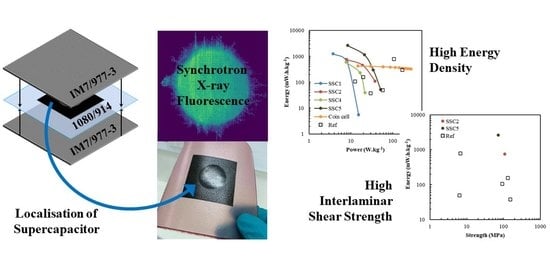
Composite Structural Supercapacitors: High-Performance Carbon Nanotube Supercapacitors through Ionic Liquid Localisation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Manufacture of Supercapacitor Panels
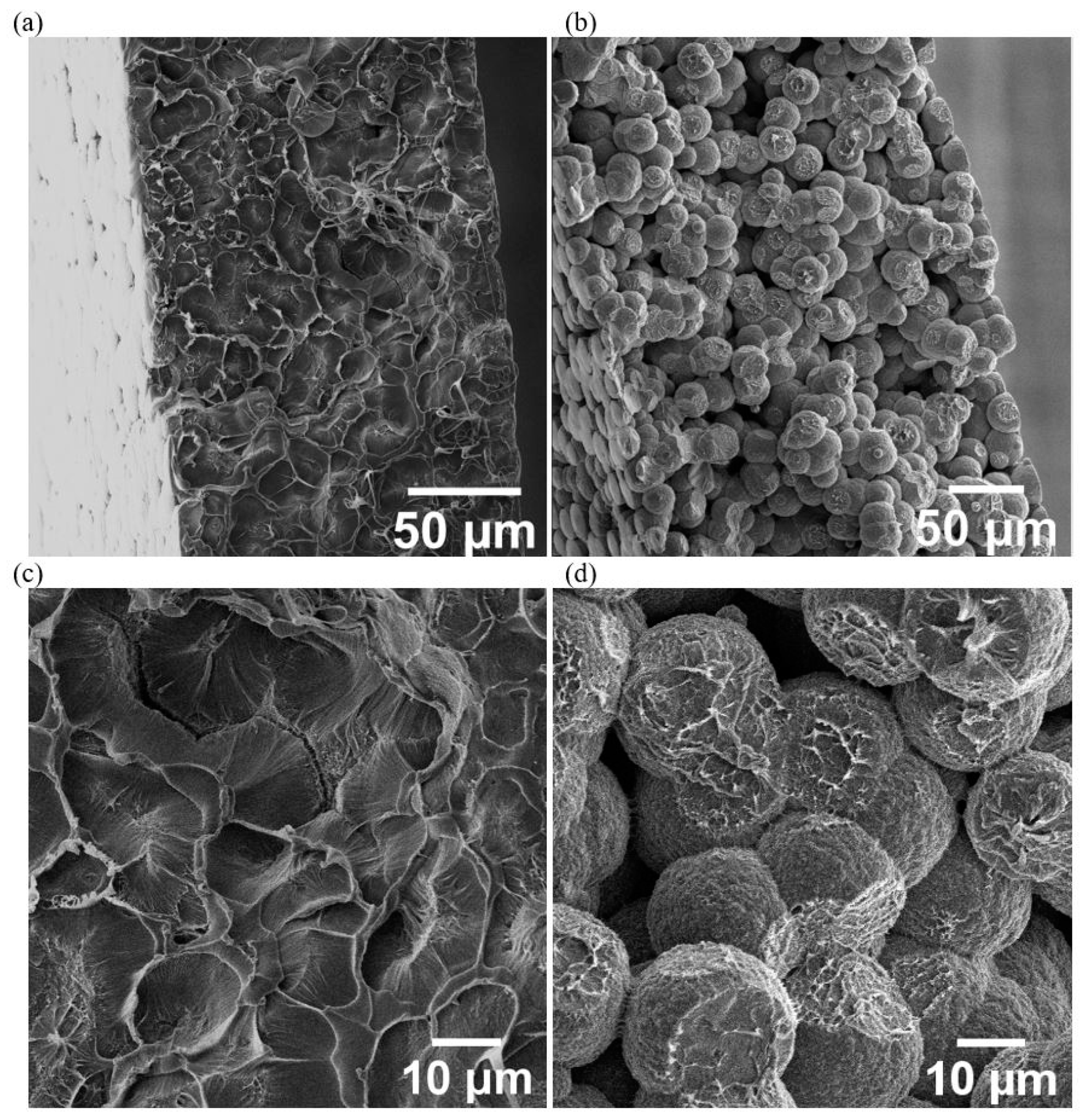
3.2. Ionic Liquid Localisation
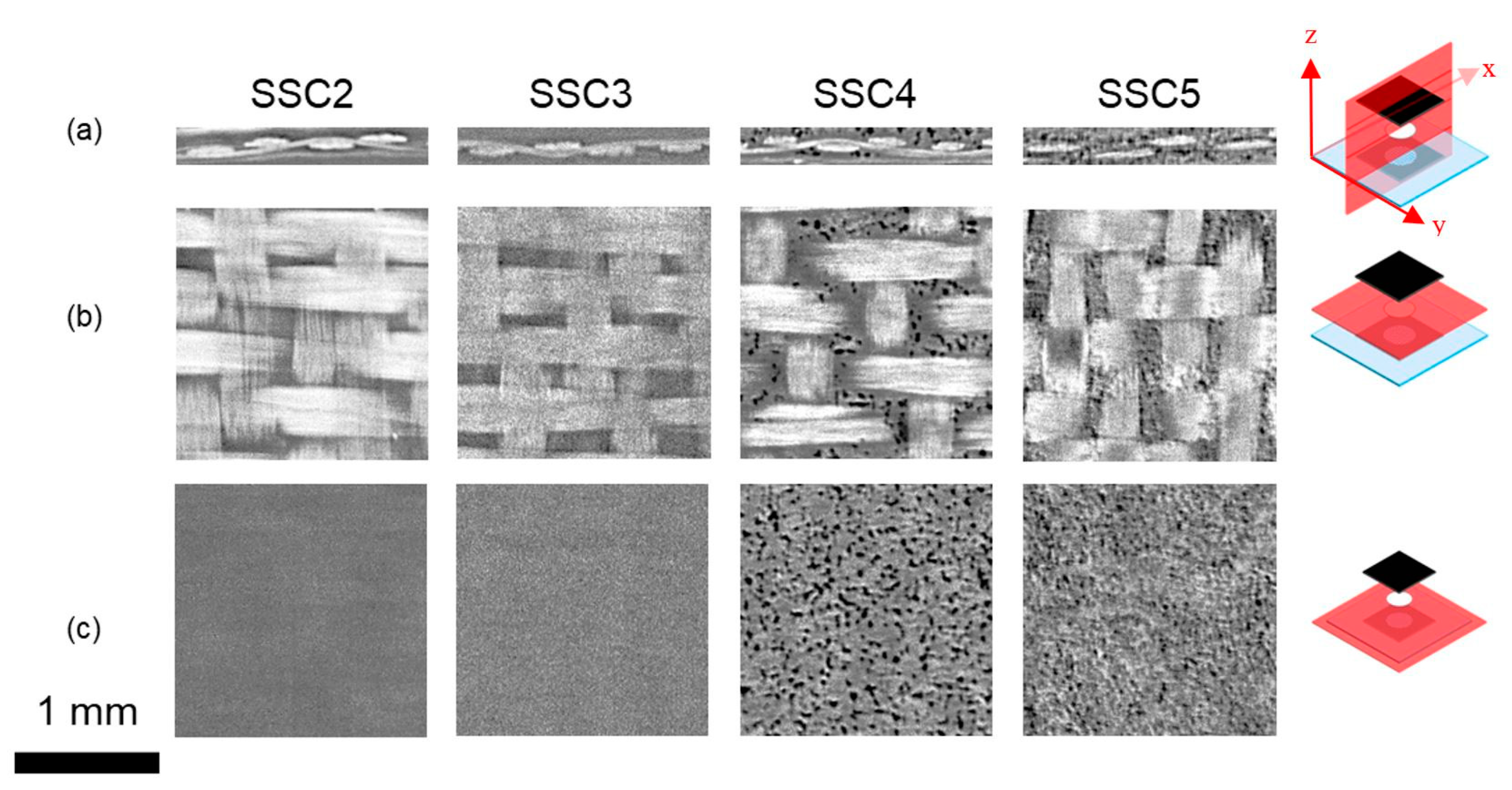
3.3. Synchrotron X-ray Micro Computed Tomography (XµCT)
3.4. Electrochemical Performance
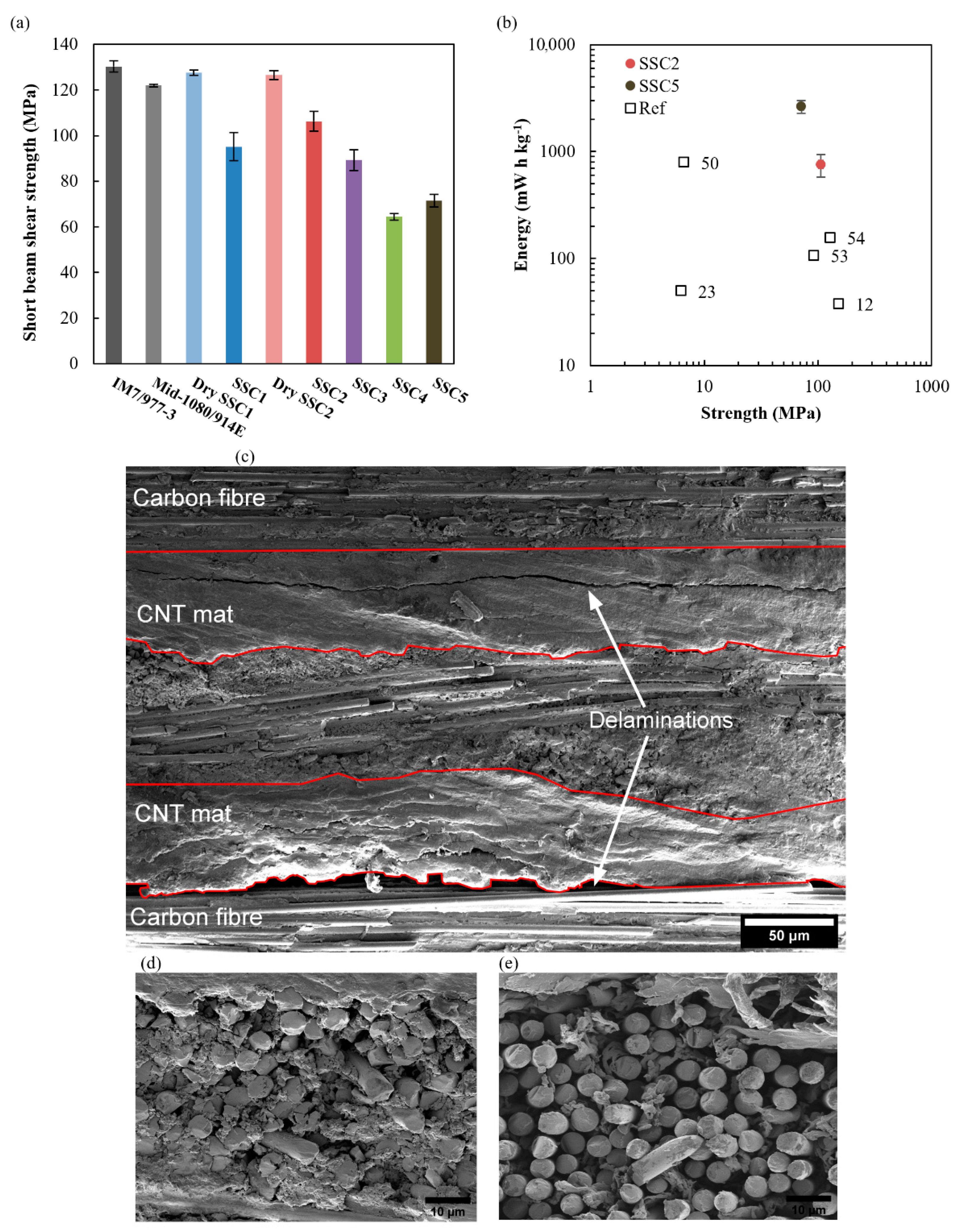
3.5. SSC Mechanical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rider, A.N. Hierarchical Nanocomposites/Multi-Scale Composites. In Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6–8, pp. 352–379. [Google Scholar]
- Nicholson, K.J.; Baum, T.C.; Patniotis, J.E.; Ghorbani, K. Discrete Holographic Antenna Embedded in a Structural Composite Laminate. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 358–362. [Google Scholar] [CrossRef]
- Charles, A.D.M.; Rider, A.N.; Brown, S.A.; Wang, C.H. Multifunctional Magneto-Polymer Matrix Composites for Electromagnetic Interference Suppression, Sensors and Actuators. Prog. Mater. Sci. 2021, 115, 100705. [Google Scholar] [CrossRef]
- Evanoff, K.; Benson, J.; Schauer, M.; Kovalenko, I.; Lashmore, D.; Ready, W.J.; Yushin, G. Ultra Strong Silicon-Coated Carbon Nanotube Nonwoven Fabric as a Multifunctional Lithium-Ion Battery Anode. ACS Nano 2012, 6, 9837–9845. [Google Scholar] [CrossRef]
- Snyder, J.F.; Wetzel, E.D.; Watson, C.M. Improving Multifunctional Behavior in Structural Electrolytes through Copolymerization of Structure- and Conductivity-Promoting Monomers. Polymer 2009, 50, 4906–4916. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon Fiber Reinforced Structural Lithium-Ion Battery Composite: Multifunctional Power Integration for CubeSats. Energy Storage Mater. 2019, 24, 676–681. [Google Scholar] [CrossRef]
- Carlson, T.; Asp, L.E. Structural Carbon Fibre Composite/PET Capacitors—Effects of Dielectric Separator Thickness. Compos. Part B Eng. 2013, 49, 16–21. [Google Scholar] [CrossRef]
- Chan, K.-Y.; Yang, D.; Demir, B.; Mouritz, A.P.; Lin, H.; Jia, B.; Lau, K.-T. Boosting the Electrical and Mechanical Properties of Structural Dielectric Capacitor Composites via Gold Nanoparticle Doping. Compos. Part B Eng. 2019, 178, 107480. [Google Scholar] [CrossRef]
