NiFeMn-Layered Double Hydroxides Linked by Graphene as High-Performance Electrocatalysts for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
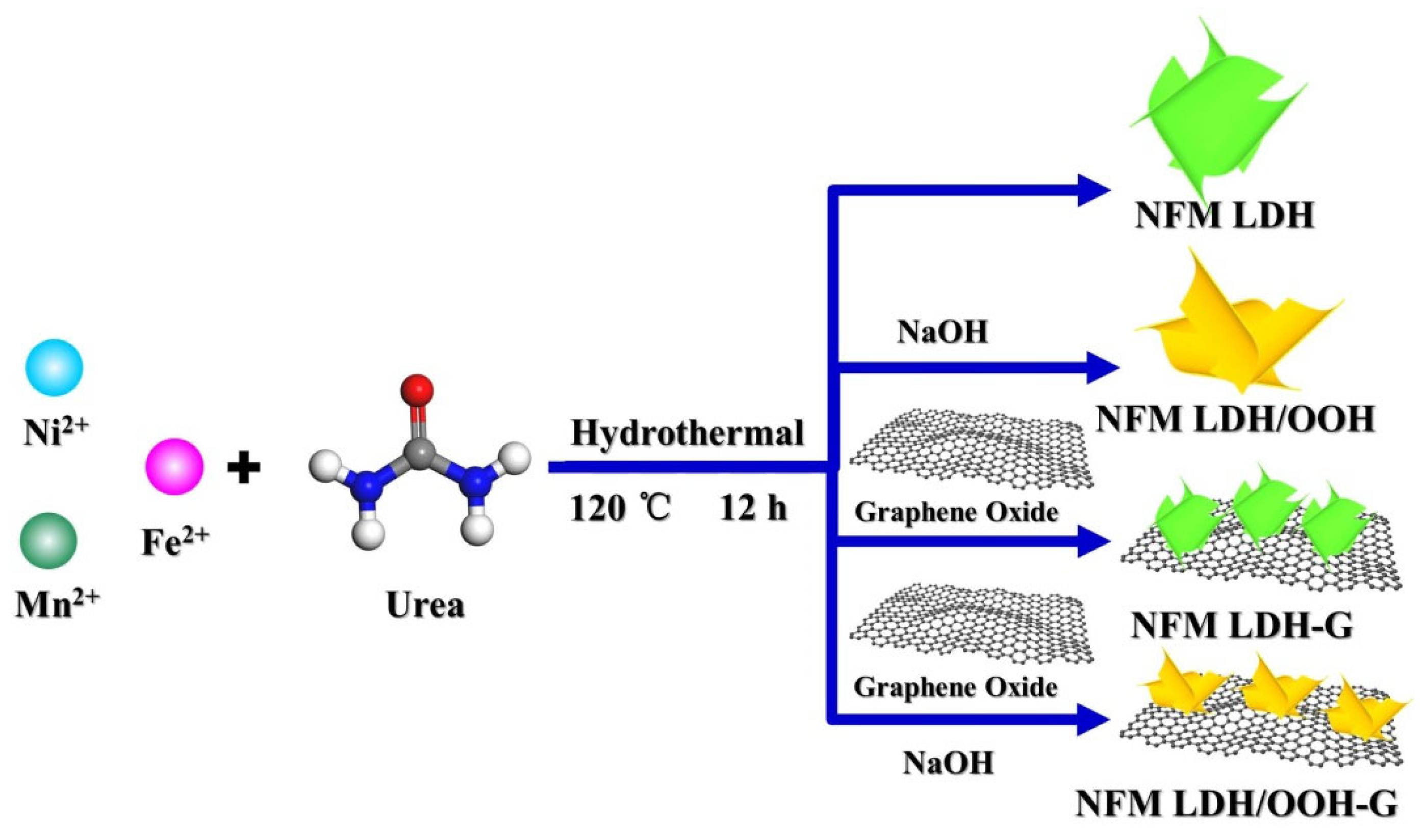
2.1. Synthesis of NFM-Layered Double Hydroxides and Their Derivatives
2.1.1. Materials
2.1.2. Preparation of NiFeMn-Layered Double Hydroxides
2.1.3. Preparation of NFM LDH with Graphene
2.1.4. Preparation of NFM LDH/OOH Heterostructures
2.1.5. Preparation of NFM LDH/OOH-G
2.2. Characterizations and Testing
2.2.1. Characterizations
2.2.2. Electrocatalytic Performance Testing
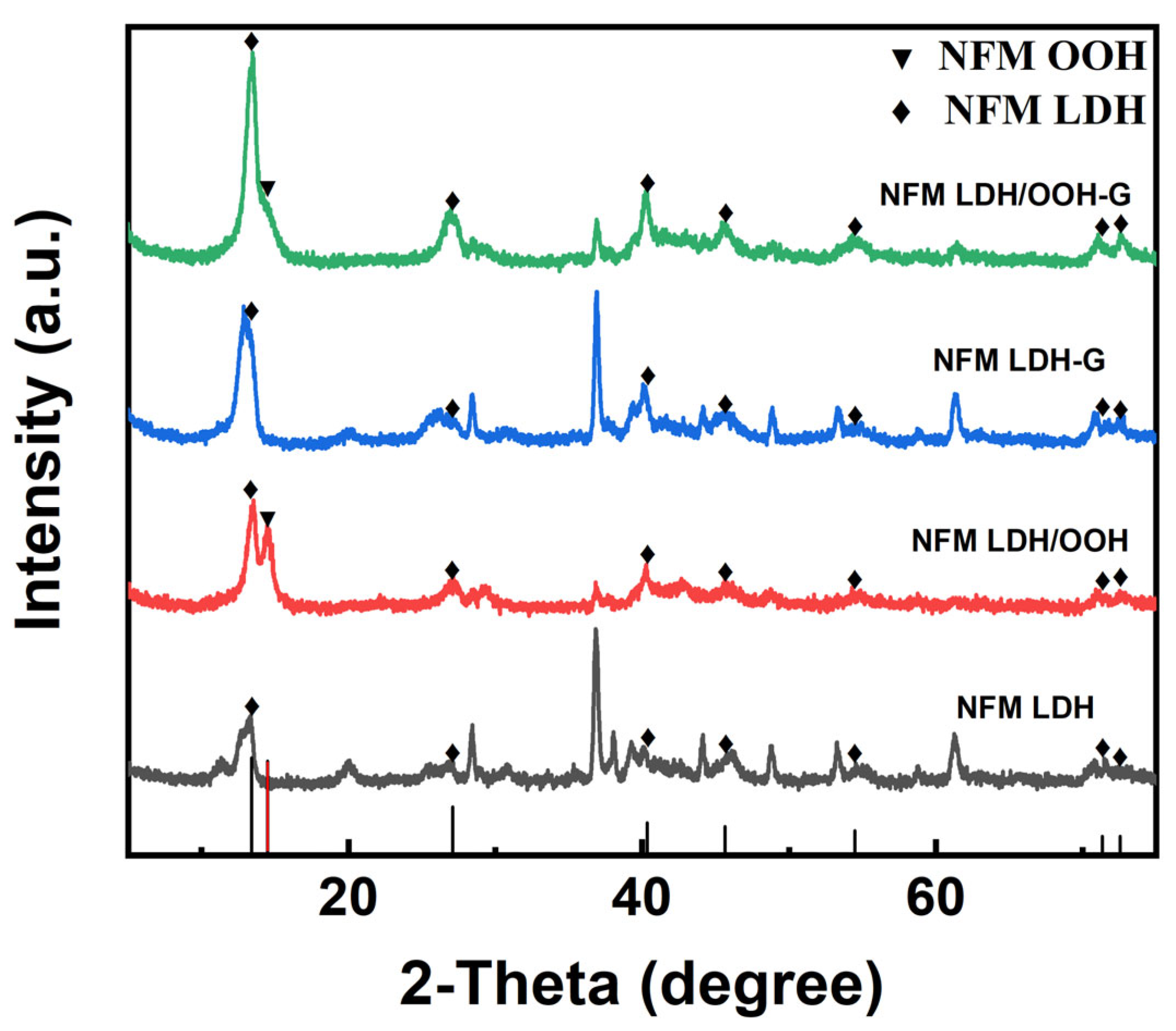
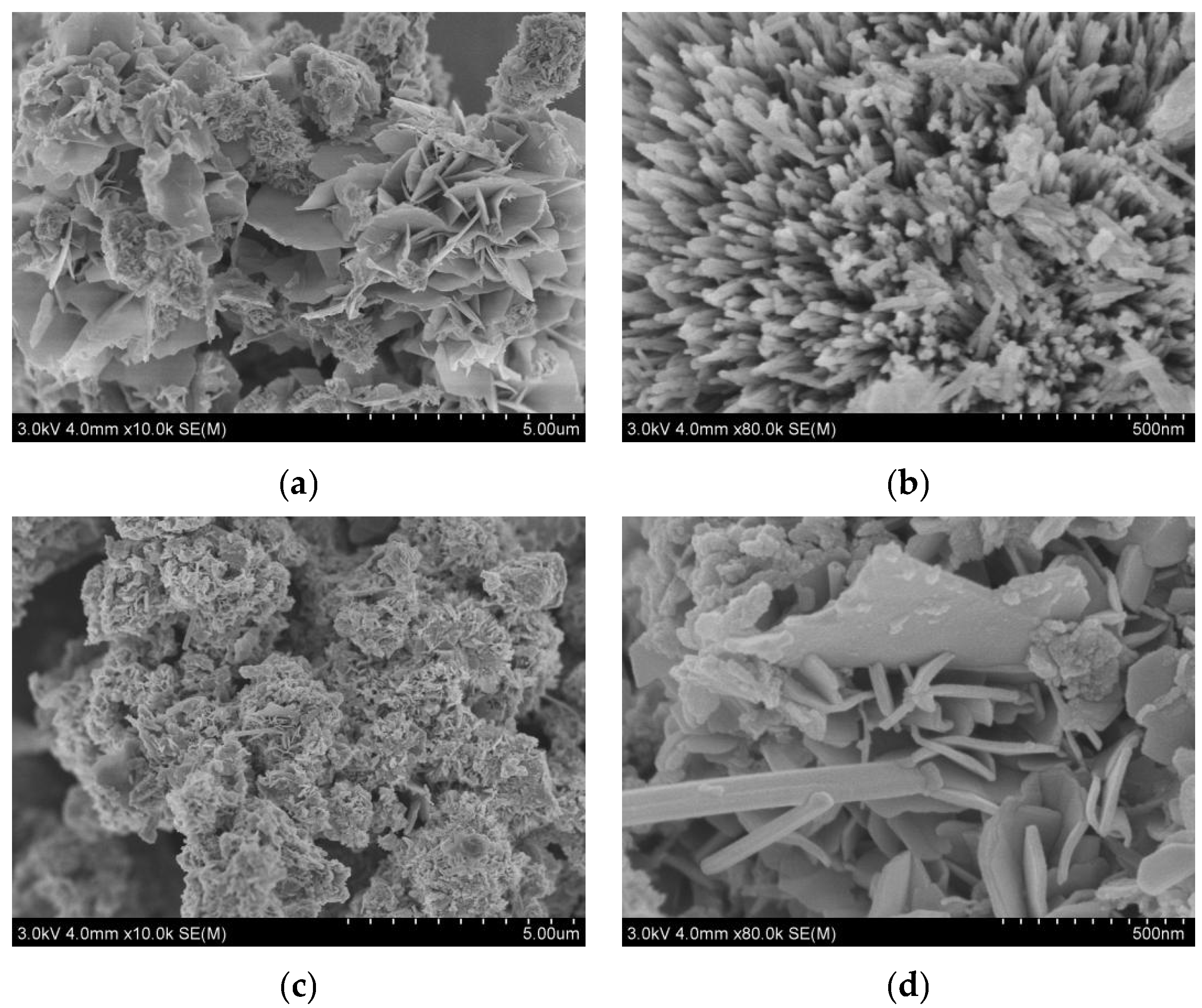
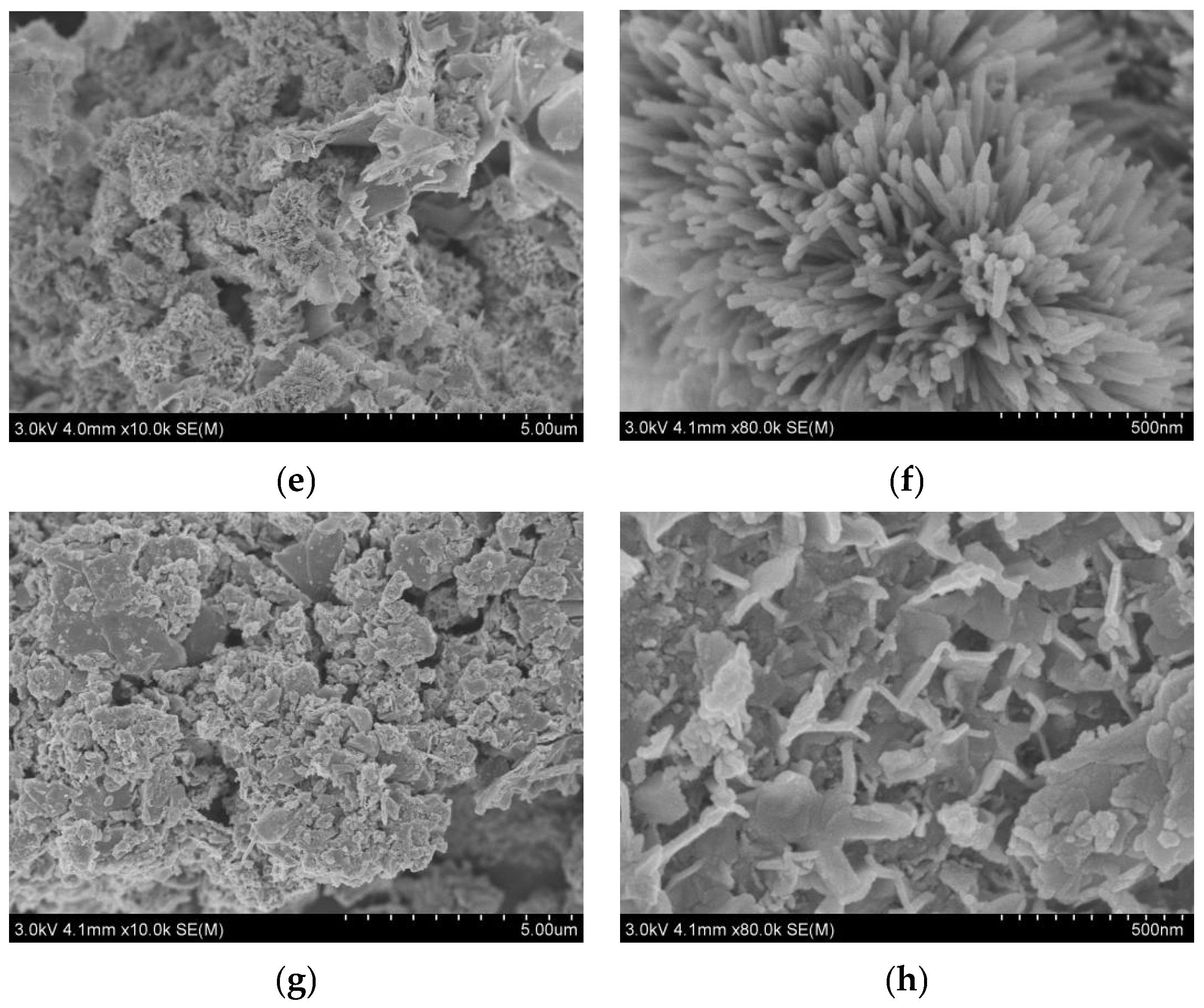
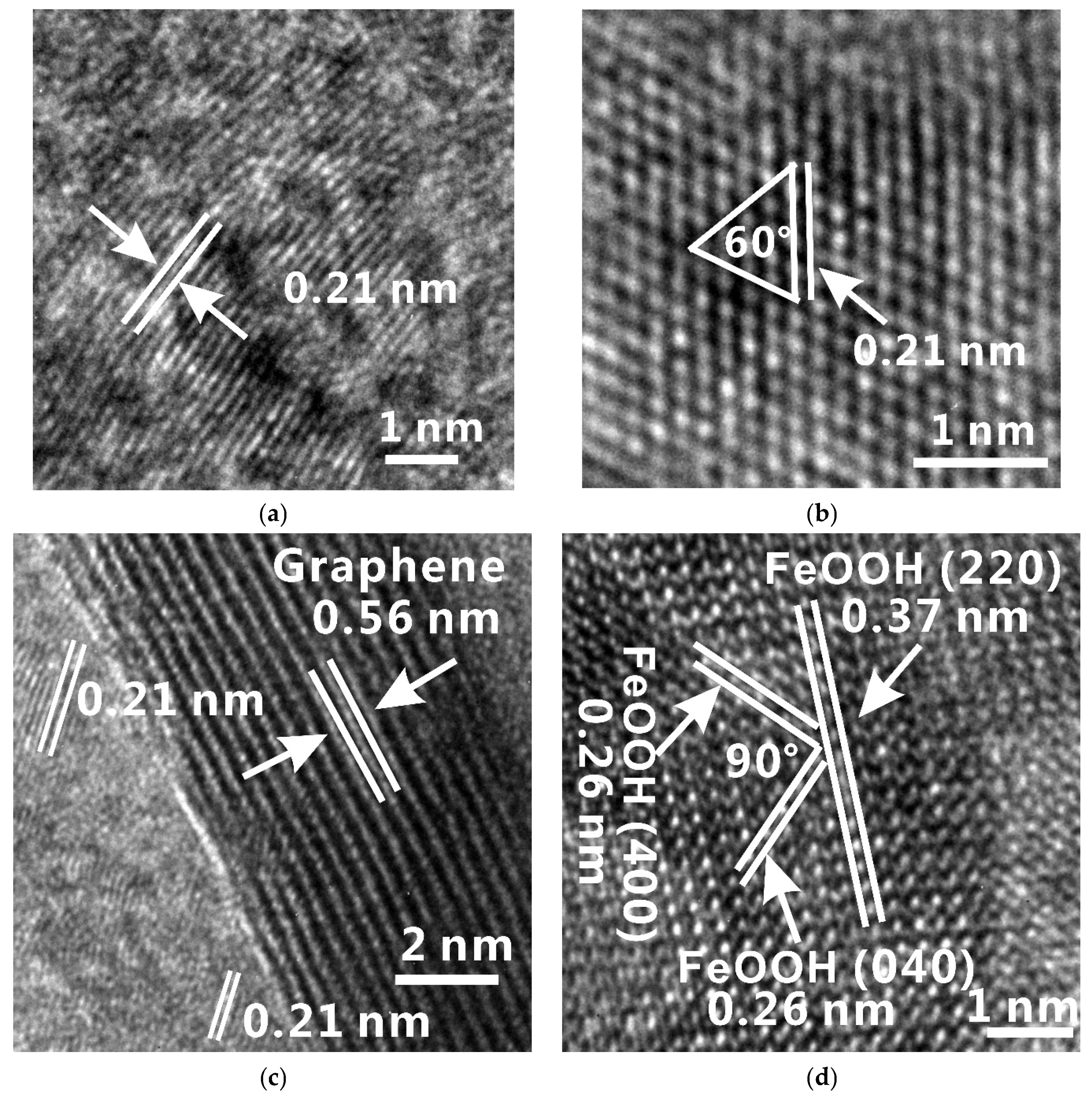
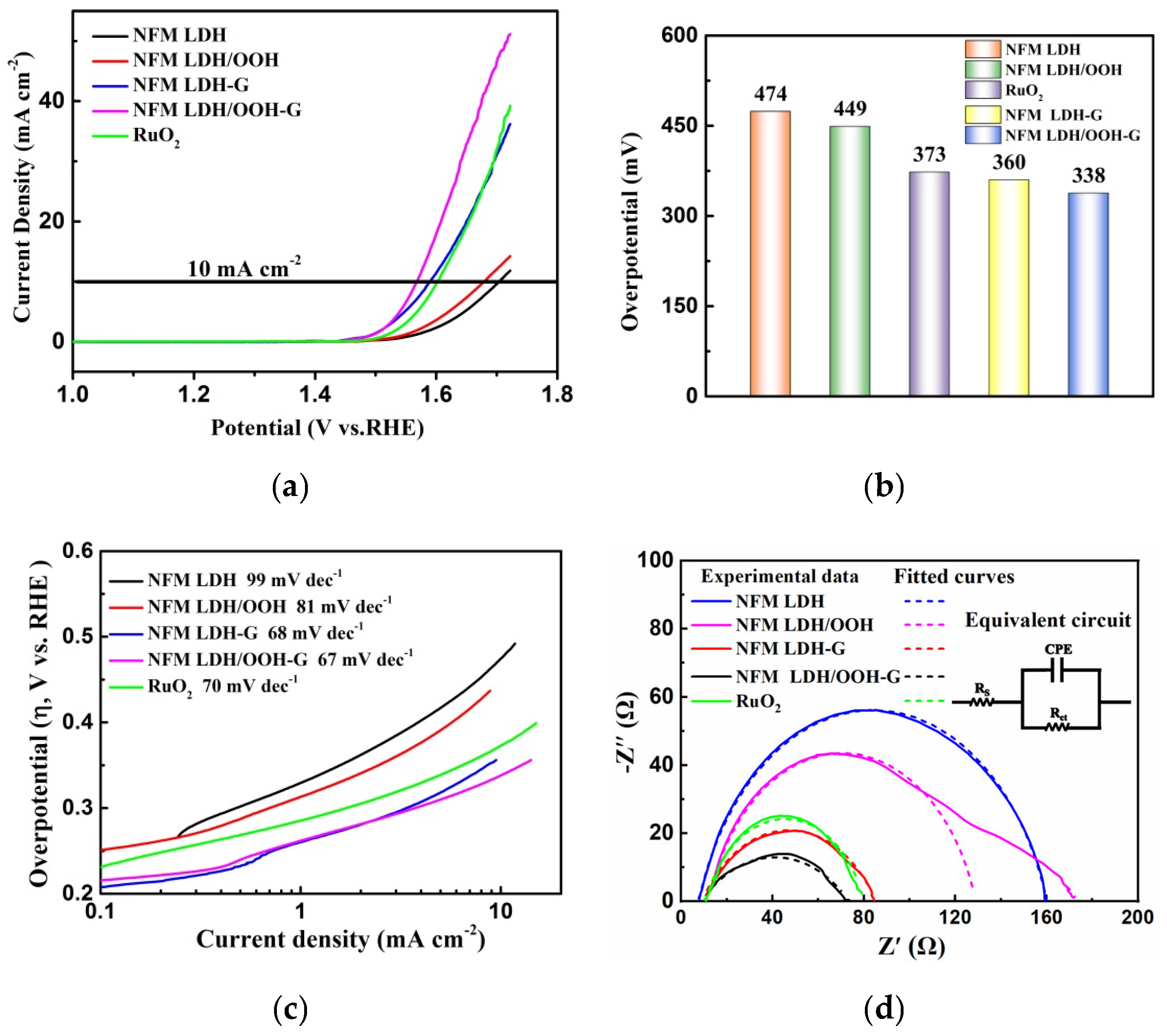
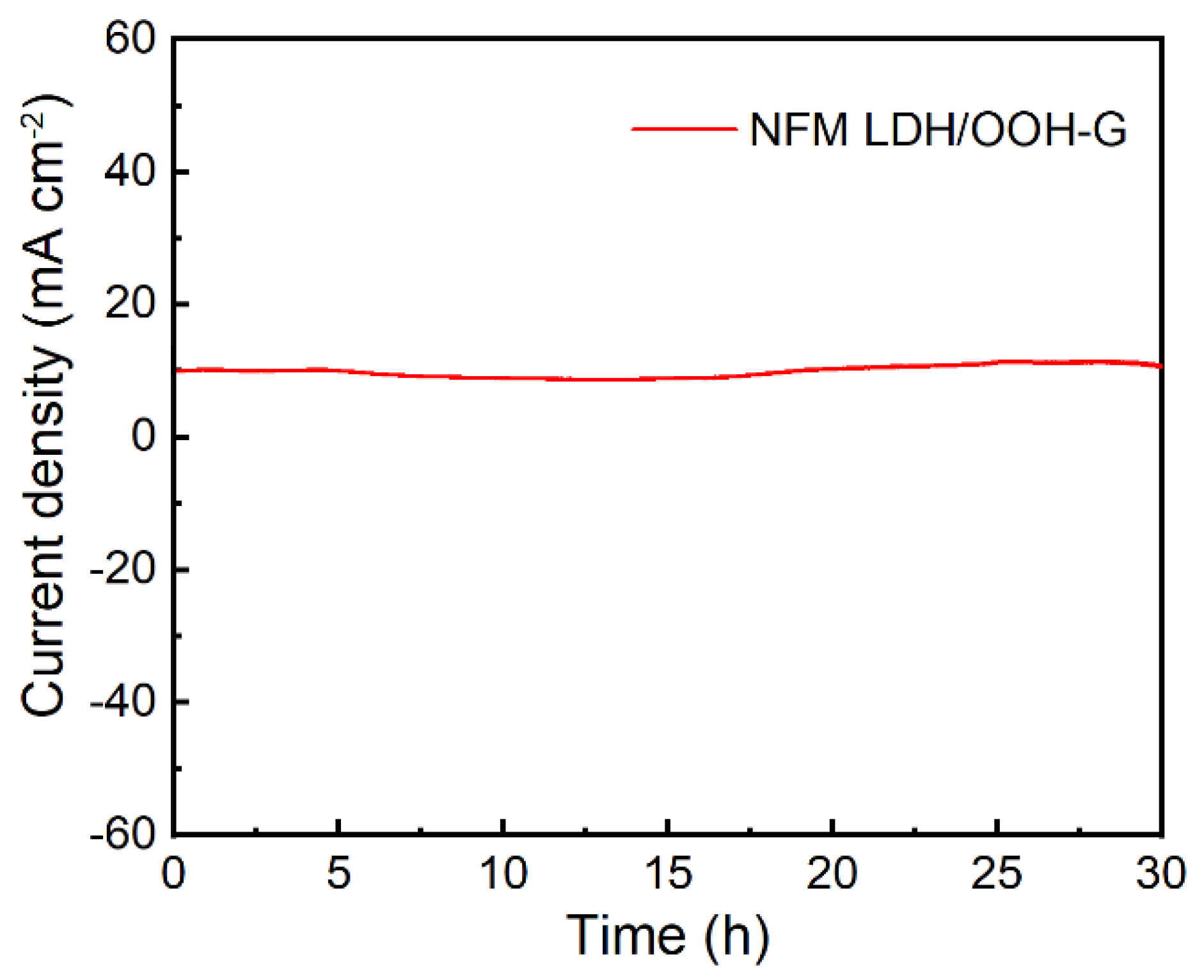
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Y.; Huang, S.; Yue, X.; Du, H.; Shen, P.K. Mo- and Fe-Modified Ni(OH)2/NiOOH Nanosheets as Highly Active and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Catal. 2018, 8, 2359–2363. [Google Scholar] [CrossRef]
- Lin, B.; Le, H.; Xu, F.; Mu, S. NiFe LDH/CuO nanosheet: A sheet-on-sheet strategy to boost the active site density towards oxygen evolution reaction. RSC Adv. 2020, 10, 27424–27427. [Google Scholar] [CrossRef] [PubMed]
- Tahira, A.; Ibupoto, Z.H.; Vagin, M.; Aftab, U.; Abro, M.I.; Willander, M.; Nur, O. An efficient bifunctional electrocatalyst based on a nickel iron layered double hydroxide functionalized Co3O4 core shell structure in alkaline media. Catal. Sci. Technol. 2019, 9, 2879–2887. [Google Scholar] [CrossRef]
