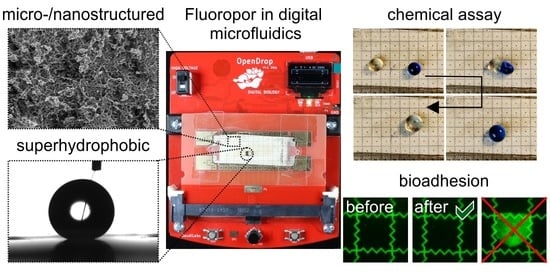
Application of Micro/Nanoporous Fluoropolymers with Reduced Bioadhesion in Digital Microfluidics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Experiments on the Wetting Behavior under EWOD
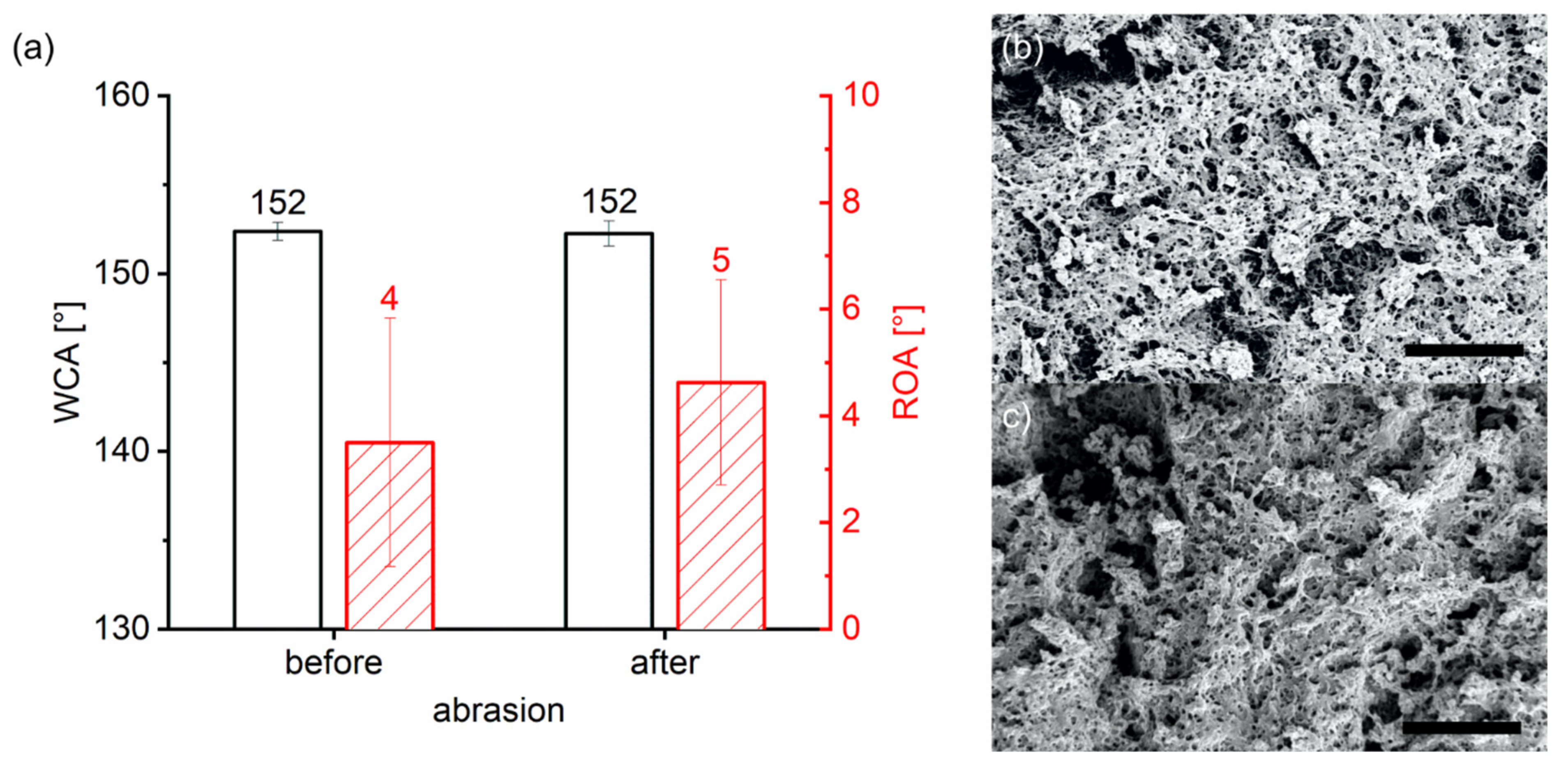
2.3.1. Surface Preparation
2.3.2. Electrowetting Experiments
- A 5 µL droplet was deposited on the Fluoropor surface and the syringe was moved up so only the thin gold wire was still in contact with the droplet. The CA was measured (CA0).
- The potential was turned on ad-hoc with the target value and the CA was measured (CAon).
- The potential was turned off and after 30 s the CA was measured (CAoff).
- The gold wire was gently removed from the droplet by moving the dispersion unit up. Then the ROA and the advancing and receding CA (ARCA) were measured upon tilting of the stage. To determine ROA, videos were recorded with 200 frames s−1 and the last frame before the drop started rolling was utilized to evaluate the advancing and receding CA (ARCA).
