Hansen Solubility Parameter Analysis on Dispersion of Oleylamine-Capped Silver Nanoinks and their Sintered Film Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
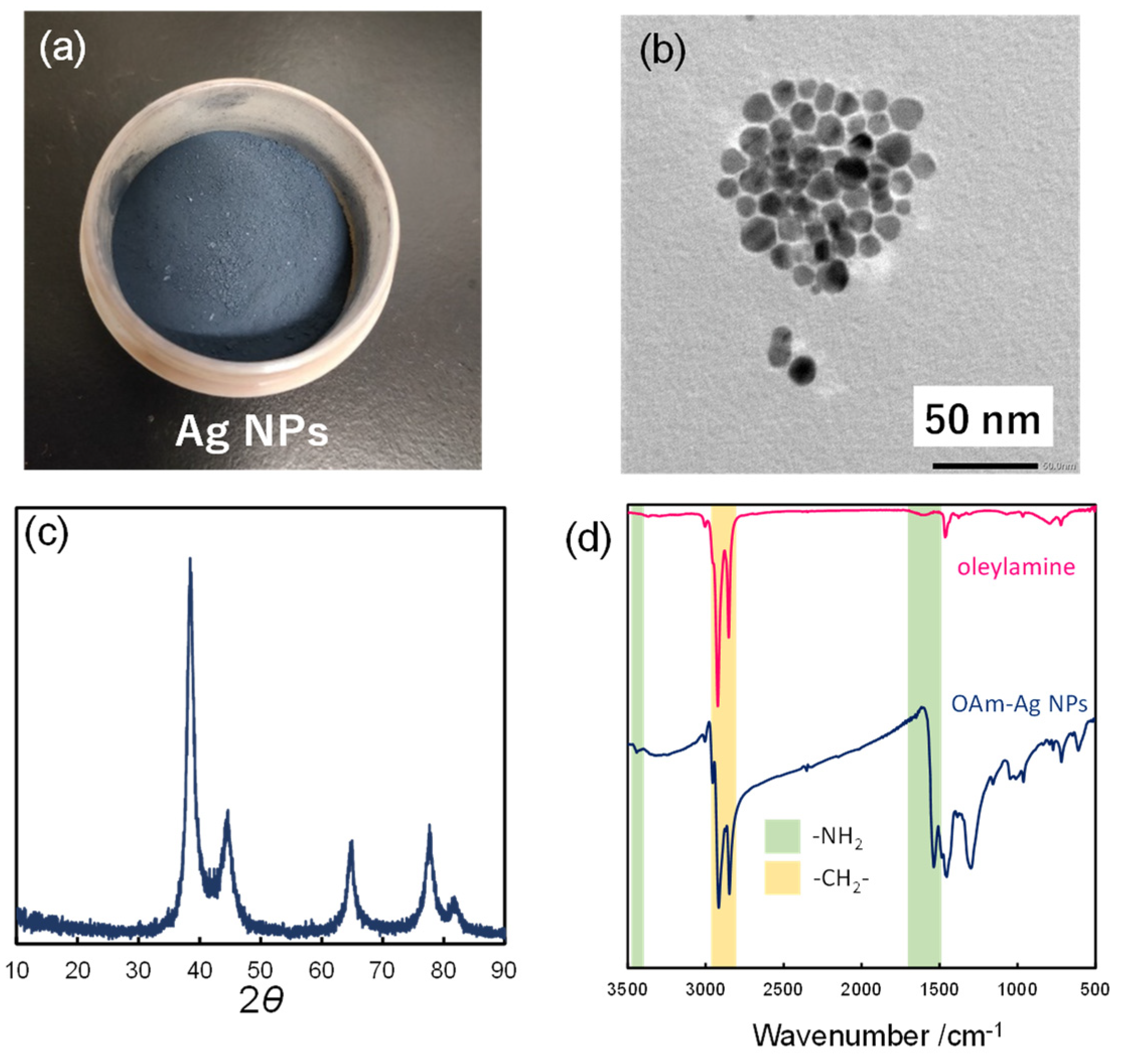
2.2. Synthesis of OAm-Ag NPs
2.3. Characterization of OAm-Ag NPs
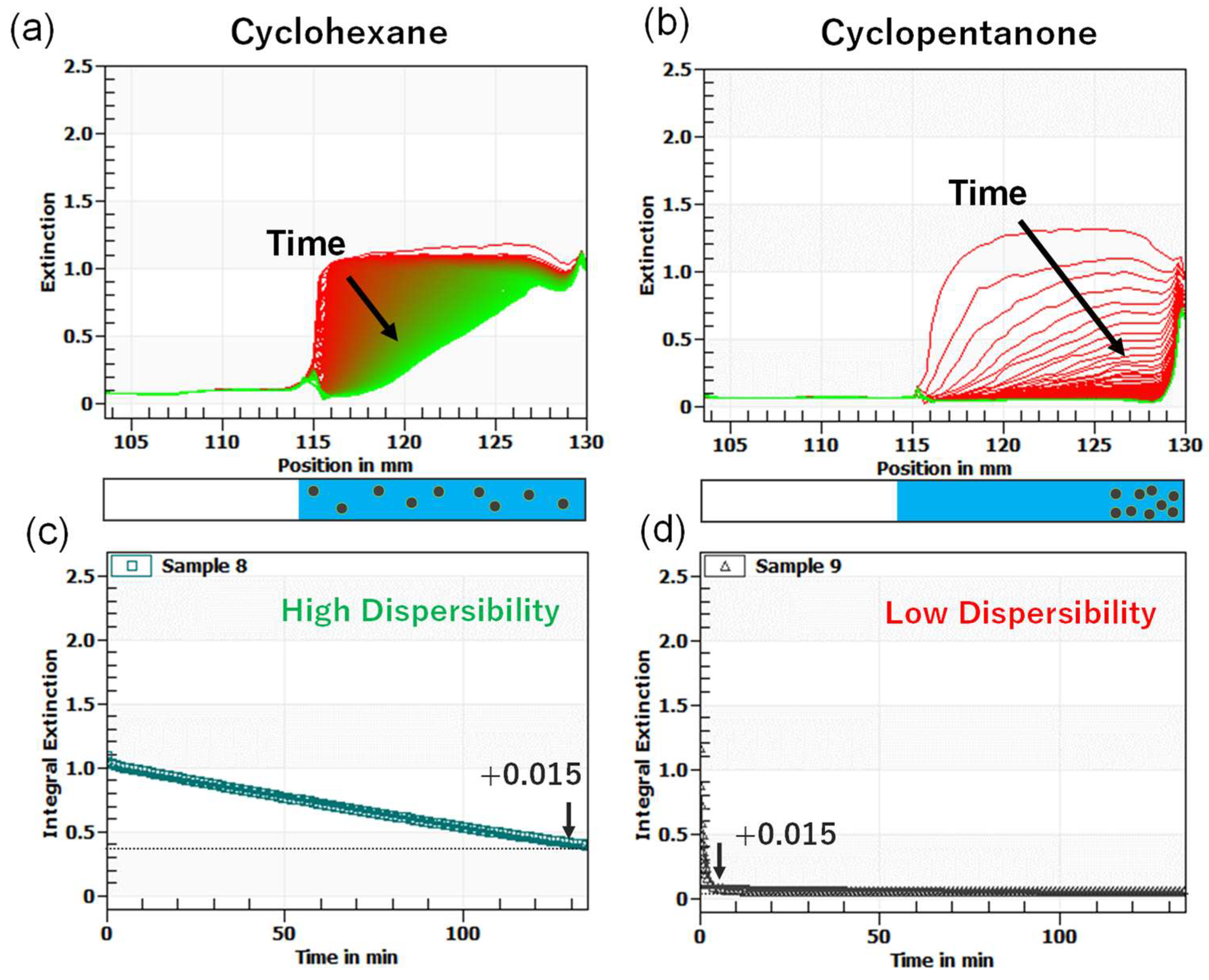
2.4. Dispersibility Evaluation of Ag NPs in Solvents by Sedimentation Experiments under Centrifugal Acceleration Field
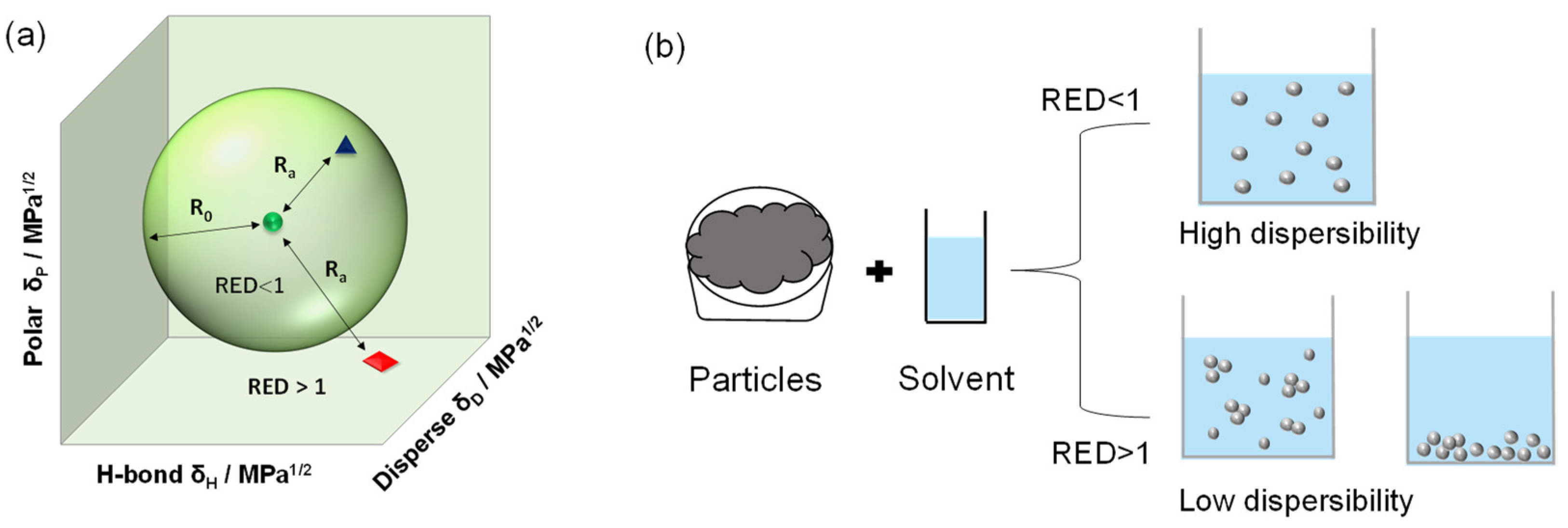
2.5. Estimation of Hansen Solubility Sphere from Particle Dispersibility
2.6. Preparation of OAm-Ag NP Inks and Sintered Ag Films from the Inks
3. Results and Discussion
3.1. Characterization of OAm-Ag NPs
