LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization
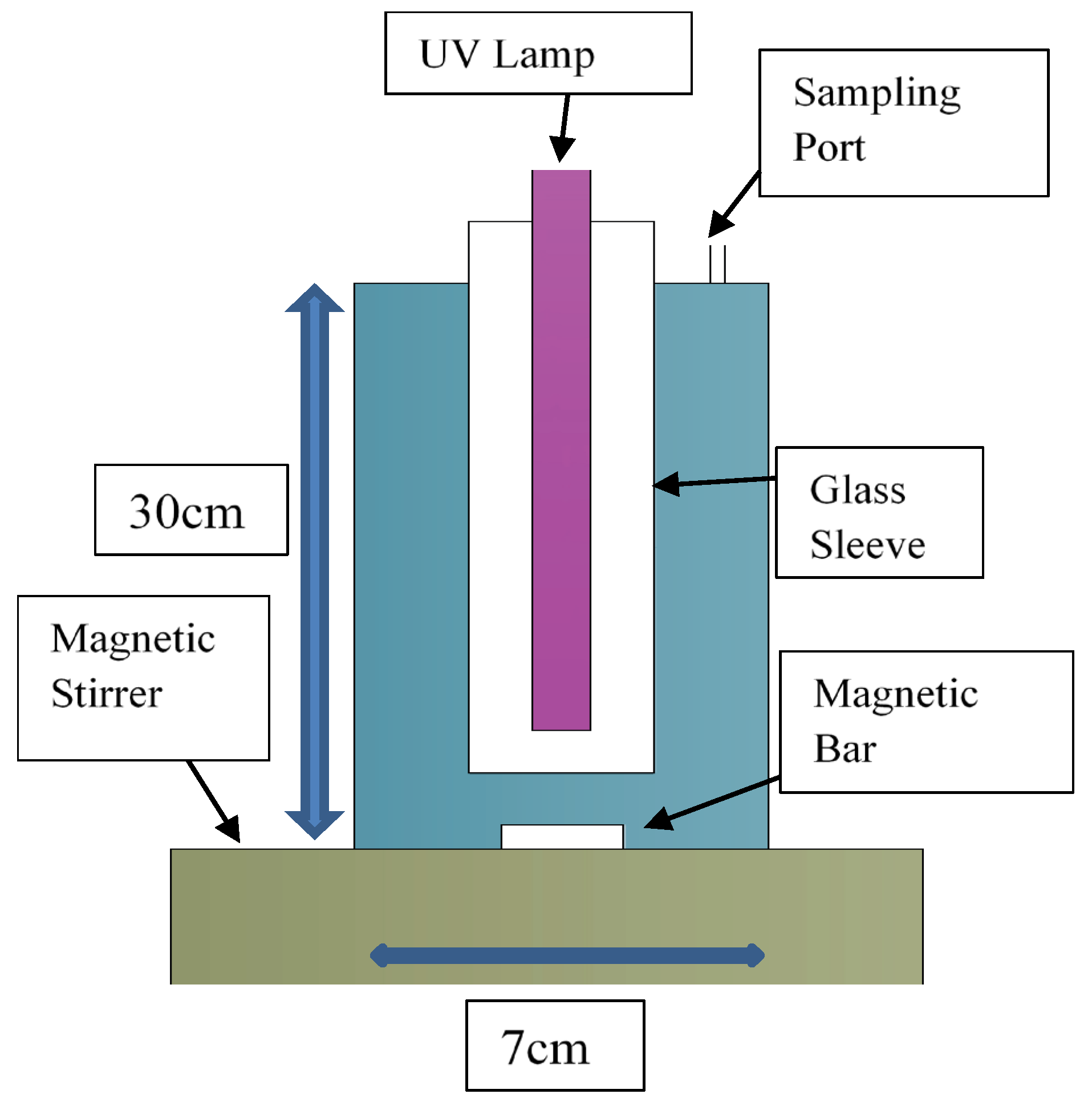
2.3. Photocatalytic Degradation (PCD) Experiments
2.4. Response Surface Methodology (RSM)
3. Results
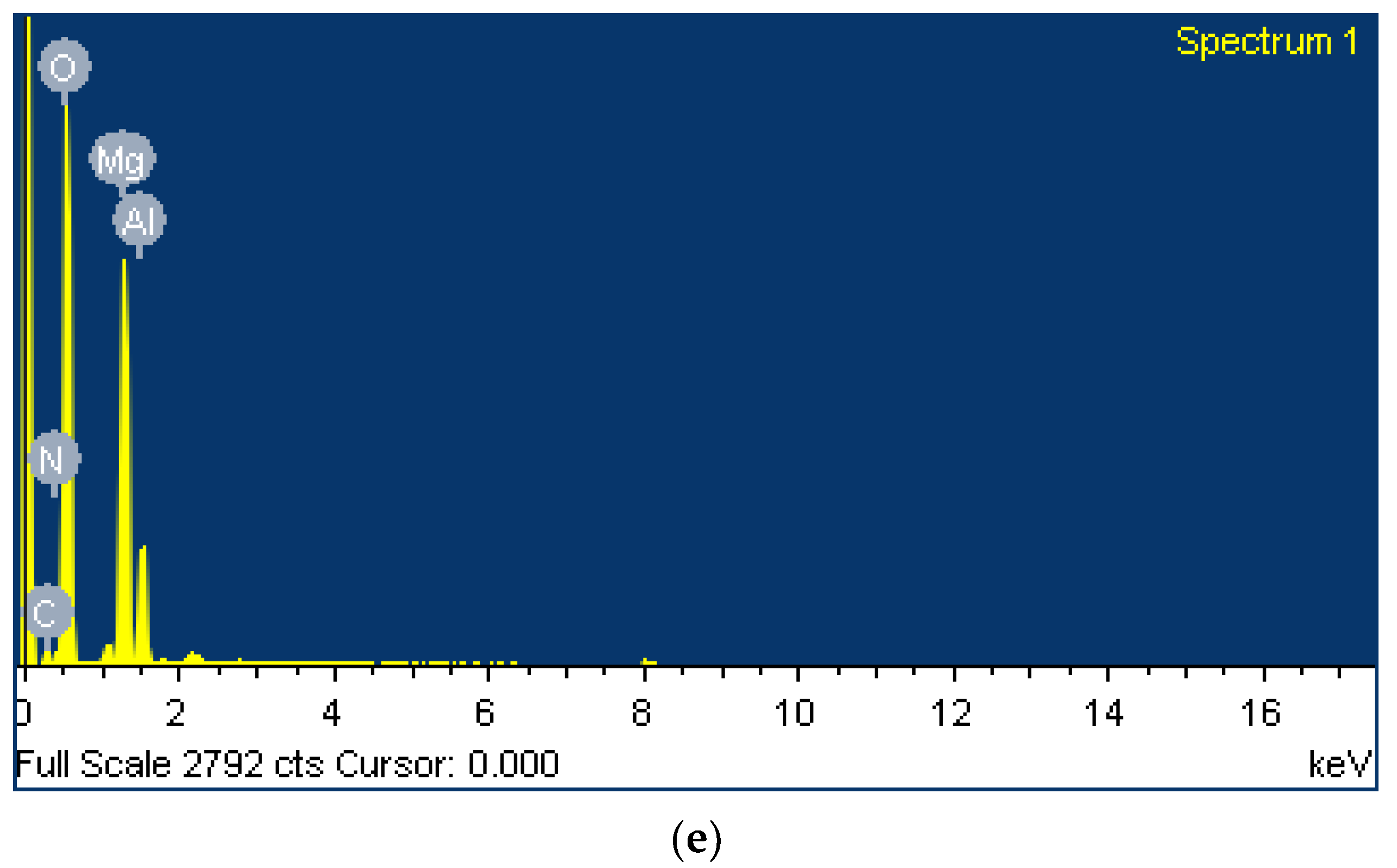
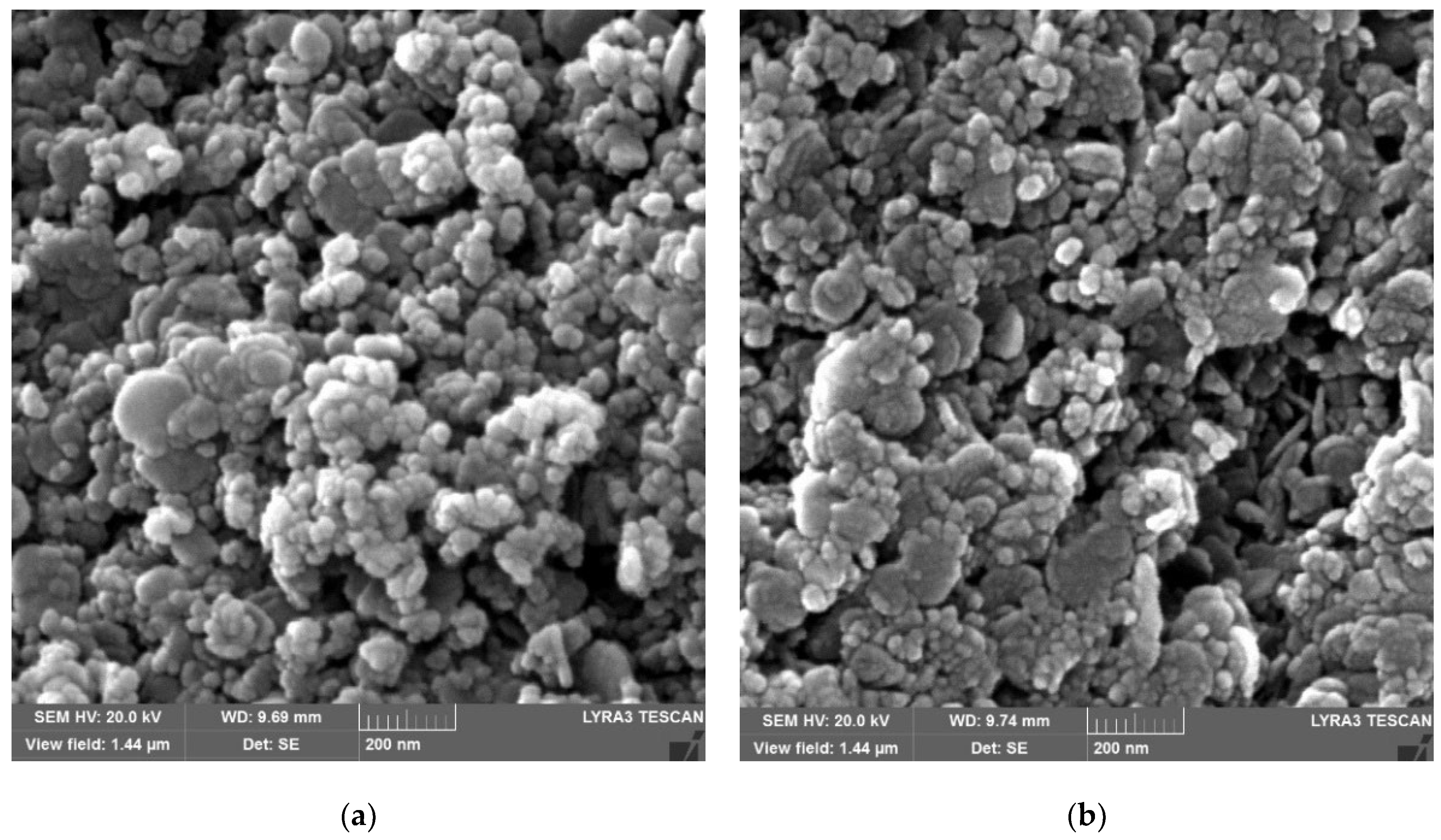
3.1. LDH and LDH-TiO2 Matrix Characterization
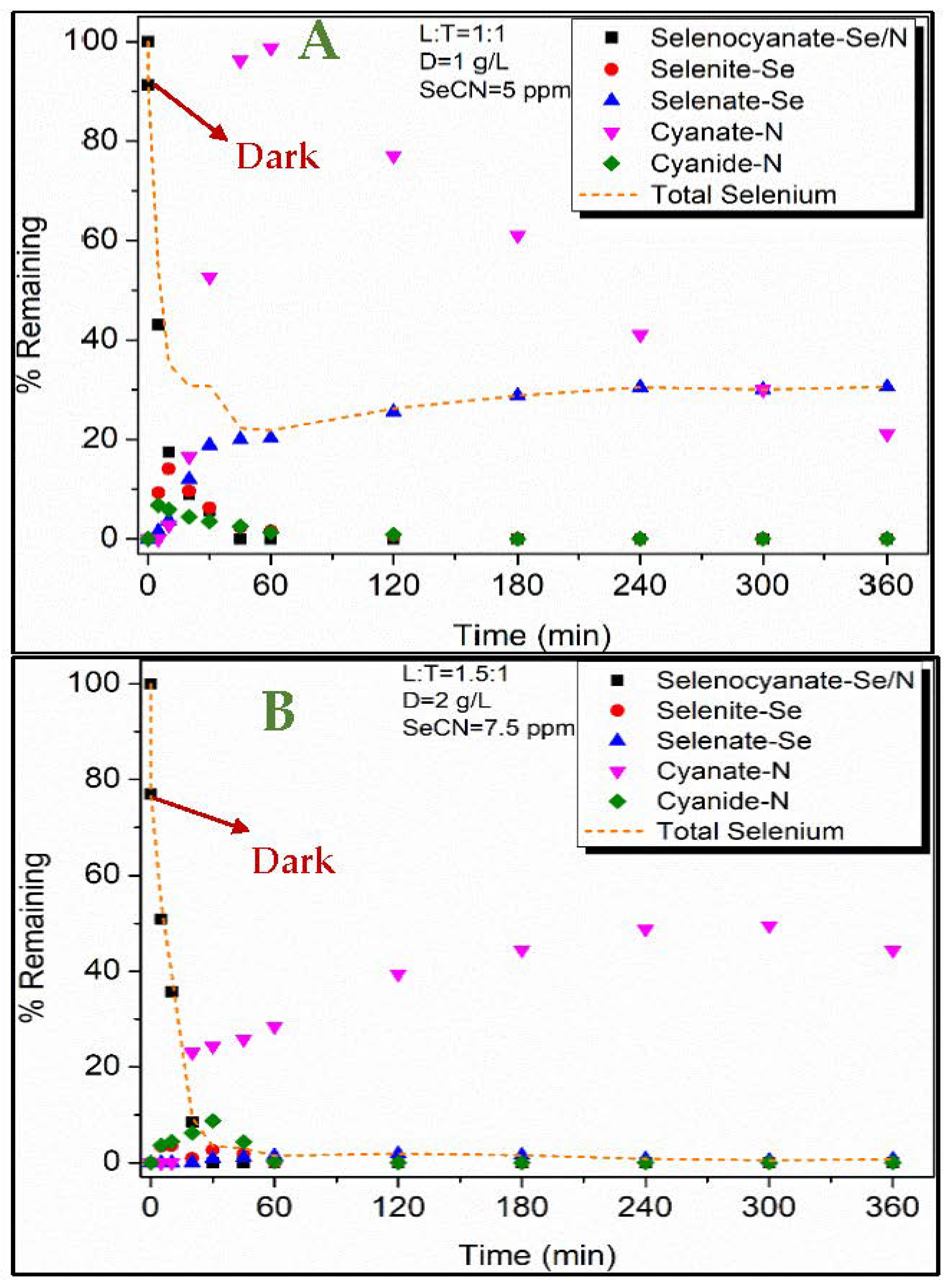
3.2. Selenocyanate Photocatalytic Degradation Using LDH:TiO2 Matrix
3.3. RSM Modeling of Photocatalytic Degradation Process
| A | = | LDH:TiO2 ratio (0.5:1.5); |
| B | = | adsorbent dosage (1:2 g/L); |
| C | = | selenocyanate concentration (2.5 to 7.5 mg/L); |
| Residual SeO42− | = | residual concentration of selenate in solution after 6 h of UV irradiation (mg/L); |
| TSR | = | selenocyanate removal efficiency expressed as total selenium removed (%). |
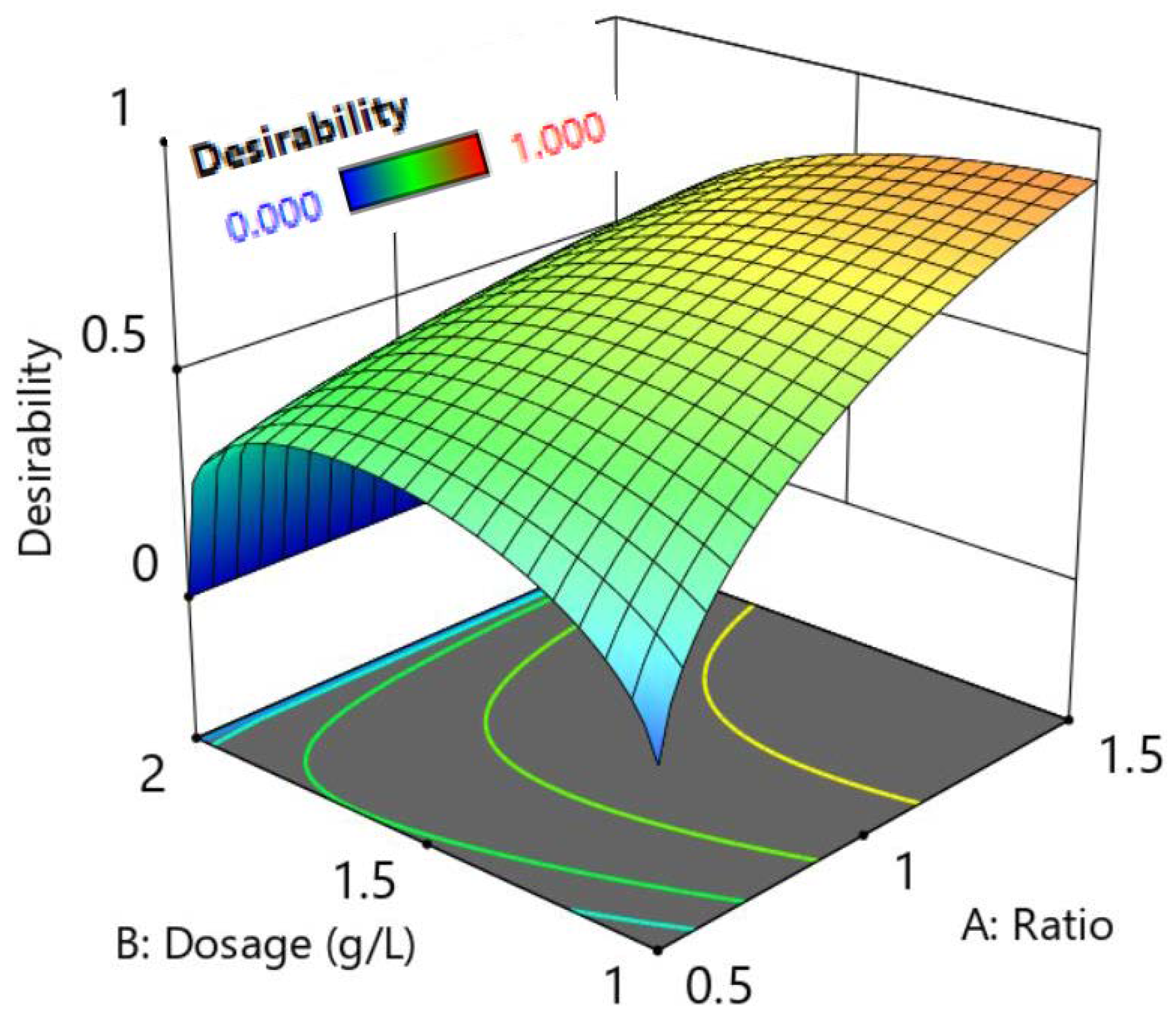
3.4. Optimization of the Photocatalytic Degradation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balaji, E.; Nagaraju, A.; Sreedhar, Y.; Thejaswi, A.; Sharifi, Z. Hydrochemical characterization of groundwater in around Tirupati Area, Chittoor District, Andhra Pradesh, South India. Appl. Water Sci. 2016, 7, 1203–1212. [Google Scholar] [CrossRef]
- Abdelkader, B.; Khan, M.; Antar, M.; Khalifa, A. Performance of bubble column humidification-dehumidification (HDH) desalination system. Desalin. Water Treat. 2020, 182, 101–112. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. ISBN 978-94-007-4375-5. [Google Scholar]
- Zhang, L.; Liu, N.; Yang, L.; Lin, Q. Sorption behavior of nano-TiO2 for the removal of selenium ions from aqueous solution. J. Hazard. Mater. 2009, 170, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Bang, S.; Korfiatis, G.P. Removal of selenocyanate from water using elemental iron. Water Res. 2002, 36, 3867–3873. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pickering, I.J.; Walla, M.; Terry, N. Selenium assimilation and volatilization from selenocyanate-treated Indian mustard and muskgrass. Plant Physiol. 2002, 128, 625–633. [Google Scholar] [CrossRef]