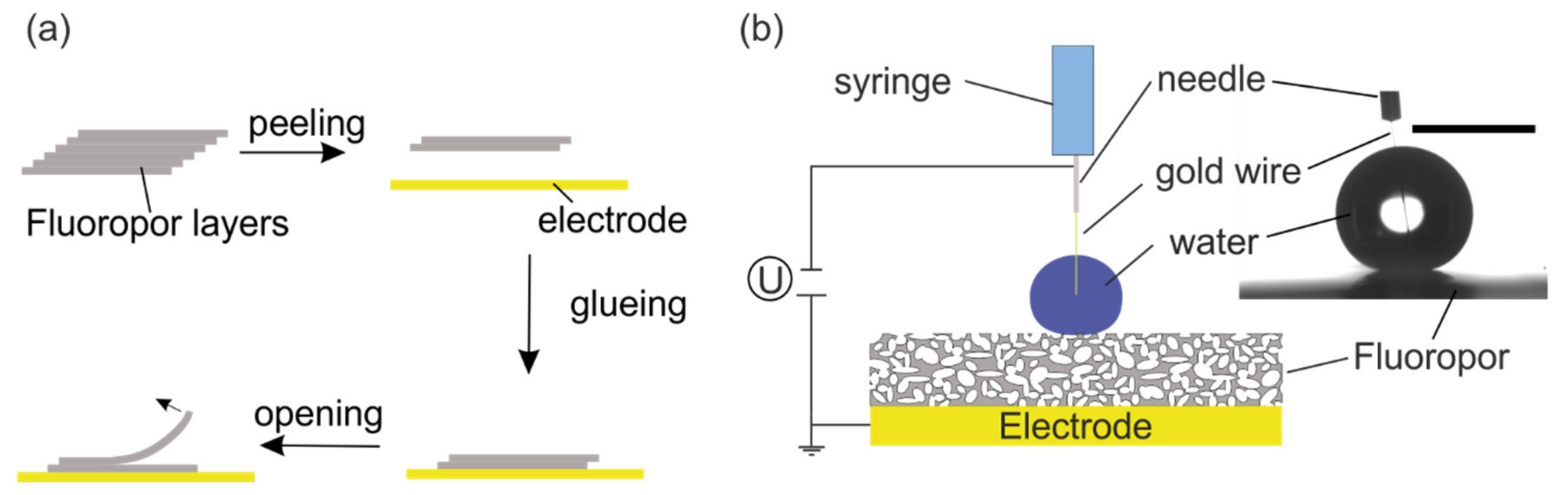
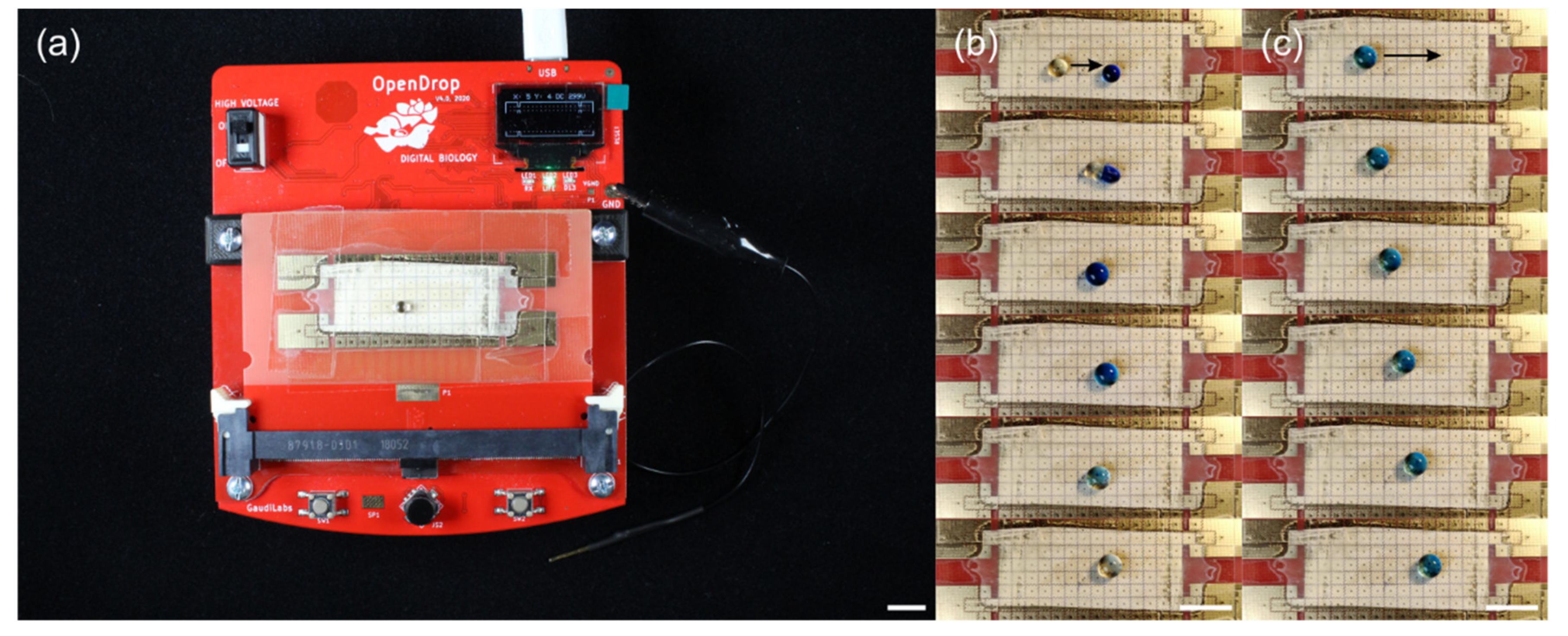
2.4. Fluoropor Film Preparation for Droplet Actuation Experiments
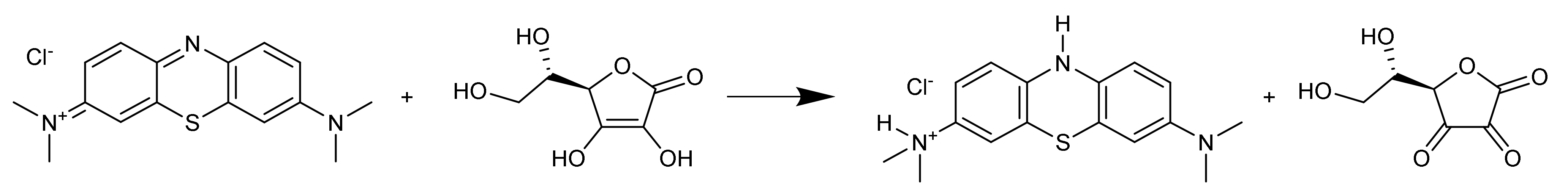
2.5. Chemical Assay
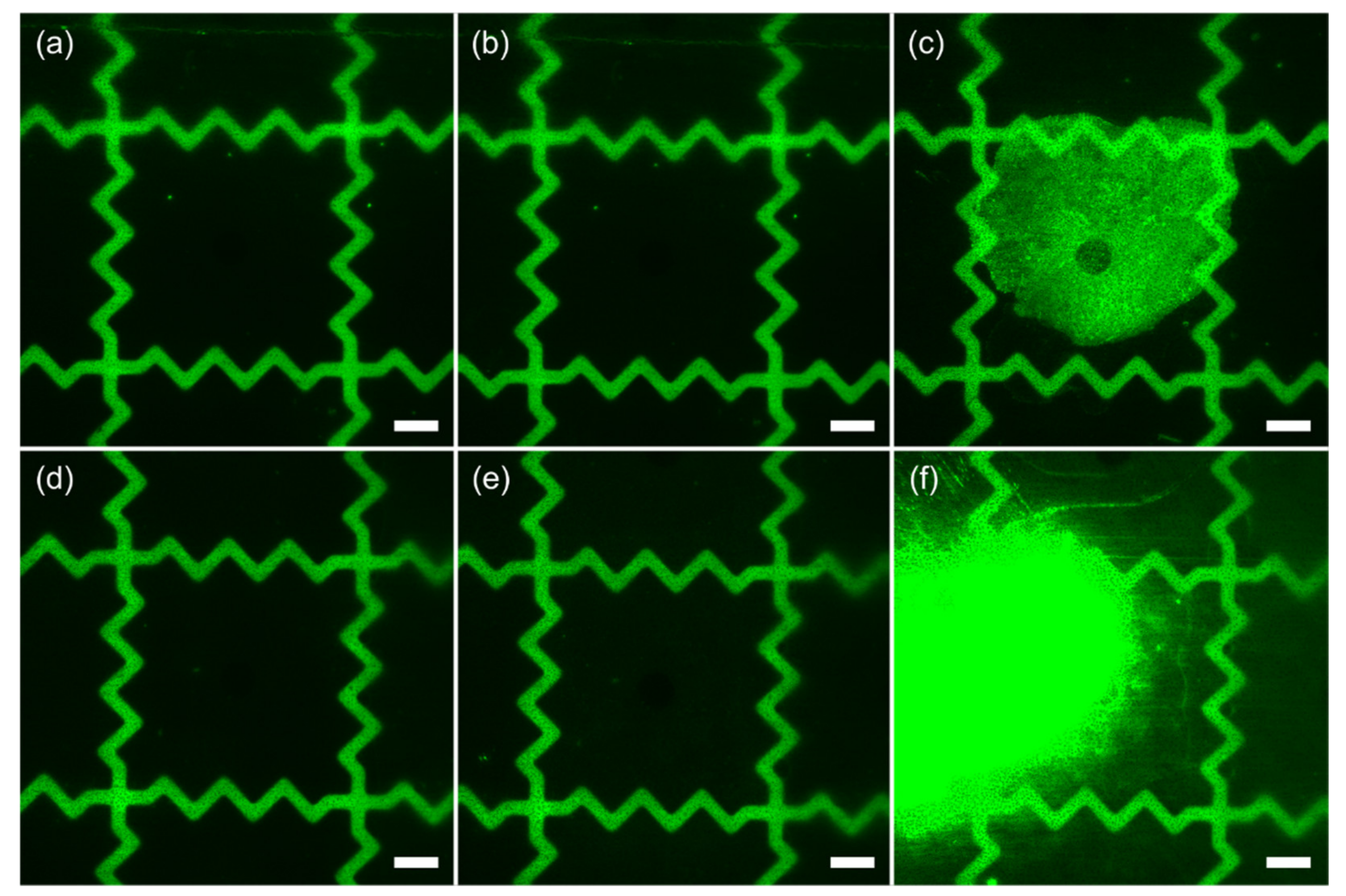
2.6. Adhesion of Biomolecules
3. Results and Discussion
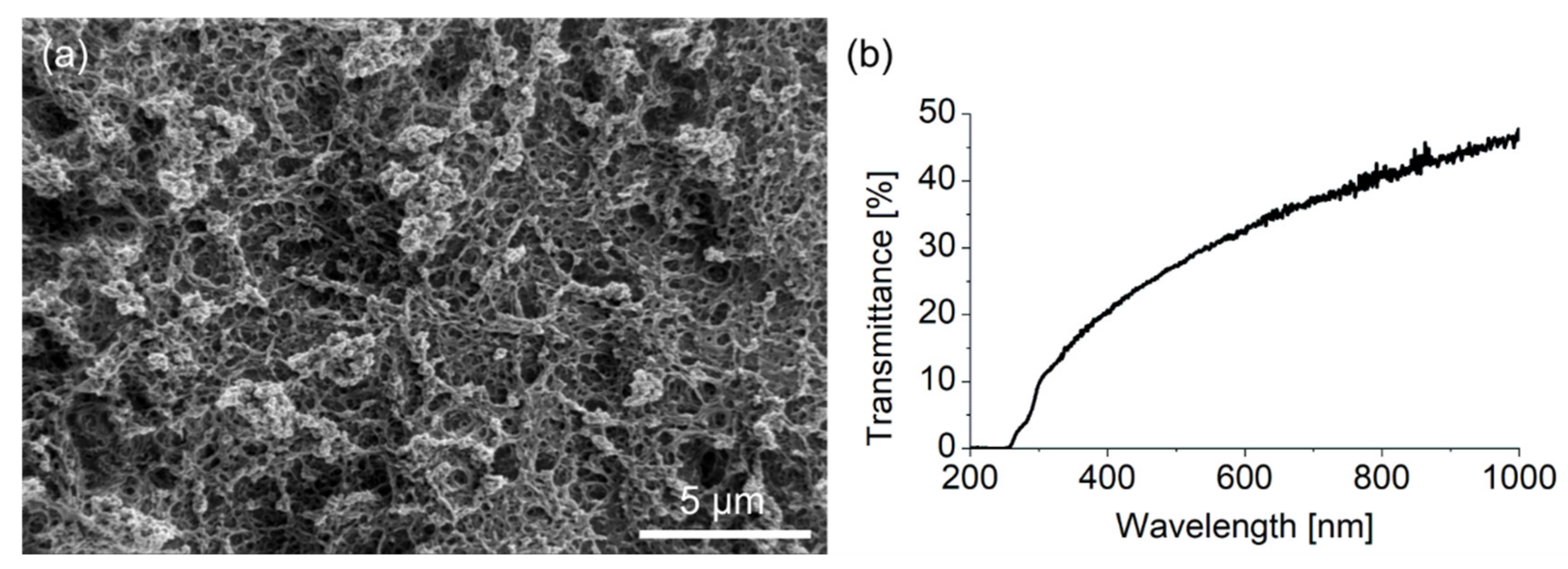
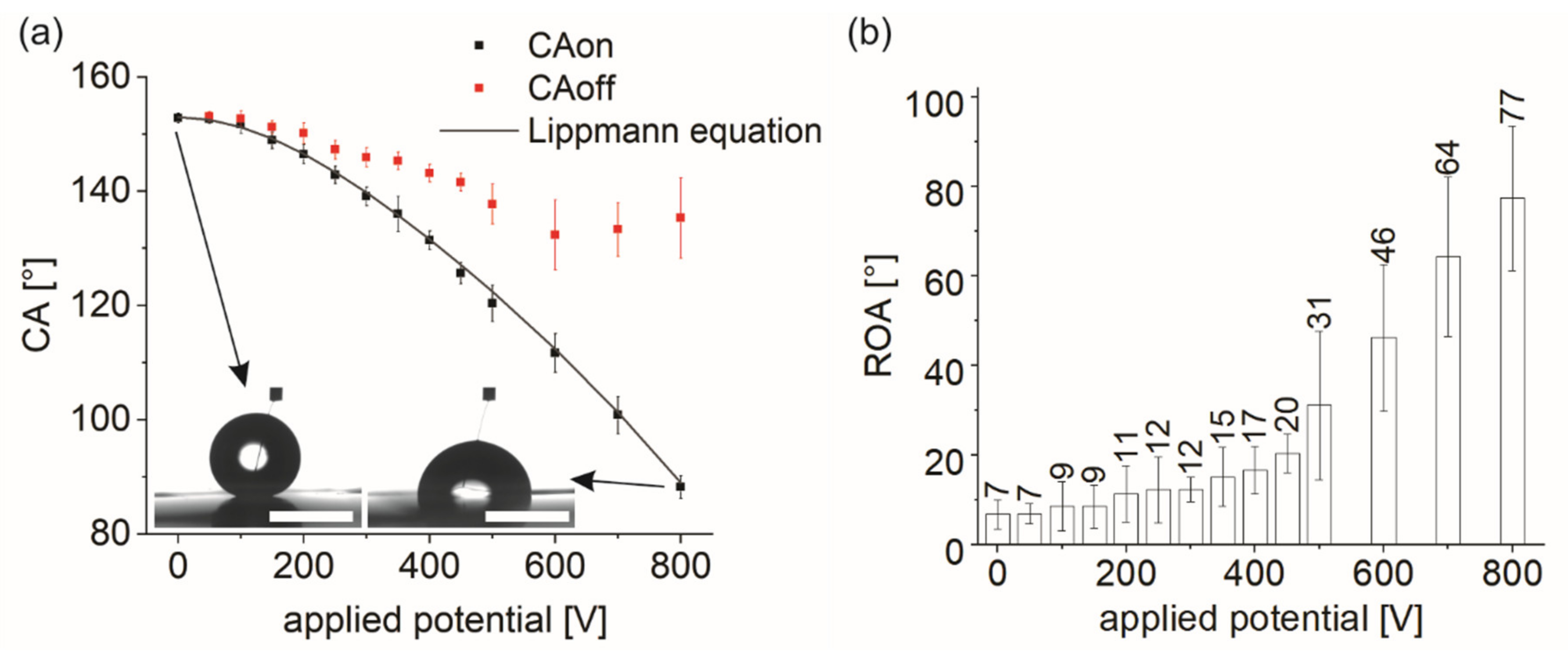
3.1. Wetting Behavior on Fluoropor under EWOD
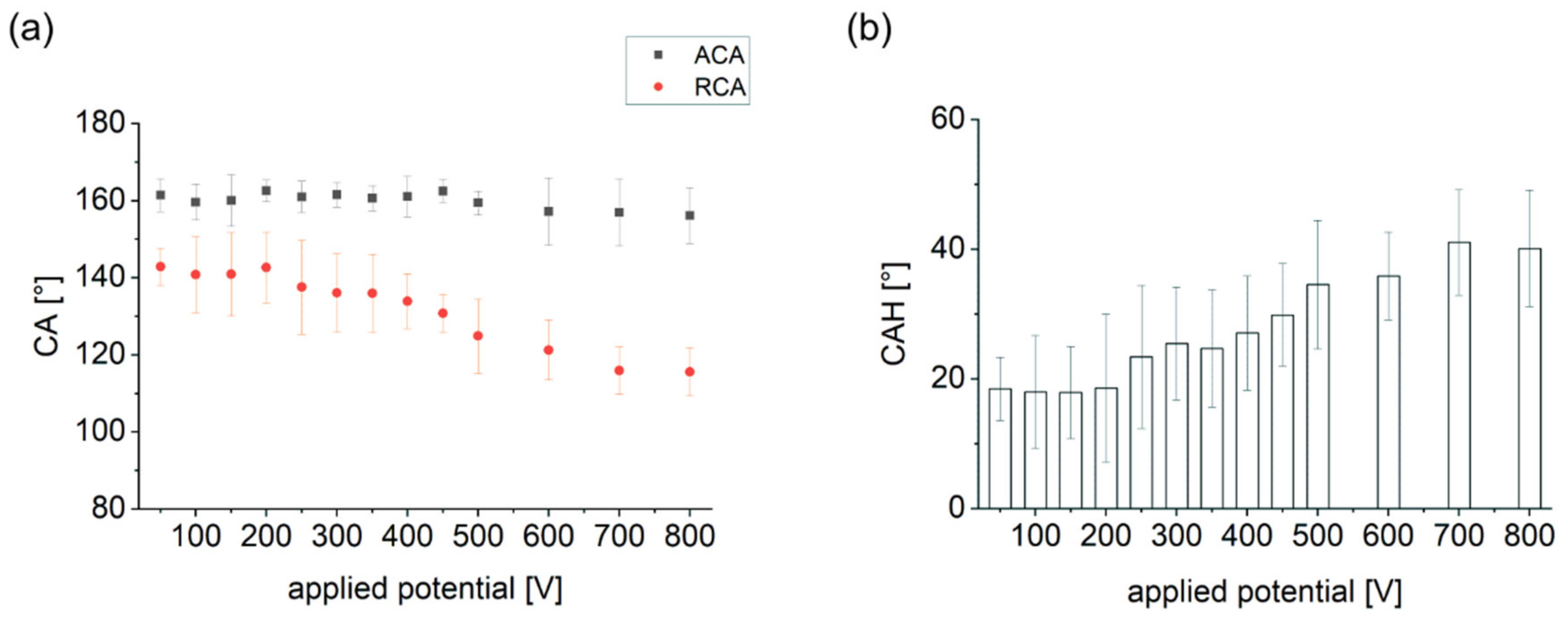
3.2. Droplet Actuation on Fluoropor Films
3.3. Adhesion of Biomolecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Applied Potential [V] | CA0 [°] | CAon [°] | CAoff [°] | ROA [°] |
|---|---|---|---|---|
| 0 | 153 ± 3° | - | - | 7 ± 3° |
| 50 | 153 ± 1° | 153 ± 1° | 153 ± 1° | 7 ± 2° |
| 100 | 153 ± 1° | 152 ± 2° | 153 ± 2° | 9 ± 6° |
| 150 | 153 ± 1° | 149 ± 2° | 151 ± 1° | 9 ± 5° |
| 200 | 153 ± 2° | 146 ± 2° | 150 ± 2° | 11 ± 6° |
| 250 | 153 ± 1° | 143 ± 2° | 147 ± 2° | 12 ± 7° |
| 300 | 153 ± 1° | 139 ± 2° | 146 ± 2° | 12 ± 3° |
| 350 | 153 ± 1° | 136 ± 3° | 145 ± 2° | 15 ± 7° |
| 400 | 153 ± 1° | 131 ± 2° | 143 ± 2° | 17 ± 5° |
| 450 | 153 ± 1° | 126 ± 2° | 142 ± 2° | 21 ± 4° |