3.2. Dispersibility of OAm-Ag NPs in Different Solvents
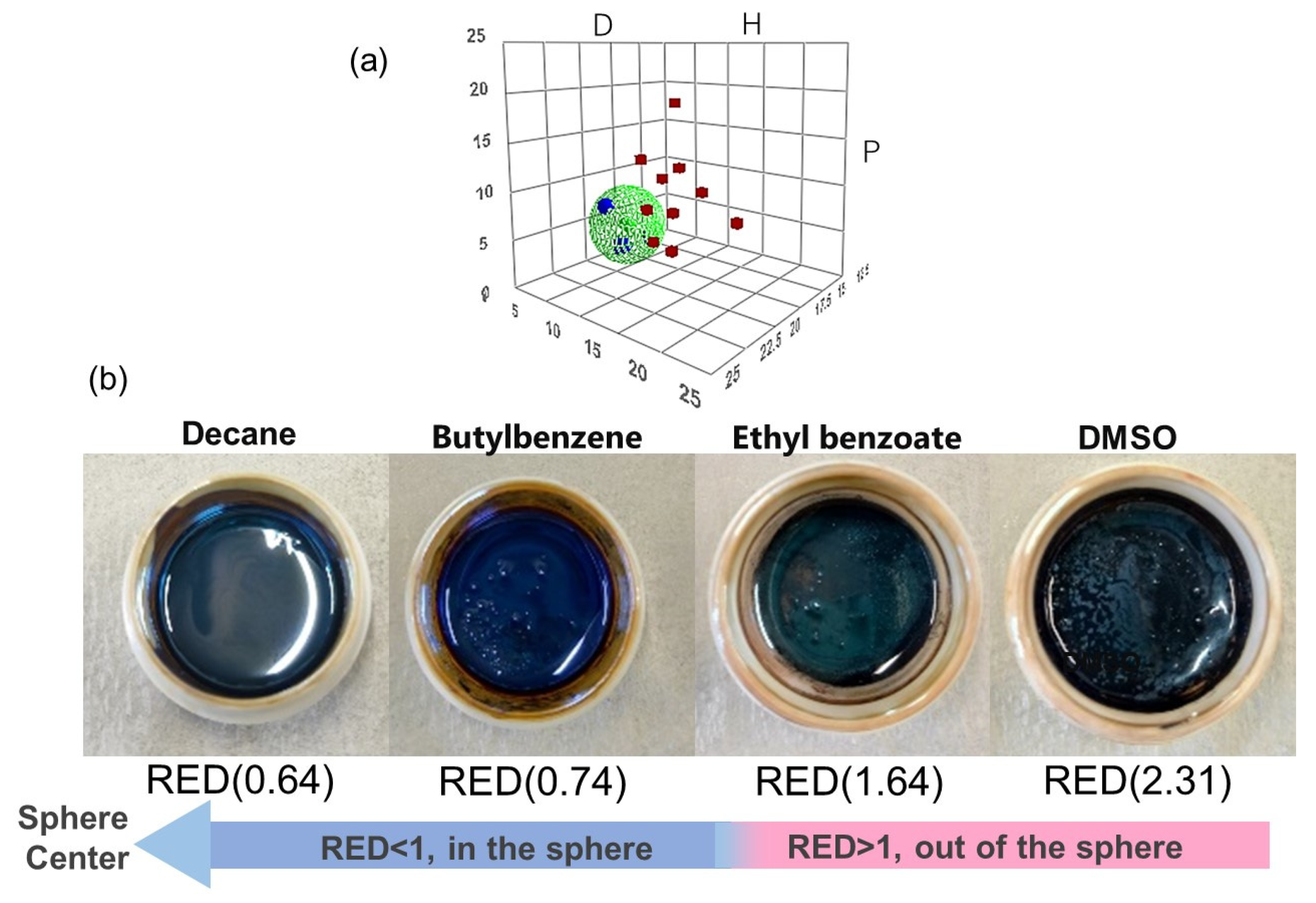
3.3. HSP Determination of OAm-Ag NPs Based on the HSP Sphere
3.4. Verification of Predictive Dispersibilities of OAm-Ag NP Inks Based on the HSP Spheres
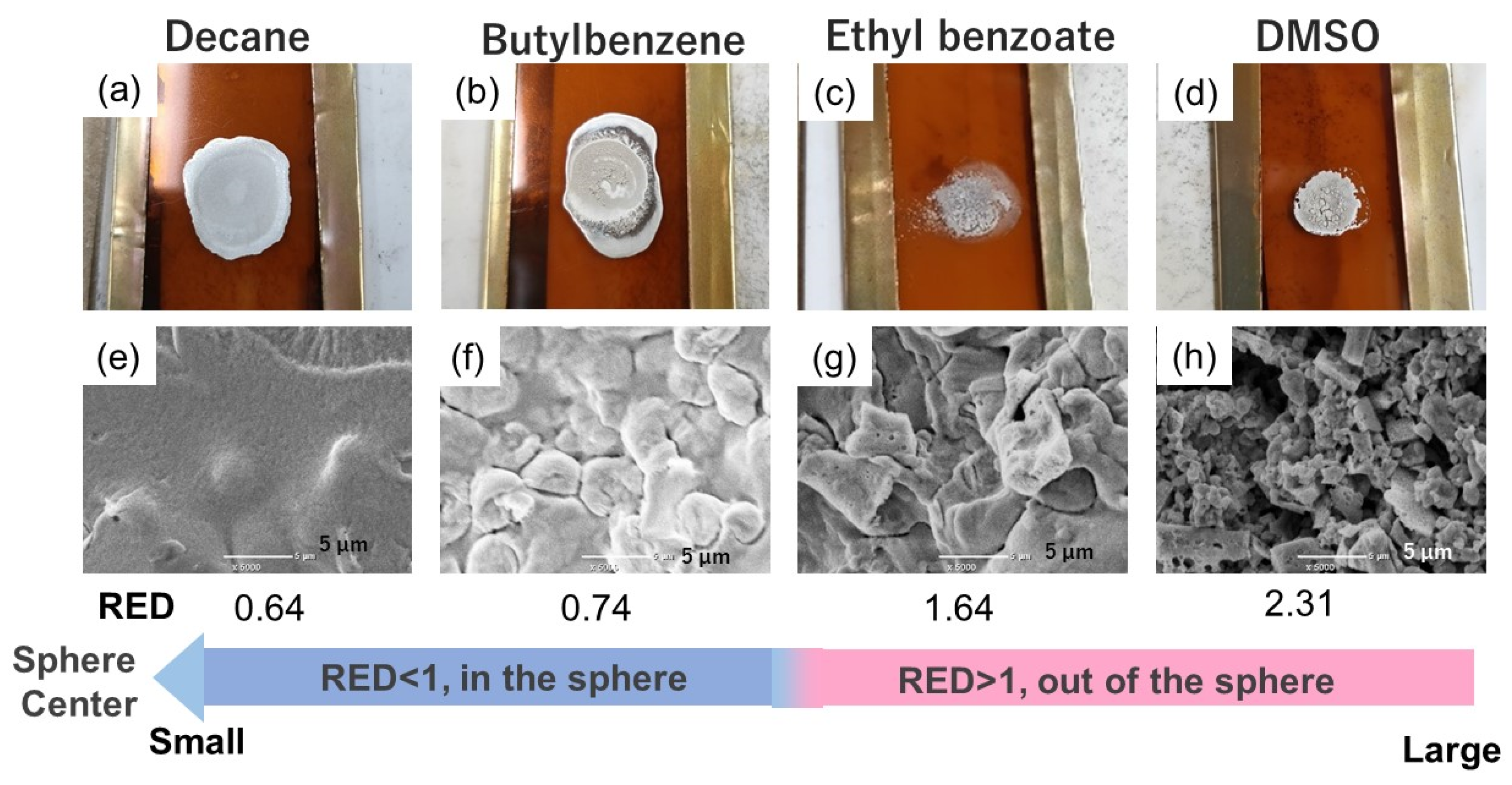
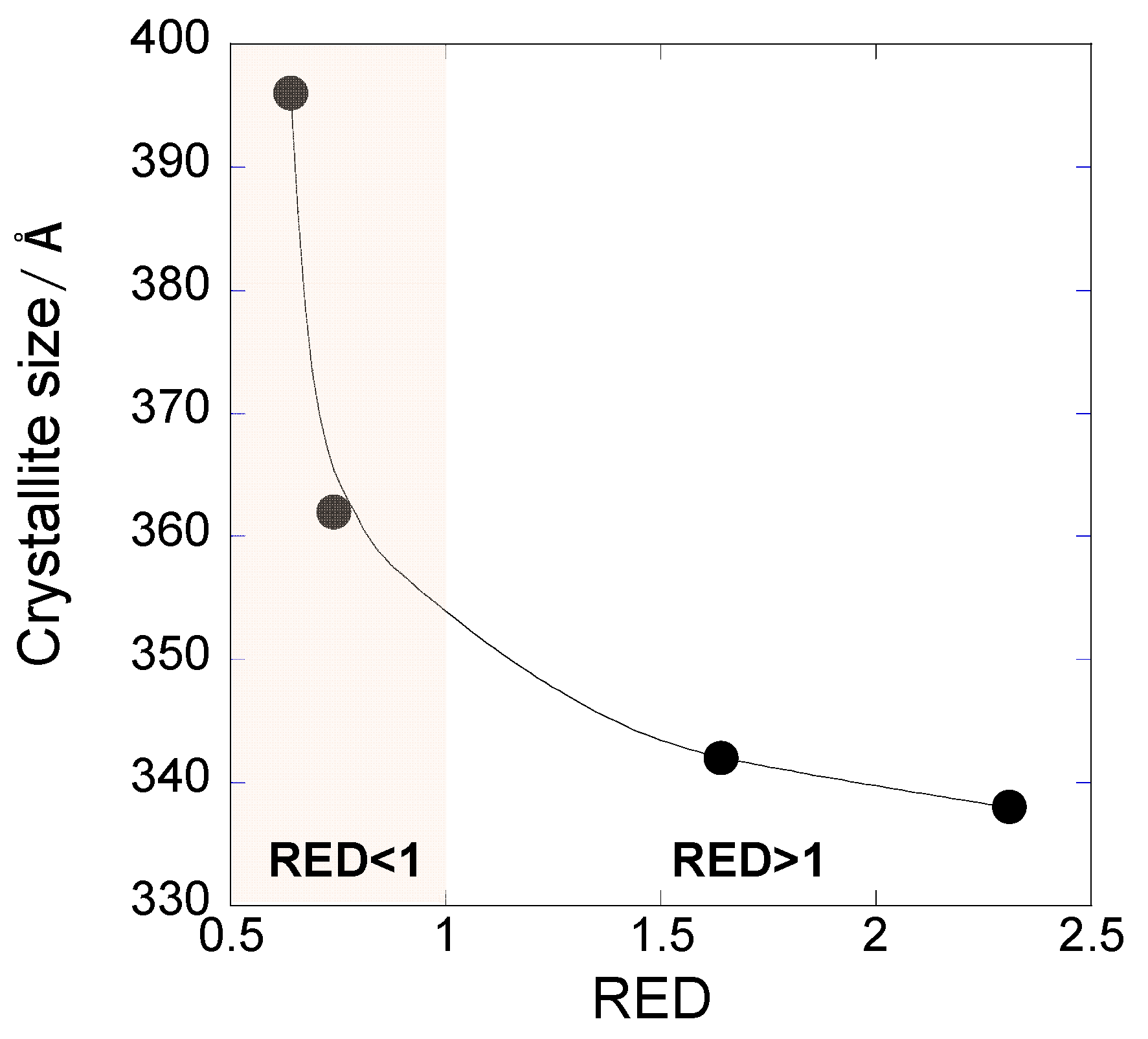
3.5. Verification of Predictive Sintered Film Morphology from OAm-Ag NP Inks Based on the HSP Spheres
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.H. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Kawawaki, T.; Negishi, Y.; Kawasaki, H. Photo/electrocatalysis and photosensitization using metal nanoclusters for green energy and medical applications. Nanoscale