- Constantino, L.V.; Quirino, J.N.; Monteiro, A.M.; Abrão, T.; Parreira, P.S.; Urbano, A.; Santos, M.J. Sorption-desorption of selenite and selenate on Mg-Al layered double hydroxide in competition with nitrate, sulfate and phosphate. Chemosphere 2017, 181, 627–634. [Google Scholar] [CrossRef]
- Guia, M.; Pappa, J.K.; Colburna, A.S.; Meeksb, N.D.; Weaverc, B.; Wilfc, I.; Bhattacharyyaa, D. Engineered Iron/Iron Oxide Functionalized Membranes for Selenium and Other Toxic Metal Removal from Power Plant Scrubber Water. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Hu, Y.; Bird, M.I.; de Nys, R. Gracilaria waste biomass (sampah rumput laut) as a bioresource for selenium biosorption. J. Appl. Phycol. 2014, 27, 611–620. [Google Scholar] [CrossRef]
- Bakather, O.Y.; Kayvani Fard, A.; Ihsanullah; Khraisheh, M.; Nasser, M.S.; Atieh, M.A. Enhanced Adsorption of Selenium Ions from Aqueous Solution Using Iron Oxide Impregnated Carbon Nanotubes. Bioinorg. Chem. Appl. 2017, 2017, 4323619. [Google Scholar] [CrossRef]
- Kazeem, T.S.; Labaran, B.A.; Ahmed, H.-R.; Mohammed, T.; Essa, M.H.; Al-Suwaiyan, M.S.; Vohra, M.S. Treatment of Aqueous Selenocyanate Anions Using Electrocoagulation. Int. J. Electrochem. Sci. 2019, 14, 10538–10564. [Google Scholar] [CrossRef]
- Murphy, A.P. Removal of selenate from water by chemical reduction. Ind. Eng. Chem. Res. 1988, 27, 187–191. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Q.; Chen, G.; Liu, H.; Qu, J. Removal of Se(IV) and Se(VI) from drinking water by coagulation. Sep. Purif. Technol. 2015, 142, 65–70. [Google Scholar] [CrossRef]
- Patwardhan, R.; Grove, L.; Ball, J.E.; Bollapragada, K.; Caskey, S.R. Removal of Selenocyanate from Industrial Water Systems with Sulfided Metal Adsorbents. US 2018/0319674 A1, 8 November 2018. [Google Scholar]
- Wang, J.; Zhang, T.; Li, M.; Yang, Y.; Lu, P.; Ning, P.; Wang, Q. Arsenic removal from water/wastewater using layered double hydroxide derived adsorbents, a critical review. RSC Adv. 2018, 8, 22694–22709. [Google Scholar] [CrossRef] [PubMed]
