Synthesis and Antibacterial Activity of Manganese-Ferrite/Silver Nanocomposite Combined with Two Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Essential Oils
2.3. Synthesis of Manganese-Ferrite/Silver Nanocomposite (MnFe2O4/Ag-NC)
2.4. Characterization of the MnFe2O4/Ag-NC
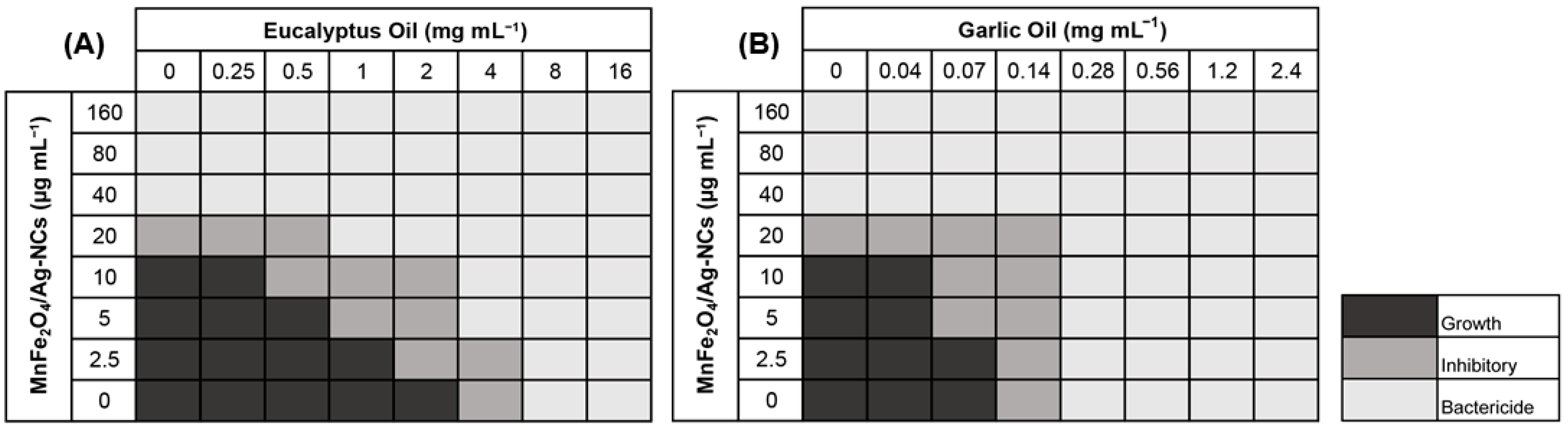
2.5. Antimicrobial Activity: Checkerboard Assay
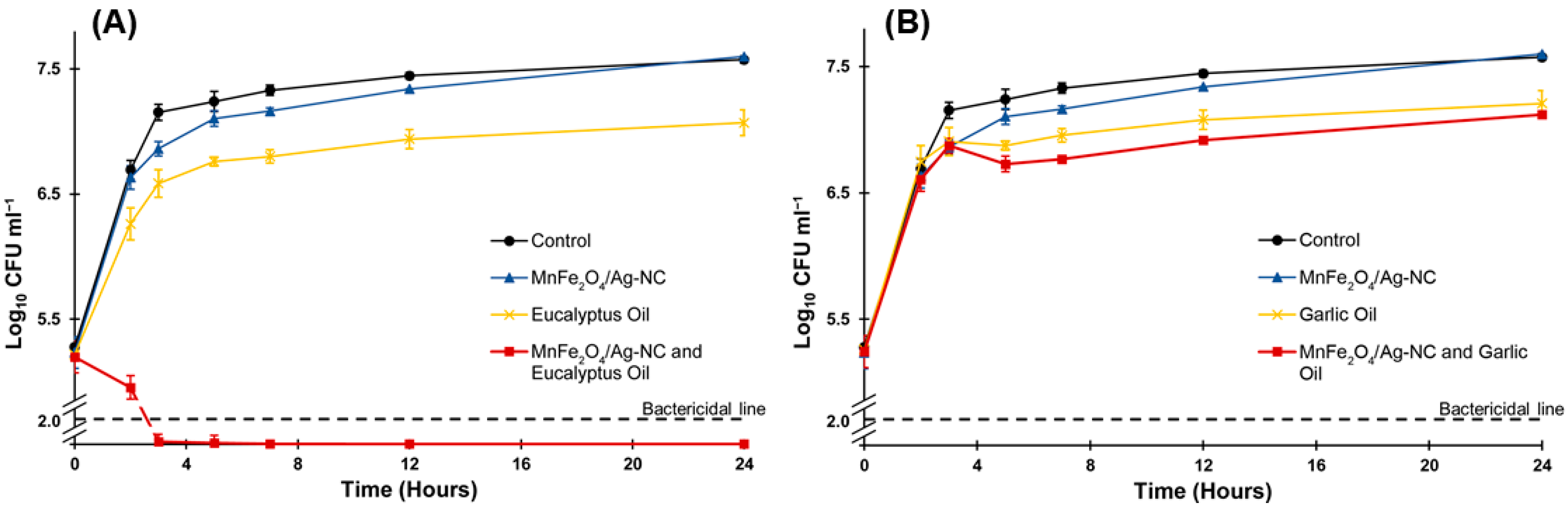
2.6. Time-Kill Curve Assay
3. Results and Discussion
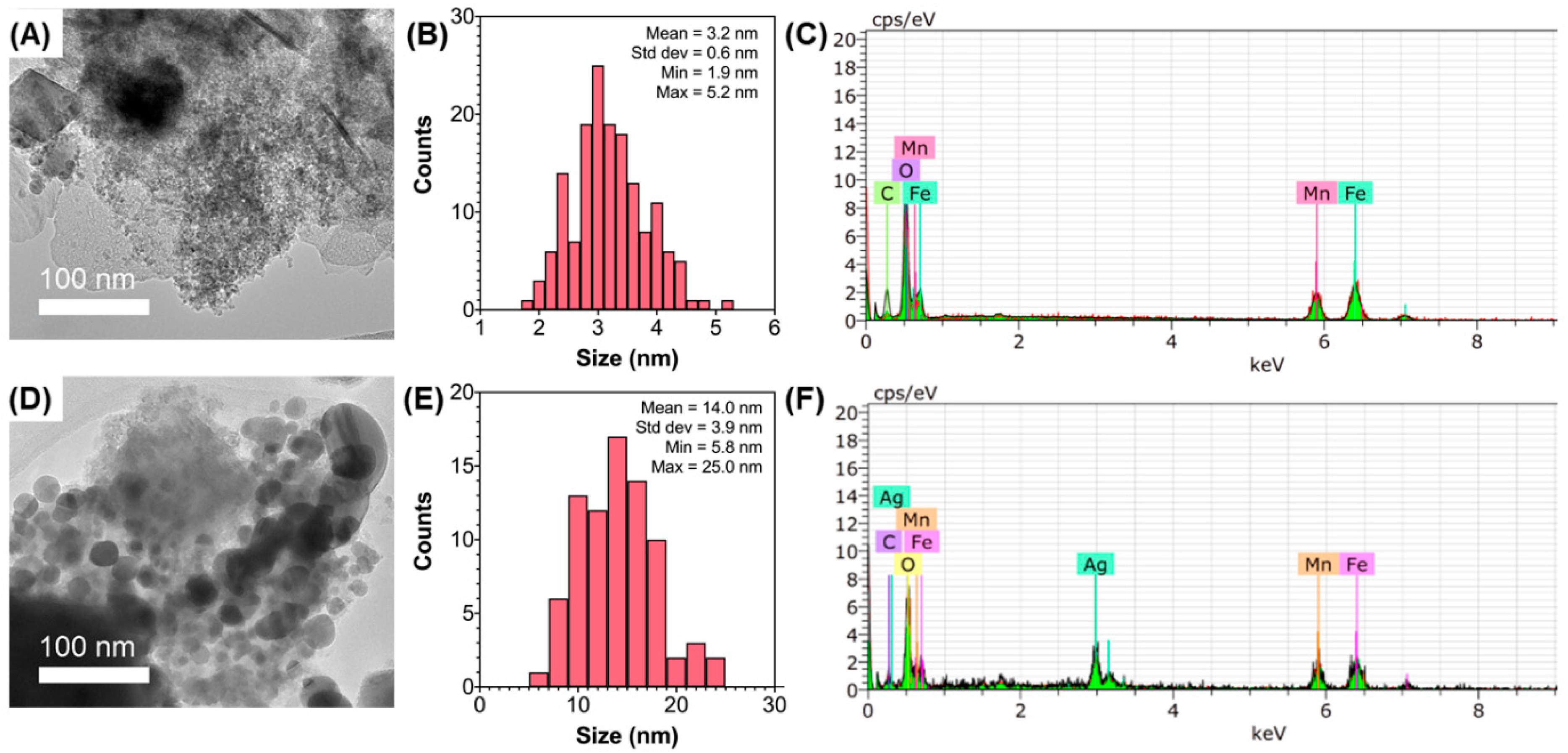
3.1. Characterization of MnFe2O4 and MnFe2O4/Ag-NC
3.2. Antimicrobial Activity of MnFe2O4/Ag-NC Combined with Essential Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guha, T.; Gopal, G.; Kundu, R.; Mukherjee, A. Nanocomposites for Delivering Agrochemicals: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kaushik, A.; Sonne, C.; Kim, K.H.; Kumar, S. Emerging nanobiotechnology in agriculture for the management of pesticide residues. J. Hazard. Mater. 2021, 401, 123369. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Salayová, A. Green-Synthesized Silver Nanoparticles and Their Potential for Antibacterial Applications. In Bacterial Pathogenesis and Antibacterial Control; InTech: London, UK, 2018. [Google Scholar]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Rubilar, O.; Diez, M.C.; Cea, M.; Sant’Ana da Silva, A.; Rodríguez-Rodríguez, C.E.; Tortella, G.R. Combined pollution of copper nanoparticles and atrazine in soil: Effects on dissipation of the pesticide and on microbiological community profiles. J. Hazard. Mater. 2019, 361, 228–236. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Liao, N.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. In-situ deposition of silver-iron oxide nanoparticles on the surface of fly ash for water purification. J. Colloid Interface Sci. 2015, 453, 159–168. [Google Scholar] [CrossRef]
- Fang, W.; Zheng, Q.; Fang, Y.; Huang, H. Facile synthesis of silver-decorated magnetic nanospheres used as effective and recyclable antibacterial agents. Curr. Appl. Phys. 2019, 19, 114–119. [Google Scholar] [CrossRef]
- Nha, T.T.N.; Nam, P.H.; Phuc, N.X.; Nguyen, V.Q.; Nam, N.H.; Manh, D.H.; Tam, L.T.; Linh, N.T.N.; Khanh, B.T.V.; Lu, L.T.; et al. Sensitive MnFe2O4–Ag hybrid nanoparticles with photothermal and magnetothermal properties for hyperthermia applications. RSC Adv. 2021, 11, 30054–30068. [Google Scholar] [CrossRef]
