Boosting the Oxygen Evolution Reaction by Controllably Constructing FeNi3/C Nanorods
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of the FeNi3/C Nanorods
2.2. Characterization
2.3. Electrochemical Measurements
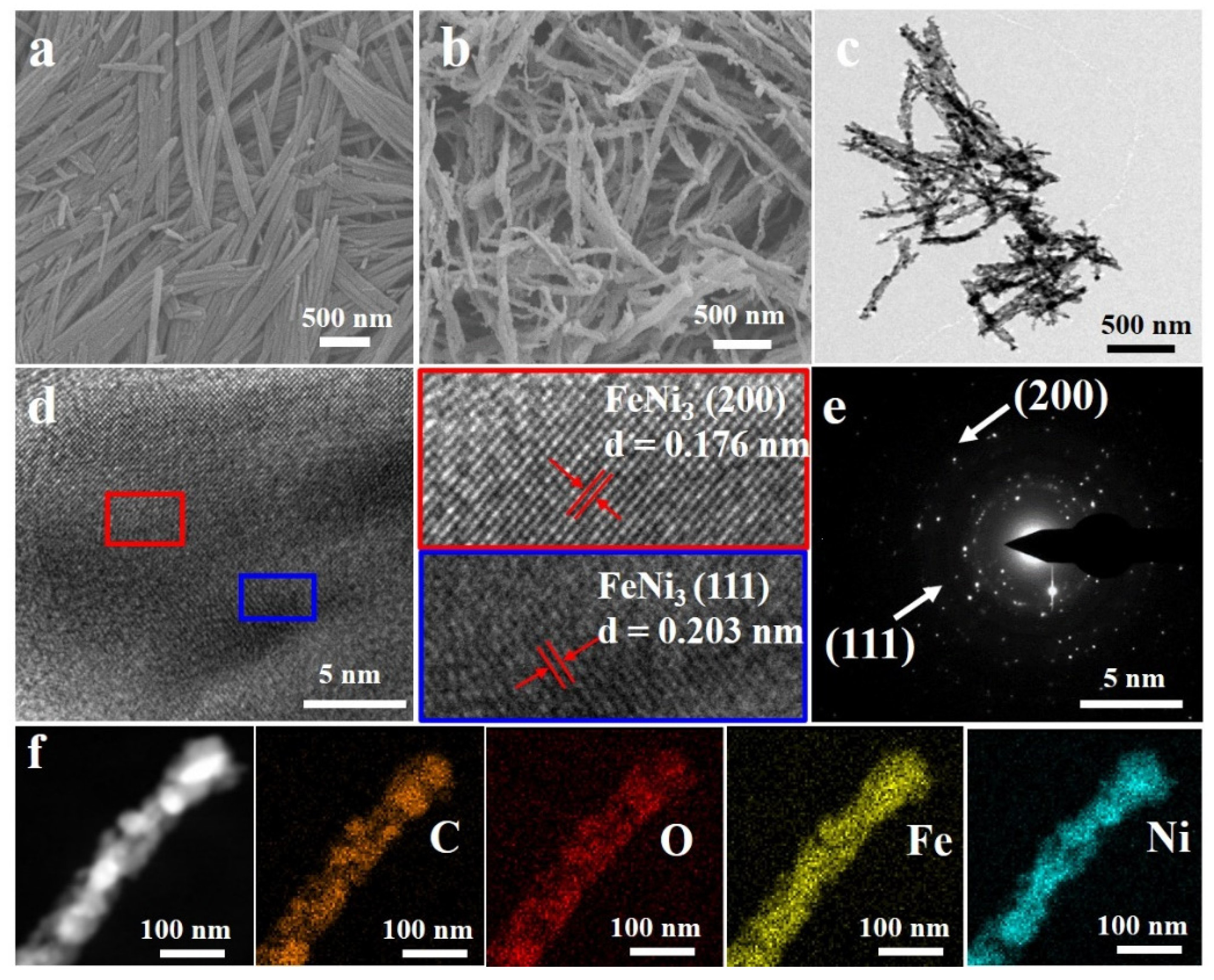
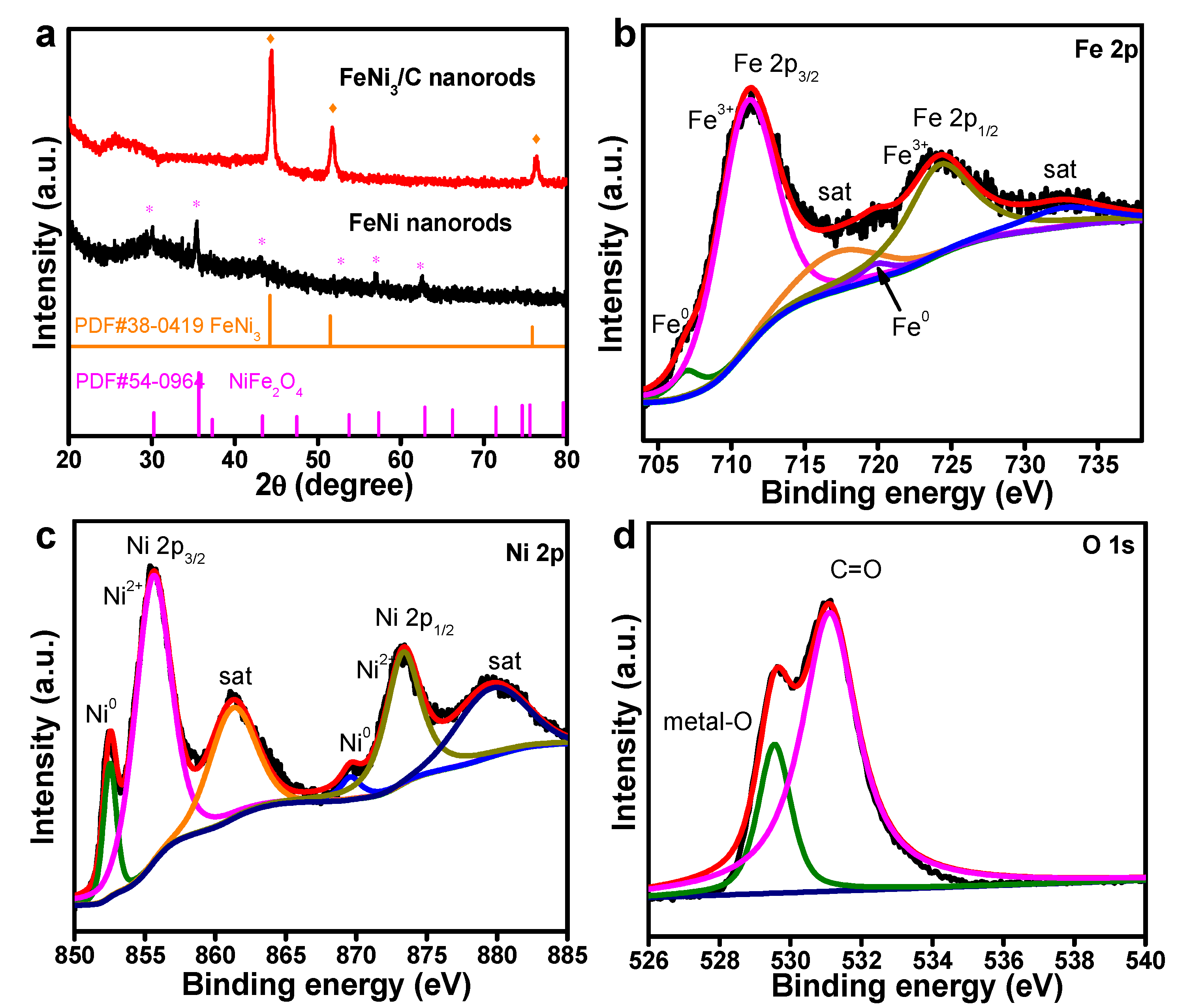
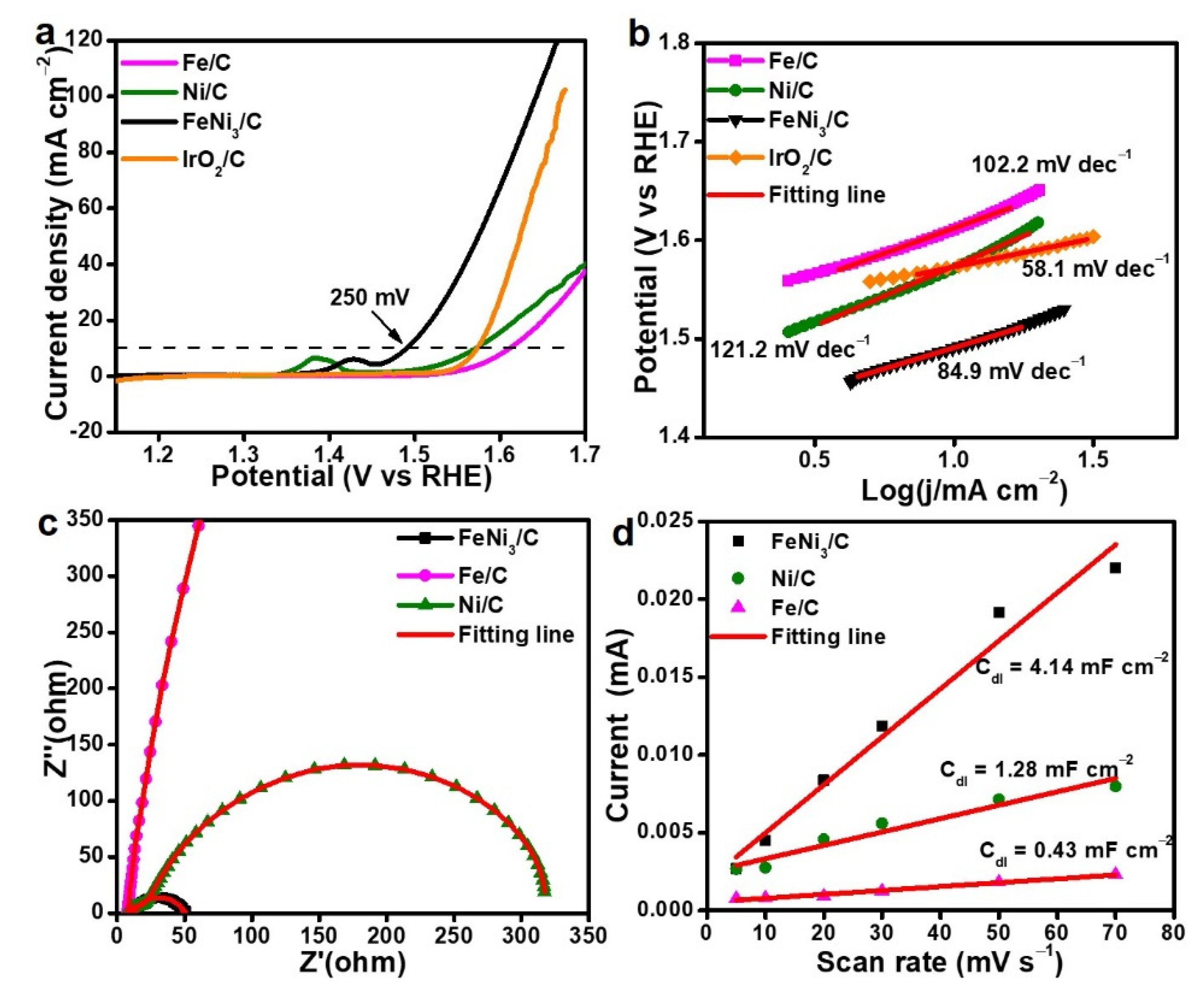
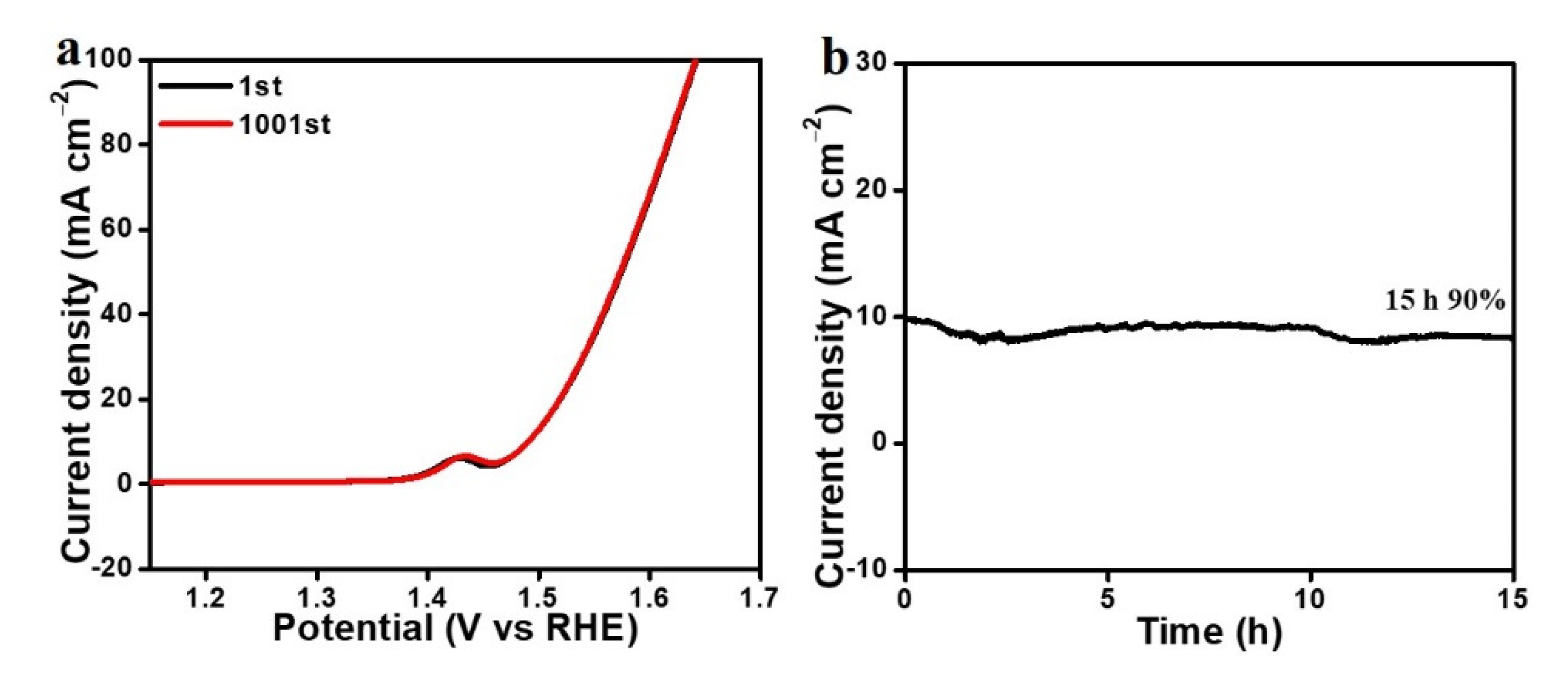
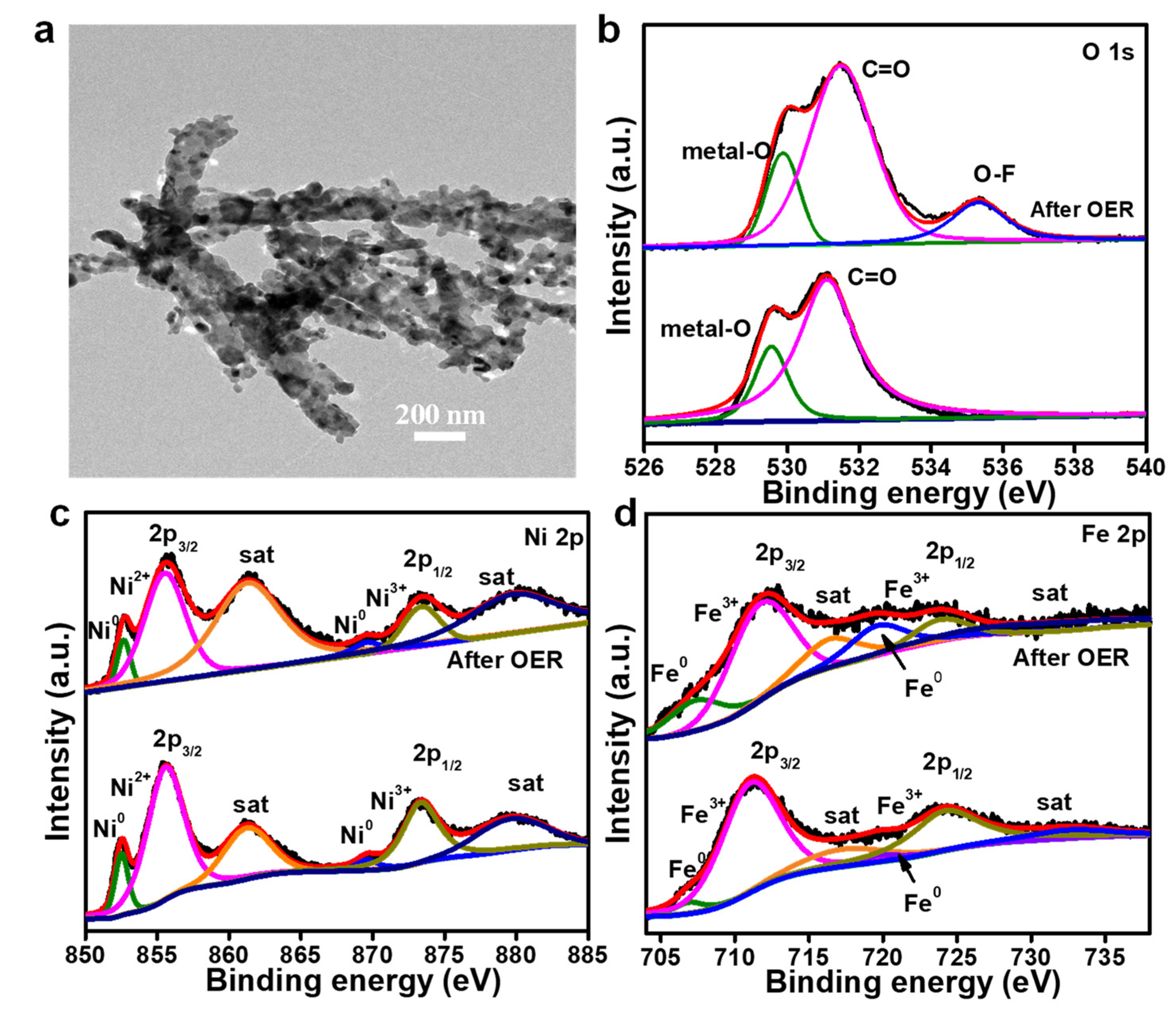
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Jiang, W.J.; Tang, T.; Zhang, Y.; Hu, J.S. Synergistic Modulation of Non-Precious-Metal Electrocatalysts for Advanced Water Splitting. Acc. Chem. Res. 2020, 53, 1111–1123. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Qian, H.; Wang, Z.; Yamauchi, Y. One-Pot Synthesis of Zeolitic Imidazolate Framework 67-Derived Hollow Co3S4@MoS2 Heterostructures as Efficient Bifunctional Catalysts. Chem. Mater. 2017, 29, 5566–5573. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Yanzhang, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B.; et al. When cubic cobalt sulfide meets layered molybdenum disulfide: A core-shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.S.; Debela, T.T.; Kwak, I.H.; Park, Y.C.; Seo, J.; Shim, J.Y.; Yoo, S.J.; Kim, J.G.; Park, J.; Kang, H.S. Ruthenium Nanoparticles on Cobalt-Doped 1T’ Phase MoS2 Nanosheets for Overall Water Splitting. Small 2020, 16, 2000081. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Lu, B.; Cao, D.; Wang, P.; Wang, G.; Gao, Y. Oxygen evolution reaction on Ni-substituted Co3O4 nanowire array electrodes. Int. J. Hydrogen Energy 2011, 36, 72–78. [Google Scholar] [CrossRef]
