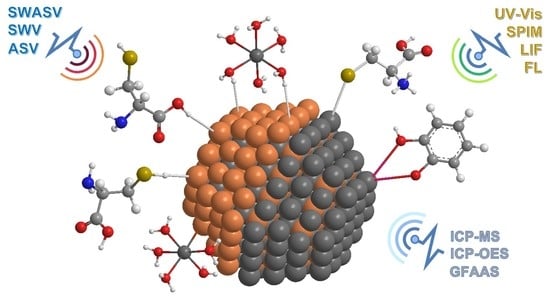
Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection
Abstract
1. Introduction
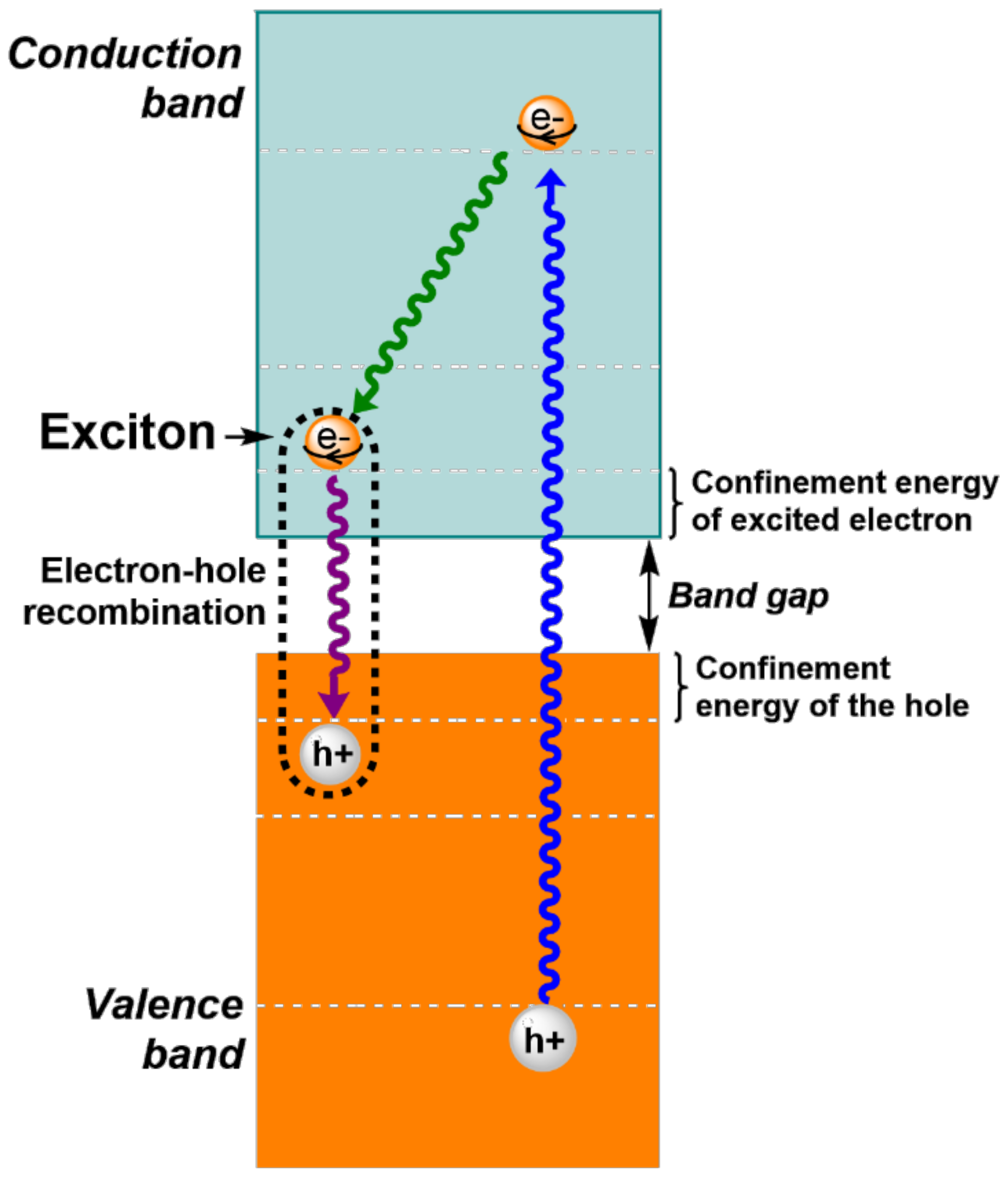
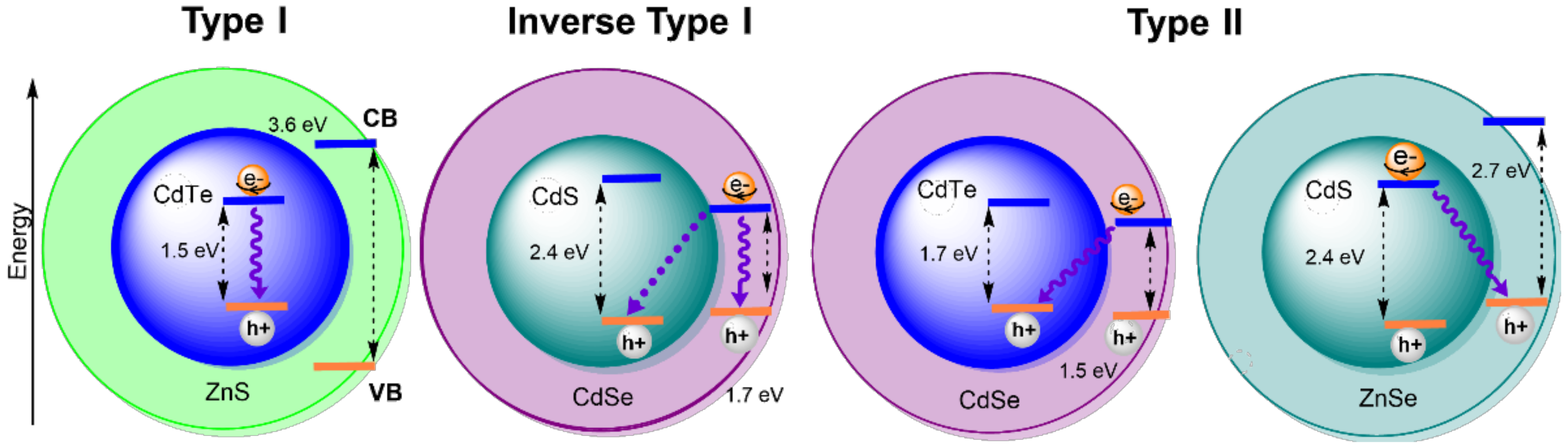
2. The Effect of Size and Composition on Properties of QDs
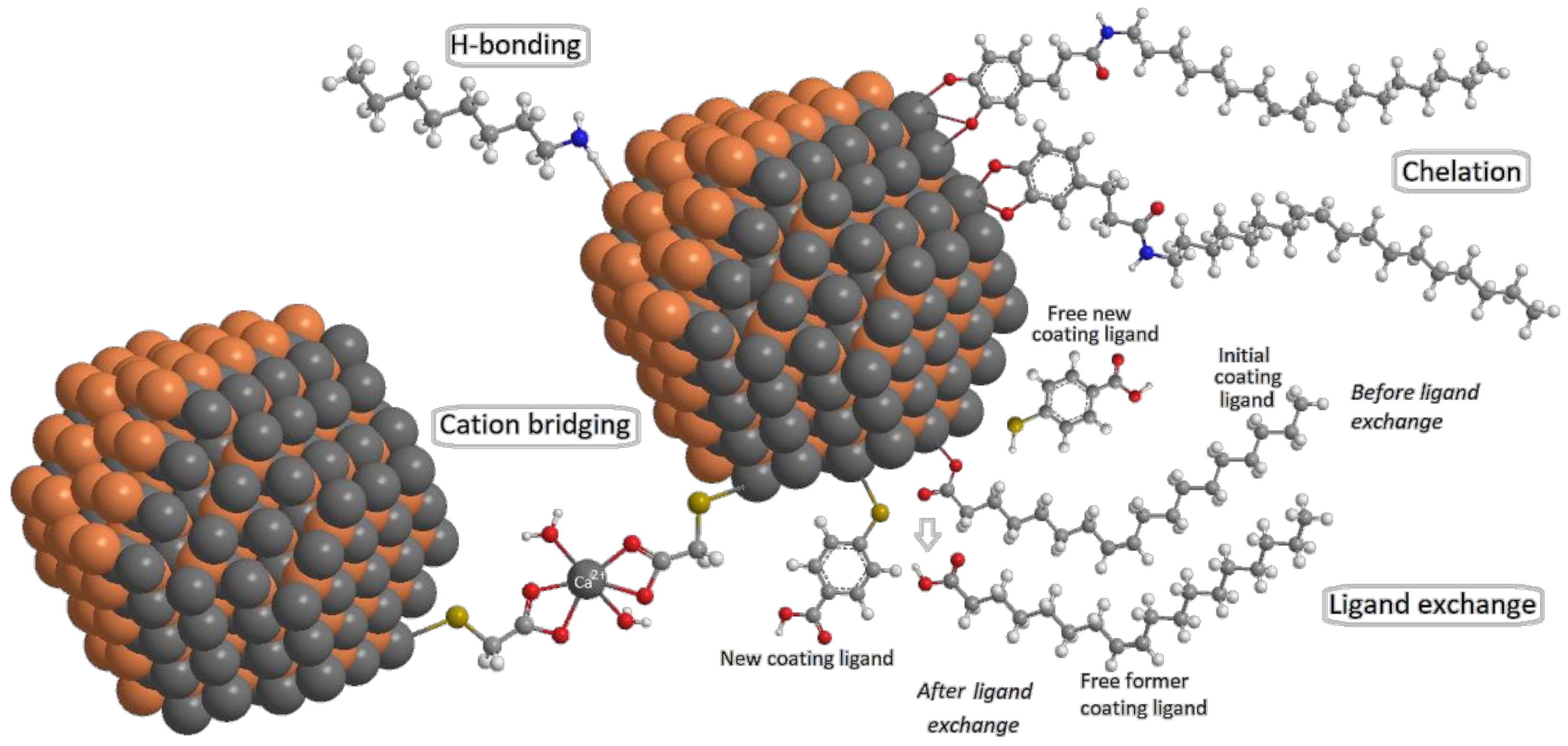
3. Surface Chemistry of QDs
3.1. Interactions between NMs and Surface Ligands
3.2. Analytical Techniques for Characterising the Interactions between NMs and Surface Ligands
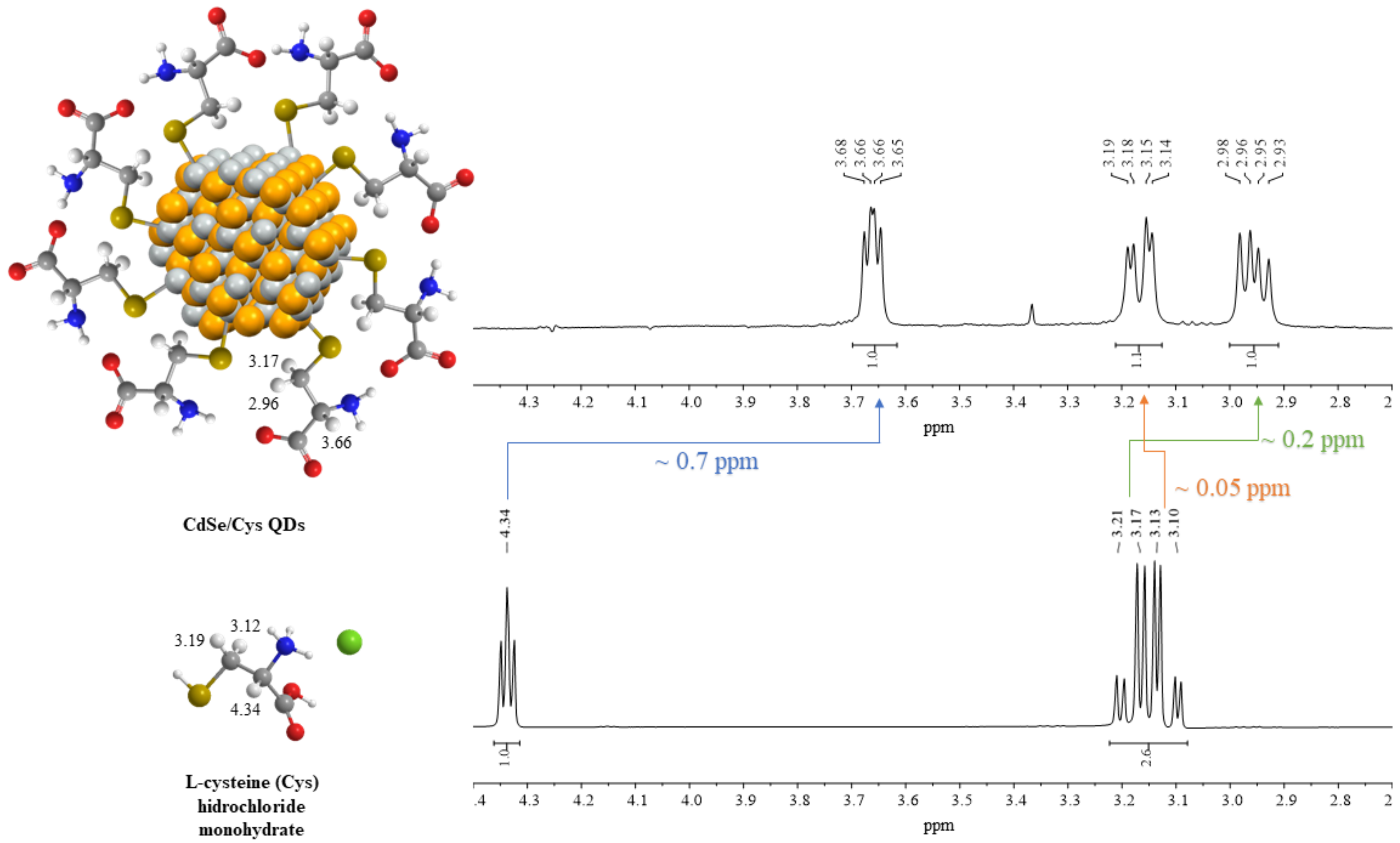
3.2.1. Nuclear Magnetic Resonance (NMR)
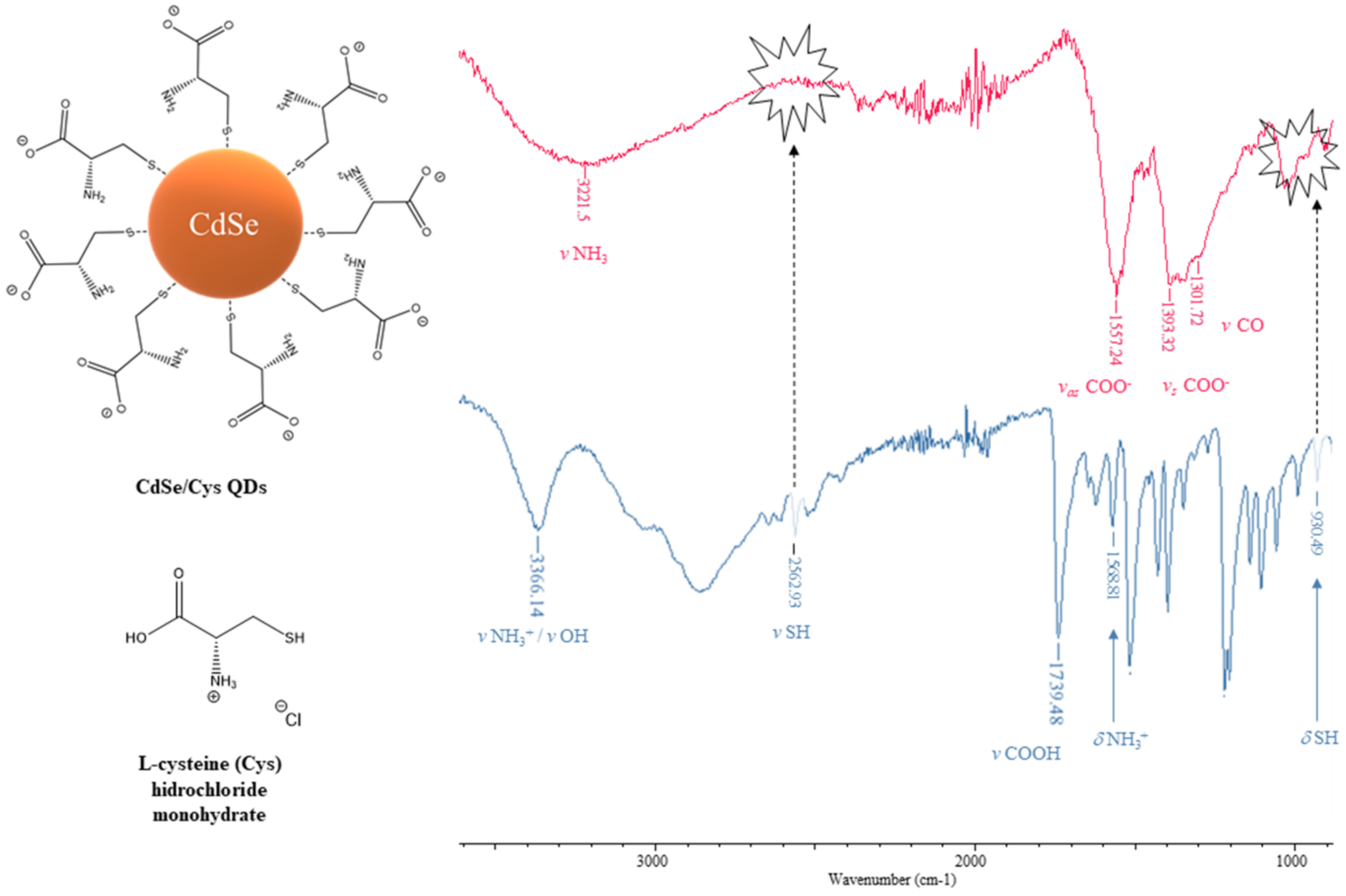
3.2.2. Fourier Transform-Infrared Spectrophotometry (FT–IR)
3.2.3. Raman Spectroscopy
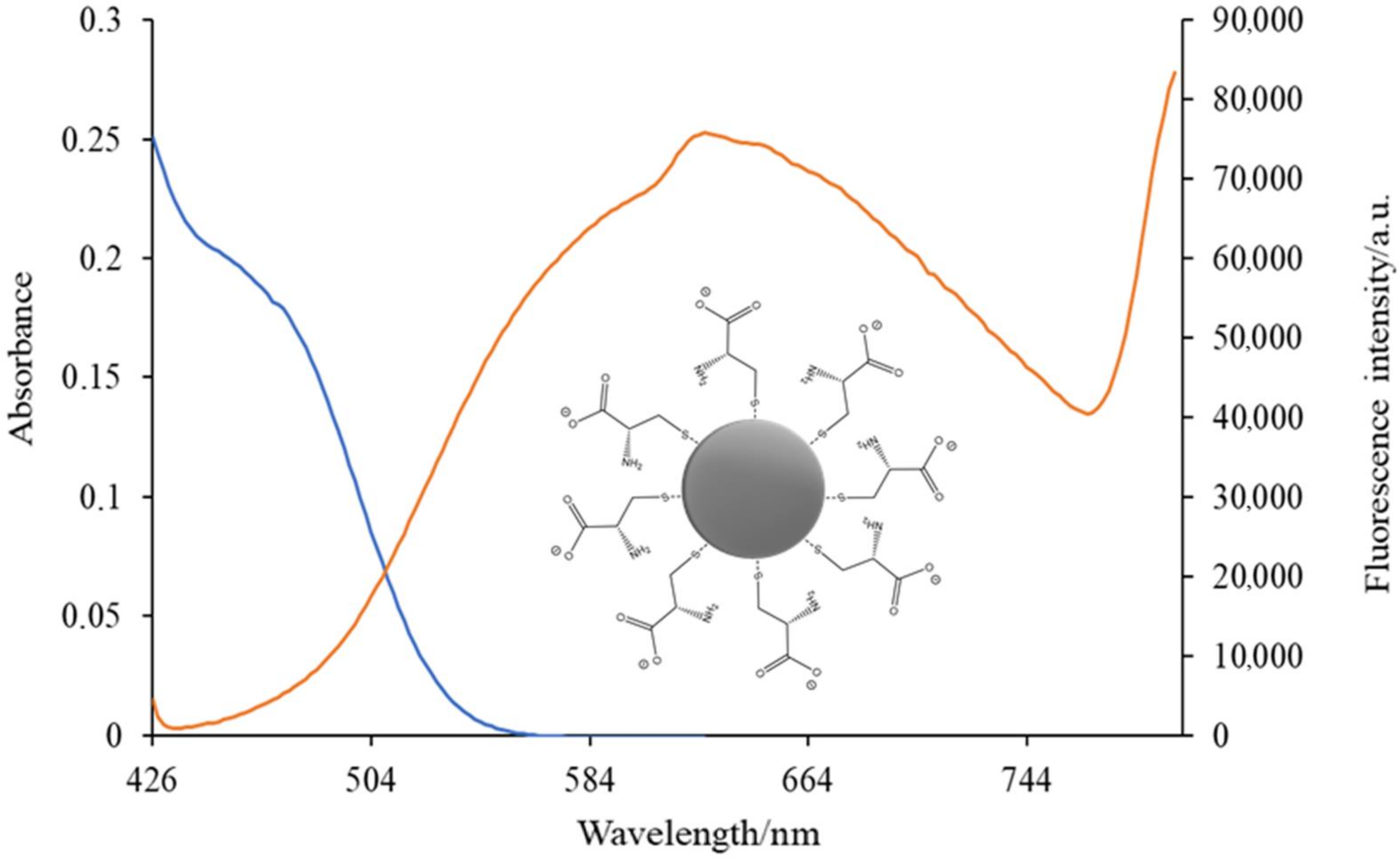
3.2.4. Spectrofluorimetry
3.2.5. UV–Vis Spectrophotometry
3.2.6. Isothermal Titration Calorimetry (ITC)
3.2.7. Size Exclusion Chromatography (SEC)
3.2.8. Zeta Potential Determination
3.2.9. Powder X-ray Diffraction (XRD)
3.2.10. X-ray Absorption Spectroscopy (XAS)
3.2.11. X-ray Photoelectron Spectroscopy (XPS)
3.2.12. X-ray Fluorescence (XRF)
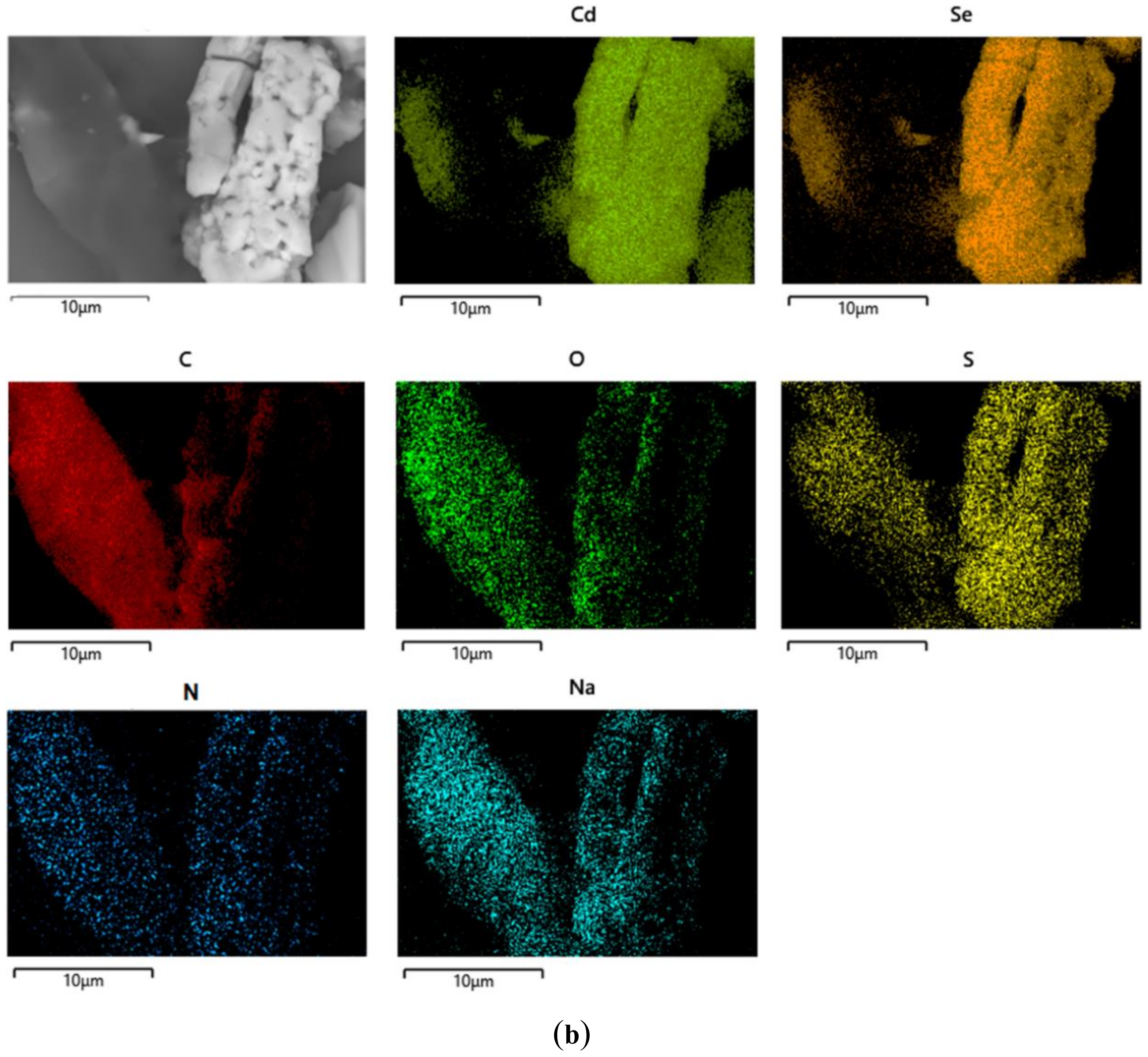
3.2.13. Scanning Electron Microscopy with Energy Dispersive X-ray Analysis (SEM–EDX)
4. Analytical Techniques for Detection and Quantification of QDs
4.1. Electroanalytical Techniques: Voltammetry
4.1.1. Square Wave Anodic Stripping Voltammetry (SWASV)
4.1.2. Square Wave Voltammetry (SWV)
4.1.3. Anodic Stripping Voltammetry (ASV)
4.2. Atomic Spectrometry Techniques
4.2.1. Inductively Coupled Plasma-Mass Spectrometry (ICP–MS)
4.2.2. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP–OES)
4.2.3. Graphite Furnace Atomic Absorption Spectrometry (GFAAS)
4.3. Molecular Spectrometry Techniques
4.3.1. Spectrofluorimetry
4.3.2. Laser-Induced Fluorescence Spectrophotometry (LIF)
4.3.3. Selective Plane Illumination Microscopy (SPIM)
4.3.4. UV–Vis Spectrophotometry
5. Concluding Remarks and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brus, L.E. Electron-Electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Vasudevan, D.; Ranganathan Gaddam, R.; Trinchi, A.; Cole, I. Core-Shell quantum dots: Properties and applications. J. Alloys Comp. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.J.D.; Stone, V. Quantum dots: An insight and perspective of their biological interaction and how this relates to their relevance for clinical use. Theranostics 2012, 2, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef]
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C.W. Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc. Chem. Res. 2013, 46, 662–671. [Google Scholar] [CrossRef]
- Benítez-Martínez, S.; Valcárcel, M. Fluorescent determination of graphene quantum dots in water samples. Anal. Chim. Acta 2015, 896, 78–84. [Google Scholar] [CrossRef]
- Chu, K.; Adsetts, J.R.; He, S.; Zhan, Z.; Yang, L.; Wong, J.M.; Love, D.A.; Ding, Z. Electrogenerated chemiluminescence and electroluminescence of N-doped graphene quantum dots fabricated from an electrochemical exfoliation process in nitrogen-containing electrolytes. Chem. Eur. J. 2020, 26, 15892–15900. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Jiang, H. A review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. J. Bioresour. Bioprod. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and applications of fungal mycelium-based advanced functional materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Lewinski, N.A.; Zhu, H.; Jo, H.-J.; Pham, D.; Kamath, R.R.; Ouyang, C.R.; Vulpe, C.D.; Colvin, V.L.; Drezek, R.A. Quantification of water solubilized CdSe/ZnS quantum dots in Daphnia magna. Environ. Sci. Technol. 2010, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sánchez, M.; Miserere, S.; Marín, S.; Aragaya, G.; Merkoçi, A. On-Chip electrochemical detection of CdS quantum dots using normal and multiple recycling flow through modes. Lab. Chip 2012, 12, 2000–2005. [Google Scholar] [CrossRef]
- Ragusa, A.; Zacheo, A.; Pellegrino, T.; Manna, L. Quantum dot nanoparticles: Properties, surface functionalization, and their applications in biosensoring and imaging. In Nanostructured Materials for Biomedical Applications; Tan, M.C., Chow, G.M., Ren, L., Eds.; Transworld Research Network: Kerala, India, 2009; pp. 151–221. [Google Scholar]
- Ma, Y.; Zhang, Y.; Yu, W.W. Near infrared emitting quantum dots: Synthesis, luminescence properties and applications. J. Mater. Chem. C 2019, 7, 13662–13679. [Google Scholar] [CrossRef]
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-Containing quantum dots: Properties, applications, and toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Bharti, S.; Bhullar, G.K.; Tripathi, S.K. I-III-VI core/shell QDs: Synthesis, characterizations and applications. J. Lumin. 2020, 219, 116912–116939. [Google Scholar] [CrossRef]
- Tsolekile, N.; Parani, S.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Evolution of ternary I–III–VI QDs: Synthesis, characterization and application. Nano Struct. Nano Objects 2017, 12, 46–56. [Google Scholar] [CrossRef]
- Ma, Q.; Su, X. Near-infrared quantum dots: Synthesis, functionalization and analytical applications. Analyst 2010, 135, 1867–1877. [Google Scholar] [CrossRef]
- Volkov, Y. Quantum dots in nanomedicine: Recent trends, advances and unresolved issues. Biochem. Biophys. Res. Commun. 2015, 468, 419–427. [Google Scholar] [CrossRef]
- Frigerio, C.; Ribeiro, D.S.M.; Rodrigues, S.S.M.; Abreu, V.L.R.G.; Barbosa, J.A.C.; Prior, J.A.V.; Marques, K.L.; Santos, J.L.M. Application of quantum dots as analytical tools in automated chemical analysis: A review. Anal. Chim. Acta 2012, 735, 9–22. [Google Scholar] [CrossRef]
- Filali, S.; Pirot, F.; Miossec, P. Biological applications and toxicity minimization of semiconductor quantum dots. Trends Biotechnol. 2020, 38, 163–177. [Google Scholar] [CrossRef]
- Ji, X.; Peng, F.; Zhong, Y.; Su, Y.; He, Y. Fluorescent quantum dots: Synthesis, biomedical optical imaging, and biosafety assessment. Colloids Surf. B 2014, 124, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int. J. Nanomed. 2018, 13, 2729–2742. [Google Scholar] [CrossRef]