- Amir, M.; Kurtan, U.; Baykal, A.; Sözeri, H. MnFe2O4@PANI@Ag Heterogeneous Nanocatalyst for Degradation of Industrial Aqueous Organic Pollutants. J. Mater. Sci. Technol. 2016, 32, 134–141. [Google Scholar] [CrossRef]
- Huy, L.T.; Tam, L.T.; Phan, V.N.; Trung, T.; Tung, L.M.; Thanh, D.T.N.; Hoa, N.Q.; Vinh, L.K.; Ngo, D.T.; Mølhave, K.; et al. Effect of synthesis parameters on the structure and magnetic properties of magnetic manganese ferrite/silver composite nanoparticles synthesized by wet chemistry method. J. Nanosci. Nanotechnol. 2016, 16, 7919–7928. [Google Scholar] [CrossRef]
- Desai, H.B.; Hathiya, L.J.; Joshi, H.H.; Tanna, A.R. Synthesis and characterization of photocatalytic MnFe2O4 nanoparticles. Mater. Today Proc. 2020, 21, 1905–1910. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Huang, C.; Li, W. Synthesis and Antibacterial Activity of Magnetic MnFe2O4/Ag Composite Particles. Nanosci. Nanotechnol. Lett. 2014, 6, 385–391. [Google Scholar] [CrossRef]
- Huy, L.T.; Tam, L.T.; Van Son, T.; Cuong, N.D.; Nam, M.H.; Vinh, L.K.; Huy, T.Q.; Ngo, D.T.; Phan, V.N.; Le, A.T. Photochemical Decoration of Silver Nanocrystals on Magnetic MnFe2O4 Nanoparticles and Their Applications in Antibacterial Agents and SERS-Based Detection. J. Electron. Mater. 2017, 46, 3412–3421. [Google Scholar] [CrossRef]
- Muthukumar, H.; Palanirajan, S.K.; Shanmugam, M.K.; Gummadi, S.N. Plant extract mediated synthesis enhanced the functional properties of silver ferrite nanoparticles over chemical mediated synthesis. Biotechnol. Rep. 2020, 26, e00469. [Google Scholar] [CrossRef]
- Luka, C.; Adoga, G.; Istifanus, G. Phytochemical Studies of Different Fractions of Galega officinalis Extract and Their Effects on Some Biochemical Parameters in Alloxan-Induced Diabetic Rats. Eur. J. Med. Plants 2017, 19, 1–10. [Google Scholar] [CrossRef][Green Version]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 35. [Google Scholar] [CrossRef]
- Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef]