- Babar, P.T.; Lokhande, A.C.; Gang, M.G.; Pawar, B.S.; Pawar, S.M.; Kim, J.H. Thermally oxidized porous NiO as an efficient oxygen evolution reaction (OER) electrocatalyst for electrochemical water splitting application. J. Ind. Eng. Chem. 2018, 60, 493–497. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, J.; An, Y.; Han, D.; Chang, S.; Liu, Y.; Yang, R. Enhanced electrochemical performance by nickel-iron layered double hydroxides (LDH) coated on Fe3O4 as a cathode catalyst for single-chamber microbial fuel cells. Sci. Total. Environ. 2020, 745, 141163. [Google Scholar] [CrossRef]
- Jin, X.; Li, J.; Xu, T.; Cui, Y.; Usoltseva, N.; An, V.; Zhang, X.; Liu, B. Hierarchical Ni2P@NiFe LDH Heterostructural Nanosheet Arrays for Highly Efficient Oxygen Evolution Reaction. Eur. J. Inorg. Chem. 2021, 2021, 3481–3487. [Google Scholar] [CrossRef]
- Kong, Y.; Li, J.; Wang, Y.; Chu, W.; Liu, Z. Tuning Interfacial Electron Transfer by Anchoring NiFe-LDH on In-situ Grown Cu2O for Enhancing Oxygen Evolution. Catal Lett. 2020, 150, 3049–3057. [Google Scholar] [CrossRef]
- Lei, C.; Li, W.; Wang, G.; Zhuang, L.; Lu, J.; Xiao, L. Improving the Catalytic Efficiency of NiFe-LDH/ATO by Air Plasma Treatment for Oxygen Evolution Reaction. Chem. Res. Chin. Univ. 2021, 37, 293–297. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Ge, R.; Ren, X.; Hao, S.; Xie, F.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; et al. Facilitating Active Species Generation by Amorphous NiFe-Bi Layer Formation on NiFe-LDH Nanoarray for Efficient Electrocatalytic Oxygen Evolution at Alkaline pH. Chem. Eur. J. 2017, 23, 11499–11503. