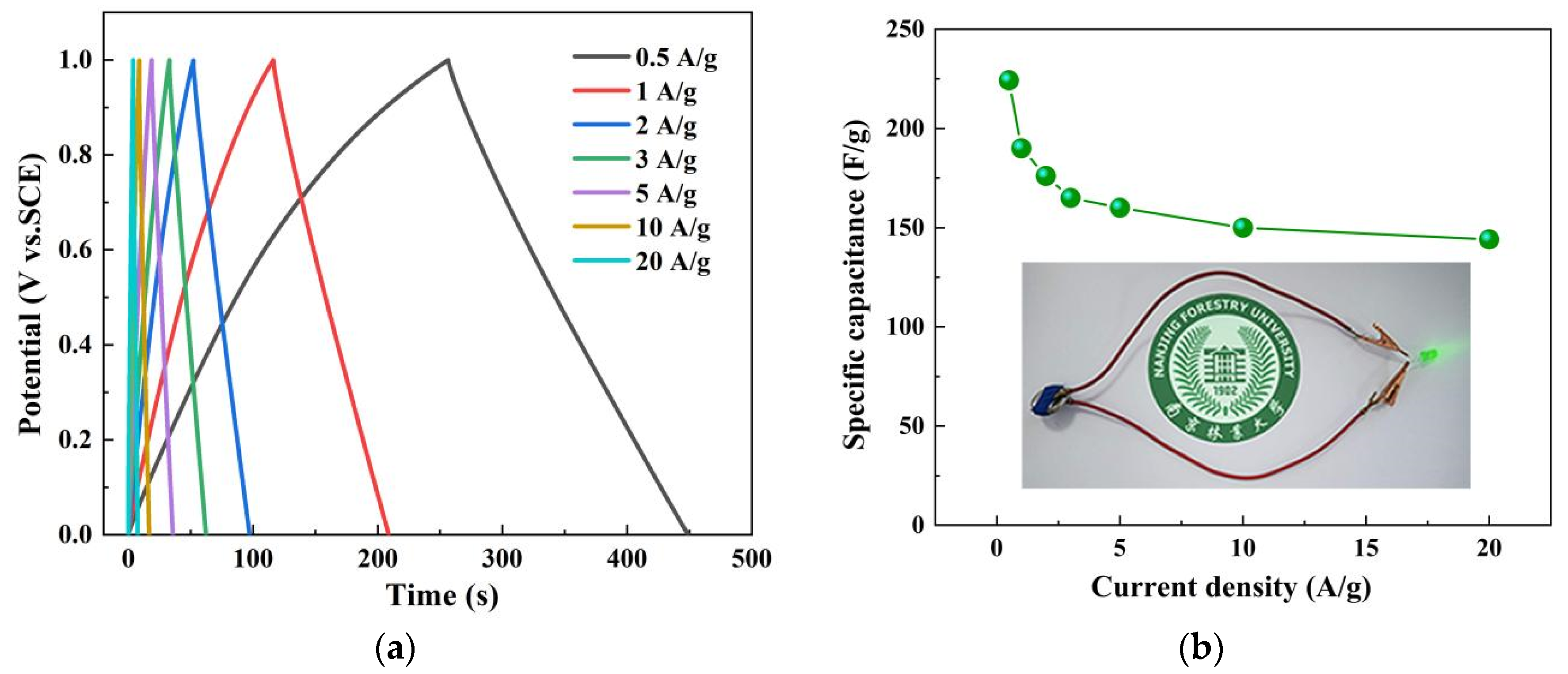
3.2.2. Constant Current Charge and Discharge
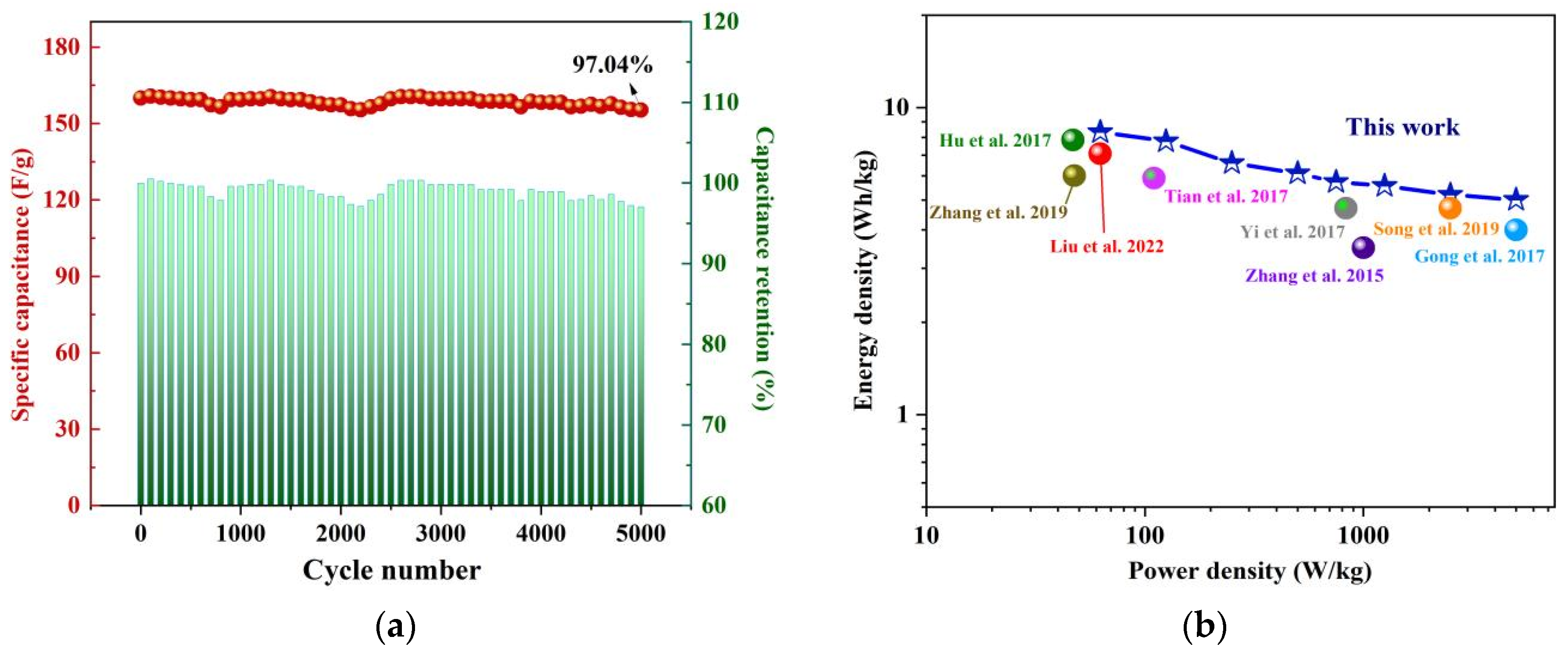
3.2.3. Cyclic Stability
3.2.4. Electrochemical Impedance Spectra
3.3. Electrochemical Performance Analysis (Two-Electrode System)
3.3.1. Cyclic Voltammetry
3.3.2. Galvanostatic Current Charge and Discharge
3.3.3. Cyclic Stability and Ragone Plot
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, S.; Mo, L.; Wang, F. One-step synthesis of O-self-doped honeycomb-like hierarchically porous carbons for supercapacitors. J. Electrochem. Energy Convers. Storage 2022, 19, 011003. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Castro, C.D.; Quintana, G. Kraft lignin as a raw material of activated carbon for supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2022, 33, 7031–7047. [Google Scholar] [CrossRef]
- Jeon, J.W.; Zhang, L.; Lutkenhaus, J.L.; Laskar, D.D.; Lemmon, J.P.; Choi, D.; Nandasiri, M.I.; Hashmi, A.; Xu, J.; Motkuri, R.K.; et al. Controlling porosity in lignin-derived nanoporous carbon for supercapacitor applications. ChemSusChem 2015, 8, 428–432. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Wang, C.; Zhao, L.; Jian, W.; Lu, K.; Lin, H.; Qiu, X.; Alshareef, H.N. Lignin derived porous carbons: Synthesis methods and supercapacitor applications. Small Methods 2021, 5, 2100896. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Doguscu, M. Preparation of porous carbons using NaOH, K2CO3, Na2CO3 and Na2S2O3 activating agents and their supercapacitor application: A comparative study. ChemistrySelect 2022, 7, e202104295. [Google Scholar] [CrossRef]
- Zhang, X.; Jian, W.; Zhao, L.; Wen, F.; Chen, J.; Yin, J.; Qin, Y.; Lu, K.; Zhang, W.; Qiu, X. Direct carbonization of sodium lignosulfonate through self-template strategies for the synthesis of porous carbons toward supercapacitor applications. Colloid. Surf. A 2022, 636, 128191. [Google Scholar] [CrossRef]
