Construction of Double-Shelled Hollow Ag2S@Polydopamine Nanocomposites for Fluorescence-Guided, Dual Stimuli-Responsive Drug Delivery and Photothermal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
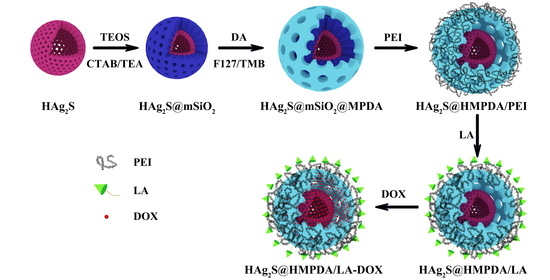
2.3. Preparation of Mesoporous Silica-Coated Hollow Ag2S NPs (HAg2S@mSiO2)
2.4. Preparation of MPDA-Coated HAg2S@mSiO2 Nanoparticles (HAg2S@mSiO2@MPDA)
2.5. Preparation of HAg2S@HMPDA/PEI Nanoparticles
2.6. Preparation of HAg2S@HMPDA/LA Nanoparticles
2.7. Loading of DOX on HAg2S@HMPDA/LA (HAg2S@HMPDA/LA-DOX)
2.8. Photothermal Performance of HAg2S@HMPDA/LA
2.9. In Vitro pH-Triggered Release of DOX from HAg2S@HMPDA/LA-DOX
2.10. In Vitro NIR-Triggered Release of DOX from HAg2S@HMPDA/LA-DOX
2.11. In Vitro Cytotoxicity Assay and Photothermal–Chemotherapy
2.12. In Vitro Targeting Ability of HAg2S@HMPDA/LA
2.13. In Vitro Imaging of HAg2S@HMPDA/LA
3. Results
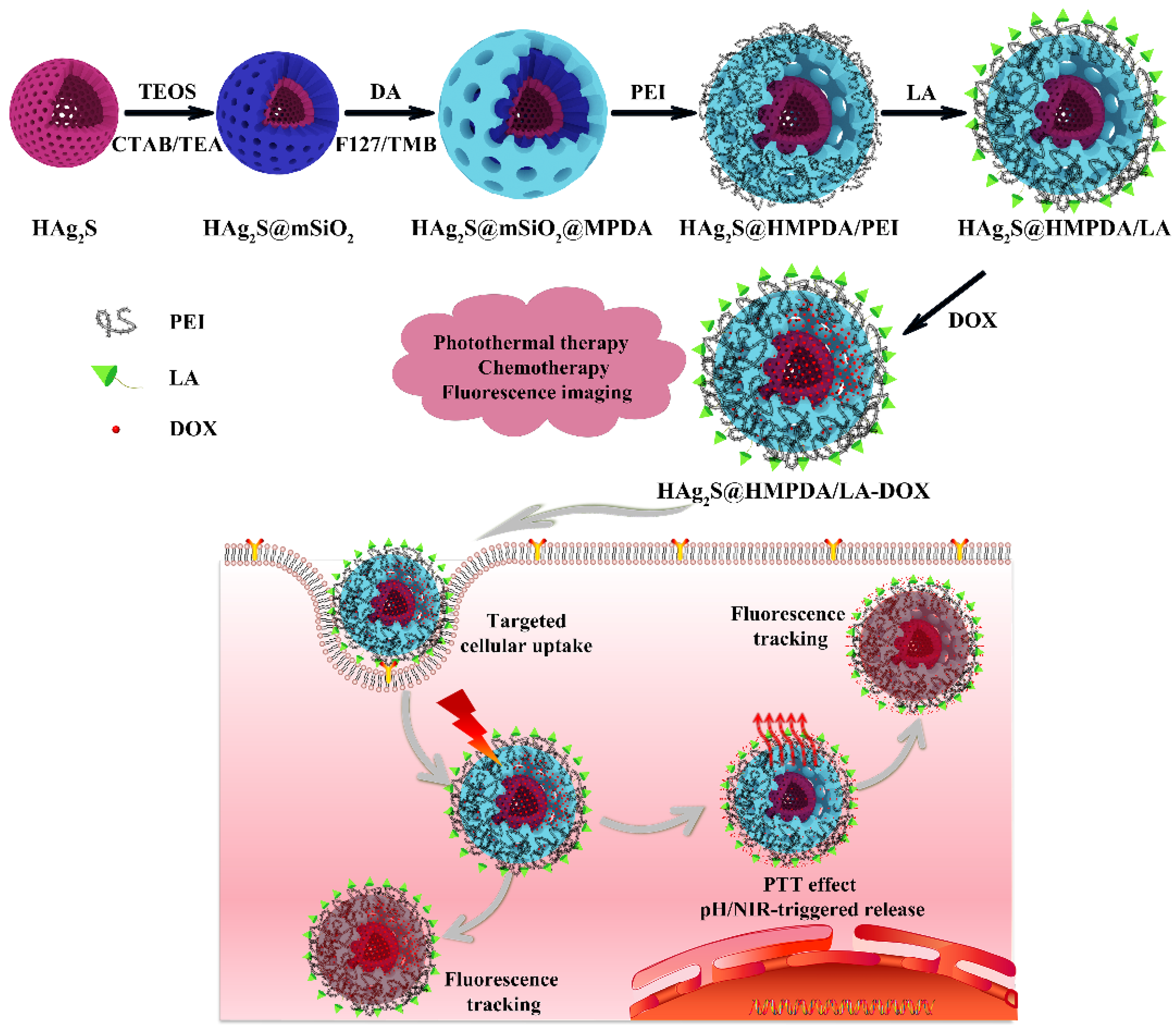
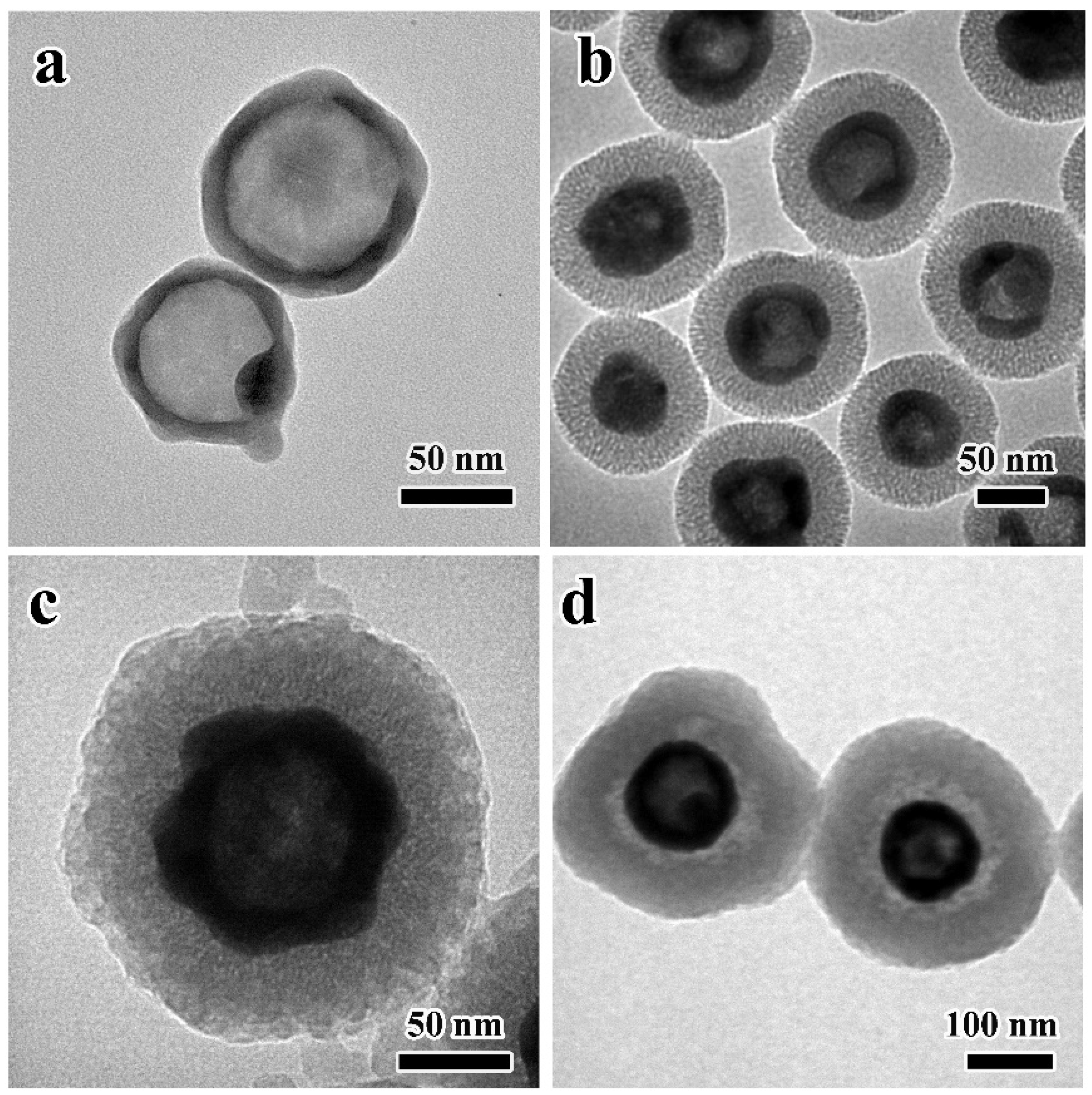
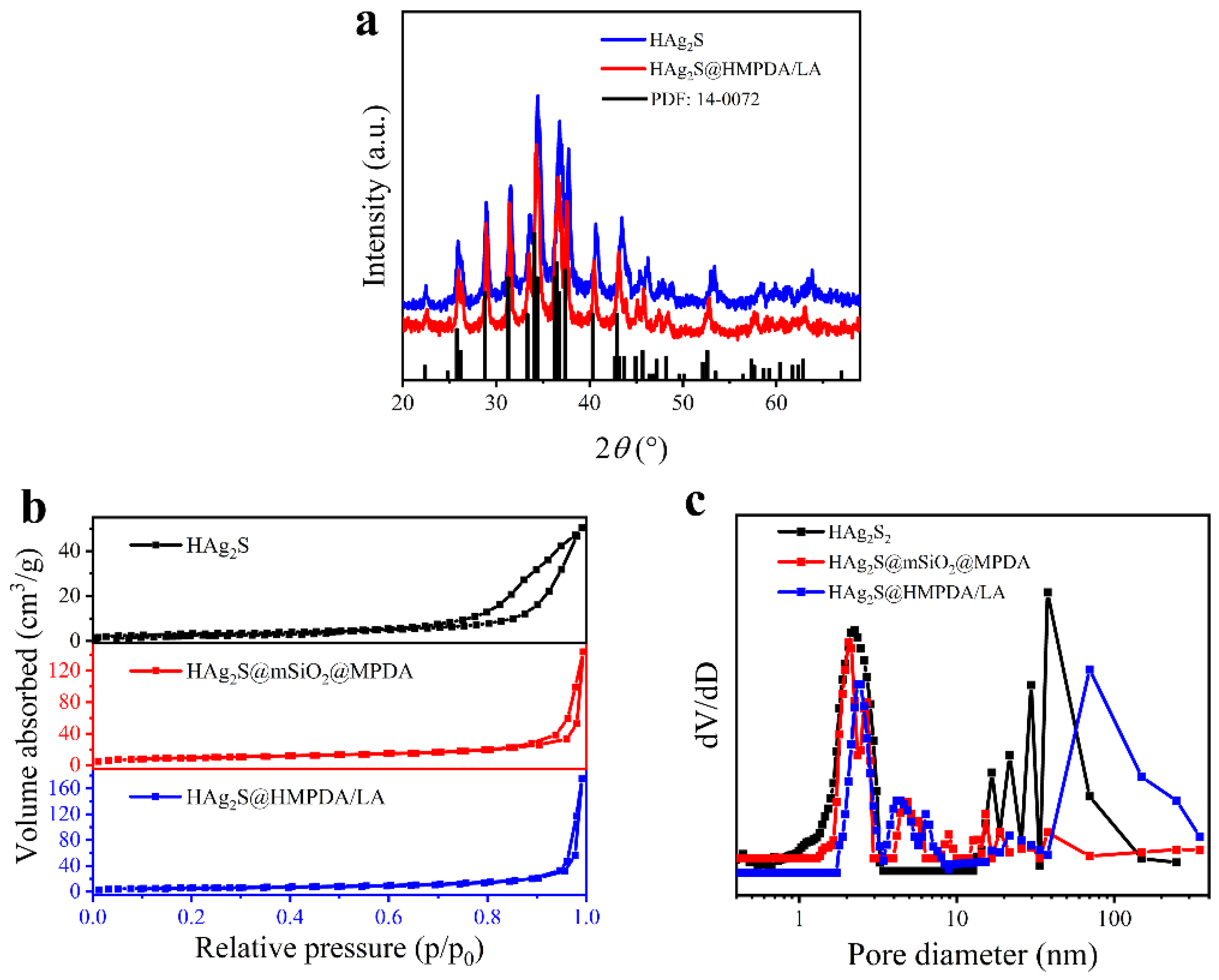
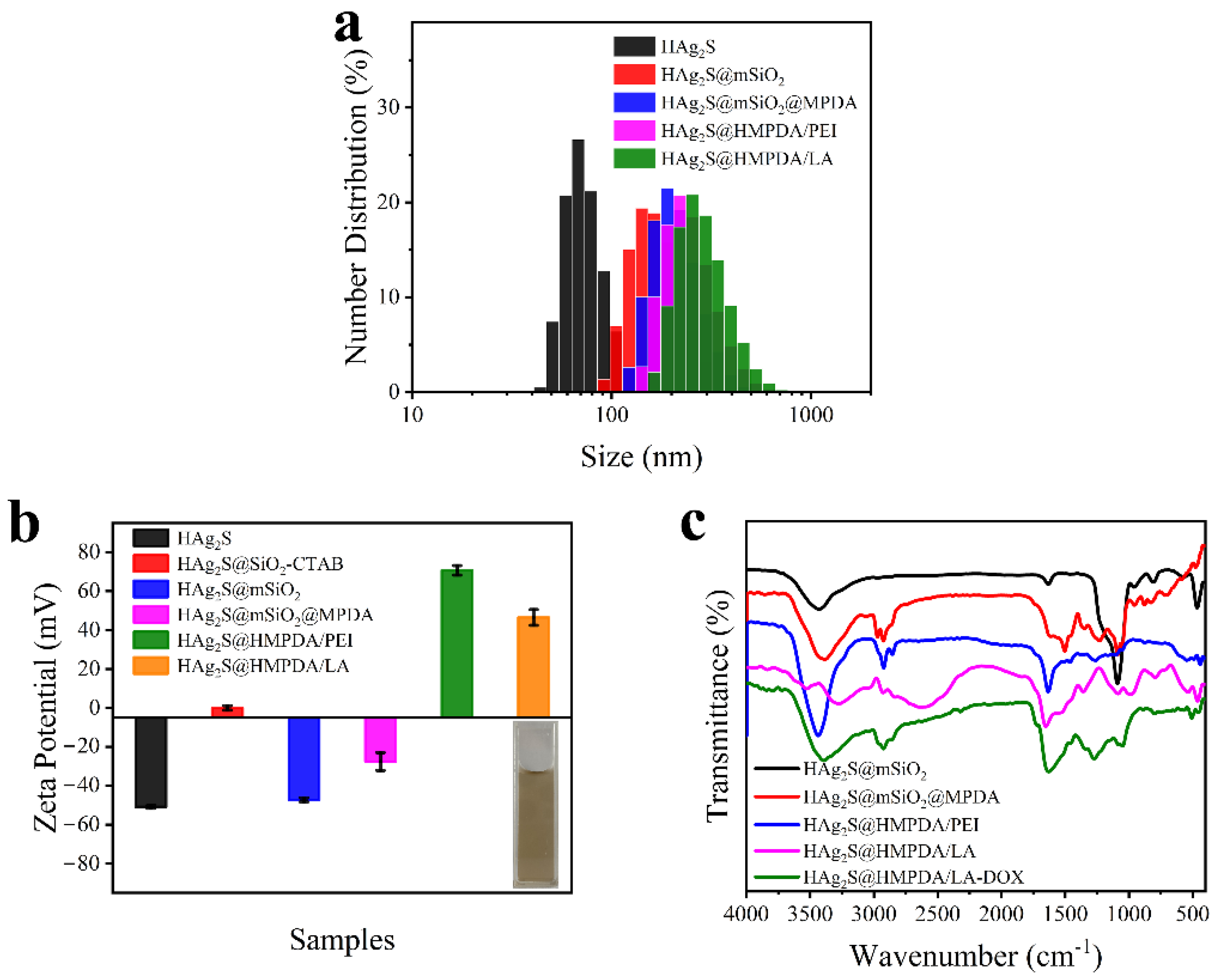
3.1. Characterization of HAg2S@HMPDA/LA
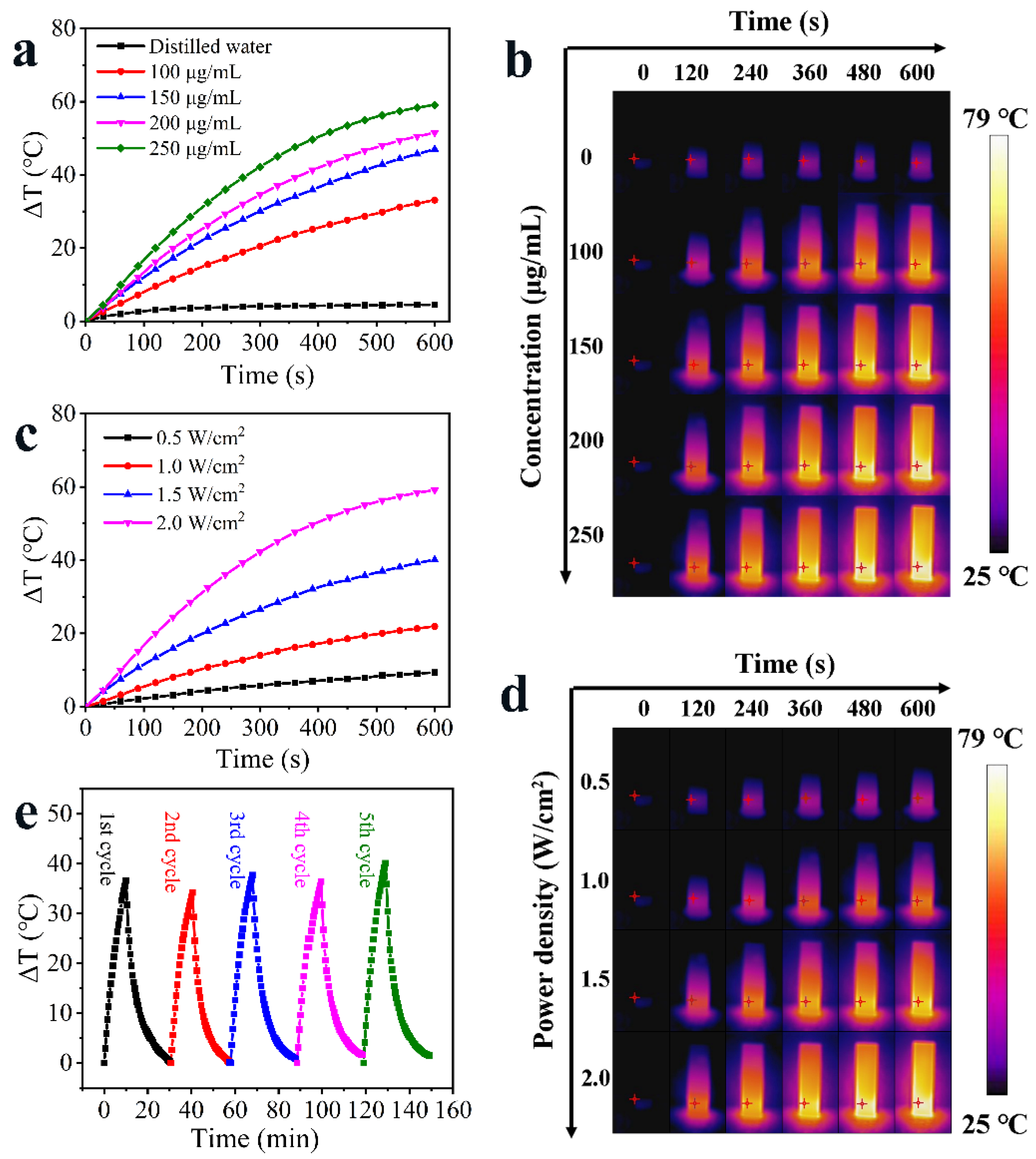
3.2. Photothermal Property of HAg2S@HMPDA/LA
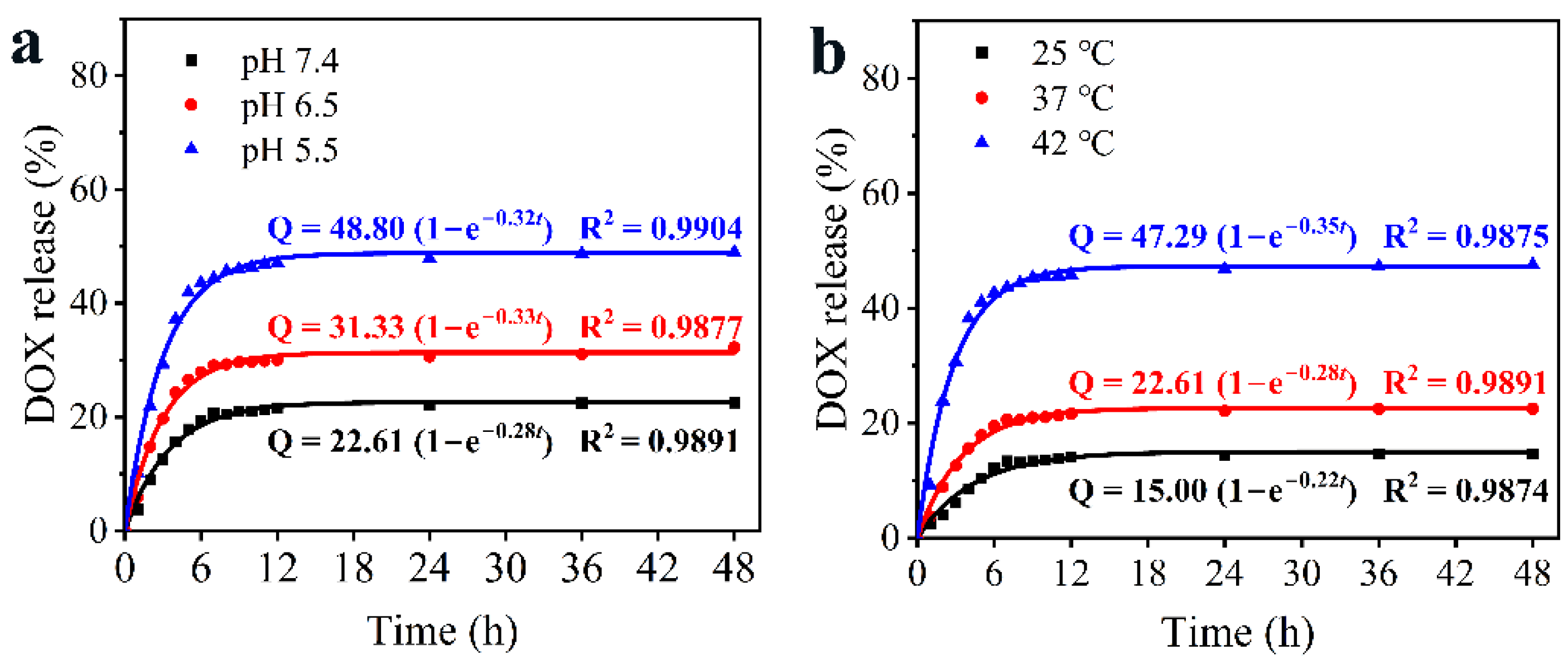
3.3. pH/NIR-Responsive Drug Release Activities
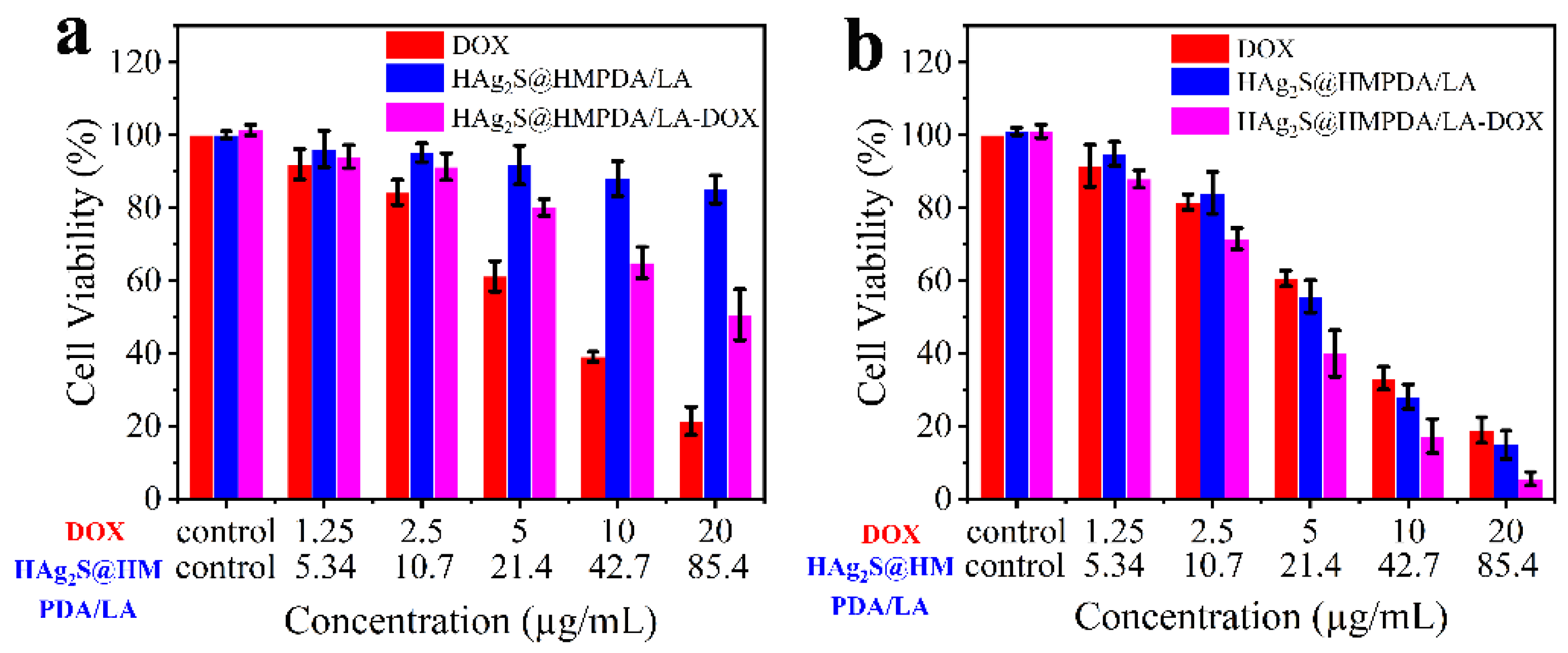
3.4. In Vitro Cytotoxicity Assay and Synergistic Photothermal–Chemotherapy
3.5. In Vitro Cancer Cell Targeting Effect
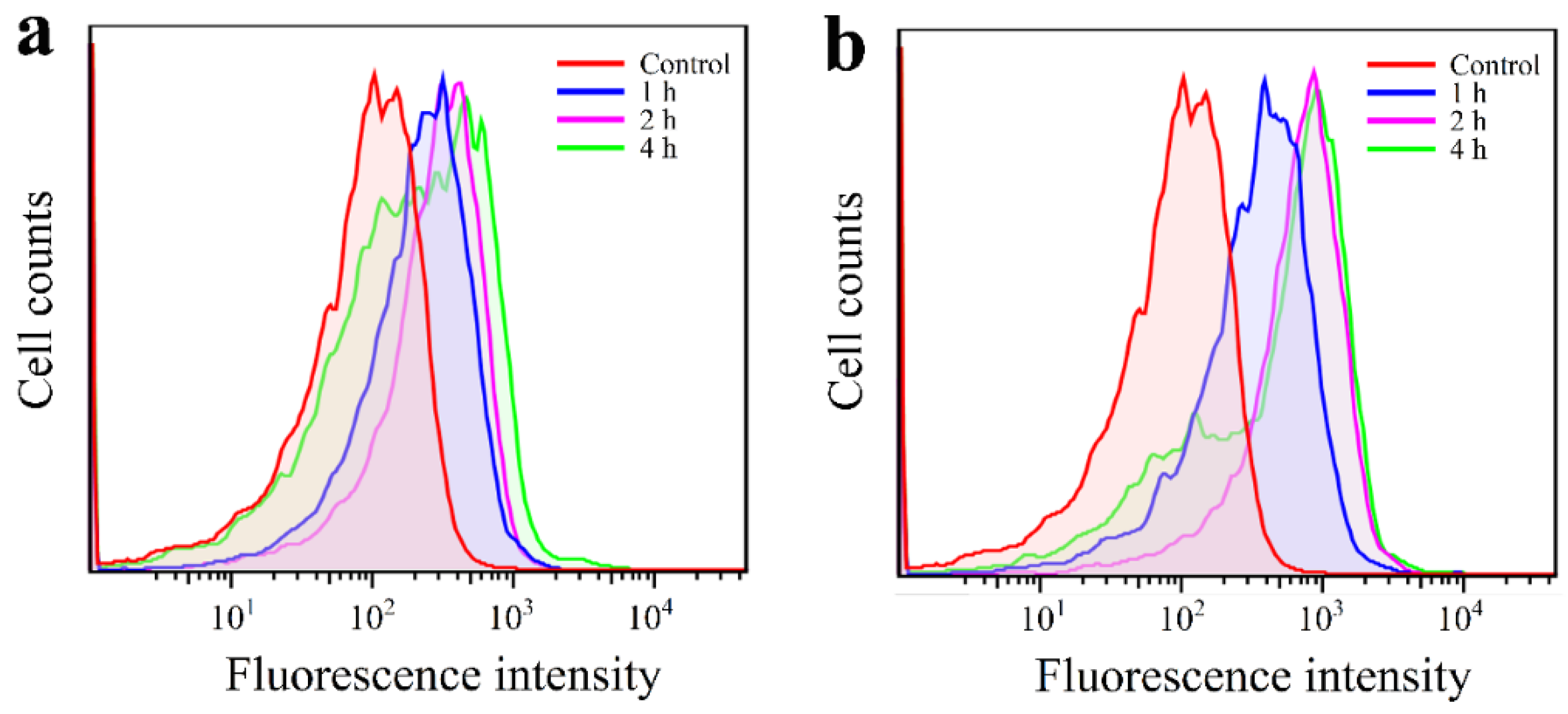