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Yang, X.; Bai, S.; Feng, Y.; Luo, R.; Li, D.; Chen, A. An integrating photoanode consisting of BiVO4, rGO and LDH for photoelectrochemical water splitting. Dalton. Trans. 2019, 48, 16091–16098. [Google Scholar] [CrossRef]
- Lu, X.; Sakai, N.; Tang, D.; Li, X.; Taniguchi, T.; Ma, R.; Sasaki, T. CoNiFe Layered Double Hydroxide/RuO2.1 Nanosheet Superlattice as Carbon-Free Electrocatalysts for Water Splitting and Li-O2 Batteries. ACS Appl. Mater. Interfaces 2020, 12, 33083–33093. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Parida, K.M. Deciphering Z-scheme Charge Transfer Dynamics in Heterostructure NiFe-LDH/N-rGO/g-C3N4 Nanocomposite for Photocatalytic Pollutant Removal and Water Splitting Reactions. Sci. Rep. 2019, 9, 2458. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Shuai, C.; Mo, Z.; Liu, N.; Liu, G.; Wang, J.; Pei, H.; Jia, Q.; Liu, W.; Guo, X. CeO2 nanoparticles@ NiFe-LDH nanosheet heterostructure as electrocatalysts for oxygen evolution reaction. J. Solid State Chem. 2021, 296, 121967. [Google Scholar] [CrossRef]
- Qi, H.; Wolfe, J.; Fichou, D.; Chen, Z. Cu2O Photocathode for Low Bias Photoelectrochemical Water Splitting Enabled by NiFe-Layered Double Hydroxide Co-Catalyst. Sci. Rep. 2016, 6, 30882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Yao, K.; Zhang, Y. MnO2-directed synthesis of NiFe-LDH@FeOOH nanosheeet arrays for supercapacitor negative electrode. Chin. Chem. Lett. 2020, 31, 2343–2346. [Google Scholar] [CrossRef]