| 500 | 152 ± 1° | 120 ± 3° | 138 ± 4° | 31 ± 17° |
| 600 | 153 ± 1° | 112 ± 3° | 132 ± 6° | 46 ± 16° |
| 700 | 153 ± 2° | 101 ± 3° | 133 ± 5° | 64 ± 18° |
| 800 | 152 ± 1° | 88 ± 2° | 135 ± 7° | 77 ± 16° |
| Applied Potential [V] | ACA [°] | RCA [°] | CAH [°] |
|---|---|---|---|
| 50 | 161 ± 4° | 143 ± 5° | 19 ± 5° |
| 100 | 160 ± 5° | 141 ± 10° | 18 ± 9° |
| 150 | 160 ± 7° | 141 ± 11° | 18 ± 7° |
| 200 | 163 ± 3° | 143 ± 10° | 19 ± 11° |
| 250 | 161 ± 4° | 138 ± 12° | 23 ± 11° |
| 300 | 162 ± 3° | 136 ± 10° | 25 ± 9° |
| 350 | 161 ± 3° | 136 ± 10° | 25 ± 9° |
| 400 | 161 ± 5° | 134 ± 7° | 27 ± 9° |
| 450 | 163 ± 3° | 131 ± 5° | 30 ± 8° |
| 500 | 159 ± 3° | 125 ± 10° | 35 ± 10° |
| 600 | 157 ± 9° | 121 ± 8° | 36 ± 7° |
| 700 | 157 ± 9° | 116 ± 6° | 41 ± 8° |
| 800 | 156 ± 7° | 116 ± 6° | 40 ± 9° |
References
- Choi, K.; Ng, A.H.C.; Fobel, R.; Wheeler, A.R. Digital Microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kim, C.-J. Current Commercialization Status of Electrowetting-on-Dielectric (EWOD) Digital Microfluidics. Lab Chip 2020, 20, 1705–1712. [Google Scholar] [CrossRef]