- Luo, G.; Long, J.; Zhang, B.; Liu, C.; Ji, S.; Xu, J.; Yu, X.; Ni, Q. Quantum dots in cancer therapy. Expert Opin. Drug Deliv. 2012, 9, 47–58. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Gao, S.; Goh, B.L.; Bin, S.I.; Lwe, K.W.; Wu, Y.; Wu, C.; Su, X. Recent advances in non-toxic quantum dots and their biomedical applications. Prog. Nat. Sci. Mater. Int. 2019, 29, 628–640. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Review of in vitro toxicological research of quantum dot and potentially involved mechanisms. Sci. Total Environ. 2018, 625, 940–962. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Galdiero, E.; Falanga, A.; Carotenuto, R.; De Alteriis, E.; Guida, M. Toxicity effects of functionalized quantum dots, gold and polystyrene nanoparticles on target aquatic biological models: A review. Molecules 2017, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.L.; Mestre, N.C.; Saboia-Morais, S.M.T.; Bebianno, M.J. Environmental behaviour and ecotoxicity of quantum dots at various trophic levels: A review. Environ. Int. 2017, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, C.; Zeng, G.; Chen, G.; Wan, J.; Guo, Z.; Wu, H.; Yu, Z.; Zhou, Y.; Liu, J. Metal-based quantum dots: Synthesis, surface modification, transport and fate in aquatic environments and toxicity to microorganisms. RSC Adv. 2016, 6, 78595–78610. [Google Scholar] [CrossRef]
- Zhan, Q.; Tang, M. Research advances on apoptosis caused by quantum dots. Biol. Trace Elem. Res. 2014, 161, 3–12. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M. Toxicity of quantum dots on respiratory system. Inhal. Toxicol. 2014, 26, 128–139. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F.; Wei, H.; Yang, B. The effects of composition and surface chemistry on the toxicity of quantum dots. J. Mater. Chem. B 2013, 1, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Law, W.-C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity assessment of quantum dots: From cellular to primate studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Pandey, A.K.; Herzog, A.B.; Rose, J.B.; Gerba, C.P.; Hashsham, S.A. Environmental applications and potential health implications of quantum dots. J. Nanopart. Res. 2012, 14, 1038. [Google Scholar] [CrossRef]
- Xu, L.; Chen, C. Physiological behavior of quantum dots. WIREs Nanomed. Nanobiotechnol. 2012, 4, 620–637. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum dots-characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro-and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef]
- Stanisavljevic, M.; Vaculovicova, M.; Kizek, R.; Adam, V. Capillary electrophoresis of quantum dots: Minireview. Electrophoresis 2014, 35, 1929–1937. [Google Scholar] [CrossRef]
- Sang, F.; Huang, X.; Ren, J. Characterization and separation of semiconductor quantum dots and their conjugates by capillary electrophoresis. Electrophoresis 2014, 35, 793–803. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, K.B. Preparation and characterization of CdS and PbS quantum dots in zeolite Y and their applications for nonlinear optical materials and solar cell. Coord. Chem. Rev. 2014, 263–264, 239–256. [Google Scholar] [CrossRef]
- Bilan, R.; Fleury, F.; Nabiev, I.; Sukhanova, A. Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjug. Chem. 2015, 26, 609–624. [Google Scholar] [CrossRef]
- Kilina, S.V.; Tamukong, P.K.; Kilin, D.S. Surface chemistry of semiconducting quantum dots: Theoretical perspectives. Acc. Chem. Res. 2016, 49, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.A.; Kamat, P.V. Recent advances in quantum dot surface chemistry. ACS Appl. Mater. Interfaces 2014, 6, 3041–3057. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Vaidya, R.; Smith, L.D.; Han, Z.; Zahid, M.U.; Winter, J.; Sarkar, S.; Chung, H.J.; Perez-Pinera, P.; Selvin, P.R.; et al. Optimizing quantum dot probe size for single-receptor imaging. ACS Nano 2020, 14, 8343–8358. [Google Scholar] [CrossRef] [PubMed]
- Enright, B.; Fitzmaurice, D. Spectroscopic determination of electron and hole effective masses in a nanocrystalline semiconductor film. J. Phys. Chem. 1996, 100, 1027–1035. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Maxwell, T.; Nogueira Campos, M.G.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Quantum dots. In Nanoparticles for Biomedical Applications. Fundamental Concepts, Biological Interactions and Clinical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdan, The Netherlands, 2020; pp. 243–265. [Google Scholar]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Soloviev, V.N.; Eichhöfer, A.; Fenske, D.; Banin, U. Molecular limit of a bulk semiconductor: Size dependence of the “band gap” in CdSe cluster molecules. J. Am. Chem. Soc. 2000, 122, 2673–2674. [Google Scholar] [CrossRef]
- Guzelturk, B.; Hernandez Martinez, P.L.; Zhang, Q.; Xiong, Q.; Sun, H.; Sun, X.W.; Govorov, A.O.; Demir, H.V. Excitonics of semiconductor quantum dots and wires for lighting and displays. Laser Photonics Rev. 2014, 8, 73–93. [Google Scholar] [CrossRef]
- Dramićanin, M. Luminescence Thermometry: Methods, Materials, and Applications; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Chaparro Barriera, E.A.; Bailón-Ruiz, S.J. Fabrication, characterization, and nanotoxicity of water stable quantum dots. MRS Adv. 2020, 5, 2231–2239. [Google Scholar] [CrossRef]
- Merkoçi, A.; Marcolino-Junior, L.H.; Marín, S.; Fatibello-Filho, O.; Alegret, S. Detection of cadmium sulphide nanoparticles by using screen-printed electrodes and a handheld device. Nanotechnology 2007, 18, 035502. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.A.; Carpenter, M.C.; Sonne, J.W.H.; Miller, C.A.; Renehan, J.R.; Odonkor, C.A.; Henry, E.M.; Miles, D.T. Reversed-Phase HPLC separation of water-soluble, monolayer-protected quantum dots. J. Phys. Chem. C 2011, 115, 18952–18957. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Buchman, J.T.; Qiu, T.A.; Zhi, B.; Lyons, T.Y.; Landy, K.M.; Rosenzweig, Z.; Haynes, C.L.; Fairbrother, D.H. Release, detection and toxicity of fragments generated during artificial accelerated weathering of CdSe/ZnS and CdSe quantum dot polymer composites. Environ. Sci. Nano 2018, 5, 1694–1710. [Google Scholar] [CrossRef]
- Becerra, L.R.; Murray, C.B.; Griffin, R.G.; Bawendi, M.G. Investigation of the surface morphology of capped CdSe nanocrystallites by 31P NMR. J. Chem. Phys. 1994, 100, 3297–3300. [Google Scholar] [CrossRef]
- Taylor, J.; Kippeny, T.; Rosenthal, S.J. Surface stoichiometry of CdSe nanocrystals determined by Rutherford backscattering spectroscopy. J. Cluster Sci. 2001, 12, 571–582. [Google Scholar] [CrossRef]
- Bowen-Katari, J.E.; Colvin, V.L.; Alivisatos, A.P. X-ray photoelectron spectroscopy of CdSe nanocrystals with application to studies of the nanocrystal surface. J. Phys. Chem. 1994, 98, 4109–4117. [Google Scholar] [CrossRef]
