Fabrication, Thermal Conductivity, and Mechanical Properties of Hexagonal-Boron-Nitride-Pattern-Embedded Aluminum Oxide Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Compositions of Thermally Conductive Composites
2.4. An Amplitude Sweep Test and a 3ITT
2.5. Fabrication of Al/h-BN/Al Composites
2.6. Thermal Diffusivity Measurement
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, Y.-J.; Li, G.; Yao, Y.-M.; Zeng, X.-L.; Zhu, P.-L.; Sun, R. Recent advances in polymer-based electronic packaging materials. Compos. Commun. 2020, 19, 154–167. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Ouyang, Y.; Bai, L.; Tian, H.; Li, X.; Yuan, F. Recent progress of thermal conductive ploymer composites: Al2O3 fillers, properties and applications. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106685. [Google Scholar] [CrossRef]
- Lim, G.; Bok, G.; Kim, Y.-S.; Kim, Y. Fabrication of h-BN Filled Epoxy-Based Thermally Conductive Adhesive Tapes Containing Cyclic Carbonate-Terminated Oligomers. Electron. Mater. Lett. 2022, 18, 145–152. [Google Scholar] [CrossRef]
- Zou, D.; Huang, X.; Zhu, Y.; Chen, J.; Jiang, P. Boron nitride nanosheets endow the traditional dielectric polymer composites with advanced thermal management capability. Compos. Sci. Technol. 2019, 177, 88–95. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Lindsay, L.; Cherns, D.; Pomeroy, J.W.; Liu, S.; Edgar, J.H.; Kuball, M. Modulating the thermal conductivity in hexagonal boron nitride via controlled boron isotope concentration. Commun. Phys. 2019, 2, 43. [Google Scholar] [CrossRef]
- Yetgin, H.; Veziroglu, S.; Aktas, O.C.; Yalçinkaya, T. Enhancing thermal conductivity of epoxy with a binary filler system of h-BN platelets and Al2O3 nanoparticles. Int. J. Adhes. Adhes. 2020, 98, 102540. [Google Scholar] [CrossRef]