- Javaid, A.; Ho, H.H.K.; Bismarck, A.; Shaffer, M.S.P.; Steinke, J.H.G.; Greenhalgh, E.S. Multifunctional Structural Supercapacitors for Electrical Energy Storage Applications. J. Compos. Mater. 2014, 48, 1409–1416. [Google Scholar] [CrossRef]
- Snyder, J.F.; Gienger, E.B.; Wetzel, E.D. Performance Metrics for Structural Composites with Electrochemical Multifunctionality. J. Compos. Mater. 2015, 49, 1835–1848. [Google Scholar] [CrossRef]
- Muralidharan, N.; Teblum, E.; Westover, A.S.; Schauben, D.; Itzhak, A.; Muallem, M.; Nessim, G.D.; Pint, C.L. Carbon Nanotube Reinforced Structural Composite Supercapacitor. Sci. Rep. 2018, 8, 17662. [Google Scholar] [CrossRef]
- Senokos, E.; Ou, Y.; Torres, J.J.; Sket, F.; González, C.; Marcilla, R.; Vilatela, J.J. Energy Storage in Structural Composites by Introducing CNT Fiber/Polymer Electrolyte Interleaves. Sci. Rep. 2018, 8, 3407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frackowiak, E.; Beguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Yang, P.; Mai, W. Flexible Solid-State Electrochemical Supercapacitors. Nano Energy 2014, 8, 274–290. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-Based Materials for Flexible All-Solid-State Supercapacitors. Adv. Mater. 2018, 30, 1705489. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The Role and Utilization of Pseudocapacitance for Energy Storage by Supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Marcilla, R. Ionic Liquids and Polymers in Energy. In Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 199–229. ISBN 978-3-662-44902-8. [Google Scholar]
- Trigueiro, J.P.C.; Lavall, R.L.; Silva, G.G. Nanocomposites of Graphene Nanosheets/Multiwalled Carbon Nanotubes as Electrodes for In-Plane Supercapacitors. Electrochim. Acta 2016, 187, 312–322. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, J.; Chen, L.; Zhu, Y.; Xu, B. Open-Ended Carbon Microtubes/Carbon Nanotubes for High-Performance Supercapacitors. Mater. Lett. 2019, 241, 80–83. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. Performance of Solid State Supercapacitors Based on Polymer Electrolytes Containing Different Ionic Liquids. J. Power Sources 2016, 326, 560–568. [Google Scholar] [CrossRef]