Adv. 2020, 2, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Gao, F.; Guo, S.; Zhang, Y.; Li, S.; You, H.; Du, T. A review of the role and mechanism of surfactants in the morphology control of metal nanoparticles. Nanoscale 2021, 13, 3895–3910. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, J.; Karakoç, A.; Palko, T.; Yiğitler, H.; Ruttik, K.; Jäntti, R.; Paltakari, J. A Review on Printed Electronics: Fabrication Methods, Inks, Substrates, Applications and Environmental Impacts. J. Manuf. Mater. Process. 2021, 5, 89. [Google Scholar] [CrossRef]
- Tomotoshi, D.; Oogami, R.; Kawasaki, H. Highly Conductive, Flexible, and Oxidation-Resistant Cu-Ni Electrodes Produced from Hybrid Inks at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 20906–20915. [Google Scholar] [CrossRef]
- Tomotoshi, D.; Kawasaki, H. Surface and Interface Designs in Copper-Based Conductive Inks for Printed/Flexible Electronics. Nanomaterials 2020, 10, 1689. [Google Scholar] [CrossRef]
- Agate, S.; Joyce, M.; Lucia, L.; Pal, L. Cellulose and nanocellulose-based flexible hybrid printed electronics and conductive composites—A review. Carbohydr. Polym. 2018, 198, 249–260. [Google Scholar] [CrossRef]
- Secor, E.B.; Hersam, M.C. Emerging Carbon and Post-Carbon Nanomaterial Inks for Printed Electronics. J. Phys. Chem. Lett. 2015, 6, 620–626. [Google Scholar] [CrossRef]