3.6. Cellular Uptake and Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cai, W. Nanomedicine for targeted photothermal cancer therapy: Where are we now? Nanomedicine 2015, 10, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Ren, Q.; Hao, R.; Sun, Z. Innovative strategies to boost photothermal therapy at mild temperature mediated by functional nanomaterials. Mater. Des. 2022, 214, 110391. [Google Scholar] [CrossRef]
- Jaque, D.; Martinez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Lu, C.; Chen, G.; Yu, B.; Cong, H. Recent advances of low biological toxicity Ag2S QDs for biomedical application. Adv. Eng. Mater. 2018, 20, 1700940. [Google Scholar] [CrossRef]
- Yang, T.; Tang, Y.; Liu, L.; Lv, X.; Wang, Q.; Ke, H.; Deng, Y.; Yang, H.; Yang, X.; Liu, G.; et al. Size-dependent Ag2S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy. ACS Nano 2017, 11, 1848–1857. [Google Scholar] [CrossRef]
- Han, R.; Xiao, Y.; Yang, Q.; Pan, M.; Hao, Y.; He, X.; Peng, J.; Qian, Z. Ag2S nanoparticle-mediated multiple ablations reinvigorates the immune response for enhanced cancer photo-immunotherapy. Biomaterials 2021, 264, 120451. [Google Scholar] [CrossRef]
- Gao, M.; Du, J.; Han, Z.; Wang, Z.; Zhao, Y.; Zou, X.; Sun, L. Precise preparation of various morphological silver sulfide nanostructures, investigation of formation mechanism, and comparative study of photothermal performance for cancer treatmen. Part. Part. Syst. Charact. 2021, 39, 1972–1982. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Jiang, C.; Zhu, Y.; Yang, X.; Hu, X.; Lin, Z.; Zhang, Y.; Peng, M.; Xia, H.; et al. Actively targeted deep tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@mesoporous silica core-shell nanoparticles. Adv. Funct. Mater. 2018, 28, 1704623. [Google Scholar] [CrossRef]