- Jin, Z.; Li, L.; Zheng, Y.; An, P. Inhibition of Bacillus cereus by garlic (Allium sativum) essential oil during manufacture of white sufu, a traditional Chinese fermented soybean curd. LWT 2020, 130, 109634. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Duarte, A.; de Menezes, I.; Bezerra Morais Braga, M.; Leite, N.; Barros, L.; Waczuk, E.; Pessoa da Silva, M.; Boligon, A.; Teixeira Rocha, J.; Souza, D.; et al. Antimicrobial Activity and Modulatory Effect of Essential Oil from the Leaf of Rhaphiodon echinus (Nees & Mart) Schauer on Some Antimicrobial Drugs. Molecules 2016, 21, 743. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Basavegowda, N.; Patra, J.K.; Baek, K.-H. Essential Oils and Mono/bi/tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Sharififard, M.; Safdari, F.; Siahpoush, A.; Kassiri, H. Evaluation of Some Plant Essential Oils against the Brown-Banded Cockroach, Supella longipalpa (Blattaria: Ectobiidae): A Mechanical Vector of Human Pathogens. J. Arthropod-Borne Dis. 2016, 10, 528–537. [Google Scholar] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Tampe, J.; Espinoza, J.; Chacón-Fuentes, M.; Quiroz, A.; Rubilar, M. Evaluation of Drimys winteri (Canelo) Essential Oil as Insecticide against Acanthoscelides obtectus (Coleoptera: Bruchidae) and Aegorhinus superciliosus (Coleoptera: Curculionidae). Insects 2020, 11, 335. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard a critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Das, T.; Ghosh, S.; Mugesh, G. A Remarkably Efficient MnFe2O4 -based Oxidase Nanozyme. Chem.-Asian J. 2016, 11, 72–76. [Google Scholar] [CrossRef]
- Pereira, V.; Dias, C.; Vasconcelos, M.C.; Rosa, E.; Saavedra, M.J. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Ind. Crops Prod. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Asghar Heydari, M.; Mobini, M.; Salehi, M. The Synergic Activity of Eucalyptus Leaf Oil and Silver Nanoparticles Against Some Pathogenic Bacteria. Arch. Pediatr. Infect. Dis. 2017, 5, e61654, in press. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and Antimicrobial Activity of the Essential Oils of Two Origanum Species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, A.; Akhondzadeh Basti, A.; Amoabediny, G.; Khanjari, A.; Tavakkoly Bazzaz, J.; Mohammadkhan, F.; Hajjar Bargh, A.; Vanaki, E. Physicochemical characteristics of nanoliposome garlic (Allium sativum L.) Essential Oil and its antibacterial effect on Escherichia coli O157:H. J. Food Qual. Hazards Control. 2017, 4, 24–28. [Google Scholar]