- Morris-Cohen, A.J.; Donakowski, M.D.; Knowles, K.E.; Weiss, E.A. The effect of a common purification procedure on the chemical composition of the surfaces of CdSe quantum dots synthesized with trioctylphosphine oxide. J. Phys. Chem. C 2009, 114, 897–906. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B.S. Adsorption of fulvic acid by carbon nanotubes from water. Environ. Pollut. 2009, 157, 1095–1100. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, H.B.; Chen, J.; Li, X.; Wang, Z.; Sheng, L. Molecular dynamics simulations on the interactions of low molecular weight natural organic acids with C-60. Chemosphere 2013, 92, 429–434. [Google Scholar] [CrossRef]
- Hyung, H.; Kim, J.H. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters. Environ. Sci. Technol. 2008, 42, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Tao, S.; Xing, B.S. Sorption and competition of aromatic compounds and humic acid on multiwalled carbon nanotubes. Environ. Sci. Technol. 2009, 43, 6214–6219. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xing, B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef]
- Liu, W.; Chen, H.; Pan, D.; Ji, X. Electrostatic interaction mediates the formation of vesicular structures from coassembly of PS-b-PAA with quantum dots. Langmuir 2019, 35, 12501–12508. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-Y.; Lin, Y.; Peng, J.; Liu, S.-L.; Zhang, Z.-L.; Tian, Z.-Q.; Pang, D.-W. Evaluation of nonspecific interactions between quantum dots and proteins. Phys. Chem. Chem. Phys. 2014, 16, 7677–7680. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Wang, K.; Yang, R.; Ji, H.; Yang, L.; Wu, C. A switchable fluorescent quantum dot probe based on aggregation/disaggregation mechanism. Chem. Commun. 2011, 47, 935–937. [Google Scholar] [CrossRef]
- Wang, X.L.; Shu, L.; Wang, Y.Q.; Xu, B.B.; Bai, Y.C.; Tao, S.; Xing, B.S. Sorption of peat humic acids to multi-walled carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9276–9283. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J.C. Stability and removal of water soluble CdTe quantum dots in water. Environ. Sci. Technol. 2008, 42, 321–325. [Google Scholar] [CrossRef]
- Mashayekhi, H.; Ghosh, S.; Du, P.; Xing, B. Effect of natural organic matter on aggregation behavior of C-60 fullerene in water. J. Colloid Interface Sci. 2012, 374, 111–117. [Google Scholar] [CrossRef]
- Kessler, M.L.; Starr, H.E.; Knauf, R.R.; Rountree, K.J.; Dempsey, J.L. Exchange equilibria of carboxylate-terminated ligands at PbS nanocrystal surfaces. Phys. Chem. Chem. Phys. 2018, 20, 23649–23655. [Google Scholar] [CrossRef]
- De Roo, J.; De Keukeleere, K.; Hens, Z.; Van Driessche, I. From ligands to binding motifs and beyond; the enhanced versatility of nanocrystal surfaces. Dalton Trans. 2016, 45, 13277–13283. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.S. The coordination chemistry of nanocrystal surfaces. Science 2015, 347, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, L.; Lei, J.; Liu, H.; Ju, H. Formation of surface traps on quantum dots by bidentate chelation and their application in low-potential electrochemiluminescent biosensing. Chem. Eur. J. 2010, 16, 10764–10770. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.A.G.; Watson, D.F.; Aga, D.S.; Banerjee, S. Natural organic matter-mediated phase transfer of quantum dots in the aquatic environment. Environ. Sci. Technol. 2009, 43, 677–682. [Google Scholar] [CrossRef]
- Navarro, D.A.; Banerjee, S.; Aga, D.S.; Watson, D.F. Partitioning of hydrophobic CdSe quantum dots into aqueous dispersions of humic substances: Influence of capping-group functionality on the phase-transfer mechanism. J. Colloid Interface Sci. 2010, 348, 119–128. [Google Scholar] [CrossRef]
- Bloemen, M.; Debruyne, D.; Demeyer, P.-J.; Clays, K.; Gils, A.; Geukens, N.; Bartic, C.; Verbiest, T. Catechols as ligands for CdSe–ZnS quantum dots. RSC Adv. 2014, 4, 10208–10211. [Google Scholar] [CrossRef]
- Hens, Z.; Martins, J.C. A solution NMR toolbox for characterizing the surface chemistry of colloidal nanocrystals. Chem. Mater. 2013, 25, 1211–1221. [Google Scholar] [CrossRef]
- Zeng, B.; Palui, G.; Zhang, C.; Zhan, N.; Wang, W.; Ji, X.; Chen, B.; Mattoussi, H. Characterization of the ligand capping of hydrophobic CdSe–ZnS quantum dots using NMR spectroscopy. Chem. Mater. 2018, 30, 225–238. [Google Scholar] [CrossRef]
- Hens, Z.; Moreels, I.; Martins, J.C. In situ 1H NMR study on the trioctylphosphine oxide capping of colloidal InP nanocrystals. ChemPhysChem 2005, 6, 2578–2584. [Google Scholar] [CrossRef]
- Ribot, F.; Escax, V.; Roiland, C.; Sanchez, C.; Martins, J.C.; Biesemans, M.; Verbruggen, I.; Willem, R. In situ evaluation of interfacial affinity in CeO2 based hybrid nanoparticles by pulsed field gradient NMR. Chem. Commun. 2005, 1019–1021. [Google Scholar] [CrossRef]
- Fritzinger, B.; Moreels, I.; Lommens, P.; Koole, R.; Hens, Z.; Martins, J.C. In situ observation of rapid ligand exchange in colloidal nanocrystal suspensions using transfer NOE nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 2009, 131, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Moreels, I.; Fritzinger, B.; Martins, J.C.; Hens, Z. Surface chemistry of colloidal PbSe nanocrystals. J. Am. Chem. Soc. 2008, 130, 15081–15086. [Google Scholar] [CrossRef] [PubMed]
- Cros-Gagneux, A.; Delpech, F.; Nayral, C.; Cornejo, A.; Coppel, Y.; Chaudret, B. Surface chemistry of InP quantum dots: A comprehensive study. J. Am. Chem. Soc. 2010, 132, 18147–18157. [Google Scholar] [CrossRef] [PubMed]
- Moreels, I.; Justo, Y.; De Geyter, B.; Haustraete, K.; Martins, J.C.; Hens, Z. Size-Tunable, bright, and stable PbS quantum dots: A surface chemistry study. ACS Nano 2011, 5, 2004–2012. [Google Scholar] [CrossRef]
- Moreels, I.; Martins, J.C.; Hens, Z. Ligand adsorption/desorption on sterically stabilized InP colloidal nanocrystals: Observation and thermodynamic analysis. ChemPhysChem 2006, 7, 1028–1031. [Google Scholar] [CrossRef]
- Van Lokeren, L.; Maheut, G.; Ribot, F.; Escax, V.; Verbruggen, I.; Sanchez, C.; Martins, J.C.; Biesemans, M.; Willem, R. Characterization of titanium dioxide nanoparticles dispersed in organic ligand solutions by using a diffusion-ordered spectroscopy-based strategy. Chem. Eur. J. 2007, 13, 6957–6966. [Google Scholar] [CrossRef]
- Shen, L.; Soong, R.; Wang, M.; Lee, A.; Wu, C.; Scholes, G.D.; Macdonald, P.M.; Winnik, M.A. Pulsed field gradient NMR studies of polymer adsorption on colloidal CdSe quantum dots. J. Phys. Chem. B 2008, 112, 1626–1633. [Google Scholar] [CrossRef]
