Buffer Components Incorporate into the Framework of Polyserotonin Nanoparticles and Films during Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Polyserotonin (PSe) Nanoparticles and Films
2.2. Characterization of PSe Nanoparticles and Films
2.3. Density Functional Theory (DFT) Calculations of PSeNP
3. Results and Discussion
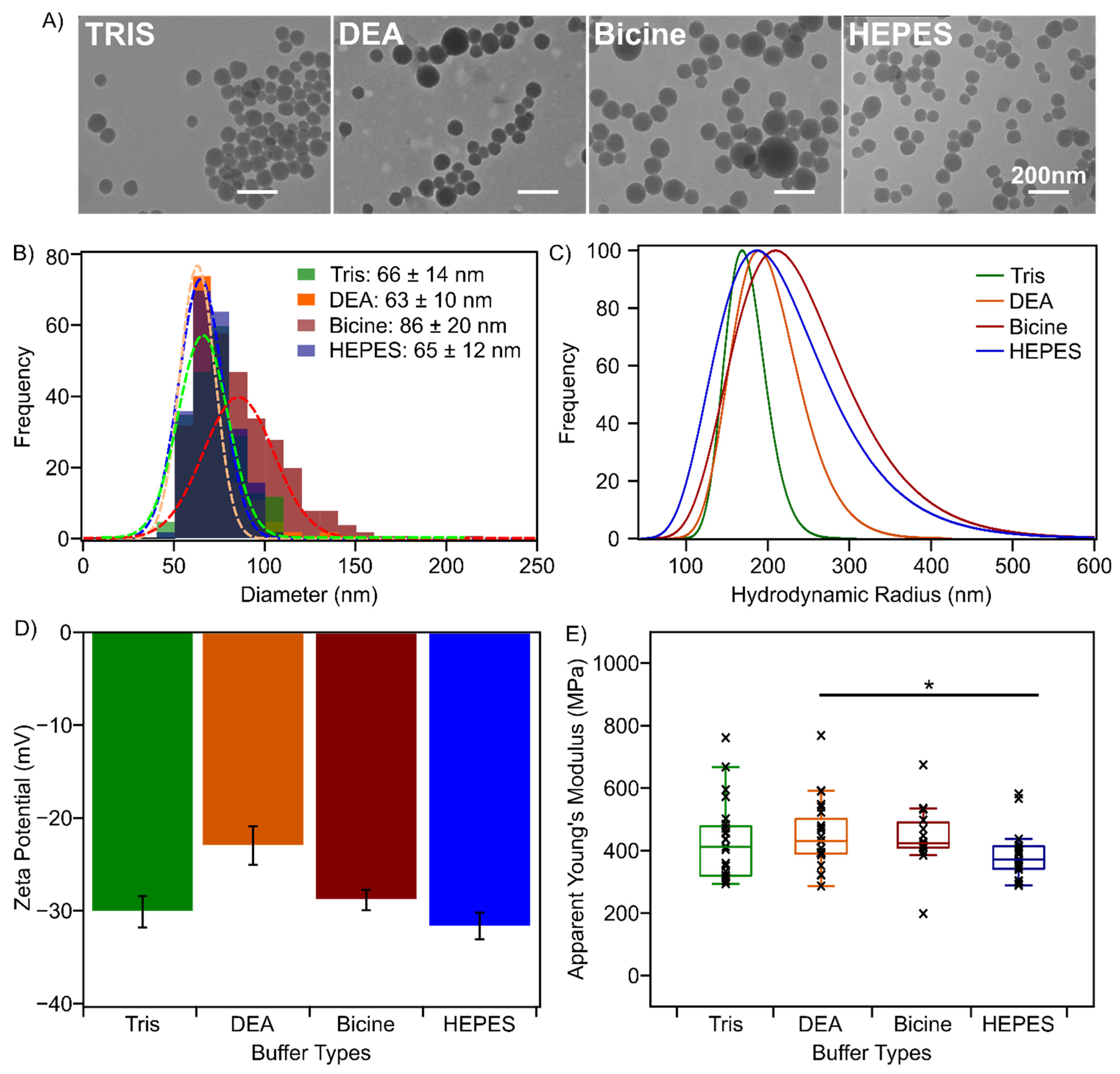
3.1. Buffer Components Impact PSeNP Size but Not Their Nanomechanical Properties
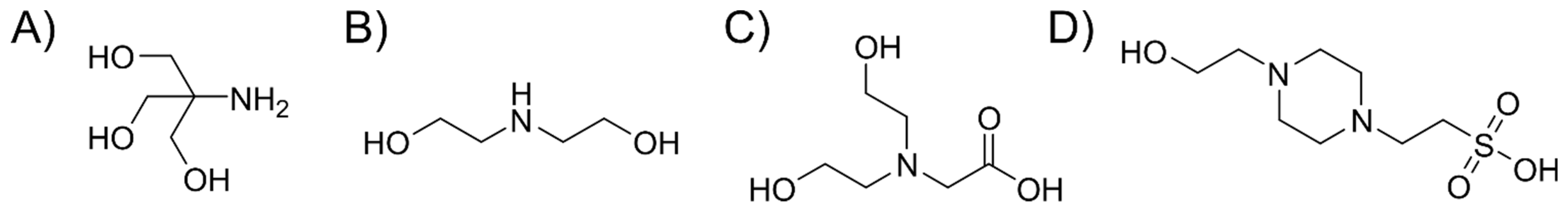
3.2. Buffer Components Can Become Incorporated into the Nanoparticle Framework
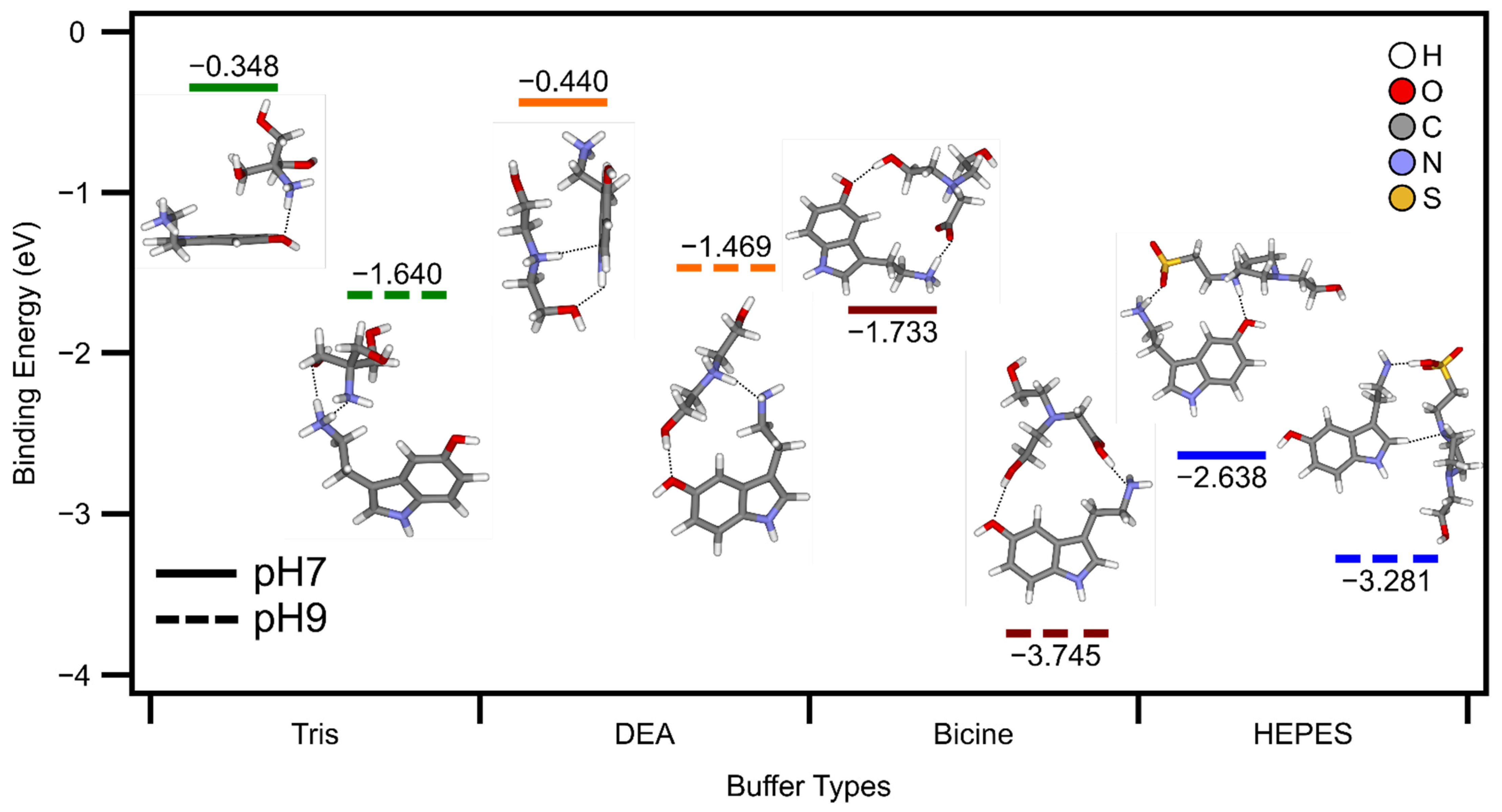
3.3. Density Functional Theory Calculations Support Incorporation of Buffer Molecules into the Nanoparticle Framework
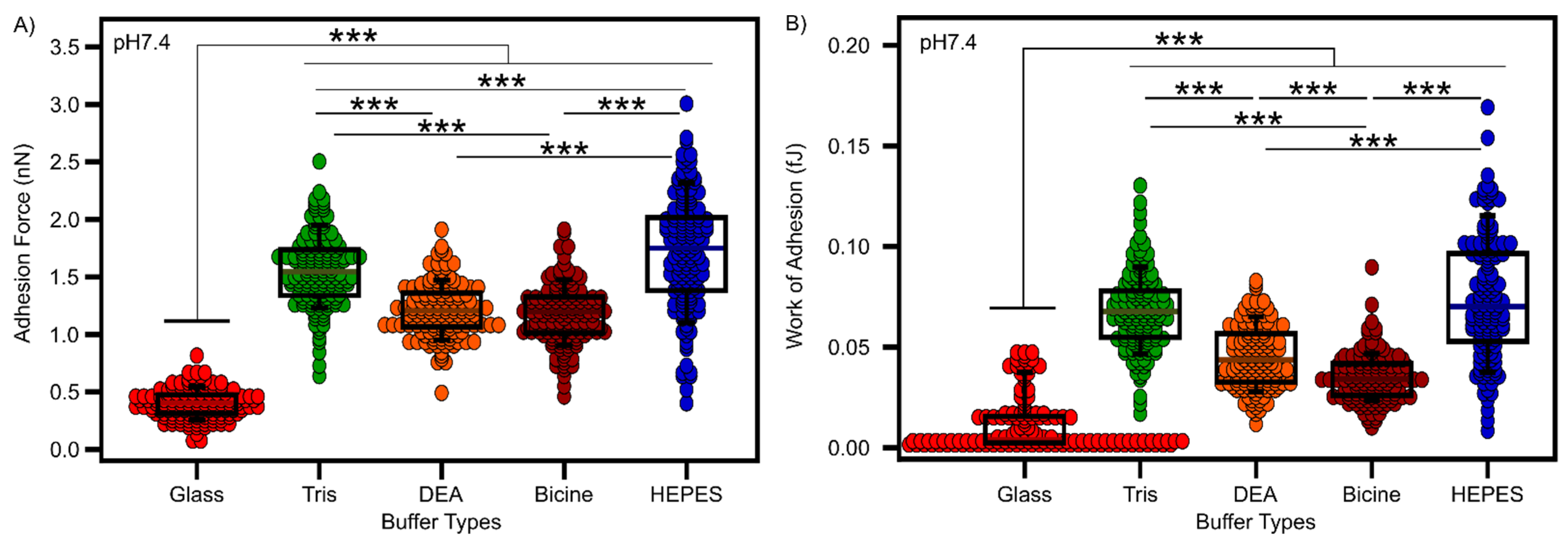
3.4. Characterization of Polyserotonin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zmerli, I.; Michel, J.; Makky, A. Multifunctional polydopamine-based nanoparticles: Synthesis, physico-chemical properties and applications for bimodal photothermal/photodynamic therapy of cancer. Multifunct. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Li, X.N.; Yeh, Y.C.; Giri, K.; Mout, R.; Landis, R.F.; Prakash, Y.S.; Rotello, V.M. Control of nanoparticle penetration into biofilms through surface design. Chem. Commun. 2015, 51, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Zelmer, C.; Zweifel, L.P.; Kapinos, L.E.; Craciun, I.; Guven, Z.P.; Palivan, C.G.; Lim, R.Y.H. Organelle-specific targeting of polymersomes into the cell nucleus. Proc. Natl. Acad. Sci. USA 2020, 117, 2770–2778. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gong, C.; Yuan, X.; Wei, G. Controlling the Self-Assembly of Biomolecules into Functional Nanomaterials through Internal Interactions and External Stimulations: A Review. Nanomaterials 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.F.; Fang, F.; Li, L.; Zhang, J.F. Self-Assembled Organic Nanomaterials for Drug Delivery, Bioimaging, and Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4816–4833. [Google Scholar] [CrossRef]
- Ball, V.; Del Frari, D.; Toniazzo, V.; Ruch, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: Insights in the polydopamine deposition mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef]
- Jiang, X.L.; Wang, Y.L.; Li, M.G. Selecting water-alcohol mixed solvent for synthesis of polydopamine nano-spheres using solubility parameter. Sci. Rep. 2014, 4, 6070. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Luchini, A.; Napolitano, A.; D’Errico, G.; Vitiello, G.; Szekely, N.; d’Ischia, M.; Paduano, L. Tris Buffer Modulates Polydopamine Growth, Aggregation, and Paramagnetic Properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef]
- Guo, P.; Liu, D.X.; Subramanyam, K.; Wang, B.R.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Song, C.; Ma, G.H.; Wei, W. Mechanical determination of particle-cell interactions and the associated biomedical applications. J. Mater. Chem. B 2018, 6, 7129–7143. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Hasani-Sadrabadi, M.M.; Cheung, K.M.; Young, T.D.; Bahlakeh, G.; Moshaverinia, A.; Weiss, P.S.; Andrews, A.M. Polyserotonin Nanoparticles as Multifunctional Materials for Biomedical Applications. ACS Nano 2018, 12, 4761–4774. [Google Scholar] [CrossRef]
- Gomaa, A. Investigating the Influence of Blood Plasma on Ultra-Thin Polydopamine Films; Universitat Ulm: Ulm, Germany, 2019. [Google Scholar]
- Jeon, K.; Andoy, N.M.O.; Schmitt, C.W.; Xue, Y.L.; Barner, L.; Sullan, R.M.A. Size-controlled synthesis of bioinspired polyserotonin nanoparticles with free radical scavenging activity. J. Mater. Chem. B 2021, 9, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.C.; Zhu, J.J.; Ding, J.S.; Zhou, W.H. Polyserotonin as a versatile coating with pH-responsive degradation for anti-tumor therapy. Chem. Commun. 2022, 58, 6713–6716. [Google Scholar] [CrossRef]
- Kreis, C.T.; Sullan, R.M.A. Interfacial nanomechanical heterogeneity of the E. coli biofilm matrix. Nanoscale 2020, 12, 16819–16830. [Google Scholar] [CrossRef]
- Zimmermann, J.L.; Nicolaus, T.; Neuert, G.; Blank, K. Thiol-based, site-specific and covalent immobilization of biomolecules for single-molecule experiments. Nat. Protoc. 2010, 5, 975–985. [Google Scholar] [CrossRef]
- Linkov, P.; Artemyev, M.; Efimov, A.E.; Nabiev, I. Comparative advantages and limitations of the basic metrology methods applied to the characterization of nanomaterials. Nanoscale 2013, 5, 8781–8798. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Zhang, M.W.