- Matusik, J.; Rybka, K. Removal of chromates and sulphates by Mg/Fe LDH and heterostructured LDH/halloysite materials: Efficiency, selectivity, and stability of adsorbents in single- and multi-element systems. Materials 2019, 12, 1373. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Leiviskä, T. Mg/Al LDH enhances sulfate removal and clarification of AMD wastewater in precipitation processes. Materials 2019, 12, 2334. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Strączek, T.; Kapusta, C.; Woch, W.M.; Tokarz, W.; Radziszewska, A.; Leiviskä, T. Highly effective magnet-responsive LDH-Fe oxide composite adsorbents for As(V) removal. Chem. Eng. J. 2019, 362, 207–216. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- Carja, G.; Nakajima, A.; Dranca, S.; Dranca, C.; Okada, K. TiO2/ZnLDH as a self-assembled nanocomposite with photoresponsive properties. J. Phys. Chem. C 2010, 114, 14722–14728. [Google Scholar] [CrossRef]
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.Z.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48. [Google Scholar] [CrossRef]
- Ren, J.; Hu, T.; Gong, Q.; Wang, Q.; Sun, B.; Gao, T.; Cao, P.; Zhou, G. Spherical bi2 wo6 /bi2 s3 /mos2 n-p heterojunction with excellent visible-light photocatalytic reduction cr(Vi) activity. Nanomaterials 2020, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, J.; Liu, L.; Yang, W.; Low, J.; Gao, X.; Fu, F. Synergistic effect of oxygen defect and doping engineering on S-scheme O-ZnIn2S4/TiO2-x heterojunction for effective photocatalytic hydrogen production by water reduction coupled with oxidative dehydrogenation. Chem. Eng. J. 2022, 430, 133125. [Google Scholar] [CrossRef]
- Sun, B.; Tao, F.; Huang, Z.; Yan, W.; Zhang, Y.; Dong, X.; Wu, Y.; Zhou, G. Ti3C2 MXene-bridged Ag/Ag3PO4 hybrids toward enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2021, 535, 147354. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Gao, T.; Li, H.; Ren, Y.; Zhou, G. Ti3C2 MXene co-catalyst assembled with mesoporous TiO2 for boosting photocatalytic activity of methyl orange degradation and hydrogen production. Chin. J. Catal. 2022, 43, 461–471. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Jianna, F.; Villaluz, A.; Daniel, M.; De Luna, G.; Colades, J.I.; Garcia-segura, S.; Lu, M. Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron (II) sulfate-doped nano-titania. Process Saf. Environ. Prot. 2019, 125, 121–128. [Google Scholar] [CrossRef]