- Hassinen, A.; Moreels, I.; Donega, C.D.M.; Martins, J.C.; Hens, Z. Nuclear magnetic resonance spectroscopy demonstrating dynamic stabilization of CdSe quantum dots by alkylamines. J. Phys. Chem. Lett. 2010, 1, 2577–2581. [Google Scholar] [CrossRef]
- Fritzinger, B.; Capek, R.K.; Lambert, K.; Martins, J.C.; Hens, Z. Utilizing self-exchange to address the binding of carboxylic acid ligands to CdSe quantum dots. J. Am. Chem. Soc. 2010, 132, 10195–10201. [Google Scholar] [CrossRef]
- Donakowski, M.D.; Godbe, J.M.; Sknepnek, R.; Knowles, K.E.; de la Cruz, M.O.; Weiss, E.A. A quantitative description of the binding equilibria of para-substituted aniline ligands and CdSe quantum dots. J. Phys. Chem. C 2010, 114, 22526–22534. [Google Scholar] [CrossRef]
- Wallner, A.; Jafri, S.H.M.; Blom, T.; Gogoll, A.; Leifer, K.; Baumgartner, J.; Ottosson, H. Formation and NMR spectroscopy of ω-thiol protected α, ω-alkanedithiol-coated gold nanoparticles and their usage in molecular charge transport junctions. Langmuir 2011, 27, 9057–9067. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.K.; Ruddy, D.A.; Blackburn, J.L.; Smith, D.K.; Bergren, M.R.; Nozik, A.J.; Johnson, J.C.; Beard, M.C. Control of PbSe quantum dot surface chemistry and photophysics using an alkylselenide ligand. ACS Nano 2012, 6, 5498–5506. [Google Scholar] [CrossRef]
- Coppel, Y.; Spataro, G.; Pages, C.; Chaudret, B.; Maisonnat, A.; Kahn, M.L. Full characterization of colloidal solutions of long-alkyl-chain-amine-stabilized ZnO nanoparticles by NMR spectroscopy: Surface state, equilibria, and affinity. Chem. Eur. J. 2012, 18, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Hassinen, A.; Szczygiel, A.; Zhao, Q.; Vantomme, A.; Martins, J.C.; Hens, Z. Binding of phosphonic acids to CdSe quantum dots: A solution NMR study. J. Phys. Chem. Lett. 2011, 2, 145–152. [Google Scholar] [CrossRef]
- Kalyuzhny, G.; Murray, R. Ligand effects on optical properties of CdSe nanocrystals. J. Phys. Chem. B 2005, 109, 7012–7021. [Google Scholar] [CrossRef] [PubMed]
- Hassinen, A.; Moreels, I.; De Nolf, K.; Smet, P.F.; Martins, J.C.; Hens, Z. Short-Chain alcohols strip X-type ligands and quench the luminescence of PbSe and CdSe quantum dots, acetonitrile does not. J. Am. Chem. Soc. 2012, 134, 20705–20712. [Google Scholar] [CrossRef] [PubMed]
- Malicki, M.; Knowles, K.E.; Weiss, E.A. Gating of hole transfer from photoexcited PbS quantum dots to aminoferrocene by the ligand shell of the dots. Chem. Commun. 2013, 49, 4400–4402. [Google Scholar] [CrossRef]
- Martínez Bonilla, C.A.; Torres Flórez, M.H.; Molina Velasco, D.R.; Kouznetsov, V.V. Surface characterization of thiol ligands on CdTe quantum dots: Analysis by 1H NMR and DOSY. New J. Chem. 2019, 43, 8452–8458. [Google Scholar] [CrossRef]
- Morris-Cohen, A.J.; Vasilenko, V.; Amin, V.A.; Reuter, M.G.; Weiss, E.A. Model for adsorption of ligands to colloidal quantum dots with concentration-dependent surface structure. ACS Nano 2012, 6, 557–565. [Google Scholar] [CrossRef]
- Huo, R.; Wehrens, R.; Van Duynhoven, J.; Buydens, L.M.C. Assessment of techniques for DOSY NMR data processing. Anal. Chim. Acta 2003, 490, 231–251. [Google Scholar] [CrossRef]
- McMurry, J.E. Organic Chemistry, 9th ed.; Cengage Learning: Andover, UK, 2016. [Google Scholar]
- Oluwafemi, S.O.; Revaprasadu, N.; Ramirez, A.J. A novel one-pot route for the synthesis of water-soluble cadmium selenide nanoparticles. J. Cryst. Growth 2008, 310, 3230–3234. [Google Scholar] [CrossRef]
- Roberts, J.D.; Caserio, M.C. Basic Principles of Organic Chemistry, 2nd ed.; W.A. Benjamin: Menlo Park, CA, USA, 1977. [Google Scholar]
- Li, L.; Zhao, F.; Zhang, T.; Lü, C. A facile method to prepare polymer functionalized carbon dots inspired by the mussel chemistry for LED application. Dye. Pigment. 2019, 162, 845–854. [Google Scholar] [CrossRef]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: San Diego, CA, USA, 2001; Volume 2, pp. 65–83. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cooper, J.K.; Franco, A.M.; Gul, S.; Corrado, C.; Zhang, J.Z. Characterization of primary amine capped CdSe, ZnSe, and ZnS quantum dots by FT-IR: Determination of surface bonding interaction and identification of selective desorption. Langmuir 2011, 27, 8486–8493. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Lim, S.J.; Wrobel, T.P.; Bhargava, R.; Smith, A.M. Measuring and predicting the internal structure of semiconductor nanocrystals through Raman spectroscopy. J. Am. Chem. Soc. 2016, 138, 10887–10896. [Google Scholar] [CrossRef]
- Tian, H.; Li, H.; Fang, Y. Binary thiol-capped gold nanoparticle monolayer films for quantitative surface-enhanced Raman scattering analysis. ACS Appl. Mater. Interfaces 2019, 11, 16207–16213. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R.; Das, A.; Kapoor, S.; Maiti, N. Surface-Induced dimerization of 2-thiazoline-2-thiol on silver and gold nanoparticles: A surface enhanced Raman scattering (SERS) and density functional theoretical (DFT) study. J. Mol. Liq. 2021, 322, 114536. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852–10865. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; Wang, F.; Fang, Y. Investigation of the microstructures of graphene quantum dots (GQDs) by surface-enhanced Raman spectroscopy. Nanomaterials 2018, 8, 964. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, K.; Chang, R.; Yi, X.; Zhang, Y.; Wang, S. Study of charge transfer at the quantum dot-graphene interface by Raman spectroscopy. J. Phys. Chem. C 2019, 123, 24943–24948. [Google Scholar] [CrossRef]
- Trajić, J.; Kostić, R.; Romčević, N.; Mitrić, M.; Lazović, V.; Balaž, P.; Stojanović, D. Raman spectroscopy of ZnS quantum dots. J. Alloys Comp. 2015, 637, 401–406. [Google Scholar] [CrossRef]
- Gong, K.; Kelley, D.F.; Kelley, A.M. Resonance Raman spectroscopy and electron-phonon coupling in zinc selenide quantum dots. J. Phys. Chem. C 2016, 120, 29533–29539. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Said, K. Germanium antimony quantum dots morphology and Raman spectroscopy fabricated by inert gas condensation. Results Phys. 2019, 13, 102311. [Google Scholar] [CrossRef]
- Hamizi, N.A.; Johan, M.R.; Chowdhury, Z.Z.; Wahab, Y.A.; Al-Douri, Y.; Saat, A.M.; Pivehzhani, O.A. Raman spectroscopy and FTIR spectroscopy studies of Mn-doped CdSe QDs at different particles size. Optik 2019, 179, 628–631. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Imam, N.G.; El-Dek, S.I.; El-Mahy, S.K. Fluorescence and spectroscopic characterization of multiferroic quantum dots of La:BiFeO3. J. Supercond. Nov. Magn. 2015, 28, 2417–2424. [Google Scholar] [CrossRef]