- Hong, J.; Kim, Y.K.; Won, D.-J.; Kim, J.; Lee, S.J. Three-Dimensional Digital Microfluidic Manipulation of Droplets in Oil Medium. Sci. Rep. 2015, 5, 10685. [Google Scholar] [CrossRef]
- Miller, E.M.; Ng, A.H.C.; Uddayasankar, U.; Wheeler, A.R. A Digital Microfluidic Approach to Heterogeneous Immunoassays. Anal. Bioanal. Chem. 2011, 399, 337–345. [Google Scholar] [CrossRef]
- Schell, W.A.; Benton, J.L.; Smith, P.B.; Poore, M.; Rouse, J.L.; Boles, D.J.; Johnson, M.D.; Alexander, B.D.; Pamula, V.K.; Eckhardt, A.E.; et al. Evaluation of a Digital Microfluidic Real-Time PCR Platform to Detect DNA of Candida Albicans in Blood. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2237–2245. [Google Scholar] [CrossRef] [Green Version]
- Aijian, A.P.; Chatterjee, D.; Garrell, R.L. Fluorinated Liquid-Enabled Protein Handling and Surfactant-Aided Crystallization for Fully in Situ Digital Microfluidic MALDI-MS Analysis. Lab Chip 2012, 12, 2552–2559. [Google Scholar] [CrossRef]
- Lapierre, F.; Piret, G.; Drobecq, H.; Melnyk, O.; Coffinier, Y.; Thomy, V.; Boukherroub, R. High Sensitive Matrix-Free Mass Spectrometry Analysis of Peptides Using Silicon Nanowires-Based Digital Microfluidic Device. Lab Chip 2011, 11, 1620–1628. [Google Scholar] [CrossRef]