- Tan, T.T.Y.; Yip, C.K.; Beydoun, D.; Amal, R. Effects of nano-Ag particles loading on TiO2 photocatalytic reduction of selenate ions. Chem. Eng. J. 2003, 95, 179–186. [Google Scholar] [CrossRef]
- Vohra, M.S.; Al-Suwaiyan, M.S.; Essa, M.H.; Chowdhury, M.M.I.; Rahman, M.M.; Labaran, B.A. Application of Solar Photocatalysis and Solar Photo-Fenton Processes for the Removal of Some Critical Charged Pollutants: Mineralization Trends and Formation of Reaction Intermediates. Arab. J. Sci. Eng. 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- Vohra, M.S.; Selimuzzaman, S.M.; Al-Suwaiyan, M.S. Aqueous phase thiosulfate removal using photo catalysis. Int. J. Environ. Res. 2011, 5, 247–254. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Beyers, E.; Mertens, M.; Zhu, H.Y.; Vansant, E.F.; Cool, P. New TiO2/MgAl-LDH nanocomposites for the photocatalytic degradation of dyes. J. Nanosci. Nanotechnol. 2010, 10, 8227–8233. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.P.; Valenzuela, M.A.; Fetter, G.; Flores, S.O. TiO2/MgAl layered double hydroxides mechanical mixtures as efficient photocatalysts in phenol degradation. J. Phys. Chem. Solids 2011, 72, 914–919. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Lu, Y.; Wang, X.; Zhu, N.; Dang, Z. Enhancement of photocatalytic degradation of dimethyl phthalate with nano-TiO2 immobilized onto hydrophobic layered double hydroxides: A mechanism study. J. Hazard. Mater. 2013, 246–247, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Hatami, M. LDH-VB9-TiO2 and LDH-VB9-TiO2/crosslinked PVA nanocomposite prepared via facile and green technique and their photo-degradation application for methylene blue dye under ultraviolet illumination. Appl. Clay Sci. 2018, 163, 235–248. [Google Scholar] [CrossRef]
- Suh, M.J.; Shen, Y.; Chan, C.K.; Kim, J.H. Titanium Dioxide-Layered Double Hydroxide Composite Material for Adsorption-Photocatalysis of Water Pollutants. Langmuir 2019, 35, 8699–8708. [Google Scholar] [CrossRef]
- Djeda, R.; Mailhot, G.; Prevot, V. Porous layered double hydroxide/TiO2 photocatalysts for the photocatalytic degradation of orange II. ChemEngineering 2020, 4, 39. [Google Scholar] [CrossRef]