- Liu, D.; Ma, C.; Chi, H.; Li, S.; Zhang, P.; Dai, P. Enhancing thermal conductivity of polyimide composite film by electrostatic self-assembly and two-step synergism of Al2O3 microspheres and BN nanosheets. RSC Adv. 2020, 10, 42584–42595. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, G.; Wang, L.; Tian, L.; Chen, S.; Wu, G.; Kong, B.; Cheng, Y. Simultaneously enhanced dielectric properties and through-plane thermal conductivity of epoxy composites with alumina and boron nitride nanosheets. Sci. Rep 2021, 11, 2495. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, W.; Hu, H.; Liu, D.; Shen, H.; Wang, Z. The combination of Al2O3 and BN for enhancing the thermal conductivity of PA12 composites prepared by selective laser sintering. RSC Adv. 2021, 11, 1984–1991. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The synergistic effects of the micro-BN and nano-Al2O3 in micro-nano composites on enhancing the thermal conductivity for insulating epoxy resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Lim, G.; Bok, G.; Park, S.-D.; Kim, Y. Thermally conductive hexagonal boron nitride/spherical aluminum oxide hybrid composites fabricated with epoxyorganosiloxane. Ceram. Int. 2022, 48, 1408–1414. [Google Scholar] [CrossRef]
- Yun, H.; Han, C.J.; Park, J.B.; Kim, Y. Thermal conductivity and mechanical properties of thermally conductive composites based on multifunctional epoxyorganosiloxanes and hexagonal boron nitride. Ceram. Int. 2022, 48, 24431–24438. [Google Scholar] [CrossRef]
- Zalewski, K.; Chyłek, Z.; Trzciński, W.A. A Review of Polysiloxanes in Terms of Their Application in Explosives. Polymers 2021, 13, 1080. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Borodin, O.; Smith, G.D. A Quantum Chemistry Based Force Field for Poly(dimethylsiloxane). J. Phys. Chem. B 2004, 108, 20340–20350. [Google Scholar] [CrossRef]
- Weinhold, F.; West, R. The Nature of the Silicon–Oxygen Bond. Organometallics 2011, 30, 5815–5824. [Google Scholar] [CrossRef]
- Poon, L.; Hum, J.R.; Weiss, R.G. Neat Linear Polysiloxane-Based Ionic Polymers: Insights into Structure-Based Property Modifications and Applications. Macromol 2021, 1, 2–17. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X.; Ding, F.; Bai, L.; Yuan, F. Simultaneously enhance thermal conductive property and mechanical properties of silicon rubber composites by introducing ultrafine Al2O3 nanospheres prepared via thermal plasma. Compos. Sci. Technol. 2020, 190, 108019. [Google Scholar] [CrossRef]