- Adam, T.J.; Liao, G.; Petersen, J.; Geier, S.; Finke, B.; Wierach, P.; Kwade, A.; Wiedemann, M. Multifunctional Composites for Future Energy Storage in Aerospace Structures. Energies 2018, 11, 335. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lu, W.; Xu, G.; Chou, T.-W. Structural Supercapacitor Composites: A Review. Compos. Sci. Technol. 2021, 204, 108636. [Google Scholar] [CrossRef]
- Javaid, A.; Khalid, O.; Shakeel, A.; Noreen, S. Multifunctional Structural Supercapacitors Based on Polyaniline Deposited Carbon Fiber Reinforced Epoxy Composites. J. Energy Storage 2021, 33, 102168. [Google Scholar] [CrossRef]
- Lota, G.; Krawczyk, P.; Lota, K.; Sierczyńska, A.; Kolanowski, Ł.; Baraniak, M.; Buchwald, T. The Application of Activated Carbon Modified by Ozone Treatment for Energy Storage. J. Solid State Electrochem. 2016, 20, 2857–2864. [Google Scholar] [CrossRef] [Green Version]
- Young, C.; Park, T.; Yi, J.W.; Kim, J.; Hossain, S.A.; Kaneti, Y.V.; Yamauchi, Y. Advanced Functional Carbons and Their Hybrid Nanoarchitectures towards Supercapacitor Applications. ChemSusChem 2018, 11, 3546–3558. [Google Scholar] [CrossRef]
- Vilatela, J.J.; Marcilla, R. Tough Electrodes: Carbon Nanotube Fibers as the Ultimate Current Collectors/Active Material for Energy Management Devices. Chem. Mater. 2015, 27, 6901–6917. [Google Scholar] [CrossRef]
- Cheng, Q.; Bao, J.; Park, J.G.; Liang, Z.; Zhang, C.; Wang, B. High Mechanical Performance Composite Conductor: Multi-Walled Carbon Nanotube Sheet/Bismaleimide Nanocomposites. Adv. Funct. Mater. 2009, 19, 3219–3225. [Google Scholar] [CrossRef]
- Senokos, E.; Reguero, V.; Cabana, L.; Palma, J.; Marcilla, R.; Vilatela, J.J. Large-Area, All-Solid, and Flexible Electric Double Layer Capacitors Based on CNT Fiber Electrodes and Polymer Electrolytes. Adv. Mater. Technol. 2017, 2, 1600290. [Google Scholar] [CrossRef] [Green Version]
- Mapleback, B.J.; Simons, T.J.; Shekibi, Y.; Ghorbani, K.; Rider, A.N. Structural Composite Supercapacitor Using Carbon Nanotube Mat Electrodes with Interspersed Metallic Iron Nanoparticles. Electrochim. Acta 2020, 331, 135233. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A. Solid-State Supercapacitors with Ionic Liquid Based Gel Polymer Electrolyte: Effect of Lithium Salt Addition. J. Power Sources 2013, 243, 211–218. [Google Scholar] [CrossRef]