- Srigunapalan, S.; Eydelnant, I.A.; Simmons, C.A.; Wheeler, A.R. A Digital Microfluidic Platform for Primary Cell Culture and Analysis. Lab Chip 2011, 12, 369–375. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Yao, D.-J.; Lin, H.-C.; Chang, W.-Y.; Chang, H.-Y. DNA Ligation of Ultramicro Volume Using an EWOD Microfluidic System with Coplanar Electrodes. J. Micromech. Microeng. 2008, 18, 045017. [Google Scholar] [CrossRef]
- Dixon, C.; Lamanna, J.; Wheeler, A.R. Direct Loading of Blood for Plasma Separation and Diagnostic Assays on a Digital Microfluidic Device. Lab Chip 2020, 20, 1845–1855. [Google Scholar] [CrossRef]
- Jain, V.; Muralidhar, K. Electrowetting-on-Dielectric System for COVID-19 Testing. Trans. Indian Natl. Acad. Eng. 2020, 5, 251–254. [Google Scholar] [CrossRef]
- Lippmann, G. Relations Entre Les Phénomènes Électriques et Capillaires. Ann. Chim. Phys. 1875, 5, 494–549. [Google Scholar]
- Recent Progress of Electrowetting for Droplet Manipulation: From Wetting to Superwetting Systems—Materials Chemistry Frontiers (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2020/QM/C9QM00458K#!divAbstract (accessed on 20 June 2020).