- Yang, L.; Shahrivari, Z.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. Removal of Trace Levels of Arsenic and Selenium from Aqueous Solutions by Calcined and Uncalcined Layered Double Hydroxides (LDH). Ind. Eng. Chem. Res. 2005, 44, 6804–6815. [Google Scholar] [CrossRef]
- Li, M.; Chowdhury, T.; Kraetz, A.; Jing, H.; Dopilka, A.; Farmen, L.; Sinha, S.; Chan, C. Layered Double Hydroxide Sorbents for Removal of Selenium from Power Plant Wastewaters. ChemEngineering 2019, 3, 20. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.; Tian, X.; Yang, C.; Li, Y. Superior capability of MgAl2O4 for selenite removal from contaminated groundwater during its reconstruction of layered double hydroxides. Sep. Purif. Technol. 2017, 176, 66–72. [Google Scholar] [CrossRef]
- Li, M.; Farmen, L.M.; Chan, C.K. Selenium Removal from Sulfate-Containing Groundwater Using Granular Layered Double Hydroxide Materials. Ind. Eng. Chem. Res. 2017, 56, 2458–2465. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; Mccarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 4. [Google Scholar] [CrossRef]
- Pang, X.; Chen, L.; Liu, Y.; Chi, M.; Li, Z.; Plank, J. Growth behavior of water dispersed MgAl layered double hydroxide nanosheets. RSC Adv. 2017, 7, 14989–14997. [Google Scholar] [CrossRef]
- Seftel, E.M.; Niarchos, M.; Mitropoulos, C.; Mertens, M.; Vansant, E.F.; Cool, P. Photocatalytic removal of phenol and methylene-blue in aqueous media using TiO2@LDH clay nanocomposites. Catal. Today 2015, 252, 120–127. [Google Scholar] [CrossRef]
- Han, E.; Vijayarangamuthu, K.; Youn, J.-S.; Park, Y.K.; Jung, S.C.; Jeon, K.J. Degussa P25 TiO2 modified with H2O2 under microwave treatment to enhance photocatalytic properties. Catal. Today 2018, 303, 305–312. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, K.; Pavlovic, I.; Barriga, C. Nanohybrid Layered Double Hydroxides Used to Remove Several Dyes from Water. ChemEngineering 2019, 3, 41. [Google Scholar] [CrossRef]
- Nejati, K.; Mokhtari, A.; Khodam, F.; Rezvani, Z. Syntheses of Mg-Al-NO3 layered double hydroxides with high crystallinity in the presence of amines. Can. J. Chem. 2015, 94, 66–71. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Aşçı, Y.S. Removal of textile dye mixtures by using modified Mg–Al–Cl layered double hydroxide (LDH). J. Dispers. Sci. Technol. 2017, 38, 923–929. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Q.; Hou, J.; Pei, L.; Li, Y.; Ai, S. Platinum nanocrystals supported on CoAl mixed metal oxide nanosheets derived from layered double hydroxides as catalysts for selective hydrogenation of cinnamaldehyde. J. Catal. 2015, 331, 193–202. [Google Scholar] [CrossRef]