- Jeong, S.; Song, H.C.; Lee, W.W.; Choi, Y.; Ryu, B.-H. Preparation of aqueous Ag Ink with long-term dispersion stability and its inkjet printing for fabricating conductive tracks on a polyimide film. J. Appl. Phys. 2010, 108, 102805. [Google Scholar] [CrossRef]
- Khandavalli, S.; Park, J.H.; Kariuki, N.N.; Myers, D.J.; Stickel, J.J.; Hurst, K.; Neyerlin, K.C.; Ulsh, M.; Mauger, S.A. Rheological Investigation on the Microstructure of Fuel Cell Catalyst Inks. ACS Appl. Mater. Interfaces 2018, 10, 43610–43622. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive Nanomaterials for Printed Electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Vincent, B. The effect of adsorbed polymers on dispersion stability. Adv. Colloid Interface Sci. 1974, 4, 192–277. [Google Scholar] [CrossRef]
- Overbeek, J.T.G. Recent developments in the understanding of colloid stability. J. Colloid Interface Sci. 1977, 58, 408–422. [Google Scholar] [CrossRef]
- Hansen, C.M. The Three-Dimensional Solubility Parameter—Key to Paint Component Affinities: Solvents, Plasticizers, Polymers, and Resins. II. Dyes, Emulsifiers, Mutual Solubility and Compatibility, and Pigments. III. Independent Calculation of the Parameter Components. J. Paint Technol. 1967, 39, 505–510. Available online: https://www.hansen-solubility.com/contents/HSP1967-OCR.pdf (accessed on 1 January 2022).