- Lee, J.; Kim, W.; Kim, W. Stretchable Carbon Nanotube/Ion–Gel Supercapacitors with High Durability Realized through Interfacial Microroughness. ACS Appl. Mater. Interfaces 2014, 6, 13578–13586. [Google Scholar] [CrossRef]
- Ortega, P.F.R.; Trigueiro, J.P.C.; Silva, G.G.; Lavall, R.L. Improving Supercapacitor Capacitance by Using a Novel Gel Nanocomposite Polymer Electrolyte Based on Nanostructured SiO2, PVDF and Imidazolium Ionic Liquid. Electrochim. Acta 2016, 188, 809–817. [Google Scholar] [CrossRef]
- Mapleback, B.J.; Brack, N.; Thomson, L.; Spencer, M.J.S.; Osborne, D.A.; Doshi, S.; Thostenson, E.T.; Rider, A.N. Development of Stable Boron Nitride Nanotube and Hexagonal Boron Nitride Dispersions for Electrophoretic Deposition. Langmuir 2020, 36, 3425–3438. [Google Scholar] [CrossRef]
- Ortega, P.F.R.; dos Santos, G.A.; Trigueiro, J.P.C.; Silva, G.G.; Quintanal, N.; Blanco, C.; Lavall, R.L.; Santamaría, R. Insights on the Behavior of Imidazolium Ionic Liquids as Electrolytes in Carbon-Based Supercapacitors: An Applied Electrochemical Approach. J. Phys. Chem. C 2020, 124, 15818–15830. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, B.; Zhang, C.; Liang, Z. Functionalized Carbon-Nanotube Sheet/Bismaleimide Nanocomposites: Mechanical and Electrical Performance Beyond Carbon-Fiber Composites. Small 2010, 6, 763–767. [Google Scholar] [CrossRef]
- Coromina, H.M.; Adeniran, B.; Mokaya, R.; Walsh, D.A. Bridging the Performance Gap between Electric Double-Layer Capacitors and Batteries with High-Energy/High-Power Carbon Nanotube-Based Electrodes. J. Mater. Chem. A 2016, 4, 14586–14594. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Li, Q.; Smith, A.D.; Kuzmenko, V.; Köhler, E.; Lundgren, P.; Enoksson, P. Thermal Influence on the Electrochemical Behavior of a Supercapacitor Containing an Ionic Liquid Electrolyte. Electrochim. Acta 2018, 263, 249–260. [Google Scholar] [CrossRef]
