In Vitro and In Vivo Cytotoxicity of Boron Nitride Nanotubes: A Systematic Review
Abstract
:1. Introduction
2. Methodology
2.1. Eligibility Criteria
2.2. Types of Interventions
2.3. Information Source
2.4. Data Collection
3. Results
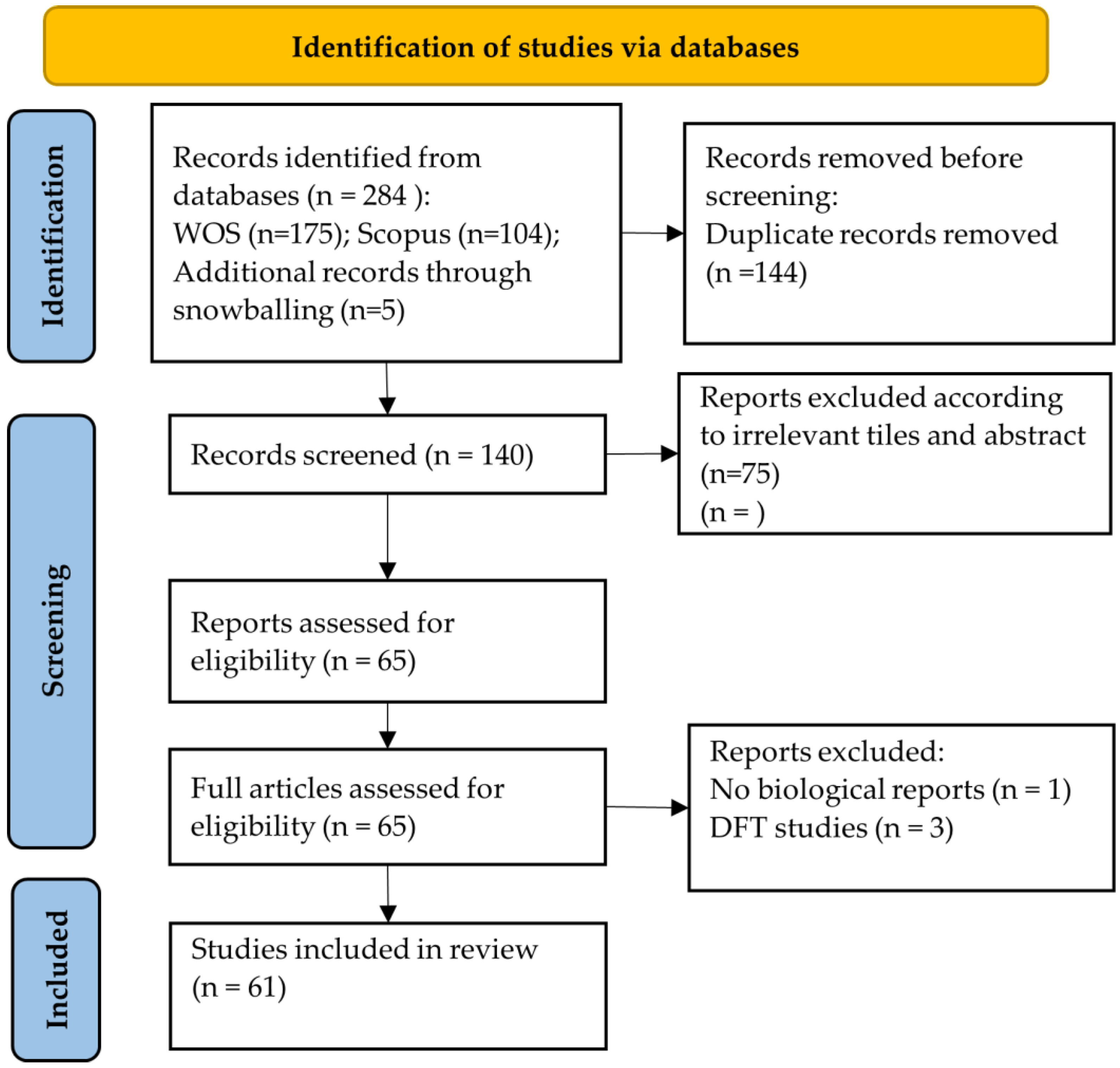
3.1. Study Selection
3.2. The Synthesis of BNNTs
3.2.1. Arc Discharge Method
3.2.2. Ball Milling Method
3.2.3. Laser Ablation
3.2.4. Thermal Plasma
3.2.5. Chemical Vapour Deposition (CVD) and Thermal Annealing
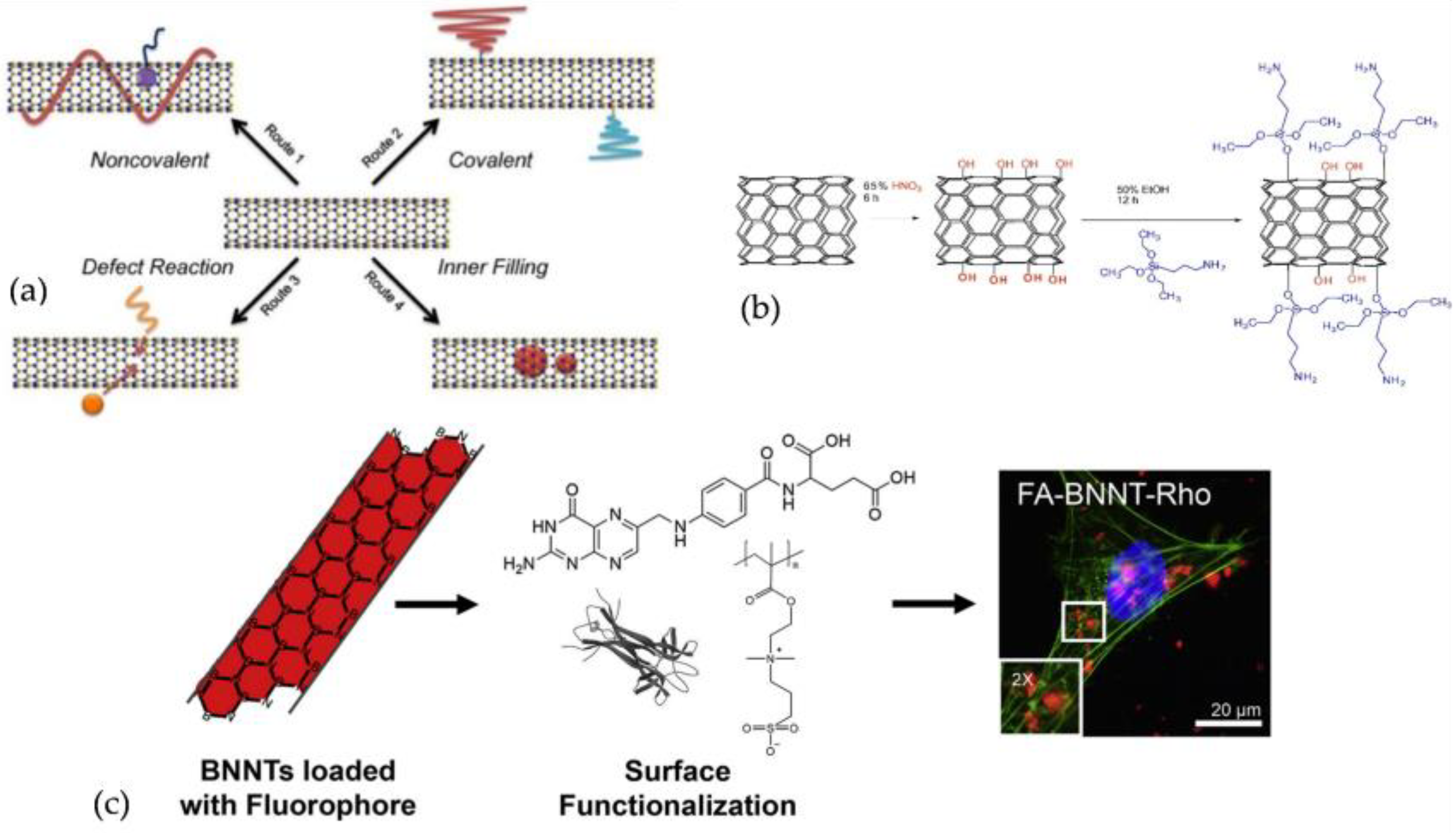
3.3. BNNTs Functionalisation, Modification, and Types of Composites
3.4. Cell Sources
3.5. Methods Evaluating In Vitro Biocompatibility of BNNTs
3.5.1. Cell Viability Assays
3.5.2. Total Reactive Oxygen Species
3.5.3. Genotoxicity Evaluation
3.6. BNNTs Biocompatibility In Vitro
3.6.1. Human Embryonic Kidney Cells
3.6.2. T98g and Fibroblast Cells
3.6.3. Human Osteoblasts Cells
3.6.4. Fibroblast Cells
3.6.5. HeLa Cells
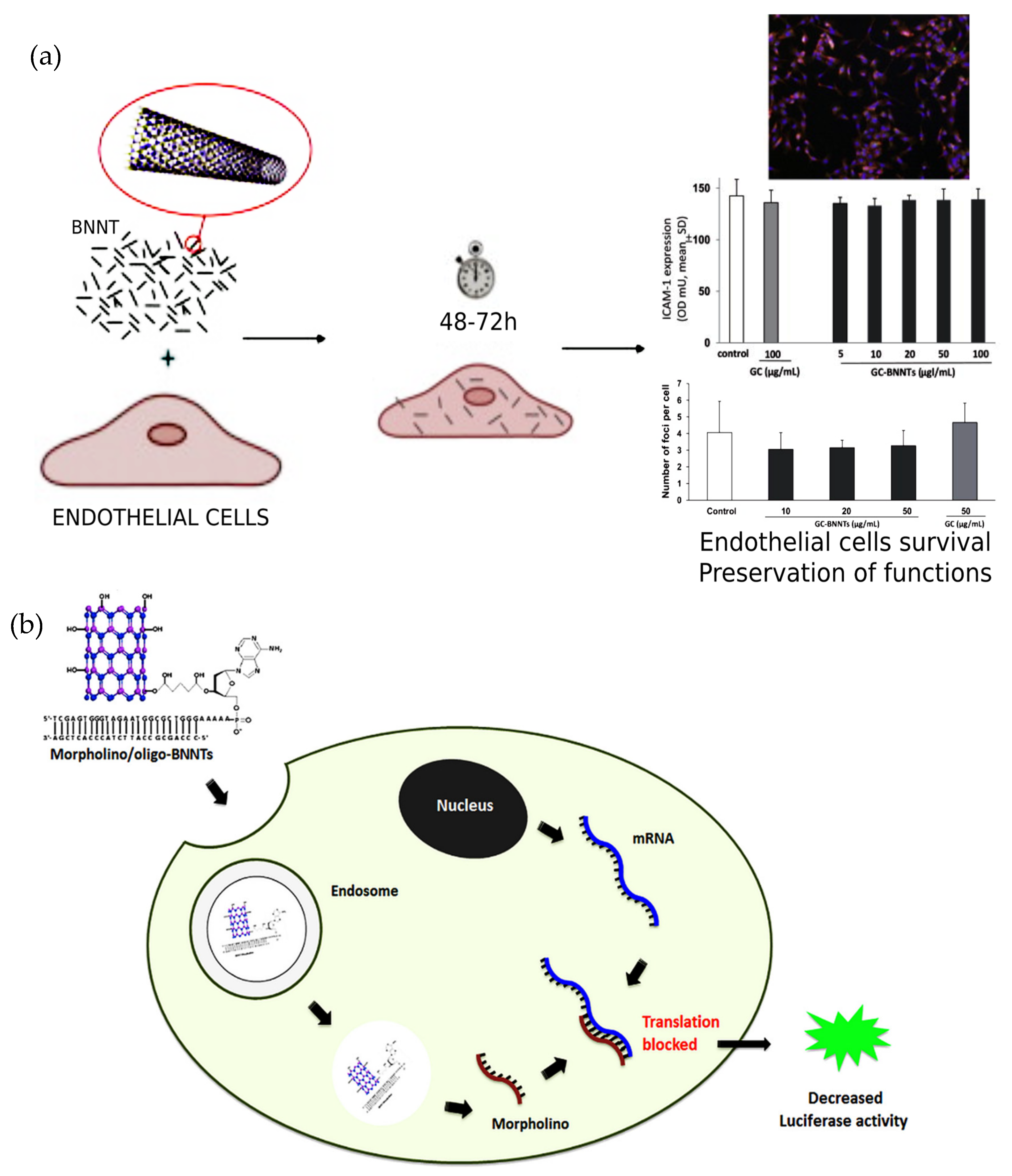
3.6.6. Human Umbilical Vein Endothelial Cells
3.6.7. Human Osteosarcoma Cells
3.6.8. Mesenchymal Stem Cells
3.6.9. MDA-MB-231-luc2 Cells
3.6.10. Glioblastoma Cells
3.6.11. Vero, Chang Liver, MCF7, and A549 Cells
3.6.12. Other Types of Cells
3.6.13. In Vitro Studies Stated BNNTs Are Cytotoxic
3.7. BNNTs Biocompatibility In Vivo
4. Biomedical and Tissue-Engineering Applications
4.1. Boron Neutron Capture Therapy (BNCT)
4.2. Nanovectors
4.3. Tissue Engineering
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubio, A.; Corkill, J.L.; Cohen, M.L. Theory of graphitic boron nitride nanotubes. Phys. Rev. B 1994, 49, 5081–5084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blase, X.; Rubio, A.; Louie, S.G.; Cohen, M.L. Stability and Band Gap Constancy of Boron Nitride Nanotubes. Europhys. Lett. 1994, 28, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron Nitride Nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron Nitride Nanotubes and Nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Xie, M.; Kayastha, V.; Wang, J.; Yap, Y.K. Patterned Growth of Boron Nitride Nanotubes by Catalytic Chemical Vapor Deposition. Chem. Mater. 2010, 22, 1782–1787. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Liu, Y.; Fu, L.; Huang, C.; Llewellyn, D. Over 1.0 mm-long boron nitride nanotubes. Chem. Phys. Lett. 2008, 463, 130–133. [Google Scholar] [CrossRef]
- Golberg, D.; Han, W.; Bando, Y.; Bourgeois, L.; Kurashima, K.; Sato, T. Fine structure of boron nitride nanotubes produced from carbon nanotubes by a substitution reaction. J. Appl. Phys. 1999, 86, 2364–2366. [Google Scholar] [CrossRef]
- Bi, X.; Yin, Y.; Li, J.; Chen, Y.; Li, J.; Su, Q. A co-precipitation and annealing route to the large-quantity synthesis of boron nitride nanotubes. Solid State Sci. 2013, 25, 39–44. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Boron nitride nanotubes. Mater. Sci. Eng. R Rep. 2010, 70, 92–111. [Google Scholar] [CrossRef]
- Köken, D.; Sungur, P.; Cebeci, H.; Cebeci, F.Ç. Revealing the Effect of Sulfur Compounds for Low-Temperature Synthesis of Boron Nitride Nanotubes from Boron Minerals. ACS Appl. Nano Mater. 2022, 5, 2137–2146. [Google Scholar] [CrossRef]
- TARHAN, T. Synthesis and characterization of boron nitride nanotubes (BNNTs) with a new method and precursor materials. Gümüşhane Üniversitesi Fen Bilim. Enstitüsü Derg. 2021, 11, 1217–1224. [Google Scholar] [CrossRef]
- Wang, J.; Lee, C.H.; Yap, Y.K. Recent advancements in boron nitride nanotubes. Nanoscale 2010, 2, 2028. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.G.; Zettl, A. Measurement of the elastic modulus of a multi-wall boron nitride nanotube. Solid State Commun. 1998, 105, 297–300. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Kurashima, K.; Sato, T. Synthesis and characterization of ropes made of {BN} multiwalled nanotubes. Scr. Mater. 2001, 44, 1561–1565. [Google Scholar] [CrossRef]
- Chang, C.W.; Fennimore, A.M.; Afanasiev, A.; Okawa, D.; Ikuno, T.; Garcia, H.; Li, D.; Majumdar, A.; Zettl, A. Isotope Effect on the Thermal Conductivity of Boron Nitride Nanotubes. Phys. Rev. Lett. 2006, 97, 085901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golberg, D.; Bando, Y.; Tang, C.C.; Zhi, C.Y. Boron Nitride Nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Bai, X.; Golberg, D.; Bando, Y.; Zhi, C.; Tang, C.; Mitome, M.; Kurashima, K. Deformation-Driven Electrical Transport of Individual Boron Nitride Nanotubes. Nano Lett. 2007, 7, 632–637. [Google Scholar] [CrossRef]
- Lee, C.; Bhandari, S.; Tiwari, B.; Yapici, N.; Zhang, D.; Yap, Y. Boron Nitride Nanotubes: Recent Advances in Their Synthesis, Functionalization, and Applications. Molecules 2016, 21, 922. [Google Scholar] [CrossRef]
- Ghazizadeh, M.; Estevez, J.E.; Kelkar, A.D. Boron Nitride Nanotubes for Space Radiation Shielding. Int. J. Nano Stud. Technol. 2015, 4, 1–2. [Google Scholar] [CrossRef]
- Piazza, V.; Gemmi, M. Optical properties of boron nitride nanotubes: Potential exploitation in nanomedicine. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 139–147. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, M.J.; Ahn, S.; Koh, B. Purification of Boron Nitride Nanotubes Enhances Biological Application Properties. Int. J. Mol. Sci. 2020, 21, 1529. [Google Scholar] [CrossRef] [Green Version]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Boron nitride nanotubes: An innovative tool for nanomedicine. Nano Today 2009, 4, 8–10. [Google Scholar] [CrossRef]
- Lee, C.H.; Zhang, D.; Yap, Y.K. Functionalization, Dispersion, and Cutting of Boron Nitride Nanotubes in Water. J. Phys. Chem. C 2012, 116, 1798–1804. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Turek, I.; Kong, C.; Irving, H. Printable gelatin, alginate and boron nitride nanotubes hydrogel-based ink for 3D bioprinting and tissue engineering applications. Mater. Des. 2022, 213, 110362. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Kong, C.; Irving, H. Extrusion-Based Bioprinted Boron Nitride Nanotubes Reinforced Alginate Scaffolds: Mechanical, Printability and Cell Viability Evaluation. Polymers 2022, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Evariste, L.; Flahaut, E.; Baratange, C.; Barret, M.; Mouchet, F.; Pinelli, E.; Galibert, A.M.; Soula, B.; Gauthier, L. Ecotoxicological assessment of commercial boron nitride nanotubes toward Xenopus laevis tadpoles and host-associated gut microbiota. Nanotoxicology 2021, 15, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xie, X.; Wu, T.; Yang, H.; Peng, Y.; Luo, L.; Chen, Y. Targeted delivery of Auristatin PE to Hep G2 cells using folate-conjugated boron nitride nanotubes. Mater. Sci. Eng. C 2020, 109, 110509. [Google Scholar] [CrossRef]
- Li, W.; Xie, X.; Wu, T.; Lin, H.; Luo, L.; Yang, H.; Li, J.; Xin, Y.; Lin, X.; Chen, Y. Loading Auristatin PE onto boron nitride nanotubes and their effects on the apoptosis of Hep G2 cells. Colloids Surfaces B Biointerfaces 2019, 181, 305–314. [Google Scholar] [CrossRef]
- Xin, X.; Barger, M.; Roach, K.A.; Bowers, L.; Stefaniak, A.B.; Kodali, V.; Glassford, E.; Dunn, K.H.L.; Dunn, K.H.L.; Wolfarth, M.; et al. Toxicity evaluation following pulmonary exposure to an as-manufactured dispersed boron nitride nanotube (BNNT) material in vivo. NanoImpact 2020, 19, 100235. [Google Scholar] [CrossRef]
- De Pasquale, D.; Marino, A.; Tapeinos, C.; Pucci, C.; Rocchiccioli, S.; Michelucci, E.; Finamore, F.; McDonnell, L.; Scarpellini, A.; Lauciello, S.; et al. Homotypic targeting and drug delivery in glioblastoma cells through cell membrane-coated boron nitride nanotubes. Mater. Des. 2020, 192, 108742. [Google Scholar] [CrossRef]
- da Silva, W.M.; de Andrade Alves e Silva, R.H.; Cipreste, M.F.; Andrade, G.F.; Gastelois, P.L.; de Almeida Macedo, W.A.; de Sousa, E.M.B. Boron nitride nanotubes radiolabeled with 153Sm and 159Gd: Potential application in nanomedicine. Appl. Radiat. Isot. 2020, 157, 109032. [Google Scholar] [CrossRef]
- Ferreira, T.H.; de Oliveira Freitas, L.B.; Fernandes, R.S.; dos Santos, V.M.; Resende, J.M.; Cardoso, V.N.; de Barros, A.L.B.; de Sousa, E.M.B. Boron nitride nanotube-CREKA peptide as an effective target system to metastatic breast cancer. J. Pharm. Investig. 2020, 50, 469–480. [Google Scholar] [CrossRef]
- Ferreira, C.; Leitune, V.; Balbinot, G.; Degrazia, F.; Arakelyan, M.; Sauro, S.; Mezzomo Collares, F. Antibacterial and Remineralizing Fillers in Experimental Orthodontic Adhesives. Materials 2019, 12, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohns, F.R.; Degrazia, F.W.; de Souza Balbinot, G.; Leitune, V.C.B.; Samuel, S.M.W.; García-Esparza, M.A.; Sauro, S.; Collares, F.M. Boron Nitride Nanotubes as Filler for Resin-Based Dental Sealants. Sci. Rep. 2019, 9, 7710. [Google Scholar] [CrossRef] [PubMed]
- Çal, T.; Bucurgat, Ü.Ü. In vitro investigation of the effects of boron nitride nanotubes and curcumin on DNA damage. DARU J. Pharm. Sci. 2019, 27, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Fujie, T.; Vazão, H.; Ciofani, G.; Marotta, R.; Brescia, R.; Filippeschi, C.; Corradini, I.; Matteoli, M.; Mattoli, V.; et al. Boron Nitride Nanotube-Mediated Stimulation of Cell Co-Culture on Micro-Engineered Hydrogels. PLoS ONE 2013, 8, e71707. [Google Scholar] [CrossRef]