- Liu, H.; Dharmatilleke, S.; Maurya, D.K.; Tay, A.A.O. Dielectric Materials for Electrowetting-on-Dielectric Actuation. Microsyst. Technol. 2009, 16, 449. [Google Scholar] [CrossRef]
- Yi, U.-C.; Kim, C.-J. Characterization of Electrowetting Actuation on Addressable Single-Side Coplanar Electrodes. J. Micromech. Microeng. 2006, 16, 2053–2059. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Luk, V.N.; Abelgawad, M.; Barbulovic-Nad, I.; Wheeler, A.R. A World-to-Chip Interface for Digital Microfluidics. Anal. Chem. 2009, 81, 1061–1067. [Google Scholar] [CrossRef]
- Yi, Z.; Feng, H.; Zhou, X.; Shui, L. Design of an Open Electrowetting on Dielectric Device Based on Printed Circuit Board by Using a Parafilm M. Front. Phys. 2020, 8, 193. [Google Scholar] [CrossRef]
- Jones, T.B.; Gunji, M.; Washizu, M.; Feldman, M.J. Dielectrophoretic Liquid Actuation and Nanodroplet Formation. J. Appl. Phys. 2001, 89, 1441–1448. [Google Scholar] [CrossRef]
- Cooney, C.G.; Chen, C.-Y.; Emerling, M.R.; Nadim, A.; Sterling, J.D. Electrowetting Droplet Microfluidics on a Single Planar Surface. Microfluid Nanofluid 2006, 2, 435–446. [Google Scholar] [CrossRef]
- Yi, U.C.; Kim, C.J.C.J. EWOD Actuation with Electrode-Free Cover Plate. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, 2005, Digest of Technical Papers, TRANSDUCERS ’05, 5–9 June 2005, Seoul, Korea; IEEE: New York, NY, USA; Taylor & Francis: Oxfordshire, UK, 2005; Volume 1, pp. 89–92. [Google Scholar]
- Srinivasan, V.K.; Pamula, V.; Fair, R.B. An Integrated Digital Microfluidic Lab-on-a-Chip for Clinical Diagnostics on Human Physiological Fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.; Kim, C.-J. Creating, Transporting, Cutting, and Merging Liquid Droplets by Electrowetting-Based Actuation for Digital Microfluidic Circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Pollack, M.G.; Shenderov, A.D.; Fair, R.B. Electrowetting-Based Actuation of Droplets for Integrated Microfluidics. Lab Chip 2002, 2, 96–101. [Google Scholar] [CrossRef]
- Moon, H.; Cho, S.K.; Garrell, R.L.; Kim, C.-J. Low Voltage Electrowetting-on-Dielectric. J. Appl. Phys. 2002, 92, 4080–4087. [Google Scholar] [CrossRef]
- Yi, U.-C.; Kim, C.-J. Soft Printing of Droplets Pre-Metered by Electrowetting. Sens. Actuators A Phys. 2004, 114, 347–354. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L. Electrowetting-on-Dielectric Based Economical Digital Microfluidic Chip on Flexible Substrate by Inkjet Printing. Micromachines 2020, 11, 1113. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, M.; Zhang, D.; Pang, W.; Zhang, H. Island-Ground Single-Plate Electro-Wetting on Dielectric Device for Digital Microfluidic Systems. Appl. Phys. Lett. 2014, 105, 013509. [Google Scholar] [CrossRef]
- Samad, M.F.; Kouzani, A.Z.; Rahman, M.M.; Magniez, K.; Kaynak, A. Design and Fabrication of an Electrode for Low-Actuation-Voltage Electrowetting-on-Dielectric Devices. Procedia Technol. 2015, 20, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do We Need for a Superhydrophobic Surface? A Review on the Recent Progress in the Preparation of Superhydrophobic Surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Mognetti, B.M.; Kusumaatmaja, H.; Yeomans, J.M. Drop Dynamics on Hydrophobic and Superhydrophobic Surfaces. Faraday Discuss. 2010, 146, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Jönsson-Niedziółka, M.; Lapierre, F.; Coffinier, Y.; Parry, S.J.; Zoueshtiagh, F.; Foat, T.; Thomy, V.; Boukherroub, R. EWOD Driven Cleaning of Bioparticles on Hydrophobic and Superhydrophobic Surfaces. Lab Chip 2011, 11, 490–496. [Google Scholar] [CrossRef]
- Krupenkin, T.N.; Taylor, J.A.; Schneider, T.M.; Yang, S. From Rolling Ball to Complete Wetting: The Dynamic Tuning of Liquids on Nanostructured Surfaces. Langmuir 2004, 20, 3824–3827. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, J.; Xiu, Y.; Sun, Y.; Hess, D.W.; Wong, C.-P. Electrowetting of Aligned Carbon Nanotube Films. J. Phys. Chem. B 2006, 110, 15945–15950. [Google Scholar] [CrossRef] [PubMed]
- Krupenkin, T.N.; Taylor, J.A.; Wang, E.N.; Kolodner, P.; Hodes, M.; Salamon, T.R. Reversible Wetting-Dewetting Transitions on Electrically Tunable Superhydrophobic Nanostructured Surfaces. Langmuir 2007, 23, 9128–9133. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Tay, B.; Tan, C.; Shakerzadeh, M.; Ostrikov, K. Electrowetting Control of Cassie-to-Wenzel Transitions in Superhydrophobic Carbon Nanotube-Based Nanocomposites. ACS Nano 2009, 3, 3031–3036. [Google Scholar] [CrossRef]