- Nikparast, Y.; Saliani, M. Synergistic Effect between Phyto-Syntesized Silver Nanoparticles and Ciprofloxacin Antibiotic on some Pathogenic Bacterial Strains. J. Med. Bacteriol. 2018, 7, 36–43. [Google Scholar]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, B.; Kumar, R.; Upadhyay, J.; Borah, D. Facile biogenic synthesis of silver nanoparticles (AgNPs) by Citrus grandis (L.) Osbeck fruit extract with excellent antimicrobial potential against plant pathogens. SN Appl. Sci. 2020, 2, 1723. [Google Scholar] [CrossRef]
- Shahryari, F.; Rabiei, Z.; Sadighian, S. Antibacterial activity of synthesized silver nanoparticles by sumac aqueous extract and silver-chitosan nanocomposite against Pseudomonas syringae pv. syringae. J. Plant Pathol. 2020, 102, 469–475. [Google Scholar] [CrossRef]
- He, Y.; Sang, S.; Tang, H.; Ou, C. In vitro mechanism of antibacterial activity of eucalyptus essential oil against specific spoilage organisms in aquatic products. J. Food Process. Preserv. 2022, 46, e16349. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Babu, S.; Krishna, N. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]

| Essential Oil | RT (min) | Compound | Area (%) | KI Exp. | KI Lib. |
|---|---|---|---|---|---|
| Eucalyptus | 9.76 | a-Pinene | 20.9 | 936 | 929 |
| 10.83 | β-Pinene | 3.4 | 970 | 979 | |
| 11.48 | β-Myrcene | 1.4 | 990 | 991 | |
| 12.42 | Eucalyptol | 46.0 | 1021 | 1032 | |
| 16.76 | Terpinen-4-ol | 1.3 | 1166 | 1177 | |
| 16.99 | Verbenyl ethyl ether | 3.7 | 1173 | 1186 | |
| 17.11 | α-Terpineol | 0.2 | 1177 | 1189 | |
| 21.14 | exo-Hydroxycineole acetate | 0.5 | 1321 | 1344 | |
| 21.47 | α-Terpenyl acetate | 5.9 | 1334 | 1350 | |
| 22.42 | Isoledene | 0.5 | 1370 | 1375 | |
| 22.50 | Copaene | 0.3 | 1373 | 1376 | |
| 23.34 | β-Maatiene | 1.0 | 1404 | 1405 | |
| 24.08 | Aromadendrene | 4.9 | 1435 | 1440 | |
| 24.54 | 9-epi-β-Caryophyllene | 1.8 | 1453 | 1466 | |
| 25.40 | Ledene | 0.8 | 1487 | 1493 | |
| 26.85 | Epiglobulol | 1.2 | 1549 | 1580 | |
| 27.41 | Globulol | 4.1 | 1572 | 1585 | |
| 27.56 | Viridiflorol | 1.7 | 1579 | 1591 | |
