Zn and Zn-Fe Nanostructures with Multifunctional Properties as Components for Food Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
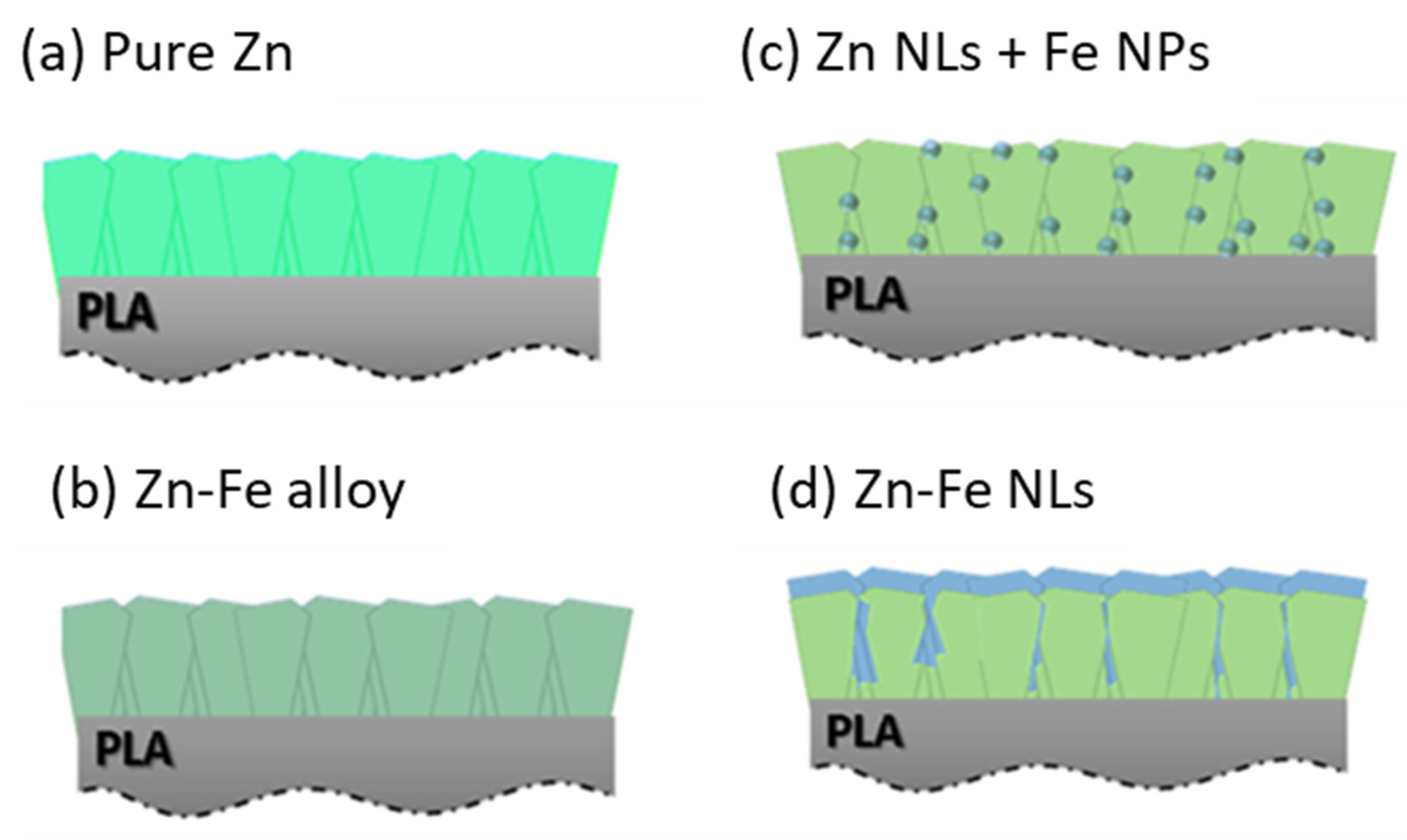
2.1. Production of the Materials
2.1.1. Classical Magnetron Sputtering
2.1.2. Hybrid Magnetron Sputtering—Cluster Gun
2.2. Methodology
2.2.1. Morphology, Composition, and Structure
2.2.2. Functional Properties
2.2.3. Statistical Analysis
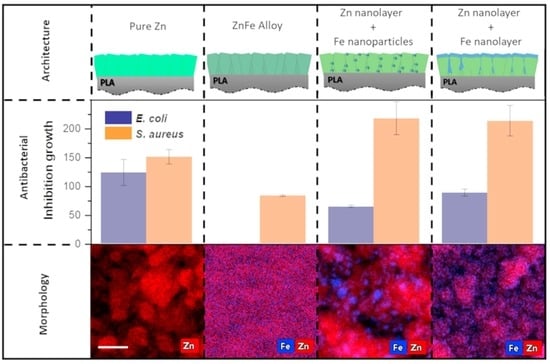
3. Results and Discussion
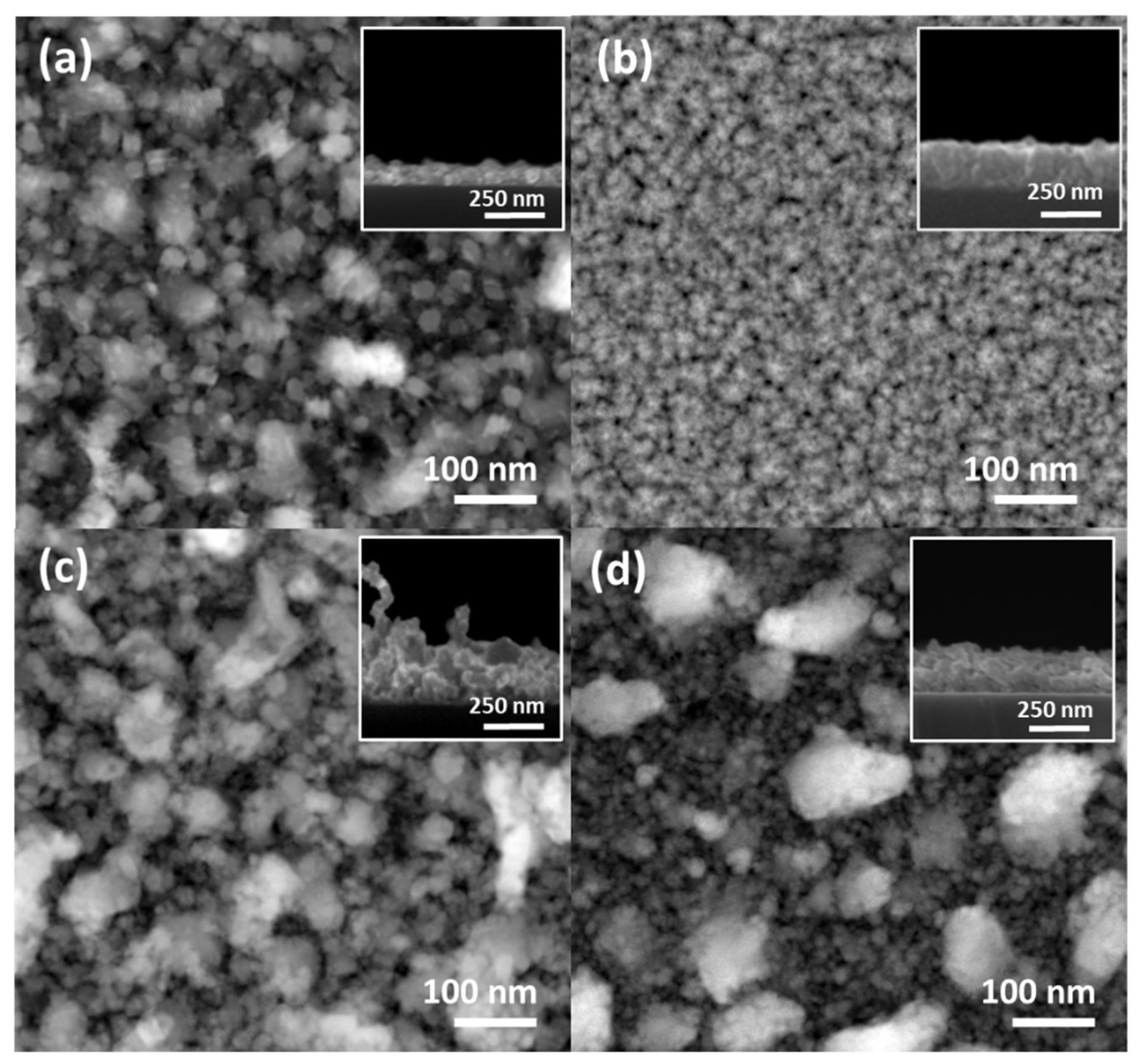
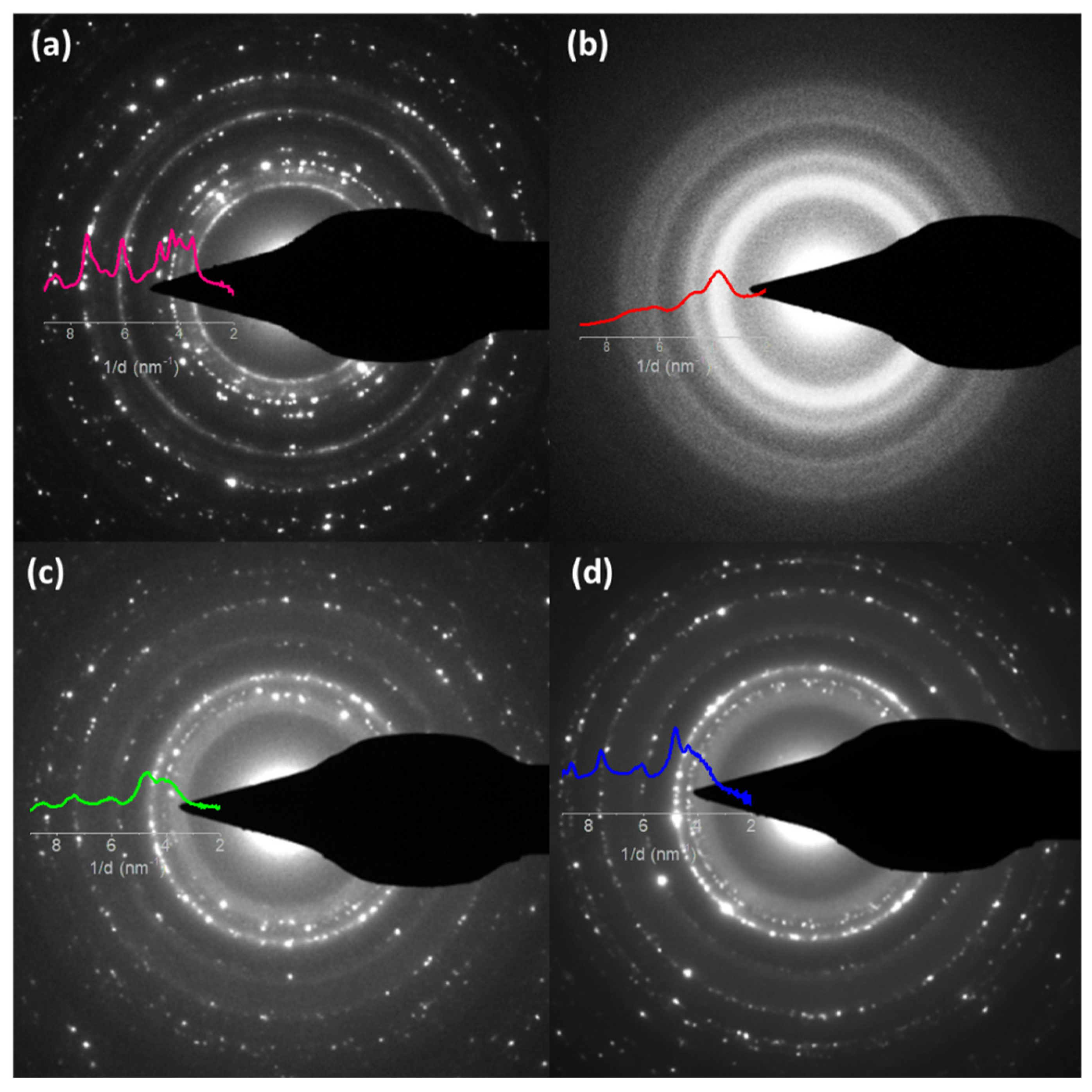
3.1. Morphology, Structure, and Chemical Composition Characterizations
3.2. Functional Properties
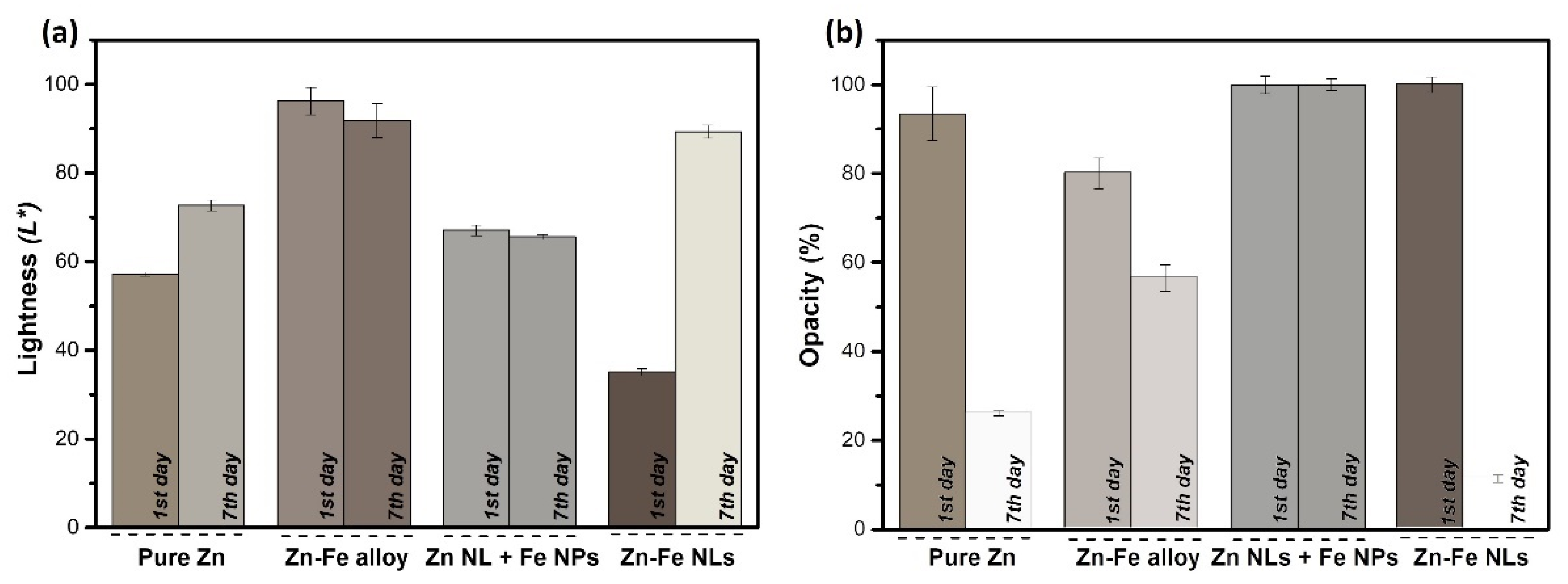
3.2.1. Chromatic Properties
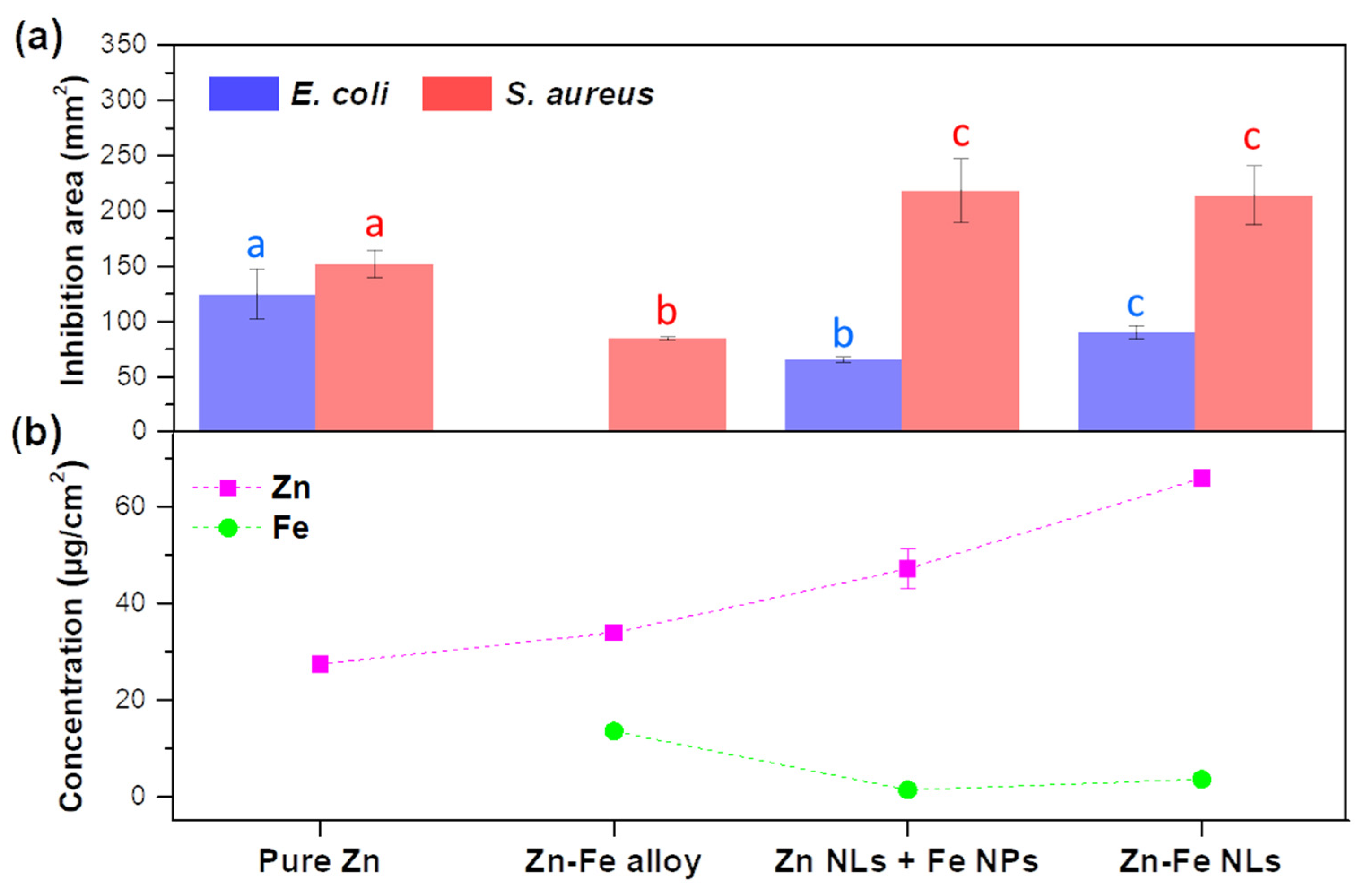
3.2.2. Antimicrobial Properties
- (i)
- Effect of Zn-Fe concentration
- (ii)
- Effect of Fe concentration
- (iii)
- Effect of Zn and Fe distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros, L.F.; Lamsaf, H.; Calderon, S.V.; Cerqueira, M.A.; Pastrana, L.; Teixeira, J.A. Active Packaging Systems Based on Metal and Metal Oxide Nanoparticles. In Nanotechnology-Enhanced Food Packaging, 1st