- Asp, L.E.; Greenhalgh, E.S. Structural Power Composites. Compos. Sci. Technol. 2014, 101, 41–61. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Tu, Q.-M.; Geng, C.-L.; Wang, Y.-L.; Sun, S.-J.; Huang, Y.-F.; Wu, J.-H. Improved Redox-Active Ionic Liquid-Based Ionogel Electrolyte by Introducing Carbon Nanotubes for Application in All-Solid-State Supercapacitors. Int. J. Hydrogen Energy 2020, 45, 17131–17139. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Hashmi, S.A. Ionic Liquid Incorporated, Redox-Active Blend Polymer Electrolyte for High Energy Density Quasi-Solid-State Carbon Supercapacitor. J. Power Sources 2020, 451, 227771. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chung, H.; Han, C.-H.; Kim, W. All-Solid-State Flexible Supercapacitors Based on Papers Coated with Carbon Nanotubes and Ionic-Liquid-Based Gel Electrolytes. Nanotechnology 2012, 23, 065401. [Google Scholar] [CrossRef]
- Kumar, Y.; Pandey, G.P.; Hashmi, S.A. Gel Polymer Electrolyte Based Electrical Double Layer Capacitors: Comparative Study with Multiwalled Carbon Nanotubes and Activated Carbon Electrodes. J. Phys. Chem. C 2012, 116, 26118–26127. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Arof, A.K. Investigation of Ionic Liquid-Doped Ion Conducting Polymer Electrolytes for Carbon-Based Electric Double Layer Capacitors (EDLCs). Mater. Des. 2016, 92, 829–835. [Google Scholar] [CrossRef]
- Lee, S.J.; Yang, H.M.; Cho, K.G.; Seol, K.H.; Kim, S.; Hong, K.; Lee, K.H. Highly Conductive and Mechanically Robust Nanocomposite Polymer Electrolytes for Solid-State Electrochemical Thin-Film Devices. Org. Electron. 2019, 65, 426–433. [Google Scholar] [CrossRef]
- Adusei, P.K.; Gbordzoe, S.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Alvarez, N.T.; Fang, Y.; Johnson, K.; McConnell, C.; Shanov, V. Fabrication and Study of Supercapacitor Electrodes Based on Oxygen Plasma Functionalized Carbon Nanotube Fibers. J. Energy Chem. 2020, 40, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.; Kumar, Y.; Hashmi, S.A. ‘Bucky Gel’ of Multiwalled Carbon Nanotubes as Electrodes for High Performance, Flexible Electric Double Layer Capacitors. Nanotechnology 2013, 24, 465704. [Google Scholar] [CrossRef]