- Liu, S.; Ma, M.G. Lignin-derived nitrogen-doped polyacrylonitrile/polyaniline carbon nanofibers by electrospun method for energy storage. Ionics 2020, 26, 4651–4660. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Sieben, J.M.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Morallón, E. Asymmetric hybrid capacitors based on activated carbon and activated carbon fibre–PANI electrodes. Electrochim. Acta 2013, 89, 326–333. [Google Scholar] [CrossRef]
- Herou, S.; Ribadeneyra, M.C.; Madhu, R.; Araullo-Peters, V.; Jensen, A.; Schlee, P.; Titirici, M. Ordered mesoporous carbons from lignin: A new class of biobased electrodes for supercapacitors. Green Chem. 2019, 21, 550–559. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Ruiz-Rosas, R.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T.; Morallón, E.; Cazorla-Amorós, D. Asymmetric capacitors using lignin-based hierarchical porous carbons. J. Power Sources 2016, 326, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Huang, L.; Linhardt, R.J.; Koratkar, N.; Simmons, T. Repurposing paper by-product lignosulfonate as a sulfur donor/acceptor for high performance lithium–sulfur batteries. Sustain. Energy Fuels 2018, 2, 422–429. [Google Scholar] [CrossRef]
- Yun, X.; Zhang, Q.; Luo, B.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Fabricating flexibly resistive humidity sensors with ultra-high sensitivity using carbonized lignin and sodium alginate. Electroanalysis 2020, 32, 2282–2289. [Google Scholar] [CrossRef]
- Lee, D.W.; Jin, M.H.; Park, J.H.; Lee, Y.J.; Choi, Y.C. Flexible synthetic strategies for lignin-derived hierarchically porous carbon materials. ACS Sustain. Chem. Eng. 2018, 6, 10454–10462. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Xu, H.; Wang, G. Construction of polyaniline/lignin composite with interpenetrating fibrous networks and its improved electrochemical capacitance performances. Synth. Met. 2019, 249, 40–46. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Y.; Gu, Z.; Fan, Q.H. Symmetric supercapacitors using urea-modified lignin derived N-doped porous carbon as electrode materials in liquid and solid electrolytes. J. Power Sources 2016, 332, 180–186. [Google Scholar] [CrossRef]