- Hayashi, A.; Kubota, M.; Okamura, M.; Nakayama, H. Complex formation with layered double hydroxides for the remediation of hygroscopicity. Chem. Pharm. Bull. 2015, 63, 13–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sikhwivhilu, L.M.; Sinha Ray, S.; Coville, N.J. Influence of bases on hydrothermal synthesis of titanate nanostructures. Appl. Phys. A Mater. Sci. Process. 2009, 94, 963–973. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.L.; Fino, D.; Russo, N.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Nope, E.; Sathicq, Á.G.; Martínez, J.J.; Rojas, H.A.; Luque, R.; Romanelli, G.P. Ternary hydrotalcites in the multicomponent synthesis of 4H-pyrans. Catalysts 2020, 10, 70. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef]
- Teixeira, T.P.F.; Aquino, S.F.; Pereira, S.I.; Dias, A. Use of calcined layered double hydroxides for the removal of color and organic matter from textile effluents: Kinetic, equilibrium and recycling studies. Braz. J. Chem. Eng. 2014, 31, 19–26. [Google Scholar] [CrossRef]
- Anbia, M.; Amirmahmoodi, S. Removal of Hg (II) and Mn (II) from aqueous solution using nanoporous carbon impregnated with surfactants. Arab. J. Chem. 2016, 9, S319–S325. [Google Scholar] [CrossRef]
- De Almeida, M.F.; Bellato, C.R.; Mounteer, A.H.; Ferreira, S.O.; Milagres, J.L.; Miranda, L.D.L. Enhanced photocatalytic activity of TiO2-impregnated with MgZnAl mixed oxides obtained from layered double hydroxides for phenol degradation. Appl. Surf. Sci. 2015, 357, 1765–1775. [Google Scholar] [CrossRef]
- Paikaray, S.; Hendry, M.J.; Essilfie-Dughan, J. Controls on arsenate, molybdate, and selenate uptake by hydrotalcite-like layered double hydroxides. Chem. Geol. 2013, 345, 130–138. [Google Scholar] [CrossRef]