- Rana, M.; Ou, Y.; Meng, C.; Sket, F.; González, C.; Vilatela, J.J. Damage-Tolerant, Laminated Structural Supercapacitor Composites Enabled by Integration of Carbon Nanotube Fibres. Multifunct. Mater. 2020, 3, 015001. [Google Scholar] [CrossRef]
- Sha, Z.; Huang, F.; Zhou, Y.; Zhang, J.; Wu, S.; Chen, J.; Brown, S.A.; Peng, S.; Han, Z.; Wang, C.-H. Synergies of Vertical Graphene and Manganese Dioxide in Enhancing the Energy Density of Carbon Fibre-Based Structural Supercapacitors. Compos. Sci. Technol. 2021, 201, 108568. [Google Scholar] [CrossRef]
- Subhani, K.; Jin, X.; Mahon, P.J.; Lau, A.K.T.; Salim, N.V. Graphene Aerogel Modified Carbon Fiber Reinforced Composite Structural Supercapacitors. Compos. Commun. 2021, 24, 100663. [Google Scholar] [CrossRef]
- Javaid, A.; Irfan, M. Multifunctional Structural Supercapacitors Based on Graphene Nanoplatelets/Carbon Aerogel Composite Coated Carbon Fiber Electrodes. Mater. Res. Express 2018, 6, 016310. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, S.; Yan, Y.; Wang, L.; Xu, G.; Yarlagadda, S.; Chou, T.-W. High-Performance Structural Supercapacitors Based on Aligned Discontinuous Carbon Fiber Electrodes and Solid Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 11774–11782. [Google Scholar] [CrossRef]
- Sánchez-Romate, X.F.; Del Bosque, A.; Artigas-Arnaudas, J.; Muñoz, B.K.; Sánchez, M.; Ureña, A. A Proof of Concept of a Structural Supercapacitor Made of Graphene Coated Woven Carbon Fibers: EIS Study and Mechanical Performance. Electrochim. Acta 2021, 370, 137746. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Park, Y.-B.; Park, H.W. Multifunctional CuO Nanowire Embodied Structural Supercapacitor Based on Woven Carbon Fiber/Ionic Liquid–Polyester Resin. Compos. Part A Appl. Sci. Manuf. 2016, 87, 256–262. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kwon, O.; Kim, D.; Park, Y.-B.; Park, H.W. Multifunctional Enhancement of Woven Carbon Fiber/ZnO Nanotube-Based Structural Supercapacitor and Polyester Resin-Domain Solid-Polymer Electrolytes. Chem. Eng. J. 2017, 325, 672–680. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Kim, N.; Jeong, H.E.; Park, Y.-B.; Park, H.W. Bimetallic Copper Cobalt Selenide Nanowire-Anchored Woven Carbon Fiber-Based Structural Supercapacitors. Chem. Eng. J. 2019, 355, 551–559. [Google Scholar] [CrossRef]