- Estupiñán-López, C.; Tolentino Dominguez, C.; Cabral Filho, P.E.; Santos, B.S.; Fontes, A.; de Araujo, R.E. A pH dependence study of CdTe quantum dots fluorescence quantum yields using eclipsing thermal lens spectroscopy. J. Lumin. 2016, 174, 17–21. [Google Scholar] [CrossRef]
- Yashima, S.; Sugimoto, H.; Takashina, H.; Fujii, M. Fluorescence enhancement and spectral shaping of silicon quantum dot monolayer by plasmonic gap resonances. J. Phys. Chem. C 2016, 120, 28795–28801. [Google Scholar] [CrossRef]
- Munro, T.; Liu, L.; Glorieux, C.; Ban, H. CdSe/ZnS quantum dot fluorescence spectra shape-based thermometry via neural network reconstruction. J. Appl. Phys. 2016, 119, 214903. [Google Scholar] [CrossRef]
- Lyons, T.Y.; Williams, D.N.; Rosenzweig, Z. Addition of fluorescence lifetime spectroscopy to the tool kit used to study the formation and degradation of luminescent quantum dots in solution. Langmuir 2017, 33, 3018–3027. [Google Scholar] [CrossRef]
- Saha, D.; Negi, D.P.S. Spectroscopic investigations on the interaction of thioacetamide with ZnO quantum dots and application for its fluorescence sensing. Spectrochim. Acta A 2018, 189, 516–521. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Kaur, R.; Kumar, N.; Deep, A.; Rajesh, C.; Kim, K.-H. Synthesis and spectroscopic studies of functionalized graphene quantum dots with diverse fluorescence characteristics. RSC Adv. 2018, 8, 11446–11454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Yang, F.; Li, D.; Wang, J.-P.; Xu, J.-H. pH dependent time-resolved fluorescence spectra of ZnSe quantum dots based on glutathione ligands. Spectrosc. Spectr. Anal. 2021, 41, 3178–3183. [Google Scholar] [CrossRef]
- Kim, S.H.; Man, M.T.; Lee, J.W.; Park, K.-D.; Lee, H.S. Influence of size and shape anisotropy on optical properties of CdSe quantum dots. Nanomaterials 2020, 10, 1589. [Google Scholar] [CrossRef]
- Schneider, G.; Decher, G.; Nerambourg, N.; Praho, R.; Werts, M.H.V.; Blanchard-Desce, M. Distance-Dependent fluorescence quenching on gold nanoparticles ensheathed with layer-by-layer assembled polyelectrolytes. Nano Lett. 2006, 6, 530–536. [Google Scholar] [CrossRef]
- Noblet, T.; Dreesen, L.; Hottechamps, J.; Humbert, C. A global method for handling fluorescence spectra at high concentration derived from the competition between emission and absorption of colloidal CdTe quantum dots. Phys. Chem. Chem. Phys. 2017, 19, 26559–26565. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xiong, Q.; Lorenzini, G.; Yue, Y. The pH effect on thermal response of fluorescence spectroscopy of graphene quantum dots for nanoscale thermal characterization. J. Eng. Thermophys. 2018, 27, 345–356. [Google Scholar] [CrossRef]
- Yang, J.; Ling, Z.; Li, B.Q.; Li, R.; Mei, X. Nanoscale 3D temperature gradient measurement based on fluorescence spectral characteristics of the CdTe quantum dot probe. Opt. Express 2019, 27, 6770–6791. [Google Scholar] [CrossRef]
- de Thomaz, A.A.; Almeida, D.B.; Pelegati, V.B.; Carvalho, H.F.; Cesar, C.L. Measurement of the hydrodynamic radius of quantum dots by fluorescence correlation spectroscopy excluding blinking. J. Phys. Chem. B 2015, 119, 4294–4299. [Google Scholar] [CrossRef]
- Green, M. The nature of quantum dot capping ligands. J. Mater. Chem. 2010, 20, 5797–5809. [Google Scholar] [CrossRef]
- Yang, Q.; Wan, X.; Chen, Y.; Luo, H.; Zheng, Y.; Li, L. Theoretical investigation complexation characteristics and UV–Vis absorption spectral properties of CdTe QDs with four capping agents. J. Mol. Model. 2022, 28, 28. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Bahramian, A.R. Self-Assembly of graphene quantum dots into hydrogels and cryogels: Dynamic light scattering, UV–Vis spectroscopy and structural investigations. J. Mol. Liq. 2018, 265, 172–180. [Google Scholar] [CrossRef]
- Budyka, M.F. Semiempirical study on the absorption spectra of the coronene-like molecular models of graphene quantum dots. Spectrochim. Acta A 2019, 207, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.S.; Tavernaro, I.; Machka, F.; Dakischew, O.; Lips, K.S.; Wickleder, M.S. Tuning optical properties of water-soluble CdTe quantum dots for biological applications. J. Nanopart. Res. 2017, 19, 70. [Google Scholar] [CrossRef]
- Pesika, N.S.; Stebe, K.J.; Searson, P.C. Determination of the particle size distribution of quantum nanocrystals from absorbance spectra. Adv. Mater. 2003, 15, 1289–1291. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.H.; Guo, W.Z.; Peng, X.G. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2013, 15, 2854–2860. [Google Scholar] [CrossRef]
- Ferreira, D.L.; Sousa, J.C.L.; Maronesi, R.N.; Bettini, J.; Schiavon, M.A.; Teixeira, A.V.N.C.; Silva, A.G. Size-Dependent bandgap and particle size distribution of colloidal semiconductor nanocrystals. J. Chem. Phys. 2017, 147, 154102. [Google Scholar] [CrossRef]
- Pesika, N.S.; Stebe, K.J.; Searson, P.C. Relationship between absorbance spectra and particle size distributions for quantum-sized nanocrystals. J. Phys. Chem. 2003, 107, 10412–10415. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Shendrik, R.; Sukhov, B.G. Relation between excitation dependent luminescence and particle size distributions for the selenium nanoparticles in κ-carrageenan shell. J. Lumin. 2019, 211, 305–313. [Google Scholar] [CrossRef]
- Xing, C.; Huang, W.; Xie, Z.; Zhao, J.; Ma, D.; Fan, T.; Liang, W.; Ge, Y.; Dong, B.; Li, J.; et al. Ultrasmall bismuth quantum dots: Facile liquid-phase exfoliation, characterization, and application in high-performance UV–Vis photodetector. ACS Photonics 2018, 5, 621–629. [Google Scholar] [CrossRef]
- Yuan, Y.; Han, Y.; Huang, B.; Zhang, L.; Yang, H.; Gu, B.; Cui, Y.; Zhang, J. Single-Channel UV/vis dual-band detection with ZnCdS:Mn/ZnS core/shell quantum dots. Nanotechnology 2019, 30, 075501. [Google Scholar] [CrossRef]
- Yang, B.; Liu, R.; Hao, X.; Wu, Y.; Du, J. Effect of CdTe quantum dots size on the conformational changes of human serum albumin: Results of spectroscopy and isothermal titration calorimetry. Biol. Trace Elem. Res. 2013, 155, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cui, E.; Tan, Z.; Liu, R. Effects of N-acetyl-L-cysteine-capped CdTe quantum dots on bovine serum albumin and bovine hemoglobin: Isothermal titration calorimetry and spectroscopic investigations. J. Biochem. Mol. Toxicol. 2014, 28, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.Y.; Shen, Y.; Greytak, A.B. Isothermal titration calorimetry resolves sequential ligand exchange and association reactions in treatment of oleate-capped CdSe quantum dots with alkylphosphonic acid. J. Phys. Chem. C 2020, 124, 23964–23975. [Google Scholar] [CrossRef]