- Augustine, J.; Cheung, T.; Gies, V.; Boughton, J.; Chen, M.; Jakubek, Z.J.; Walker, S.; Martinez-Rubi, Y.; Simard, B.; Zou, S. Assessing size-dependent cytotoxicity of boron nitride nanotubes using a novel cardiomyocyte AFM assay. Nanoscale Adv. 2019, 1, 1914–1923. [Google Scholar] [CrossRef] [Green Version]
- Poudel, A.; Fernandez, M.A.; Tofail, S.A.M.; Biggs, M.J.P. Boron Nitride Nanotube Addition Enhances the Crystallinity and Cytocompatibility of PVDF-TrFE. Front. Chem. 2019, 7, 364. [Google Scholar] [CrossRef]
- Genchi, G.G.; Sinibaldi, E.; Ceseracciu, L.; Labardi, M.; Marino, A.; Marras, S.; De Simoni, G.; Mattoli, V.; Ciofani, G. Ultrasound-activated piezoelectric P(VDF-TrFE)/boron nitride nanotube composite films promote differentiation of human SaOS-2 osteoblast-like cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2421–2432. [Google Scholar] [CrossRef]
- Demir, E.; Marcos, R. Antigenotoxic potential of boron nitride nanotubes. Nanotoxicology 2018, 12, 868–884. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Long-term stability of dental adhesive incorporated by boron nitride nanotubes. Dent. Mater. 2018, 34, 427–433. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Faria, J.A.Q.A.; Gonzalez, I.J.; Outon, L.E.F.; Macedo, W.A.A.; Gomes, D.A.; Sousa, E.M.B. BNNT/Fe3O4 System as an Efficient Tool for Magnetohyperthermia Therapy. J. Nanosci. Nanotechnol. 2018, 18, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Miranda, M.; Rocha, Z.; Leal, A.; Gomes, D.; Sousa, E. An Assessment of the Potential Use of BNNTs for Boron Neutron Capture Therapy. Nanomaterials 2017, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponraj, S.B.; Chen, Z.; Li, L.H.; Shankaranarayanan, J.S.; Rajmohan, G.D.; du Plessis, J.; Sinclair, A.J.; Chen, Y.; Wang, X.; Kanwar, J.R.; et al. Fabrication of Boron Nitride Nanotube–Gold Nanoparticle Hybrids Using Pulsed Plasma in Liquid. Langmuir 2014, 30, 10712–10720. [Google Scholar] [CrossRef]
- Kodali, V.K.; Roberts, J.R.; Shoeb, M.; Wolfarth, M.G.; Bishop, L.; Eye, T.; Barger, M.; Roach, K.A.; Friend, S.; Schwegler-Berry, D.; et al. Acute in vitro and in vivo toxicity of a commercial grade boron nitride nanotube mixture. Nanotoxicology 2017, 11, 1040–1058. [Google Scholar] [CrossRef] [PubMed]
- Şen, Ö.; Çobandede, Z.; Emanet, M.; Bayrak, Ö.F.; Çulha, M. Boron nitride nanotubes for gene silencing. Biochim. Biophys. Acta -Gen. Subj. 2017, 1861, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Farshid, B.; Lalwani, G.; Shir Mohammadi, M.; Simonsen, J.; Sitharaman, B. Boron nitride nanotubes and nanoplatelets as reinforcing agents of polymeric matrices for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Emanet, M.; Kazanç, E.; Çobandede, Z.; Çulha, M. Boron nitride nanotubes enhance properties of chitosan-based scaffolds. Carbohydr. Polym. 2016, 151, 313–320. [Google Scholar] [CrossRef]
- Rocca, A.; Marino, A.; Del Turco, S.; Cappello, V.; Parlanti, P.; Pellegrino, M.; Golberg, D.; Mattoli, V.; Ciofani, G. Pectin-coated boron nitride nanotubes: In vitro cyto-/immune-compatibility on RAW 264.7 macrophages. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 775–784. [Google Scholar] [CrossRef]
- Niskanen, J.; Zhang, I.; Xue, Y.; Golberg, D.; Maysinger, D.; Winnik, F.M. Boron nitride nanotubes as vehicles for intracellular delivery of fluorescent drugs and probes. Nanomedicine 2016, 11, 447–463. [Google Scholar] [CrossRef]
- Şen, Ö.; Culha, M. Boron nitride nanotubes included thermally cross-linked gelatin–glucose scaffolds show improved properties. Colloids Surfaces B Biointerfaces 2016, 138, 41–49. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Jiang, X.; Yamaguchi, M.; Ito, A.; Bando, Y.; Golberg, D. Boron nitride nanotube-enhanced osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. PEGylated boron nitride nanotube-reinforced poly(propylene fumarate) nanocomposite biomaterials. RSC Adv. 2016, 6, 79507–79519. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Larrañaga, A.; Gladkovskaya, O.; Stanley, A.; Tadayyon, G.; Guo, Y.; Sarasua, J.-R.; Tofail, S.A.M.; Zeugolis, D.I.; Pandit, A.; et al. Effects of Polydopamine Functionalization on Boron Nitride Nanotube Dispersion and Cytocompatibility. Bioconjug. Chem. 2015, 26, 2025–2037. [Google Scholar] [CrossRef]
- Emanet, M.; Şen, Ö.; Çobandede, Z.; Çulha, M. Interaction of carbohydrate modified boron nitride nanotubes with living cells. Colloids Surfaces B Biointerfaces 2015, 134, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; Ciofani, G.; Pertici, G.; Moscato, S.; D’Alessandro, D.; Ciabatti, E.; Chiellini, F.; D’Acunto, M.; Mattoli, V.; Berrettini, S. Boron nitride nanotube-functionalised myoblast/microfibre constructs: A nanotech-assisted tissue-engineered platform for muscle stimulation. J. Tissue Eng. Regen. Med. 2015, 9, 847–851. [Google Scholar] [CrossRef]
- Salvetti, A.; Rossi, L.; Iacopetti, P.; Li, X.; Nitti, S.; Pellegrino, T.; Mattoli, V.; Golberg, D.; Ciofani, G. In vivo biocompatibility of boron nitride nanotubes: Effects on stem cell biology and tissue regeneration in planarians. Nanomedicine 2015, 10, 1911–1922. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, T.H.; Marino, A.; Rocca, A.; Liakos, I.; Nitti, S.; Athanassiou, A.; Mattoli, V.; Mazzolai, B.; de Sousa, E.M.B.; Ciofani, G. Folate-grafted boron nitride nanotubes: Possible exploitation in cancer therapy. Int. J. Pharm. 2015, 481, 56–63. [Google Scholar] [CrossRef]
- Nakamura, H.; Koganei, H.; Miyoshi, T.; Sakurai, Y.; Ono, K.; Suzuki, M. Antitumor effect of boron nitride nanotubes in combination with thermal neutron irradiation on BNCT. Bioorg. Med. Chem. Lett. 2015, 25, 172–174. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Rocca, A.; Marino, A.; Mattoli, V.; de Sousa, E.M.B.; Ciofani, G. Evaluation of the effects of boron nitride nanotubes functionalized with gum arabic on the differentiation of rat mesenchymal stem cells. RSC Adv. 2015, 5, 45431–45438. [Google Scholar] [CrossRef]
- Li, X.; Hanagata, N.; Wang, X.; Yamaguchi, M.; Yi, W.; Bando, Y.; Golberg, D. Multimodal luminescent-magnetic boron nitride nanotubes@NaGdF 4: Eu structures for cancer therapy. Chem. Commun. 2014, 50, 4371–4374. [Google Scholar] [CrossRef]
- Barachini, S.; Danti, S.; Pacini, S.; D’Alessandro, D.; Carnicelli, V.; Trombi, L.; Moscato, S.; Mannari, C.; Cei, S.; Petrini, M. Plasticity of human dental pulp stromal cells with bioengineering platforms: A versatile tool for regenerative medicine. Micron 2014, 67, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Maria Nithya, J.S.; Pandurangan, A. Aqueous dispersion of polymer coated boron nitride nanotubes and their antibacterial and cytotoxicity studies. RSC Adv. 2014, 4, 32031–32046. [Google Scholar] [CrossRef]
- Ciofani, G.; Del Turco, S.; Rocca, A.; de Vito, G.; Cappello, V.; Yamaguchi, M.; Li, X.; Mazzolai, B.; Basta, G.; Gemmi, M.; et al. Cytocompatibility evaluation of gum Arabic-coated ultra-pure boron nitride nanotubes on human cells. Nanomedicine 2014, 9, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; Soares, D.C.F.; Moreira, L.M.C.; da Silva, P.R.O.; dos Santos, R.G.; de Sousa, E.M.B. Boron nitride nanotubes coated with organic hydrophilic agents: Stability and cytocompatibility studies. Mater. Sci. Eng. C 2013, 33, 4616–4623. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; Ciofani, G.; Moscato, S.; D’Alessandro, D.; Ciabatti, E.; Nesti, C.; Brescia, R.; Bertoni, G.; Pietrabissa, A.; Lisanti, M.; et al. Boron nitride nanotubes and primary human osteoblasts: In vitro compatibility and biological interactions under low frequency ultrasound stimulation. Nanotechnology 2013, 24, 465102. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, S.; Ciofani, G.; Cappello, V.; Gemmi, M.; Cervelli, T.; Saponaro, C.; Nitti, S.; Mazzolai, B.; Basta, G.; Mattoli, V. Cytocompatibility evaluation of glycol-chitosan coated boron nitride nanotubes in human endothelial cells. Colloids Surfaces B Biointerfaces 2013, 111, 142–149. [Google Scholar] [CrossRef]
- Ciofani, G.; Boni, A.; Calucci, L.; Forte, C.; Gozzi, A.; Mazzolai, B.; Mattoli, V. Gd-doped BNNTs as T 2 -weighted MRI contrast agents. Nanotechnology 2013, 24, 315101. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; Nitti, S.; Mazzolai, B.; Mattoli, V.; Giorgi, M. Biocompatibility of boron nitride nanotubes: An up-date of in vivo toxicological investigation. Int. J. Pharm. 2013, 444, 85–88. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Athanassiou, A.; Dinucci, D.; Chiellini, F.; Mattoli, V. A simple approach to covalent functionalization of boron nitride nanotubes. J. Colloid Interface Sci. 2012, 374, 308–314. [Google Scholar] [CrossRef]