- Que, R.; Liu, S.; He, P.; Yang, Y.; Pan, Y. Hierarchical heterostructure CoCO3@NiFe LDH nanowires array as outstanding bifunctional electrocatalysts for overall water splitting. Mater. Lett. 2020, 277, 128285. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Xie, R.; Chen, L.; Li, M.; Li, J.; Ji, B.; Hu, Z.; Li, J.; Song, L.; et al. Controlled Self-Assembled NiFe Layered Double Hydroxides/Reduced Graphene Oxide Nanohybrids Based on the Solid-Phase Exfoliation Strategy as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 13545–13556. [Google Scholar] [CrossRef]
- Sanati, S.; Rezvani, Z. Ultrasound-assisted synthesis of NiFe- layered double hydroxides as efficient electrode materials in supercapacitors. Ultrason. Sonochem. 2018, 48, 199–206. [Google Scholar] [CrossRef]
- Tian, M.; Liu, C.; Neale, Z.G.; Zheng, J.; Long, D.; Cao, G. Chemically Bonding NiFe-LDH Nanosheets on rGO for Superior Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2019, 11, 35977–35986. [Google Scholar] [CrossRef]
- Fei, W.; Gao, J.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. A visible-light active p-n heterojunction NiFe-LDH/Co3O4 supported on Ni foam as photoanode for photoelectrocatalytic removal of contaminants. J. Hazard Mater. 2021, 402, 123515. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, S.; Lin, H.; Wang, G.; Zhao, K.; Cai, R.; Tao, K.; Zhang, C.; Sun, M.; Hu, J.; et al. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy 2021, 81, 105606. [Google Scholar] [CrossRef]