- Pitkänen, L.; Striegel, A.M. Size-Exclusion chromatography of metal nanoparticles and quantum dots. Trends Anal. Chem. 2016, 80, 311–320. [Google Scholar] [CrossRef]
- Wu, J.-K.; Tian, Z.-Q.; Zhang, Z.-L.; Liu, A.-A.; Tang, B.; Zhang, L.-J.; Chen, Z.-L.; Pang, D.-W. Purification of quantum dot-based bioprobes via high-performance size exclusion chromatography. Talanta 2016, 159, 64–73. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H. Targeting of somatostatin receptors using fluorescent nanoparticles. J. Nanomed. Nanotechnol. 2018, 9, 1000506. [Google Scholar] [CrossRef]
- Radchanka, A.; Hrybouskaya, V.; Antanovich, A.; Artemyev, M. Poly (maleicanhydride) shell modified with negatively and positively charged groups to control zeta potential and hydrodynamic size of encapsulated quantum dots at variable pH. ChemNanoMat 2022, 8, e20210053. [Google Scholar] [CrossRef]
- Voráčová, I.; Klepárník, K.; Lišková, M.; Foret, F. Determination of ζ-potential, charge, and number of organic ligands on the surface of water soluble quantum dots by capillary electrophoresis. Electrophoresis 2015, 36, 867–874. [Google Scholar] [CrossRef]
- Wang, A.; Fu, L.; Ng, H.P.; Cai, W.; Zheng, Y.; Han, F.; Wang, Z.; Peng, F. Monitoring fluorescence lifetime changes of CdTe QDs synthesized with different stabilizers by photoluminscence and zeta potential measurement. J. Non Oxide Glasses 2015, 7, 1–12. [Google Scholar]
- Rastogi, A.; Pandey, F.P.; Parmar, A.S.; Singh, S.; Hegde, G.; Manohar, R. Effect of carbonaceous oil palm leaf quantum dot dispersion in nematic liquid crystal on zeta potential, optical texture and dielectric properties. J. Nanostruct. Chem. 2021, 11, 527–548. [Google Scholar] [CrossRef]
- Pandey, F.P.; Rastogi, A.; Singh, S. Optical properties and zeta potential of carbon quantum dots (CQDs) dispersed nematic liquid crystal 4′-heptyl-4-biphenylcarbonitrile (7CB). Opt. Mater. 2020, 105, 109849. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Calvin, J.J.; Kaufman, T.M.; Sedlak, A.B.; Crook, M.F.; Alivisatos, A.P. Observation of ordered organic capping ligands on semiconducting quantum dots via powder X-ray diffraction. Nat. Comm. 2021, 12, 2663. [Google Scholar] [CrossRef]
- Vega-Macotela, L.G.; Torchynska, T.V.; Polupan, G. Emission and HR-XRD study of InGaAs/GaAs quantum wells with InAs quantum dots grown at different temperatures. J. Mater. Sci. Mater. Electron. 2017, 28, 17778–17783. [Google Scholar] [CrossRef]
- Torchynska, T.; Cisneros-Tamayo, R.; Vega-Macotela, L.; Polupan, G.; Escobosa-Echavarria, A. Emission and HR-XRD study of MBE structures with InAs quantum dots and AlGaInAs strain reducing layers. Superlat. Microst. 2018, 124, 153–159. [Google Scholar] [CrossRef]
- Polupan, G.; Torchynska, T.; Vega-Macotela, L.; Cisneros-Tamayo, R.; Escobosa-Echavarria, A. Emission and HR XRD varying in GaAs/AlGaInAs heterostructures with InAs quantum dots at annealing. J. Mater. Sci. Mater. Electron. 2020, 31, 2643–2649. [Google Scholar] [CrossRef]
- Zhang, Y.; Ersoy, O.; Karatutlu, A.; Little, W.; Sapelkin, A. Local structure of Ge quantum dots determined by combined numerical analysis of EXAFS and XANES data. J. Synchrotron Rad. 2016, 23, 253–259. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, L.; Cibin, G.; Gianolio, D.; Han, S.; Yu, K.; Dove, M.T.; Sapelkin, A.V. Analysis of the atomic structure of CdS magic-size clusters by X-ray absorption spectroscopy. Nanoscale 2020, 12, 19325–19332. [Google Scholar] [CrossRef]
- Wei, H.; Zhou, J.; Zhang, L.; Wang, F.; Wang, J.; Jin, C. The core/shell structure of CdSe/ZnS quantum dots characterized by X-ray absorption fine spectroscopy. J. Nanomater. 2015, 2015, 764712. [Google Scholar] [CrossRef]
- Marmiroli, M.; Lepore, G.O.; Pagano, L.; d’Acapito, F.; Gianoncelli, A.; Villani, M.; Lazzarini, L.; White, J.C.; Marmiroli, N. The fate of CdS quantum dots in plants as revealed by extended X-ray absorption fine structure (EXAFS) analysis. Environ. Sci. Nano 2020, 7, 1150–1162. [Google Scholar] [CrossRef]
- Manikandan, D.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Boukhvalov, D.W.; Murugan, R. XANES, EXAFS, EPR, and first-principles modeling on electronic structure and ferromagnetism in Mn doped SnO2 quantum dots. J. Phys. Chem. C 2019, 123, 3067–3075. [Google Scholar] [CrossRef]
- Erenburg, S.B.; Trubina, S.V.; Zvereva, V.V.; Zinov’ev, V.A.; Dvurechenskiy, A.V.; Kuchinskaya, P.A.; Kvashnina, K.O. Microstructure of multilayer heterosystems containing molecules of Ge quantum dots in Si on the stages of nucleation and growth as revealed by EXAFS spectroscopy. J. Struct. Chem. 2016, 57, 1407–1416. [Google Scholar] [CrossRef]
- Chang, H.-W.; Fu, J.-X.; Huang, Y.-C.; Lu, Y.-R.; Kuo, C.-H.; Chen, J.-L.; Chen, C.-L.; Lee, J.-F.; Chen, J.-M.; Tsai, Y.-C.; et al. NiCo2O4/graphene quantum dots (GQDs) for use in efficient electrochemical energy devices: An electrochemical and X-ray absorption spectroscopic investigation. Catal. Today 2020, 348, 290–298. [Google Scholar] [CrossRef]
- Clark, P.C.J.; Flavell, W.R. Surface and interface chemistry in colloidal quantum dots for solar applications studied by X-ray photoelectron spectroscopy. Chem. Rec. 2019, 19, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Iacono, F.; de la Cueva, L.; Gallego, J.M.; Juarez, B.H.; Otero, R. Thermal ligand desorption in CdSe quantum dots by correlated XPS and STM. Part. Part. Syst. Charact. 2016, 33, 358–362. [Google Scholar] [CrossRef]
- He, S.; Turnbull, M.J.; Nie, Y.; Sun, X.; Ding, Z. Band structures of blue luminiscent nitrogen-doped graphene quantum dots by synchrotron-based XPS. Surface Sci. 2018, 676, 51–55. [Google Scholar] [CrossRef]
- Babu, B.; Reddy, I.N.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Hamizi, N.A.; Johan, M.R.; Wahab, Y.A.; Chowdhury, Z.Z.; Akbarzadeh, O.; Sagadevan, S.; Badruddin, I.A.; Khan, T.M.Y.; Kamangar, S. Investigation on Surface Properties of Mn-Doped CdSe Quantum Dots Studied by X-ray Photoelectron Spectroscopy. Symmetry 2019, 11, 1250. [Google Scholar] [CrossRef]
- Futamura, Y.; Nakashima, Y.; Ohta, A.; Ikeda, M.; Makihara, K.; Miyazaki, S. Evaluation of the potential distribution in a multiple stacked Si quantum dots structure by hard X-ray photoelectron spectroscopy. Jpn. J. Appl. Phys. 2019, 58, SAAE01. [Google Scholar] [CrossRef]