- Nguyen, S.; Anthony, D.B.; Qian, H.; Yue, C.; Singh, A.; Bismarck, A.; Shaffer, M.S.P.; Greenhalgh, E.S. Mechanical and Physical Performance of Carbon Aerogel Reinforced Carbon Fibre Hierarchical Composites. Compos. Sci. Technol. 2019, 182, 107720. [Google Scholar] [CrossRef]
- Patel, A.; Loufakis, D.; Flouda, P.; George, I.; Shelton, C.; Harris, J.; Oka, S.; Lutkenhaus, J.L. Carbon Nanotube/Reduced Graphene Oxide/Aramid Nanofiber Structural Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11763–11771. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Zhang, C.; Zhou, X. Development of Structural Supercapacitors with Epoxy Based Adhesive Polymer Electrolyte. J. Energy Storage 2019, 26, 100968. [Google Scholar] [CrossRef]
- Ryan, C.G.; Cousens, D.R.; Etschmann, B.E. GeoPIXE Software; CSIRO Earth Science and Resource Engineering: Clayton, VIC, Australia, 2012. [Google Scholar]
- Object Research Systems (ORS) Inc. Dragonfly 2020.2 [Computer Software]; Object Research Systems (ORS) Inc.: Montreal, QC, Canada, 2020. [Google Scholar]
- D30 Committee. Test Method for Short-Beam Strength of Polymer Matrix Composite Materials and Their Laminates; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Matsumoto, K.; Endo, T. Confinement of Ionic Liquid by Networked Polymers Based on Multifunctional Epoxy Resins. Macromolecules 2008, 41, 6981–6986. [Google Scholar] [CrossRef]
- Shirshova, N.; Bismarck, A.; Carreyette, S.; Fontana, Q.P.V.; Greenhalgh, E.S.; Jacobsson, P.; Johansson, P.; Marczewski, M.J.; Kalinka, G.; Kucernak, A.R.J.; et al. Structural Supercapacitor Electrolytes Based on Bicontinuous Ionic Liquid–Epoxy Resin Systems. J. Mater. Chem. A 2013, 1, 15300–15309. [Google Scholar] [CrossRef]
- Solvay CYCOM 977-3. Available online: https://www.solvay.com/en/product/cycom-977-3 (accessed on 18 June 2021).
- Wang, S.; Downes, R.; Young, C.; Haldane, D.; Hao, A.; Liang, R.; Wang, B.; Zhang, C.; Maskell, R. Carbon Fiber/Carbon Nanotube Buckypaper Interply Hybrid Composites: Manufacturing Process and Tensile Properties. Adv. Eng. Mater. 2015, 17, 1442–1453. [Google Scholar] [CrossRef]