- Mom, R.V.; Cheng, J.; Koper, M.T.M.; Sprik, M. Modeling the oxygen evolution reaction on metal oxides: The infuence of unrestricted DFT calculations. J. Phys. Chem. C 2014, 118, 4095–4102. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. Recent Advances in Noble Metal (Pt, Ru, and Ir)-Based Electrocatalysts for Efficient Hydrogen Evolution Reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: Status and perspective. Energy Environ. Sci. 2012, 5, 9331–9344. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.-Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Evans, D.G.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Structure–property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.-J.; Sokaras, D.; Weng, T.-C.; Alonso-Mori, R.; et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Yang, D.; Yu, X.; Xue, H.; Feng, L. NiMn layered double hydroxide nanosheets/NiCo2O4 nanowires with surface rich high valence state metal oxide as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2018, 392, 23–32. [Google Scholar] [CrossRef]
- Chen, G.-F.; Ma, T.Y.; Liu, Z.-Q.; Li, N.; Su, Y.-Z.; Davey, K.; Qiao, S.-Z. Efficient and stable bifunctional electrocatalysts ni/nixmy (M = P, S) for overall water splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, J.; Xia, W.; Peng, Z.; Wu, Z.; Lei, W.; Xia, K.; Xin, H.L.; Wang, D. Porous Structured Ni–Fe–P Nanocubes Derived from a Prussian Blue Analogue as an Electrocatalyst for Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 26134–26142. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zou, Y.; Ye, Y.-Q.; Ouyang, T.; Xiao, K.; Liu, Z.-Q. Unveiling the active sites of Ni–Fe phosphide/metaphosphate for efficient oxygen evolution under alkaline conditions. Chem. Commun. 2019, 55, 7687–7690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Guo, X.; Xue, H.; Pan, K.; Liu, C.; Pang, H. Facile one-pot generation of metal oxide/hydroxide@metal–organic framework composites: Highly efficient bifunctional electrocatalysts for overall water splitting. Chem. Commun. 2019, 55, 10904–10907. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zhang, L.; Xu, Y.X.; Li, Q.; Xue, H.; Pang, H. Smart Yolk/Shell ZIF-67@POM Hybrids as Efficient Electrocatalysts for the Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 5027–5033. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Ma, W.; Xu, S.; Lu, Y.; Wei, W.; Zhu, J.; Jiang, D. Fe-doped NiCoP/Prussian blue analog hollow nanocubes as an efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2021, 367, 137492. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.; Dong, B.; Yu, X.; Xue, H.; Feng, L. A FeP powder electrocatalyst for the hydrogen evolution reaction. Electrochem. Commun. 2018, 92, 33–38. [Google Scholar] [CrossRef]
- Gao, M.-R.; Cao, X.; Gao, Q.; Xu, Y.-F.; Zheng, Y.-R.; Jiang, J.; Yu, S.-H. Nitrogen-doped graphene supported CoSe2 nanobelt composite catalyst for efficient water oxidation. ACS Nano 2014, 8, 3970–3978. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Huo, J.; Wang, S.; Dai, L. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326. [Google Scholar] [CrossRef]
- Liu, H.; Zha, M.; Liu, Z.; Tian, J.; Hu, G.; Feng, L. Synergistically boosting the oxygen evolution reaction of an Fe-MOF via Ni doping and fluorination. Chem. Commun. 2020, 56, 7889–7892. [Google Scholar] [CrossRef]
- Zha, M.; Pei, C.; Wang, Q.; Hu, G.; Feng, L. Electrochemical oxygen evolution reaction efficiently boosted by selective fluoridation of FeNi3 alloy/oxide hybrid. J. Energy Chem. 2020, 47, 166–171. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef] [Green Version]
- Lyons, M.E.G.; Brandon, M.P. A comparative study of the oxygen evolution reaction on oxidised nickel, cobalt and iron electrodes in base. J. Electroanal. Chem. 2010, 641, 119–130. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Trześniewski, B.J.; Diaz-Morales, O.; Vermaas, D.A.; Longo, A.; Bras, W.; Koper, M.T.M.; Smith, W.A. In situ observation of active oxygen species in Fe-containing Ni-based oxygen evolution catalysts: The effect of pH on electrochemical activity. J. Am. Chem. Soc. 2015, 137, 15112–15121. [Google Scholar] [CrossRef] [PubMed]
- Swierk, J.R.; Klaus, S.; Trotochaud, L.; Bell, A.T.; Tilley, T.D. Electrochemical study of the energetics of the oxygen evolution reaction at nickel iron (Oxy)hydroxide catalysts. J. Phys. Chem. C 2015, 119, 19022–19029. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhang, C.; Ai, L. Hierarchical iron nickel oxide architectures derived from metal-organic frameworks as efficient electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2016, 208, 17–24. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.; Xu, R.; Zhang, L.; Gao, J.; Xia, Z.; Tian, Z.; Wei, N.; Rümmeli, M.H.; Zou, X.; et al. Biotemplating Growth of Nepenthes-like N-Doped Graphene as a Bifunctional Polysulfide Scavenger for Li–S Batteries. ACS Nano 2018, 12, 10240–10250. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Li, Q.; Pang, H. Graphene/Co3O4 composites in application of electrochemical energy conversion and storage. FlatChem 2019, 16, 100107. [Google Scholar] [CrossRef]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.; Yu, H.; Xue, H.; Feng, L. Nanostructured FeNi3 Incorporated with Carbon Doped with Multiple Nonmetal Elements for the Oxygen Evolution Reaction. ChemSusChem 2018, 11, 2703–2709. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, F.; Li, K.; Zhu, C.; Xie, Y.; Zhang, X.; Chen, Y. Ultrasmall FeNi3N particles with an exposed active (110) surface anchored on nitrogen-doped graphene for multifunctional electrocatalysts. J. Mater. Chem. A 2019, 7, 1083–1091. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Pei, C. Surface oxidized iron-nickel nanorods anchoring on graphene architectures for oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 3579–3583. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, C.; Xie, S.; Liang, J.; Chen, X.; Tang, Y. Iron–Nickel Nitride Nanostructures in Situ Grown on Surface-Redox-Etching Nickel Foam: Efficient and Ultrasustainable Electrocatalysts for Overall Water Splitting. Chem. Mater. 2016, 28, 6934–6941. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Liu, H.; Yu, X.; Xue, H.; Feng, L. Electrochemical oxygen evolution reaction catalyzed by a novel nickel–cobalt-fluoride catalyst. Chem. Commun. 2018, 54, 6204–6207. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lopes, P.P.; Farinazzo Bergamo Dias Martins, P.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, H.B.; Kuang, M.; Yang, H.B.; Cai, W.; Yan, Q.; Mao, Q.; Liu, B. Advances in Thermodynamic-Kinetic Model for Analyzing the Oxygen Evolution Reaction. ACS Catal. 2020, 10, 8597–8610. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, K.; Zhong, H.-X.; Xu, D.; Wang, Z.-L.; Jiang, Z.; Wu, Z.-J.; Zhang, X.-B. Synergistic Effect between Met-al–Nitrogen–Carbon Sheets and NiO Nanoparticles for Enhanced Electrochemical Water—Oxidation Performance. Angew. Chem. Int. Ed. 2015, 54, 10530–10534. [Google Scholar] [CrossRef] [PubMed]
- Narendra Kumar, A.V.; Li, Y.; Yu, H.; Yin, S.; Xue, H.; Xu, Y.; Li, X.; Wang, H.; Wang, L. 3D graphene aerogel sup-ported FeNi-P derived from electroactive nickel hexacyanoferrate as efficient oxygen evolution catalyst. Electrochim. Acta 2018, 292, 107–114. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Zhao, Y.; Song, L.; Zhang, Z. FeNi layered double-hydroxide nanosheets on a 3D carbon network as an efficient electrocatalyst for the oxygen evolution reaction. Part. Part. Syst. Char. 2016, 33, 158–166. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Sun, B.; Su, D.; Huang, X.; Liu, H.; Yan, Y.; Sun, K.; Wang, G. Graphene-Co3O4 nanocomposite as electrocatalyst with high performance for oxygen evolution reaction. Sci. Rep. 2015, 5, 7629. [Google Scholar]
- Yang, J.; Zhu, G.; Liu, Y.; Xia, J.; Ji, Z.; Shen, X.; Wu, S. Fe3O4-decorated Co9S8 nanoparticles in situ grown on re-duced graphene oxide: A new and efficient electrocatalyst for oxygen evolution reaction. Adv. Funct. Mater. 2016, 6, 4712–4721. [Google Scholar] [CrossRef]
- Elizabeth, I.; Nair, A.K.; Singh, B.P.; Gopukumar, S. Multifunctional Ni-NiO-CNT composite as high performing free standing anode for Li ion batteries and advanced electro catalyst for oxygen evolution reaction. Electrochim. Acta 2017, 230, 98–105. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Highly efficient and robust oxygen evolution catalysts achieved by anchoring nanocrystalline cobalt oxides onto mildly oxidized multiwalled carbon nanotubes. J. Mater. Chem. A 2013, 1, 12053–12059. [Google Scholar] [CrossRef]
- Han, G.-Q.; Liu, Y.-R.; Hu, W.-H.; Dong, B.; Li, X.; Shang, X.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. Three dimensional nickel oxides/nickel structure by in situ electro-oxidation of nickel foam as robust electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2015, 359, 172–176. [Google Scholar] [CrossRef]