- Weigert, F.; Müller, A.; Häusler, I.; Geiβler, D.; Skroblin, D.; Krumrey, M.; Unger, W.; Radnik, J.; Resch-Genger, U. Combining HR-TEM and XPS to elucidate the core-shell structure of ultrabright CdSe/CdS semiconductor quantum dots. Sci. Rep. 2020, 10, 20712. [Google Scholar] [CrossRef]
- Ma, J.; Liu, M.; Li, Z.; Li, L. Synthesis of highly photo-stable CuInS2/ZnS core/shell quantum dots. Opt. Mater. 2015, 47, 56–61. [Google Scholar] [CrossRef]
- Buso, D.; Jasieniak, J.; Lay, M.D.H.; Schiavuta, P.; Scopece, P.; Laird, J.; Amenitsch, H.; Hill, A.J.; Falcaro, P. Highly luminescent metal-organic frameworks through quantum dot doping. Small 2012, 8, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.S.; Harteveld, C.A.M.; Vancso, G.J.; Huskens, J.; Cloetens, P.; Vos, W.L. Targeted positioning of quantum dots inside 3D silicon photonic crystals revealed by synchrotron X-ray fluorescence tomography. ACS Nano 2022, 16, 3674–3683. [Google Scholar] [CrossRef]
- Song, J.-T.; Yang, X.-Q.; Zhang, X.-S.; Yan, D.-M.; Yao, M.-H.; Qin, M.-Y.; Zhao, Y.-D. Composite silica coated gold nanosphere and quantum dots nanoparticles for X-ray CT and fluorescence bimodal imaging. Dalton Trans. 2015, 44, 11314–11320. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-T.; Yang, X.-Q.; Zhang, X.-S.; Yan, D.-M.; Yao, M.-H.; Qin, M.-Y.; Zhao, Y.-D. Composite nanoparticle of Au and quantum dots for X-ray computed tomography and fluorescence dual-mode imaging in vivo. J. Nanopart. Res. 2015, 17, 479. [Google Scholar] [CrossRef]
- Zhao, F.; Kim, J.S. Fabrication of CdSeS alloyed quantum dots and study on fluorescence lifetime. Mol. Cryst. Liq. Cryst. 2012, 566, 120–125. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Tirbandpay, R. Synthesis, optical properties and tuning size of CdSe quantum dots by variation capping agent. Spectrochim. Acta A 2021, 250, 119369. [Google Scholar] [CrossRef]
- Begum, A.; Sonkar, S.K.; Saxena, M.; Sarkar, S. Nanocomposites of carbon quantum dots-nickel (II) dithiolene as nanolights. J. Mater. Chem. 2011, 21, 19210–19213. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Mousavi, M.; Ghasemi, J.B.; Le, Q.V.; Delbari, S.A.; Namini, A.S.; Asl, M.S.; Shokouhimehr, M.; Mohammadi, M. Novel p-n heterojunction nanocomposite: TiO2 QDs/ZnBi2O4 photocatalyst with considerably enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. C 2020, 124, 27519–27528. [Google Scholar] [CrossRef]
- Cryer, M.E.; Halper, J.E. Room temperature Mid-IR detection through localized surface vibrational states of SnTe nanocrystals. ACS Sens. 2018, 3, 2087–2094. [Google Scholar] [CrossRef]
- Friedrich, M.; Nozadze, R.; Gan, Q.; Zelman-Femiak, M.; Ermolayev, V.; Wagner, T.U.; Harms, G.S. Detection of single quantum dots in model organisms with sheet illumination microscopy. Biochem. Biophys. Res. Commun. 2009, 390, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrión, C.; Moliner-Martínez, Y.; Simonet, B.M.; Valcárcel, M. Capillary electrophoresis method for the characterization and separation of CdSe quantum dots. Anal. Chem. 2011, 83, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Nozadze, R.; de Keijzer, S.; Steinmeyer, R.; Ermolayev, V.; Harms, G.S. Detection of single quantum dots in model systems with sheet illumination microscopy. J. Fluoresc. 2018, 27, 29–39. [Google Scholar] [CrossRef]
- Paydary, P.; Larese-Casanova, P. Separation and quantification of quantum dots and dissolved metal cations by size exclusion chromatography-ICP-MS. Int. J. Environ. Anal. Chem. 2015, 95, 1450–1470. [Google Scholar] [CrossRef]
- Peng, L.; He, M.; Chen, B.; Qiao, Y.; Hu, B. Metallomics study of CdSe/ZnS quantum dots in HepG2 cells. ACS Nano 2015, 9, 10324–10334. [Google Scholar] [CrossRef]
- Meng, P.; Xiong, Y.; Wu, Y.; Hu, Y.; Wang, H.; Pang, Y.; Jiang, S.; Han, S.; Huang, P. A novel strategy to evaluate the degradation of quantum dots: Identification and quantification of CdTe quantum dots and corresponding ionic species by CZE-ICP-MS. Chem. Commun. 2018, 54, 5342–5345. [Google Scholar] [CrossRef]
- Kuçur, E.; Boldt, F.M.; Cavaliere-Jaricot, S.; Ziegler, J.; Nann, T. Quantitative analysis of cadmium selenide nanocrystal concentration by comparative techniques. Anal. Chem. 2007, 79, 8987–8993. [Google Scholar] [CrossRef]
- Gopee, N.V.; Roberts, D.W.; Webb, P.; Cozart, C.R.; Siitonen, P.H.; Latendresse, J.R.; Warbitton, A.R.; Yu, W.W.; Colvin, V.L.; Walker, N.J.; et al. Quantitative determination of skin penetration of PEG-Coated CdSe quantum dots in dermabraded but not intact SKH-1 hairless mouse skin. Toxicol. Sci. 2009, 111, 37–48. [Google Scholar] [CrossRef]
- Montoro Bustos, A.R.; Ruiz Encinar, J.; Fernández-Argüelles, M.T.; Costa-Fernández, J.M.; Sanz-Medel, A. Elemental mass spectrometry: A powerful tool for an accurate characterisation at elemental level of quantum dots. Chem. Commun. 2009, 3107–3109. [Google Scholar] [CrossRef]
- Sewell, S.L.; Higgins, M.M.; Bell, C.S.; Giorgio, T.D. Quantification of quantum dot concentration using inductively coupled plasma-mass spectrometry (ICP-MS). J. Biomed. Nanotechnol. 2011, 7, 685–690. [Google Scholar] [CrossRef]
- Liu, J.; Katahara, J.; Li, G.; Coe-Sullivan, S.; Hurt, R.H. Degradation products from consumer nanocomposites: A case study on quantum dots lighting. Environ. Sci. Technol. 2012, 46, 3220–3227. [Google Scholar] [CrossRef]
- Zheng, L.-N.; Wang, M.; Wang, B.; Chen, H.-Q.; Ouyang, H.; Zhao, Y.-L.; Chai, Z.-F.; Feng, W.-Y. Determination of quantum dots in single cells by inductively coupled plasma mass spectrometry. Talanta 2013, 116, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, W.G.; Batchelor-McAuley, C.; Tschulik, K.; Kachoosangi, R.T.; Ness, D.; Compton, R.G. Use of the capping agent for the electrochemical detection and quantification of nanoparticles: CdSe quantum dots. Sens. Act. B 2014, 204, 445–449. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Bouzas-Ramos, D.; Menéndez-Miranda, M.; Montoro Bustos, A.R.; Ruiz Encinar, J.; Costa-Fernández, J.M.; Sanz-Medel, A.; Costa-García, A. Voltammetric determination of size and particle concentration of Cd-Based quantum dots. Electrochim. Acta 2015, 166, 100–106. [Google Scholar] [CrossRef]