- Xu, M.; Iglesia, E.; Apestegu, C.R.; Cosimo, D.I.; Al, E.T. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1−xAl3+x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Alanis, C.; Natividad, R.; Barrera-Diaz, C.; Martínez-Miranda, V.; Prince, J.; Valente, J.S. Photocatalytically enhanced Cr(VI) removal by mixed oxides derived from MeAl (Me: Mg and/or Zn) layered double hydroxides. Appl. Catal. B Environ. 2013, 140–141, 546–551. [Google Scholar] [CrossRef]
- Forano, C. Environmental remediation involving layered double hydroxides. Clay Surf. Fundam. Appl. 2004, 425–458. [Google Scholar]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Vohra, M.S. Selenocyanate (SeCN−) contaminated wastewater treatment using TiO2 photocatalysis: SeCN− complex destruction, intermediates formation, and removal of selenium species. Fresenius Environ. Bull. 2015, 24, 8–11. [Google Scholar]
- Vohra, M.S.; Labaran, B.A. Photocatalytic treatment of mixed selenocyanate and phenol streams: Process modeling, optimization, and kinetics. Environ. Prog. Sustain. Energy 2020, 39, e13401. [Google Scholar] [CrossRef]
- You, Y.; Vance, G.F.; Zhao, H. Selenium adsorption on Mg-Al and Zn-Al layered double hydroxides. Appl. Clay Sci. 2001, 20, 13–25. [Google Scholar] [CrossRef]
- Isaacs-Paez, E.D.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Martinez-Rosales, J.M.; Flores-Cano, J.V. Adsorption of boron on calcined AlMg layered double hydroxide from aqueous solutions. Mechanism and effect of operating conditions. Chem. Eng. J. 2014, 245, 248–257. [Google Scholar] [CrossRef]
- Raissi, S.; Farsani, R.E. Statistical process optimization Through multi-response surface methodology. World Acad. Sci. Eng. Technol. 2009, 39, 280–284. [Google Scholar]




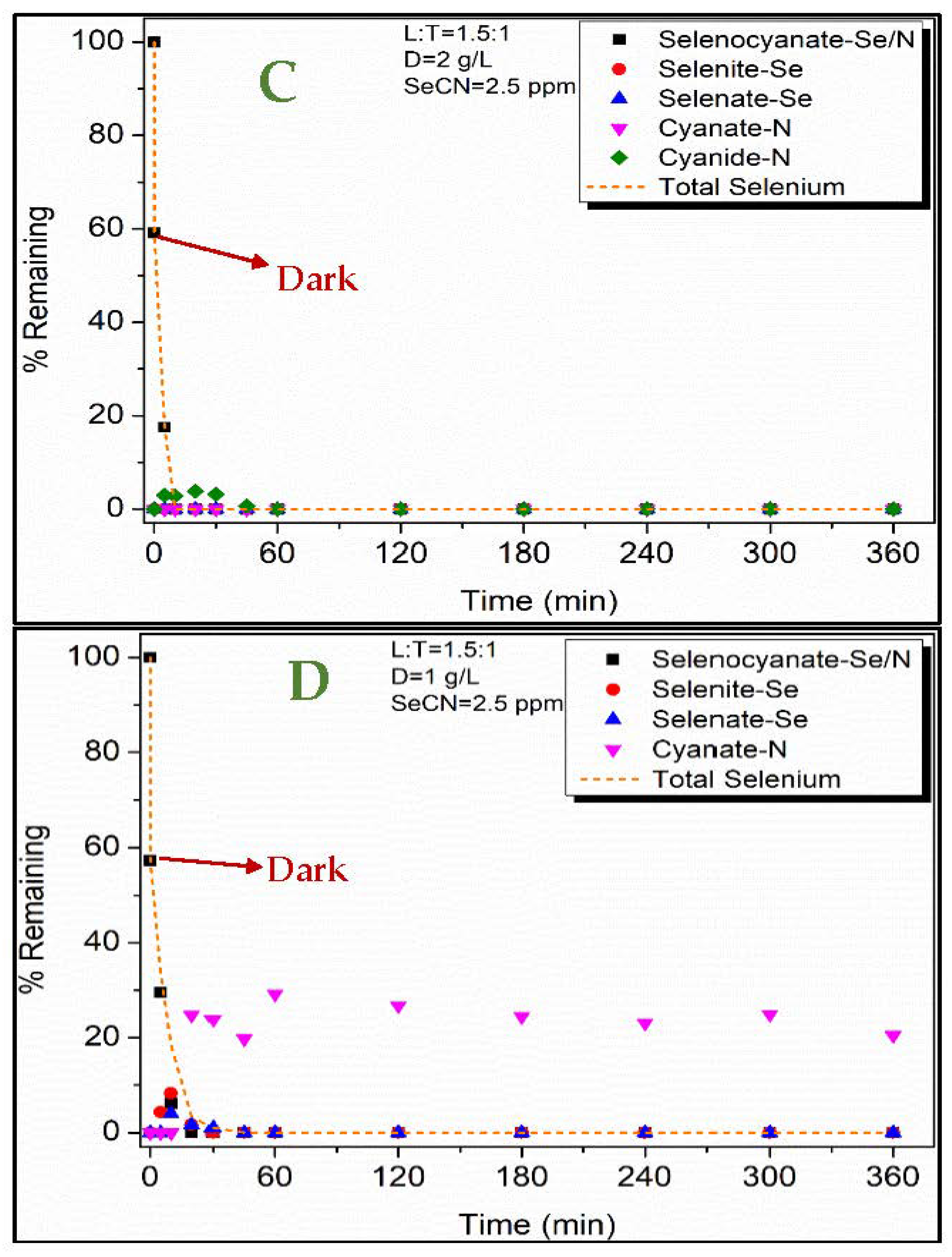
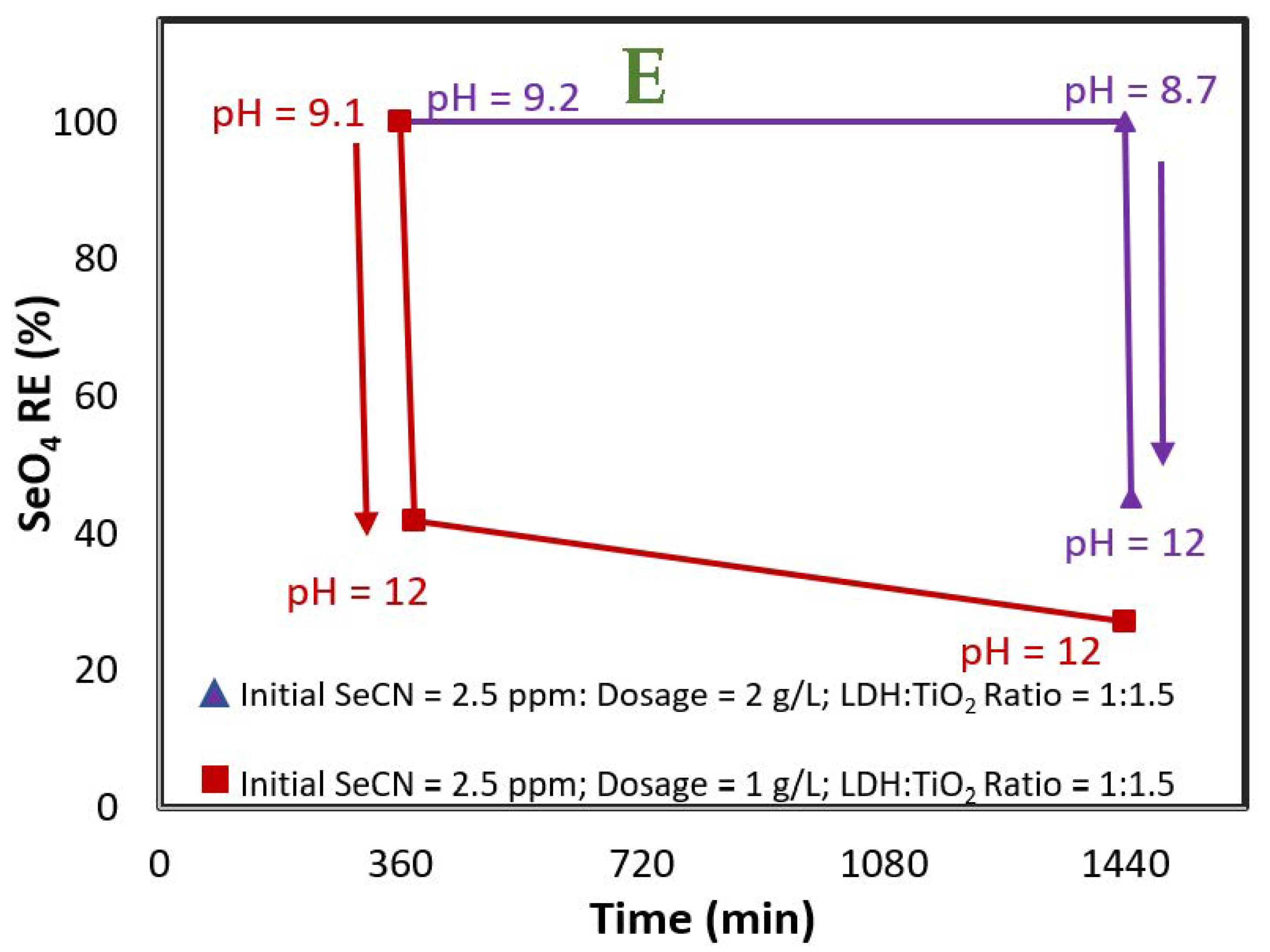
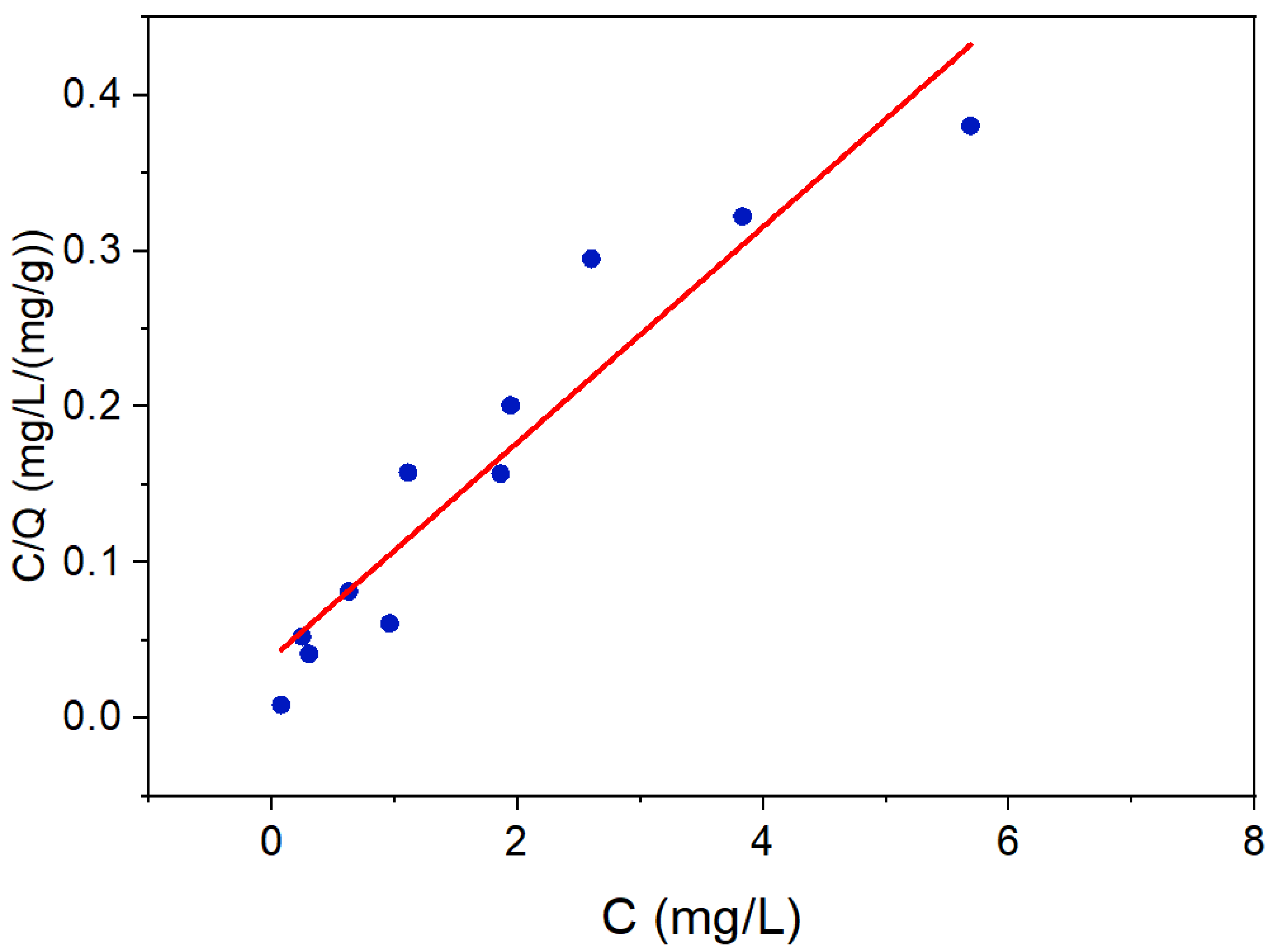


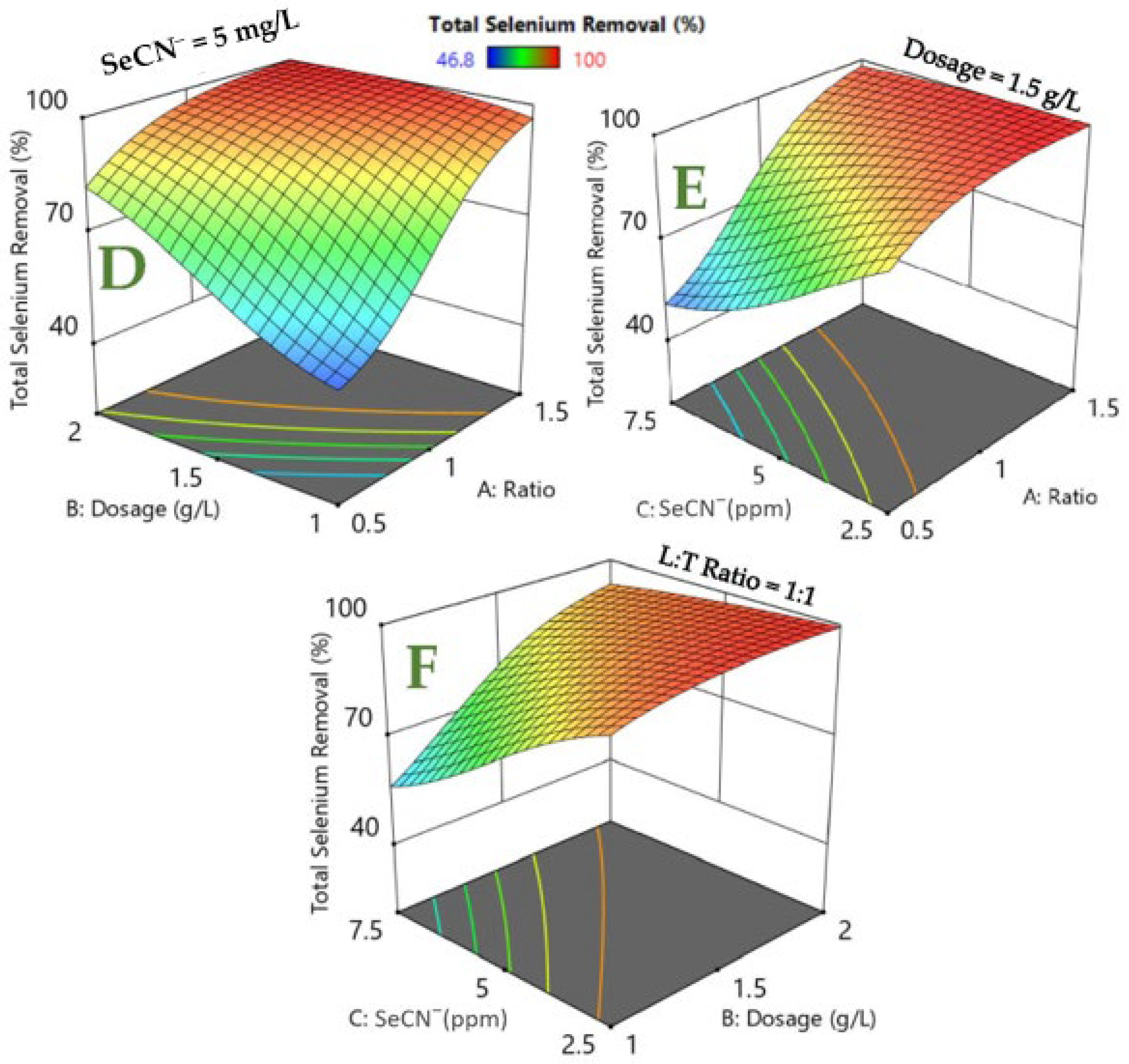
| Factors | Level −1 | Level 0 | Level 1 |
|---|---|---|---|
| A (w/w ratio of LDH:TiO2 matrix) | 0.5 | 1 | 1.5 |
| B (dosage of LDH:TiO2 matrix (g/L)) | 1.0 | 1.50 | 2.0 |
| C (selenocyanate (mg/L)) | 2.50 | 5.0 | 7.50 |
| Exp No. | LDH:TiO2 Ratio | Dosage (g/L) | SeCN− (mg/L) | Residual SeO42− (mg/L) | Total Selenium Removal (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1.5 | 5 | 0.63 | 90.2 |
| 2 | 1 | 2 | 5 | 0.15 | 98 |
| 3 | 1 | 1 | 5 | 1.94 | 71.4 |