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanopartides Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Beningo, K.A.; Wang, Y.L. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002, 115, 849–856. [Google Scholar] [CrossRef]
- Eshaghi, B.; Alsharif, N.; An, X.D.; Akiyama, H.; Brown, K.A.; Gummuluru, S.; Reinhard, B.M. Stiffness of HIV-1 Mimicking Polymer Nanoparticles Modulates Ganglioside-Mediated Cellular Uptake and Trafficking. Adv. Sci. 2020, 7, 2000649. [Google Scholar] [CrossRef]
- Zmerli, I.; Michel, J.P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef]
- Mostert, A.B.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Powell, B.J.; Meredith, P. Hydration-controlled X-band EPR spectroscopy: A tool for unravelling the complexities of the solid-state free radical in eumelanin. J. Phys. Chem. B 2013, 117, 4965–4972. [Google Scholar] [CrossRef]
- Kiratitanavit, W.; Bruno, F.F.; Xia, Z.Y.; Yu, S.R.; Kumar, J.; Nagarajan, R. Biocatalytic Synthesis of Fluorescent Conjugated Polyserotonin. J. Renew. Mater. 2019, 7, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef]
- Pratuangdejkul, J.; Nosoongnoen, W.; Guerin, G.A.; Loric, S.; Conti, M.; Launay, J.M.; Manivet, P. Conformational dependence of serotonin theoretical pK(a) prediction. Chem. Phys. Lett. 2006, 420, 538–544. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Bourmaud, A.; Riviere, J.; Le Duigou, A.; Raj, G.; Baley, C. Investigations of the use of a mussel-inspired compatibilizer to improve the matrix-fiber adhesion of a biocomposite. Polym. Test. 2009, 28, 668–672. [Google Scholar] [CrossRef]