- Guo, N.; Luo, W.; Zhou, D.; Xu, M.; Qiu, D.; Gao, A.; Ma, R.; Sheng, R.; Jia, D.; Wang, L. In-situ expansion strategy towards hierarchical mesoporous carbon: Formation mechanism and application in supercapacitors. Int. J. Energ. Res. 2022, 46, 7249–7260. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; González-Gaitán, C.; Morallón, E.; Cazorla-Amorós, D. Effect of carbonization conditions of polyaniline on its catalytic activity towards ORR. Some insights about the nature of the active sites. Carbon 2017, 119, 62–71. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Castro-Muñiz, A.; Nishihara, H.; Kyotani, T.; Morallón, E.; Cazorla-Amorós, D. Ultraporous nitrogen-doped zeolite-templated carbon for high power density aqueous-based supercapacitors. Carbon 2018, 129, 510–519. [Google Scholar] [CrossRef]
- Park, S.; Choi, M.S.; Park, H.S. Nitrogen-doped nanoporous carbons derived from lignin for high CO2 capacity. Carbon Lett. 2019, 29, 289–296. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Z.; Li, Z.; Wang, W.; Zhang, H. Hierarchical S-doped porous carbon derived from by-product lignin for high-performance supercapacitors. RSC Adv. 2017, 7, 12089–12097. [Google Scholar] [CrossRef] [Green Version]
- Demir, M.; Farghaly, A.A.; Decuir, M.J.; Collinson, M.M.; Gupta, R.B. Supercapacitance and oxygen reduction characteristics of sulfur self-doped micro/mesoporous bio-carbon derived from lignin. Mater. Chem. Phys. 2018, 216, 508–516. [Google Scholar] [CrossRef]
- Tian, J.; Liu, C.; Lin, C.; Ma, M. Constructed nitrogen and sulfur codoped multilevel porous carbon from lignin for high-performance supercapacitors. J. Alloys Compd. 2019, 789, 435–442. [Google Scholar] [CrossRef]