- Soares, D.C.F.; Ferreira, T.H.; de Aguiar Ferreira, C.; Cardoso, V.N.; de Sousa, E.M.B. Boron nitride nanotubes radiolabeled with 99mTc: Preparation, physicochemical characterization, biodistribution study, and scintigraphic imaging in Swiss mice. Int. J. Pharm. 2012, 423, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Ciofani, G.; Graziana, G.G.; Mattoli, V.; Danti, S.; Giorgi, M. Pilot in vivo toxicological investigation of boron nitride nanotubes. Int. J. Nanomed. 2012, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menichetti, L.; De Marchi, D.; Calucci, L.; Ciofani, G.; Menciassi, A.; Forte, C. Boron nitride nanotubes for boron neutron capture therapy as contrast agents in magnetic resonance imaging at 3T. Appl. Radiat. Isot. 2011, 69, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Horváth, L.; Magrez, A.; Golberg, D.; Zhi, C.; Bando, Y.; Smajda, R.; Horváth, E.; Forró, L.; Schwaller, B. In Vitro Investigation of the Cellular Toxicity of Boron Nitride Nanotubes. ACS Nano 2011, 5, 3800–3810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiri, D.; Singh, V.; Benaduce, A.P.; Seal, S.; Kos, L.; Agarwal, A. Boron nitride nanotube reinforced hydroxyapatite composite: Mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J. Mech. Behav. Biomed. Mater. 2011, 4, 44–56. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Menciassi, A. Assessing cytotoxicity of boron nitride nanotubes: Interference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405–411. [Google Scholar] [CrossRef]
- Lahiri, D.; Rouzaud, F.; Richard, T.; Keshri, A.K.; Bakshi, S.R.; Kos, L.; Agarwal, A. Boron nitride nanotube reinforced polylactide–polycaprolactone copolymer composite: Mechanical properties and cytocompatibility with osteoblasts and macrophages in vitro. Acta Biomater. 2010, 6, 3524–3533. [Google Scholar] [CrossRef]
- Ciofani, G.; Ricotti, L.; Danti, S.; Moscato, S.; Nesti, C.; D’Alessandro, D.; Dinucci, D.; Chiellini, F.; Pietrabissa, A.; Petrini, M.; et al. Investigation of interactions between poly-L-lysine-coated boron nitride nanotubes and C2C12 cells: Up-take, cytocompatibility, and differentiation. Int. J. Nanomed. 2010, 5, 285. [Google Scholar] [CrossRef] [Green Version]
- Raffa, V.; Ciofani, G.; Cuschieri, A. Enhanced low voltage cell electropermeabilization by boron nitride nanotubes. Nanotechnology 2009, 20, 075104. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Folate Functionalized Boron Nitride Nanotubes and their Selective Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy. Nanoscale Res. Lett. 2009, 4, 113. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef] [Green Version]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Cytocompatibility, interactions, and uptake of polyethyleneimine-coated boron nitride nanotubes by living cells: Confirmation of their potential for biomedical applications. Biotechnol. Bioeng. 2008, 101, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Dario, P. Preparation of Boron Nitride Nanotubes Aqueous Dispersions for Biological Applications. J. Nanosci. Nanotechnol. 2008, 8, 6223–6231. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Pham, T.V.; Hwang, J.H.; Kim, C.S.; Kim, M.J. Boron nitride nanotubes: Synthesis and applications. Nano Converg. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumings, J.; Zettl, A. Mass-production of boron nitride double-wall nanotubes and nanococoons. Chem. Phys. Lett. 2000, 316, 211–216. [Google Scholar] [CrossRef]
- Saito, Y.; Maida, M.; Matsumoto, T. Structures of Boron Nitride Nanotubes with Single-Layer and Multilayers Produced by Arc Discharge. Jpn. J. Appl. Phys. 1999, 38, 159–163. [Google Scholar] [CrossRef]
- Yeh, Y.-W.; Raitses, Y.; Koel, B.E.; Yao, N. Stable synthesis of few-layered boron nitride nanotubes by anodic arc discharge. Sci. Rep. 2017, 7, 3075. [Google Scholar] [CrossRef]
- Chen, Y.; Fitz Gerald, J.; Williams, J.S.; Bulcock, S. Synthesis of boron nitride nanotubes at low temperatures using reactive ball milling. Chem. Phys. Lett. 1999, 299, 260–264. [Google Scholar] [CrossRef]
- Chen, Y.; Chadderton, L.T.; Gerald, J.F.; Williams, J.S. A solid-state process for formation of boron nitride nanotubes. Appl. Phys. Lett. 1999, 74, 2960–2962. [Google Scholar] [CrossRef] [Green Version]
- Fitz Gerald, J.D.; Chen, Y.; Conway, M.J. Nanotube growth during annealing of mechanically milled Boron. Appl. Phys. A 2003, 76, 107–110. [Google Scholar] [CrossRef]
- Yong Bae, S.; Won Seo, H.; Park, J.; Sang Choi, Y.; Chul Park, J.; Young Lee, S. Boron nitride nanotubes synthesized in the temperature range 1000–1200 °C. Chem. Phys. Lett. 2003, 374, 534–541. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, Y.; Glushenkov, A.M. Synthesis of boron nitride nanotubes by boron ink annealing. Nanotechnology 2010, 21, 105601. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.-C.; Feng, J.; Xu, H.; Li, L.; Liu, X.-W. Synthesis of boron nitride nanotube films with a nanoparticle catalyst. Chinese Chem. Lett. 2016, 27, 871–874. [Google Scholar] [CrossRef]
- Zhu, G.; Dong, S.; Hu, J.; Kan, Y.; He, P.; Gao, L.; Zhang, X.; Zhou, H. In situ growth behavior of boron nitride nanotubes on the surface of silicon carbide fibers as hierarchical reinforcements. RSC Adv. 2016, 6, 14112–14119. [Google Scholar] [CrossRef]
- Zhu, G.; Xue, Y.; Hu, J.; Yang, J.; Zhou, H.; Gao, L.; Shan, Q.; Dong, S. Influence of boron nitride nanotubes on the damage evolution of SiCf/SiC composites. J. Eur. Ceram. Soc. 2018, 38, 4614–4622. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Eremets, M.; Takemura, K.; Kurashima, K.; Yusa, H. Nanotubes in boron nitride laser heated at high pressure. Appl. Phys. Lett. 1996, 69, 2045–2047. [Google Scholar] [CrossRef]
- Yu, D.P.; Sun, X.S.; Lee, C.S.; Bello, I.; Lee, S.T.; Gu, H.D.; Leung, K.M.; Zhou, G.W.; Dong, Z.F.; Zhang, Z. Synthesis of boron nitride nanotubes by means of excimer laser ablation at high temperature. Appl. Phys. Lett. 1998, 72, 1966–1968. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.W.; Jordan, K.C.; Park, C.; Kim, J.-W.; Lillehei, P.T.; Crooks, R.; Harrison, J.S. Very long single- and few-walled boron nitride nanotubes via the pressurized vapor/condenser method. Nanotechnology 2009, 20, 505604. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.; Pham, T.V.; Hwang, J.H.; Ahn, S.; Jang, S.G.; Lee, H.; Park, C.; Kim, C.S.; Kim, M.J. Dual growth mode of boron nitride nanotubes in high temperature pressure laser ablation. Sci. Rep. 2019, 9, 15674. [Google Scholar] [CrossRef]
- Kim, K.S.; Kingston, C.T.; Hrdina, A.; Jakubinek, M.B.; Guan, J.; Plunkett, M.; Simard, B. Hydrogen-Catalyzed, Pilot-Scale Production of Small-Diameter Boron Nitride Nanotubes and Their Macroscopic Assemblies. ACS Nano 2014, 8, 6211–6220. [Google Scholar] [CrossRef]
- Fathalizadeh, A.; Pham, T.; Mickelson, W.; Zettl, A. Scaled Synthesis of Boron Nitride Nanotubes, Nanoribbons, and Nanococoons Using Direct Feedstock Injection into an Extended-Pressure, Inductively-Coupled Thermal Plasma. Nano Lett. 2014, 14, 4881–4886. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.H.; Oh, J.-H.; Hong, S.-H.; Min, B.-I.; Kim, T.-H.; Choi, S. Synthesis of boron nitride nanotubes using triple DC thermal plasma reactor with hydrogen injection. Chem. Eng. J. 2020, 395, 125148. [Google Scholar] [CrossRef]
- Lee, C.M.; Choi, S.I.; Choi, S.S.; Hong, S.H. Synthesis of boron nitride nanotubes by arc-jet plasma. Curr. Appl. Phys. 2006, 6, 166–170. [Google Scholar] [CrossRef]
- Shiratori, T.; Yamane, I.; Nodo, S.; Ota, R.; Yanase, T.; Nagahama, T.; Yamamoto, Y.; Shimada, T. Synthesis of Boron Nitride Nanotubes Using Plasma-Assisted CVD Catalyzed by Cu Nanoparticles and Oxygen. Nanomaterials 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wang, J.; Kayatsha, V.K.; Huang, J.Y.; Yap, Y.K. Effective growth of boron nitride nanotubes by thermal chemical vapor deposition. Nanotechnology 2008, 19, 455605. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Zhang, K.; Cai, Q.; Wang, N.; Wu, L.; He, Q.; Wang, H.; Zhang, Y.; Xie, Y.; Yao, Y.; et al. Advances in synthesis and applications of boron nitride nanotubes: A review. Chem. Eng. J. 2022, 431, 134118. [Google Scholar] [CrossRef]
- Cai, P.; Chen, L.; Shi, L.; Yang, Z.; Zhao, A.; Gu, Y.; Huang, T.; Qian, Y. One convenient synthesis route to boron nitride nanotube. Solid State Commun. 2005, 133, 621–623. [Google Scholar] [CrossRef]
- Loiseau, A.; Willaime, F.; Demoncy, N.; Hug, G.; Pascard, H. Boron Nitride Nanotubes with Reduced Numbers of Layers Synthesized by Arc Discharge. Phys. Rev. Lett. 1996, 76, 4737–4740. [Google Scholar] [CrossRef]
- Terauchi, M.; Tanaka, M.; Matsumoto, T.; Saito, Y. Electron energy-loss spectroscopy study of the electronic structure of boron nitride nanotubes. J. Electron Microsc. 1998, 47, 319–324. [Google Scholar] [CrossRef]
- Narita, I.; Oku, T. Synthesis of boron nitride nanotubes by using YB6 powder. Solid State Commun. 2002, 122, 465–468. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Eremets, M.; Kurashima, K.; Tamiya, T.; Takemura, K.; Yusa, H. High-resolution analytical electron microscopy of boron nitrides laser heated at high pressure. J. Electron Microsc. 1997, 46, 281–292. [Google Scholar] [CrossRef]
- Louchev, O.A. Surface diffusion growth and stability mechanism of BN nanotubes produced by laser beam heating under superhigh pressures. Appl. Phys. Lett. 1997, 71, 3522–3524. [Google Scholar] [CrossRef]
- Zhou, G.W.; Zhang, Z.; Bai, Z.G.; Yu, D.P. Catalyst effects on formation of boron nitride nano-tubules synthesized by laser ablation. Solid State Commun. 1999, 109, 555–559. [Google Scholar] [CrossRef]