- Guo, X.; Hu, X.; Wu, D.; Jing, C.; Liu, W.; Ren, Z.; Zhao, Q.; Jiang, X.; Xu, C.; Zhang, Y.; et al. Tuning the Bifunctional Oxygen Electrocatalytic Properties of Core-Shell Co3O4@NiFe LDH Catalysts for Zn-Air Batteries: Effects of Interfacial Cation Valences. ACS Appl. Mater. Interfaces 2019, 11, 21506–21514. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, C.; Niu, J.; Zhang, S.; Ou, X.; Feng, P.; Garcia, H. Porous NiFe-LDH grown on graphene oxide towards highly efficient OER electrocatalysis. Mater. Lett. 2021, 290, 129517. [Google Scholar] [CrossRef]
- Xie, X.; Shang, L.; Shi, R.; Waterhouse, G.I.N.; Zhao, J.; Zhang, T. Tubular assemblies of N-doped carbon nanotubes loaded with NiFe alloy nanoparticles as efficient bifunctional catalysts for rechargeable zinc-air batteries. Nanoscale 2020, 12, 13129–13136. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yu, C.; Zhou, S.; Zhao, C.; Huang, H.; Yang, J.; Liu, Z.; Zhao, J.; Qiu, J. Ultrasensitive Iron-Triggered Nanosized Fe-CoOOH Integrated with Graphene for Highly Efficient Oxygen Evolution. Adv. Energy Mater. 2017, 7, 1602148. [Google Scholar] [CrossRef]
- Lu, Z.; Qian, L.; Tian, Y.; Li, Y.; Sun, X.; Duan, X. Ternary NiFeMn layered double hydroxides as highly-efficient oxygen evolution catalysts. Chem. Commun. 2016, 52, 908–911. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Yang, X.L.; Lv, Y.W.; Hu, J.; Zhao, J.R.; Xu, G.Y.; Hao, X.Q.; Chen, P.; Yan, M.Q. A three-dimensional nanostructure of NiFe(OH)X nanoparticles/nickel foam as an efficient electrocatalyst for urea oxidation. RSC Adv. 2021, 11, 17352. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A Review Unveiling the Critical Roles of Fe in Enhancing OER Activity of Ni and Co Based Catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, F.; Zhang, S.J.; Fisher, A.; Zhou, Y.; Wang, Z.; Li, Y.; Xu, B.B.; Li, J.T.; Sun, S.G. Interfacial Interaction between FeOOH and Ni-Fe LDH to Modulate the Local Electronic Structure for Enhanced OER Electrocatalysis. ACS Catal. 2018, 8, 11342–11351. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Men, Y.L.; Li, Y.; Peng, C.; Xi, S.; Pan, Y.X. Incorporating MoO3 Patches into a Ni Oxyhydroxide Nanosheet Boosts the Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 26064–26073. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olmos, C.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Optimization of Cu-Ni-Mn-catalysts for the conversion of ethanol to butanol. Catal. Today 2020, 357, 132–142. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Yu, L.; Liu, S.; Ruan, B.; Hu, H.; Zhu, N.; Lin, Z. FeOOH-loaded MnO2 nano-composite: An efficient emergency material for thallium pollution incident. J. Environ. Manag. 2017, 192, 31–38. [Google Scholar] [CrossRef]
- Ham, K.; Lee, J.; Lee, K.; Lee, J. Boosting the oxygen evolution reaction performance of wrinkled Mn(OH)2 via conductive activation with a carbon binder. J. Energy Chem. 2022, 71, 580–587. [Google Scholar] [CrossRef]
- Huang, H.H.; De Silva, K.K.H.; Kumara, G.R.A.; Yoshimura, M. Structural Evolution of Hydrothermally Derived Reduced Graphene Oxide. Sci. Rep. 2018, 8, 6849. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Wang, Y.; Zhang, H. Ultrafine PdCu Nanoclusters by Ultrasonic-Assisted Reduction on the LDHs/rGO Hybrid with Significantly Enhanced Heck Reactivity. ACS Appl. Mater. Interfaces 2020, 12, 50365–50376. [Google Scholar] [CrossRef]