- Yan, H.; Dai, X.; Ruan, K.; Zhang, S.; Shi, X.; Guo, Y.; Cai, H.; Gu, J. Flexible thermally conductive and electrically insulating silicone rubber composite films with BNNS@Al2O3 fillers. Adv. Compos. Hybrid Mater. 2021, 4, 36–50. [Google Scholar] [CrossRef]
- Bouville, F.; Deville, S. Dispersion of Boron Nitride Powders in Aqueous Suspensions with Cellulose. J. Am. Ceram. Soc. 2014, 97, 394–398. [Google Scholar] [CrossRef]
- Bashir, A.; Maqbool, M.; Lv, R.; Usman, A.; Guo, H.; Aftab, W.; Niu, H.; Liu, M.; Bai, S.-L. Surface modified boron nitride towards enhanced thermal and mechanical performance of thermoplastic polyurethane composite. Compos. B. Eng. 2021, 218, 108871. [Google Scholar] [CrossRef]
- Muratov, D.S.; Kuznetsov, D.V.; Il’inykh, I.A.; Burmistrov, I.N.; Mazov, I.N. Thermal conductivity of polypropylene composites filled with silane-modified hexagonal BN. Compos. Sci. Technol. 2015, 111, 40–43. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2021, 56, 4053–4079. [Google Scholar] [CrossRef]
- Bi, J.C.; Yun, H.; Cho, M.; Kwak, M.-G.; Ju, B.-K.; Kim, Y. Thermal conductivity and mechanical durability of graphene composite films containing polymer-filled connected multilayer graphene patterns. Ceram. Int. 2022, 48, 17789–17794. [Google Scholar] [CrossRef]
- Yu, C.; Gong, W.; Zhang, J.; Lv, W.; Tian, W.; Fan, X.; Yao, Y. Hot pressing-induced alignment of hexagonal boron nitride in SEBS elastomer for superior thermally conductive composites. RSC Adv. 2018, 8, 25835–25845. [Google Scholar] [CrossRef]