- Turhan, E.A.; Pazarçeviren, A.E.; Evis, Z.; Tezcaner, A. Properties and Applications of Boron Nitride Nanotubes. Nanotechnology. 2022, 30, 242001. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Chen, Y.; Glushenkov, A.M. Boron nitride nanotube films grown from boron ink painting. J. Mater. Chem. 2010, 20, 9679. [Google Scholar] [CrossRef] [Green Version]
- Li, L.L.H.; Li, L.L.H.; Chen, Y.; Dai, X.J.; Xing, T.; Petravic, M.; Liu, X. Mechanically activated catalyst mixing for high-yield boron nitride nanotube growth. Nanoscale Res. Lett. 2012, 7, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Conway, M.; Williams, J.S.; Zou, J. Large-quantity production of high-yield boron nitride nanotubes. J. Mater. Res. 2002, 17, 1896–1899. [Google Scholar] [CrossRef]
- Chadderton, L.T.; Chen, Y. A model for the growth of bamboo and skeletal nanotubes: Catalytic capillarity. J. Cryst. Growth 2002, 240, 164–169. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Wuhrer, R.; Liu, Z.; Ringer, S.P. In Situ Formation of BN Nanotubes during Nitriding Reactions. Chem. Mater. 2005, 17, 5172–5176. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.C.P.; Zou, J.; Chen, Y. Influence of nitriding gases on the growth of boron nitride nanotubes. J. Mater. Sci. 2007, 42, 4025–4030. [Google Scholar] [CrossRef]
- Velázquez-Salazar, J.J.; Muñoz-Sandoval, E.; Romo-Herrera, J.M.; Lupo, F.; Rühle, M.; Terrones, H.; Terrones, M. Synthesis and state of art characterization of BN bamboo-like nanotubes: Evidence of a root growth mechanism catalyzed by Fe. Chem. Phys. Lett. 2005, 416, 342–348. [Google Scholar] [CrossRef]
- Bechelany, M.; Bernard, S.; Brioude, A.; Cornu, D.; Stadelmann, P.; Charcosset, C.; Fiaty, K.; Miele, P. Synthesis of Boron Nitride Nanotubes by a Template-Assisted Polymer Thermolysis Process. J. Phys. Chem. C 2007, 111, 13378–13384. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cao, L.; Gao, J.; Wang, Y.Y. Graphitic Foam with High Strength and High Thermal Conductivity Doped by Tungsten. Cell. Polym. 2008, 27, 251–260. [Google Scholar] [CrossRef]
- Shelimov, K.B.; Moskovits, M. Composite Nanostructures Based on Template-Grown Boron Nitride Nanotubules. Chem. Mater. 2000, 12, 250–254. [Google Scholar] [CrossRef]
- Tay, R.Y.; Li, H.; Tsang, S.H.; Jing, L.; Tan, D.; Wei, M.; Teo, E.H.T. Facile Synthesis of Millimeter-Scale Vertically Aligned Boron Nitride Nanotube Forests by Template-Assisted Chemical Vapor Deposition. Chem. Mater. 2015, 27, 7156–7163. [Google Scholar] [CrossRef]
- Han, W.; Bando, Y.; Kurashima, K.; Sato, T. Synthesis of boron nitride nanotubes from carbon nanotubes by a substitution reaction. Appl. Phys. Lett. 1998, 73, 3085–3087. [Google Scholar] [CrossRef]
- Han, W.-Q.; Todd, P.J.; Strongin, M. Formation and growth mechanism of B10N nanotubes via a carbon nanotube–substitution reaction. Appl. Phys. Lett. 2006, 89, 173103. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Kurashima, K.; Sato, T. Ropes of BN multi-walled nanotubes. Solid State Commun. 2000, 116, 1–6. [Google Scholar] [CrossRef]
- Zhong, B.; Song, L.; Huang, X.X.; Wen, G.W.; Xia, L. Synthesis of boron nitride nanotubes with SiC nanowire as template. Mater. Res. Bull. 2011, 46, 1521–1523. [Google Scholar] [CrossRef]
- Kim, K.S.; Couillard, M.; Shin, H.; Plunkett, M.; Ruth, D.; Kingston, C.T.; Simard, B. Role of Hydrogen in High-Yield Growth of Boron Nitride Nanotubes at Atmospheric Pressure by Induction Thermal Plasma. ACS Nano 2018, 12, 884–893. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, T.; Ling, L.; Luo, J.; Zhang, K.; Xu, Y.; Lu, H.; Yao, Y. Remote catalyzation for growth of boron nitride nanotubes by low pressure chemical vapor deposition. Chem. Phys. Lett. 2016, 652, 27–31. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Khan, Z.R.; Amin, Y.M. Synthesis of boron nitride nanotubes via chemical vapour deposition: A comprehensive review. Rsc Adv. 2015, 5, 35116–35137. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Zou, J.; Li, F.; Liu, G.; Tang, D.-M.; Li, D.; Liu, C.; Ma, X.; Cheng, H.-M.; Lu, G.Q.; et al. Growth of Magnetic Yard-Glass Shaped Boron Nitride Nanotubes with Periodic Iron Nanoparticles. Adv. Funct. Mater. 2007, 17, 3371–3376. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Long, X.; Wang, X.; Xu, Y.; Yao, Y. Bimetallic catalytic growth of boron nitride nanotubes. Nanoscale 2017, 9, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Yin, Y.; Chen, Y.; Bi, X. Water-assisted chemical vapor deposition synthesis of boron nitride nanotubes and their photoluminescence property. Nanotechnology 2013, 24, 365605. [Google Scholar] [CrossRef]
- Songfeng, E.; Long, X.; Li, C.; Geng, R.; Han, D.; Lu, W.; Yao, Y. Boron nitride nanotubes grown on stainless steel from a mixture of diboron trioxide and boron. Chem. Phys. Lett. 2017, 687, 307–311. [Google Scholar] [CrossRef]
- Tang, D.-M.; Liu, C.; Cheng, H.-M. Controlled synthesis of quasi-one-dimensional boron nitride nanostructures. J. Mater. Res. 2007, 22, 2809–2816. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.; Sato, T.; Kurashima, K. Thin boron nitride nanotubes with unusual large inner diameters. Chem. Phys. Lett. 2001, 350, 434–440. [Google Scholar] [CrossRef]
- Pakdel, A.; Zhi, C.; Bando, Y.; Nakayama, T.; Golberg, D. A comprehensive analysis of the CVD growth of boron nitride nanotubes. Nanotechnology 2012, 23, 215601. [Google Scholar] [CrossRef]
- Wang, X.Z.; Wu, Q.; Hu, Z.; Chen, Y. Template-directed synthesis of boron nitride nanotube arrays by microwave plasma chemical reaction. Electrochim. Acta 2007, 52, 2841–2844. [Google Scholar] [CrossRef]
- Su, C.-Y.; Chu, W.-Y.; Juang, Z.-Y.; Chen, K.-F.; Cheng, B.-M.; Chen, F.-R.; Leou, K.-C.; Tsai, C.-H. Large-Scale Synthesis of Boron Nitride Nanotubes with Iron-Supported Catalysts. J. Phys. Chem. C 2009, 113, 14732–14738. [Google Scholar] [CrossRef]
- Kim, M.J.; Chatterjee, S.; Kim, S.M.; Stach, E.A.; Bradley, M.G.; Pender, M.J.; Sneddon, L.G.; Maruyama, B. Double-Walled Boron Nitride Nanotubes Grown by Floating Catalyst Chemical Vapor Deposition. Nano Lett. 2008, 8, 3298–3302. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M. Synthesis of boron nitride nanotubes by Argon supported Thermal Chemical Vapor Deposition. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 67, 33–37. [Google Scholar] [CrossRef]
- Xu, L.; Peng, Y.; Meng, Z.; Yu, W.; Zhang, S.; Liu, X.; Qian, Y. A Co-pyrolysis Method to Boron Nitride Nanotubes at Relative Low Temperature. Chem. Mater. 2003, 15, 2675–2680. [Google Scholar] [CrossRef]
- Dai, J.; Xu, L.; Fang, Z.; Sheng, D.; Guo, Q.; Ren, Z.; Wang, K.; Qian, Y. A convenient catalytic approach to synthesize straight boron nitride nanotubes using synergic nitrogen source. Chem. Phys. Lett. 2007, 440, 253–258. [Google Scholar] [CrossRef]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Functionalization of boron nitride nanotubes for applications in nanobiomedicine. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–40. [Google Scholar] [CrossRef]
- Ribeiro, H.; von Cardoso Randow, P.; Vilela, D.N.; Adriane Luciano, M.; Maria de Andrade, L. Functionalized Boron Nitride Applications in Biotechnology. In Recent Advances in Boron-Containing Materials; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, Q.; Liu, J.; Li, H.; Zheng, S. Covalent Surface Functionalization of Boron Nitride Nanotubes Fabricated with Diazonium Salt. J. Nanomater. 2018, 2018, 6717046. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Noncovalent Functionalization of Boron Nitride Nanotubes in Aqueous Media Opens Application Roads in Nanobiomedicine. Nanobiomedicine 2014, 1, 7. [Google Scholar] [CrossRef]
- Zhi, C.Y.; Bando, Y.; Tang, C.C.; Huang, Q.; Golberg, D. Boron nitride nanotubes: Functionalization and composites. J. Mater. Chem. 2008, 18, 3900. [Google Scholar] [CrossRef]
- Wu, X.; An, W.; Zeng, X.C. Chemical Functionalization of Boron−Nitride Nanotubes with NH3 and Amino Functional Groups. J. Am. Chem. Soc. 2006, 128, 12001–12006. [Google Scholar] [CrossRef]
- Niskanen, J.; Zhang, I.; Xue, Y.; Golberg, D.; Maysinger, D.; Winnik, F.M. Dually-functionalized boron nitride nanotubes to target glioblastoma multiforme. Mater. Today Chem. 2020, 16, 100270. [Google Scholar] [CrossRef]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Isolation of Individual Boron Nitride Nanotubes via Peptide Wrapping. J. Am. Chem. Soc. 2010, 132, 4976–4977. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Khan, A.; Khandaker, M.U.; Kebaili, I.; Alam, M.M.; Din, I.U.; Muhammad, S.; Razzaq, Z.; Rehman, I.U.; et al. Cytotoxic and photocatalytic studies of hexagonal boron nitride nanotubes: A potential candidate for wastewater and air treatment. RSC Adv. 2022, 12, 6592–6600. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S. Evaluation of Cytocompatibility and Cell Response to Boron Nitride Nanotubes. In Nanotechnology in Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2012; pp. 193–206. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Silva, P.R.O.; Santos, R.G.; Sousa, E.M.B. A Novel Synthesis Route to Produce Boron Nitride Nanotubes for Bioapplications. J. Biomater. Nanobiotechnol. 2011, 2, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Şen, Ö.; Emanet, M.; Çulha, M. Biocompatibility evaluation of boron nitride nanotubes. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–58. [Google Scholar] [CrossRef]