- Shirshova, N.; Bismarck, A.; Greenhalgh, E.S.; Johansson, P.; Kalinka, G.; Marczewski, M.J.; Shaffer, M.S.P.; Wienrich, M. Composition as a Means To Control Morphology and Properties of Epoxy Based Dual-Phase Structural Electrolytes. J. Phys. Chem. C 2014, 118, 28377–28387. [Google Scholar] [CrossRef] [Green Version]
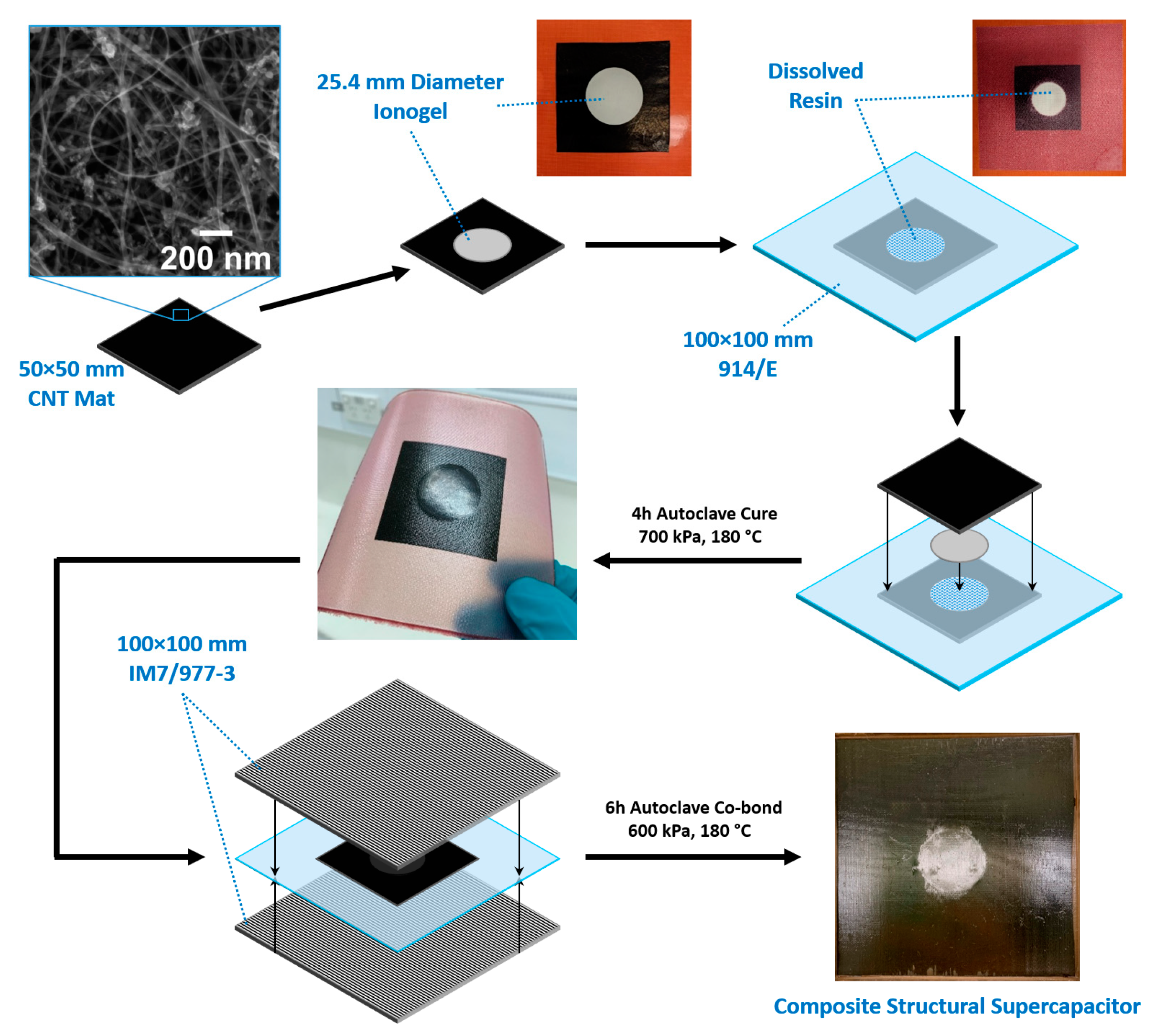



| Designation | 1080/914 Separator | Ionogel (12 mg cm−2) | Additional IL (50 µL) |
|---|---|---|---|
| SSC1 | unmodified | - | Electrodes + separator |
| SSC2 | central 25.4 mm diam. resin disk removed | - | Electrodes + separator |
| SSC3 | 2 × 25.4 mm disks either side of separator | - | |
| SSC4 | Ionogels | ||
| SSC5 | Electrodes + Separator + ionogels |
| Component | Physical Interpretation | SSC1 | SSC2 | SSC4 | SSC5 | Coin Cell |
|---|---|---|---|---|---|---|
| R1 (Ω) | Equivalent series resistance | ~0.10 | ~0.10 | ~0.10 | ~0.10 | 8.2 |
| C1 (uF) | EIS equilibrium EDLC | 32 | 44 | 18 | 260 | 4800 |
| Rd3 (Ω) | Diffuse layer resistance | 25 | 8.5 | 34 | 13 | 20 |
| t3 (s) | RC time constant | 0.25 | 0.14 | 0.40 | 0.33 | 0.060 |
| a3 | Limited diffusion exponent | 0.80 | 0.87 | 0.88 | 0.89 | 0.94 |
| C4 (nF) | EIS equilibrium pseudocapacitance | 9.7 | 3.9 | 3.1 | 29 | 19 |
| R4 (Ω) | Charge transfer resistance | 44 | 19 | 86 | 14 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapleback, B.; Dao, V.; Webb, L.; Rider, A. Composite Structural Supercapacitors: High-Performance Carbon Nanotube Supercapacitors through Ionic Liquid Localisation. Nanomaterials 2022, 12, 2558. https://doi.org/10.3390/nano12152558
Mapleback B, Dao V, Webb L, Rider A. Composite Structural Supercapacitors: High-Performance Carbon Nanotube Supercapacitors through Ionic Liquid Localisation. Nanomaterials. 2022; 12(15):2558. https://doi.org/10.3390/nano12152558
Chicago/Turabian StyleMapleback, Benjamin, Vu Dao, Lachlan Webb, and Andrew Rider. 2022. "Composite Structural Supercapacitors: High-Performance Carbon Nanotube Supercapacitors through Ionic Liquid Localisation" Nanomaterials 12, no. 15: 2558. https://doi.org/10.3390/nano12152558
APA StyleMapleback, B., Dao, V., Webb, L., & Rider, A. (2022). Composite Structural Supercapacitors: High-Performance Carbon Nanotube Supercapacitors through Ionic Liquid Localisation. Nanomaterials, 12(15), 2558. https://doi.org/10.3390/nano12152558