- Liu, Y.; Zhen, W.; Jin, L.; Zhang, S.; Sun, G.; Zhang, T.; Xu, X.; Song, S.; Wang, Y.; Liu, J.; et al. All-in-One Theranostic Nanoagent with Enhanced Reactive Oxygen Species Generation and Modulating Tumor Microenvironment Ability for Effective Tumor Eradication. ACS Nano 2018, 12, 4886–4893. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Su, H.; Sun, W.; Cai, J.; Liu, S.; Chai, Y.; Zhang, C. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics 2018, 8, 1966–1984. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yin, W.; Zhang, X.; Zhu, S.; He, X.; Yu, J.; Xie, J.; Guo, Z.; Yan, L.; Liu, X.; et al. Intelligent MoS2 nanotheranostic for targeted and enzyme-/pH-/NIR-responsive drug delivery to overcome cancer chemotherapy resistance guided by PET imaging. ACS Appl. Mater. Interfaces 2018, 10, 4271–4284. [Google Scholar] [CrossRef] [PubMed]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yu, C.; Wei, J.; Li, L.; Zhang, C.; Wu, Q.; Liu, J.; Yao, S.; Huang, W. Rational design of nanocarriers for intracellular protein delivery. Adv. Mater. 2019, 31, 1902791. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, S.; Dong, S.; Gai, S.; Zhang, F.; Dong, Y.; Yang, D.; He, F.; Zhong, L.; Yang, P. A smart nanoplatform for synergistic starvation, hypoxia-active prodrug treatment and photothermal therapy mediated by near-infrared-II light. Chem. Eng. J. 2021, 405, 127027. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.Q.; An, J.; Cheng, K.; Hou, X.L.; Zhang, X.S.; Song, X.L.; Huang, K.C.; Chen, W.; Liu, B.; et al. A near-infrared light-controlled smart nanocarrier with reversible polypeptide-engineered valve for targeted fluorescence-photoacoustic bimodal imaging-guided chemo-photothermal therapy. Theranostics 2019, 9, 7666–7679. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Mahapatra, C.; Parthiban, S.P.; Kim, T.H.; Kim, H.W. Combinatory cancer therapeutics with nanoceria-capped mesoporous silica nanocarriers through pH-triggered drug release and redox activity. ACS Appl. Mater. Interfaces 2019, 11, 288–299. [Google Scholar] [CrossRef]
- Li, Q.; Sun, L.; Hou, M.; Chen, Q.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Phase-change material packaged within hollow copper sulfide nanoparticles carrying doxorubicin and chlorin e6 for fluorescence-guided trimodal therapy of cancer. ACS Appl. Mater. Interfaces 2019, 11, 417–429. [Google Scholar] [CrossRef]
- Li, X.; Xie, C.; Xia, H.; Wang, Z. pH and ultrasound dual-responsive polydopamine-coated mesoporous silica nanoparticles for controlled drug delivery. Langmuir 2018, 34, 9974–9981. [Google Scholar] [CrossRef]
- Qiang, W.; Li, W.; Li, X.; Chen, X.; Xu, D. Bioinspired polydopamine nanospheres: A superquencher for fluorescence sensing of biomolecules. Chem. Sci. 2014, 5, 3018–3024. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Kwon, S.-H.; Lee, S.; Kim, G.C.; Han, A.M.; Kwon, Y.H.K.; Kim, B.-S. Enhanced neuronal differentiation of pheochromocytoma 12 cells on polydopamine-modified surface. Biochem. Biophys. Res. Commun. 2013, 430, 1294–1300. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.; Zhang, L.; Han, Q.; Liu, H.; Shen, J.; Li, G.; Zhang, L.; Yang, Y. Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem. Eng. J. 2020, 399, 125795. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Jang, M.; Lee, S.; Hardy, J.G.; Lee, J.Y. Electrically Conductive Polydopamine–Polypyrrole as High Performance Biomaterials for Cell Stimulation In Vitro and Electrical Signal Recording in Vivo. ACS Appl. Mater. Inter. 2018, 10, 33032–33042. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Shi, Y.; Xia, Y. Polydopamine nanobottles with photothermal capability for controlled release and related applications. Adv. Mater. 2021, 33, 2104729. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Prabhakar, N.; Xing, Y.; Rosenholm, J.M.; Zhang, J.; Cai, K. CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability for combined photothermal and siRNA therapy. Acta Biomater. 2019, 86, 416–428. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Zhang, J.; Zhang, P.; Zhou, X.; Yalamarty, S.S.K.; Liang, X.; Liu, Y.; Zheng, Q.; Gao, J. Self-assembled dual-targeted epirubicin-hybrid polydopamine nanoparticles for combined chemo-photothermal therapy of triple-negative breast cancer. Int. J. Nanomed. 2020, 15, 6791–6811. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, K.; Liu, J.; Ren, X.; Jiang, C.; Lu, L. Polydopamine-based coordination nanocomplex for T1/T2 dual mode magnetic resonance imaging-guided chemo-photothermal synergistic therapy. Biomaterials 2016, 77, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, N.; Zhang, T.; Yin, X.; Shen, J. Fabrication of doxorubicin-gated mesoporous polydopamine nanoplatforms for multimode imaging-guided synergistic chemophotothermal therapy of tumors. Drug Deliv. 