- Allard, C.; Schué, L.; Fossard, F.; Recher, G.; Nascimento, R.; Flahaut, E.; Loiseau, A.; Desjardins, P.; Martel, R.; Gaufrès, E. Confinement of Dyes inside Boron Nitride Nanotubes: Photostable and Shifted Fluorescence down to the Near Infrared. Adv. Mater. 2020, 32, 2001429. [Google Scholar] [CrossRef] [PubMed]

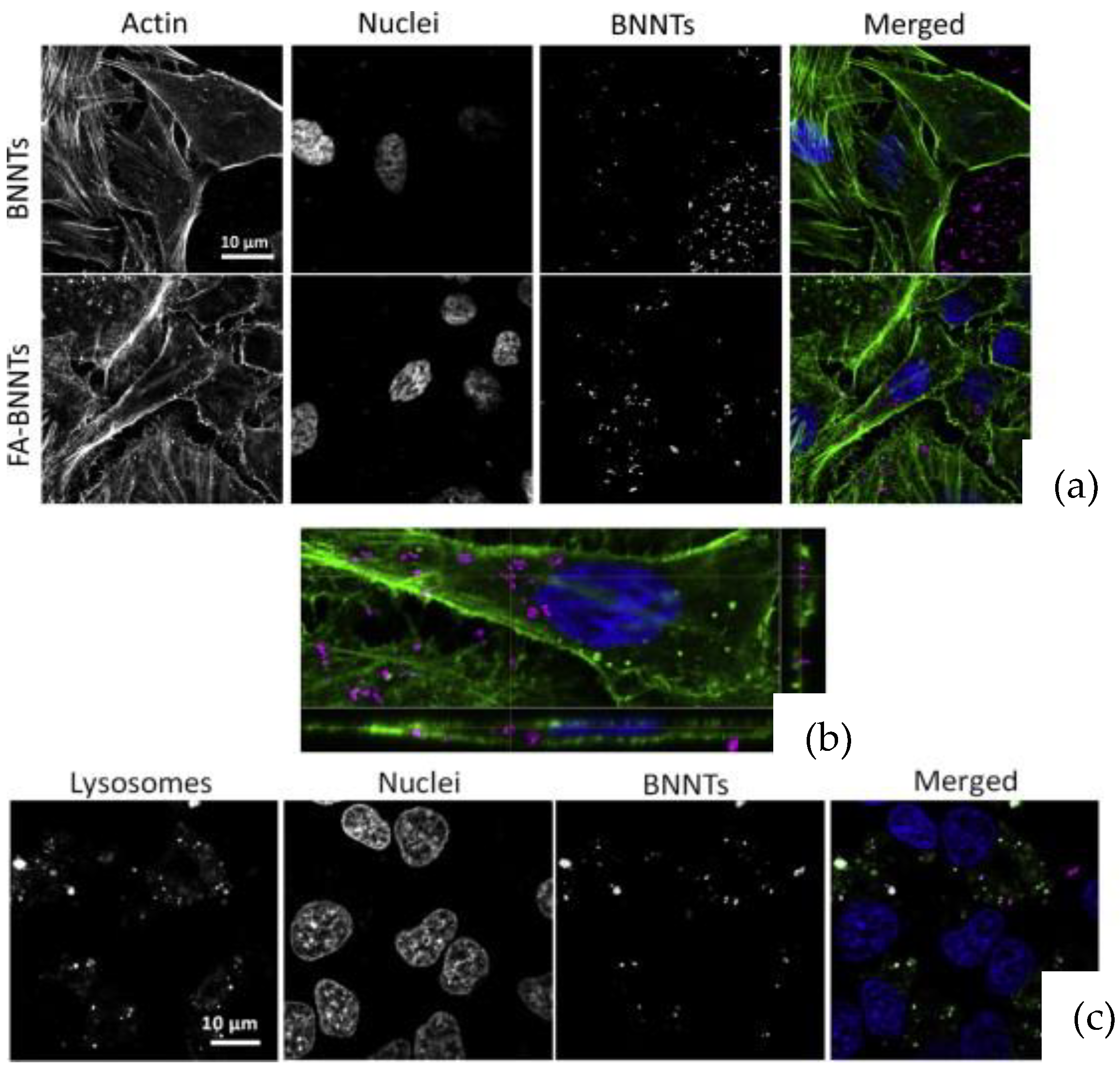
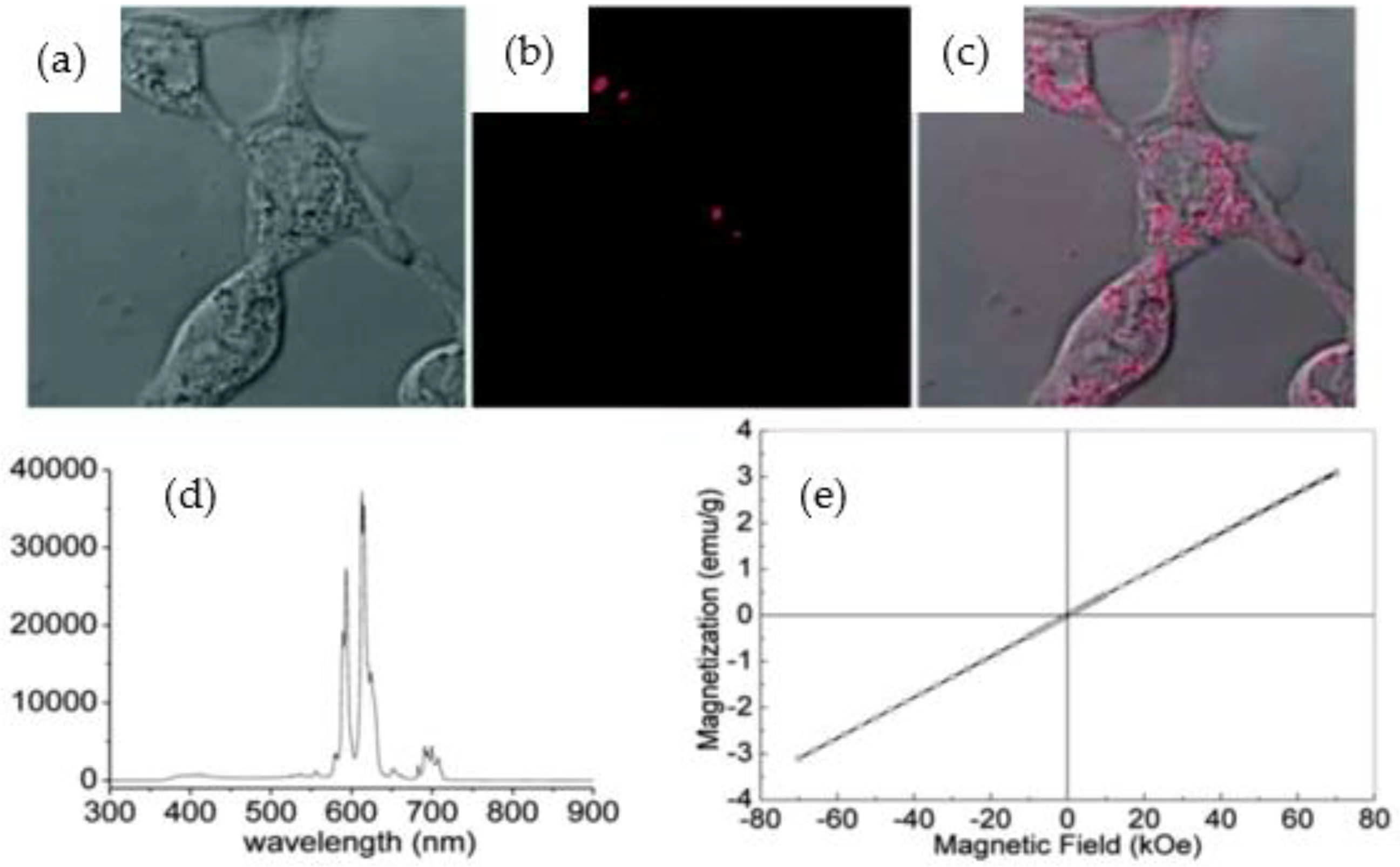
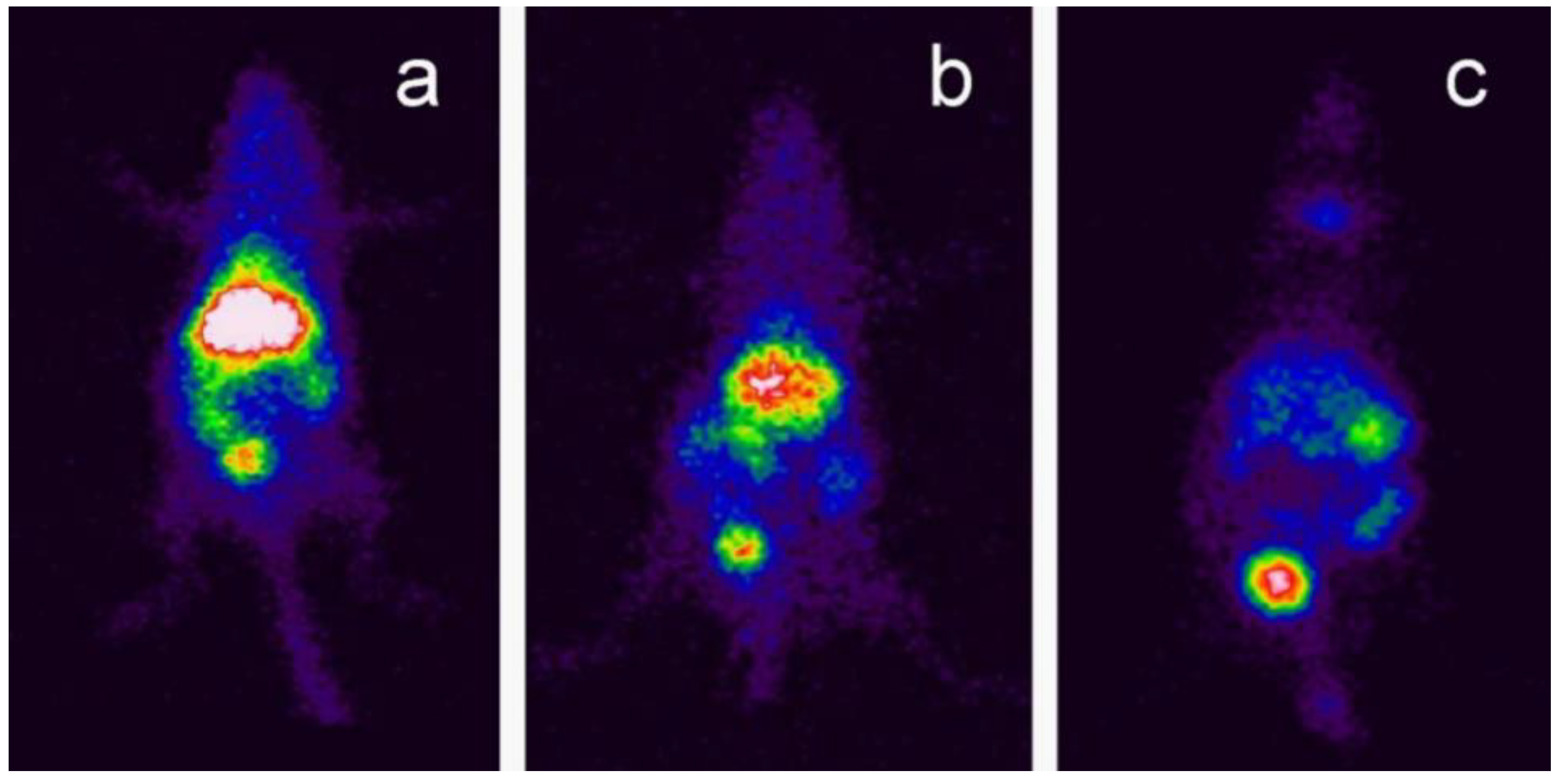

| Keywords |
|---|
| “Boron nitride nanotubes” or “bnnts” and “toxicity” or “in vivo” or “in vitro” or “tissue engineering” or “biomedical”) and (limit—to (doctype, “ar”)) and (limit—to (srctype, “j”)) and (limit—to (language, “English”)) and (limit—to (pub stage,” final”) |
| “Boron nitride nanotubes” (topic) and “biomedical” (topic) and review articles or proceedings papers or book chapters or early access (exclude—document types) and articles (document types) and English (languages) |
| “Boron nitride nanotubes” (topic) and “tissue engineering” (topic) and review articles or proceedings papers or book chapters or early access (exclude—document types) and articles (document types) and English (languages) |
| “Boron nitride nanotubes” (topic) and “toxicity” (topic) and review articles or proceedings papers or book chapters or early access (exclude—document types) and articles (document types) and English (languages) |
| “Boron nitride nanotubes” (topic) and “in vivo” (topic) and review articles or proceedings papers or book chapters or early access (exclude—document types) and articles (document types) and English (languages) |
| “Boron nitride nanotubes” (topic) and “in vitro” (topic) and review articles or proceedings papers or book chapters or early access (exclude—document types) and articles (document types) and English (languages) |
| Authors and References | Synthesis/Source of BNNTs | Geometrical Dimensions of BNNTs | Functionalisation/Composition of BNNTs | Dosage and Time of Exposure | Animal Model/Cell Line | Physiochemical Characterisation | Biocompatibility and Toxicity Assays | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Kakarla et al. [24,25] | Co-precipitation and annealing | Diameter: 70 to 130 nm | Hydroxyl-BNNTs (BNNTs-OH)/BNNTs reinforced alginate and gelatin/BNNTs reinforced alginate hydrogel scaffolds | 0.05 to 0.1 w·v−1%; up to 72 h | HEK 293T | Scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform spectroscopy (FTIR), mechanical, thermogravimetric analysis (TGA) | Viability: Trypan blue and Ready Probes™ Cell Viability Imaging Kit (blue/green) | Good printability, mechanical strength, and thermal stability with the addition of BNNTs. Minimal toxicity at higher concentrations of BNNTs. |
| Evariste et al. [26] | Commercial BNNTs (B and N > 99.9%) | Diameter: 2 to 14 nm | – | 0, 0.1, 1, and 10 mg·L−1; up to 24 h. Larvae were fed twice daily with BNNTs ground aquarium fish food | Xenopus laevis | SEM, TEM, TGA, XRD, and Raman spectroscopy | Micronucleus test, cell cycle analysis, analysis of sequences from gut microbiota survey | The specific surface area of BNNTs was 163 m2·g−1. Micrographs displayed 2 to 10 walls of nanotubes with a mean outer diameter of 6 ± 2.6 nm. BNNTs possessed minor threat to amphibians. |
| Li et al. [27] | Solid-state reaction | Folate-conjugated BNNTs and coated with auristatin-phenethylamine (PE) (BNNTs-FA@PE) | 0–100 μg·mL−1 | Hep G2 and L02 | TEM, FTIR, ultraviolet-visible (UV-vis) absorption spectroscopy, X-ray photoelectron spectroscopy (XPS), size distribution and zeta potential | CCK-8 assay, cellular uptake, actin staining, in vitro anticancer effects, Annexin V-FITC/ propidium iodide (PI), mitochondrial membrane potential, Western blot analysis, detection of Caspase 3/7 activity | The morphology showed bamboo-like shaped nanotubes with diameter of ≈90 nm. BNNTs displayed photoluminescence emission bands at 419, 489, and 594 nm. FTIR analysis displayed BNNTs-FA and BNNTs-FA@PE had absorption bands at 2937–2829 cm−1 and 1250–950 cm−1 related to the methylene bands of PE molecules. No toxicity in both cell lines and significant increase in metabolic and cellular uptake. | |
| Li et al. [28] | Solid-state reaction | PE-loaded BNNTs | 0–100 μg·mL−1 | HeP G2 Cells | SEM, TEM, Z-potential, FTIR, UV–vis, XPS | Intracellular uptake, lysosomal staining, actin staining, cell viability, flow cytometry, western blot, Capase-3/7 activity | The morphology images displayed BNNTs bamboo-like structures with good dispersive behaviour. Furthermore, BNNTs showed strong emission bands related to B-N and excellent PL properties in the visible light range. The in vivo analysis displayed good internalisation and stimulated cell apoptosis of BNNTs-PE. | |
| Xin et al. [29] | Commercial BNNTs that contain 50% BNNTs with 5 nm wide and 200 μm long | Length: 200 µm; diameter: 5 nm | – | 4 or 40 μg; 4 h, 1–7 days, 1–2 months. The mice were fed with BNNT mixed in dispersion media through an oropharyngeal aspiration. | Male C57BL/6 J mice | SEM, TEM, electron paramagnetic response spectra | Lung lavage, BAL cell differentiation, lactic dehydrogenase activity (LDH), BAL fluid protein analysis, lymphocyte phenotypic quantification, mediastinal lymph node and spleen analysis, white blood cell differentiation, histopathology, macrophage uptake, pulmonary clearance, RNA isolation and gene expression | The micrographic analysis of BNNTs showed an ideal length of nanotubes. The specific surface area of BNNTs was 182.6 ± 2.4 m2·g−1 with the density of 0.03 g·cm−3. Only a higher dosage of BNNTs caused the inflammation and a lower dose did not show any effects in the lung. |
| Lee et al. [21] | Commercial BNNTs | – | Purified BNNTs | 0–100 µg | CHO-K1 and 3T3-L1 | SEM, XRD, dispersion stability | Cell viability, drug delivery | The SEM and dispersion stability analysis confirmed the nanotubes in tubular structures with stable dispersion in aqueous media. The XRD analysis observed the hexagonal lattice of B−N in BNNTs. Purified BNNTs showed lower cytotoxic at a higher dosage and efficiently carried the drugs than as synthesised BNNTs |
| Pasquale et al. [30] | – | BNNTs loaded with dox and coated with cell membranes (CM) (Dox-CM-BNNTs) | 25, 50, 100, and 200 μg·mL−1; up to 72 h | U87 | TEM, FTIR, size distribution, zeta potential, TGA, dynamic light scattering (DLS), bicinchoninic acid assay | Cell uptake mechanism, cell viability | The morphology of BNNTs coated with CM was not precise due to low thickness. The FTIR confirmed that BNNTs coated with CM with presence of peaks related to amino acids of CM proteins. The TGA analysis indicated that the total weight loss of CM-BNNTs was 20%. DLS analysis indicated that negative Z-potential related to stable colloidal solution. Dox-CM-BNNTs and free drug were able to substantially decline the cell viability compared to non-treated controls and BNNT controls. | |
| Marcos da Silva et al. [31] | Chemical vapor deposition (CVD) | – | BNNTs doped in situ with samarium (Sm) and gadolinium (Gd) (SmBO3-BNNTs and GdBO3-BNNTs) | 10 and 50 μg·mL−1; up to 24 h | HDF and Sarcoma osteogenic (SAOS-2) | XPS, FTIR, SEM, TEM, X-ray fluorescence spectroscopy (XRF), electron energy loss spectroscopy (EELS), vibrational sample magnetometry (VSM), neutron activation | MTT assay, Calcein/Hoechst assay | SEM and TEM images confirmed that the BNNTs were successfully modified with Sm and Gd with uniform distribution on their surfaces. The XPS and EELS analysis further confirmed the presence of Sm and Gd in the BNNTs. In addition, VSM analysis stated that coated BNNTs exhibited magnetic properties. 50 μg·mL−1 of GdBO3-BNNTs suggested low biocompatibility with fibroblasts (50% of cell viability), but high biocompatibility with SAOS-2 cells (80% of cell viability). |
| Ferreira et al. [32] | CVD | Diameter: 30 nm; length: 1 µm | BNNT with the CREKA peptide/99mTc-BNNT-CREKA | 100 µL; 1, 4 and 8 h | 4T1 tumour cells | SEM, TEM, TGA, zeta potential, FTIR | Biodistribution histopathological and blood clearance analysis; fluorescence microscopy cell images | The SEM and TEM micrographs revealed several nanotubes with ≈10 nm outer wall thickness. BNNTs and coated BNNTs showed good thermal stability. FTIR analysis showed B-N stretching vibrations and additional C—H, O—H and O—C bands in coated BNNTs. BNNTs-CREKA as an effective material for targeting the primary tumour tissues and metastatic tumour sites. |
| Ferreira et la. [33] | Commercial BNNTs | BNNTs incorporated with alkyl trimethyl ammonium bromide (ATAB) | 0 to 0.2 wt%; up to 72 h | HaCaT | FTIR (degree of conversion (DC) analysis), microhardness, contact angle, mineral deposition | Cytotoxicity assay and antibacterial assay | No DC was noted in the samples. The contact angle was higher for functionalised BNNTs. The minerals deposition analysis was displayed higher peak intensities in BNNTs-ATAB. No significant cell viability reduction was observed in the BNNTs-ATAB compared with control groups (90%). | |