- Deng, Q.; Abbas, S.C.; Li, Z.; Lv, J.; Ma, X.; Cao, S.; Ni, Y.; Zhao, W. Chemically modified self-doped biocarbon via novel sulfonation assisted sacrificial template method for high performance flexible all solid-state supercapacitor. J. Colloid. Interf. Sci. 2020, 574, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dianat, N.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Chang, X.; Kaner, R.B.; Mousavi, M.F. Polyaniline-lignin interpenetrating network for supercapacitive energy storage. Nano Lett. 2021, 21, 9485–9493. [Google Scholar] [CrossRef]
- Jiang, Q.; Jing, Y.; Wang, J.; Ni, Y.; Gao, R.; Wang, Y.; Zhang, J.; Yin, H. Lignosulfonate for improving electrochemical performance of chitin derived carbon materials as a superior anode for lithium-ion batteries. J. Alloys Compd. 2021, 885, 160973. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Li, J.; Li, M.; Lu, C. Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous Mesoporous Mater. 2017, 243, 291–300. [Google Scholar] [CrossRef]
- Arslanoğlu, H. Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J. Hazard. Mater. 2019, 374, 238–247. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Hunsom, M.; Autthanit, C. Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH. Chem. Eng. J. 2013, 229, 334–343. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710. [Google Scholar] [CrossRef]
- Arias-Ferreiro, G.; Ares-Pernas, A.; Lasagabáster-Latorre, A.; Dopico-García, M.S.; Ligero, P.; Pereira, N.; Costa, P.; Lanceros-Mendez, S.; Abad, M.J. Photocurable printed piezocapacitive pressure sensor based on an acrylic resin modified with polyaniline and lignin. Adv. Mater. Technol. 2022, 7, 2101503. [Google Scholar] [CrossRef]
- Fu, F.; Wang, H.; Yang, D.; Qiu, X.; Li, Z.; Qin, Y. Lamellar hierarchical lignin-derived porous carbon activating the capacitive property of polyaniline for high-performance supercapacitors. J. Colloid. Interf. Sci. 2022, 617, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Zhao, Y.; Li, Z.; Lai, S.; Zeng, Q.; Liu, C.; Liu, Y. Preparation of lignin-based carbon/polyaniline composites for advanced microwave absorber. Diam. Relat. Mater. 2021, 111, 108219. [Google Scholar] [CrossRef]