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group Oxfordshire: Abingdon, UK, 2007. [Google Scholar] [CrossRef]
- Lerche, D. Dispersion Stability and Particle Characterization by Sedimentation Kinetics in a Centrifugal Field. J. Dispers. Sci. Technol. 2002, 23, 699–709. [Google Scholar] [CrossRef]
- Wang, S.H.; Liu, J.H.; Pai, C.T.; Chen, C.W.; Chung, P.T.; Chiang, A.S.T.; Chang, S.J. Hansen Solubility Parameter Analysis on the Dispersion of Zirconia Nanocrystals. J. Colloid Interface Sci. 2013, 407, 140–147. [Google Scholar] [CrossRef]
- Petersen, J.B.; Meruga, J.; Randle, J.S.; Cross, W.M.; Kellar, J.J. Hansen Solubility Parameters of Surfactant-Capped Silver Nanoparticles for Ink and Printing Technologies. Langmuir 2014, 30, 15514–15519. [Google Scholar] [CrossRef]
- Süß, S.; Sobisch, T.; Peukert, W.; Lerche, D.; Segets, D. Determination of Hansen Parameters for Particles: A Standardized Routine Based on Analytical Centrifugation. Adv. Powder Technol. 2018, 29, 1550–1561. [Google Scholar] [CrossRef]
- Stauch, C.; Süß, S.; Luxenhofer, R.; Binks, B.P.; Segets, D.; Mandel, K. Quantifying Surface Properties of Silica Particles by Combining Hansen Parameters and Reichardt’s Dye Indicator Data. Part. Part. Syst. Charact. 2018, 35, 1800328. [Google Scholar] [CrossRef]
- Fujiwara, N.; Imai, S.; Yamamoto, H. Evaluation of the Influence of Fine Particle Surface Modification with the Hansen Solubility Parameters. Mater. Chem. Phys. 2019, 229, 139–148. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Kondo, K.; Kato, Y.; Fujiwara, N.; Yamamoto, H. Determination of Hansen Solubility Parameters of Particles Using a Capillary Penetration Method. Chem. Phys. 2019, 521, 115–122. [Google Scholar] [CrossRef]
- Süß, S.; Lin, W.; Getmanenko, O.; Pflug, L.; Sobisch, T.; Peukert, W.; Lerche, D.; Segets, D. Suspension- and Powder-Based Derivation of Hansen Dispersibility Parameters for Zinc Oxide Quantum Dots. Particuology 2019, 44, 71–79. [Google Scholar] [CrossRef]
- Lerche, D. Comprehensive Characterization of Nano-and Microparticles by in-Situ Visualization of Particle Movement Using Advanced Sedimentation Techniques. KONA Powder Part. J. 2019, 36, 156–186. [Google Scholar] [CrossRef] [Green Version]
- Tomai, T.; Tajima, N.; Kimura, M.; Yoko, A.; Seong, G.; Adschiri, T. Solvent Accommodation Effect on Dispersibility of Metal Oxide Nanoparticle with Chemisorbed Organic Shell. J. Colloid Interface Sci. 2021, 587, 574–580. [Google Scholar] [CrossRef]
- Gilliam, M.S.; Yousaf, A.; Guo, Y.; Li, D.O.; Momenah, A.A.; Wang, Q.H.; Green, A.A. Evaluating the Exfoliation Efficiency of Quasi-2D Metal Diboride Nanosheets Using Hansen Solubility Parameters. Langmuir 2021, 37, 1194–1205. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Xing, S.; Liu, L.; Zou, G.; Zhang, P. Structure Inheritance in Nanoparticle Ink Direct-Writing Processes and Crack-Free Nano-Copper Interconnects Printed by a Single-Run Approach. Materials 2019, 12, 1559. [Google Scholar] [CrossRef] [Green Version]
- Carazzone, J.R.; Martin, C.L.; Cordero, Z.C. Crack Initiation, Propagation, and Arrest in Sintering Powder Aggregates. J. Am. Ceram. Soc. 2020, 103, 4754–4773. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L.A.C. Silver Nanoparticle Conductive Inks: Synthesis, Characterization, and Fabrication of Inkjet-Printed Flexible Electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef]
- Chen, M.; Feng, Y.G.; Wang, X.; Li, T.C.; Zhang, J.Y.; Qian, D.J. Silver Nanoparticles Capped by Oleylamine: Formation, Growth, and Self-Organization. Langmuir 2007, 23, 5296–5304. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Pérez-Mirabet, L.; Solano, E.; Martínez-Julián, F.; Guzmán, R.; Arbiol, J.; Puig, T.; Obradors, X.; Pomar, A.; Yáñez, R.; Ros, J.; et al. One-Pot Synthesis of Stable Colloidal Solutions of MFe2O4 Nanoparticles Using Oleylamine as Solvent and Stabilizer. Mater. Res. Bull. 2013, 48, 966–972. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Sun, X.; Zhu, W.; Li, X.Z.; Sellmyer, D.J.; Sun, S. Monodisperse MPt (M = Fe, Co, Ni, Cu, Zn) Nanoparticles Prepared from a Facile Oleylamine Reduction of Metal Salts. Nano Lett. 2014, 14, 2778–2782. [Google Scholar] [CrossRef] [Green Version]