ed.; Parameswaranpillai, J., Krishnankutty, R.E., Jayakumar, A., Rangappa, S.M., Siengchin, S., Eds.; Wiley-VCH GmbH: Hoboken, NJ, USA, 2022; pp. 143–181. [Google Scholar]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium oxide nanoparticles in biology and medicine. Antioxidants 2012, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaei-Ghomi, J.; Ghasemzadeh, M.A. Zinc oxide nanoparticles: A highly efficient and readily recyclable catalyst for the synthesis of xanthenes. Chin. Chem. Lett. 2012, 23, 1225–1229. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Nanoparticle Catalysed Redox Reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Technol. 2017, 52, 1862–1868. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; Garrigós, M.D.C.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Li, F.; Chen, G.; Fang, Y.; An, X.; Zheng, Y.; Xin, Z.; Zhang, M.; Yang, Y.; Hu, Q. Effect of nanocomposite-based packaging on preservation quality of green tea. Int. J. Food Sci. 2012, 47, 572–578. [Google Scholar] [CrossRef]
- Pietroiusti, L.; Magrini, A.; Campagnolo, A. New frontiers in nanotoxicology: Gut microbiota/microbiome-mediated effects of engineered nanomaterials. Toxicol. Appl. Pharmacol. 2016, 299, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.S.; Khalilzadeh, M.A.; Mohseni, M.; Hargalani, F.Z.; Getso, M.I.; Raissi, V.; Raiesi, O. Green synthesis of Ag nanoparticles from pomegranate seeds extract and synthesis of Ag-Starch nanocomposite and characterization of mechanical properties of the films. Biocatal. Agric. Biotechnol. 2020, 25, 101569. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific opinion on the safety assessment of the substance zinc oxide, nanoparticles, for use in food contact materials. EFSA J. 2017, 14, 4408. [Google Scholar]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Calderon, S.V.; Gomes, B.; Ferreira, P.J.; Carvalho, S. Zinc nanostructures for oxygen scavenging. Nanoscale 2017, 9, 5254–5262. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. Npj Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Foltynowicz, Z.; Bardenshtein, A.; Sängerlaub, S.; Antvorskov, H.; Kozak, W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag. Shelf Life 2017, 11, 74–83. [Google Scholar] [CrossRef]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Castro, A.; Carvalho, I.; Marques, L.; Ferreira, P.J.; Cavaleiro, A.; Carvalho, S.; Calderon, S.V. Galvanic Oxidation of Bimetallic Zn-Fe Nanoparticles for Oxygen Scavenging. Appl. Surf. Sci. 2020, 537, 147896. [Google Scholar] [CrossRef]