| Bohns [34] | Commercial BNNTs | Length: 200 µm | BNNTs reinforced resin-based dental sealants (RBSs) | 0.1 and 0.2 wt% | Pulp fibroblasts and human keratinocytes | FTIR, tensile strength, contact angle, surface roughness, colour assessment, Mineral deposition | Sulforhodamine B (SRB) cytotoxicity assay | No evidence of DC in the BNNTs-RBSs. The additions of BNNTs to RBSs did not show a significant difference in tensile strength from RBSs. The contact angle values were adequate even though the incorporation of nanotubes. Lower surface energy was noticed for BNNTs comprising RBSs. BNNTs at 0.1 and 0.2 wt% in RBSs did not show any cytotoxicity effects. |
| Çal [35] | Commercial BNNTs | Diameter:5 nm | BNNTs incorporated with curcumin | 10–300 μg·mL−1; up to 24 h | HeLa, V79 and CD34+ | TEM, zeta potential | MTT assay, comet assay | The TEM images showed the BNNTs with micrometres length and Z-potential with positive z signals for curcumin in the BNNTs. BNNTs and BNNTs-curcumin showed minimal toxicity in all cell lines |
| Ricotti et al. [36] | Annealing | – | Glycol-chitosan (GC)-BNNTs | 10 μg·mL−1; for 24 h | HDF and C2C12 | Focused ion beam (FIB), ICP-MS, EELS | Quantitative real-time polymerase chain reaction (qRT-PCR), cytokine measurements, calcium transients imaging | FIB images revealed evenly dispersed GC-BNNTs in cell culture medium. The ICP-MS showed highest content of boron in cells treated with GC-BNNTs. EEL spectrum confirmed the presence of GC-BNNTs in sections of C2C12 cells. BNNTs were internalised on the top layer of cells and localised inside C2C12 cells, while no particles were internalised by the HDF cells. In addition, BNNTs stimulate cell differentiation at both gene and protein levels. |
| Augustine et al. [37] | Thermal plasma | – | – | 5 to 10 mg of BNNT in 20 mL glass scintillation vial | NB4, HepG2, U87, and A549 | AFM, and probe sonication | WST-8, MTT and monitoring beating behaviour of cardiomyocytes | The AFM analysis of BNNTs displayed that the tubes were ≈300 to 500 nm in length with 2 to 3 nm in height. While after probe sonication, the length of nanotubes decreased to 191.9 ± 5.2 nm. BNNTs displayed cytotoxic to the cells measured through AFM-based cardiomyocyte assay. |
| Poudel et al. [38] | Commercial BNNTs | 20–30 µm thickness | Polyvinylidene fluoride (PVDF) and the trifluoroethylene (TrFE) reinforced with BNNTs (PVDF-TrFE-BNNTs) | 10 days | Human tendon derived cells | DSC, FTIR, differential scanning calorimetry (DSC), tensile analysis, electrical poling, quasi-static measurement of piezoelectric coefficient | Fibronectin functionalisation, live/dead assay, cell proliferation assay | Addition of BNNTs was evident in enhancing mechanical properties, melting and crystallisation temperatures, and crystallinity. PVDF-TrFE-BNNTs nanocomposite displayed enhanced cell attachment and proliferation compared to pure PVDF-TrFE |
| Genchi et al. [39] | Pressurised vapor/condenser (PVC) | – | PVDF-TrFE-BNNTs | – | Saos-2 | SEM, TEM, AFM, piezo response, piezoelectric transduction, numerical simulation | Cell differentiation, cell stimulation, alizarin red and collagen staining, quantitative real-time reverse transcriptase polymerase chain reaction | The micrographs of BNNTs revealed bundles of nanotube ranging up to µm in length. AFM topographic maps of the PVDF-TrFE-BNNTs showed ~30 nm of mean surface roughness with good piezo electric properties. The piezoelectric films of PVDF-TrFE-BNNTs indicated increased cell differentiation. |
| Demir et al. [40] | Commercial BNNTs | Average diameter 239.7 ± 6.48 nm | – | 0.0003, 0.003, 0.027, 0.135, and 0.270 mg·g−1 | Drosophila (D) melanogaster adults and larvae | SEM, TEM, DLS, laser doppler velocimetry (LDV) | Endotoxin assay, drosophila strain, exposure, and toxicity, hemocytes collection, ROS, gene expression changes, genotoxicity, antigenotoxicity, comet assay | SEM and TEM images of BNNTs revealed that the average nanotubes length was 245 ± 65.72 nm. The DLS and LDV analysis showed lower zeta potential that indicated the propensity of BNNTs to aggregates. BNNTs treated larvae increased the genotoxicity and antigenotoxicity. |
| Degrazia et al. [41] | PVC | – | BNNTs incorporated with bisphenol A glycerolate dimethacrylate (BisGMA) and hydroxyethyl methacrylate | 0.05, 0.075, 0.1 and 0.15 wt% | Fibroblasts | FTIR, contact angle, micro tensile bond strength, failure pattern analysis | Cytotoxicity sulforhodamine B (SRB) colorimetric assay, cell viability | The successful incorporation of 0.1 wt% BNNTs into adhesive resin increased the tensile and longer stability. BNNTs treated with cells did not show any cytotoxicity. |
| Ferreira et al. [42] | CVD | – | BNNTs–OH– ferric oxide (Fe3O4) | 0–2 µg·mL−1; 48 h | HeLa | XRD, TEM, XPS, vibrating sample magnetometer (VSM) | WST-8 and CCK-8 assay, internalisation tests, magneto hyperthermia assay, cell death assay (calcein-AM and PI), cell imaging | Micrograph imaging revealed a bundle of nanotubes with tube like structures. The XPS analysis showed that BNNTs consisted mostly of B and N atoms. Magnetic measurements displayed that coercivity and magnetisation were not agitated with the addition of BNNTs. The results showed excellent viability of cells treated with OH-BNNT-Fe3O4 and validated the internalisation capability of BNNTs by the cells. |
| Ferreira et al. [43] | CVD | – | BNNTs-OH covered with radioactive C-39 detectors | 0–200 µg·mL−1; up to 48 h | HeLa | SEM, FTIR, XRD | WST-8, CCK-8, performance test, cells irradiation | The outcomes showed no evidence of changes in crystallinity of the material and intense solid B-N bands. No substantial differences after irradiation in the microstructures of the BNNTs compared to pure BNNTs. BNNTs had appropriate cell viability and that irradiation with a suitable flux of thermal neutron without adverse damage in the cells. |
| Ponraj et al. [44] | Ball milling | – | Gold nanoparticles functionalised on BNNTs and loaded with dox | 30, 60, and 90 μL | DU145 | TEM, XPS | Cyquant assay | Micrographs images showed long and medium BNNTs. XPS analysis displayed the BNNT surface with oxygen rate from 8 to 27.4%. The dox loaded BNNTs killed ~99% of cancer cells, which resulted in good drug carrier for cancer treatment. |
| Kodali et al. [45] | Commercial BNNTs | Length:0.6 to 1.6 µm | – | 0–100 µg·mL−1 | THP-1 cells, NLRP3 and c57BL/6J mice | SEM, TEM, DLS | ROS, high content epifluorescence microscopy, lysosomal membrane permeabilisation, cytokine analysis, cathepsin B and caspase 1 activity inside the cells, phagocytosis and lipopolysaccharide (LPS) functional assays | The morphology images showed BNNTs with a diameter ranging from 13–23 nm observed with a minimum agglomerate rate. BNNTs showed acute inflammation and toxicity both in vitro and in vivo condition. |
| Sen et al. [46] | CVD | – | Hydroxylated BNNTs modified with oligonucleotides (BNNTs- OH-oligo) and further doped with morpholino | – | MDA-MB-231-luc2 | TEM, FTIR, agarose gel electrophoresis | Cell viability assay, luciferase activity | FTIR spectrum showed the B-N and –OH bands in the BNNTs and TEM images displayed some damaged nanotubes due to hydroxylation. The luciferase activity decreased when MDA-MB-231-luc2 cells were incubated with morpholino/oligo-BNNTs. The cell viability results almost similar to control. |
| Farshid et al. [47] | Commercial BNNTs | Length 1–2 μm and diameter ~100 nm | BNNTs reinforced propylene fumarate (PPF-BNNTs) nanocomposites | 24 h | MC3T3 | TEM, X-ray spectroscopy, Raman spectroscopy, sol-fraction analysis, compressive test | Presto Blue® assay, LDH, Calcein-AM staining, osmolarity of degradation, cell attachment and spreading | BNNTs displayed a tubular morphology with a diameter of 100 nm and length of 1-2 µm. The spectroscopy analysis showed good bands of B-N. Furthermore, the compressive modulus increased up to 6% with the incorporation of BNNTs in PPF. BNNTs reinforced polymer nanocomposite was non-cytotoxic. |
| Emanet et al. [48] | CVD | Length: 5 μm; and diameter 10 nm | BNNTs-OH reinforced chitosan | Up to 7 days | HDF | SEM, TEM, fluorescent microscopy, mechanical, in vitro biodegradation | WST-1 colorimetric assay, cell proliferation and adhesion | The micrograph images showed large pores in the BNNTs-chitosan scaffolds. FTIR spectra of the BNNTs showed the -OH and B-N bands of the modified BNNTs BNNT-OH-chitosan scaffolds showed enhanced mechanical strength and reduced water absorption. The cell viability results showed that increase in viability rate over the incubation time in BNNTs-OH reinforced chitosan |
| Rocca et al. [49] | CVD | Length: 2 μm and diameter ~50 nm | Pectin coated BNNTs (P-BNNTs) | 0 to 50 μg·mL−1; up to 24 h | RAW 264.7 | SEM, TEM, zeta potential | WST-1 assay, quant-iT PicoGreen dsDNA assay, reactive oxygen species, annexin V-FITC apoptosis detection, cytokine detection, qRT-PCR | The results indicated that pectin coated BNNTs significantly improved the dispersibility of BNNTs. Furthermore, the micrograph analysis showed that 65% of cells positively internalised of P-BNNTs without any effects BNNTs with pectin did not show any adverse effects on cells. |
| Niskanen et al. [50] | Boron oxide-assisted chemical vapor deposition (BOCVD) | Length: 15 μm | BNNTs modified with isopropanol, glycine coated BNNTs loaded with curcumin | 0–50 μg·mL−1; up to 72 h | N9 murine microglia | TEM | Confocal and non-confocal fluorescence microscopy, cellular uptake, cell viability, mitochondrial metabolic activity assay, Griess test, ELISA assay | The micrograph analysis reported that BNNTs were successfully coated with glycine and loaded with curcumin. However, the sonication resulted in shortened length and damaged some nanotubes. Non-cytotoxic. |