- Menéndez-Miranda, M.; Ruiz Encinar, J.; Costa-Fernández, J.M.; Sanz-Medel, A. Asymmetric flow field-flow fractionation coupled to inductively coupled plasma mass spectrometry for the quantification of quantum dots bioconjugation. J. Chromatogr. A 2015, 1422, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; Costa Rama, E.; Costa-García, A. Electrochemical study and applications of selective electrodeposition of silver on quantum dots. Anal. Chem. 2016, 88, 3739–3746. [Google Scholar] [CrossRef]
- Garcia-Cortés, M.; Sotelo González, E.; Fernández-Argüelles, M.T.; Ruiz Encinar, J.; Costa-Fernández, J.M.; Sanz-Medel, A. Capping of Mn-Doped ZnS quantum dots with DHLA for their stabilization in aqueous media: Determination of the nanoparticle number concentration and surface ligand density. Langmuir 2017, 33, 6333–6341. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Costa-García, A. Electrochemical detection of quantum dots by stabilization of electrogenerated copper species. Electrochem. Commun. 2017, 74, 53–56. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Fanjul-Bolado, P.; Hernández-Santos, D.; Costa-García, A. Enhanced detection of quantum dots by the magnetohydrodynamic effect for electrochemical biosensing. Analyst 2017, 142, 1591–1600. [Google Scholar] [CrossRef]
- Sýs, M.; Metelka, R.; Korecká, L.; Pokorná, H.; Švancara, I. Comparison of various bismuth film electrodes in simultaneous electrochemical detection of heavy metals for application in quantum dot-linked immunoassays. Monatsh. Chem. 2017, 148, 505–510. [Google Scholar] [CrossRef]
- Čadková, M.; Kovářová, A.; Dvořáková, V.; Bílková, Z.; Korecká, L. Optimization of anodic stripping voltammetry conditions for efficient detection of quantum dots at micro flow-cell electrodes. Monatsh. Chem. 2017, 148, 571–575. [Google Scholar] [CrossRef]
- Yu, X.; Chen, B.; He, M.; Wang, H.; Hu, B. Chip-Based magnetic solid phase microextraction coupled with ICP-MS for the determination of Cd and Se in HepG2 cells incubated with CdSe quantum dots. Talanta 2018, 179, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Amor-Gutiérrez, O.; Iglesias-Mayor, A.; Llano-Suárez, P.; Costa-Fernández, J.M.; Soldado, A.; Podadera, A.; Parra, F.; Costa-García, A.; de la Escosura-Muñiz, A. Electrochemical quantification of Ag2S quantum dots: Evaluation of different surface coating ligands for bacteria determination. Microchim. Acta 2020, 187, 169. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Yang, K. Accurate detection of on-state quantum dot and biomolecules in a micro fluidic flow with single-molecule two-color coincidence detection. Anal. Bioanal. Chem. 2010, 397, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeen, T.; Tran, M.V.; Algar, W.R. Polyacrylamide gel electrophoresis of semiconductor quantum dots and their bioconjugates: Materials characterization and physical insights from spectrofluorimetric detection. Analyst 2018, 143, 1104–1116. [Google Scholar] [CrossRef]
- Schmelz, O.; Mews, A.; Basché, T.; Herrmann, A.; Müllen, K. Supramolecular complexes from CdSe nanocrystals and organic fluorophors. Langmuir 2001, 17, 2861–2865. [Google Scholar] [CrossRef]
- Striolo, A.; Ward, J.; Prausnitz, J.M.; Parak, W.J.; Zanchet, D.; Gerion, D.; Milliron, D.; Alivisatos, A.P. Molecular weight, osmotic second virial coefficient, and extinction coefficient of colloidal CdSe nanocrystals. J. Phys. Chem. B 2002, 106, 5500–5505. [Google Scholar] [CrossRef]


| CdSe | Vis. Absorption | Fluorescence Emission | |
|---|---|---|---|
| Diameter (nm) | λabs (nm) | λem (nm) | COLOUR * |
| Bulk material | 671 | 713 |  |
| 7.3 | 645 | 653 |  |
| 4.1 | 586 | 596 |  |
| 2.9 | 541 | 549 |  |
| 2.2 | 484 | 499 |  |
| 1.8 | 427 | 449 |  |
| IV–VI QDs | Bulk Bandgap (eV) [ref.] | Bulk Exciton Bohr Radius (nm) [ref.] |
|---|---|---|
| PbS | 0.41 [14] | 18 [14] |
| PbSe | 0.28 [14] | 46 [14] |
| PbTe | 0.31 [14] | 150 [14] |
| II–VI QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| ZnS | 3.6 [50] | 2.5 [51] |
| ZnSe | 2.72 [52] | 4.5 [52] |
| ZnTe | 2.25 [52] | 6.7 [52] |
| CdS | 2.42 [50] | 2.9 [51] |
| CdSe | 1.73 [50] | 5.6 [51] |
| CdTe | 1.5 [14] | 10 [14] |
| HgTe | −0.15 * [14] | 80 [14] |
| III–V QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| AlN | 6.2 [52] | 1.6 [52] |
| AlP | 2.45 [52] | 37 [52] |
| AlAs | 2.15 [52] | 41 [52] |
| AlSb | 1.6 [52] | 64 [52] |
| GaN | 3.4 [52] | 3.1 [52] |
| GaP | 2.27 [52] | 29 [52] |
| GaAs | 1.43 [52] | 12.4 [52] |
| GaSb | 0.7 [52] | 60 [52] |
| InN | 0.65 [52] | 11.4 [52] |
| InP | 1.27 [14]/1.42 [52] | 15 [14]/11 [52] |
| InAs | 0.36 [14] | 34 [14] |
| InSb | 018 [52] | 54 [52] |
| IV QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| Si | 1.17 [52] | 4.3 [52] |
| Ge | 0.67 [52] | 11.5 [52] |
| II–V QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| Cd3P2 | 0.55 [14] | 18 [14] |
| Cd3As2 | −0.19 * [14] | 47 [14] |
| I–VI QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| Ag2S | 0.9–1.1 [14,52] | 2.2 [52] |
| Ag2Se | 0.15 [14,52] | 2.9 [52] |
| I–III–VI QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| CuInS2 | 1.53 [14,52] | 4.1 [14,52] |
| CuInSe2 | 1.04 [14,52] | 10.6 [14,52] |
| AgInS2 | 1.87 [52] | 5.5 [52] |
| AgInSe2 | 1.24 [14,52] | 5–6 [52] |
| II–II–VI QDs | Bulk bandgap (eV) | Bulk exciton Bohr radius (nm) [ref.] |
| CdHgTe | 0–1.5 [11] | Not found |
| Diameter of QDs (nm) | No. of Surface Atoms | Ratio of Surface Atoms to Total Atoms (%) |
|---|---|---|
| 10 | 3 × 104 | 20 |
| 4 | 4 × 103 | 40 |
| 2 | 2.5 × 102 | 80 |
| 1 | 30 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín-Matalobos, J.; Bermejo-Barrera, P.; Aboal-Somoza, M.; Fondo, M.; García-Deibe, A.M.; Corredoira-Vázquez, J.; Alves-Iglesias, Y. Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection. Nanomaterials 2022, 12, 2501. https://doi.org/10.3390/nano12142501
Sanmartín-Matalobos J, Bermejo-Barrera P, Aboal-Somoza M, Fondo M, García-Deibe AM, Corredoira-Vázquez J, Alves-Iglesias Y. Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection. Nanomaterials. 2022; 12(14):2501. https://doi.org/10.3390/nano12142501
Chicago/Turabian StyleSanmartín-Matalobos, Jesús, Pilar Bermejo-Barrera, Manuel Aboal-Somoza, Matilde Fondo, Ana M. García-Deibe, Julio Corredoira-Vázquez, and Yeneva Alves-Iglesias. 2022. "Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection" Nanomaterials 12, no. 14: 2501. https://doi.org/10.3390/nano12142501
APA StyleSanmartín-Matalobos, J., Bermejo-Barrera, P., Aboal-Somoza, M., Fondo, M., García-Deibe, A. M., Corredoira-Vázquez, J., & Alves-Iglesias, Y. (2022). Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection. Nanomaterials, 12(14), 2501. https://doi.org/10.3390/nano12142501