- Accardo, A.; Mecarini, F.; Leoncini, M.; Brandi, F.; Cola, E.D.; Burghammer, M.; Riekel, C.; Fabrizio, E.D. Fast, Active Droplet Interaction: Coalescence and Reactive Mixing Controlled by Electrowetting on a Superhydrophobic Surface. Lab Chip 2013, 13, 332–335. [Google Scholar] [CrossRef]
- Mats, L.; Bramwell, A.; Dupont, J.; Liu, G.; Oleschuk, R. Electrowetting on Superhydrophobic Natural (Colocasia) and Synthetic Surfaces Based upon Fluorinated Silica Nanoparticles. Microelectron. Eng. 2015, 148, 91–97. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Tan, B.T.; Wu, P.; Kim, S.S. Electrowetting-on-Dielectric Characteristics of ZnO Nanorods. Sci. Rep. 2020, 10, 14194. [Google Scholar] [CrossRef]
- Hou, J.; Ding, W.; Feng, Y.; Shui, L.; Wang, Y.; Li, H.; Li, N.; Zhou, G. Electrowetting Performances of Novel Fluorinated Polymer Dielectric Layer Based on Poly(1H,1H,2H,2H-Perfluoroctylmethacrylate) Nanoemulsion. Polymers 2017, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Chao, L.; Zhou, J. Biocompatible/Biodegradable Electrowetting on Dielectric Microfluidic Chips with Fluorinated CTA/PLGA. Materials 2018, 11, 1332. [Google Scholar] [CrossRef] [Green Version]
- Herbertson, D.L.; Evans, C.R.; Shirtcliffe, N.J.; McHale, G.; Newton, M.I. Electrowetting on Superhydrophobic SU-8 Patterned Surfaces. Sens. Actuators A Phys. 2006, 130–131, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.-T.; Wang, F.-C.; Zhao, Y.-P. Electrowetting on a Lotus Leaf. Biomicrofluidics 2009, 3, 022406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wei, H.; Zhu, X. Electrically Modulated Adhesive Hydrophobicity for Patterning Various Microstructures. Microelectron. Eng. 2019, 209, 1–5. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Lai, C.-H.; Wu, P.-W.; Fan, S.-K. Electrowetting of Superhydrophobic ZnO Inverse Opals. J. Electrochem. Soc. 2011, 158, P93. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.; Banerjee, D.; Marin Quintero, J.S.; Fishlock, S.J.; McLaughlin, J.; Waghmare, P.R.; Roy, S.S. Polarity Dependent Electrowetting for Directional Transport of Water through Patterned Superhydrophobic Laser Induced Graphene Fibers. Carbon 2021, 182, 605–614. [Google Scholar] [CrossRef]
- Lapierre, F.; Brunet, P.; Coffinier, Y.; Thomy, V.; Blossey, R.; Boukherroub, R. Electrowetting and Droplet Impalement Experiments on Superhydrophobic Multiscale Structures. Faraday Discuss. 2010, 146, 125–139. [Google Scholar] [CrossRef]
- Teh, K.S.; Takahashi, Y.; Yao, Z.; Lu, Y.-W. Influence of Redox-Induced Restructuring of Polypyrrole on Its Surface Morphology and Wettability. Sens. Actuators A Phys. 2009, 155, 113–119. [Google Scholar] [CrossRef]
- Lee, C.; Kim, C.-J. Underwater Restoration and Retention of Gases on Superhydrophobic Surfaces for Drag Reduction. Phys. Rev. Lett. 2011, 106, 014502. [Google Scholar] [CrossRef] [Green Version]
- Vourdas, N.; Tserepi, A.; Stathopoulos, V.N. Reversible Pressure-Induced Switching of Droplet Mobility after Impingement on Porous Surface Media. Appl. Phys. Lett. 2013, 103, 111602. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Chen, C.-H. Restoring Superhydrophobicity of Lotus Leaves with Vibration-Induced Dewetting. Phys. Rev. Lett. 2009, 103, 174502. [Google Scholar] [CrossRef] [Green Version]
- Lapierre, F.; Coffinier, Y.; Boukherroub, R.; Thomy, V. Electro-(de)Wetting on Superhydrophobic Surfaces. Langmuir 2013, 29, 13346–13351. [Google Scholar] [CrossRef]
- Perry, G.; Coffinier, Y.; Boukherroub, R.; Thomy, V. Investigation of the Anti-Biofouling Properties of Graphene Oxide Aqueous Solutions by Electrowetting Characterization. J. Mater. Chem. A 2013, 1, 12355–12360. [Google Scholar] [CrossRef]
- Helmer, D.; Keller, N.; Kotz, F.; Stolz, F.; Greiner, C.; Nargang, T.M.; Sachsenheimer, K.; Rapp, B.E. Transparent, Abrasion-Insensitive Superhydrophobic Coatings for Real-World Applications. Sci. Rep. 2017, 7, 15078. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Bruchmann, J.; Sollich, T.; Richter, C.; Thelen, R.; Kotz, F.; Schwartz, T.; Helmer, D.; Rapp, B.E. Study of Biofilm Growth on Slippery Liquid-Infused Porous Surfaces Made from Fluoropor. ACS Appl. Mater. Interfaces 2019, 11, 4480–4487. [Google Scholar] [CrossRef] [PubMed]