- Nan, B.; Gołębiewski, P.; Buczyński, R.; Galindo-Rosales, F.J.; Ferreira, J.M.F. Direct Ink Writing Glass: A Preliminary Step for Optical Application. Materials 2020, 13, 1636. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, G.; Yang, E.; Kim, Y.-S.; Kwak, M.-G.; Kim, Y. Thermally Conductive Film Fabricated Using Perforated Graphite Sheet and UV-Curable Pressure-Sensitive Adhesive. Nanomaterials 2021, 11, 93. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Weng, C.; Zhang, H.; Zhang, Z. High thermal conductive epoxy based composites fabricated by multi-material direct ink writing. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105684. [Google Scholar] [CrossRef]
- Li, J.; Leng, J.; Jiang, Y.; Zhang, J. Experimental characterization of 3D printed PP/h-BN thermally conductive composites with highly oriented h-BN and the effects of filler size. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106586. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Luo, F.; Jin, Y.; Huang, B.; Qian, Q. Bio-based flexible phase change composite film with high thermal conductivity for thermal energy storage. Compos. Part A Appl. Sci. Manuf. 2021, 151, 106638. [Google Scholar] [CrossRef]
- Luo, F.; Yang, S.; Yan, P.; Li, H.; Huang, B.; Qian, Q.; Chen, Q. Orientation behavior and thermal conductivity of liquid crystal polymer composites based on Three-Dimensional printing. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107059. [Google Scholar] [CrossRef]
- del-Mazo-Barbara, L.; Ginebra, M.-P. Rheological characterisation of ceramic inks for 3D direct ink writing: A review. J. Eur. Ceram. Soc. 2021, 41, 18–33. [Google Scholar] [CrossRef]
- Raymond, Y.; Thorel, E.; Liversain, M.; Riveiro, A.; Pou, J.; Ginebra, M.-P. 3D printing non-cylindrical strands: Morphological and structural implications. Addit. Manuf. 2021, 46, 102129. [Google Scholar] [CrossRef]
- Toker, O.S.; Karasu, S.; Yilmaz, M.T.; Karaman, S. Three interval thixotropy test (3ITT) in food applications: A novel technique to determine structural regeneration of mayonnaise under different shear conditions. Food Res. Int. 2015, 70, 125–133. [Google Scholar] [CrossRef]
- Luo, S.; Weinell, C.E.; Okkels, F.; Østergård, A.L.; Kiil, S. On-line, non-Newtonian capillary rheometry for continuous and in-line coatings production. J. Coat. Technol. Res. 2021, 18, 611–626. [Google Scholar] [CrossRef]