- Lamsaf, H.; Lenzi, V.; Marques, L.; Rebouta, L.; Carvalho, S.; Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Pastrana, L.; Calderon, S.V. Zn-Fe Flower-like nanoparticles growth by gas condensation. Mater. Lett. 2021, 297, 129916. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Cioffi, N.; Di Niso, F.; Sibillano, T.; Ancona, A.; Conte, A.; Del Nobile, M.A.; Sabbatini, L.; Torsi, L. Analytical characterization of laser-generated copper nanoparticles for antibacterial composite food packaging. Anal. Bioanal. Chem. 2012, 403, 1179–1186. [Google Scholar] [CrossRef]
- Aghababazadeh, R.; Mazinani, B.; Mirhabibi, A.; Tamizifar, M. ZnO nanoparticles synthesised by mechanochemical processing. In Journal of Physics: Conference Series; IOP Publishing: Leeds, UK, 2006; p. 312. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Šupová, M.; Martynková, G.S.; Barabaszová, K. Effect of Nanofillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Santagiuliana, G.; Picot, O.T.; Crespo, M.; Porwal, H.; Zhang, H.; Li, Y.; Rubini, L.; Colonna, S.; Fina, A.; Barbieri, E.; et al. Breaking the Nanoparticle Loading–Dispersion Dichotomy in Polymer Nanocomposites with the Art of Croissant-Making. ACS Nano 2018, 12, 9040–9050. [Google Scholar] [CrossRef]
- Zawadzka, K.; Kisielewska, A.; Piwonski, I.; Kadziola, K.; Felczak, A.; Rozalska, S.; Wronska, N.; Lisowska, K. Mechanisms of antibacterial activity and stability of silver nanoparticles grown on magnetron sputtered TiO2 coatings. Bull. Mater. Sci. 2016, 39, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and physicochemical properties of carboxymethylcellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- CLSI—Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; Approved Standard. Document M02-A12; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- El Mel, A.-A.; Buffiere, M.; Ewels, C.P.; Molina-Luna, L.; Faulques, E.; Colomer, J.-F.; Kleebe, H.-J.; Konstantinidis, S.; Snyders, R.; Bittencourt, C. Zn based nanoparticle–carbon nanotube hybrid materials: Interaction and charge transfer. Carbon 2014, 66, 442–449. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Dang, Z.; Zeng, S.; Li, C. Enhancement of heterogeneous photo-Fenton performance of core-shell structured boron-doped reduced graphene oxide wrapped magnetical Fe3O4 nanoparticles: Fe (II)/Fe (III) redox and mechanism. Appl. Surf. Sci. 2020, 544, 148886. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wang, H.; Xie, C. The effects of oxygen partial pressure on the microstructures and photocatalytic property of ZnO nanoparticles. Physica E Low Dimens. Syst. Nanostruct. 2008, 40, 2724–2729. [Google Scholar] [CrossRef]
- Mursal; Irhamni; Bukhari; Jalil, Z. Structural and Optical Properties of Zinc Oxide (ZnO) based Thin Films Deposited by Sol-Gel Spin Coating Method. J. Phys. Conf. Ser. 2018, 1116, 032020. [Google Scholar] [CrossRef]
- Yadav, A.P.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Effect of Fe–Zn alloy layer on the corrosion resistance of galvanized steel in chloride containing environments. Corros. Sci. 2007, 49, 3716–3731. [Google Scholar] [CrossRef]
- Appenzeller, B.M.R.; Yanez, C.; Jorand, F.; Block, J.-C. Advantage provided by iron for Escherichia coli growth and cultivability in drinking water. Appl. Environ. Microbiol. 2005, 71, 5621–5623. [Google Scholar] [CrossRef] [Green Version]
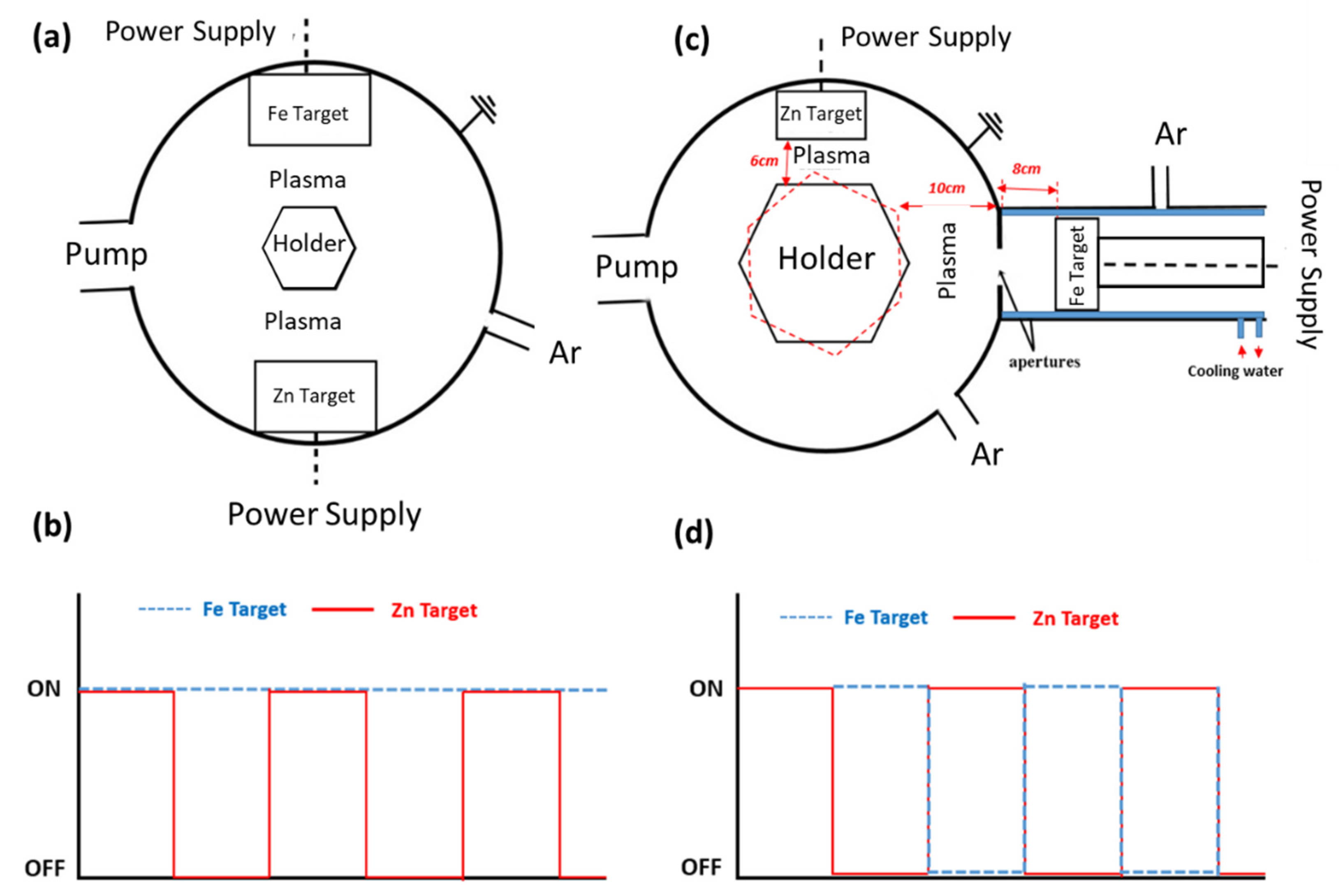