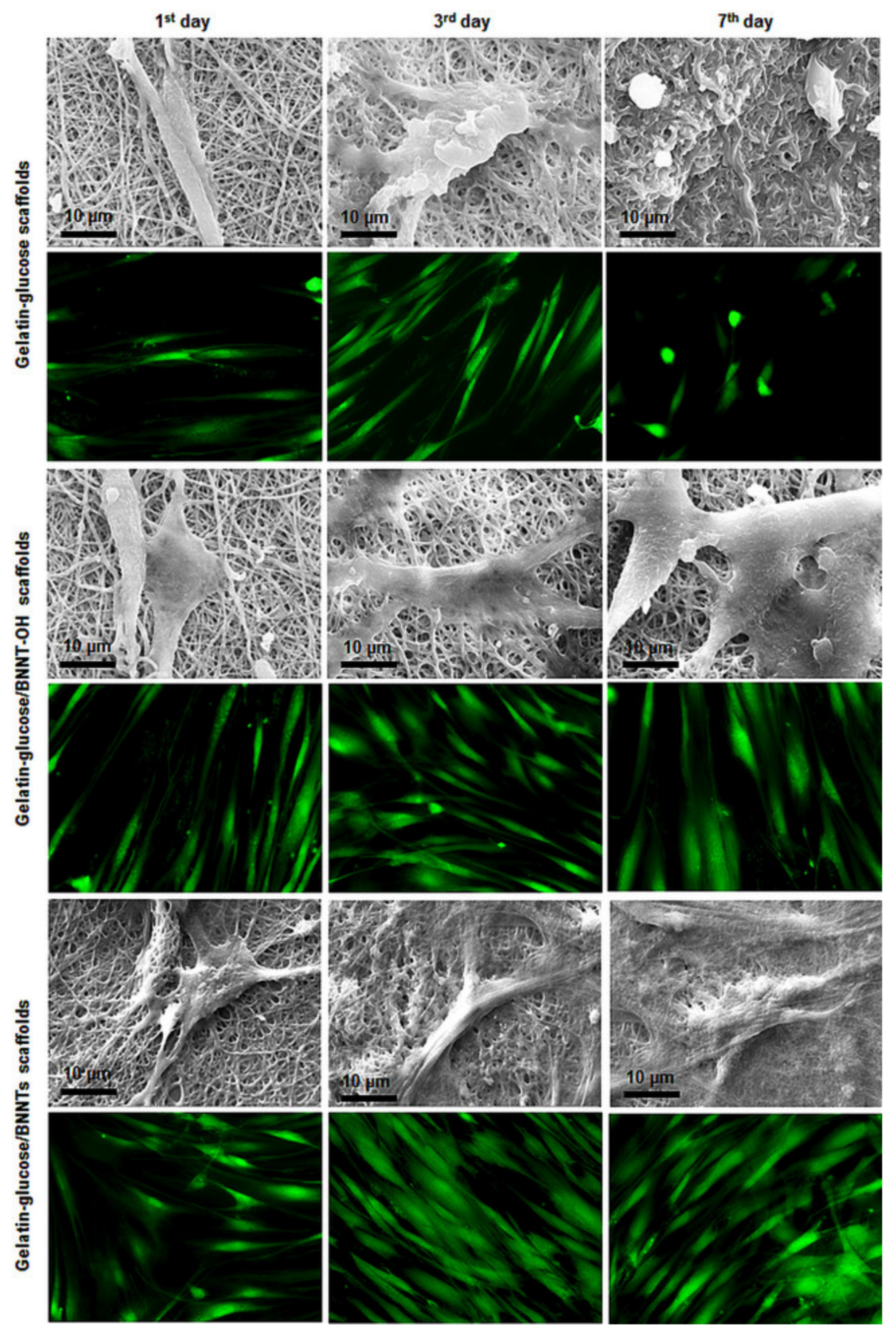
| Sen et al. [51] | CVD | – | BNNTs-OH reinforced gelatine and glucose | 7 days | HDF | SEM, TEM, contact angle, tensile test, in vitro biodegradation | Cell viability, adhesion, and proliferation | The results indicated that the biodegradation amount of the scaffolds was slower with the incorporation of BNNTs. The SEM and fluorescence microscopy images showed that the BNNTs positively impacted cell adhesion and proliferation. The cells retained their own morphology and increased the proliferation rat with inclusion of BNNTs. |
| Li et al. [52] | CVD | Length: 1–2 μm; diameter: 80 nm | – | 0–50 μg·mL−1; up to 14 days | MSCs | SEM, TEM, AFM, protein absorption | Cell viability | The SEM and TEM images of BNNTs showed the nanotubes of 1–2 μm length. The AFM analysis confirmed that BNNTs were uniformly distributed on the surface of piranha solution treated substrate. The protein absorption measurement indicated highest absorption ability with BNNTs on the substrate. BNNTs showed good biocompatibility with MSCs. |
| Diez-Pascual et al. [53] | CVD | – | Polyethylene glycol grafted BNNTs reinforced poly(propylene fumarate) (PEG-g-BNNTs-PPF) | 0, 0.1, 0.5, 1.0, 2.0, 4.0 wt%; up to 24 h | HDF | FESEM, TGA, water uptake, tensile tests, antibacterial action, biodegradability, protein absorption, tribological analysis | Cell viability (alamarBlue assay) | SEM micrographs displayed a random and uniform dispersion of the PEG-g-BNNTs in the PPF. The degree of hydrophilicity, water absorption, protein absorption and biodegradability enhanced with increasing PEG-g-BNNTs content. In addition, the BNNTs nanocomposites did not show toxicity for the adhesion and growth of HDF cells. |
| Fernandez-Yague et al. [54] | PVC | – | Polydopamine (PD) functionalised BNNTs (PD-BNNTs) | 1, 10, 30 µg·mL−1; up to 72 h | Osteoblasts | XPS, TEM, DLS | Live/dead assay | The TEM images indicated that the BNNTs were successfully coated with PD, and XPS analysis confirmed the presence of elemental composition of PD in BNNTs varied from BNNTs. The dispersion of PD-BNNTs in media without any precipitation was confirmed with DLS. The PD-BNNTs do not show any cytotoxic effects on cells. |
| Emanet et al. [55] | CVD | – | BNNTs-OH combined with glucose, lactose and starch | 5 to 200 µg·mL−1; up to 3 days | HDF and A549 | TEM, FTIR, TGA, protein interaction | Cellular uptake, ROS, cell viability, genotoxicity assay | The TEM images displayed the smooth nanotubes, and FTIR analysis confirmed the -OH and B-N bands in modified BNNTs. Furthermore, the results indicated no negative of cells treated with BNNTs. |
| Danti et al. [56] | – | – | BNNTs functionalised myoblast/microfibre mesh constructs | 108 h | C2C12 | SEM | Cellular viability, protein expression, spatial distribution, 4′-6′-diamidino-2-phenylindole staining, phalloidin-Alexa 488 stanning | Micrographs displayed the myotubes on the surface of the BNNTs. The results stated that cells were able to differentiate and to internalise upon treating with BNNTs. |
| Salvetti et al. [57] | CVD | Length: 10 µm; diameter: 10–80 nm | Gum Arabic coated BNNTs (GA− BNNTs) | 100 or 200 µg·g−1; 4 and 24 h; Injected GA-BNNTs | Planarians | TEM, morphometric analysis, Inductive coupled plasma (ICP)-AES | DNA diffusion and comet assay, propidium iodide/JC1 staining, qRT-PCR, phototactic assay, analysis of mitosis | The morphological analysis demonstrated micrometres length of BNNTs, and there were no abnormalities observed after injecting GA-BNNTs into planarians. BNNTs did not induce DNA damage or apoptosis or does not show harmful effects on planarian stem cells. |
| Ferreira et al. [58] | CVD | Length–1 µm | BNNTs functionalised with folic acid (FA-BNNTs) | 0–50 µg·mL−1; 1 and 3 days | HeLA | FTIR, XPS, TGA, TEM, ICP microscopy | WST-1 assay, cell uptake, lysosome staining | The FTIR analysis demonstrated bands related to B-N and C=O in Fa-BNNTs. The XPS analysis displayed strong B and N bonds in FA-BNNTs. The microscopy analysis displayed a hallow inner channel with a detailed tubular structure of nanotubes. FA-BNNTs displayed increased in cellular uptake compared to pure BNNTs. |
| Nakamura et al. [59] | – | – | Poly(ethyleneglycol)–1,2–distearoyl–sn–glycero–3–phosphoethanolamine (mPEG–DSPE) functionalised BNNTs (BNNTs–DSPE–PEG2000) | – | B16 | – | MTT assay | BNNTs-DSPE-PEG2000 displayed antitumor effect on cells incubated over the time. |
| Ferreria et al. [60] | CVD | Diameter: 70 nm | Gum Arabic (GA) functionalised BNNTs (GA-BNNTs) | 0–50 µg·mL−1; 1, 3, and 7 days | Rat MSCs | TEM, FTIR, Raman spectroscopy, DLS | Cell viability, metabolic activity, cytoskeleton conformation, differentiation of stem cells into adipocytes and osteocytes at gene and phenotype | TEM images of the BNNTs displayed hallow inner channels of nanotubes, and spectroscopy results showed the presence of B and N bands. The toxicity analysis showed that BNNTs were cyto-compatible with non-toxic effects on cells. |
| Li et al. [61] | CVD | – | Europium functionalised BNNTs and doped with sodium gadolinium (BNNTs@NaGdF4:Eu) | 0–50 µg·mL−1; 3 and 20 h | Human LNcap prostate cancer cells | X-ray spectrometry (XRS), TEM | Cellular uptake | Micrograph images showed nanotube with inner shells coated with EU and GD. BNNTs@NaGdF4:Eu displayed higher cell uptake and displayed improvement of chemotherapy efficacy through magnetic fields. |
| Barachini et al. [62] | Ball milling and annealing | – | PLL functionalised BNNTs | 0–10 µg·mL−1; up to 72 h | Human dental pulp stromal cells | UV–vis spectrophotometer, SEM, TEM | Cell viability, double stranded (ds-DNA) and glycosaminoglycan (GAG) contents, histological analysis | The micrographs showed that PLL-BNNTs internalised inside cytoplasm vesicles of a single DPSC. Non-cytotoxic. |
| Nitya et al. [63] | CVD | – | BNNTs functionalised with four surfactants: Pluronic (P123), polyethyleneimine (PEI), Pluronic (F127), and ammonium oleate (A.O.) | 15.62, 31.25, 62.5, 125, 250, 500 and 1000 µg·mL−1; 24 h | Vero, Chang liver, MCF7 and A549 | XRD, TEM, XPS | MTT assay, DNA fragmentation assay, acridine orange staining, ethidium bromide stanning | The XRD showed the hexagonal lattice of boron nitride and TEM images confirmed the presence of multiwalled BNNTs. BNNTs functionalised with four surfactants resulted in good cytocompatibility. |
| Ciofani et al. [64] | CVD | Length: 10 µm; diameter: 1.5 nm | GA-BNNTs | 0–50 µg·mL−1; up to 72 h | SH-SY5Y and HUVECs | SEM | WST-1 assay, annexin V-FITC/propidium iodide (PI) apoptosis analysis, ROS, cytoskeleton analysis, immunofluorescence, qRT-PCR, detection of endothelial adhesion molecule expression | The morphology images showed that BNNTs were internalised in the cells. BNNTs with high purity about 20 µg·mL−1 displayed good biocompatibility. |
| Ferreria et al. [65] | CVD | – | BNNTs functionalised with glucosamine (GA), polyethylene glycol (PEG) 1000, and chitosan (CH) | 0 to 100 µg·mL−1; 48 h | MRC-5 | FTIR, TGA, TEM, XRD, photon correlation spectroscopy and zeta potential analysis, physical stability study, fluorescence microscope | MTT assay, ROS | The results indicated that BNNTs were successfully obtained and functionalised, achieving a standard size and dispersity considered satisfactory for in vitro studies. BNNTs functionalised with PEG and chitosan showed significant cell damage and increase cytotoxicity at higher concentration (above 50 µg·mL−1). However, the results stated that no considerable changes in cell morphology or increase in ROS. |
| Danti et al. [66] | Ball milling and annealing | – | poly-L-lysine-(PLL) coated BNNTs | 0–20 µg·mL−1; up to 72 h | hOB | UV–vis/NIR spectrophotometer, TEM, Zeta potential distribution | MTT assay, ROS, annexin V-FITC/PI, cellular uptake, investigation of BNNTs-treated hOB cells under ultrasound irradiation, gene expression, biochemical assay, histologic analyses | The evaluation with TEM or spectroscopy confirmed that PLL-BNNTs were internalised at cytoplasm level and were noticed in membranal vesicles. The results stated PLL-BNNTs were non-cytotoxic. |
| Turco et al. [67] | Annealing | – | Glycol (G)-chitosan (C)-coated boron nitride nanotubes(GC-BNNTs) | 0–100 µg·mL−1; up to 72 h | HUVECs | TEM, SEM, XRS and immunofluorescence microscopy | Cell viability, cell proliferation, surface enzyme immunoassay, cytoskeleton organisation and focal adhesions analysis, endothelial adhesion molecule expression | The SEM and TEM images displayed non-continuous nanotubes with no presence of regular stacking single units. TEM analysis indicated cellular internalisation after treating cells with GC-BNNTs. GC-BNNTs did not show adverse effects on cell biology or DNA damage, which resulted in non-cytotoxicity. |