2020, 27, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, J.; Chen, X.; Chen, Y.; Hou, S.; Qian, H.; Zhang, L.; Tang, G.; Chen, Z.; Ping, Y.; et al. Mesoporous polydopamine with built-in plasmonic core: Traceable and NIR triggered delivery of functional proteins. Biomaterials 2020, 238, 119847. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liang, C.; Xu, L.; Liu, G.; Gao, N.; Tao, W.; Luo, L.; Zuo, Y.; Wang, X.; Zhang, X.; et al. TPGS-functionalized polydopamine-modified mesoporous silica as drug nanocarriers for enhanced lung cancer chemotherapy against multidrug resistance. Small 2017, 13, 1700623. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, C.; Lin, A.; Pan, J.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J.; et al. Responsive functionalized MoSe2 nanosystem for highly efficient synergistic therapy of breast cancer. Colloids Surf. B Biointerfaces 2020, 189, 110820. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhang, X.S.; An, J.; Li, C.; Zhang, R.Y.; Ye, R.; Ye, B.J.; Liu, B.; Zhao, Y.D. Hitherto-unexplored photodynamic therapy of Ag2S and enhanced regulation based on polydopamine in vitro and vivo. Chemistry 2019, 25, 7553–7560. [Google Scholar] [CrossRef]
- Chu, C.; Bao, Z.; Sun, M.; Wang, X.; Zhang, H.; Chen, W.; Sui, Y.; Li, J.; Zhuang, Y.; Wang, D. NIR Stimulus-Responsive PdPt Bimetallic Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy. Pharmaceutics 2020, 12, 675. [Google Scholar] [CrossRef]