- Jiang, J.H.; Zhu, L.P.; Zhu, L.J.; Zhu, B.K.; Xu, Y.Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hydrophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, K.; Asuncion, J.A.; Corbett, A.L.; Yuan, T.; Patel, M.; Andoy, N.M.O.; Kreis, C.T.; Voznyy, O.; Sullan, R.M.A. Buffer Components Incorporate into the Framework of Polyserotonin Nanoparticles and Films during Synthesis. Nanomaterials 2022, 12, 2027. https://doi.org/10.3390/nano12122027
Jeon K, Asuncion JA, Corbett AL, Yuan T, Patel M, Andoy NMO, Kreis CT, Voznyy O, Sullan RMA. Buffer Components Incorporate into the Framework of Polyserotonin Nanoparticles and Films during Synthesis. Nanomaterials. 2022; 12(12):2027. https://doi.org/10.3390/nano12122027
Chicago/Turabian StyleJeon, Keuna, Justin Andrei Asuncion, Alexander Lucien Corbett, Tiange Yuan, Meera Patel, Nesha May Octavio Andoy, Christian Titus Kreis, Oleksandr Voznyy, and Ruby May Arana Sullan. 2022. "Buffer Components Incorporate into the Framework of Polyserotonin Nanoparticles and Films during Synthesis" Nanomaterials 12, no. 12: 2027. https://doi.org/10.3390/nano12122027
APA StyleJeon, K., Asuncion, J. A., Corbett, A. L., Yuan, T., Patel, M., Andoy, N. M. O., Kreis, C. T., Voznyy, O., & Sullan, R. M. A. (2022). Buffer Components Incorporate into the Framework of Polyserotonin Nanoparticles and Films during Synthesis. Nanomaterials, 12(12), 2027. https://doi.org/10.3390/nano12122027