| 4 | 1 | 1.5 | 7.5 | 1.86 | 82.7 |
| 5 | 1.5 | 2 | 7.5 | 0.07 | 99.3 |
| 6 | 1.5 | 2 | 2.5 | 0 | ~100 |
| 7 | 0.5 | 1.5 | 5 | 2.6 | 63 |
| 8 | 1.5 | 1 | 7.5 | 0.96 | 90.8 |
| 9 | 0.5 | 1 | 7.5 | 5.69 | 46.8 |
| 10 | 1.5 | 1 | 2.5 | 0 | ~100 |
| 11 | 0.5 | 1 | 2.5 | 1.11 | 67.7 |
| 12 | 1 | 1.5 | 2.5 | 0 | ~100 |
| 13 | 1.5 | 1.5 | 5 | 0 | ~100 |
| 14 | 0.5 | 2 | 7.5 | 3.43 | 67.6 |
| 15 | 0.5 | 2 | 2.5 | 0.25 | 92.8 |
| Element | Weight% | Atomic% | Mg/Al Ratio |
|---|---|---|---|
| C | 5.56 | 7.9 | - |
| N | 5.27 | 6.4 | - |
| O | 64.57 | 68.9 | - |
| Mg | 18.55 | 13.0 | 3.4 |
| Al | 6.05 | 3.8 | |
| Totals | 100.00 | - | - |
| Models | Significance Values for the Model Terms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | A: L:T Ratio | B: Dosage | C: SeCN− | AB | AC | BC | A2 | B2 | C2 | |
| RS | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.021 | <0.0001 | 0.0187 | 0.0115 | -- | -- |
| TS | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | 0.0017 | 0.0035 | 0.0468 | 0.0512 | -- |
| Statistic | RS Model | TS Model |
|---|---|---|
| R2 | 0.9868 | 0.9917 |
| Adjusted R2 | 0.9736 | 0.9806 |
| Predicted R2 | 0.9265 | 0.9188 |
| Adequate Precision | 29.99 | 34.46 |
| Name | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| A: LDH:TiO2 | within range | 0.5 | 1.5 |
| B:Dose (g/L) | Reduce | 1 | 2 |
| C: Selenocyanate (mg/L) | 7.5 | 2.5 | 7.5 |
| Selenate remaining (mg/L) | reduce | 0 | 5.7 |
| Removal of selenate (percentage) | increase | 47 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussaini, M.; Vohra, M. LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling. Nanomaterials 2022, 12, 2035. https://doi.org/10.3390/nano12122035
Hussaini M, Vohra M. LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling. Nanomaterials. 2022; 12(12):2035. https://doi.org/10.3390/nano12122035
Chicago/Turabian StyleHussaini, Minaam, and Muhammad Vohra. 2022. "LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling" Nanomaterials 12, no. 12: 2035. https://doi.org/10.3390/nano12122035
APA StyleHussaini, M., & Vohra, M. (2022). LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling. Nanomaterials, 12(12), 2035. https://doi.org/10.3390/nano12122035