| 27.84 | Rosifoliol | 0.5 | 1590 | 1649 | |
| Garlic | 8.89 | Diallyl sulfide | 7.3 | 905 | 861 |
| 10.69 | Methyl 2-propenyl disulfide | 13.7 | 966 | 920 | |
| 11.75 | 1,2-Dithiole | 19.7 | 997 | 952 | |
| 12.53 | Dimethyl trisulfide | 1.4 | 1025 | 970 | |
| 16.46 | Diallyl disulfide | 15.5 | 1156 | 1081 | |
| 17.02 | 1(E)-1-Propen-1-yl 2-propenyl disulfide | 0.8 | 1174 | 1103 | |
| 18.12 | Allyl methyl trisulfide | 4.4 | 1211 | 1142 | |
| 19.56 | 3-Vinyl-1,2-dithiacyclohex-4-ene | 0.2 | 1264 | 1198 | |
| 19.63 | 1,2,3-Trithia-4-cyclohexene | 4.1 | 1266 | 1202 | |
| 24.53 | 5-Methyl-1,2,3,4-tetrathiane | 14.1 | 1446 | 1364 |
| Combination | MIC | FIC | Interaction Type | |
|---|---|---|---|---|
| Alone | Combined | |||
| MnFe2O4/Ag-NC and Eucalyptus oil | 20 µg mL−1 | 5 µg mL−1 | 0.5 | Synergistic |
| 4 mg mL−1 | 1 mg mL−1 | |||
| MnFe2O4/Ag-NC and Garlic oil | 20 µg mL−1 | 5 µg mL−1 | 0.75 | Additive |
| 0.14 mg mL−1 | 0.07 mg mL−1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parada, J.; Díaz, M.; Hermosilla, E.; Vera, J.; Tortella, G.; Seabra, A.B.; Quiroz, A.; Hormazábal, E.; Rubilar, O. Synthesis and Antibacterial Activity of Manganese-Ferrite/Silver Nanocomposite Combined with Two Essential Oils. Nanomaterials 2022, 12, 2137. https://doi.org/10.3390/nano12132137
Parada J, Díaz M, Hermosilla E, Vera J, Tortella G, Seabra AB, Quiroz A, Hormazábal E, Rubilar O. Synthesis and Antibacterial Activity of Manganese-Ferrite/Silver Nanocomposite Combined with Two Essential Oils. Nanomaterials. 2022; 12(13):2137. https://doi.org/10.3390/nano12132137
Chicago/Turabian StyleParada, Javiera, Marcela Díaz, Edward Hermosilla, Joelis Vera, Gonzalo Tortella, Amedea B. Seabra, Andrés Quiroz, Emilio Hormazábal, and Olga Rubilar. 2022. "Synthesis and Antibacterial Activity of Manganese-Ferrite/Silver Nanocomposite Combined with Two Essential Oils" Nanomaterials 12, no. 13: 2137. https://doi.org/10.3390/nano12132137
APA StyleParada, J., Díaz, M., Hermosilla, E., Vera, J., Tortella, G., Seabra, A. B., Quiroz, A., Hormazábal, E., & Rubilar, O. (2022). Synthesis and Antibacterial Activity of Manganese-Ferrite/Silver Nanocomposite Combined with Two Essential Oils. Nanomaterials, 12(13), 2137. https://doi.org/10.3390/nano12132137