- Pei, C.; Chen, H.; Dong, B.; Yu, X.; Feng, L. Electrochemical oxygen evolution reaction efficiently catalyzed by a novel porous iron-cobalt-fluoride nanocube easily derived from 3-dimensional Prussian blue analogue. J. Power Sources 2019, 424, 131–137. [Google Scholar] [CrossRef]
- Mao, S.; Wen, Z.; Huang, T.; Hou, Y.; Chen, J. High-performance bi-functional electrocatalysts of 3D crumpled graphene–cobalt oxide nanohybrids for oxygen reduction and evolution reactions. Energ. Environ. Sci. 2014, 7, 609–616. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Pan, Z.; Zhao, Z.; Zhou, Y.; Pei, C.; Ma, Y.; Park, H.S.; Wang, M. Boosting the Oxygen Evolution Reaction by Controllably Constructing FeNi3/C Nanorods. Nanomaterials 2022, 12, 2525. https://doi.org/10.3390/nano12152525
Yu X, Pan Z, Zhao Z, Zhou Y, Pei C, Ma Y, Park HS, Wang M. Boosting the Oxygen Evolution Reaction by Controllably Constructing FeNi3/C Nanorods. Nanomaterials. 2022; 12(15):2525. https://doi.org/10.3390/nano12152525
Chicago/Turabian StyleYu, Xu, Zhiqiang Pan, Zhixin Zhao, Yuke Zhou, Chengang Pei, Yifei Ma, Ho Seok Park, and Mei Wang. 2022. "Boosting the Oxygen Evolution Reaction by Controllably Constructing FeNi3/C Nanorods" Nanomaterials 12, no. 15: 2525. https://doi.org/10.3390/nano12152525
APA StyleYu, X., Pan, Z., Zhao, Z., Zhou, Y., Pei, C., Ma, Y., Park, H. S., & Wang, M. (2022). Boosting the Oxygen Evolution Reaction by Controllably Constructing FeNi3/C Nanorods. Nanomaterials, 12(15), 2525. https://doi.org/10.3390/nano12152525