- Yu, S.; Liu, D.; Zhao, S.; Bao, B.; Jin, C.; Huang, W.; Chen, H.; Shen, Z. Synthesis of wood derived nitrogen-doped porous carbon–polyaniline composites for supercapacitor electrode materials. RSC Adv. 2015, 5, 30943–30949. [Google Scholar] [CrossRef]
- Wan, X.; Shen, F.; Hu, J.; Huang, M.; Zhao, L.; Zeng, Y.; Tian, D.; Yang, G.; Zhang, Y. 3-D hierarchical porous carbon from oxidized lignin by one-step activation for high-performance supercapacitor. Int. J. Biol. Macromol. 2021, 180, 51–60. [Google Scholar] [CrossRef]
- Gindl-Altmutter, W.; Köhnke, J.; Unterweger, C.; Gierlinger, N.; Keckes, J.; Zalesak, J.; Rojas, O.J. Lignin-based multiwall carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2019, 121, 175–179. [Google Scholar] [CrossRef]
- Chang, Z.; Dai, J.; Xie, A.; He, J.; Zhang, R.; Tian, S.; Yan, Y.; Li, C.; Xu, W.; Shao, R. From lignin to three-dimensional interconnected hierarchically porous carbon with high surface area for fast and superhigh-efficiency adsorption of sulfamethazine. Ind. Eng. Chem. Res. 2017, 56, 9367–9375. [Google Scholar] [CrossRef]
- Duan, B.; Gao, X.; Yao, X.; Fang, Y.; Huang, L.; Zhou, J.; Zhang, L. Unique elastic N-doped carbon nanofibrous microspheres with hierarchical porosity derived from renewable chitin for high rate supercapacitors. Nano Energy 2016, 27, 482–491. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, S.; Wu, W.; Yang, J.; Li, J.; Shi, K.; Liu, H. Influence of Lignin units on the properties of Lignin/PAN-derived carbon fibers. J. Appl. Polym. Sci. 2020, 137, 49274. [Google Scholar] [CrossRef]
- Yin, W.M.; Tian, L.F.; Pang, B.; Guo, Y.R.; Li, S.J.; Pan, Q.J. Fabrication of dually N/S-doped carbon from biomass lignin: Porous architecture and high-rate performance as supercapacitor. Int. J. Biol. Macromol. 2020, 156, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wan, S.; Gao, S. Self-assembly-template engineering nitrogen-doped carbon aerogels for high-rate supercapacitors. Nano Energy 2016, 28, 206–215. [Google Scholar] [CrossRef]