- Togashi, T.; Nakayama, M.; Hashimoto, A.; Ishizaki, M.; Kanaizuka, K.; Kurihara, M. Solvent-Free Synthesis of Monodisperse Cu Nanoparticles by Thermal Decomposition of an Oleylamine-Coordinated Cu Oxalate Complex. Dalton Trans. 2018, 47, 5342–5347. [Google Scholar] [CrossRef]
- Chen, B.; Kong, W.; Wang, N.; Zhu, G.; Wang, F. Oleylamine-Mediated Synthesis of Small NaYbF4 Nanoparticles with Tunable Size. Chem. Mater. 2019, 31, 4779–4786. [Google Scholar] [CrossRef]
- Baranov, D.; Lynch, M.J.; Curtis, A.C.; Carollo, A.R.; Douglass, C.R.; Mateo-Tejada, A.M.; Jonas, D.M. Purification of Oleylamine for Materials Synthesis and Spectroscopic Diagnostics for Trans Isomers. Chem. Mater. 2019, 31, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Kakuta, T.; Nagaoka, M.; Koyama, Y.; Sakamoto, M.; Kawasaki, S.; Umeda, N.; Kurihara, M. Direct transformation into silver nanoparticles via thermal decomposition of oxalate-bridging silver oleylamine complexes. J. Nanosci. Nanotechnol. 2009, 9, 6655–6660. [Google Scholar] [CrossRef]
- Zapka, W. Handbook of Industrial Inkjet Printing: A Full System Approach, Polymeric Nonabsorbing Substrates for Industrial Inkjet Printing Applications; Wiley-VCH: Weinheim, Germany, 2017; pp. 373–390. [Google Scholar] [CrossRef]
- Volkman, S.K.; Yin, S.; Bakhishev, T.; Puntambekar, K.; Subramanian, V.; Toney, M.F. Mechanistic Studies on Sintering of Silver Nanoparticles. Chem. Mater. 2011, 23, 4634–4640. [Google Scholar] [CrossRef]
- Venkatram, S.; Kim, C.; Chandrasekaran, A.; Ramprasad, R. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194. [Google Scholar] [CrossRef]
| Solvent | δd [MPa1/2] | δp [MPa1/2] | δh [MPa1/2] | t* [min] | RSTnor | RED |
|---|---|---|---|---|---|---|
| pentane | 14.5 | 0 | 0 | 86.4 | 1.0 | 0.99 |
| ethylbenzene | 17.8 | 0.6 | 1.4 | 156.0 | 0.63 | 0.77 |
| toluene | 18 | 1.4 | 2 | 114.8 | 0.50 | 0.81 |
| tetrachloroethylene | 18.3 | 5.7 | 0 | 160.6 | 0.43 | 0.99 |
| cyclohexane | 16.8 | 0 | 0.2 | 130.7 | 0.33 | 0.58 |
| diacetone alcohol | 15.8 | 8.2 | 10.8 | 67.5 | 0.056 | 2.5 |
| 1-butanol | 16 | 5.7 | 15.8 | 48.5 | 0.047 | 3.4 |
| ethylbenzoate | 17.9 | 6.2 | 6 | 33.1 | 0.036 | 1.6 |
| acetone | 15.5 | 10.4 | 7 | 3.7 | 0.025 | 2.2 |
| acetonitrile | 15.3 | 18 | 6.1 | 2.7 | 0.019 | 3.5 |
| 1,4-dioxane | 17.5 | 1.8 | 9 | 7.2 | 0.013 | 1.9 |
| cyclopentanone | 17.9 | 11.9 | 5.2 | 7.0 | 0.013 | 2.3 |
| tetrahydrofuran | 16.8 | 5.7 | 8 | 1.5 | 0.008 | 1.8 |
| methylethyl ketone | 16 | 9 | 5.1 | 0.16 | 0.0009 | 1.7 |
| furan | 17 | 1.8 | 5.3 | 0.17 | 0.0003 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saita, S.; Takeda, S.-i.; Kawasaki, H. Hansen Solubility Parameter Analysis on Dispersion of Oleylamine-Capped Silver Nanoinks and their Sintered Film Morphology. Nanomaterials 2022, 12, 2004. https://doi.org/10.3390/nano12122004
Saita S, Takeda S-i, Kawasaki H. Hansen Solubility Parameter Analysis on Dispersion of Oleylamine-Capped Silver Nanoinks and their Sintered Film Morphology. Nanomaterials. 2022; 12(12):2004. https://doi.org/10.3390/nano12122004
Chicago/Turabian StyleSaita, Satoshi, Shin-ichi Takeda, and Hideya Kawasaki. 2022. "Hansen Solubility Parameter Analysis on Dispersion of Oleylamine-Capped Silver Nanoinks and their Sintered Film Morphology" Nanomaterials 12, no. 12: 2004. https://doi.org/10.3390/nano12122004
APA StyleSaita, S., Takeda, S.-i., & Kawasaki, H. (2022). Hansen Solubility Parameter Analysis on Dispersion of Oleylamine-Capped Silver Nanoinks and their Sintered Film Morphology. Nanomaterials, 12(12), 2004. https://doi.org/10.3390/nano12122004