- Bok, G.; Lim, G.; Park, K.; Kim, Y. Mechanical properties and fracture toughness of fumed silica epoxy composites containing glycidyl terminated polysiloxanes. Ceram. Int. 2021, 47, 25738–25743. [Google Scholar] [CrossRef]
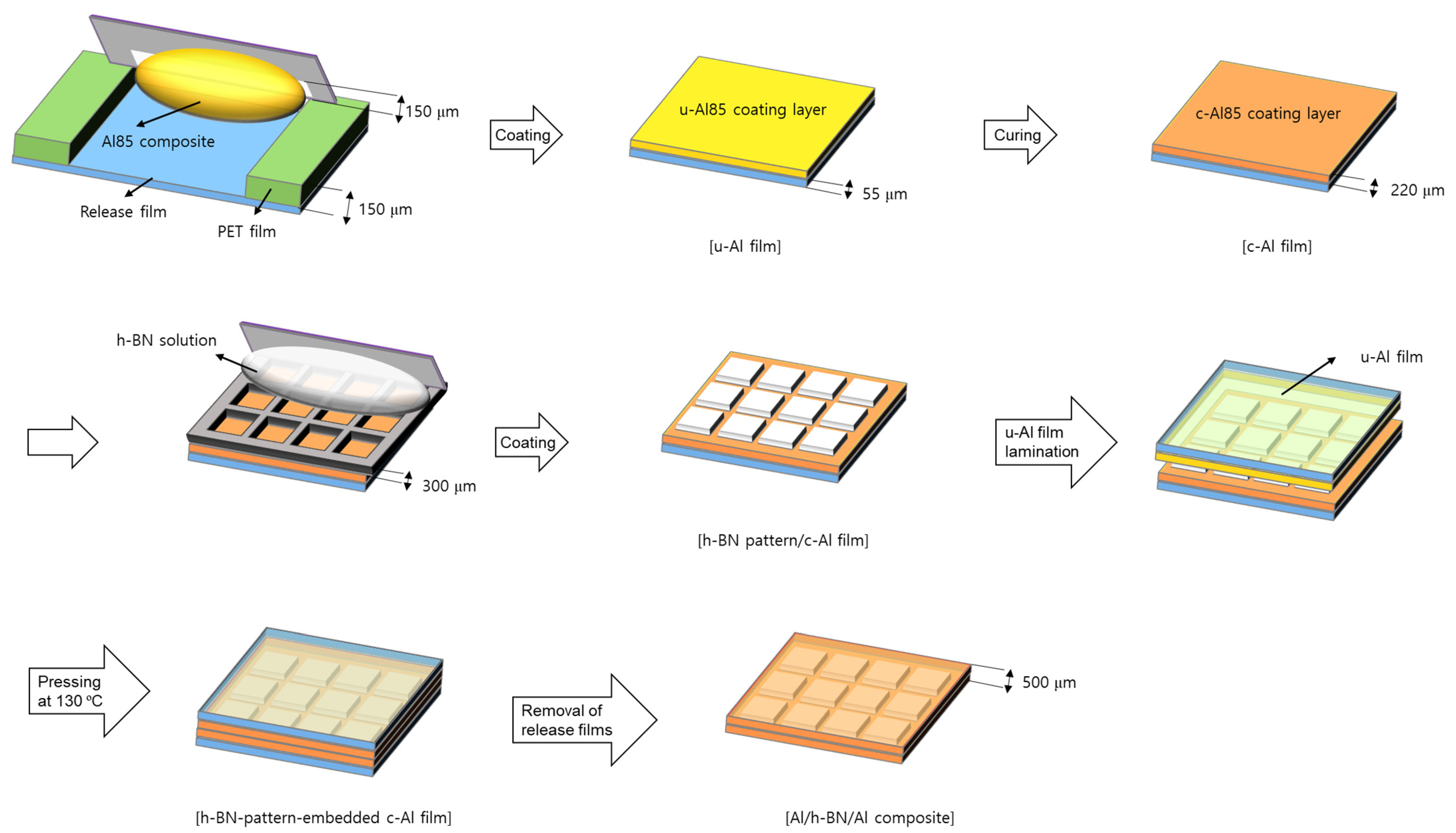

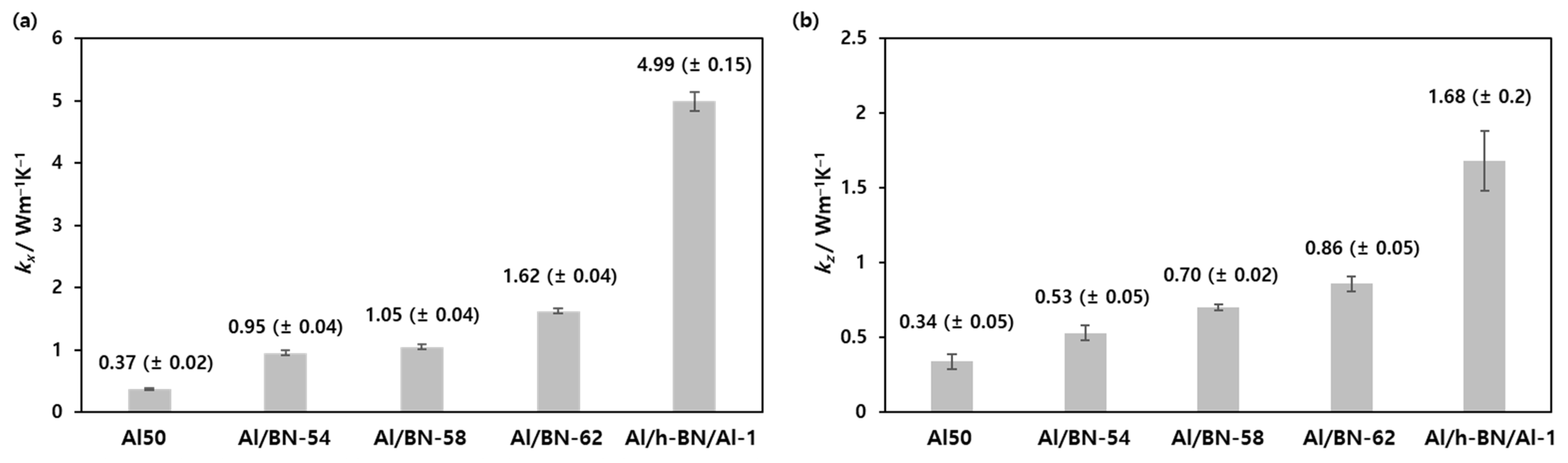




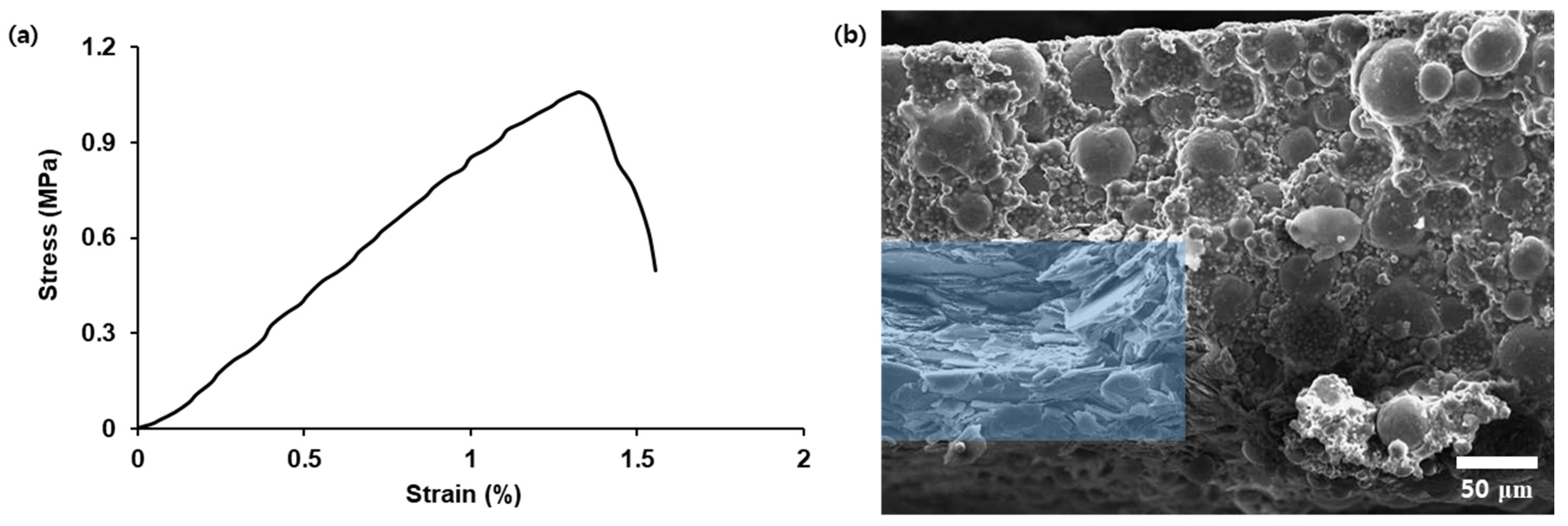
| Composites | Silicone Resin (g) | Al2O3 Mixture (g) | h-BN Mixture (g) | Filler Content (wt.%) |
|---|---|---|---|---|
| Al50 | 2.22 | 2.22 | 50 | |
| Al/BN-54 | 2.22 | 2.22 | 0.38 | 54 |
| Al/BN-58 | 2.22 | 2.22 | 0.84 | 58 |
| Al/BN-62 | 2.22 | 2.22 | 1.39 | 62 |
| Al80 | 2.22 | 8.88 | 80 | |
| Al85 | 2.22 | 12.47 | 85 | |
| Al90 | 2.22 | 19.80 | 90 |
| Composites | Al2O3 (wt.%) | h-BN (wt.%) |
|---|---|---|
| Al/h-BN/Al-1 | 74.1 | 12.8 |
| Al/h-BN/Al-2 | 71.6 | 15.8 |
| Al/h-BN/Al-3 | 70.2 | 17.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.; Kwak, M.-G.; Park, K.; Kim, Y. Fabrication, Thermal Conductivity, and Mechanical Properties of Hexagonal-Boron-Nitride-Pattern-Embedded Aluminum Oxide Composites. Nanomaterials 2022, 12, 2815. https://doi.org/10.3390/nano12162815
Yun H, Kwak M-G, Park K, Kim Y. Fabrication, Thermal Conductivity, and Mechanical Properties of Hexagonal-Boron-Nitride-Pattern-Embedded Aluminum Oxide Composites. Nanomaterials. 2022; 12(16):2815. https://doi.org/10.3390/nano12162815
Chicago/Turabian StyleYun, Hyesun, Min-Gi Kwak, KeumHwan Park, and Youngmin Kim. 2022. "Fabrication, Thermal Conductivity, and Mechanical Properties of Hexagonal-Boron-Nitride-Pattern-Embedded Aluminum Oxide Composites" Nanomaterials 12, no. 16: 2815. https://doi.org/10.3390/nano12162815
APA StyleYun, H., Kwak, M.-G., Park, K., & Kim, Y. (2022). Fabrication, Thermal Conductivity, and Mechanical Properties of Hexagonal-Boron-Nitride-Pattern-Embedded Aluminum Oxide Composites. Nanomaterials, 12(16), 2815. https://doi.org/10.3390/nano12162815