- Gu, W.; Sevilla, M.; Magasinski, A.; Fuertes, A.B.; Yushin, G. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: A case study for pseudocapacitance detection. Energy Environ. Sci. 2013, 6, 2465–2476. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Zhang, H.; Jin, L.; Chu, X.; Gu, B.; Huang, H.; Yang, W. Nitrogen, oxygen and sulfur co-doped hierarchical porous carbons toward high-performance supercapacitors by direct pyrolysis of kraft lignin. Carbon 2019, 149, 105–116. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.L. Lignin derived activated carbon particulates as an electric supercapacitor: Carbonization and activation on porous structures and microstructures. RSC Adv. 2017, 7, 30459–30468. [Google Scholar] [CrossRef]
- Kijima, M.; Hirukawa, T.; Hanawa, F.; Hata, T. Thermal conversion of alkaline lignin and its structured derivatives to porous carbonized materials. Bioresour. Technol. 2011, 102, 6279–6285. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Liu, W.; Shao, Y.; Ren, X.; Lv, C.; Liu, Q. Hierarchical porous carbon/Kraft lignin composite with significantly improved superior pseudocapacitive behavior. Electrochim. Acta 2021, 398, 139307. [Google Scholar] [CrossRef]
- Yang, S.; Wu, X.; Chen, C.; Dong, H.; Hu, W.; Wang, X. Spherical alpha-Ni(OH)2 nanoarchitecture grown on graphene as advanced electrochemical pseudocapacitor materials. Chem. Commun. 2012, 48, 2773–2775. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Chen, M.; Shi, S.; Cao, Y. Preparing hierarchical porous carbon aerogels based on enzymatic hydrolysis lignin through ambient drying for supercapacitor electrodes. Microporous Mesoporous Mater. 2018, 265, 258–265. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Wang, X.; Xu, K.; Zhong, J. Biomass-derived activated carbon nanoarchitectonics with hibiscus flowers for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Y.; Li, Y.; Xiao, H. Heteroatom-doped porous carbon microspheres derived from ionic liquid-lignin solution for high performance supercapacitors. J. Colloid. Interf. Sci. 2022, 614, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Wang, Y.; Yang, D.; Zhang, Z.; Liu, W.; Li, Q.; Qiu, X. K2CO3 activation enhancing the graphitization of porous lignin carbon derived from enzymatic hydrolysis lignin for high performance lithium-ion storage. J. Alloy Compd. 2019, 785, 706–714. [Google Scholar] [CrossRef]
- Nasser, R.; Jian, T.T.; Song, J.M. Hierarchical porous activated carbon derived from olives: Preparation, (N, S) co-doping, and its application in supercapacitors. J. Energy Storage 2022, 51, 104348. [Google Scholar] [CrossRef]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef]
- Zeng, L.; Lou, X.; Zhang, J.; Wu, C.; Liu, J.; Jia, C. Carbonaceous mudstone and lignin-derived activated carbon and its application for supercapacitor electrode. Surf. Coat. Technol. 2019, 357, 580–586. [Google Scholar] [CrossRef]
- Li, P.; Wei, Y.; Wu, C.; Yang, C.; Jiang, B.; Wu, W. Lignin-based composites for high-performance supercapacitor electrode materials. RSC Adv. 2022, 12, 19485–19494. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, H.; Lin, Z.; Yin, J.; Lu, H.; Liu, D.; Zhao, M. 3D hierarchical porous carbon for supercapacitors prepared from lignin through a facile template-free method. ChemSusChem 2015, 8, 2114–2122. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Shen, X.; Sun, Y.; Li, Z.; Yuan, A.; Zhai, L.; Zhang, D. Dissolution-assistant all-in-one synthesis of N and S dual-doped porous carbon for high-performance supercapacitors. Adv. Powder Technol. 2019, 30, 2211–2217. [Google Scholar] [CrossRef]
- Yi, J.; Qing, Y.; Wu, C.; Zeng, Y.; Wu, Y.; Lu, X.; Tong, Y. Lignocellulose-derived porous phosphorus-doped carbon as advanced electrode for supercapacitors. J. Power Sources 2017, 351, 130–137. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, P.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.; Deng, S. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2019, 355, 309–319. [Google Scholar] [CrossRef]