- Gang, C.; Chen, J.; Chen, Q.; Chen, Y. Heterostructure of ultrafine FeOOH nanodots supported on CoAl-layered double hydroxide nanosheets as highly efficient electrocatalyst for water oxidation. J. Colloid Interface Sci. 2021, 600, 594–601. [Google Scholar] [CrossRef]
- Navadeepthy, D.; Rebekah, A.; Viswanthan, C.; Ponpandian, N. Boosting the kinetics of oxygen and hydrogen evolution in alkaline water splitting using nickel ferrite /N-graphene nanocomposite as a bifunctional electrocatalyst. Int. J. Hydrogen Energy 2021, 46, 21512–21524. [Google Scholar] [CrossRef]
- Jebaslinhepzybai, B.T.; Partheeban, T.; Gavali, D.S.; Thapa, R.; Sasidharan, M. One-pot solvothermal synthesis of Co2P nanoparticles: An effificient HER and OER electrocatalysts. Int. J. Hydrogen Energy 2021, 46, 21924–21938. [Google Scholar] [CrossRef]
- Tang, L.; Fan, T.; Chen, Z.; Tian, J.; Guo, H.; Peng, M.; Zuo, F.; Fu, X.; Li, M.; Bu, Y.; et al. Binary-dopant promoted lattice oxygen participation in OER on cobaltate electrocatalyst. Chem. Eng. J. 2021, 417, 129324. [Google Scholar] [CrossRef]
- Bao, W.; Xiao, L.; Zhang, J.; Jiang, P.; Zou, X.; Yang, C.; Hao, X.; Ai, T. Electronic and structural engineering of NiCo2O4/Ti electrocatalysts for effificient oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 10259–10267. [Google Scholar] [CrossRef]