| Ciofani et al. [68] | Annealing | – | Gadolinium coated BNNTs (Gd-BNNTs) | 0–100 µg·mL−1; up to 72 h | SH-SY5Y | ICP-MS, XRS, TEM | WTS-1 assay and DNA content quantification, cell labelling using MRI experiments | The TEM images displayed defects on the nanotubes due to functionalisation. The ICP-MS and XRS confirmed the presence of B and N elements in BNNTs. Furthermore, the EDX and ICP analyses showed Gd-BNNTs as a favourable negative contrast agent. It was stated that Gd-BNNTs were biocompatible with their ability to efficiently label and distinguish in MRI images at 7 T. |
| Ciofani et al. [69] | Annealing | Length–500 nm | GC-BNNTs | 5 and 10 mg·kg−1; up to 7 days; injected into the marginal ear vein of animals | New Zealand male rabbits | DLS, SEM, TEM, X-ray spectroscopy | Blood analysis, pharmacokinetic analysis, objective symptoms such as sweating, excitement, trembling, and head nodding were analysed | The morphology images displayed bamboo-like nanotubes. The DLS confirmed good dispersion in aqueous media after modification with GC.Results stated that all doses were extremely endured by the animals, with no indication of major effects. |
| Ciofani et al. [70] | Annealing | – | BNNTs-OH coated with 3-aminopropyl-triethoxysilane (APTES) | 0–100 µg·mL−1; up to 24 h | NIH/3T3 | Z-potential analysis, X-ray spectroscopy, SEM, TEM, XPS | WST-1 assay, ds-DNA quantification, cell internalisation analysis, actin staining | The atomic composition analysis confirmed the maximum percentage of B and N atoms present in BNNTs. The SEM/TEM images displayed nanotubes with small bundles of nanotubes. The functionalised BNNTs resulted in good cytocompatibility at higher concentration (100 µg·mL−1). |
| Soares et al. [71] | Metallic oxide-assisted chemical vapor transport | – | GC-BNNTs coated with radioelement 99mTc | 5 and 40 mg·kg−1; 10 and 30 min, 1 and 24 h; Injected intravenously into the tail of Swiss mice | Swiss mice | SEM, TGA, FTIR, photon correlation spectroscopy, zeta potential analysis | Radioactivity analysis, scintigraphy imaging biodistribution analysis | The morphology images confirmed the nanotubes coated with GC. The FTIR spectrum confirmed strong bands of B-N in BNNTs and −OH, C=H and C=H in GC-BNNTs. The TGA results displayed that BNNTs had less weight loss compared to GC-BNNTs. The in vivo distribution analysis indicated that major elimination of BNNTs by renal excretion and accumulation in the liver, spleen, and intestines. |
| Ciofani et al. [72] | Annealing | – | GC-BNNTs | 1 mg·kg−1; 2, 24, and 72 h; injected into the marginal ear vein of animals | New Zealand male rabbits | FIB, TEM, AFM, Size distribution, Z-potential analysis | Blood analysis to evaluate hematic parameters and live and kidney functionality | The FIB and TEM images of BNNTs showed the presence of bamboo-like shape nanotube structures with diameter ranging between 30 and 100 nm. The AFM images revealed that nanotubes edges decorated with globular structures. Z-potential analysis demonstrated good stability of GC-BNNTs dispersion in aqueous medium. GC-BNNTs did not cause any organ failure or effects on blood parameters. |
| Menichetti et al. [73] | Ball milling | – | PLL-BNNTs | 1–100 µg·mL−1; up to 72 h | SH–SY5Y | MRI experiments, UV–vis/NIR spectrophotometry | MTT assay, metabolic activity testing, cell adhesion | The PLL-BNNTsnoted at 3T showed considerable signal attenuation with increasing the concentration of BNNTs. The PLL-BNNTs compatibility in vitro at least up to 100 μg·mL−1. |
| Horvath et al. [74] | – | – | – | 0.05, 1, and 2 µg·mL−1; up to 6 days | A549, RAW 264.7, 3T3-L1, HEK 293 | SEM, TEM | Cytopathological analyses, MTT assay, FMCA assays, DNA assays | The BNNTs morphology images showed multiwalled nanotubes found in the plasmathe membrane of the cells. BNNTs induced higher toxicity in all the cell lines. |
| Lahiri et al. [75] | Commercial | Length–0.4–5.8 µm; diameter 10–145 nm | BNNTs reinforced hydroxyapatite (BNNTs-HA) | 1, 3, and 5 days | Osteoblasts | SEM, TEM, XRD, nanoindentation, Vickers indent impression | Cell viability | The SEM/TEM images showed the nodular and cylindrical shaped BNNTs. The XRD results confirmed the hexanol lattice of B and N atoms. The composite with the highest BNNTs concentration displayed excellent mechanical properties. The BNNTs-HA did not induce any significant effects on cells. |
| Ciofani et al. [76] | Annealing | – | GC-BNNTs | 0–100 µg·mL−1; up to 48 h | SH-SY5Y cells | SEM, TEM, UV–vis | MTT assay, WST-1 assay, DNA content assessment, ROS, annexin V-FITC with PI Early apoptosis detection | The SEM/TEM images showed a bamboo-shaped nanotube. Furthermore, the UV-vis spectrum confirmed strong absorption at 5.5 eV related to BNNTs. The cytotoxicity results with MTT-assay interfered the toxicity results and resulted in wrong toxicity data at low concentrationwhile WST-1 showed non-toxicity above 50 µg·mL−1. The results stated no significant ROS or apoptosis up to 100 µg·mL−1. |
| Lahiri et al. [77] | Commercial | – | Polylactide-polycaprolactone copolymer (PLC) reinforced with BNNTs (PLC−BNNTs) | 0, 2 and 5 wt% | Osteoblasts, murine macrophages | SEM, XRD, micro-Raman spectroscopy, tensile tests | Cell viability, gene expression, nucleic acid isolation, qRT-PCR | The SEM images displayed both tubular and bamboo-shaped nanotubes. The spectroscopy strong BNNTs as well as co-polymer peaks in PLC-BNNT. The elastic modulus of PLC-BNNTs increased up to 1370% with an increase in BNNTs concentration. PLC-BNNTs incubated with cells did not increase in rate of cell death and hence resulted in non-cytotoxicity. |
| Ciofani et al. [78] | Ball milling and annealing | – | PLL-BNNTs | Up to 72 h | C2C12 | TEM | MTT assay, live/dead assay using annexin V-FITC, metabolic activity, apoptosis detection, double stranded (ds)-DNA and protein quantification, qRT-PCR, gel electrophoresis, Western blot analysis, immunocytochemistry | The TEM images confirmed the stable dispersion with a small amount of aggregates nanotubes in dispersion agents. PLL-BNNTs did not show any difference in MyOD and Cx43 gene expression. The viability results indicated excellent cell proliferation and metabolic activity up to concentration of 10 µg·mL−1. |
| Raffa et al. [79] | Ball milling and annealing | Radius–40 nm | PLL-BNNTs | Up 24 h | SH-SY5Y | UV–vis/NIR (near-infrared) spectrophotometer, focused ion beam (FIB) microscopy, electroporation analysis | MTT assay | The UV–vis/NIR quantification reported the best and repeatability absorption of PLL-BNNTs. The microscopy images showed the bundles of nanotubes. The cells exposed to BNNTs facilitated electroporation displayed excellent cell viability, metabolism, and proliferation. |
| Ciofani et al. [80] | Ball milling and annealing method | – | Folic acid (FA)-PLL-BNNTs | 10 µg·mL−1; 24 h | T98G | FIB microscopy, UV-vis spectroscopy, Z-potential | MTT assay, cellular uptake, lysosome tracking assay, Quantum dots labelling images | The FIB images showed that the FA-PLL-BNNTs could be internalised by tumour cells. The UV-vis analysis displayed firm peaks for BNNTs and PLL-BNNTs. The Z-potential evaluation showed the strong positive Z-signals for FA-PLL-BNNTs. The functionalised BNNTs indicated ability to treat malignant cerebral tumours. |
| Chen et al. [81] | CVD | – | – | 100 mg·mL−1; up to 4 days | HEK 293 | TEM | Cell count and cell viability using annexin V-FITC/PI assay | The microscopy images showed high purity multiwalled BNNTs. BNNTs demonstrated non-cytotoxicity. |
| Ciofani et al. [82,83] | Ball milling and annealing | – | Polyethyleneimine (PEI)-coated BNNTs | 10 µg·mL−1; up to 72 h | SH-SY5Y | TEM, UV–vis/NIR spectrophotometer | Trypan blue exclusion viability assay, MTT cell proliferation assay, cell uptake, cell imaging using fluorescent microscope | The morphology images showed a bundle of nanotubes. Furthermore, the cell treated with BNNTs did not show any evidence of cell morphology changes. BNNTs treated with cells indicated no considerable effects on viability, metabolism, and cellular replication of this cell line. |
| Methods | Temperature (°C) | References |
|---|---|---|
| Arc-discharge | >3426.85 | [3,85,87,108,109,110] |
| Laser ablation | 1200–5000 | [96,98,111,112,113] |
| Ball mill/annealing | 1000–1300 | [6,88,89,90,91,92,114,115,116,117,118,119,120,121] |
| Template synthesis | 500–1580 | [122,123,124,125,126,127,128,129] |
| Thermal plasma | >526.85 | [84,100,101,102,130] |
| CVD | 1100–1700 | [131,132,133,134,135,136,137,138,139,140,141,142,143] |
| Autoclave | 450–600 | [107,144,145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakarla, A.B.; Kong, I. In Vitro and In Vivo Cytotoxicity of Boron Nitride Nanotubes: A Systematic Review. Nanomaterials 2022, 12, 2069. https://doi.org/10.3390/nano12122069
Kakarla AB, Kong I. In Vitro and In Vivo Cytotoxicity of Boron Nitride Nanotubes: A Systematic Review. Nanomaterials. 2022; 12(12):2069. https://doi.org/10.3390/nano12122069
Chicago/Turabian StyleKakarla, Akesh Babu, and Ing Kong. 2022. "In Vitro and In Vivo Cytotoxicity of Boron Nitride Nanotubes: A Systematic Review" Nanomaterials 12, no. 12: 2069. https://doi.org/10.3390/nano12122069
APA StyleKakarla, A. B., & Kong, I. (2022). In Vitro and In Vivo Cytotoxicity of Boron Nitride Nanotubes: A Systematic Review. Nanomaterials, 12(12), 2069. https://doi.org/10.3390/nano12122069