- Chen, F.; Xing, Y.; Wang, Z.; Zheng, X.; Zhang, J.; Cai, K. Nanoscale polydopamine (PDA) meets π–π interactions: An Interface-directed coassembly approach for mesoporous nanoparticles. Langmuir 2016, 32, 12119–12128. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Lou, X.W. Formation of asymmetric bowl-like mesoporous particles via emulsion-induced interface anisotropic assembly. J. Am. Chem. Soc. 2016, 138, 11306–11311. [Google Scholar] [CrossRef]
- Peng, L.; Hung, C.T.; Wang, S.; Zhang, X.; Zhu, X.; Zhao, Z.; Wang, C.; Tang, Y.; Li, W.; Zhao, D. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Zhang, T.; Yue, Q.; Elzatahry, A.A.; Alghamdi, A.; Cheng, X.; Deng, Y. Interface coassembly and polymerization on magnetic colloids: Toward core-shell functional mesoporous polymer microspheres and their carbon derivatives. Adv. Sci. 2020, 7, 2000443. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Chen, T.; Liu, H.; Su, S. Design of biocompatible Fe3O4@MPDA mesoporous core-shell nanospheres for drug delivery. Microporous Mesoporous Mater. 2020, 293, 109823. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Geng, H.; Wang, Y.; Gao, Y.; Huang, J.; Wang, Y.; Zhang, J.; Wang, S. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20290–20299. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [PubMed]
- Lin, Z.; Wei, J.; Geng, L.; Mei, D.; Liao, L. Adsorption of carbon dioxide by a novel amine impregnated ZSM-5/KIT-6 composite. RSC Adv. 2017, 7, 54422–54430. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Wang, D.; Yu, Q.