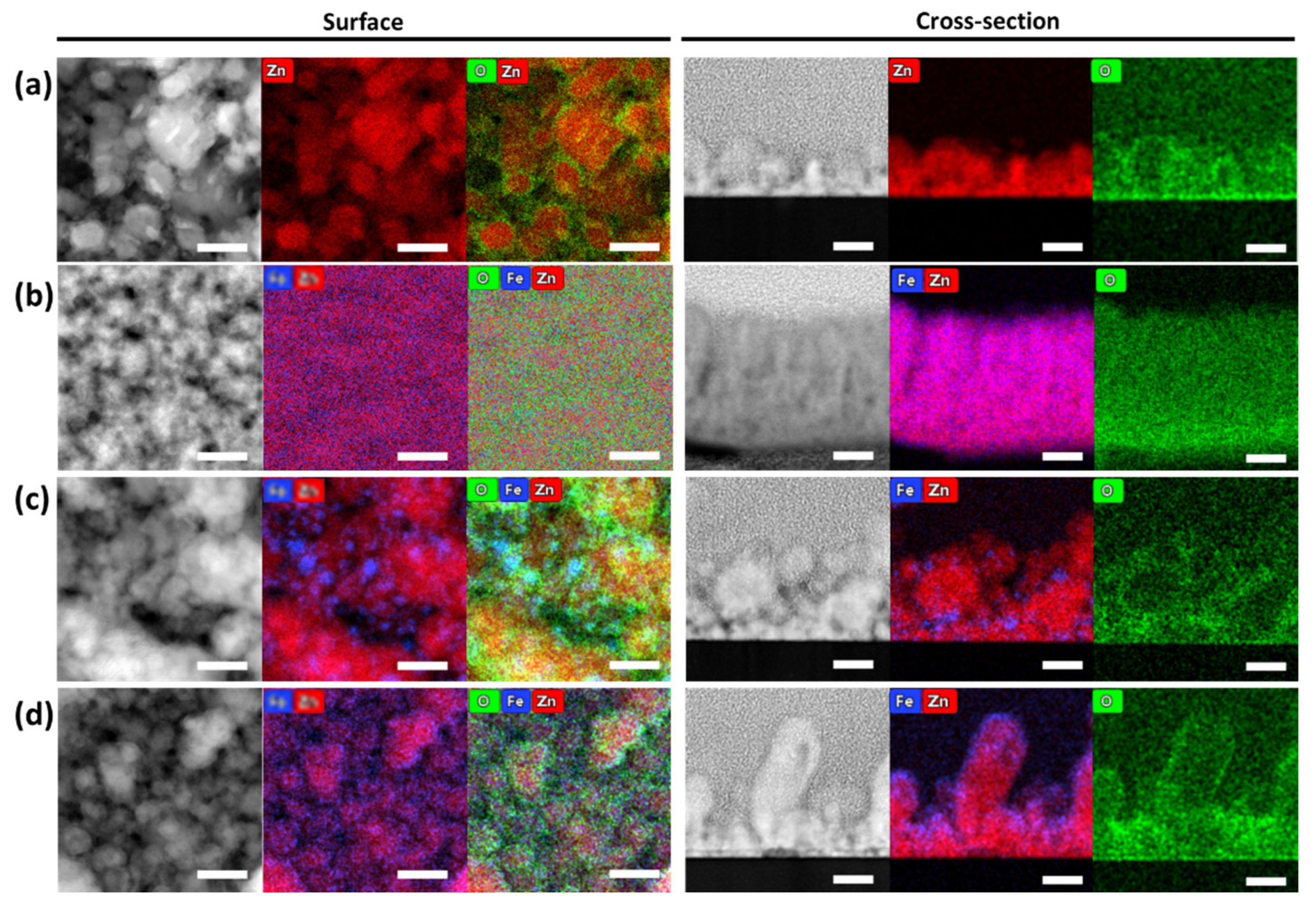

| Process | Coating | JZn (mA/cm2) | JFe (mA/cm2) | Zn + Fe Layer Time (min) | Fe Layer Time (min) | N. of Layers |
|---|---|---|---|---|---|---|
| Classical | Pure Zn | 0.5 | — | 14.5 * | 1 | |
| Classical | Zn-Fe alloy | 0.5 | 2.5 | 1 | 4.17 | 15 |
| Cluster | Zn NLs + Fe NPs | 1.9 | 3.2 | 0.5* | 10 | 24 |
| Classical | Zn-Fe NLs | 1.0 | 2.5 | 15 | 10 | 1 |
| Deposit Conditions | Characteristics of the Produced Coatings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coatings | Deposition time (min) | Current Density (J) (mA/cm2) | Deposition Rate (nm/s) | Thickness (nm) | Atomic Percent (at.%) | Metal Concentration by ICP-OES (µg/cm2) | ||||
| Jzn | JFe | Zn | Fe | O | Zn | Fe | ||||
| Pure Zn | Zn = 14.5 | 0.5 | — | 0.13 | 109 | 86 | — | 14 | 27.40 ± 0.43 | — |
| Zn-Fe alloy | Zn = 15 Fe = 62.5 | 0.5 | 2.5 | 0.30 | 175 | 46 | 24 | 30 | 33.90 ± 0.60 | 13.51 ± 0.97 |
| Zn NLs + Fe NPs | Zn = 12 Fe = 240 | 2.5 | 3.2 | 0.02 | 238 | 81 | 8 | 11 | 47.13 ± 4.21 | 1.40 ± 0.52 |
| Zn-Fe NLs | Zn = 15 Fe = 25 | 1 * | 2.5 * | 0.09 | 207 | 89 | 7 | 4 | 69.20 ± 1.34 | 3.77 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamsaf, H.; Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Pastrana, L.M.; Rebouta, L.; Carvalho, S.; Calderon, S. Zn