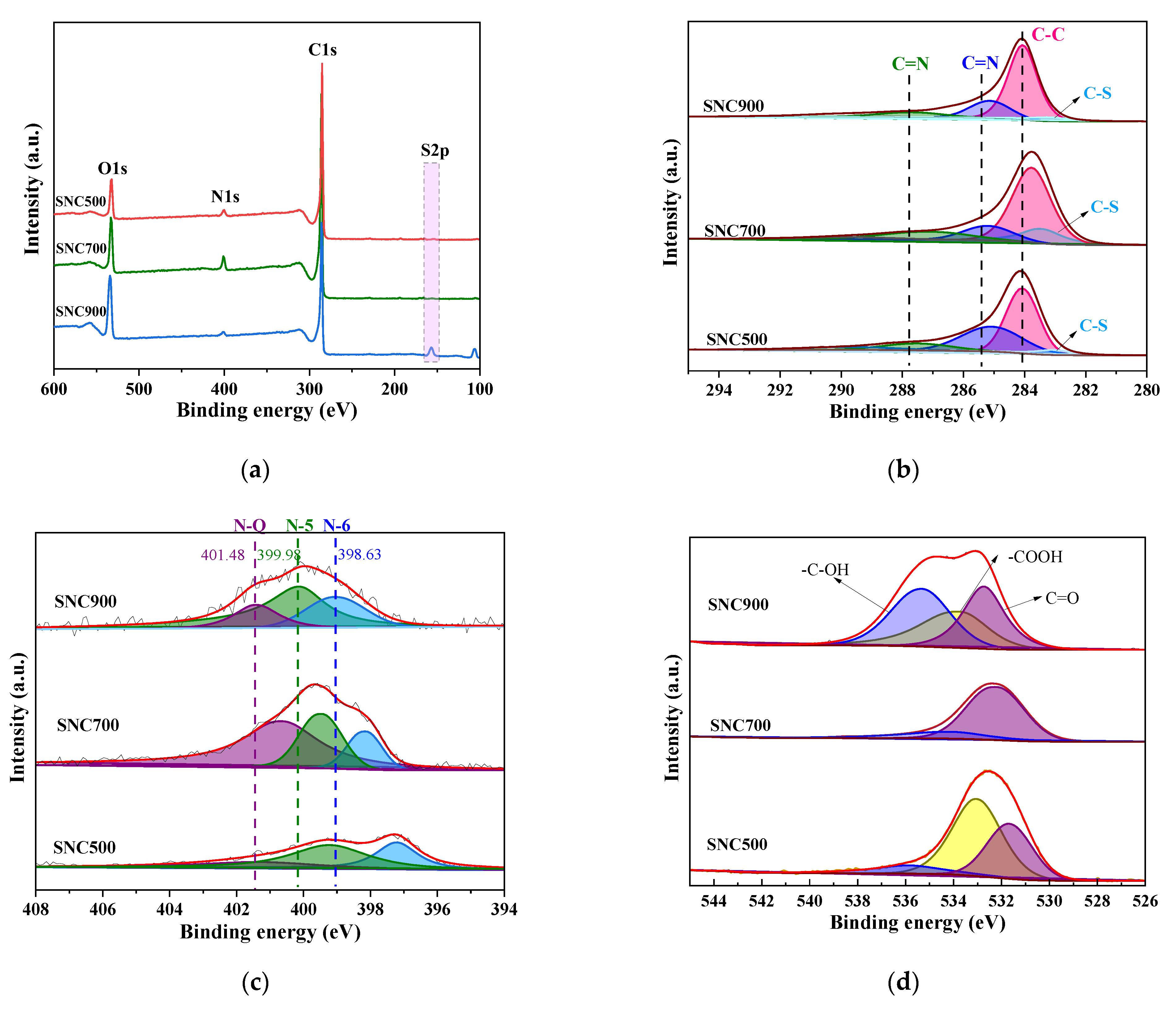
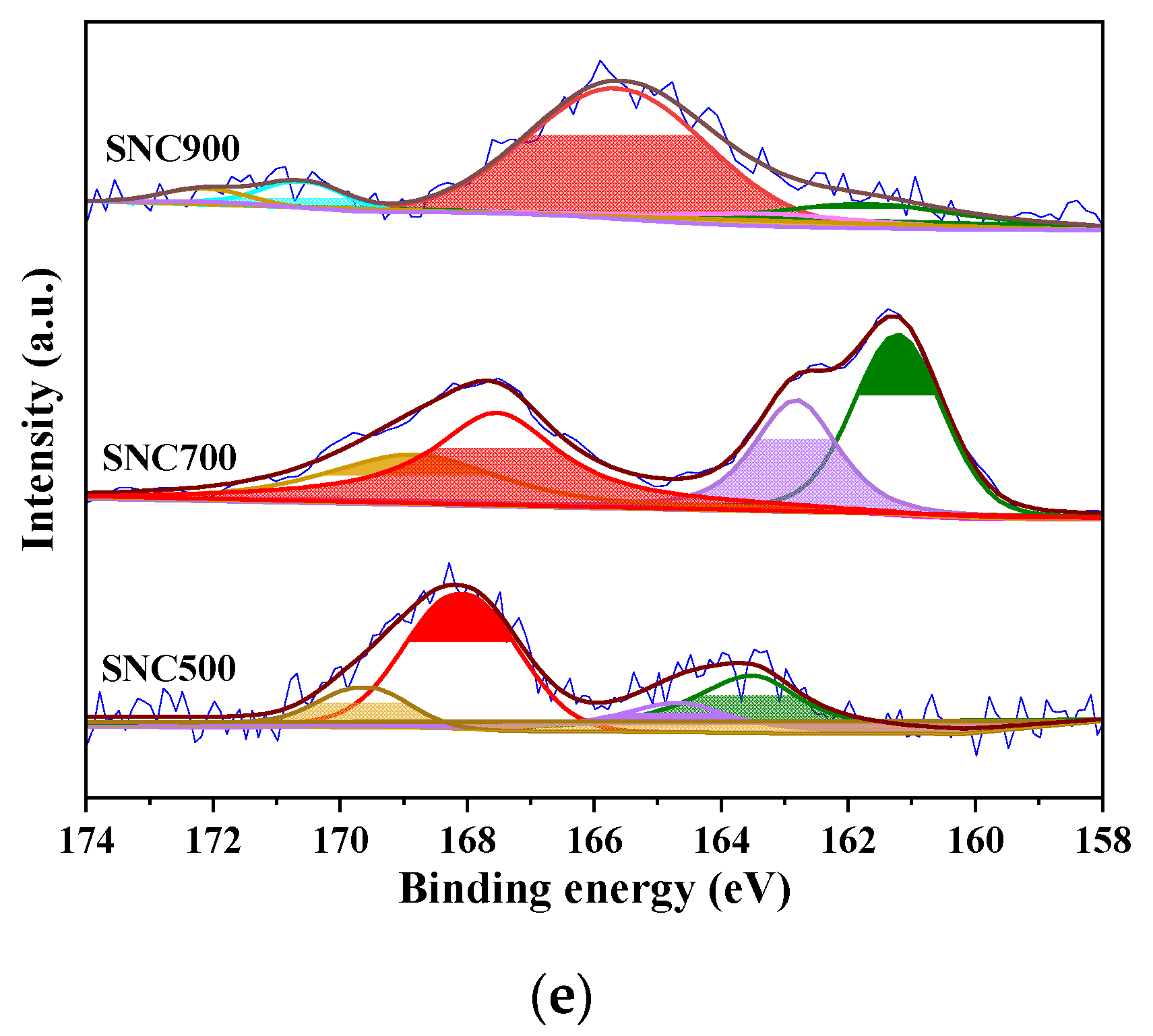





| Samples | N Content (at.%) | S Content (at.%) | O Content (at.%) | Content (at.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-5 | N-6 | N-Q | 2p1/2 | 2p2/3 | O-1 | O-2 | O-3 | C | N | S | O | |
| SNC500 | 29.20 | 60.12 | 10.66 | 15.11 | 84.88 | 34.58 | 54.73 | 10.68 | 90.44 | 5.97 | 0.56 | 3.01 |
| SNC700 | 27.48 | 17.23 | 55.23 | 20.42 | 79.57 | 78.98 | 19.01 | 1.96 | 86.98 | 7.27 | 1.01 | 4.72 |
| SNC900 | 24.95 | 36.52 | 38.51 | 7.56 | 92.43 | 31.66 | 29.35 | 38.98 | 86.50 | 5.61 | 0.89 | 6.98 |
| Sample | SBET (m2/g) | Vmic (cm3/g) | Vtot (cm3/g) | Dpor (nm) | Vmic/Vtotal (%) |
|---|---|---|---|---|---|
| NC700 | 875.0 | 0.37 | 1.20 | 2.27 | 31.2 |
| SNC500 | 184.3 | 0.05 | 0.76 | 3.96 | 59.3 |
| SNC700 | 511.4 | 0.26 | 1.25 | 2.75 | 20.5 |
| SNC900 | 383.2 | 0.12 | 0.80 | 2.72 | 15.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Yang, C.; Wu, C.; Wei, Y.; Jiang, B.; Jin, Y.; Wu, W. Bio-Based Carbon Materials for High-Performance Supercapacitors. Nanomaterials 2022, 12, 2931. https://doi.org/10.3390/nano12172931
Li P, Yang C, Wu C, Wei Y, Jiang B, Jin Y, Wu W. Bio-Based Carbon Materials for High-Performance Supercapacitors. Nanomaterials. 2022; 12(17):2931. https://doi.org/10.3390/nano12172931
Chicago/Turabian StyleLi, Penghui, Chi Yang, Caiwen Wu, Yumeng Wei, Bo Jiang, Yongcan Jin, and Wenjuan Wu. 2022. "Bio-Based Carbon Materials for High-Performance Supercapacitors" Nanomaterials 12, no. 17: 2931. https://doi.org/10.3390/nano12172931
APA StyleLi, P., Yang, C., Wu, C., Wei, Y., Jiang, B., Jin, Y., & Wu, W. (2022). Bio-Based Carbon Materials for High-Performance Supercapacitors. Nanomaterials, 12(17), 2931. https://doi.org/10.3390/nano12172931