-P.; Yu, Z.; Wang, H.; Wu, C.; Du, S.; Chen, X.; Li, J.; Zhou, Z.; et al. Near-infrared light-controllable multifunction mesoporous polydopamine nanocomposites for promoting infected wound healing. ACS Appl. Mater. Interfaces 2022, 14, 2534–2550. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Lei, Z.; Liu, L.; Zhang, J.; You, H.; Zhang, H.; Wang, Z. Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv. Healthc. Mater. 2015, 4, 559–568. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Han, Z.; Zhang, X.; Zou, X.; Peng, L.; Zhao, Y.; Sun, L. Construction of Double-Shelled Hollow Ag2S@Polydopamine Nanocomposites for Fluorescence-Guided, Dual Stimuli-Responsive Drug Delivery and Photothermal Therapy. Nanomaterials 2022, 12, 2068. https://doi.org/10.3390/nano12122068
Gao M, Han Z, Zhang X, Zou X, Peng L, Zhao Y, Sun L. Construction of Double-Shelled Hollow Ag2S@Polydopamine Nanocomposites for Fluorescence-Guided, Dual Stimuli-Responsive Drug Delivery and Photothermal Therapy. Nanomaterials. 2022; 12(12):2068. https://doi.org/10.3390/nano12122068
Chicago/Turabian StyleGao, Minjie, Zehua Han, Xu Zhang, Xueyan Zou, Lichao Peng, Yanbao Zhao, and Lei Sun. 2022. "Construction of Double-Shelled Hollow Ag2S@Polydopamine Nanocomposites for Fluorescence-Guided, Dual Stimuli-Responsive Drug Delivery and Photothermal Therapy" Nanomaterials 12, no. 12: 2068. https://doi.org/10.3390/nano12122068
APA StyleGao, M., Han, Z., Zhang, X., Zou, X., Peng, L., Zhao, Y., & Sun, L. (2022). Construction of Double-Shelled Hollow Ag2S@Polydopamine Nanocomposites for Fluorescence-Guided, Dual Stimuli-Responsive Drug Delivery and Photothermal Therapy. Nanomaterials, 12(12), 2068. https://doi.org/10.3390/nano12122068