and Zn-Fe Nanostructures with Multifunctional Properties as Components for Food Packaging Materials. Nanomaterials 2022, 12, 2104. https://doi.org/10.3390/nano12122104
Lamsaf H, Ballesteros LF, Cerqueira MA, Teixeira JA, Pastrana LM, Rebouta L, Carvalho S, Calderon S. Zn and Zn-Fe Nanostructures with Multifunctional Properties as Components for Food Packaging Materials. Nanomaterials. 2022; 12(12):2104. https://doi.org/10.3390/nano12122104
Chicago/Turabian StyleLamsaf, Hafsae, Lina F. Ballesteros, Miguel A. Cerqueira, José A. Teixeira, Lorenzo M. Pastrana, Luís Rebouta, Sandra Carvalho, and Sebastian Calderon. 2022. "Zn and Zn-Fe Nanostructures with Multifunctional Properties as Components for Food Packaging Materials" Nanomaterials 12, no. 12: 2104. https://doi.org/10.3390/nano12122104
APA StyleLamsaf, H., Ballesteros, L. F., Cerqueira, M. A., Teixeira, J. A., Pastrana, L. M., Rebouta, L., Carvalho, S., & Calderon, S. (2022). Zn and Zn-Fe Nanostructures with Multifunctional Properties as Components for Food Packaging Materials. Nanomaterials, 12(12), 2104. https://doi.org/10.3390/nano12122104