- Mayoussi, F.; Doeven, E.H.; Kick, A.; Goralczyk, A.; Thomann, Y.; Risch, P.; Guijt, R.M.; Kotz, F.; Helmer, D.; Rapp, B.E. Facile Fabrication of Micro-/Nanostructured, Superhydrophobic Membranes with Adjustable Porosity by 3D Printing. J. Mater. Chem. A 2021, 9, 21379–21386. [Google Scholar] [CrossRef] [PubMed]
- Goralczyk, A.; Sachsenheimer, K.; Kotz, F.; Helmer, D.; Rapp, B.E. Electrowetting Induced Μ-Droplet Actuation Using Fluoropor in Digital Microfluidics (DMF). In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XIX, 5 March 2021, Online Only; Gray, B.L., Becker, H., Eds.; SPIE: Bellingham WA, USA, 2021; p. 3. [Google Scholar]
- Seyrat, E.; Hayes, R.A. Amorphous Fluoropolymers as Insulators for Reversible Low-Voltage Electrowetting. J. Appl. Phys. 2001, 90, 1383–1386. [Google Scholar] [CrossRef]
- Kotz, F.; Risch, P.; Helmer, D.; Rapp, B.E. High-Performance Materials for 3D Printing in Chemical Synthesis Applications. Adv. Mater. 2019, 31, 1805982. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Evans, R.D.; Welch, E.; Hsu, B.-N.; Madison, A.C.; Fair, R.B. Low Voltage Electrowetting-on-Dielectric Platform Using Multi-Layer Insulators. Sens. Actuators B Chem. 2010, 150, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Zulkepli, S.N.I.S.; Hamid, N.H.; Shukla, V. Droplet Velocity Measurement Based on Dielectric Layer Thickness Variation Using Digital Microfluidic Devices. Biosensors 2018, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Manukyan, G.; Oh, J.M.; van den Ende, D.; Lammertink, R.G.H.; Mugele, F. Electrical Switching of Wetting States on Superhydrophobic Surfaces: A Route Towards Reversible Cassie-to-Wenzel Transitions. Phys. Rev. Lett. 2011, 106, 014501. [Google Scholar] [CrossRef] [Green Version]
- Vrancken, R.J.; Kusumaatmaja, H.; Hermans, K.; Prenen, A.M.; Pierre-Louis, O.; Bastiaansen, C.W.M.; Broer, D.J. Fully Reversible Transition from Wenzel to Cassie-Baxter States on Corrugated Superhydrophobic Surfaces. Langmuir 2010, 26, 3335–3341. [Google Scholar] [CrossRef]
- Im, M.; Kim, D.-H.; Lee, J.-H.; Yoon, J.-B.; Choi, Y.-K. Electrowetting on a Polymer Microlens Array. Langmuir 2010, 26, 12443–12447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.-P. Wetting and Electrowetting on Corrugated Substrates. Phys. Fluids 2017, 29, 067101. [Google Scholar] [CrossRef] [Green Version]
- Goralczyk, A.; Zhu, M.; Mayoussi, F.; Lallemang, M.; Tschaikowsky, M.; Warmbold, A.; Caliaro, S.; Tauber, F.; Balzer, B.N.; Kotz-Helmer, F.; et al. Study of Repellence on Polymeric Surfaces with Two Individually Adjustable Pore Hierarchies. Chem. Eng. J. 2022, 437, 135287. [Google Scholar] [CrossRef]
- He, X.; Zhang, B.-X.; Wang, S.-L.; Wang, Y.-F.; Yang, Y.-R.; Wang, X.-D.; Lee, D.-J. Electrowetting-Based Control of Wetting Transition of a Nanodroplet on Pillar-Arrayed Surfaces. J. Mol. Liq. 2022, 345, 117049. [Google Scholar] [CrossRef]
- Torabinia, M.; Farzbod, A.; Moon, H. Electromechanical Model to Predict the Movability of Liquids in an Electrowetting-on-Dielectric Microfluidic Device. J. Appl. Phys. 2018, 123, 154902. [Google Scholar] [CrossRef] [Green Version]
- Mowry, S.; Ogren, P.J. Kinetics of Methylene Blue Reduction by Ascorbic Acid. J. Chem. Educ. 1999, 76, 970. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goralczyk, A.; Bhagwat, S.; Mayoussi, F.; Nekoonam, N.; Sachsenheimer, K.; Hou, P.; Kotz-Helmer, F.; Helmer, D.; Rapp, B.E. Application of Micro/Nanoporous Fluoropolymers with Reduced Bioadhesion in Digital Microfluidics. Nanomaterials 2022, 12, 2201. https://doi.org/10.3390/nano12132201
Goralczyk A, Bhagwat S, Mayoussi F, Nekoonam N, Sachsenheimer K, Hou P, Kotz-Helmer F, Helmer D, Rapp BE. Application of Micro/Nanoporous Fluoropolymers with Reduced Bioadhesion in Digital Microfluidics. Nanomaterials. 2022; 12(13):2201. https://doi.org/10.3390/nano12132201
Chicago/Turabian StyleGoralczyk, Andreas, Sagar Bhagwat, Fadoua Mayoussi, Niloofar Nekoonam, Kai Sachsenheimer, Peilong Hou, Frederik Kotz-Helmer, Dorothea Helmer, and Bastian E. Rapp. 2022. "Application of Micro/Nanoporous Fluoropolymers with Reduced Bioadhesion in Digital Microfluidics" Nanomaterials 12, no. 13: 2201. https://doi.org/10.3390/nano12132201
APA StyleGoralczyk, A., Bhagwat, S., Mayoussi, F., Nekoonam, N., Sachsenheimer, K., Hou, P., Kotz-Helmer, F., Helmer, D., & Rapp, B. E. (2022). Application of Micro/Nanoporous Fluoropolymers with Reduced Bioadhesion in Digital Microfluidics. Nanomaterials, 12(13), 2201. https://doi.org/10.3390/nano12132201