- Sam, D.K.; Gong, S.; Durairaj, A.; Sam, E.K.; Liu, J.; Lv, X. Fabrication of highly dispersed Mo2C coupled with Co-N-C via self-template as bifunctional electrocatalysts. Int. J. Energy Res. 2021, 45, 10989–11001. [Google Scholar] [CrossRef]




| Electrocatalysts | Overpotential at 10 mA cm−2 | Tafel Slope (mV dec−1) | Reference |
|---|---|---|---|
| NGNF | 340 mV | 93.2 | [41] |
| Co2P NP | 364 mV | 78 | [42] |
| LSCFM | 343 mV | 63 | [43] |
| NiCo2O4/Ti | 353 mV | 61 | [44] |
| VB12Mo@NC | 396 mV | 79 | [45] |
| NFM LDH/OOH-G | 338 mV | 67 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, Q.; Zhu, Y.; Du, Y.; Yang, W.; Chen, Y.; Li, Y.; Wang, S. NiFeMn-Layered Double Hydroxides Linked by Graphene as High-Performance Electrocatalysts for Oxygen Evolution Reaction. Nanomaterials 2022, 12, 2200. https://doi.org/10.3390/nano12132200
Wang Z, Zhou Q, Zhu Y, Du Y, Yang W, Chen Y, Li Y, Wang S. NiFeMn-Layered Double Hydroxides Linked by Graphene as High-Performance Electrocatalysts for Oxygen Evolution Reaction. Nanomaterials. 2022; 12(13):2200. https://doi.org/10.3390/nano12132200
Chicago/Turabian StyleWang, Ze, Qianyu Zhou, Yanni Zhu, Yangfan Du, Weichun Yang, Yuanfu Chen, Yong Li, and Shifeng Wang. 2022. "NiFeMn-Layered Double Hydroxides Linked by Graphene as High-Performance Electrocatalysts for Oxygen Evolution Reaction" Nanomaterials 12, no. 13: 2200. https://doi.org/10.3390/nano12132200
APA StyleWang, Z., Zhou, Q., Zhu, Y., Du, Y., Yang, W., Chen, Y., Li, Y., & Wang, S. (2022). NiFeMn-Layered Double Hydroxides Linked by Graphene as High-Performance Electrocatalysts for Oxygen Evolution Reaction. Nanomaterials, 12(13), 2200. https://doi.org/10.3390/nano12132200







